Chapter 46 Sphincter of Oddi Dysfunction
Introduction
Since its original description by Oddi in 1887, the sphincter of Oddi has been the subject of much study and controversy. Its very existence as a distinct anatomic or physiologic entity has been disputed. Not surprisingly, the clinical syndrome of sphincter of Oddi dysfunction (SOD) and its therapy are controversial areas.1,2 Nevertheless, SOD is commonly diagnosed and treated by physicians. This chapter reviews the epidemiology and clinical presentation of SOD and currently available diagnostic and therapeutic modalities.
Definitions
Postcholecystectomy pain resembling the patient’s preoperative biliary colic occurs in at least 10% to 20% of patients.3 These patients should have appropriate noninvasive and invasive (when clinically appropriate) evaluation to rule out common bile duct (CBD) stones, tumors, or strictures near the cholecystectomy site. Patients in whom these entities are ruled out have a high frequency of SOD. SOD refers to an abnormality of sphincter of Oddi contractility. It is a benign, noncalculous obstruction to flow of bile or pancreatic juice through the pancreaticobiliary junction (i.e., the sphincter of Oddi) resulting from a dyskinetic or stenotic sphincter of Oddi. SOD may be manifested clinically by “pancreaticobiliary” pain, pancreatitis, abnormal liver function tests, or abnormal pancreatic enzymes. Sphincter of Oddi dyskinesia refers to a motor abnormality of the sphincter of Oddi, which may result in a hypotonic sphincter but, more commonly, causes a hypertonic sphincter. In contrast, sphincter of Oddi stenosis refers to a structural alteration of the sphincter, probably from an inflammatory process, with subsequent fibrosis.
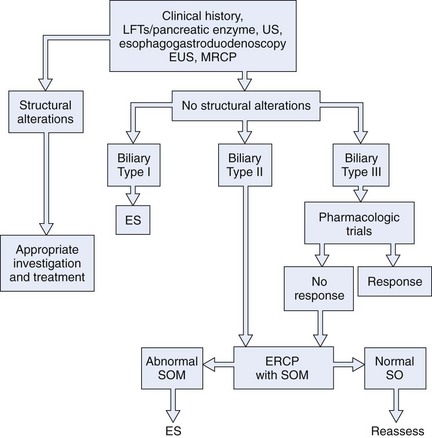
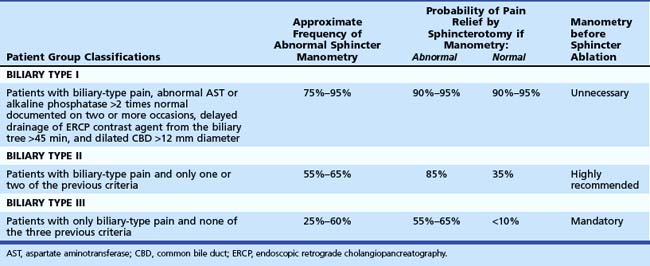
Because it is often impossible to distinguish patients with sphincter of Oddi dyskinesia from patients with sphincter of Oddi stenosis, the term SOD has been used to incorporate both groups of patients. In an attempt to deal with this overlap in etiology and to determine the appropriate use of sphincter of Oddi manometry (SOM), a biliary clinical classification system has been developed for patients with suspected SOD (Hogan-Geenen SOD classification system; Table 46.1) based on clinical history, laboratory results, and endoscopic retrograde cholangiopancreatography (ERCP) findings.4 A pancreatic classification has also been developed, but it is less commonly used (Box 46.1).5 Both the biliary and the pancreatic classification systems have been modified,6 making them more applicable for clinical use because biliary and pancreatic drainage times have been generally abandoned. Various less accurate terms—papillary stenosis, ampullary stenosis, biliary dyskinesia, and postcholecystectomy syndrome—are used in the medical literature to describe this entity. The last term, postcholecystectomy syndrome, is a misnomer because SOD may occur with an intact gallbladder.
Table 46.1 Hogan-Geenen Biliary Sphincter of Oddi Classification System (Postcholecystectomy) Related to the Frequency of Abnormal Sphincter of Oddi Manometry and Pain Relief by Biliary Sphincterotomy
Box 46.1 Pancreatic Sphincter of Oddi Classification System
Patient Group Classification
Pancreatic Type III
Patients with pancreatic-type pain only and no other abnormalities
ERCP, endoscopic retrograde cholangiopancreatography; PD, pancreatic duct.
Adapted from Sherman S, Troiano FP, Hawes RH, et al: Frequency of abnormal sphincter of Oddi manometry compared with the clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol 86:586–590, 1991.
Anatomy, Physiology, and Pathophysiology
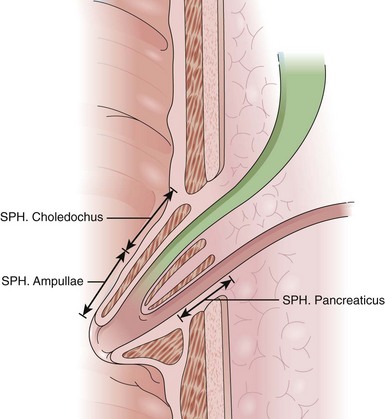
The anatomy, physiology, and pharmacology of the sphincter of Oddi have been reviewed by Bosch and Pena.7 The sphincter of Oddi is a small complex of smooth muscles surrounding the terminal CBD, main (ventral) pancreatic duct (of Wirsung), and common channel (ampulla of Vater), when present (Fig. 46.1). It has both circular and figure-eight components. The high-pressure zone generated by the sphincter is variably 4 to 10 mm in length. Its role is to regulate bile and pancreatic exocrine juice flow and to prevent duodenum-to-duct reflux (i.e., maintain a sterile intraductal environment). The sphincter of Oddi possesses both a variable basal pressure and phasic contractile activity. The former seems to be the predominant mechanism, regulating outflow of pancreaticobiliary secretion into the intestine. Although phasic sphincter of Oddi contractions may aid in regulating bile and pancreatic juice flow, their primary role seems to be maintaining a sterile intraductal milieu.
Sphincter regulation is under neural and hormonal control. Phasic wave activity of the sphincter is closely tied to the migrating motor complex of the duodenum. Innervation of the bile duct does not seem to be essential because sphincter function has been reported to be preserved after liver transplantation.8 Although regulatory processes vary among species, cholecystokinin (CCK) and secretin seem to be most important in causing sphincter relaxation, whereas nonadrenergic, noncholinergic neurons, which at least partially transmit vasoactive intestinal peptide and nitric oxide, also relax the sphincter.9 The role of cholecystectomy in altering these neural pathways needs further definition.
Luman and colleagues10 reported that cholecystectomy, at least in the short-term, suppresses the normal inhibitory effect of pharmacologic doses of CCK on the sphincter of Oddi. The mechanism of this effect is unknown, however. Wedge specimens of the sphincter of Oddi obtained at surgical sphincteroplasty from patients with SOD show evidence of inflammation, muscular hypertrophy, fibrosis, or adenomyosis within the papillary zone in approximately 60% of patients.11 In the remaining 40% with normal histology, a motor disorder is suggested. Less commonly, infections with cytomegalovirus or Cryptosporidium, as may occur in patients with acquired immunodeficiency syndrome (AIDS), or Strongyloides have caused SOD.
How does SOD cause pain? From a theoretical point of view, abnormalities of sphincter of Oddi pressure can give rise to pain by (1) impeding the flow of bile and pancreatic juice resulting in ductal hypertension, (2) inducing ischemia arising from spastic contractions, and (3) resulting in “hypersensitivity” of the papilla. Although unproved, these mechanisms may act alone or in concert to explain the genesis of pain. Patients with SOD have been shown to have lower perception thresholds in the referred pain area. Visceral and referred hyperalgesia may be important features in the pathogenesis of pain in patients with SOD.12
Epidemiology
SOD may occur in children or adults of any age; however, patients with SOD are typically middle-aged women.13,14 Although SOD most commonly occurs after cholecystectomy, it may be present with the gallbladder in situ.15 In a survey on functional gastrointestinal (GI) disorders, SOD seemed to have a significant impact on quality of life because it was highly associated with work absenteeism, disability, and health care use.16 Using a brief symptom inventory and the 12-item short form health survey (SF-12), Winstead and Wilcox17 found that patients with biliary SOD and unexplained recurrent pancreatitis had a significantly worse quality of life than nonpatients, high levels of somatic complaints, and a common history of sexual and physical abuse (20%).
Limited studies of the frequency of manometrically documented SOD in patients before cholecystectomy have been done. Guelrud and colleagues18 evaluated 121 patients with symptomatic gallstones and a normal CBD diameter (by transcutaneous ultrasound) by SOM before cholecystectomy. An elevated basal sphincter pressure was found in 14 (11.6%) patients. SOD was diagnosed in 4.1% (4 of 96) of patients with a normal serum alkaline phosphatase and in 40% (10 of 25) with an elevated serum alkaline phosphatase. Ruffolo and associates19 evaluated 81 patients with symptoms suggestive of biliary disease but normal ERCP and no gallbladder stones on transcutaneous ultrasound by scintigraphic gallbladder ejection fraction and endoscopic SOM. Of patients, 53% had SOD, and 49% had an abnormal gallbladder ejection fraction. SOD occurred with a similar frequency in patients with an abnormal gallbladder ejection fraction (50%) and a normal ejection fraction (57%).
The frequency of diagnosing SOD varies considerably in reported series with the patient selection criteria, the definition of SOD, and the diagnostic tools used. In a British report, SOD was diagnosed in 41 (9%) of 451 consecutive patients being evaluated for postcholecystectomy pain.20 Roberts-Thomson and Toouli21 evaluated 431 similar patients and found SOD in 47 (11%). In a subpopulation of patients with normal ERCP (except dilated ducts in 28%) and recurrent pain of more than 3 months’ duration, SOD was diagnosed in 68%. Sherman and colleagues5 used SOM to evaluate 115 patients with pancreaticobiliary pain with and without liver function test abnormalities. Patients with bile duct stones and tumors were excluded from analysis. Of 115 patients, 59 (51%) showed abnormal basal sphincter of Oddi pressure greater than 40 mm Hg. These patients were categorized further by the Hogan-Geenen SOD classification system based on clinical presentation, laboratory results, imaging tests, and ERCP findings (see Table 46.1). The frequency of abnormal manometry of one or both sphincter segments was 86%, 55%, and 28% for biliary type I, II, and III patients. These abnormal manometric frequencies were very similar to the frequencies reported by others for type I and type II patients.22,23 In biliary type III patients, the finding of an abnormal basal sphincter pressure has ranged from 12% to 59%.6,24 Patient selection factors may be one explanation for this great variability.
SOD can involve abnormalities in the biliary sphincter, pancreatic sphincter, or both.6,25 The true frequency of SOD depends on whether one or both sphincters are studied. Eversman and colleagues6 performed manometry of the biliary and pancreatic sphincter segments in 360 patients with pancreatobiliary pain and intact sphincters. In this large series, 19% had abnormal pancreatic sphincter basal sphincter pressure alone, 11% had abnormal biliary basal sphincter pressure alone, and 31% had abnormal basal sphincter pressure in both segments (overall frequency of sphincter dysfunction was 61%). Among the 214 patients labeled type III by a modified Hogan-Geenen SOD classification system, 17%, 11%, and 31% had elevated basal sphincter pressure in the pancreatic sphincter alone, biliary sphincter alone, or both segments (overall frequency of SOD was 59%). In 123 type II patients, SOD was diagnosed in 65%; 22%, 11%, and 32% had elevated basal sphincter pressure in the pancreatic sphincter only, biliary sphincter only, or both sphincter segments. Similar findings were reported by Aymerich and colleagues.26 In a series of 73 patients with suspected SOD, basal pressures were normal in both segments in 19%, abnormal in both segments in 40%, and abnormal in one segment but normal in the other segment in 41%. The negative predictive value of normal biliary basal sphincter pressure in excluding SOD was 0.42; when the pancreatic basal sphincter pressure was normal, the negative predictive value was 0.58. These two studies suggest that both the bile duct and the pancreatic duct should be evaluated when assessing the sphincter by SOM.
Although SOM has traditionally been thought to be reproducible,27 two more recent studies have shown abnormal sphincter pressures in 42% and 60% in symptomatic patients restudied about 1 year after a normal study.28,29 Dysfunction may occur in the pancreatic duct portion of the sphincter of Oddi and cause recurrent pancreatitis. As noted earlier, a pancreatic SOD classification system has been developed (see Box 46.1), but it has not been widely used.5,6 Manometrically documented SOD has been reported in 15% to 72% of patients with recurrent pancreatitis, previously labeled as idiopathic5,24,30; this is discussed later in this chapter.
Clinical Presentation
A symposium on functional disorders of the pancreas and biliary tree established the Rome III diagnostic criteria31 for SOD. These criteria include episodes of severe abdominal pain located in the epigastrium or right upper quadrant or both and all of the following: (1) symptom episodes last 30 minutes or more with pain-free intervals, (2) recurrent symptoms occur at different intervals (not daily), (3) the pain builds up to a steady level, (4) the pain is moderate to severe enough to interrupt the patient’s daily activities or lead to an emergency department visit, (5) the pain is not relieved by bowel movements, (6) the pain is not relieved by postural change, (7) the pain is not relieved by antacids, (8) other structural diseases that would explain the symptoms are excluded. The pain may manifest in one or more of the following ways: pain with nausea and vomiting, pain radiation to the back or right subscapular region or both, and pain awakens patient from sleep.
Physical examination is typically characterized only by mild epigastric or right upper quadrant tenderness. The pain is not relieved by trial medications for acid peptic disease or irritable bowel syndrome. Laboratory abnormalities consisting of transient elevation of liver function tests, typically during episodes of pain, are present in less than 50% of patients. Patients with SOD may present with typical pancreatic pain (epigastric or left upper quadrant radiating to the back) with or without pancreatic enzyme elevation and recurrent pancreatitis. The pain is often indistinguishable from biliary pain.31 SOD may exist in the presence of an intact gallbladder.18,19,32 Because the symptoms of SOD or gallbladder dysfunction cannot be reliably separated, the diagnosis of SOD is commonly made after cholecystectomy or less often after gallbladder abnormalities have been excluded.31
Clinical Evaluation
General Initial Evaluation
Evaluation of patients with suspected SOD (i.e., patients with upper abdominal pain with characteristics suggestive of a pancreatobiliary origin) should be initiated with standard serum liver chemistries; serum amylase or lipase or both; and abdominal ultrasound, magnetic resonance (MR) imaging or magnetic resonance cholangiopancreatography (MRCP), or computed tomography (CT) scans. Serum enzyme studies should be drawn during bouts of pain, if possible. Mild elevations (<2 times upper limits of normal) are frequent in SOD, whereas greater abnormalities are more suggestive of stones, tumors, and liver parenchymal disease. Although the diagnostic sensitivity and specificity of abnormal serum liver chemistries are low,33 evidence suggests that the presence of abnormal liver tests in type II biliary SOD patients may predict a favorable response to endoscopic sphincterotomy.34
Diagnostic Methods (Noninvasive)
Morphine-Prostigmin Provocative Test (Nardi Test)
Morphine has been shown to cause sphincter of Oddi contraction, as assessed manometrically. Neostigmine (Prostigmin), 1 mg subcutaneously, is added as a vigorous cholinergic secretory stimulant to morphine, 10 mg subcutaneously, to make this challenge test. The morphine-Prostigmin test was used extensively in the past to diagnose SOD. Reproduction of the patient’s typical pain associated with a fourfold increase in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, amylase, or lipase levels constitutes a positive response. The usefulness of this test is limited by its low sensitivity and specificity in predicting the presence of SOD and its poor correlation with outcome after sphincter ablation.35,36 This test has largely been replaced by tests thought to be more sensitive.
Radiographic Assessment of Extrahepatic Bile Duct and Main Pancreatic Duct Diameter after Secretory Stimulation
After a lipid-rich meal or CCK administration, the gallbladder contracts, bile flow from the hepatocytes increases, and the sphincter of Oddi relaxes, resulting in bile entry into the duodenum. Similarly, after a lipid-rich meal or secretin administration, pancreatic exocrine juice flow is stimulated, and the sphincter of Oddi relaxes. If the sphincter of Oddi is dysfunctional and causes obstruction to flow, the CBD or main pancreatic duct may dilate under secretory pressure; this can be monitored by transcutaneous ultrasound. Sphincter and terminal duct obstruction from other causes (stones, tumors, strictures) may similarly cause ductal dilation and need to be excluded. Pain provocation should also be noted if present. Limited studies comparing these noninvasive tests with SOM or outcome after sphincter ablation37–42 show only modest correlation. Because of overlying intestinal gas, the pancreatic duct may not be visualized on standard transcutaneous ultrasound. Despite the superiority of EUS in visualizing the pancreas, Catalano and coworkers43 reported the sensitivity of secretin-stimulated EUS in detecting SOD to be only 57%.
MRCP can also be performed to monitor the pancreatic duct noninvasively after secretin stimulation. It is also the best noninvasive test to obtain a cholangiogram and pancreatogram and evaluate for other structural causes for the patient’s symptoms. Aisen and colleagues44 showed that the pancreatic diameter increased significantly after secretin injection (monitored by MRCP), but the amount of increase and the duration of increase were similar for patients with normal and abnormal basal sphincter pressure. Pereira and colleagues45 and Baillie and Kimberly46 also found secretin-stimulated MRCP to be insensitive in predicting abnormal manometry. In contrast, in a pilot study of 15 patients with idiopathic pancreatitis, secretin-stimulated MRCP and SOM were concordant in 87%.47 However, Testoni and colleagues48 reported a disappointing negative predictive value for SOD and clinical success of sphincter ablation.
Quantitative Hepatobiliary Scintigraphy
Hepatobiliary scintigraphy (HBS) assesses bile flow through the biliary tract. Impairment to bile flow from sphincter disease, tumors, or stones (and parenchymal liver disease) results in impaired radionuclide flow. The precise criteria to define a positive (abnormal) study are controversial, but a prolonged duodenal arrival time, a prolonged hepatic hilum-to-duodenal transit time, and a high Johns Hopkins scintigraphic score are most widely used.49–51 Four studies49,52–54 showed a correlation between HBS and SOM. Taking these four studies as a whole, totaling 105 patients, the overall sensitivity of HBS using SOM as the “gold standard” was 78% (range 44% to 100%), specificity was 90% (range 80% to 100%), positive predictive value was 92% (range 82% to 100%), and negative predictive value was 81% (range 62% to 100%). These promising results have not been reproduced by others, however.
Overall, patients with dilated bile ducts and high-grade obstruction seem likely to have a positive scintigraphic study. Esber and colleagues55 found that patients with lower grade obstruction (Hogan-Geenen classification types II and III) generally have normal scintigraphy, even if done after CCK provocation. Pineau and coworkers56 reported that 8 of 20 asymptomatic control subjects had an abnormal CCK-stimulated study. Using SOM as the “gold standard” in 29 patients with suspected SOD, two independent reviewers found the Johns Hopkins scintigraphic score to have a sensitivity of 25% to 38%, specificity of 85% to 90%, positive predictive value of 40% to 60%, and negative predictive value of 75% to 79% for diagnosing SOD.57 The hepatic hilum-to-duodenal transit time had a sensitivity of 13%, specificity of 95%, positive predictive value of 50%, and negative predictive value of 74%. The duodenal arrival time mirrored the hepatic hilum-to-duodenal transit time findings. The value of adding morphine provocation to HBS was reported.54 In 34 patients with a clinical diagnosis of type II and type III SOD, scintigraphy with and without morphine and subsequent biliary manometry were performed. The standard HBS scan did not distinguish between patients with normal and abnormal SOM. However, after provocation with morphine, there were significant differences in the time to maximal activity and the percentage of excretion at 45 minutes and 60 minutes. Using a cutoff value of 15% excretion at 60 minutes, the use of morphine during HBS increased the sensitivity and specificity for SOD detection to 83% and 81%.
The Milwaukee group reported their retrospective review of fatty meal sonography (FMS) and HBS as potential predictors of SOD.58 In this study, 304 postcholecystectomy patients suspected to have SOD were evaluated by SOM, FMS, and HBS. A diagnosis of SOD was made in 73 patients (24%) by using SOM as the reference standard. The sensitivity of FMS was 21%, and the sensitivity of HBS was 49%, whereas specificities were 97% and 78%. FMS, HBS, or both were abnormal in 90%, 50%, and 44% of patients with Hogan-Geenen SOD types I, II, and III. Of 73 patients who underwent biliary sphincterotomy, 40 had a good long-term response. Among these patients with SOD, 11 (85%) of 13 patients with abnormal HBS and FMS had a good long-term response. This study suggested that although noninvasive tests are unable to predict an abnormal SOM with high sensitivity, they may be of assistance in predicting response to sphincter ablation in patients with SOD.
Cicala and colleagues59 compared the reliability of HBS (hepatic hilum-to-duodenal transit time was measured) with SOM of the biliary sphincter in 30 postcholecystectomy patients (8 type I, 22 type II; 40% were men). HBS was abnormal in all 15 patients with abnormal maximal basal sphincter pressures and in 7 of 15 patients with normal maximal basal sphincter pressures. Of 14 patients with abnormal HBS who agreed to undergo biliary sphincterotomy, 13 were asymptomatic and had normal liver function tests, amylase levels, and lipase levels at 10 to 13 months of follow-up. All eight patients with abnormal HBS who refused to undergo sphincterotomy remained symptomatic. A favorable postsphincterotomy outcome was predicted by hepatic hilum-to-duodenal transit in 93% and by SOM in 57% of patients.
Although this study suggested that HBS is a useful and noninvasive test to diagnose SOD and is a reliable predictor of sphincterotomy outcome in postcholecystectomy biliary type I and type II patients, several concerns exist. Of enrolled patients, 40% were men, which is unusually high in the SOD population, and the frequency of abnormal maximal basal sphincter pressures in biliary type II patients was exceedingly low (36%). If the authors had used the mean basal sphincter pressure, which is the more commonly recommended manometric parameter for diagnosing SOD, the frequency of an abnormal SOM would likely have been even lower. This calls into question the authors’ SOM technique and interpretation. In the absence of more definitive data, we and others conclude that use of HBS as a screening tool for SOD should not be recommended for general clinical use.60 Abnormal results may be found in asymptomatic controls.56 HBS does not address the pancreatic sphincter, which may be dysfunctional and a cause for patient symptoms. Use of HBS and other noninvasive methods should be reserved for situations in which more definitive testing (manometry) is unsuccessful or unavailable.
Diagnostic Methods (Invasive)
Endoscopic Retrograde Cholangiopancreatography
Although some controversy exists, extrahepatic ducts that are greater than 12 mm in diameter (postcholecystectomy) when corrected for magnification are considered dilated. Drugs that affect the rate of bile flow and relaxation or contraction of the sphincter of Oddi influence drainage of contrast material and must be avoided to obtain accurate drainage times (if drainage time is desired). Because the extrahepatic bile duct angulates from anterior (the hilum) to posterior (the papilla), the patient must be supine to assess gravitational drainage through the sphincter. Although definitive normal supine drainage times have not been well defined,61 a postcholecystectomy biliary tree that fails to empty all contrast material by 45 minutes is generally considered abnormal. Endoscopic evaluation of the papilla and peripapillary area can yield important information that can influence the diagnosis and treatment of patients with suspected SOD. Occasionally, ampullary cancer may simulate SOD. The endoscopist should do tissue sampling of the papilla (preferably after sphincterotomy) in suspicious cases.62
Radiographic features of the pancreatic duct are also important to assess in a patient with suspected SOD. Dilation of the pancreatic duct (>6 mm in the pancreatic head and >5 mm in the body) and delayed contrast agent drainage time (9 minutes in the prone position) may give indirect evidence for the presence of SOD. ERCP alone is generally not indicated in the evaluation of abdominal pain of obscure origin in the absence of objective findings that suggest a biliary or pancreatic disease (i.e., type III patient).63 Sherman and colleagues64 found that only 10% of 197 patients with pancreaticobiliary pain, normal liver function tests, serum amylase, upper GI tract evaluation, and abdominal ultrasound or CT scan had an ERCP finding that might affect their therapy, including chronic pancreatitis (7%), gallbladder stones or sludge (2%), and a choledochal cyst (1%). Most of these findings could have been identified on noninvasive (MR imaging or MRCP) and less invasive (EUS) imaging tests. In view of the high procedure-related complication rate in these patients, the investigators concluded that ERCP alone could not be justified. In the National Institutes of Health State-of-the-Science Conference on ERCP, it was concluded that ERCP, if performed in type III patients, should be coupled with SOM.65
Intraductal Ultrasound
Intraductal ultrasound makes it possible to assess sphincter of Oddi morphology during endoscopy. The sphincter appears as a thin hypoechoic circular structure on intraductal ultrasound.66 Limited studies so far reveal no correlation between the basal sphincter pressures (as detected at SOM) and the thickness of the hypoechoic layer.67 Although intraductal ultrasound may provide additional information at the level of the sphincter, it cannot be used as a substitute for SOM.
Sphincter of Oddi Manometry
The most definitive development in understanding of the pressure dynamics of the sphincter of Oddi occurred with the advent of SOM. SOM is the only available method to measure sphincter of Oddi motor activity directly. Although SOM can be performed intraoperatively and percutaneously, it is most commonly done in the ERCP setting. SOM is considered by most authorities to be the “gold standard” for evaluating patients for SOD.68,69 The use of manometry to detect motility disorders of the sphincter of Oddi is similar to its use in other parts of the GI tract. However, performance of SOM is more technically demanding and hazardous, with complication rates (in particular, pancreatitis) of 30% reported. Questions remain as to whether these short-term observations (2- to 10-minute recordings per pull-through) reflect the 24-hour pathophysiology of the sphincter. Despite some problems, SOM is gaining more widespread clinical application.
Technique and Indications for Sphincter of Oddi Manometry
SOM is usually performed at the time of ERCP. All drugs that relax (anticholinergics, nitrates, calcium channel blockers, glucagon) or stimulate (most narcotics, cholinergic agents) the sphincter should be avoided for at least 8 to 12 hours before manometry and during the manometric session. Current data indicate that benzodiazepines do not affect the sphincter pressure and are acceptable sedatives for SOM. Meperidine, at a dose of 1 mg/kg or less, does not affect the basal sphincter pressure but does alter phasic wave characteristics.70 Because the basal sphincter pressure is generally the only manometric criterion used to diagnose SOD and determine therapy, it was suggested that meperidine could be used to facilitate conscious sedation for manometry. Droperidol71 and propofol72 are increasingly used for SOM, and it appears that these agents also do not affect the basal sphincter pressure. Similarly, ketamine, used in combination with meperidine and diazepam or midazolam, does not significantly alter the biliary and pancreatic basal sphincter pressure.73 If glucagon must be used to achieve cannulation, an 8- to 15-minute waiting period is required to restore the sphincter to its basal condition.
Catheters sized 5-Fr should be used because virtually all standards have been established with catheters of this size. Triple-lumen catheters are state-of-the-art and are available from several manufacturers. Various catheter types can be used. Catheters with a long intraductal tip may help secure the catheter within the bile duct, but such a long nose is commonly a hindrance if pancreatic manometry is desired. Over-the-wire (monorail) catheters can be passed after first securing one’s position within the duct with a guidewire. Whether this guidewire influences basal sphincter pressure is unknown. Some triple-lumen catheters accommodate a 0.018- to 0.021-inch diameter guidewire passed through the entire length of the catheter and can be used to facilitate cannulation or maintain position in the duct. A study in our unit found, however, that stiffer shafted nitinol core guidewires used for this purpose commonly increase basal sphincter pressure by 50% to 100%.74 To avoid such artifacts, such wires need to be avoided, the wires need to be pulled back into the catheter during the recording period, or guidewires with a very soft core must be used.
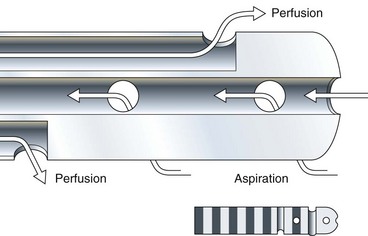
Aspiration catheters, in which one recording port is sacrificed to permit both end and side-hole aspiration of intraductal juice, are highly recommended for pancreatic manometry (Fig. 46.2). Most centers prefer to perfuse the catheters at 0.25 mL/channel using a low-compliance pump. Lower perfusion rates give accurate basal sphincter pressures but do not give accurate phasic wave information. A new water perfused sleeve system, similar to that used in the lower esophageal sphincter, awaits further study in the sphincter of Oddi.75 The perfusate is generally distilled water, although physiologic saline needs further evaluation. The latter may crystallize in the capillary tubing of perfusion pumps and must be flushed out often.
SOM requires selective cannulation of the bile duct or pancreatic duct (Fig. 46.3). The duct entered can be identified by gently aspirating on any port. The appearance of yellow fluid in the endoscopic view indicates entry into the bile duct. Clear aspirate indicates that the pancreatic duct was entered. It is preferable to obtain a cholangiogram or pancreatogram or both before performing SOM because certain findings (e.g., CBD stone) may obviate the need for SOM; this can be done simply by injecting contrast material through one of the perfusion ports. Blaut and colleagues76 showed that injection of contrast material into the biliary tree before SOM does not significantly alter sphincter pressure characteristics. Similar evaluation of the pancreatic sphincter after contrast agent injection has not been reported. To ensure accurate pressure measurements, one must ensure that the catheter is not impacted against the wall of the duct. When deep cannulation is achieved and the patient is adequately sedated, the catheter is withdrawn across the sphincter at 1- to 2-mm intervals by standard station pull-through technique.
Ideally, both the pancreatic and the bile ducts should be studied. Data indicate that an abnormal basal sphincter pressure may be confined to one side of the sphincter in 35% to 65% of patients with abnormal manometry.6,26,77–80 One sphincter may be dysfunctional, whereas the other is normal. Raddawi and colleagues77 reported that an abnormal basal sphincter was more likely to be confined to the pancreatic duct segment in patients with pancreatitis and to the bile duct segment in patients with biliary-type pain and elevated liver function tests. Abnormalities of the basal sphincter pressure ideally should be observed for at least 30 seconds in each lead and be seen on two or more separate pull-throughs. From a practical clinical standpoint, we settle for one pull-through (from each duct) if the readings are clearly normal or abnormal.
Criteria for interpretation of a sphincter of Oddi tracing are standard; however, they may vary from center to center. Some areas in which there may be disagreement in interpretation include the required duration of basal sphincter of Oddi pressure elevation, the number of leads in which basal pressure elevation is required, and the role of averaging pressures from the three (or two in an aspirating catheter) recording ports.4 Our recommended method for reading the manometry tracings is first to define the zero duodenal baseline before and after the pull-through. Alternatively, intraduodenal pressure can be continuously recorded from a separate intraduodenal catheter attached to the endoscope.
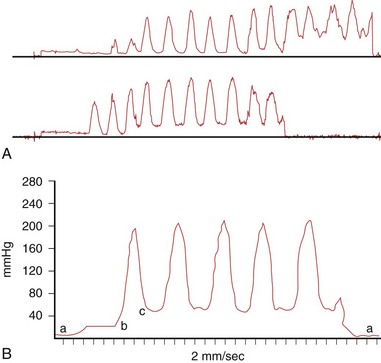
The highest basal pressure (defined as the pressure above the zero duodenal baseline; Fig. 46.4) that is sustained for at least 30 seconds is identified. The mean of the readings from the four lowest amplitude points in this zone is taken as the basal sphincter pressure for that lead for that pull-through. The basal sphincter pressure for all interpretable observations is averaged; this is the final basal sphincter pressure. The amplitude of phasic wave contractions is measured from the beginning of the slope of the pressure increase from the basal pressure to the peak of the contraction wave. Four representative waves are taken for each lead, and the mean pressure is determined. The number of phasic waves per minute and the duration of the phasic waves can also be determined.
Most authorities read only the basal sphincter pressure as an indicator of pathology of the sphincter of Oddi. However, data from Kalloo and coworkers81 suggest that intraductal biliary pressure, which is easier to measure than sphincter of Oddi pressure, correlates with sphincter of Oddi basal pressure. In this study, intrabiliary pressure was significantly higher in patients with SOD than patients with normal sphincter of Oddi pressure (20 mm Hg vs. 10 mm Hg; P < .01). In a similar study, Fazel and colleagues82 found that pancreatic duct pressure correlated with pancreatic sphincter pressure (P < .01). Pancreatic duct pressure was significantly higher in patients with SOD compared with patients with normal pressure (20 mm Hg vs. 11 mm Hg; P < .001). These studies must be confirmed, but they support the theory that increased intrabiliary or intrapancreatic pressure is a cause of pain in SOD.
The best study establishing normal values for SOM was reported by Guelrud and associates.83 The study evaluated 50 asymptomatic control patients and repeated the evaluation on two occasions in 10 subjects. This study established normal values for intraductal pressure, basal sphincter pressure, and phasic wave parameters (Table 46.2). The reproducibility of SOM was confirmed. Various authorities interchangeably use 35 mm Hg or 40 mm Hg as the upper limits of normal for mean basal sphincter of Oddi pressure (this is 3 standard deviations above the mean). Although all authorities diagnose SOD when the basal sphincter pressure is 35 to 40 mm Hg or greater, some also make this diagnosis when there are greater than 50% retrograde contractions, tachyoddia (phasic wave frequency >7/min), or a paradoxical contraction response after an intravenous dose of CCK.84
Table 46.2 Suggested Standard for Abnormal Values for Endoscopic Sphincter of Oddi Manometry Obtained from 50 Volunteers without Abdominal Symptoms
| Basal sphincter pressure* | >35 mm Hg |
| Basal ductal pressure | >13 mm Hg |
| Phasic contractions | |
| Amplitude | >220 mm Hg |
| Duration | >8 sec |
| Frequency | >10/min |
Note: Values were obtained by adding 3 standard deviations to the mean (means were obtained by averaging the results on two to three station pull-throughs). Data combine pancreatic and biliary studies.
* Basal pressures determined by (1) reading the peak basal pressure (i.e., the highest single lead as obtained using a three-lumen catheter) and (2) obtaining the mean of these peak pressures from multiple station pull-throughs.
Adapted from Guelrud M, Mendoza S, Rossiter G, et al: Sphincter of Oddi manometry in healthy volunteers. Dig Dis Sci 35:38–46, 1990.
Several studies have shown that pancreatitis is the most common major complication after SOM.85–87 Using standard perfused catheters, pancreatitis rates of 31% have been reported. Such high complication rates have limited more widespread use of SOM. These data also emphasize that manometric evaluation of the pancreatic duct is associated with a particularly high complication rate. Rolny and associates86 found that patients with chronic pancreatitis were at higher risk of postprocedure pancreatitis after pancreatic duct manometry. They reported an 11% incidence of pancreatitis after manometric evaluation of the pancreatic duct. Pancreatitis developed in 26% of patients with chronic pancreatitis undergoing SOM.
Methods that have been proposed to decrease the incidence of postmanometry pancreatitis include (1) use of an aspiration catheter; (2) gravity drainage of the pancreatic duct after manometry; (3) decrease in the perfusion rate to 0.05 to 0.1 mL/lumen/min; (4) limitation of pancreatic duct manometry time to less than 2 minutes (or avoid pancreatic manometry); (5) use of the microtransducer (nonperfused) or sleeve SOM system75,88–91; and (6) placement of a pancreatic stent after manometry, sphincterotomy, or both. In a prospective randomized study, Sherman and colleagues85 found that an aspirating catheter (catheter that allows for aspiration of the perfused fluid from end and side holes while accurately recording pressure from the two remaining side ports) reduced the frequency of pancreatic duct manometry–induced pancreatitis from 31% to 4%. The reduction in pancreatitis rates with the use of this catheter in the pancreatic duct and the very low incidence of pancreatitis after bile duct manometry lend support to the notion that increased pancreatic duct hydrostatic pressure is a major cause of this complication.
When the pancreatic duct sphincter is studied by SOM, aspiration of pancreatic juice and the perfusate is strongly recommended. In a prospective randomized trial, Wehrmann and colleagues88 found that microtransducer manometry was associated with a significantly lower incidence of postmanometry pancreatitis than standard (nonaspirating) perfusion manometry (13.8% vs. 3.1%; P = .04). A sleeve SOM catheter–based system was shown more recently to have similar accuracy as the standard triple-lumen SOM catheter with less artifact.90 Because the sleeve assembly is reverse-perfused, no fluid enters the ducts potentially reducing the rate of postprocedure pancreatitis. In another prospective randomized trial, Tarnasky and colleagues92 showed that placement of a stent in the pancreatic duct decreased post-ERCP pancreatitis from 26% to 6% in a group of patients with pancreatic sphincter hypertension undergoing biliary sphincterotomy alone.
SOM is recommended in patients with idiopathic pancreatitis or unexplained disabling pancreaticobiliary pain with or without hepatic enzyme abnormalities. An attempt should be made to study both sphincters, but clinical decisions can be made when the first sphincter evaluated is abnormal. However, if the other sphincter is dysfunctional and not treated, the outcome of therapy may be suboptimal. Indications for the use of SOM have also been developed according to the Hogan-Geenen SOD classification system (see Table 46.1). In type I patients, there is a general consensus that a structural disorder of the sphincter (i.e., sphincter stenosis) exists. Although SOM may be useful in documenting SOD, it is not an essential diagnostic study before endoscopic or surgical sphincter ablation. Such patients uniformly benefit from sphincter ablation regardless of the SOM results (see the section on endoscopic therapy). Type II patients exhibit sphincter of Oddi motor dysfunction in 55% to 65% of cases. In these patients, SOM is highly recommended because the results of the study predict outcome from sphincter ablation. Type III patients have pancreaticobiliary pain without other objective evidence of sphincter outflow obstruction. SOM is mandatory to confirm the presence of SOD. Although this has not been well studied, the results of SOM may predict outcome from sphincter ablation in these patients.
Stent Trial as a Diagnostic Test
Placement of a pancreatic or biliary stent on a trial basis in hope of achieving pain relief and predicting the response to more definitive therapy (i.e., sphincter ablation) has received only limited application. Pancreatic stent trials, especially in patients with normal pancreatic ducts, are strongly discouraged because serious ductal and parenchymal injury may occur if stents are left in place for more than a few days.93,94 Goff95 reported a biliary stent trial in 21 patients with normal biliary manometry suspected to have type II and type III SOD. Stents (7-Fr) were left in place for at least 2 months if symptoms resolved and were removed sooner if they were judged ineffective. Relief of pain with the stent was predictive of long-term pain relief after biliary sphincterotomy. Pancreatitis developed in 38% of the patients (14% were graded severe) after stent placement. Because of this high complication rate, biliary stent trials are strongly discouraged. Rolny and colleagues96 also reported a series of bile duct stent placement as a predictor of outcome after biliary sphincterotomy in 23 postcholecystectomy patients (7 type II and 16 type III). Similar to the study by Goff,95 resolution of pain during at least 12 weeks of stent placement predicted a favorable outcome from sphincterotomy regardless of sphincter of Oddi pressure. In this series, there were no complications related to stent placement.
Therapy for Sphincter of Oddi Dysfunction
The therapeutic approach in patients with SOD is aimed at reducing the resistance to the flow of bile or pancreatic juice, or both, caused by the sphincter of Oddi.13 Historically, emphasis has been placed on definitive intervention (i.e., surgical sphincteroplasty or endoscopic sphincterotomy). This approach seems appropriate for patients with high-grade obstruction (type I as per Hogan-Geenen criteria). In patients with lesser degrees of obstruction, the clinician must carefully weigh the risks and benefits before recommending invasive therapy. Most reports indicate that patients with SOD have a complication rate from ERCP, manometry, and endoscopic sphincterotomy of at least twice that of patients with ductal stones.97,98
Medical Therapy
Medical therapy for documented or suspected SOD has received only limited study. Because the sphincter of Oddi is a smooth muscle structure, it is reasonable to assume that drugs that relax smooth muscle might be an effective treatment for SOD. Vardenafil (Levitra), an inhibitor of phosphodiesterase type 5 and a smooth muscle relaxant used most commonly for male erectile dysfunction, was found to reduce basal sphincter pressure and phasic wave amplitude.99,100 This drug has not been investigated in clinical trials, however. Sublingual nifedipine and nitrates have been shown to reduce basal sphincter pressures in asymptomatic volunteers and symptomatic patients with SOD.1,101 Khuroo and colleagues102 evaluated the clinical benefit of nifedipine in a placebo-controlled crossover trial. Of 28 patients with manometrically documented SOD, 21 (75%) had a reduction in pain scores, emergency department visits, and use of oral analgesics during short-term follow-up. In a similar study, Sand and associates103 found that 9 (75%) of 12 patients with type II SOD (suspected; SOM was not done) improved with nifedipine. In a study of 59 patients with postcholecystectomy pain and suspected SOD treated with medical therapy for 1 year (nitrates or an antispasmodic or both),30 51% reported complete relief and 8 (14%) reported partial relief including 45%, 67%, and 71% type I, type II, and type III patients.104
Although medical therapy may be an attractive initial approach in patients with SOD, several drawbacks exist.1 First, medication side effects may be seen in one-third of patients. Second, smooth muscle relaxants are unlikely to be of any benefit in patients with the structural form of SOD (i.e., sphincter of Oddi stenosis), and the response is incomplete in patients with a primary motor abnormality of the sphincter of Oddi (i.e., sphincter of Oddi dyskinesia). Finally, long-term outcome from medical therapy has not been reported. In a pilot randomized controlled trial, Craig and Toouli105 found no benefit for extended-release nifedipine. Nevertheless, because of the relative safety of medical therapy and the benign (although painful) character of SOD, this approach should be considered in all patients with type III SOD and in patients with type II SOD and less severe symptoms before considering more aggressive sphincter ablation therapy.
Guelrud and colleagues106 showed that transcutaneous electrical nerve stimulation reduces the basal sphincter pressure in patients with SOD by a mean of 38%, although generally not into the normal range. This stimulation was associated with an increase in serum vasoactive intestinal peptide levels. Electroacupuncture applied at acupoint GB 34 (a specific acupoint that affects the hepatobiliary system) was shown to relax the sphincter of Oddi in association with increased plasma CCK levels.107 The role of electroacupuncture in the management of SOD has not been investigated.
Surgical Therapy
Historically, surgery was the traditional therapy of SOD. Most commonly, the surgical approach is a transduodenal biliary sphincteroplasty with a transampullary septoplasty (pancreatic septoplasty). During a 1- to 10-year follow-up, 60% to 70% of patients were reported to have benefited from this therapy.108–111 Patients with an elevated basal sphincter pressure, determined by intraoperative SOM, were more likely to improve from surgical sphincter ablation than patients with a normal basal pressure.109 Some reports have suggested that patients with biliary-type pain have a better outcome than patients with idiopathic pancreatitis, whereas others suggested no difference.108,109 However, most studies found that symptom improvement after surgical sphincter ablation alone was uncommon in patients with established chronic pancreatitis.109
Morgan and associates111 reported that chronic pancreatitis and younger age were independent predictors of poor outcome from surgical sphincter ablation. The surgical approach for SOD has largely been replaced by endoscopic therapy. Patient tolerance, cost of care, morbidity, mortality, and cosmetic results are some factors that favor an initial endoscopic approach. At the present time, surgical therapy is reserved for patients with restenosis after endoscopic sphincterotomy and when endoscopic evaluation or therapy is unavailable or not technically feasible (e.g., Roux-en-Y gastrojejunostomy). Among 68 surgical sphincteroplasties done at Medical University of South Carolina over a 5-year period, 51 had prior endoscopic sphincterotomy, and 17 had endoscopically inaccessible papillae because of prior gastric surgery. There was a trend toward improved outcome after surgical sphincteroplasty (P = .06) in patients who had previous gastric surgery and no prior ERCP compared with patients who had endoscopic sphincterotomy before surgery.111 In many centers, however, operative therapy continues to be the standard treatment of pancreatic sphincter hypertension.13,112
Endoscopic Therapy
Endoscopic Sphincterotomy
Endoscopic sphincterotomy is the standard therapy for patients with SOD.113 Most data on endoscopic sphincterotomy relate to biliary sphincter ablation alone. Clinical improvement after therapy has been reported to occur in 55% to 95% of patients (see Table 46.1). These variable outcomes are reflective of the different criteria used to document SOD, the degree of obstruction (type I biliary patients appear to have a better outcome than type II and type III patients), the methods of data collection (retrospective vs. prospective), and the techniques used to determine benefit. Rolny and colleagues114 studied 17 type I postcholecystectomy biliary patients by SOM (Table 46.3). In this series, 65% had abnormal SOM (although not specifically stated, the biliary sphincter apparently was studied alone). Nevertheless, during a mean follow-up interval of 2.3 years, all patients benefited from biliary sphincterotomy. The results of this study suggested that because type I biliary patients invariably benefit from biliary sphincterotomy, SOM in this patient group not only is unnecessary but also may be misleading. However, the results of this study have never been validated at another center.
Table 46.3 Biliary Sphincter Ablation in Type I Sphincter of Oddi Dysfunction (28-Month Follow-up)*
| Basal Sphincter of Oddi Pressure | N | Asymptomatic or Improved after ES or SS |
|---|---|---|
| <40 mm Hg | 6 (35%) | 6 (100%) |
| >40 mm Hg | 11 (65%) | 11 (100%) |
ES, endoscopic sphincterotomy; SS, surgical sphincterotomy.
Adapted from Rolny P, Geenen JE, Hogan WJ: Post-cholecystectomy patients with ‘objective signs’ of partial bile outflow obstruction: Clinical characteristics, sphincter of Oddi manometry findings, and results of therapy. Gastrointest Endosc 39:778–781, 1993.
In contrast, results of several nonrandomized controlled trials23,32,58,115,116 suggest that performance of SOM is highly recommended in biliary type II and type III patients because clinical benefit is less certain (Table 46.4). Several other case series have reported symptom improvement in 75% to 100% of type I patients undergoing biliary sphincterotomy.59,117–120 Although most of the studies reporting efficacy of endoscopic therapy in SOD have been retrospective, three notable randomized trials have been reported. In a landmark study, Geenen and associates121 randomly assigned 47 postcholecystectomy type II biliary patients to biliary sphincterotomy or sham sphincterotomy. SOM was performed in all patients but was not used as a criterion for randomization. During a 4-year follow-up, 95% of patients with an elevated basal sphincter benefited from sphincterotomy. In contrast, only 30% to 40% of patients with an elevated sphincter pressure treated by sham sphincterotomy or with a normal sphincter pressure treated by endoscopic sphincterotomy or sham sphincterotomy benefited from this therapy. The two important findings of this study were that SOM predicted the outcome from endoscopic sphincterotomy and that endoscopic sphincterotomy offered long-term benefit in type II biliary patients with SOD.
Table 46.4 Biliary Sphincterotomy for Type II and Type III Sphincter of Oddi Dysfunction Documented by Sphincter of Oddi Manometry: Results of Five Nonrandomized Trials
| Author (Year) | Clinical Benefit | |
|---|---|---|
| Type II | Type III | |
| Choudhry et al (1993)32* | 10/18 (56%) | 9/16 (56%) |
| Botoman et al (1994)23 | 13/19 (68%) | 9/16 (56%) |
| Bozkurt et al (1996)115 | 14/19 (78%) | 5/5 (100%) |
| Wehrmann et al (1996)116 | 12/20 (60%) | 1/13 (8%) |
| Rosenblatt et al (2001)58 | 22/30 (73%) | 11/32 (34%) |
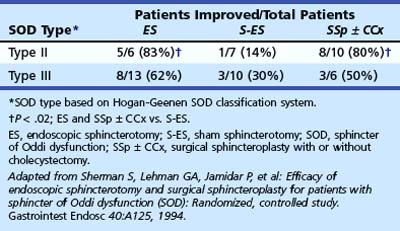
Confirming data were seen in a 2-year follow-up study by Toouli and coworkers.122,123 In this study, postcholecystectomy patients with biliary-type pain (mostly type II) were prospectively randomly assigned to endoscopic sphincterotomy or sham after stratification according to SOM. At 2 years after endoscopic sphincterotomy, 85% (11 of 13) of patients with elevated basal pressure improved, whereas 38% (5 of 13) of patients improved after a sham procedure (P = .041). Patients with normal SOM were also randomly assigned to sphincterotomy or sham. The outcome was similar for the two groups (8 of 13 improved after sphincterotomy, and 8 of 19 improved after sham; P = .47). Sherman and associates124 reported their preliminary results of a randomized study comparing endoscopic sphincterotomy and surgical biliary sphincteroplasty with pancreatic septoplasty (with or without cholecystectomy) with sham sphincterotomy for type II and type III biliary patients with manometrically documented SOD. The results are shown in Tables 46.5 and 46.6. During a 3-year follow-up period, 69% of patients undergoing endoscopic or surgical sphincter ablation improved compared with 24% in the sham sphincterotomy group (P = .009). There was a trend for type II patients to benefit more often from sphincter ablation than type III patients (13 of 16 [81%] vs. 11 of 19 [58%]; P = .14).
Table 46.5 Change in the Mean Pain Score*, Number of Hospital Days per Month Required for Pain, and the Percentage Improved in Patients with Manometrically Documented Sphincter of Oddi Dysfunction Randomly Assigned to Endoscopic Sphincterotomy, Sham Sphincterotomy, and Surgical Sphincteroplasty with or without Cholecystectomy
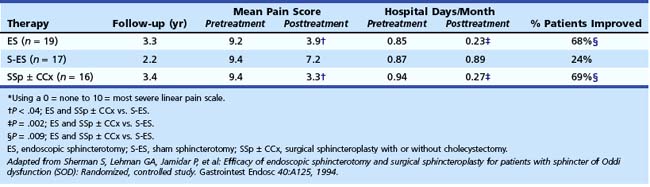
Fig. 46.5 shows the recommended approach of the Rome III committee on functional disorders of the sphincter of Oddi31 for the therapy of patients with types I, II, and II biliary SOD. Evidence is now accumulating that the addition of a pancreatic sphincterotomy to an endoscopic biliary sphincterotomy in patients with pancreatic sphincter disease may improve the outcome, as preliminarily reported by Guelrud and coworkers.125 Soffer and Johlin126 reported that 25 of 26 patients (mostly type II) who failed to respond to biliary sphincterotomy had elevated pancreatic sphincter pressure. Pancreatic sphincter therapy was performed with overall symptomatic improvement in two-thirds of patients. Eversman and colleagues127 found that 90% of patients with persistent pain or pancreatitis after biliary sphincterotomy had residual abnormal pancreatic basal pressure. Data from 5 years of follow-up revealed that patients with untreated pancreatic sphincter hypertension were much less likely to improve after biliary sphincterotomy than patients with isolated biliary sphincter hypertension. Elton and colleagues128 performed pancreatic sphincterotomy on 43 type I and type II SOD patients who failed to benefit from biliary sphincterotomy alone. During the follow-up period, 72% were symptom-free, and 19% had partial or transient improvement.
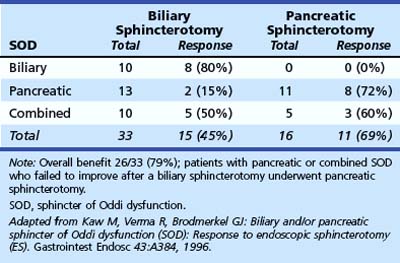
Kaw and colleagues129 presented preliminary data showing that response to sphincterotomy also depends on treating the diseased sphincter segment. Patients with pancreatic sphincter hypertension who fail to respond to biliary sphincterotomy can be “rescued” by undergoing pancreatic sphincterotomy (Table 46.7). More recent data from our unit130 examined the outcome of endoscopic therapy in patients with SOD with initial pancreatic sphincter hypertension (with or without biliary sphincter hypertension). Patients were followed for a mean of 43.1 months (range 11 to 77 months); reintervention was offered for sustained or recurrent symptoms at a median of 8 months after initial therapy. Performance of an initial dual pancreatobiliary sphincterotomy was associated with a lower reintervention rate (70 of 285 [24.6%]) than biliary sphincterotomy alone (31 of 95 [33%]; P < .05). Confirmatory outcome studies, preferably in randomized trials, are awaited.
Table 46.7 Response to Sphincterotomy in Relation to Sphincter of Oddi Segment Treated (Follow-up 17 Months)
Many authorities argue that the current SOD classification systems might not be a good predictor of outcome.84,131–133 In a study of 121 patients classified by the modified Milwaukee biliary classification system (18 type I, 53 type II, and 50 type III patients) and treated by biliary sphincterotomy with (49 patients) or without (72 patients) pancreatic sphincterotomy, Freeman and colleagues131 reported a good to excellent response in 69%. The response was not significantly different between biliary types I, II, and III. The authors found that significant predictors of a poor response to therapy were normal pancreatic manometry, delayed gastric emptying, daily opioid use, and age younger than 40 years. Abnormal liver function tests or dilated bile duct were not significant predictors of outcome. These results indicate that the response rate and enthusiasm for sphincter ablation must be correlated with patient presentation and results of manometry and balanced against the high complication rates reported for endoscopic therapy of SOD.
Most studies indicate that patients undergoing ERCP, manometry, and endoscopic sphincterotomy for SOD have complication rates two to five times higher than patients undergoing ERCP and endoscopic sphincterotomy for ductal stones.97,98 Pancreatitis is the most common complication, occurring in up to 30% of patients in some series. Several prospective, multicenter studies examining risk factors for post-ERCP pancreatitis identified suspected SOD as an independent factor by multivariate analysis.134 A suspicion of SOD tripled the risk of postprocedure pancreatitis to a frequency (23%) that was comparable to that found in other more recent prospective studies.92,98,135–137 Placement of a prophylactic pancreatic duct stent has been shown to limit such complications.92,138–140 These studies have also shown that the risk of pancreatitis is intrinsic to the patient group (patient-related factors) and events occurring during the procedure (procedure-related factors) rather than the SOM when the SOM is performed with an aspirating catheter. In multivariate analysis, SOM has not been shown to be a risk factor for pancreatitis.141,142
Balloon Dilation and Stent Placement
Balloon dilation of strictures in the GI tract has become a common procedure. In an attempt to be less invasive and possibly to preserve sphincter function, adaptation of this technique to treat SOD has been described. Because of the unacceptably high complication rates, primarily pancreatitis, this technology has little role in the primary management of SOD.143 Similarly, although biliary stent placement might offer short-term symptom benefit in patients with SOD and predict outcome from sphincter ablation, it too has unacceptably high complication rates and cannot be advocated in this setting.95
Botulinum Toxin Injection
Botulinum toxin (Botox), a potent inhibitor of acetylcholine release from nerve endings, has been successfully applied to smooth muscle disorders of the GI tract such as achalasia. In a preliminary clinical trial, botulinum toxin injection into the sphincter of Oddi resulted in a 50% reduction in the basal biliary sphincter pressure and improved bile flow.144 This reduction in pressure may be accompanied by symptom improvement in some patients. Although further study is warranted, botulinum toxin may serve as a therapeutic trial for SOD with responders undergoing permanent sphincter ablation. In a small series,145 22 postcholecystectomy type III patients with manometric evidence of SOD underwent botulinum toxin injection into the intraduodenal sphincter segment. Of the 12 patients who responded to botulinum toxin injection, 11 later benefited from endoscopic sphincterotomy, whereas only 2 of 10 patients who did not benefit from botulinum toxin injection later responded to sphincter ablation. Such an approach requires two endoscopies to achieve symptom relief. Patients must have relatively frequent episodes of pain to assess the benefit from botulinum toxin. Further studies are needed before this technique can be recommended.
Failure to Achieve Symptomatic Improvement after Biliary Sphincterotomy
There are several potential explanations as to why patients may fail to experience symptom relief after biliary sphincterotomy is performed for well-documented SOD. First, biliary sphincterotomy may have been inadequate, or restenosis may have occurred. Although the biliary sphincter is commonly not totally ablated,146 Manoukian and coworkers147 indicated that clinically significant biliary restenosis occurs infrequently. If no “cutting space” remains in such a patient, balloon dilation to 8 to 10 mm may suffice, but long-term outcome from such therapy is unknown, and the risks may be considerable.143 Prophylactic pancreatic stent placement may reduce the frequency of post–balloon dilation pancreatitis.148 Second, as noted previously,125–130 the importance of pancreatic sphincter ablation is being increasingly recognized. Third, patients may fail to respond to sphincterotomy because they have chronic pancreatitis. Tarnasky and colleagues149 reported that patients with SOD were four times more likely to have evidence of chronic pancreatitis than patients without SOD (P = .01). Although SOD seems to be associated with chronic pancreatitis, a causal relationship has not been proven. These patients may or may not have abnormal pancreatograms. Intraductal pancreatic juice aspiration after secretin stimulation may help make this diagnosis.150–152 EUS may show parenchymal and ductular changes of the pancreas in some of these patients suggesting chronic pancreatitis.153 Fourth, some patients may be having pain from altered gut motility of the stomach, small bowel, or colon (irritable bowel or pseudo-obstruction variants). There is increasing evidence that upper GI motility disorders may masquerade as pancreatobiliary-type pain (i.e., discrete right upper quadrant pain). Multiple preliminary studies show disordered duodenal motility in such patients.154–156 Soffer and Johlin157 found that small bowel dysmotility occurred with greater frequency in type II and type III SOD patients who failed to benefit from sphincterotomy than in patients who did respond. This area needs much more study to determine the frequency, significance, and coexistence of these motor disorders along with SOD.
DeSautels and colleagues158 suggested that type III patients have duodenal-specific visceral hyperalgesia with pain reproduction by duodenal distention. These patients were also shown to have high levels of somatization, depression, obsessive-compulsive behavior, and anxiety compared with control subjects.159 Patients with SOD appear to have a higher than expected prevalence of irritable bowel syndrome,160 and SOD may occur as part of a more generalized functional disorder of the gut. Patients with SOD not only have more somatization than controls, but also they may have an antecedent history of sexual or physical abuse similar to patients with irritable bowel syndrome.161 Wald15 suggested that selective treatment of the sphincter of Oddi cannot be expected to provide symptom resolution in such patients, and this may account for the high failure rate of sphincterotomy in many patients with type III SOD.
Sphincter of Oddi Dysfunction in Recurrent Pancreatitis
Disorders of the pancreatic sphincter may give rise to unexplained (idiopathic) pancreatitis or episodic pain suggestive of a pancreatic origin.112 Although the pathogenesis of acute pancreatitis in SOD is uncertain, it is believed that the combination of pancreatic duct obstruction and increased exocrine juice flow are needed.84 SOD is a frequent cause of recurrent pancreatitis previously labeled as idiopathic acute recurrent pancreatitis (IARP). It has been documented with manometry in 15% to 72% of such patients (Table 46.8).6,24,30,162–171 Pancreatic sphincter manometry should be done in patients with IARP, particularly patients with normal biliary manometry and patients who have recurrent attacks after a biliary sphincterotomy. Isolated pancreatic sphincter hypertension is common among patients with IARP found to have SOD.77,172 In addition, pancreatic sphincter hypertension may explain recurrent pancreatitis despite biliary sphincterotomy or surgical biliary sphincteroplasty.172
Table 46.8 Manometrically Documented Sphincter of Oddi Dysfunction Causing Idiopathic Acute Recurrent Pancreatitis
| Author (Year) | Frequency |
|---|---|
| Toouli et al (1985)165 | 16/26 (57%) |
| Guelrud et al (1986)166 | 17/42 (40%) |
| Gregg (1989)167 | 38/125 (30%) |
| Venu et al (1989)164 | 17/116 (15%) |
| Sherman et al (1993)168 | 18/55 (33%) |
| Choudari et al (1998)163 | 79/225 (35%) |
| Kaw and Brodmerkel (2002)169 | 67/126 (53%) |
| Coyle et al (2002)170 | 28/90 (31%) |
| Total | 781/2046 (38%) |
Biliary sphincterotomy alone has been reported to prevent further pancreatitis episodes in more than 50% of patients in some series. From a scientific, but not practical, viewpoint, care must be taken to separate out subtle biliary pancreatitis173 that would similarly respond to biliary sphincterotomy. Because IARP is an episodic illness, long-term follow-up is necessary to conclude that a patient is “cured.” Sphincter ablation is the recommended therapy for patients with IARP resulting from SOD. Historically, ablation has been accomplished surgically.109 However, with increasing experience, endoscopic sphincterotomy has become the treatment of choice. Controversy continues to exist about the type of sphincterotomy that should be performed.174
The value of ERCP, SOM, and sphincter ablation therapy was studied in 51 patients with idiopathic pancreatitis.69 An elevated basal sphincter pressure was present in 24 (47.1%) patients. There were 30 patients treated by biliary sphincterotomy (n = 20) or surgical sphincteroplasty with septoplasty (n =10). Of 18 patients, 15 (83%) with an elevated basal sphincter pressure had long-term benefit (mean follow-up 38 months) from sphincter ablation therapy (including 10 of 11 treated by biliary sphincterotomy), in contrast to only 4 (33.3%; P < .05) of 12 patients with a normal basal sphincter pressure (including 4 of 9 patients treated by biliary sphincterotomy).
Guelrud and colleagues125 found, however, that severance of the pancreatic sphincter was necessary to resolve pancreatitis (Table 46.9). In this series, 69 patients with idiopathic pancreatitis resulting from SOD underwent treatment by standard biliary sphincterotomy (n = 18), biliary sphincterotomy with pancreatic sphincter balloon dilation (n = 24), biliary sphincterotomy followed by pancreatic sphincterotomy in separate sessions (n = 13), or combined pancreatic and biliary sphincterotomy in the same session (n = 14). Of patients undergoing pancreatic and biliary sphincterotomy, 81% had resolution of pancreatitis compared with 28% of patients undergoing biliary sphincterotomy alone (P < .005). Sherman and colleagues168 reported that only 44% of patients with SOD and IARP had no further attacks during a 5-year follow-up interval after biliary sphincterotomy alone. These data are consistent with the theory that many such patients who benefit from biliary sphincterotomy alone have subtle gallstone pancreatitis.
Table 46.9 Pancreatic Sphincter Dysfunction and Recurrent Pancreatitis: Response to Sphincter Therapy
| Treatment | Patients Improved/Total Patients |
|---|---|
| Biliary sphincterotomy alone | 5/18 (28%) |
| Biliary sphincterotomy followed by pancreatic sphincter balloon dilation | 13/24 (54%) |
| Biliary sphincterotomy plus pancreatic sphincterotomy at later session | 10/13 (77%)* |
| Biliary sphincterotomy and pancreatic sphincterotomy at same session | 12/14 (86%)* |
* P < .005 vs. biliary sphincterotomy alone.
Adapted from Guelrud M, Plaz J, Mendoza S, et al: Endoscopic treatment in type II pancreatic sphincter dysfunction. Gastrointest Endosc 41:A398, 1995.
The results of Guelrud and colleagues125 also support the anatomic findings of separate biliary and pancreatic sphincters and the manometry findings of residual pancreatic sphincter hypertension in more than 50% of persistently symptomatic patients who undergo biliary sphincterotomy alone. Kaw and Brodmerkel169 reported that among patients with idiopathic pancreatitis secondary to SOD, 78% had persistent manometric evidence of pancreatic sphincter hypertension despite a biliary sphincterotomy. Toouli and coworkers175 also showed the importance of pancreatic and biliary sphincter ablation in patients with idiopathic pancreatitis. In this series, 23 of 26 patients (88%) undergoing surgical ablation of both the biliary and the pancreatic sphincter were either asymptomatic or had minimal symptoms at a median follow-up of 24 months (range 9 to 105 months). Okolo and colleagues176 retrospectively evaluated the long-term results of endoscopic pancreatic sphincterotomy in 55 patients with manometrically documented or presumed pancreatic sphincter hypertension (presumption based on recurrent pancreatitis with pancreatic duct dilation and contrast medium drainage time from the pancreatic duct >10 minutes). During a median follow-up of 16 months (range 3 to 52 months), 34 patients (62%) reported significant pain improvement. Patients with normal pancreatograms were more likely to respond to therapy than patients with pancreatographic evidence of chronic pancreatitis (73% vs. 58%).
Jacob and coworkers177 postulated that SOD might cause recurrent episodes of pancreatitis, even though SOM was normal, and pancreatic stent placement might prevent further attacks. In a randomized study, 34 patients with unexplained recurrent pancreatitis; normal pancreatic duct SOM, ERCP, and secretin testing; and no biliary crystals were treated with pancreatic stents (n = 19; 5-Fr to 7-Fr, with stents exchanged three times over a 1-year period) or conservative therapy (n = 15). During a 3-year follow-up, pancreatitis recurred in 53% of the patients in the control group and only 11% of the patients with stent placement (P < .02). This study suggests that SOM may be an imperfect test because patients may have SOD, but it may not be detected at the time of SOM. Long-term studies are needed to evaluate the outcome after removal of stents, and concern remains regarding stent-induced ductal and parenchymal changes.93,94 Because of the concern of stent-induced injury to the pancreas, trial pancreatic duct stent placement to predict outcome from pancreatic sphincterotomy is not recommended.178
Wehrmann and colleagues179 evaluated the feasibility and effectiveness of botulinum toxin injection in patients with recurrent pancreatitis resulting from pancreatic sphincter hypertension. No side effects of the injection were noted in any of the 15 treated patients. At 3-month follow-up, 12 patients (80%) remained asymptomatic, but 11 developed a relapse at a follow-up period of 6 ± 2 months. These 11 patients underwent pancreatic or combined pancreatobiliary sphincterotomy with subsequent remission after a median follow-up of 15 months. This study showed that injection of botulinum toxin is safe, may be effective in the short-term, and may predict the outcome from pancreatic sphincter ablation in patients having frequent episodes of pancreatitis, but the need for definitive sphincter ablation in most patients limits its clinical use.
1 Kalloo AN, Pasricha PJ. Therapy of sphincter of Oddi dysfunction. Gastrointest Endosc Clin N Am. 1996;6:117-125.
2 Baille J. Sphincter of Oddi dysfunction: Overdue for an overhaul. Am J Gastroenterol. 2005;100:1217-1220.
3 Black NA, Thompson E, Sanderson CF. Symptoms and health status before and six weeks after open cholecystectomy: A European cohort study. ECHSS Group. European Collaborative Health Services Study Group. Gut. 1994;35:1301-1305.
4 Hogan W, Sherman S, Pasricha P, et al. Position paper on sphincter of Oddi manometry. Gastrointest Endosc. 1997;45:342-348.
5 Sherman S, Troiano FP, Hawes RH, et al. Frequency of abnormal sphincter of Oddi manometry compared with the clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol. 1991;86:586-590.
6 Eversman D, Fogel EL, Rusche M, et al. Frequency of abnormal pancreatic and biliary sphincter manometry compared with clinical suspicion of sphincter of Oddi dysfunction. Gastrointest Endosc. 1999;50:637-641.
7 Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci. 2007;52:1211-1218.
8 Richards RD, Yeaton P, Shaffer HA, et al. Human sphincter of Oddi motility and cholecystokinin response following liver transplantation. Dig Dis Sci. 1993;38:462-468.
9 Becker JM, Parodi JM. Basic control mechanisms of sphincter of Oddi motor function. Gastrointest Endosc Clin N Am. 1993;3:41-66.
10 Luman W, Williams AJ, Pryde A, et al. Influence of cholecystectomy on sphincter of Oddi motility. Gut. 1997;41:371-374.
11 Anderson TM, Pitt HA, Longmire WPJr. Experience with sphincteroplasty and sphincterotomy in pancreatobiliary surgery. Ann Surg. 1985;201:399-406.
12 Kurucsai G, Joo I, Fejes R, et al. Somatosensory hypersensitivity in the referred pain area in patients with chronic biliary pain and a sphincter of Oddi dysfunction: New aspects of an almost forgotten pathogenetic mechanism. Am J Gastroenterol. 2008;103:2717-2725.
13 Corazziari E, Shaffer EA, Hogan W, et al. Functional disorders of the biliary tract and pancreas. Gut. 1999;45:48-54.
14 Misra S, Treanor MR, Vegunta RK, et al. Sphincter of Oddi dysfunction in children with recurrent abdominal pain: 5-year follow-up after endoscopic sphincterotomy. J Gastroenterol Hepatol. 2007;22:2246-2250.
15 Wald A. Functional biliary-type pain: Update and controversies. J Clin Gastroenterol. 2005;39:S217-S222.
16 Drossman DA, Zhiming L, Andruzzi E, et al. US Householder Survey of functional gastrointestinal disorders—prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569-1580.
17 Winstead NS, Wilcox CM. Health-related quality of life, somatization, and abuse in sphincter of Oddi dysfunction. J Clin Gastroenterol. 2007;41:773-776.
18 Guelrud M, Mendoza S, Mujica V, et al. Sphincter of Oddi (SO) motor function in patients with symptomatic gallstones. Gastroenterology. 1993;104:A361.
19 Ruffolo TA, Sherman S, Lehman GA, et al. Gallbladder ejection fraction and its relationship to sphincter of Oddi dysfunction. Dig Dis Sci. 1994;39:289-292.
20 Neoptolemos JP, Bailey IS, Carr-Locke DL. Sphincter of Oddi dysfunction: Results of treatment by endoscopic sphincterotomy. Br J Surg. 1988;75:454-459.
21 Roberts-Thomson IC, Toouli J. Is endoscopic sphincterotomy for disabling biliary-type pain after cholecystectomy effective? Gastrointest Endosc. 1985;31:370-373.
22 Meshkinpoor H, Mollot M. Sphincter of Oddi dysfunction and unexplained abdominal pain: Clinical and manometric study. Dig Dis Sci. 1992;37:257-261.
23 Botoman VA, Kozarek RA, Novell LA, et al. Long term outcome after endoscopic sphincterotomy in patients with biliary colic and suspected sphincter of Oddi dysfunction. Gastrointest Endosc. 1994;40:165-170.
24 Lehman GA, Sherman S. Sphincter of Oddi dysfunction. Int J Pancreatol. 1996;20:11-25.
25 Linder JD, Geels W, Wilcox CM. Prevalence of sphincter of Oddi dysfunction: Can results from specialized centers be generalized? Dig Dis Sci. 2002;47:2411-2415.
26 Aymerich RR, Prakash C, Aliperti G. Sphincter of Oddi manometry: Is it necessary to measure both biliary and pancreatic sphincter pressure? Gastrointest Endosc. 2000;52:183-186.
27 Thune A, Scicchitano J, Roberts-Thompson I, et al. Reproducibility of endoscopic sphincter of Oddi manometry. Dig Dis Sci. 1991;36:1401-1405.
28 Varadarajulu S, Hawes RH, Cotton PB. Determination of sphincter of Oddi dysfunction in patients with prior normal manometry. Gastrointest Endosc. 2003;58:341-344.
29 Khashab MA, Watkins JL, McHenry LJr, et al. Frequency of sphincter of Oddi dysfunction in patients with previously normal sphincter of Oddi manometry studies. Endoscopy. 2010;42:369-374.
30 Geenen JE, Nash JA. The role of sphincter of Oddi manometry and biliary microscopy in evaluating idiopathic recurrent pancreatitis. Endoscopy. 1998;30:237-241.
31 Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
32 Choudhry U, Ruffolo T, Jamidar P, et al. Sphincter of Oddi dysfunction in patients with intact gallbladder: Therapeutic response to endoscopic sphincterotomy. Gastrointest Endosc. 1993;39:492-495.
33 Steinberg WM. Sphincter of Oddi dysfunction: A clinical controversy. Gastroenterology. 1988;95:1409-1415.
34 Lin OS, Soetikno RM, Young HS. The utility of liver function test abnormalities concomitant with biliary symptoms in predicting a favorable response to endoscopic sphincterotomy in patients with presumed sphincter of Oddi dysfunction. Am J Gastroenterol. 1998;93:1833-1836.
35 Steinberg WM, Salvato RF, Toskes PP. The morphine-prostigmin provocative test: Is it useful for making clinical decisions? Gastroenterology. 1980;78:728-731.
36 Lobo DN, Takhar AS, Thaper A, et al. The morphine prostigmine provocation (Nardi) test for sphincter of Oddi dysfunction: Results in healthy volunteers and in patients before and after transduodenal sphincteroplasty and transampullary septectomy. Gut. 2007;56:1472-1473.
37 Darweesh RM, Dodds WJ, Hogan WJ, et al. Efficacy of quantitative hepatobiliary scintigraphy and fatty-meal sonography for evaluating patients with suspected partial common duct obstruction. Gastroenterology. 1988;94:779-786.
38 Simeone JF, Mueller PR, Ferrucci JTJr, et al. Sonography of the bile ducts after a fatty meal: An aid in detection of obstruction. Radiology. 1982;143:211-215.
39 Troiano F, O’Connor K, Lehman GA, et al. Comparison of secretin-stimulated ultrasound and sphincter of Oddi manometry in evaluating sphincter of Oddi dysfunction. Gastrointest Endosc. 1989;35:A166.
40 Warshaw AL, Simeone J, Schapiro RH, et al. Objective evaluation of ampullary stenosis with ultrasonography and pancreatic stimulation. Am J Surg. 1985;149:65-72.
41 DiFrancesco V, Brunori MR, Rigo L, et al. Comparison of ultrasound-secretin test and sphincter of Oddi manometry in patients with recurrent acute pancreatitis. Dig Dis Sci. 1999;44:336-340.
42 Silverman WB, Johlin FC, Crowe G. Does secretin stimulated ultrasound (SSUS) predict results of sphincter of Oddi manometry (SOM) basal sphincter pressure (BSP) in patients suspected of having sphincter of Oddi dysfunction (SOD)? Gastrointest Endosc. 2001;53:A100.
43 Catalano MF, Lahoti S, Alcocer E, et al. Dynamic imaging of the pancreas using real-time endoscopic ultrasonography with secretin stimulation. Gastrointest Endosc. 1998;48:580-587.
44 Aisen A, Sherman S, Jennings SG, et al. Comparison of secretin-stimulated magnetic resonance pancreatography and manometry results in patients with suspected sphincter of Oddi dysfunction. Acad Radiol. 2008;15:601-609.
45 Pereira SP, Gillams A, Sgouros SN, et al. Prospective comparison of secretin-stimulated magnetic resonance cholangiopancreatography with manometry in the diagnosis of sphincter of Oddi dysfunction types II and III. Gut. 2007;56:809-813.
46 Baillie J, Kimberly J. Prospective comparison of secretin-stimulated MRCP with manometry in the diagnosis of sphincter of Oddi dysfunction types II and III. Gut. 2007;56:742-744.
47 Mariani A, Curioni S, Zanello A, et al. Secretin MRCP and endoscopic pancreatic manometry in the evaluation of sphincter of Oddi function: A comparative pilot study in patients with idiopathic recurrent pancreatitis. Gastrointest Endosc. 2003;58:847-852.
48 Testoni PA, Mariani A, Curioni S, et al. MRCP-secretin test-guided management of idiopathic recurrent pancreatitis: Long-term outcomes. Gastrointest Endosc. 2008;67:1028-1034.
49 Sostre S, Kalloo AN, Spiegler EJ, et al. A noninvasive test of sphincter of Oddi dysfunction in postcholecystectomy patients: The scintigraphic score. J Nucl Med. 1992;33:1216-1222.
50 Kalloo AN, Sostre S, Pasricha PJ. The Hopkins scintigraphic score: A noninvasive, highly accurate screening test for sphincter of Oddi dysfunction. Gastroenterology. 1994;106:A342.
51 Cicala M, Scopinaro F, Corazziari E, et al. Quantitative cholescintigraphy in the assessment of choledochoduodenal bile flow. Gastroenterology. 1991;100:1106-1113.
52 Corazziari E, Cicala M, Habib FI, et al. Hepatoduodenal bile transit in cholecystectomized subjects: Relationship with sphincter of Oddi dysfunction and diagnostic value. Dig Dis Sci. 1994;39:1985-1993.
53 Peng NJ, Lai KH, Tsay DG, et al. Efficacy of quantitative cholescintigraphy in the diagnosis of sphincter of Oddi dysfunction. Nucl Med Commun. 1994;15:899-904.
54 Thomas PD, Turner JG, Dobbs BR, et al. Use of 99m Tc-DISIDA biliary scanning with morphine provocation in the detection of elevated sphincter of Oddi basal pressure. Gut. 2000;46:838-841.
55 Esber E, Ruffolo TA, Park H, et al. Prospective assessment of biliary scintigraphy in patients with suspected sphincter of Oddi dysfunction. Gastrointest Endosc. 1995;41:A396.
56 Pineau BC, Knapple WL, Spicer KM, et al. Cholecystokinin-stimulated mebrofenin (99mTc-Choletec) hepatobiliary scintigraphy in asymptomatic postcholecystectomy individuals: Assessment of specificity, interobserver reliability, and reproducibility. Am J Gastroenterol. 2001;96:3106-3109.
57 Craig AG, Peter D, Saccone GT, et al. Scintigraphy versus manometry in patients with suspected biliary sphincter of Oddi dysfunction. Gut. 2003;52:352-357.
58 Rosenblatt ML, Catalano MF, Alcocer E, et al. Comparison of sphincter of Oddi manometry, fatty meal sonography, and hepatobiliary scintigraphy in the diagnosis of sphincter of Oddi dysfunction. Gastrointest Endosc. 2001;54:697-704.
59 Cicala M, Habib FI, Vavassori P, et al. Outcome of endoscopic sphincterotomy in post cholecystectomy patients with sphincter of Oddi dysfunction as predicted by manometry and quantitative choledochoscintigraphy. Gut. 2002;50:665-668.
60 Toouli J. Biliary scintigraphy versus sphincter of Oddi manometry in patients with post-cholecystectomy pain: Is it time to disregard the scan. Curr Gastroenterol Rep. 2005;7:154-159.
61 Elta GH, Barnett JL, Ellis JH, et al. Delayed biliary drainage is common in asymptomatic post-cholecystectomy volunteers. Gastrointest Endosc. 1992;38:435-439.
62 Ponchon T, Aucia N, Mitchell R, et al. Biopsies of the ampullary region in patients suspected to have sphincter of Oddi dysfunction. Gastrointest Endosc. 1995;42:296-300.
63 ASGE Standards of Practice Committee. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc. 2000;52:831-837.
64 Sherman S, Fogel E, Phillips S, et al. Yield of diagnostic ERCP for pancreatobiliary pain alone—can we justify the procedure? Gastrointest Endosc. 1999;49:84A.
65 Cohen S, Bacon B, Berlin JA, et al. NIH State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14–16, 2002. Gastrointest Endosc. 2002;56:803-816.
66 Itoh A, Tsukamoto Y, Naitoh Y, et al. Intraductal ultrasonography for the examination of duodenal papillary region. J Ultrasound Med. 1994;13:679-684.
67 Wehrmann T, Stergiou N, Riphaus A, et al. Correlation between sphincter of Oddi manometry and intraductal ultrasound morphology in patients with suspected sphincter of Oddi dysfunction. Endoscopy. 2001;33:773-777.
68 Lehman GA. Endoscopic sphincter of Oddi manometry: A clinical practice and research tool. Gastrointest Endosc. 1991;37:490-492.
69 Lans JL, Parikh NP, Geenen JE. Application of sphincter of Oddi manometry in routine clinical investigations. Endoscopy. 1991;23:139-143.
70 Sherman S, Gottlieb K, Uzer MF, et al. Effects of meperidine on the pancreatic and biliary sphincter. Gastrointest Endosc. 1996;44:239-242.
71 Fogel EL, Sherman S, Bucksot L, et al. Effects of droperidol on the pancreatic and biliary sphincter. Gastrointest Endosc. 2003;58:488-492.
72 Goff JS. Effect of propofol on human sphincter of Oddi. Dig Dis Sci. 1995;40:2364-2367.
73 Varadarajulu S, Tamhane A, Wilcox CM. Prospective evaluation of adjunctive ketamine on sphincter of Oddi motility in humans. J Gastroenterol Hepatol. 2008;23:e405-e409.
74 Blaut U, Sherman S, Fogel EL, et al. The influence of variable stiffness guidewires on basal biliary sphincter of Oddi pressure measured at ERCP. Gastrointest Endosc. 2002;55:83A.
75 Craig AG, Omari T, Lingenfelser T, et al. Development of a sleeve sensor for measurement of sphincter of Oddi motility. Endoscopy. 2001;33:651-657.
76 Blaut U, Sherman S, Fogel E, et al. Influence of cholangiography on biliary sphincter of Oddi manometric parameters. Gastrointest Endosc. 2000;52:624-629.
77 Raddawi HM, Geenen JE, Hogan WJ, et al. Pressure measurements from biliary and pancreatic segments of sphincter of Oddi: Comparison between patients with functional abdominal pain, biliary, or pancreatic disease. Dig Dis Sci. 1991;36:71-74.
78 Rolny P, Arleback A, Funch-Jensen P, et al. Clinical significance of manometric assessment of both pancreatic duct and bile duct sphincter in the same patient. Scand J Gastroenterol. 1989;24:751-754.
79 Silverman WB, Ruffolo TA, Sherman S, et al. Correlation of basal sphincter pressures measured from both the bile duct and pancreatic duct in patients with suspected sphincter of Oddi dysfunction. Gastrointest Endosc. 1992;38:440-443.
80 Chan YK, Evans PR, Dowsett JF, et al. Discordance of pressure recordings from biliary and pancreatic duct segments in patients with suspected sphincter of Oddi dysfunction. Dig Dis Sci. 1997;42:1501-1506.
81 Kalloo AN, Tietjen TG, Pasricha PJ. Does intrabiliary pressure predict basal sphincter of Oddi pressure? A study in patients with and without gallbladders. Gastrointest Endosc. 1996;44:696-699.
82 Fazel A, Geenen JE, MoezArdalan K, et al. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas. 2005;30:359-362.
83 Guelrud M, Mendoza S, Rossiter G, et al. Sphincter of Oddi manometry in healthy volunteers. Dig Dis Sci. 1990;35:38-46.
84 McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol. 2007;13:6333-6343.
85 Sherman S, Troiano FP, Hawes RH, et al. Sphincter of Oddi manometry: Decreased risk of clinical pancreatitis with the use of a modified aspirating catheter. Gastrointest Endosc. 1990;36:462-466.
86 Rolny P, Anderberg B, Ihse I, et al. Pancreatitis after sphincter of Oddi manometry. Gut. 1990;31:821-824.
87 Maldonado ME, Brady PG, Mamel JJ, et al. Incidence of pancreatitis in patients undergoing sphincter of Oddi manometry (SOM). Am J Gastroenterol. 1999;94:387-390.
88 Wehrmann T, Stergiou N, Schmitt T, et al. Reduced risk for pancreatitis after endoscopic microtransducer manometry of the sphincter of Oddi: A randomized comparison with perfusion manometry technique. Endoscopy. 2003;35:472-477.
89 Frenz MB, Wehrmann T. Solid state biliary manometry catheter: Impact on diagnosis and post-study pancreatitis. Curr Gastroenterol Rep. 2007;9:171-174.
90 Kawamoto M, Geenen J, Omari T, et al. Sleeve sphincter of Oddi (SO) manometry: A new method for characterizing the motility of the sphincter of Oddi. J Hepatobiliary Pancreat Surg. 2008;15:391-396.
91 Draganov PV, Kowalczyk L, Forsmark CE. Prospective trial comparing solid-state catheter and water-perfusion triple-lumen catheter for sphincter of Oddi manometry done at the time of ERCP. Gastrointest Endosc. 2009;70:92-95.
92 Tarnasky PR, Palesch YY, Cunningham JT, et al. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518-1524.
93 Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointest Endosc. 1990;36:93-95.
94 Smith MT, Sherman S, Ikenberry S, et al. Alterations in pancreatic ductal morphology following polyethylene pancreatic duct stenting. Gastrointest Endosc. 1996;44:268-275.
95 Goff JS. Common bile duct sphincter of Oddi stenting in patients with suspected sphincter of Oddi dysfunction. Am J Gastroenterol. 1995;90:586-589.
96 Rolny P. Endoscopic bile duct stent placement as a predictor of outcome following endoscopic sphincterotomy in patients with suspected sphincter of Oddi dysfunction. Eur J Gastroenterol Hepatol. 1997;9:467-471.
97 Sherman S, Ruffolo TA, Hawes RH, et al. Complications of endoscopic sphincterotomy: A prospective series with emphasis on the increased risk associated with sphincter of Oddi dysfunction and nondilated bile ducts. Gastroenterology. 1991;101:1068-1075.
98 Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy: A prospective, multicenter study. N Engl J Med. 1996;335:909-918.
99 Cheon YK, Cho YD, Moon JH, et al. Effects of vardenafil, a phosphodiesterase type-5 inhibitor, on sphincter of Oddi motility in patients with suspected biliary sphincter of Oddi dysfunction. Gastrointest Endosc. 2009;69:1111-1116.
100 Affronti J. Levitra for sphincter of Oddi dysfunction? Are you kidding? Gastrointest Endosc. 2009;69:1117-1119.
101 Guelrud M, Mendoza S, Rossiter G, et al. Effect of nifedipine on sphincter of Oddi motor activity: Studies in healthy volunteers and patients with biliary dyskinesia. Gastroenterology. 1988;95:1050-1055.
102 Khuroo MS, Zargar SA, Yattoo GN. Efficacy of nifedipine therapy in patients with sphincter of Oddi dysfunction: A prospective, double-blind, randomized, placebo-controlled, cross over trial. Br J Clin Pharmacol. 1992;33:477-485.
103 Sand J, Nordback I, Koskinen M, et al. Nifedipine for suspected type II sphincter of Oddi dyskinesia. Am J Gastroenterol. 1993;88:530-535.
104 Vitton V, Delpy R, Gasmi M, et al. Is endoscopic sphincterotomy avoidable in patients with sphincter of Oddi dysfunction? Eur J Gastroenterol Hepatol. 2008;20:15-21.
105 Craig AG, Toouli J. Slow release nifedipine for patients with sphincter of Oddi dysfunction: Results of a pilot study. Int Med J. 2002;32:119-120.
106 Guelrud M, Rossiter A, Souney P, et al. The effect of transcutaneous nerve stimulation on sphincter of Oddi pressure in patients with biliary dyskinesia. Am J Gastroenterol. 1991;86:581-585.
107 Lee SK, Kim MH, Kim HJ, et al. Electroacupuncture may relax the sphincter of Oddi in humans. Gastrointest Endosc. 2001;53:211-216.
108 Moody FG, Vecchio R, Calabuig R, et al. Transduodenal sphincteroplasty with transampullary septectomy for stenosing papillitis. Am J Surg. 1991;161:213-218.
109 Sherman S, Hawes RH, Madura J, et al. Comparison of intraoperative and endoscopic manometry of the sphincter of Oddi. Surg Gynecol Obstet. 1992;175:410-418.
110 Madura JA, Madura JAII, Sherman S, et al. Surgical sphincteroplasty in 446 patients. Arch Surg. 2005;140:504-513.
111 Morgan KA, Romagnuolo J, Adams DB. Transduodenal sphincteroplasty in the management of sphincter of Oddi dysfunction and pancreas divisum in the modern era. J Am Coll Surg. 2008;206:908-914.
112 Chen JW, Saccone GT, Toouli J. Sphincter of Oddi dysfunction and acute pancreatitis. Gut. 1998;43:305-308.
113 Sherman S. What is the role of ERCP in the setting of abdominal pain of pancreatic or biliary origin (suspected sphincter of Oddi dysfunction)? Gastrointest Endosc. 2002;56(Suppl):258-266.
114 Rolny P, Geenen JE, Hogan WJ. Post-cholecystectomy patients with ‘objective signs’ of partial bile outflow obstruction: Clinical characteristics, sphincter of Oddi manometry findings, and results of therapy. Gastrointest Endosc. 1993;39:778-781.
115 Bozkurt T, Orth KH, Butsch B, et al. Long-term clinical outcome of post-cholecystectomy patients with biliary-type pain: Results of manometry, non-invasive techniques and endoscopic sphincterotomy. Eur J Gastroenterol Hepatol. 1996;8:245-249.
116 Wehrmann T, Wiemer K, Lembcke B, et al. Do patients with sphincter of Oddi dysfunction benefit from endoscopic sphincterotomy? A 5-year prospective trial. Eur J Gastroenterol Hepatol. 1996;8:251-256.
117 Thatcher BS, Snak MV, Tedesco FJ, et al. Endoscopic sphincterotomy for suspected dysfunction of the sphincter of Oddi. Gastrointest Endosc. 1987;33:91-95.
118 Boender J, VanBlankenstein M, Nix GA, et al. Endoscopic papillotomy in biliary tract pain and fluctuating cholestasis with common bile duct dilation and small gallbladder stones. Endoscopy. 1992;24:203-207.
119 Elmi F, Silverman WB. Biliary sphincter of Oddi dysfunction type I versus occult biliary microlithiasis in post-cholecystectomy patients: Are they both part of the same clinical entity? Dig Dis Sci. 2010;55:842-846.
120 Sgouros SN, Pereira SP. Systematic review: Sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther. 2006;24:237-246.
121 Geenen JE, Hogan WJ, Dodds WJ, et al. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter of Oddi dysfunction. N Engl J Med. 1989;320:82-87.
122 Toouli J, Roberts-Thomson I, Kellow J, et al. Prospective randomized trial of endoscopic sphincterotomy for treatment of sphincter of Oddi dysfunction. J Gastroenterol Hepatol. 1996;11:A115.
123 Toouli J, Roberts-Thomson IC, Kellow J, et al. Manometry based randomized trial of endoscopic sphincterotomy for sphincter of Oddi dysfunction. Gut. 2000;46:98-102.
124 Sherman S, Lehman GA, Jamidar P, et al. Efficacy of endoscopic sphincterotomy and surgical sphincteroplasty for patients with sphincter of Oddi dysfunction (SOD): Randomized, controlled study. Gastrointest Endosc. 1994;40:A125.
125 Guelrud M, Plaz J, Mendoza S, et al. Endoscopic treatment in type II pancreatic sphincter dysfunction. Gastrointest Endosc. 1995;41:A398.
126 Soffer EE, Johlin FC. Intestinal dysmotility in patients with sphincter of Oddi dysfunction: A reason for failed response to sphincterotomy. Dig Dis Sci. 1994;39:1942-1946.
127 Eversman D, Fogel E, Philips S, et al. Sphincter of Oddi dysfunction (SOD): Long-term outcome of biliary sphincterotomy (BES) correlated with abnormal biliary and pancreatic sphincters. Gastrointest Endosc. 1999;49:A78.
128 Elton E, Howell DA, Parsons WG, et al. Endoscopic pancreatic sphincterotomy: Indications, outcome, and a safe stentless technique. Gastrointest Endosc. 1998;47:240-249.
129 Kaw M, Verma R, Brodmerkel GJ. Biliary and/or pancreatic sphincter of Oddi dysfunction (SOD): Response to endoscopic sphincterotomy (ES). Gastrointest Endosc. 1996;43:A384.
130 Park SH, Watkins JL, Fogel EL, et al. Long-term outcome of endoscopic dual pancreatobiliary sphincterotomy in patients with manometry-documented sphincter of Oddi dysfunction and normal pancreatogram. Gastrointest Endosc. 2003;57:483-491.
131 Freeman ML, Gill M, Overby C, et al. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol. 2007;41:94-102.
132 Petersen BT. An evidence-based review of sphincter of Oddi dysfunction, part I: Presentations with “objective” biliary findings (types I and II). Gastrointest Endosc. 2004;59:525-534.
133 Petersen BT. Sphincter of Oddi dysfunction, part 2: Evidence-based review of the presentations, with “objective” pancreatic findings (types I and II) and of presumptive type III. Gastrointest Endosc. 2004;59:670-687.
134 Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434.
135 Gottlieb K, Sherman S. ERCP- and endoscopic sphincterotomy-induced pancreatitis. Gastrointest Endosc Clin N Am. 1998;8:87-114.
136 Tarnasky P, Cunningham T, Cotton P, et al. Pancreatic sphincter hypertension increases the risk of post-ERCP pancreatitis. Endoscopy. 1997;29:252-257.
137 Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: A prospective multicenter study. Am J Gastroenterol. 2006;101:139-147.
138 Rashdan A, Fogel EL, McHenry L, et al. Improved stent characteristics for post-ERCP pancreatitis prophylaxis. Clin Gastroenterol Hepatol. 2004;2:322-329.
139 Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis: A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550.
140 Saad AM, Fogel EL, McHenry L, et al. Pancreatic duct stent placement prevents post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction but normal manometry results. Gastrointest Endosc. 2008;67:255-261.
141 Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: A prospective multicenter study. Am J Gastroenterol. 2006;101:139-147.
142 Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc. 2004;59:499-505.
143 Kozarek RA. Balloon dilation of the sphincter of Oddi. Endoscopy. 1988;20:207-210.
144 Pasricha PJ, Miskovsky EP, Kalloo AN. Intrasphincteric injection of botulinum toxin for suspected sphincter of Oddi dysfunction. Gut. 1994;35:1319-1321.
145 Wehrmann T, Seifert H, Seipp M, et al. Endoscopic injection of botulinum toxin for biliary sphincter of Oddi dysfunction. Endoscopy. 1998;30:702-707.
146 Heinerman PM, Graf AH, Boeckl O. Does endoscopic sphincterotomy destroy the function of Oddi’s sphincter? Arch Surg. 1994;129:876-880.
147 Manoukian AV, Schmalz MJ, Geenen JE, et al. The incidence of post-sphincterotomy stenosis in group II patients with sphincter of Oddi dysfunction. Gastrointest Endosc. 1993;39:496-498.
148 Yoo BM, Barkay O, McHenry L, et al. Biliary orifice dilation (BD) after prior biliary sphincterotomy in patients with sphincter of Oddi dysfunction (SOD): Is pancreatic duct (PD) stent placement necessary to prevent post-ERCP pancreatitis? Gastrointest Endosc. 2009;69:156A.
149 Tarnasky PR, Hoffman B, Aabakken L, et al. Sphincter of Oddi dysfunction is associated with chronic pancreatitis. Am J Gastroenterol. 1997;92:1125-1129.
150 Gregg JA. The intraductal secretin test (IDST)—an adjunct to the diagnosis of pancreatic disease and pancreatic physiology. In: Sivak MV, editor. Gastroenterologic endoscopy. Philadelphia: Saunders; 1987:794-807.
151 Sherman S, Hawes RH, Lehman GA. Pure pancreatic juice analysis in patients with pancreatic sphincter stenosis who have undergone sphincter ablation. Am J Gastroenterol. 1990;85:A1261.
152 Abdel Aziz AM, Watkins JL, Fogel EL, et al. Intraductal secretin stimulation test: What is the proper collection time? Gastrointest Endosc. 2007;65:114A.
153 Wiersema MJ, Hawes RH, Lehman GA, et al. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555-564.
154 Gottlieb K, Nowak T, Sherman S, et al. Sphincter of Oddi dysfunction (SOD) and abnormal small bowel motility: Analysis of 32 patients. Gastrointest Endosc. 1994;40:A109.
155 Evans PR, Bak YT, Dowsett JF, et al. Small bowel motor dysfunction occurs in patients with biliary dyskinesia. Gastroenterology. 1994;106:A496.
156 Koussayer T, Ducker TE, Clench MH, et al. Ampulla of Vater/duodenal wall spasm diagnosed by antroduodenal manometry. Dig Dis Sci. 1995;40:1710-1719.
157 Soffer EE, Johlin FL. Intestinal dysmotility in patients with sphincter of Oddi dysfunction: A reason for failed response to sphincterotomy. Dig Dis Sci. 1994;39:1942-1946.
158 DeSautels SG, Slivka A, Hutson WR, et al. Postcholecystectomy pain syndrome: Pathophysiology of abdominal pain in sphincter of Oddi type III. Gastroenterology. 1999;116:900-905.
159 Chun A, Desautels S, Slivka A, et al. Visceral algesia in irritable bowel syndrome, fibromyalgia, and sphincter of Oddi dysfunction, type III. Dig Dis Sci. 1999;44:631-636.
160 Evans PR, Dowsett JF, Kak Y-T, et al. Abnormal sphincter of Oddi response to cholecystokinin in post-cholecystectomy syndrome patients with irritable bowel syndrome: The “irritable sphincter.”. Dig Dis Sci. 1995;40:1149-1156.
161 Abraham HD, Anderson C, Lee D. Somatization disorder in sphincter of Oddi dysfunction. Psychosom Med. 1997;59:553-557.
162 Kuo WH, Pasricha PJ, Kalloo AN. The role of sphincter of Oddi manometry in the diagnosis and therapy of pancreatic disease. Gastrointest Endosc Clin N Am. 1998;8:79-85.
163 Choudari CP, Fogel EL, Sherman S, et al. Idiopathic pancreatitis: Yield of ERCP correlated with patient age. Am J Gastroenterol. 1998;93:1654A.
164 Venu RP, Geenen JE, Hogan W, et al. Idiopathic recurrent pancreatitis: An approach to diagnosis and treatment. Dig Dis Sci. 1989;34:56-60.
165 Toouli J, Roberts-Thomson IC, Dent J, et al. Sphincter of Oddi motility disorders in patients with idiopathic recurrent pancreatitis. Br J Surg. 1985;72:859-863.
166 Guelrud M, Mendoz S, Viera L. Idiopathic recurrent pancreatitis and hypercontractile sphincter of Oddi: Treatment with endoscopic sphincterotomy and pancreatic duct dilation [abstract]. Gastroenterology. 1986;90:1443.
167 Gregg JA. Function and dysfunction of the sphincter of Oddi. In: Jacobson IM, editor. ERCP: Diagnostic and therapeutic applications. New York: Elsevier; 1989:137-170.
168 Sherman S, Jamidar P, Reber H. Idiopathic acute pancreatitis (IAP): Endoscopic diagnosis and therapy. Am J Gastroenterol. 1993;88:1541A.
169 Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic pancreatitis. Gastrointest Endosc. 2002;55:157-162.
170 Coyle WJ, Pineau BC, Tarnasky PR, et al. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry, and endoscopic ultrasound. Endoscopy. 2002;34:617-623.
171 Fischer M, Hassan A, Sipe BW, et al. Endoscopic retrograde cholangiopancreatography and manometry findings in 1,241 idiopathic pancreatitis patients. Pancreatology. 2010;10:444-452.
172 Tarnasky PR, Hawes RH. Endoscopic diagnosis and therapy of unexplained (idiopathic) acute pancreatitis. Gastrointest Endosc Clin N Am. 1998;8:13-37.
173 Ros E, Navarro S, Bru C, et al. Occult microlithiasis in “idiopathic” acute pancreatitis: Prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701-1709.
174 Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol. 2008;14:1023-1026.
175 Toouli J, Di Francesco V, Saccone G, et al. Division of the sphincter of Oddi for treatment of dysfunction associated with recurrent pancreatitis. Br J Surg. 1996;83:1205-1210.
176 Okolo PI3rd, Pasricha PJ, Kalloo AN. What are the long-term results of endoscopic pancreatic sphincterotomy? Gastrointest Endosc. 2000;52:15-19.
177 Jacob L, Geenen JE, Catalano MF, et al. Prevention of pancreatitis in patients with idiopathic recurrent pancreatitis: A prospective nonblinded randomized study using endoscopic stents. Endoscopy. 2001;33:559-562.
178 Testoni PA, Caporuscio S, Bagnolo F, et al. Idiopathic recurrent pancreatitis: Long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol. 2000;95:1702-1707.
179 Wehrmann T, Schmitt TH, Arndt A, et al. Endoscopic injection of botulinum toxin in patients with recurrent acute pancreatitis due to pancreatic sphincter of Oddi dysfunction. Aliment Pharmacol Ther. 2000;14:1469-1477.