16 Spinal cord
Descending pathways
Anatomy of the Anterior Gray Horn
Cell columns
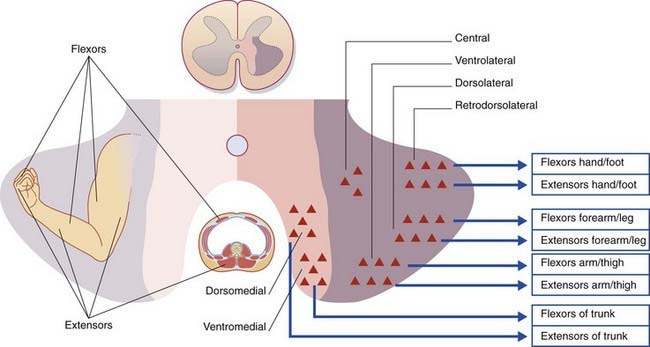
Each of the columns of motor neurons in the anterior gray horn supplies a group of muscles having similar functions. The individual muscles are supplied from cell groups (nuclei) within the columns. Axial (trunk) muscles are supplied from medially placed columns, proximal limb segment muscles from the midregion, and distal limb segment muscles from lateral columns (Figure 16.1). Columns supplying extensor muscles lie anterior to columns supplying flexors; hence the presence of ventromedial and dorsomedial columns for the trunk, and ventrolateral and dorsolateral columns for the limbs. A retrodorsolateral nucleus is devoted to the intrinsic muscles of the hand and foot. An isolated, central nucleus supplies the diaphragm.
The segmental levels of the six somatomotor cell columns are listed in Table 16.1. The autonomic nervous system is represented by the intermediolateral cell column.
| Cell column | Muscles |
|---|---|
| Ventromedial (all segments) | Erector spinae |
| Dorsomedial (T1–L2) | Intercostals, abdominals |
| Ventrolateral (C5–C8, L2–S2) | Arm/thigh |
| Dorsolateral (C6–C8, L3–S3) | Forearm/leg |
| Retrodorsolateral (C8, T1, S1–S2) | Hand/foot |
| Central (C3–C5) | Diaphragm |
Cell types
Renshaw cells
The axons of the α motor neurons give off recurrent branches which form excitatory, cholinergic synapses upon inhibitory internuncial neurons called Renshaw cells in the medial part of the anterior horn. The Renshaw cells form inhibitory, glycinergic synapses upon the α motor neurons. This is a classic example of negative feedback, or recurrent inhibition, through which the discharges of α motor neurons are self-limiting (cf. Clinical Panel 8.1).
Segmental-level inputs to α motor neurons
Segmental-level inputs to a flexor α motor neuron include the following:
Descending Motor Pathways
Important pathways descending to the spinal cord are the following:
Corticospinal tract
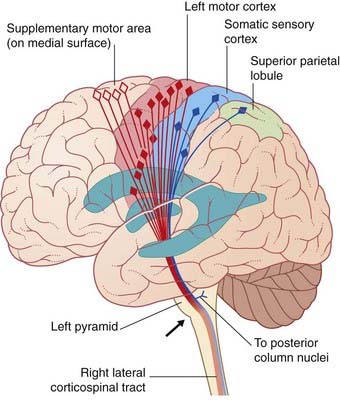
The corticospinal tract is the great voluntary motor pathway. About 40% of its fibers take their origin from the primary motor cortex in the precentral gyrus. Other sources include the supplementary motor area on the medial side of the hemisphere, the premotor cortex on the lateral side, the somatic sensory cortex, the parietal lobe, and the cingulate gyrus (Figure 16.2). The contributions from the two sensory areas mentioned terminate in sensory nuclei of the brainstem and spinal cord, where they modulate sensory transmission.
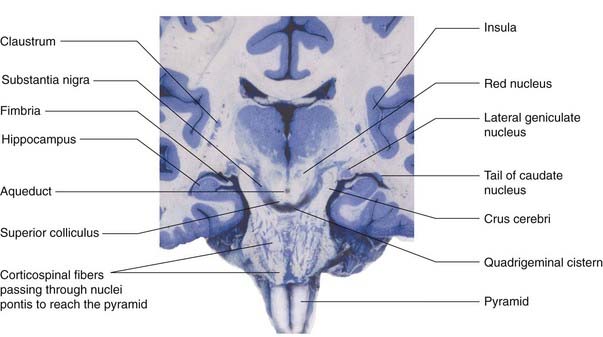
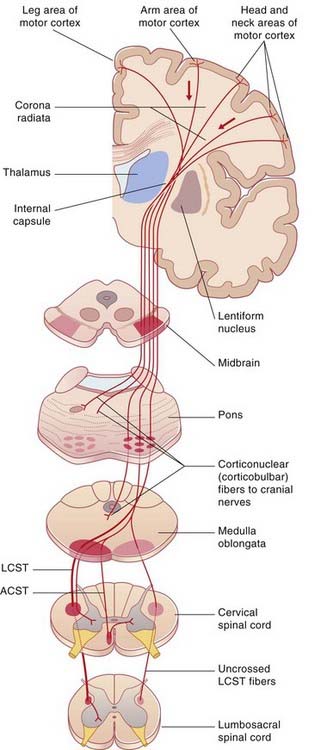
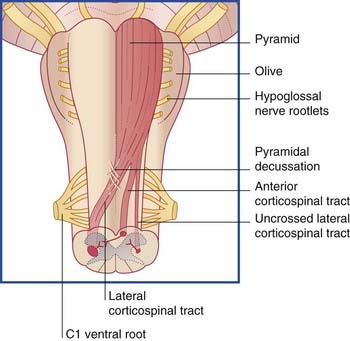
The corticospinal tract descends through the corona radiata and posterior limb of the internal capsule to reach the brainstem. It continues through the crus of the midbrain and the basilar pons to reach the medulla oblongata (Figure 16.3). Here it forms the pyramid (hence the synonym, pyramidal tract).
During its descent through the brainstem, the corticospinal tract gives off fibers which activate motor cranial nerve nuclei, notably those serving the muscles of the face, jaw, and tongue. These fibers are called corticonuclear (Figure 16.4). (The term ‘corticobulbar’ is sometimes used, but ‘bulb’ is open to different interpretations.)
Just above the spinomedullary junction (Figure 16.5):
The corticospinal tract contains about one million nerve fibers. The average conduction velocity is 60 m/s, indicating an average fiber diameter of 10 µm (‘rule of six’ in Ch. 6). About 3% of the fibers are extra large (up to 20 µm); they arise from giant neurons (cells of Betz), located mainly in the leg area of the motor cortex (Ch. 26). All corticospinal fibers are excitatory and appear to use glutamate as their transmitter substance.
Targets of the lateral corticospinal tract
Distal limb motor neurons
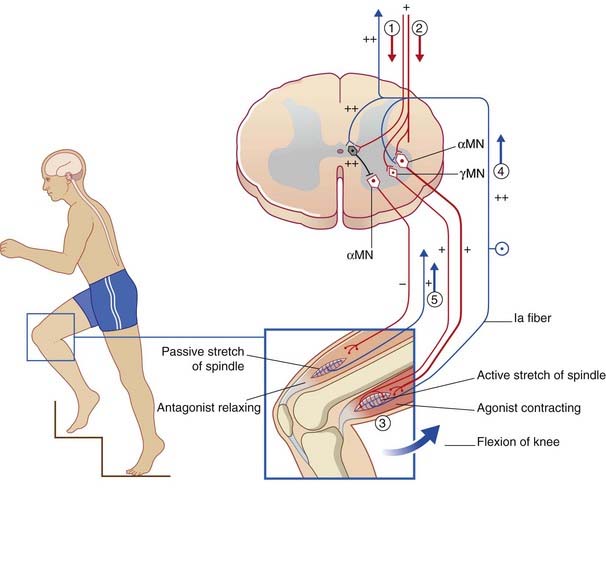
As mentioned already in Chapter 10, the α and γ motor neurons are coactivated by the LCST during a given movement, so that spindles in the prime movers are signaling active stretch while those in the antagonists are signaling passive stretch.
Ia inhibitory internuncials
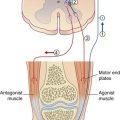
Also located in the intermediate gray matter are the Ia inhibitory internuncials, and these are the first neurons to be activated by the LCST during voluntary movements. Activity of the Ia internuncials causes the antagonist muscles to relax before the prime movers (agonists) contract. In addition, it renders the antagonists’ motor neurons refractory to stimulation by spindle afferents passively stretched by the movement. The sequence of events is shown in Figure 16.6 and its caption for voluntary flexion of the knee.
(Note on terminologies: During quiet standing, the knees are ‘locked’ in slight hyperextension and the quadriceps is inactive, as indicated by the patellae being ‘loose’. Any tendency of one or both knees to go into flexion is counteracted by a twitch of quadriceps in response to passive stretching of dozens of muscle spindles there. Because the flexion movement is resisted in this way, the reflex concerned is called a resistance reflex. During voluntary flexion of the knee, on the other hand, the movement is helped along in the manner described in the caption to Figure 16.6, through an assistance reflex. The change of sign, from negative to positive, is called reflex reversal.)
Presynaptic inhibitory neurons serving the stretch reflex
Consider a sprinter. At each stride, gravity pulls the body out of the air onto a knee extended by the quadriceps muscle. At the moment of impact, all of the muscle spindles in the contracted quadriceps are thrown into active stretch. The obvious danger is that the quadriceps may rupture. Golgi tendon endings (Ch. 10) offer some protection through autogenetic inhibition, but the main protection seems to be through presynaptic inhibition by the LCST of spindle afferents close to their contact points with motor neurons. At the same time, preservation of the ankle jerk is advantageous in this situation, giving immediate recruitment of calf motor neurons for the next take-off. The extent of suppression of the stretch reflex by the LCST in fact appears to depend upon the particular motor program being executed.
Upper and lower motor neurons
In the context of disease, clinicians refer to the corticospinal (and corticonuclear) neurons as upper motor neurons (Clinical Panel 16.1), and those of the brainstem and spinal cord as lower motor neurons (Clinical Panel 16.2).
Clinical Panel 16.1 Upper motor neuron disease
Sudden interruption of the corticospinal tract is characterized by the following features:
The above features are most commonly observed after a vascular stroke interrupting the corticospinal tract on one side of the cerebrum or brainstem. The usual picture here is one of initial flaccid hemiplegia (‘half-paralysis’), followed by a permanent spastic hemiparesis (‘half-weakness’). As illustrated in Clinical Panel 35.3, the spasticity following a stroke characteristically affects the antigravity muscles. In the lower limb, these are the extensors of the knee and the plantar flexors of the foot; in the upper limb, they are the flexors of the elbow and of the wrist and fingers. Following complete transection of the spinal cord, on the other hand, there may be a paraplegia in flexion of the lower limbs, owing to concurrent interruption of the vestibulospinal tract (Clinical Panel 16.3).
Why are motor neurons hyperexcitable?
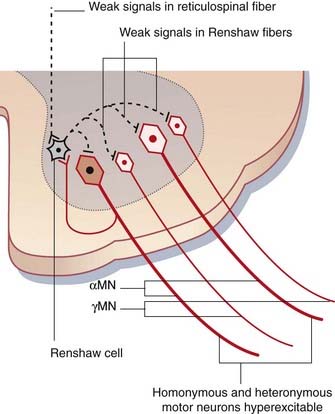
Figure CP 16.1.2 shows the distribution of inhibitory nerve endings derived from Renshaw cells. Not alone do they normally have a tonic breaking action on α and γ motor neurons at their own segmental level: they also tonically inhibit heteronymous motor neurons (i.e. those serving other muscle groups). For example, they act simultaneously upon motor neurons controlling knee and ankle movements, as part of the executive arm of central motor programs regulating successive muscle engagements and disengagements during locomotion. Locomotion is controlled by reticulospinal rather than corticospinal neurons, and any reduction in reticulospinal drive will render motor neurons hyperexcitable, and accounts for the frequent occurrence of ill-timed contractions produced by heteronymous motor neurons.
How do voluntary movements recover?
Multiple explanations are discussed in the final Clinical Panel in the final chapter.
Dietz V. Spastic gait disorder. In: Bronstein AM, Brandt T, Woollacott M, editors. Clinical disorders of balance posture and gait. London: Arnold; 1996:1-17.
Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity—from a basic science point of view. Acta physiol. 2007;189:171-180.
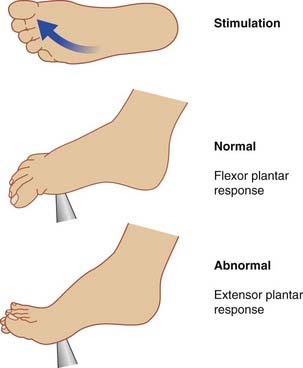
Singermann J, Lee L. Consistency of the Babinski reflex and its variants. J Neurol. 2008;15:960-964.
Clinical Panel 16.2 Lower motor neuron disease
The search for etiological clues is intense. Damage to motor neurons by free radicals has long been suspected, and it is of interest that mutation of a free-radical scavenging enzyme has been detected in some of the 10% of patients who inherit MND in an autosomal dominant mode. Because it is known that retrograde transport of neurotrophins is essential for long-term neuronal survival, recent research has focused upon retrograde transport of signaling endosomes. In particular, failure of the dynein retrograde motor (Ch. 6) has been implicated in the development of several neurodegenerative disorders.
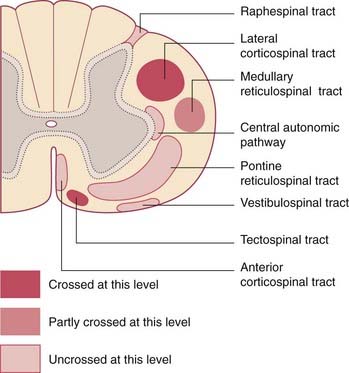
Reticulospinal tracts
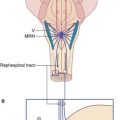
The pontine reticulospinal tract descends ipsilaterally in the anterior funiculus, and the medullary reticulospinal tract descends, partly crossed, in the lateral funiculus (Figure 16.7). Both tracts act, via internuncials shared with the corticospinal tract, upon motor neurons supplying axial (trunk) and proximal limb muscles. Information from animal experiments indicates that the pontine reticulospinal tract acts upon extensor motor neurons and the medullary reticulospinal tract upon flexor motor neurons. Both pathways exert reciprocal inhibition.
Posture
Definitions of posture vary with the context in which the term is used. In the general context of standing, sitting, and recumbency, posture may be defined as the position held between movements. In the local context of a single hand or foot, the term signifies postural fixation – the immobilization of proximal limb joints by co-contraction of the surrounding muscles, leaving the distal limb parts free to do voluntary business. As will be noted in Chapter 29, there is reason to believe that the human premotor cortex is programmed to select appropriate proximal muscle groups by way of the reticulospinal tracts, to set the stage for any particular movement of the hand or foot.
Tectospinal tract
The tectospinal tract is a crossed pathway descending from the tectum of the midbrain to the medial part of the anterior gray horn at cervical and upper thoracic levels. It is strategically placed for access to axial motor neurons (Figure 16.7).
Vestibulospinal tract
The vestibulospinal tract is an important uncrossed pathway whereby the tone of appropriate antigravity muscles is automatically increased when the head is tilted to one side. It descends in the anterior funiculus (Figure 16.7) and its function is to keep the center of gravity between the feet. It originates in the vestibular nucleus in the medulla oblongata. (Note: As explained in Ch. 17, there are in fact two vestibulospinal tracts on each side. The unqualified term refers to the lateral vestibulospinal tract.)
Raphespinal tract
The raphespinal tract originates in and beside the raphe nucleus situated in the midline in the medulla oblongata. It descends on both sides within the posterolateral tract of Lissauer. Its function is to modulate sensory transmission between first- and second-order neurons in the posterior gray horn – particularly with respect to pain (see Ch. 24).
Aminergic pathways
Aminergic pathways descend from specialized cell groups in the pons and medulla oblongata (Ch. 24). The principal neurotransmitters involved are norepinephrine and serotonin, both of which are classed as biogenic amines. The aminergic pathways descend in the outer parts of the anterior and lateral funiculi, and are distributed widely in the spinal gray matter. In general terms, they have inhibitory effects on sensory neurons and facilitatory effects on motor neurons.
Central autonomic pathways
Central sympathetic and parasympathetic fibers descend beside the intermediate gray matter (Figure 16.7). They originate in part from autonomic control centers in the hypothalamus and in part from several nuclear groups in the brainstem. They terminate in the intermediolateral cell columns that give rise to the preganglionic sympathetic and parasympathetic fibers of the peripheral autonomic system.
The central parasympathetic pathway is required for normal bladder (and rectal) function. The fibers concerned originate in the reticular formation, mainly at the level of the pons (Ch. 24). The pontine micturition center has a tonic inhibitory action on the sacral parasympathetic system. Severe injury to the spinal cord or cauda equina results in reflex voiding when the bladder is only half full (Clinical Panel 16.3).
Clinical Panel 16.3 Spinal cord injury
Blood Supply of the Spinal Cord
Arteries
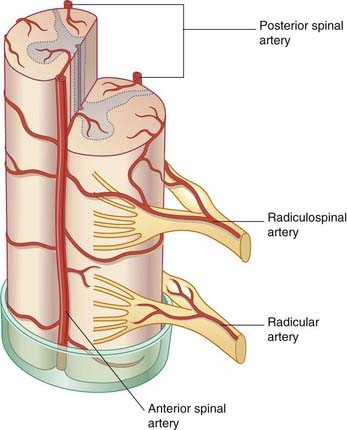
Close to the foramen magnum, the two vertebral arteries give off anterior and posterior spinal branches. The anterior branches fuse to form a single anterior spinal artery in front of the anterior median fissure (Figure 16.8). Branches are given alternately to the left and right sides of the spinal cord. The posterior spinal arteries descend along the line of attachment of the dorsal nerve roots on each side.
Busches A, El Manira A. Sensory pathways and their modulation in the control of locomotion. Current Opin Neurobiol. 1998;8:733-739.
Crone C, Nielson J. Central control of disynaptic inhibition in humans. Acta Physiol Scand. 1994;162:351-363.
Ellingson BM, Kurpad SN, Schmit BD. Functional correlates of diffusion tensor imaging in spinal cord injury. Biomed Sci Instrum. 2008;44:28-33.
Gruener G, Biller J. Spinal cord anatomy, localization, and overview of spinal cord syndromes. Continuum. 2008;14:11-35.
Halezparast M, Klocke R. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808-812.
Katz R, Pierrot-Deseilligny E. Recurrent inhibition in man. Prog Neurobiol. 1998;57:325-355.
Lemon RN. Descending pathways in motor control. Ann Rev Neurosci. 2008;31:195-218.
Levy LM. Brain γ-aminobutyric acid changes in stiff-person syndrome. Arch Neurol. 2005;62:970-974.
Martaens de Nordhout A, Rapisarda G, Bogacz D, et al. Corticomotoneural synaptic connections in man. Brain. 1999;122:1327-1340.
Schieber MH. Comparative anatomy of the corticospinal system. Handbook Clin Neurol. 2007;82:15-37.