10 Spinal Cord
The spinal cord is pretty small, but it’s important out of proportion to its size. It’s the home of all the motor neurons that work your body, and of a large percentage of the autonomic motor neurons as well. It’s also the recipient of nearly all the sensory information taken in by your body. Beyond that, many of the organizing principles of spinal cord reflexes and pathways apply to other parts of the CNS.
The Spinal Cord Is Segmented
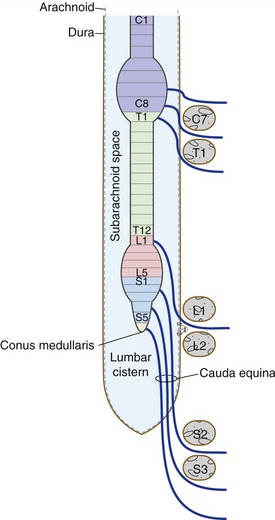
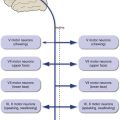
Segments of the spinal cord (Fig. 10-1)—8 cervical, 12 thoracic, 5 lumbar, 5 sacral, 1 coccygeal—are defined by the spinal nerves formed from dorsal and ventral roots attached bilaterally to each segment. The spinal cord has a cervical and a lumbar enlargement, serving the needs of the arms and legs respectively, and ends at the pointed conus medullaris. The conus medullaris is located at vertebral level L1/L2, even though the dural sac surrounding the spinal cord extends to vertebral level S2. So dorsal and ventral roots from progressively more caudal levels need to travel progressively longer distances through spinal subarachnoid space before reaching their intervertebral foramina of entry or exit. The collection of spinal nerves in subarachnoid space caudal to the conus medullaris is the cauda equina (Latin for “horse’s tail”).
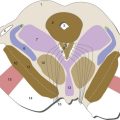
All Levels of the Spinal Cord Have a Similar Cross-Sectional Structure
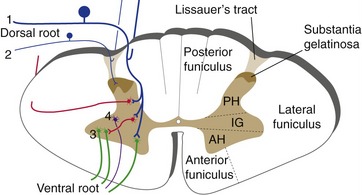
The gray matter core of the spinal cord is roughly in the shape of an H with a dorsal-ventral orientation at all levels (Fig. 10-2). The dorsally directed limbs of the H are the posterior (or dorsal) horns, and the ventrally directed limbs are the anterior (or ventral) horns. The posterior horn, derived from the alar plate of the neural tube, is a sensory processing area that receives most of the afferents that arrive in ipsilateral dorsal roots. The anterior horn is derived from the basal plate and contains the motor neurons whose axons form the ventral roots. The intermediate gray matter between the anterior and posterior horns is a mixture of interneurons and tract cells in sensory and motor circuits; at some levels it also contains autonomic motor neurons with axons that leave in the ventral roots.
As dorsal roots approach the spinal cord, the afferent fibers sort themselves out so that large-diameter fibers enter medial to small-diameter fibers. The large-diameter fibers, carrying touch and position information, have some branches that travel rostrally in the posterior funiculus and others that end in deeper portions of the posterior horn. The small-diameter fibers, primarily carrying pain and temperature information, travel in the dorsolateral fasciculus (Lissauer’s tract) to termination sites in a superficial zone of the posterior horn called the substantia gelatinosa.
The Spinal Cord Is Involved in Sensory Processing, Motor Outflow, and Reflexes
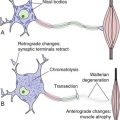
The wiring principles discussed in Chapter 3 are pretty apparent in the spinal cord. Central processes of primary afferents (cell bodies in dorsal root ganglia) are the only routes through which sensory information from the body can reach the spinal cord. They give rise to branches that feed into reflex circuits, into pathways to the thalamus, and into pathways to the cerebellum. These branches end in ipsilateral gray matter, mostly but not entirely in the posterior horn. Lower motor neurons in the anterior horn receive inputs from reflex circuits, as well as through descending pathways (i.e., axons of upper motor neurons), and project to ipsilateral muscles. They are the only routes through which the spinal cord can tell skeletal muscles to contract, and loss of lower motor neurons or their axons is followed by flaccid paralysis of the muscles they used to innervate—profound weakness, loss of tone and reflexes, and atrophy.
Reflex Circuitry Is Built into the Spinal Cord
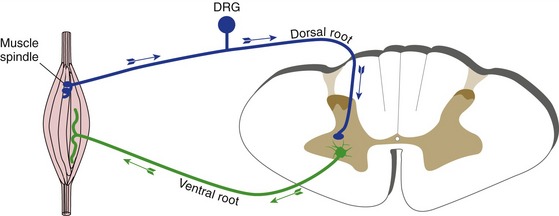
Reflexes are involuntary, stereotyped responses to sensory inputs, and every kind of sensory input is involved in reflex circuitry of one or more types. The simplest kind of reflex imaginable (other than the axon reflex) involves a primary afferent and a lower motor neuron, with a synapse in the CNS connecting the two. This is the circuit underlying stretch reflexes (Fig. 10-3), through which a muscle contracts in response to being stretched. (Stretch reflexes are tested by tapping tendons and so they’re often called deep tendon reflexes, even though the receptors that initiate them are located in muscle spindles and not tendons.)
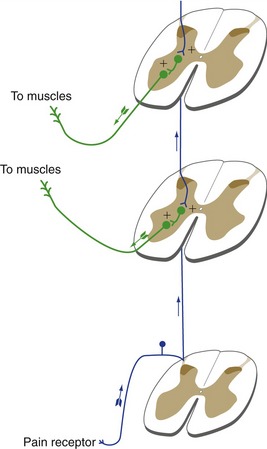
Stretch reflexes are the only monosynaptic reflexes. All others involve one or more interneurons. An example is the flexor reflex (or withdrawal reflex), through which a limb is removed from a painful stimulus (Fig. 10-4). This reflex is considerably more complex than a stretch reflex because all the muscles of a limb, and therefore motor neurons in several spinal segments, come into play.
Ascending and Descending Pathways Have Defined Locations in the Spinal White Matter
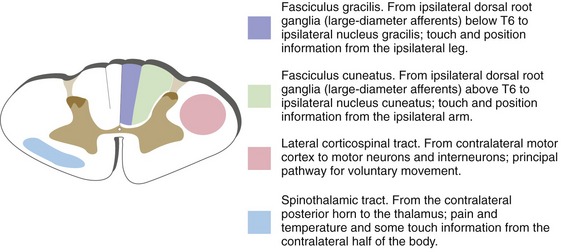
There are only three pathways of major clinical significance in the spinal cord: the posterior columns (touch and position), spinothalamic tract (pain and temperature), and lateral corticospinal tract (commands for voluntary movements). Each has a consistent location at all cord levels (see Fig. 10-9 later in this chapter).
The Posterior Column–Medial Lemniscus System Conveys Information about Touch and Limb Position
Key Concepts
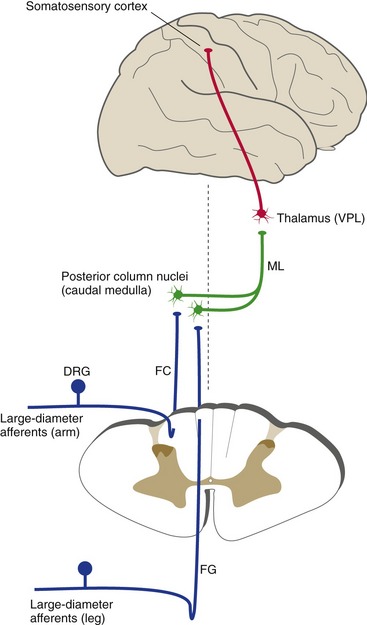
Large-diameter primary afferents, carrying touch and position information, have collaterals that ascend through the ipsilateral posterior funiculus. Incoming collaterals add on lateral to those already present in the posterior column, so by cervical levels each posterior column is subdivided into a medial fasciculus gracilis, representing the leg, and a more lateral fasciculus cuneatus, representing the arm (Fig. 10-5). Fasciculi gracilis and cuneatus then terminate in nuclei gracilis and cuneatus, the posterior column nuclei of the caudal medulla. Second order fibers from neurons of the posterior column nuclei cross the midline and form the medial lemniscus, which ascends through the brainstem to the thalamus (to a thalamic nucleus called the ventral posterolateral nucleus (VPL), for reasons explained in Chapter 16). VPL neurons then project to somatosensory cortex of the postcentral gyrus and adjacent areas.
The Spinothalamic Tract Conveys Information about Pain and Temperature
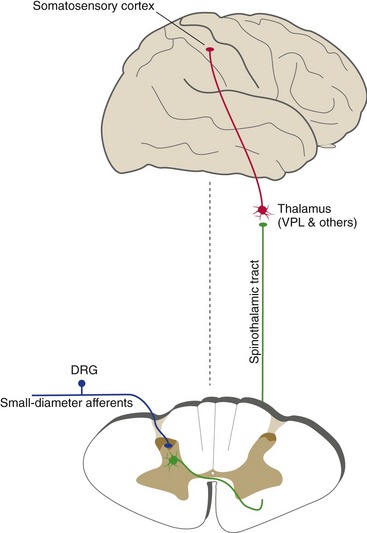
The most important pathway for pain and temperature information is the spinothalamic tract (Fig. 10-6); it also carries some touch information. Spinothalamic neurons (i.e., the second order cells) are located in the posterior horn, so this pathway, unlike the posterior column-medial lemniscus pathway, crosses the midline in the spinal cord. Small-diameter primary afferents, carrying pain and temperature and some touch information, traverse Lissauer’s tract and end on spinothalamic neurons in the posterior horn. Small interneurons of the substantia gelatinosa regulate the transmission of information at this synapse. The axons of spinothalamic neurons then cross the midline and form the spinothalamic tract, which ascends through the brainstem to VPL and to other thalamic nuclei as well (corresponding to pain usually having a more widespread effect on attention and mood than touch does). These thalamic neurons then project to somatosensory cortex of the postcentral gyrus and some other cortical areas (again, more widespread than touch information). Intermingled with these direct spinothalamic fibers are other axons that convey pain and temperature information from the posterior horn to the thalamus indirectly, by way of the reticular formation, and to other sites as well. The entire collection of ascending pain and temperature fibers is often referred to collectively as the anterolateral pathway because of its position in the spinal cord.
Descending Pathways Influence the Activity of Lower Motor Neurons
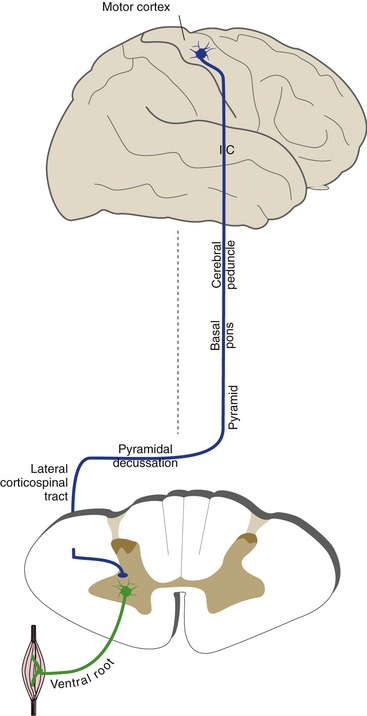
The most important pathway for the control of voluntary movement is the corticospinal tract (also called the pyramidal tract because its fibers travel through the pyramids on the ventral surface of the medulla). Corticospinal neurons are located in the precentral gyrus and adjacent cortical areas. Their axons descend through the internal capsule (bypassing the thalamus) and travel in a ventral location in the brainstem, passing through the cerebral peduncle (on the ventral surface of the midbrain), the basal pons, and the medullary pyramids (Fig. 10-7). Most of these fibers then cross the midline in the pyramidal decussation at the medulla-spinal cord junction and enter the lateral corticospinal tract. (The few uncrossed fibers enter the anterior corticospinal tract in the anterior funiculus.) Corticospinal fibers then end on motor neurons or interneurons of the spinal gray matter. Just as in the case of sensory pathways, there are additional, parallel routes for some of this input to reach the spinal cord, so destruction of the corticospinal tract causes weakness but not total paralysis. For example, projections from the vestibular nuclei of the brainstem to the spinal cord (vestibulospinal tracts) provide one alternate route.
The Autonomic Nervous System Monitors and Controls Visceral Activity
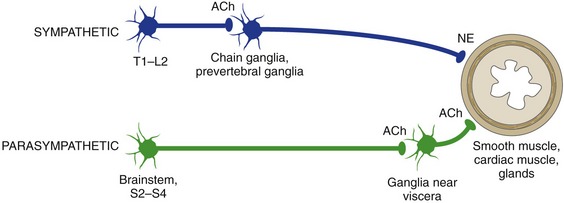
The autonomic nervous system (ANS) does not project efferents from the CNS directly to smooth or cardiac muscles or to glands. Instead, preganglionic autonomic neurons in the CNS project through the ventral roots to postganglionic neurons in ganglia outside the CNS (Fig. 10-8). These postganglionic neurons then project to target organs. (The only exception is the adrenal medulla, which receives autonomic (sympathetic) projections directly from the CNS.) Preganglionic axons are thinly myelinated, while postganglionic axons are unmyelinated.
Spinal Cord Damage Causes Predictable Deficits
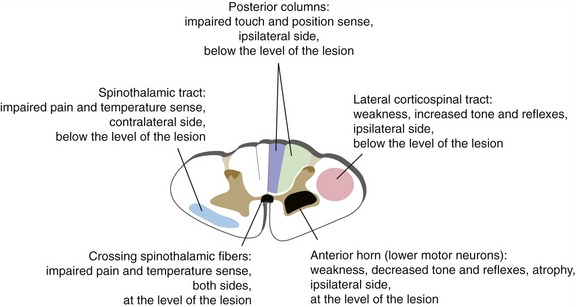
Spinal cord damage is typically followed by an acute phase of spinal shock, in which reflexes and muscle tone are depressed. The consistent locations of tracts and cell groups at all levels of the spinal cord (Fig. 10-9) make it possible to predict the subsequent chronic effects of partial cord damage (Fig. 10-10). Sensory deficits will be on the side ipsilateral to the damage if a pathway is affected before it crosses (e.g., posterior column), and on the contralateral side if affected after it crosses (e.g., spinothalamic tract). Weakness will be on the side ipsilateral to the damage because (1) lower motor neurons project to ipsilateral muscles, and (2) most corticospinal fibers cross in the pyramidal decussation (at the spinal cord-medulla junction) and are therefore on their way to ipsilateral lower motor neurons. So after unilateral damage there’s a seemingly weird mix of ipsilateral (touch, position, strength) and contralateral (pain, temperature) deficits.