CHAPTER 109 Spinal Ablative Techniques for the Treatment of Chronic Pain Conditions
INTRODUCTION
The interruption of pathways of the nervous system concerned with pain is a classical approach to the relief of intractable pain disorders, which stems from neurosurgeons having been trained with a primary understanding of anatomy and, to a lesser extent, an appreciation of neurophysiology. While successful when implemented in the correct clinical setting, there have also been significant complications with these procedures, including return of pain and sometimes worsening of pain, and/or the evolution of neuropathic pain syndromes in many patients treated with these procedures.
Historical aspects of pain management
The origin of the word ‘pain’ is the Latin word poena, meaning punishment. The early concept of pain as a form of punishment for sinful activities is as old as humankind.1 Early healers used a wide range of modalities to attempt the control of pain, including herbal medicines and the application of electric fish.2 Decartes’ description of pain conduction from peripheral damage through nerves to the brain led to the first scientific understanding of pain.3 The first plausible surgical management of pain was on an anatomic basis where nerves were cut in attempts to denervate the painful areas of the body. These methods were a direct byproduct of the understanding of pain transmission during the scientific revolution. It was not until the Wall and Melzack Gate Control Theory that a sound scientific basis for pain mechanism was formulated.4 Shortly thereafter, initial efforts were made to modulate pain at peripheral nerve, spinal cord,5,6 and other targets such as brain stem7 and, more recently, the cerebral cortex.8
The diagnosis of pain
Long before the different mechanisms of chronic pain were defined, neurosurgeons distinguished between chronic pain due to cancer and chronic pain of ‘benign’ origin. Currently, a more helpful way to differentiate chronic pain is through physiology: namely, nociceptive and neuropathic pain syndromes. The difference between these two categories is the absence of a continuous nociceptive input through pain receptors in neuropathic pain syndromes. It is key to recognize that a given patient may have features of more than one pain physiology. Weir Mitchell first described and named causalgia as a regional pain disorder associated with both motor and sensory disturbance.9 Since then, many and often confusing terms have been used to describe chronic neuropathic pain syndromes. In 1994, the International Association for the Study of Pain (IASP) adopted the term complex regional pain syndrome (CRPS) to replace the terms reflex sympathetic dystrophy (RSD) and causalgia.10 Complex regional pain syndrome is further divided into type 1 and type 2, representing RSD and causaglia, respectively. CRPS may exist in a state of flux and, regardless of whether the patient suffers from CRPS 1 or 2, there may be sympathetically maintained and independent features involved. While many theories exist, the definitive pathophysiology and etiology of CPRS remains unclear.
A correct physiologic diagnosis can only be obtained through detailed history and physical examination. It may be necessary to obtain diagnostic studies such as computed tomography (CT) scan, margnetic resonance imaging (MRI), and electromyogram/nerve conduction velocity (EMG/NCV). Diagnostic spinal procedures such as selective root blocks, sympathetic blocks, and discography will often further aid the clinician. There is no confirmatory test or procedure for CRPS 1, and this diagnosis can only be attained through a clinical examination. An early and specific refinement of the pain diagnosis is necessary and valuable, as it directs care of the patient.
SPINAL RHIZOTOMIES AND GANGLIONECTOMIES
Historical background
Dorsal root rhizotomy was first attempted for the relief of intractable pain by Abbe in 1889.11 The operation was based on the concept that afferent signals are conveyed via the dorsal roots while efferent signals are conveyed via the ventral roots.12,13 There now exists a large body of evidence supporting the existence of as much as 30% afferent axons passing through the ventral roots.14–16 Dorsal rhizotomies or ganglionectomies are primarily indicated for treatment of pain syndromes involving the neck, trunk, abdomen, and perineal region. Persistent post-thoracotomy or postlaparotomy pain is a frequent indication for these procedures, as well as treating malignant pain syndromes from pleural based or apical lung lesions. Several variations of spinal root surgery have been developed over the century, though the methods have experienced a long decline due to the disappointing outcomes of most series.17,18 The procedure is contraindicated for treating extremity pain in a functional limb, as complete or near-complete functional paralysis will occur because of absence of sensory input. Extensive sectioning of sacral posterior root also needs to be performed with selectivity as interference with sphincter and sexual function can occur.
Pertinent anatomy
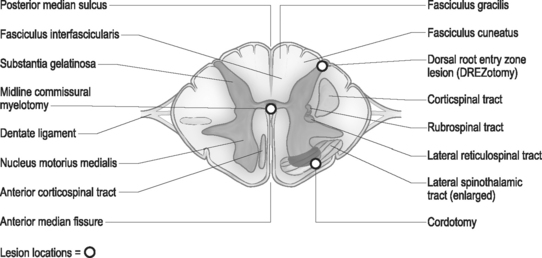
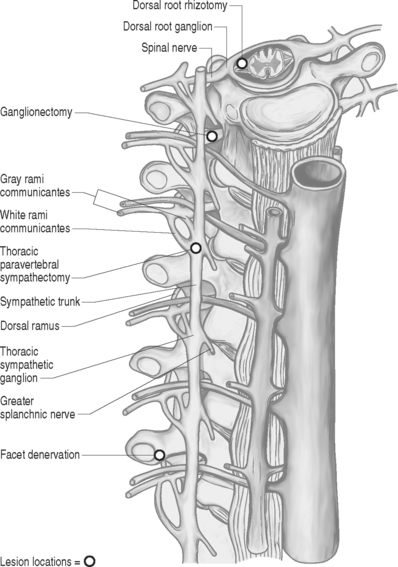
The spinal root anatomy begins with the formation of the anterior roots, which are formed by three to five rootlets emerging from the anterolateral sulcus; the posterior roots are formed by three to ten rootlets which penetrate into the dorsolateral sulcus (Fig. 109.1). The dorsal and ventral roots are separated by the dentate ligaments, though they are grouped together prior to leaving the thecal sac. Microscopic anastomotic branches exist that pass from one rootlet to another. As the roots approach the intervertebral foramen, both the ventral and dorsal roots are situated in a common dural sleeve. In the intervertebral foramen the dorsal ganglion can be identified. At this point the subarachnoid space is sealed by the arachnoid trabeculae and no cerebrospinal fluid (CSF) is contained.19 Distal to the dorsal ganglion and lateral to the foramen, the spinal nerve is formed which will then bifurcate into ventral and dorsal branches.
Procedure
Prior to the procedure, a selective nerve root block may aid in determining if rhizotomy or ganglionectomy may provide any benefit. The operation is performed under general anesthesia with the patient in the prone position and may be performed via an intradural (dorsal rhizotomy) or extradural (ganglionectomy) approach. The desired spinal segments are exposed. In the case of dorsal rhizotomies, laminectomies are performed and a midline dural opening is made. The dorsal rootlets of the desired segments are then sectioned sharply. A technique described by Sindou in 1972 aimed to interrupt selectively small-diameter nociceptive fibers at their radicular entrance into the spinal cord.20 This technique of selective dorsal root rhizotomy allows for preservation of lemniscal fibers that may avoid secondary appearance of pain and leave intact substrates that can be utilized for neuromodulatory procedures.21 For ganglionectomies, foraminotomies are performed to expose the desired dorsal root ganglia. The ganglia are then carefully dissected free from the surrounding tissue and the dorsal root is divided proximal to the ganglion. Following this, the ganglion is gently elevated and the distal connection to the root is sectioned. In most cases, the ventral root can be spared. Important aspects from a purely technical point of view include verification of the level of lamienctomy, root identification by anatomical, radiographic, and physiological means, selection of roots by stimulation, recognition of anastomoses between roots, and sparing of radicular arteries. Other technical variants exist such as extradural spinal root ganglion resection as described by Scoville in 1966, which avoid the opening of the dura.
An alternative to the open surgical technique’s proximal radiofrequency thermocoagulation of primary spinal trunk and ganglion has been described; Uematse et al. proposed a percutaneous technique for lesioning the dorsal root ganglion and rootlets.22
Outcomes
The variability of clinical results of posterior rhizotomy and ganglionectomy warrants the cautious use of these denervation interventions and should only be used after nondestructive techniques have been exhausted. For lumbar radiculopathies, there is about a 30% success rate at 1 year.23 The results for postherpetic neuralgia are no better, with success rates at less than 30%.24 And the results for non-specific relief of chronic pain are extremely poor in the long term.25 A review of the literature reveals that results are generally less than 50% for good pain reduction with limited long-term follow-up. Unfortunately, these procedures produce a complete denervation of one or more spinal segments, thus precluding the patient from potential future neuromodulation. Because of this, and the general availability of long-acting opiate analgesics, these procedures are not generally recommended.
DORSAL ROOT ENTRY ZONE LESIONS (DREZOTOMY)
Historical background
During the early 1960s, research into pain focused attention on the dorsal root entry zone (DREZ) as the first level of modulation for the cessation of pain.26 What is known of these pathological mechanisms is that the cells in the dorsal root ganglion become hyperactive and send nociceptive impulses via the spinothalamic pathways. Based on this understanding, Sindou performed the first DREZ operation in 1972 for pain caused by Pancoast-Tobias syndrome.27 Others such as Nashold and Ostdahl placed thermal lesions into the substantia gelatinosa of the spinal cord for the treatment of nonmalignant pain.28 In view of the complex anatomy and lack of histological confirmation, the target was referred to as the DREZ. This region in the spinal cord is currently recognized as a sophisticated structure for the modulation of pain and continues to be utilized for ablative techniques, either open or percutaneous.
Pertinent anatomy
Using cytoarchitectural criteria, in 1952 Rexed divided the gray matter of the spinal cord of the cat into ten separate cell layers. Similar laminar patterns were confirmed by Schoenen.29 The uppermost lamina (I–V) are pertinent to the DREZ procedure. The major nociceptive input is distributed to layers I, II, and V (layers I and II representing the substantia gelatinosa), with their second-order neurons giving rise to the spinothalamic tract. Neurons in layers III and IV receive non-noxious inputs from the periphery and project to the dorsal column nuclei. Situated dorsolateral to the dorsal horn is the tract of Lissauer, an intersegmental longitudinal spinal tract with multiple collaterals to Rexed layers I and II. This tract plays an important role in the intersegmental modulation of the nociceptive afferents. Its medial part transmits the excitatory effects of each dorsal root to the adjacent segments and its lateral part conveys inhibitory influences to the substantia gelatinosa.30 The DREZ procedure is directed at destroying the Rexed layers I, II, and V and the medial portion of the tract of Lissauer which coordinates sensory information.
Procedure
The surgical technique of thermal coagulation for DREZ lesions has been described in detail by Nashold and collaborators and extensively published.31 With the patient placed under general anesthesia and in the prone position, laminectomies or hemilaminectomies are performed over the involved regions of the spinal cord. In the cervical region, the localization of the appropriate level is one level rostal to the dermatomal localization, and in the thoracic region two to three veterbral segments rostal to the affected dermatomes (Fig. 109.2). Lumbar and sacral segments are localized through laminectomies at T10 through L1 for exposure of the conus medullaris. The operating microscope is utilized throughout the procedure following the dural opening. Each dorsal root is composed of several small rootlets which enter the cord at the postointermediolateral sulcus at the margin of the dorsal columns. Identification of the root and corresponding cord level can be confirmed with electrophysiologic monitoring. Following the identification of the rootlets of each root, though this may be difficult at the level of the conus, the lesions are made in the dorsal root entry zone. The lesions are created over an additional one to two segments above and below the affected roots to ensure adequate coverage of the painful segments. Bilateral lesioning should be avoided in patients with good neurologic function below the lesion, as deterioration in proprioception, motor, or bladder and bowel function can occur.
Outcomes
The DREZ procedure has been implemented for a large series of deafferentation pain syndromes. Results from several series report a 54–79% success rate for the procedure.32–34 Probably the single best indication for the DREZ lesioning is pain following brachial plexus avulsion. Long-term pain relief for this procedure for brachial plexus avulsion pain reaches 70%.35 The major complication with thoracic spinal DREZ operation is weakness in the ipsilateral leg due to injury of the corticospinal tract; this is seen in 5–10% of patients,36–38 though this may occur with other sites of DREZotomy.
While the DREZ operation has been used in an attempt to treat a multitude of pain conditions, its current indications are very specific. It is best used to treat deafferentation pain (as seen with brachial plexus avulsion), limited cancer pain (as seen with a Pancoast tumor), segmental pain after spinal cord injury, peripheral nerve pain (seen with nerve injuries and phantom limb or stump pain), and postherpetic pain.39 These authors do not utilize this procedure, however, for other than segmental pain following spinal cord injury because neuromodulation is commonly usable in most of the other painful phenomenon listed and neuromodulation has a superior safety profile.
OPEN ANTEROLATERAL CORDOTOMY
Historical background
In 1889, Edinger40 first described the anatomy of the spinothalamic tracts, though functional correlation was not discovered until Spiller reported his findings in 1905.41 The first anterolateral tractotomy was performed by Martin at the suggestion of Spiller in 1911 for management of pain in man.42 Eventually, both in Europe and America, the surgical cordotomy became a standard neurosurgical procedure for the treatment of pain. Mullan43 developed the technique for percutaneous cordotomy using a radioactive strontium needle in 1963 where the lesion can be made without the necessity of general anesthesia. Rosomoff44 further refined the technique using a radiofrequency needle electrode system. Additional refinements to the procedure have included myelography45 to outline landmarks, CT guidance,46 and electrical impedance monitoring.47 While the operation has remained in essence the same as that introduced by Spiller and Martin, much effort over the decades has continued to be devoted to lesioning the anterolateral quadrant of the spinal cord. Both open and percutaneous techniques will be discussed.
Pertinent anatomy
Neuroanatomical and physiological aspects of nociceptive pathways have been extensively studied in animals and humans. Within close proximity to the spinothalamic tract lie many other ascending and descending tracts, damage to which leads to many of the complications of cordotomy. The corticospinal tract is located posteriorly and injury to it results in ipsilateral weakness. Overlying the spinothalamic tract is the ventral spinocerebellar tract and injury of that pathway may produce an ipsilateral ataxia of the arm. Knowledge of the location of descending respiratory pathways is not just theoretical, as one of the most feared complications of a high bilateral cordotomy is respiratory dysfunction. Typically, the patient is capable of voluntary but not involuntary respiration, and consequently dies during sleep because of destruction of the system for automatic respiration (Ondine’s Curse).48
Surgical cordotomy procedure
The basic open surgical cordotomy has not changed much since it was first described by Spiller and Martin in 1912.42 The technique is relatively simple; however, it is important to note that the vertebral and spinal cord segments do not correspond. Functional segments of the spinal cord tend to lie two to three levels lower than the vertebrae. The authors’ practice is to perform open cordotomy at the T1–2 or T2–3 segments. Following the laminectomy, the dura is opened in a semicircular manner paramedian to the midline. In order to facilitate rotation of the spinal cord, a dorsal root may be incised as the dentate ligaments above and below the segment are sectioned. The cut end of the dentate ligament is then grasped and the cord is rotated 45° to allow visualization of the anterolateral surface. The origin of the dentate ligament from the cord is a valid landmark for the dorsal extent of the crossed spinothalamic tract. If necessary, a dental mirror can be used to better identify the anterior spinal artery or the medial limits of the ventral rootlets. A cordotomy knife is then inserted at the origin of the ligament, and an incision is made to a point just medial to the emergence of the most medial fibers of the ventral rootlets. Poletti’s technique has increased safety, as he incises the tough pia with a knife and then completes a tractotomy with a ball hook.49 The incision should be deep enough into the cord to transect a pie-shaped segment of about 90°.
Percutaneous cervical cordotomy procedure
Mullan et al., in 1963, introduced a procedure to percutaneously perform an anterolateral cordotomy.43 Since the first description, there are have been few technical advances such as CT guidance17 and impedence monitoring.18
Originally, strontium radioactive source was used, later modified by Rosomoff50 to incorporate radiofrequency ablation. Many authors currently recommend impedance monitoring. An electrode can be inserted through the needle, and impedance measurements taken to confirm the location as the needle passes from CSF into the spinal cord. Electrical impedance measures about 400 ohms when the electrode is in CSF and rises to about 1000 ohms once the cord is entered. Further physiologic localization can then be performed with stimulation. With appropriate localization, contralateral warm or cold sensory effects are induced at 100 Hz. Ideal placement is generally 1–3 mm anterior to the dentate ligament, with upper extremity fibers tending to be more anterior. A test lesion is then generally made with an electrode tip temperature of 50–60°C. Close monitoring of contralateral motor function must be performed. If no untoward side effects are noted, a permanent lesion is then made at 70–80°C for 60 seconds. It is highly recommended following high cervical cordotomy to monitor these patients’ respiratory status for approximately 5–7 days, as an unintended unilateral lesion may actually represent a bilateral lesion.
Lower cervical cordotomy procedure
Lin et al. describe an anterior approach for percutaneous lower cervical cordotomy, thus attempting to avoid phrenic nerve fibers and the dreaded respiratory complications of high cervical cordotomies.51 It involves an anterior transdiscal approach to the lower anterolateral cervical cord, producing a lesion in the spinothalamic tract. This transdiscal technique can be difficult to achieve if the needle should be redirected in the event it does not hit the target.
Outcomes
The results for the various cordotomy procedures are fairly similar; however, lower complication rates are seen with the percutaneous approach.52 For open surgical cordotomy, approximately 50% of patients with cancer pain have complete relief and an additional 25% will have significant reduction in pain.20 By 6 months, however, pain will return in half of the initially successfully treated patients.21 After high percutaneous cordotomy, 60–70% have complete relief and 80–90% have significant relief.53–55 As with surgical cordotomy, these results tend to drop at 1 year.28,56 The results for low anterior cordotomy are similar, with 75–80% of patients experiencing significant pain relief.57
Although cordotomy has initially been used to treat all types of pain, it is now reserved for pain of malignant origin. Studies show that 80% of patients undergoing cordotomy have significant relief of their pain, though at 1-year follow-up only 40% have any pain reduction.36,58 The highest level of analgesia that can be reliably and persistently obtained with high cervical cordotomy is the C5 dermatome and is generally not effective for pain due to head and neck cancers. Lesions above C5 may be treated with a mesencephalic tractotomy. Bilateral cordotomy is reserved primarily for midline abdominal and pelvic pain or bilateral lower extremity pain, though not recommend secondary to the risk of Ondine’s Curse.37,59
The complication rates of the various techniques differ, though with surgical cordotomy there is an 8% mortality rate27 and 13% incidence of lower extremity weakness.38 Complications for high cervical cordotomy include a 1–5% mortality rate,60 4–8% incidence of lower extremity paresis or ataxia,34 and a 5% incidence of bladder dysfunction.34 The complications for low cervical cordotomy are similar to the higher posterior approaches with except of the phrenic and respiratory tract injury.32
MIDLINE/COMMISSURAL MYELOTOMY
Historical background
Midline or commissural myelotomy is a procedure in which the decussating fibers of the spinothalamic tract are interrupted as they cross in the anterior white commissure of the spinal cord. The procedure was conceived as an alternative to bilateral anterolateral cordotomy in patients in whom a bilateral area on analgesia was sought. The procedure was first described by Hitchcock in 1970.61 During a planned percutaneous cervical cordotomy, the electrode was inadvertently inserted into the center of the spinal cord in the upper cervical region. The patient had immediate relief of pain, and the relief lasted when only a small lesion was made at the site. This target was adopted for central myelotomy, and other patients had similar successful relief of pain with this technique. The results tended to be best in those patients with midline or visceral pain. This led some to conclude that a previously undescribed pathway ascends at the center of the spinal cord and carries predominately visceral pain perception. The technique was later refined by Gildenberg and Hirshberg,62 who felt that there was no advantage to making a high cervical lesion for patients with pelvic or perineal pain. Interruption of the central cord at the lumbothoracic level provided similar results. Today, limited myelotomy is most commonly performed for pelvic pain related to rectal or uterine cancer.
Pertinent anatomy
On the anterior surface of the spinal cord there can be identified a deep anterior median fissure which penetrates the spinal cord. On the posterior surface, a small posterior median sulcus is continuous with a glial partition. The spinothalamic fibers cross in the anterior white commissure in a decussion that involves several spinal segments and ascend contralaterally. The intent of the myelotomy is to interrupt the paleospinothalamic tract, producing analgesia with preserved ability to localize and discriminate between sharp and dull sensation.63
Procedure
It is important to note that the vertebral and spinal cord segments do not correspond. Functional segments of the spinal cord tend to lie two to three levels lower than the vertebrae. With this in mind, the lamina overlying the spinal segments targeted for denervation are identified with the aid of fluoroscopy. Laminectomies are then performed and the dura is opened to expose the spinal cord. The midline can be identified by the vessels diving into the posterior median sulcus between the posterior columns. The pia is then opened sharply and the posterior median septum is identified. The septum is a single fibrous layer that lies between the posterior columns, and one can dissect along either side of it to the central canal area. Dissection continues until the anterior median septum is encountered. This represents the ventral extent of the myelotomy, as further dissection risks injury to the anterior spinal artery. Various other techniques have been reported, including radiofrequency and carbon dioxide laser techniques.64
Outcomes
Midline myelotomy is most effective for pain in the lower portion of the body, especially midline visceral pain. The overall efficacy of midline myelotomy has been reported in the order of 70%.65–67 There are, however, several undesirable side effects of the procedure, such as hyperesthesia, diminished proprioception, parasthesias, and incoordination of gait.68 A reduction in side effects has been reported by performing a limited myelotomy versus the classic midline myelotomy technique. The difficulty with comparing reports stems from the controversy of the depth of cut. Neither the limited and classic myelotomy technique is performed with any frequency, limiting the role of these techniques for chronic pain management.
CORDECTOMY
Historical background
Armour, in 1916, performed the first cordectomy for pain in a post-traumatic paraplegic.69 Prior to this, cordectomy had been formed for other indications including intramedullary spinal cord tumors, severe spasticity, and post-traumatic syringomyelia.
Outcomes
The clinical indications for cordectomy are varied, including post-traumatic syringomyelia, uncontrollable leg spasticity, and post-traumatic spontaneous neurogenic leg pain. The operation of selective spinal cordectomy is rarely performed. Jefferson, in 1983, reported his series of 19 cases of cordectomy with adequate pain relief in traumatic paraplegics.70 Clinical results in the patients with syringomyelia and uncontrollable leg spasticity have been excellent, though cordectomy did not provide permanent relief in the patients with neurogenic leg pain.71
SYMPATHECTOMY
Background
Surgery on the sympathetic nervous system dates to the late nineteenth century. The functional role of the autonomic nervous system was poorly understood, and early sympathectomies were performed for such varied disorders as epilepsy, vascular disease, and spasticity. By 1930, thoracic and lumbar sympathectomies were being performed for angina, hypertension, and pain.72 While many of these are no longer indications due to the advent of modern pharmaceuticals and surgical techniques, sympathetically maintained pain remains one of the few indications for a sympathectomy other than hyperhidrosis.
Currently, sympathetic denervation for pain is carried out for three main sites of pain: the heart, the limbs, and the abdominal viscera. Sympathetically mediated pain includes a wide spectrum of disorders that share in common the factor that the pain can be relieved through sympathetic block or interruption.73 The most common indications for the procedure are reflex sympathetic dystrophy (RSD)/Sudeck’s atrophy, causalgia, and ischemic pain states from occlusive vascular disorders. These conditions can be sympathetically mediated pain syndromes and generally respond to sympathetic blocks with local anesthetics. Sympathectomy should not be considered unless there is good relief with the diagnostic block. Often, lasting relief can be achieved with a series of blocks if they are performed early in the progression of the disease.
Visceral pain secondary to unresectable cancers of the abdominal visera and painful relapsing chronic pancreatitis are some of the indication for sympathectomy.46,74 Pancreatic afferents travel bilaterally through the splanchnic chain and into the lower thoracic sympathetic ganglia. The biliary tract is supplied by the right splanchnic nerves and each kidney is supplied unilaterally by the nerves on that side. Attempts at chemical sympathectomy of the celiac plexus and splanchnic nerves during exploratory laparotomy, or percutaneously, have had good initial pain relief though poor localization, and the diffuse target make it difficult to assess the completeness of denervation.75
Upper thoracic ganglionectomy procedure
Sympathetic activity can be interrupted by lesioning the paravertebral sympathetic chain along its course. This can be achieved either by direct/endoscopic surgical resection or through chemical or radiofrequency ablation. Resection of the T2 and T3 ganglia should result in complete sympathetic denervation to the upper extremity.76,77 The most common surgical approach is through a midline incision with the patient in the prone position, which allows for bilateral exposure and lesioning. After a subperiosteal dissection of the muscles, the T3 lamina and rib are identified with the use of fluoroscopy. The transverse process and medial portion of the rib are then resected. The second and third sympathetic ganglia should be found in the paravertebral fat and sectioned at the ganglia; a Horner’s syndrome can generally be avoided.
Alternative approaches include the anterior transthoracic, percutaneous radiofrequency, and transaxillary exposure.78 Here, a rib-spreading incision is made low in the axilla between the third and fourth ribs, and the lung must temporarily be collapsed. The thoracic chain can be identified beneath the pleura alongside the vertebrae. Lastly, the procedure can be performed through a supraclavicular approach.48 The lower cervical and upper thoracic sympathetic ganglia can be found in the fatty tissues deep and medial to the sublclavian artery.
Lumbar sympathectomy procedure
The operation is performed through a retroperioneal approach through a flank incision.46,79 The external oblique, internal oblique, and transverse muscles are divided in the direction of their fibers in order to reach the retroperitoneal space alongside the psoas muscle. The ureter is identified and carefully elevated from the vertebral column. The sympathetic chain should be encountered between the psoas and vertebral bodies. The ganglia and their rami communicantes can then be segmentally resected. Removal of the L2 and L3 sympathetic ganglion is usually adequate to remove sympathetic tone from the lower extremities. Male patients should be warned that sexual dysfunction may occur with bilateral lumbosacral sympathectomy.
Splanchnicectomy procedure
The upper abdominal viscera sends numerous amounts of nociceptive afferents into the spinal cord via the greater and lesser splanchnic nerves. This allows for the effective treatment of pain by denervation of sympathetic tone to the viscera. The patient is positioned prone and an oblique incision is made over the eleventh rib approximately 5 cm from midline. Six centimeters of rib are then resected, starting just lateral to the transverse process. The pleura is carefully stripped away to visualize the lateral aspects of the vertebral bodies. The sympathetic ganglia are identified ventral to the intercostal nerves. The sympathetic chain and T9–12 ganglia, along with the three splanchnic nerves, are then resected. For pancreatic pain, the procedure must be done bilaterally.46,47,80 Further modifications have been reported with the use of endoscopic procedures performed by thoracic surgeons.
Outcomes
The indications for open sympathectomy have decreased steadily over the past decades as pharmacological substances have supplanted these aggressive surgical techniques. The results of sympathectomy for pain disorders have yielded modest success. Most authors report sustained pain relief in less than two-thirds of patients at 2 years, and about one-third at 5 years.46,47,51,81,82 The adequate and limited long-term outcomes should give consideration to either repetitive blocks or other neuromodulatory methods that are nondestructive.
RADIOFREQUENCY FACET DENERVATION
Historical background
Shealy first described the application of radiofrequency to the facet joint for the treatment of spinal pain.83 Prior to Shealy, Goldthwait84 and others85,86 initiated interest in the neuroanatomy of the facet joint and its origin of nociceptive lumbar pain. In 1971, Rees published a specific method of attempting facet denervation to modulate back pain.87 Others later suggested that the knife that had been used by Rees was, however, too short to have produced a lesion to the innervation of the facet, being long enough to only produce a myofasciotomy.88 In 1979 and 1980, Bogduk and Long went on to describe a more anatomically correct approach to facet denervation, and their approach is the one used today.89,90
Outcomes
The only randomized, double-blind study on this topic was conducted by Wedley et al; it demonstrated that lumbar facet denervation is clearly superior to placebo.91 The results from radiofrequency facet denervation series vary greatly; however, most studies report success rates of 45–80%.92–95 The wide range of outcomes is likely due to many factors, including differing techniques and localization of target. Result from facet rhizotomies have a tendency to diminish over time96 and may be explained through the regeneration of the medial branch. This relapse of symptoms may be due to regeneration of the medial branch nerve of the posterior ramus. The most common complications are superficial infections and reactions to local anesthetic, though serious complications with root injury may occur with the malpositioning of electrodes.
1 Procacci P, Maresca M. Evolution of the concept of pain. Sicuteri F, editor. Advances in pain research and therapy, vol 20. New York: Raven Press, 1984.
2 Sheon RP. Transcutaneous electrical nerve stimulation. From electric eels to electrodes. Postgrad Med. 1984;75(5):71-74.
3 Schiff M. Lehrbuch der physiologie, muskel, and nervenphysiologie. Schavenberg, Germany: Lahr, 1848.
4 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-979.
5 Sweet WH, Wepsic JG. Treatment of chronic pain by stimulation of fibers of primary afferent neuron. Trans Am Meurol Ass. 1968;93:103-105.
6 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns. Anesth Analg. 1967;46:489-491.
7 Adams JE, Hosobuchi Y. Stimulation of internal capsule for relief of chronic pain. J Neurosurg. 1974;41:740-747.
8 Nguen JP, Lefaucher JP, Le Guerinal C, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000;31:263-265.
9 Mitchell SW. Gunshot wounds and other injury of the nerves. Philadelphia: JB Lippincott, 1864.
10 Merskey H, Bogduk N. Classification of chronic pain, 2nd edn. Seattle: IASP Press, 1994.
11 Abbe R. A contribution to the surgery of the spine. Med Rec. 1889;35:149-152.
12 Bell C. Ideas of a new anatomy of the brain. London: Strahan & Preston, 1811.
13 Magendie F. Experiences sur les functions de raciness des nerfs rachidiens. J Physiol [Paris]. 1882;2:246-279.
14 Light AR, Metz CB. The morphology of the spinal cord efferent and afferent neurons contributing to the ventral root of the cat. J Comp Neurol. 1978;179:501-516.
15 Coggeshall RE, Applebaum ML, Fazen M, et al. Unmyelinated axons in human ventral roots, a possible explanation for the failure of dorsal rhizotomy to relieve pain. Brain. 1975;98:157-166.
16 Frykholm R, Hyde J, et al. On pain sensations produced by the stimulation of ventral roots in man. Acta Physiol Scand. 1953;106:455-469.
17 Scoville WB. Extradural spinal sensory rhizotomy. J Neurosurg. 1966;24:94-95.
18 Smith FP. Trans-spinal ganglionectomy for the relief of intercostal pain. J Neurosurg. 1970;32:574-577.
19 McCabe JS, Low FN. The subarachnoid angle: an area of transition in peripheral nerves. Anat Rec. 1969;164:15-34.
20 Sindou M, Daher A. Spinal coablation procedures for pain. In: Dubner, Gebhart, Bond, eds. Pain research and clinical management, vol 3. 1988:447–495.
21 Sindou M, Kervel Y. Analgesie par la methode d’electrostimulation transcutaanee. Neurochirurgie. 1980;26:153-157.
22 Uematsu S. Percutaneous electrotherrmaocoagulation of spinal truck, ganglion and rootlets. Schmidek HH, Sweet WH, editors. Operative neurosurgical techniques; indications, methods and results, vol 2.. Grune and Stratton, New York, 1982;1177-1198.
23 Bernard TN, Broussard TS, et al. Extradural sensory rhizotomy in the management of chronic lumbar spondylosis with radiculopathy. Orthop Trans. 1987;11:23-27.
24 Onofrio B, Campa H. Evaluation of rhizotomy. J Neurosurg. 1972;36:751-755.
25 Loeser JD. Dorsal rhizotomy for the relief of chronic pain. J Neurosurg. 1972;36:745.
26 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
27 Sindou M. Etude de la jonction radiculo-medullaire posterieure: la radicellotomie posterieure selective dans la chirurgie de la douleur. Lyon: These Med, 1972.
28 Nashold BSJr, Urban B, Zorub DS. Phantom pain relief by focal destruction of the substantia gelatinosa of Rolando. Adv Pain Res Ther. 1976;1:959-963.
29 Schoenen J. Organisation neuronale de la moelle epiniere de l’homme; these, Liege. 1980.
30 Denny-Brown D, Kirk EJ, Yanagisawa N. The tract of Lissauer in relation to sensory transmission in the dorsal horn of spinal cord in the macaque monkey. J Comp Neurol. 1973;151:175-200.
31 Nashold BS, Pearlstein R, editors. The DREZ operation. The American Association of Neurological Surgeons Publications Committee, 1996.
32 Friedman AH, Nashold BS, Bronec PR. Dorsal root entry zone lesions for the treatment of brachial plexus avulsion injuries: A follow-up study. J Neurosurg. 1988;22:369-373.
33 Thomas DGT, Kitchen ND. Long-term follow-up of dorsal root entry zone lesions in brachial plexus avulsion. J Neurol Neurosurg Psychiatry. 1994;57:737-738.
34 Rath SA, et al. Results of DREZ-coagulations for pain related to plexus lesions, spinal cord injuries, and post-herpetic neuralgia. Acta Neurochir. 1996;138:364-369.
35 Ostdahl R. DREZ surgery for brachial plexus avulsion pain. The American Association of Neurolgical Surgeons Publications Committee, 1996.
36 Cowie RA, Hitchcock ER. The late results of antero-lateral cordotomy for pain relief. Acta Neurochir. 1982;64:39-50.
37 Rosomoff HL, et al. Effects of percutaneous cervical cordotomy on pulmonary function. J Neurosurg. 1969;31:620-627.
38 White JC, Sweet WH. Pain and the neurosurgeon. A forty year experience. Springfield: Charles C Thomas, 1969.
39 Iskandar BJ, Nashold BS. Spinal and trigeminal DREZ lesion. In: Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill, 1998.
40 Edinger L. Vergleichend-entwicklingsgeschichtliche und anatomische Studien im Beriche des Central-nervensystems. II Uber die Forsetzung der hinten Ruckenmarkswurzlen zum Gehirn. Anat Anz. 1889;4:121-128.
41 Spiller WG. The location within the spinal cord of the fibers for temperature and pain sensations. J Nerv Ment Dis. 1905;32:318-320.
42 Spiller WG, Martin E. The treatment of persistent pain of organic origin in the lower part of the body by division of the anterolateral column of the spinal cord. JAMA. 1912;58:1489-1490.
43 Mullan S, et al. Percutaneous interruption of spinal-pain tracts by means of strontium-90 needle. J Neurosurg. 1963;20:931-939.
44 Rosomoff HL, et al. Percutaneous radiofrequency cervical cordotomy. J Neurosurg. 1965;23:639-644.
45 Onofrio BM. Cervical spinal cord and dentate delineation in percutaneous radiofrequency cordotomy at the level of the first to second cervical vertebrae. Surg Gynecol Obstet. 1971;133:30-34.
46 Kanpolat Y, et al. CT-guided extralemniscal myelotomy. Acta Neurochir. 1988;91:151-152.
47 Gildenberg PL, et al. Impedance monitoring device for detection of penetration of the spinal cord in anterior percutaneous cordotomy. J Neurosurg. 1969;30:87-92.
48 Hitchcock E, Leece B. Somatotopic representation of the respiratory pathways in the cervical cord in man. J Neurosurg. 1967;27:320-329.
49 Poletti, CE. Open cordotomy: new technique. In: Schmidek HH, Sweet WH, eds. Operative neurosurgical techniques: indication, methods and results, vol 2. 1982:1119–1136.
50 Rosomoff HL, Carroll F, Brown J. Percutaneous radiofrequency cervical cordotomy technique. J Neurosurg. 1965;23:639-644.
51 Lin PM, et al. An anterior approach to percutaneous lower cervical cordotomy. J Neurosurg. 1966;25:553.
52 White JC, Sweet WH. Anterolateral cordotomy: Open versus closed comparison of end results. Adv Pain Res. 1979;3:911-919.
53 Lorenz R. Method of percutaneous spinothalamic tract section. Krayenbuhl H, Brihaye J, editors. Advances and technical standards in neurosurgery, vol 3.. Springer-Verlag, Vienna, 1976;123-154.
54 Sindou M, et al. Ablative neurosurgical procedures for the treatment of chronic pain. Neurophysiol Clin. 1990;20:399-423.
55 Tasker RR. Outcomes of surgery for movement disorders and pain. In: Wilden JN, ed. Outcomes of neurological and neurosurgical disorders. Cambridge, MA: Cambridge University Press;
56 Rosomoff HL, Papo I, Loeser JD, et al. Neurosurgical operations on the spinal cord. In: Bonica JJ, editor. The management of pain. 2nd edn. Philadelphia: Lea and Febiger; 1990:2067-2081.
57 Lin PM. Percutaneous lower cervical cordotomy. In: Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill; 1998:1403-1409.
58 Nathan PW. Results of antero-lateral cordotomy for pain in cancer. J Neurol Neurosurg Psychiatry. 1963;26:353-362.
59 Rosomoff HL. Bilateral percutaneous cervical radiofrequency cordotomy. J Neurosurg. 1969;31:41-46.
60 Tasker RR. Percutaneous cordotomy for persistent pain. In: Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill; 1998:1491-1505.
61 Hitchcock ER. Stereotactic cervical myelotomy. J Neurol Neurosurg Psychiatry. 1970;33:224-230.
62 Gildenberg PL, Hirshberg RM. Limited myelotomy for the treatment of intractable cancer pain. J Neurol Neurosurg Psychiatry. 1984;47:94-96.
63 Schvarcz JR. Stereotactic extralemniscal myelotomy. J Neurol Neurosurg Psychiatry. 1976;39:53.
64 Fink RA. Neurosurgical treatment of nonmalignant intractable rectal pain: microsugical commissural myelotomy with the carbon dioxide laser. Neurosurgery. 1984;14:64.
65 Adams JE, Lippert R, Hosobuchi Y. Commissural myelotomy. Schmidek HH, Sweet WH, editors. Operative neurosurgical techniques: indications, methods, and results, 2nd edn., vol. 2. Philadelphia: WB Saunders, 1988;1185-1189.
66 Broager B. Commissural myelotomy. Surg Neurol. 1974;2:71-74.
67 Cook AW, Kawakami Y. Commissural myelotomy. J Neurosurg. 1977;47:1-6.
68 Gybels JM, Sweet WH. Neurosurgical treatment of persistent pain. pain and headache, vol 11 . Karger, Basel, 1989.
69 Armour D. Surgery of the spinal cord and its membranes. Lancet. 1927;I:691-697.
70 Jefferson A. Cordectomy for intractable pain in paraplegia. In: Lipton, Miles, eds. Persistant pain: modern methods for treatment, vol. 4. 1983:115–132.
71 Durward QJ, Rice GP, et al. Selective spinal cordectomy: clinicopathogical correlation. J Neurosurg. 1982;56(3):359-367.
72 Greenwood B. The origins of sympathectomy. Med Hist. 1967;11:166-169.
73 Sweet WH. Sympathectomy for pain. In: Youmans JR, editor. Neurological surgery. 3rd edn. Philadelphia: WB Saunders; 1990:4086-4107.
74 Sadar ES, Cooperman MA. Bilateral thoracic sympathectomy–splanchnicectomy in the treatment of intractable pain due to pancreatic carcinoma. Cleve Clin Q. 1974;41:185-188.
75 Flanigan DP, Kraft RO. Continuing experience with palliative chemical splanchnicectomy. Arch Surg. 1978;113:50-51.
76 Mattassi T, Miele F. Thoracic sympathectomy: Review of indications, results, and surgical techniques. J Cardiovasc Surg 22:336–339.
77 Shih CJ, Wang YC. Thoracic sympathectomy for palmar hyperhydrosis: Report of 457 cases. Surg Neurol. 1978;10:291-296.
78 Berguer R, Smit R. Transaxillary sympathectomy (T2 to T4) for relief of vasospastic/sympathetic pain of upper extremities. Surgery. 1981;89:764-769.
79 Gybels JM, Sweet WH. Sympathectomy for pain. In: Neurosurgical treatment of persistent pain. New York: Karger; 1984:257-282.
80 Hardy RW. Surgery of the sympathetic nervous system. Schmidek HH, Sweet WH, editors. Operative neurosurgical techniques: indications, methods and results, vol. 2.. Grune and Stratton, New York, 1982;1045-1061.
81 Dawson DM, Katz M. Reflex sympathetic dystrophy. Neurol Chron. 1993;8:1-6.
82 Schwartzman RJ, McLellan TL. Reflex sympathetic dystrophy: a review. Arch Neurol. 1987;44:555-561.
83 Shealy CN. Percutaneous radiofrequency denervation of spine facets, treatment of chronic back pain and sciatica. J Neursurg. 1978;43:448-451.
84 Goldthwait JE. The lumbo-sacral articulation: an explanation of many cases of ‘lumbago,’ ‘sciatica,’ and paraplegia. Boston Med Surg J. 1911;164:365-372.
85 Putti V. New conceptions in the pathogenesis of sciatic pain. Lancet. 1927;2:53-60.
86 Ghormley RK. Low back pain. J Am Med Assoc. 1933;101:1773-1777.
87 Rees WE. Multiple bilateral subcutaneous rhizolysis of segmental nerves in the treatment of the intervertebral disc syndrome. Ann Gen Pract. 1971;26:126-127.
88 King JS, Lagger R. Sciatica viewed as a referred pain syndrome. Surg Neurol. 1976;5:46-50.
89 Bogduk N, Long DM. The anatomy of the so-called ‘articular nerves’ and their relationship to facet denervation in the treatment of low back pain. J Neurosurg. 1979;51:172-177.
90 Bogduk N, Long DM. Percutaneous lumbar medial branch neurotomy: a modification of facet denervation. Spine. 1980;5:193-200.
91 Wedley JR, Gallagherr J, Hamann W, et al. An evaluation of facet joint denervation in the management of low back pain. Pain. 1986;24:67-73.
92 Shealy CN. Percutaneous radiofrequency denervation of spinal facets. J Neurosurg. 1979;43:448-451.
93 Burton CV. Percutaneous radiofrequency facet denervation. Appl Neurophysiol. 1976;39:80-86.
94 Lora J, Long D. So-called facet denervation in the management of intractable back pain. Spine. 1976;1:121-126.
95 North RB, et al. Radiofrequency lumbar facet denervation: analysis of prognostic factors. Pain. 1994;57:77-83.
96 Ignelzi RJ, Cummings TW. A statistical analysis of percutaneous radiofrequency lesions in the treatment of chronic low back pain and sciatica. Pain. 1980;8:181-187.