Speech, Mastication, and Swallowing Considerations in the Evaluation and Treatment of Dentofacial Deformities
The physiologic and functional mechanisms of human speech, mastication, and swallowing are essential for an individual to maintain his or her normal performance levels. These are highly coordinated and complex actions that are closely interrelated. These functions are susceptible to physical impairments, such as the presence of a dentofacial deformity. This chapter will review the mechanisms of normal speech, mastication, and swallowing. The potential for negative sequelae that stem from the presence of a jaw deformity with malocclusion and the benefits of successful reconstruction are also reviewed.11,151
The Neuromuscular Masticatory System
The masticatory system is an integrated complex that is primarily made up of bones, muscles, ligaments, and teeth. Movement of the structures is neurologically coordinated for efficient function and for the maintenance of the component parts over the individual’s lifetime.1–3 Controlled contracture and relaxation of the head and neck musculature are necessary to move the mandible, the soft palate, the lips, and the tongue efficiently for effective function (i.e., speech, swallowing, and chewing). A neurologic control system regulates and coordinates the activities of the entire masticatory system.
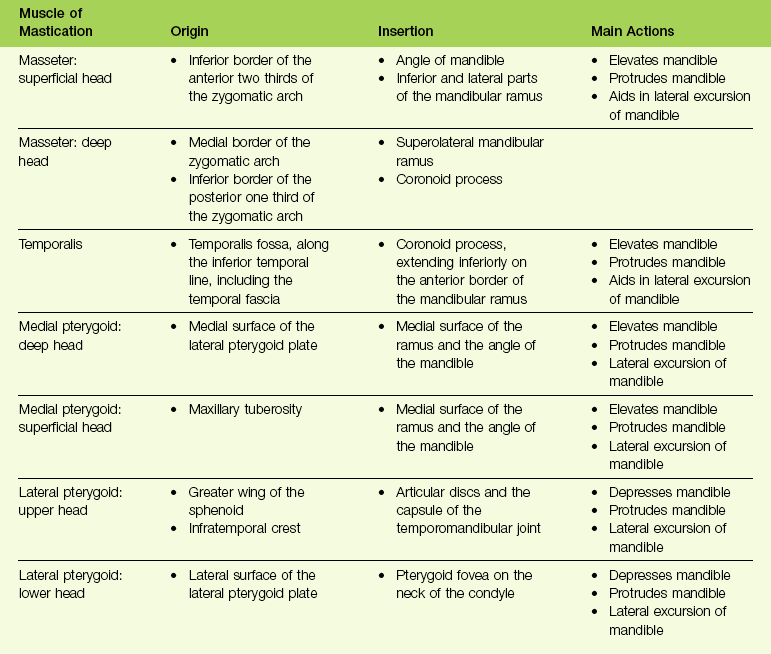
The dynamic balance of muscles in the head and neck is made possible through feedback that is provided by sensory receptors.10 To create a precise mandibular movement, input from the various sensory receptors is received by the central nervous system through the afferent fibers.41 The brain stem and cortex work together to assimilate and organize this input, and then provide modulated motor activities through the efferent nerve fibers.80 Within the brain stem, neurons control rhythmic muscle activities for effective breathing, swallowing, and chewing.25 These neurons are called the central pattern generator (CPG).96 The CPG is responsible for the timed and integrated activity among antagonistic muscles that is needed to accomplish these functions. For example, during chewing, the suprahyoid and infrahyoid muscles contract at the same time that the elevator muscles relax; this allows the mouth to open and accept food. With the bolus of food in the mouth, the CPG causes contraction of the elevator muscles while relaxing the suprahyoid and infrahyoid muscles, thereby producing closure of the mouth onto the food bolus. This chewing mechanism is repeated until the particles of food are small enough to be efficiently swallowed. All of this assumes that the individual has normal functioning of the tongue and lips, adequate numbers and locations of teeth, and intact temporomandibular joints and jaws (Fig. 8-1).43 Ideally, with all of these component structures in place, chewing can be accomplished without excess stress to any of the component parts. Although chewing is typically a subconscious activity, it can be brought into conscious control at any time. Likewise, breathing and swallowing are generally carried out as subconscious activities, but they can also be brought under voluntary control to be refined (Table 8-1).
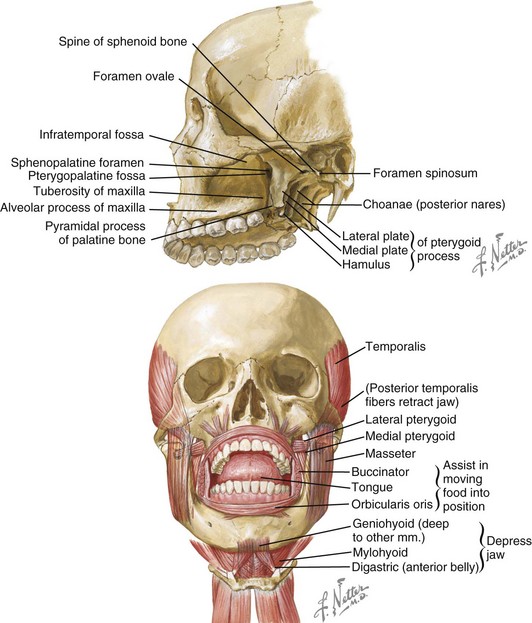
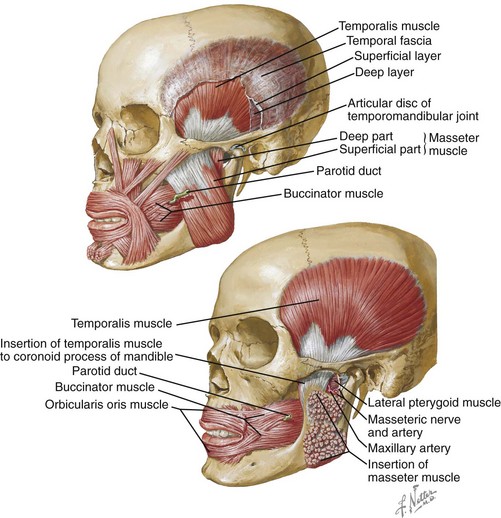
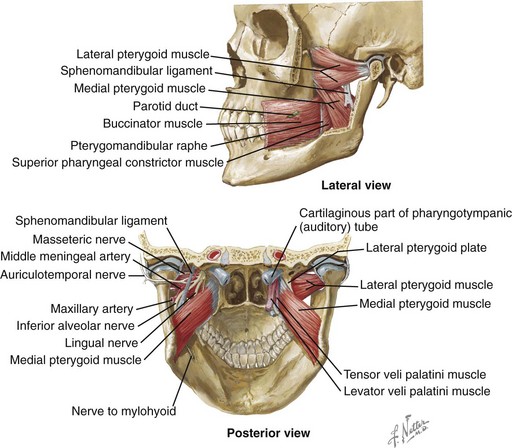
Figure 8-1 Mastication is the process of chewing food in preparation for deglutition (swallowing) and digestion. All muscles of mastication originate on the skull and insert on the mandible. All muscles of mastication are innervated by the mandibular division of the trigeminal nerve. All muscles of mastication are derivatives of the first pharyngeal arch. Movements of the mandible are classified as follows:
The bones involved include the base of the skull and the mandible. The skull base and the mandible articulate at the temporomandibular joint between the squamous portion of the temporal bone and the condyle of the mandible.
The muscles involved in the masticatory process include four paired muscles:
Netter illustration from www.netterimages.com © Elsevier Inc. All rights reserved.
Mastication Mechanism
Mastication is defined as the act of chewing food (Fig. 8-2). It represents the initial stage of digestion. During mastication, the food bolus is broken down into small particles for ease of swallowing. For most, it is considered an enjoyable activity that involves the senses of taste, touch, and smell. It is a complex function that requires the involvement of the muscles, the teeth, and the periodontal supportive structures. It also requires functioning temporomandibular joints and jaws, and it makes use of the lips, the cheeks, the tongue, the palate, and the salivary glands. It is an activity that is usually automatic and often taken for granted, although it can be easily brought into voluntary (i.e., conscious) control. It is also susceptible to pathologic conditions and damaging habits. Furthermore, modifications of this system (i.e., compensatory patterns) will be introduced when component parts are not in proper working order (e.g., in the presence of dentofacial deformity with malocclusion).
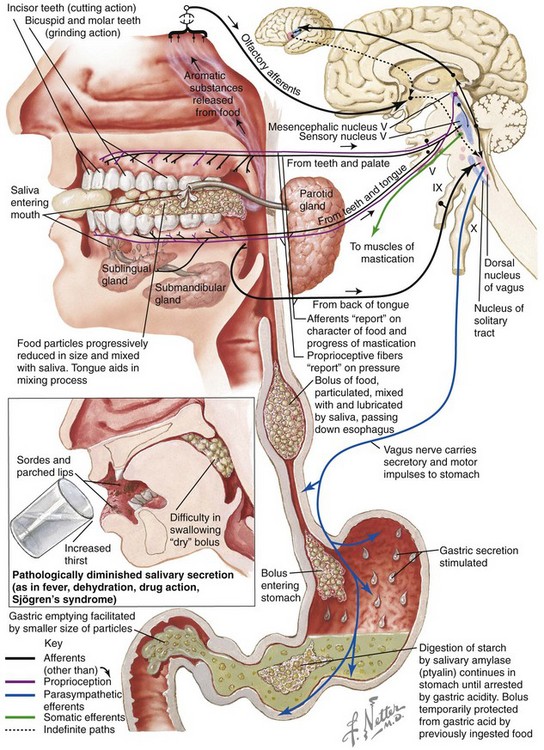
Figure 8-2 Mastication prepares food via “chewing” for deglutition and digestion. The illustrations and legends show an overview of the process. Netter illustration from www.netterimages.com © Elsevier Inc. All rights reserved.
In the normal setting, mastication is made up of rhythmic, well-controlled separations and closures of the maxillary and mandibular teeth. This activity is under the control of the CPG, which is located in the brain stem. Each opening and closing movement of the mandible represents a chewing stroke.79 The chewing stroke can be divided into the opening phase and the closing phase. The closing phase may be further subdivided into the crushing phase and the grinding phase. During mastication, the chewing stroke is repeated until the food is broken down into small enough parts for swallowing. After the food has been incised (usually with the anterior teeth) and then brought further into the mouth, the crushing of the bolus is concentrated on the posterior teeth.
It has been documented that the tooth contact ability, the number of teeth, and the quality of the teeth influence the chewing stroke. During mastication, sensory information is sent back to the central nervous system.5,158 This feedback mechanism allows for the alteration of the chewing stroke in response to the particular food being chewed and the quality of the anatomic structures that are available for chewing.65 Generally, with maximum full intercuspation (i.e., tall cusps, deep fossae, and ideal contacts), a predominantly vertical chewing stroke is seen. When there is a flattened occlusal surface (e.g., as a result of occlusal equilibrium or a grinding habit that involves a loss of enamel), a broader chewing stroke can be documented.54,81 The presence of malocclusion may produce irregular and less repeatable chewing strokes in the individual.6 Studies document that, in approximately 75% of subjects, there is a preferred chewing side.6 Chewing activities normally occur on the side with the greatest number of pain-free tooth contacts noticed during lateral glide.
The maximum biting force that can be applied to the teeth varies from individual to individual.156 With tougher foods, chewing preferably occurs predominantly on the first molar and second premolar areas. In general, males can bite more forcefully than females. Typically, in the presence of normal anatomy, the maximal amount of force applied to a molar is several times that which can be applied to an incisor.60 The maximum biting force generally increases with age, up to adolescence. Nevertheless, the amount of force placed on the teeth during mastication varies greatly from individual to individual.103,114
When food is introduced into the mouth, the lips must guide and control intake. After the food is in place, the lips must seal off the oral cavity. The lips are especially necessary when liquid is being introduced.58 This is more difficult for the individual with a long face growth pattern that causes mentalis strain and lip incompetence. When the food is in the mouth, the tongue plays a major role in maneuvering the bolus for sufficient chewing. The tongue typically initiates the process of breaking up the food by pressing the bolus against the hard palate; thus, the presence of an oronasal fistula in a patient with a cleft palate can be problematic. The tongue then pushes the food onto the occlusal surfaces of the teeth, where it can be effectively crushed during the chewing stroke. During the opening phase of the next chewing stroke, the tongue repositions the partially crushed food again onto the teeth for further breakdown. While the tongue is repositioning the food that has been displaced lingually, the buccinator muscle in the cheek region is compressing the food that has shifted into the vestibule back over the molars. The displaced food is continuously repositioned on the occlusal surfaces of the teeth until the particles are small enough to be swallowed efficiently. The tongue is also used to separate the food particles that require more chewing (and are therefore replaced over the molars) from those that are now small enough for swallowing. This sorting process is essential to prevent choking on large food particles. After the eating process is complete, the tongue is used to clean the teeth and to remove any food residue that has been trapped in locations such as the floor of the mouth (i.e., the sulcus) or in the labial vestibule.
In the presence of normal anatomy, the ingestion of a bolus of food is made easier by the active lowering of the mandible, the opening of the lips, and the depression of the tongue.25 All of these activities increase the size of the oral cavity to accommodate the bolus that is to be ingested. Not surprisingly, temporomandibular joint ankylosis or masticatory muscle pain with trismus will limit mouth opening and alter the process. The ingestion of fluid is usually via sucking (e.g., with a straw). In this case, the lips remain sealed around the delivery device, and the exit to the back of the oral cavity is closed by the tongue and the soft palate. In the individual with a long face growth pattern (i.e., lip incompetence and an anterior open bite), this will be more difficult. The lowering of the mandible and the depression and retraction of the tongue are accomplished by bracing the cheeks laterally and the mouth floor inferiorly. These actions will generate a subatmospheric pressure within the oral cavity to facilitate the flow of fluids into the oral cavity. This process will be more difficult in the presence of an oronasal fistula (i.e., an incompletely repaired cleft palate). This form of suction is also a useful mechanism for driving the entry of saliva into the oral cavity from the salivary glands.112
Swallowing Mechanism
Swallowing is a process that occurs through a series of coordinated muscular contractions that move the bolus of food from the oral cavity through the esophagus and into the stomach (Fig. 8-3). It requires voluntary, involuntary, and reflex muscular activity. The decision to swallow generally depends on the following: (1) the degree of fineness of the food (2) the intensity of the taste extracted and (3) the degree of lubrication of the bolus. During the swallowing mechanism, the lips are closed to seal the anterior aspect of the oral cavity. The teeth are brought into their maximum intercuspal position for the stabilization of the mandible. With the mandible fixed, the contraction of the suprahyoid and infrahyoid muscles will control the movement of the hyoid bone for effective swallowing.
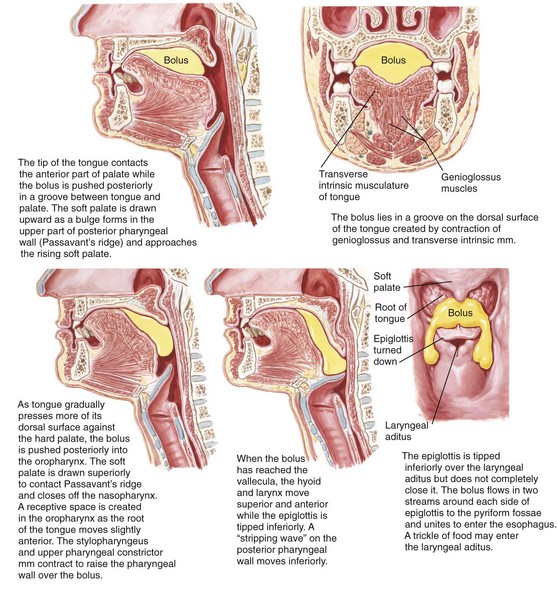
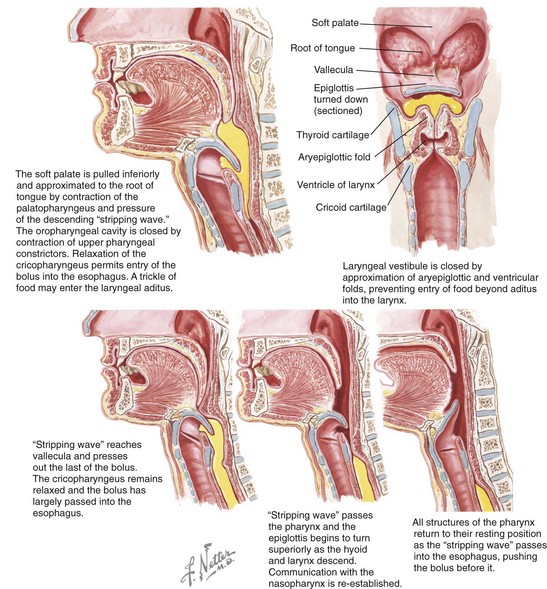
Figure 8-3 Deglutition or swallowing is a combination of voluntary and involuntary muscle contractions that move a bolus of food from the oral cavity to the esophagus. These illustrations review the details of the process. Netter illustration from www.netterimages.com © Elsevier Inc. All rights reserved.
In the normal growing child, as the posterior teeth erupt, the occluding teeth are able to brace the mandible, and the transition to an adult swallow occurs. In the presence of malocclusion, either from a developmental jaw deformity (i.e., an anterior open bite or a Class II malocclusion with significant overjet) or from the loss of teeth from either tumor or trauma, the infant swallow is maintained or reassumed (Fig. 8-4). In the presence of an anterior open bite, it is necessary for the individual to thrust the tongue forward to close the gap during the swallowing mechanism. This is an appropriate compensatory swallowing pattern.

Figure 8-4 Illustrations of a compensatory swallowing mechanism in an individual with a Class II excess overjet malocclusion. This illustration is representative of the patient shown in Figure 8-10.
Studies confirm that this repetitive swallowing cycle occurs approximately 600 times during a 24-hour cycle in the average individual.33 The general breakdown includes cycles during eating, cycles between meals while awake, and cycles during sleep. Lower levels of salivary flow during sleep result in less need for swallowing.112
Speech: Mechanism, Formation, and Assessment
The mechanism of speech is composed of several highly integrated processes (Fig. 8-5)26,31,122:
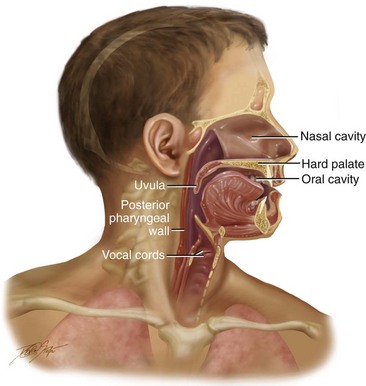
Figure 8-5 Illustration of a cross section through the head and neck that shows the pertinent anatomy required to articulate speech.
• Speech initiation, during which the content of the utterance to be spoken is converted into phonemes in the brain’s language center
• The generation of commands that go from the brain’s motor center to the speech organs
• Phonation, during which the emission of air sent from the lungs moves through the vocal tract and causes the formation of the voice as it moves past the vocal folds
• Articulation, which involves movement by the oral cavity, the oral pharynx, and the hypopharynx for the production of speech via this specific set of motor commands
During human speech production, the airflow that is sent from the lungs with a constant pressure passes between the vocal cords; the vibration of the vocal cords causes the airflow to be converted into cyclic puffs of air that then become sound (Fig. 8-6). The airstream is interrupted by movements of the jaws, the tongue, the soft palate, and the lips. These movements change the shape of the vocal tract, which in turn enables the individual to control the articulated sound and resonance characteristics.4 The pitch of the voice changes in accordance with adjustments that are made in the tension of the vocal cords. Voiced sounds incorporate the vibration of the vocal cords in addition to the placement of the articulators. Unvoiced sounds are produced by the interruption of the airstream by the articulators. Voiced sounds are produced with vocal fold vibration that accompanies articulator placement. Vocal tract resonance characteristics are controlled by the cross-sectional area of various parts of the vocal tract.36 Examples include the articulation of the upper lip to the lower lip for the bilabial sounds of /p/, /b/, and /m/ and the articulation of the tongue to the alveolar ridge for the production of the lingual-alveolar sounds /t/, /d/, /n/, /l/, /s/, and /z/.120
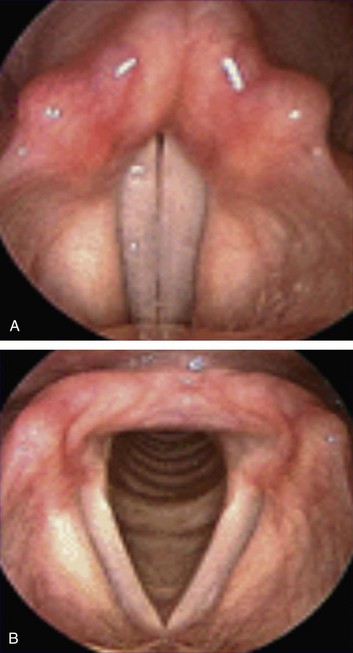
Figure 8-6 A view of vocal cords as seen via nasoendoscopic examination. A, Closed vocal cords (adduction position). B, Open vocal cords (abduction position).
Individual articulators (i.e., upper jaw, lower jaw, upper lip, lower lip, tongue, soft palate, hard palate, upper teeth, and lower teeth) are used for both the speech mechanism (Figs. 8-7 and 8-8; Table 8-2) and for the mastication and swallowing (deglutition) mechanisms (see Figs. 8-1, 8-2, and 8-3).123 Speech sounds are composed of the approximation of individual articulators within the speech mechanism and the manner in which the airflow from the vocal cords is modulated.59 This in turn produces what is called a phoneme, which is known to most as an isolated speech sound. A significant feature of articulatory movements is the phenomenon of assimilation in which the mouth position for an individual phoneme in the utterance incorporates the effects of the mouth position for the phonemes uttered immediately before and after. Interestingly, a human can imitate the sound of an utterance that he or she has heard without necessarily being able to speak the language of the utterance.66 In other words, the individual can estimate which mouth movements are necessary to produce a sound that is similar to the one that he or she has heard. By convention, the classification of speech sounds is categorized in accordance with their manner and place of production (i.e., the location of articulation).
TABLE 8-2
Kinesiologic Positions for the Oral and Pharyngeal Musculature during the Articulation of Speech Sounds
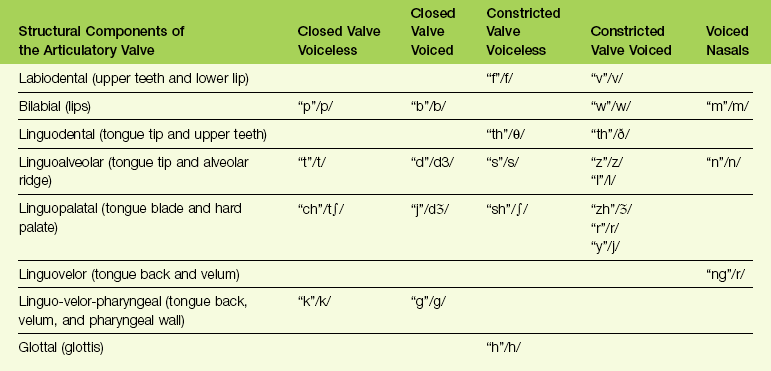
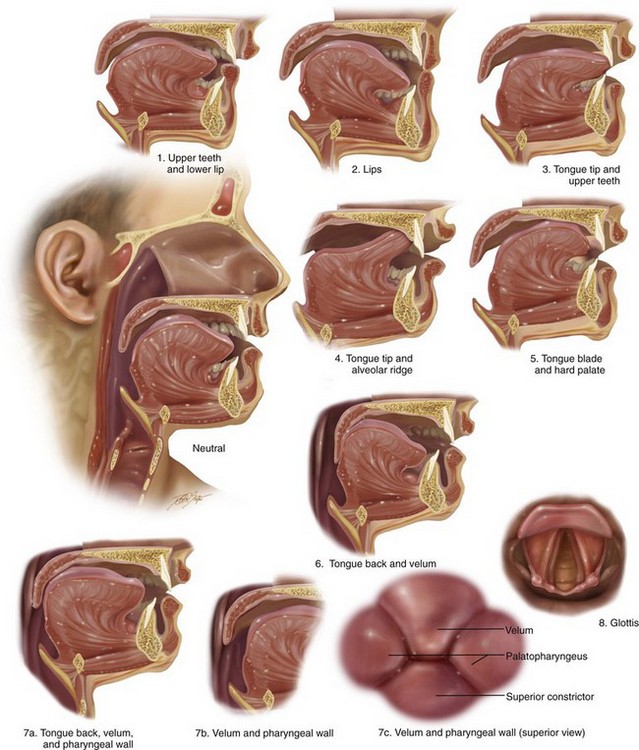
Figure 8-7 Diagram showing the kinesiologic positions for the oral and pharyngeal musculature during the articulation of speech sounds.
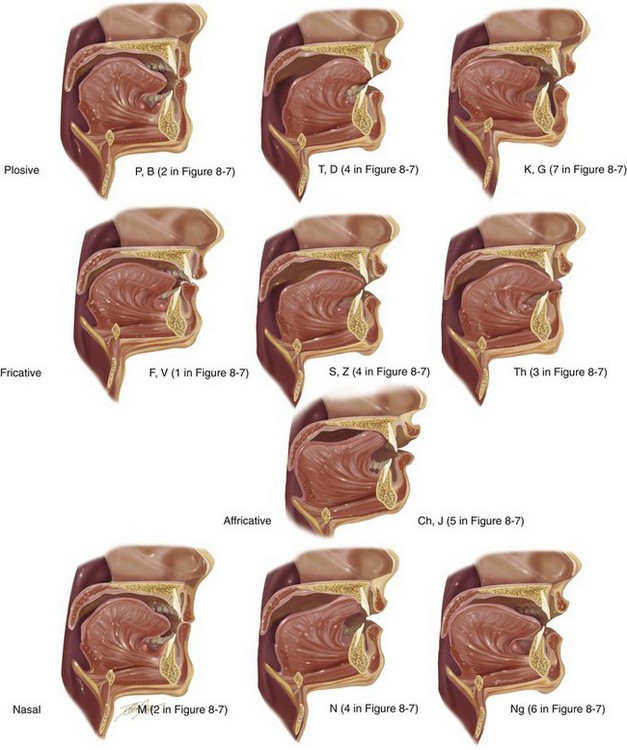
Figure 8-8 Illustrations that demonstrate the articulation of sounds created by specific positions of the lips, tongue, and teeth in the presence of harmonious jaws and occlusion. The numbering allows you to refer back to the chart shown in Figure 8-7, B, to correlate the anatomic position of the articulators with the associated speech sounds.
Vocal tract variables include the dynamic voluntary movements of the vocal organs (e.g., lower jaw, tongue, soft palate, vocal cords, upper lip, lower lip); these organs and their movements are influenced by the basic positions of the organs in relation to each other. The baseline position of the jaws and teeth vary widely; however, if they are significantly displaced (i.e., if there is malposition of the teeth or jaws), this can negatively affect articulation (Fig. 8-9). When the position of a jaws is abnormal (i.e., dentofacial deformity) and then successfully corrected via orthodontics and orthognathic surgery, including a return of normal morphology, then the tongue may or may not respond instantaneously to achieve a more normal utterance of specific consonant or vowel sounds (see Fig. 8-8).
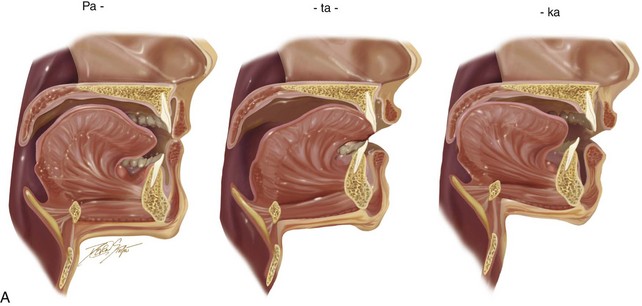
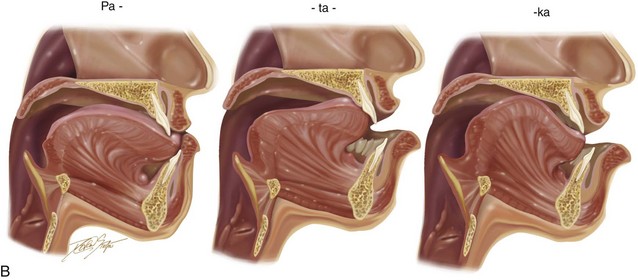
Figure 8-9 A, Drawings that show the representative stages of lip, dental, and tongue positioning during the production of the word patty-cake. B, In the presence of a jaw deformity with malocclusion, the lips and tongue must position themselves in other locations (i.e., via compensatory mechanisms) to create similar sounds in an attempt to achieve effective communication. This is demonstrated in an individual with a long face Class III negative overjet anterior open-bite malocclusion and similar to what is seen in the patient in Figure 8-11.
Speech sounds are generally divided into the categories of consonant sounds and vowel sounds. The production of consonant sounds requires the obstruction or partial closure (i.e., sphinctering) of the airstream at specific sites along the vocal tract.121 The classifications of consonant sounds are based on the extent of airstream obstruction, the site of airstream obstruction, and whether the vocal cords vibrate.16,17 Vowel sounds are generally produced by the position of the tongue and lips without airstream obstruction and with the upper and lower teeth more widely separated. All vowel sounds are voiced, and it is for this reason that vowel sounds are generally less affected by the malpositioning of the teeth or jaws than consonant sounds are.138
• Plosive/stop: Air pressure is stopped within the oral cavity at a specific location, and it is then suddenly released. Examples are /p/, /b/, /t/, /d/, /k/, and /g/.
• Fricative: Air pressure is partially blocked or obstructed within the vocal tract, thereby causing turbulent airflow. Examples are /f/, /v/, /s/, and /z/.
• Affricative: There is a sequencing of the airstream initially with complete obstruction (i.e., plosive/stop) followed by fricative (i.e., partial vocal tract obstruction) at the same location. Examples are /ch/ and /j/.
• Nasal: Air is allowed to pass freely through the nasal cavity. Examples are /m/, /n/, and /ng/.
The anatomic locations of the vocal organs that block or partially block the airflow (i.e., places of articulation) include the following: bilabial (upper lip to lower lip); labiodental (lower lip to upper teeth); interdental (tongue between the upper and lower teeth); lingual to palate alveolar (tongue to alveolar process of the upper jaw), lingual to palate (tongue to hard palate); and glottal (vocal cords to each other).124
The assessment of speech disorders involves the use of a number of tests and techniques.23,24,27,32,34,75,109,132,139 Historically, various tests have been described to assess speech and how it compares with recognized normative values. These assessments typically will evaluate sound production in the word position or within sentences. Tests that are frequently used for the assessment of speech are found in Box 8-1.
These tests are based on determining errors when the results are compared with those of normal individuals during single-word utterances. The Bzoch Error Pattern Diagnostic Articulation Test further grades each error type in accordance with its severity. All of these tests fall short for the assessment of the complexities of dynamic speech. Among educators and clinicians, there is often a lack of agreement with regard to a preferred objective method for the analysis of speech. The majority of the published studies were carried out to analyze the effects of orthodontics and orthognathic surgery on speech attempt to document articulation error rates in a traditional manner by noting sound production as simply correct or incorrect and by noting substitutions, distortions, or omissions. Some studies further describe errors as either visual or auditory in an attempt to overcome the usual problems associated with detecting mild sibilant distortions that might not be captured on audiotape.161
The classic negative effects of a jaw deformity with malocclusion on speech articulation are known.30,35 The speech benefits of successful orthodontic and surgical correction will have their most notable effects on consonant articulation within the fricative class, which includes /s/, /z/, and /f/. It is known that the /s/ sound in particular is highly sensitive to precise tongue placement and to the accurate direction of airflow across the incisal edges of the upper and lower teeth.
The characterization of speech error type is usually defined as follows:
• Substitution: when one sound is replaced by another
• Omission: when a sound is omitted from a word altogether
• Distortion: when sound is not produced appropriately but is still understood
• Visual distortion: when the visual appearance of making the sound is abnormal but the sound itself is correct
• Auditory distortion: when the perception of the sound (what is heard) is abnormal but the appearance (visual appearance) of the positioning of the anatomy is correct
• Combined visual/auditory distortion: when both the perception of the sound and the appearance of the production of the sound are abnormal
Effects of Jaw Deformities with Malocclusion on Speech
Background
Published studies show a tendency for individuals with Angle Class I occlusions to rate better with regard to speech as compared with those individuals with malocclusions.37 A review of the literature shows a significant association between misarticulation in speech and Angle Class II malocclusion (Fig. 8-10), Angle Class III malocclusion (Fig. 8-11), and anterior open-bite malocclusion (Fig. 8-12).39,43,53,54 Studies that evaluate speech articulation in individuals with jaw deformity and malocclusion confirm that as many as 90% of affected individuals have significant misarticulation errors. Several published studies report articulation errors among all jaw deformity patients analyzed.39,47,53

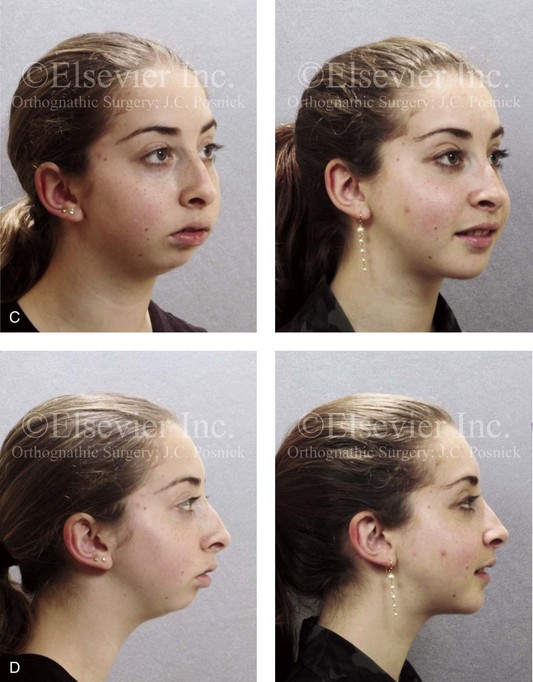
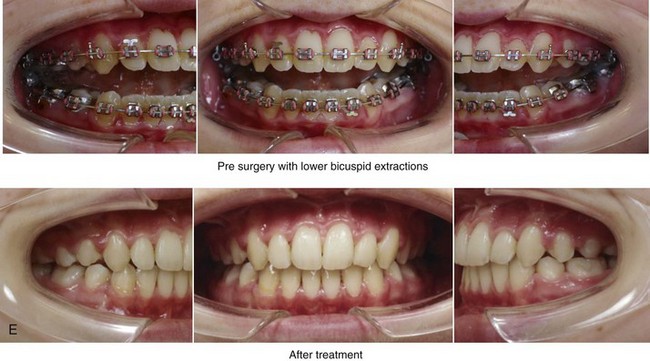
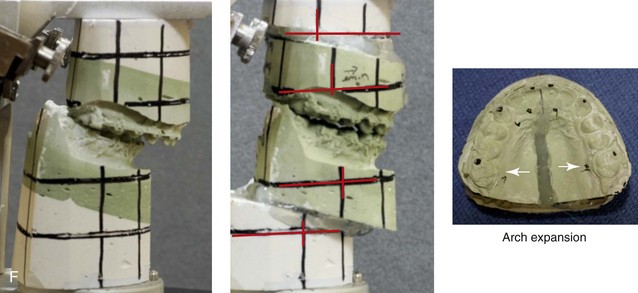
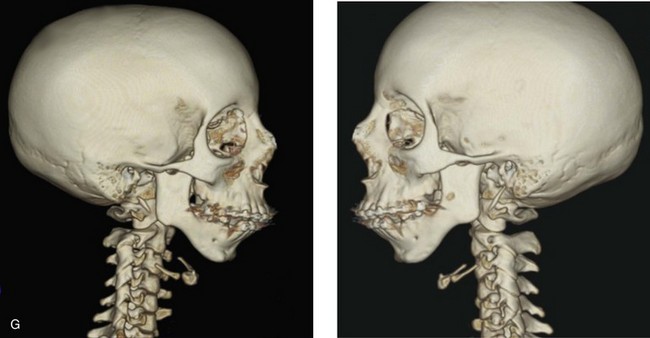
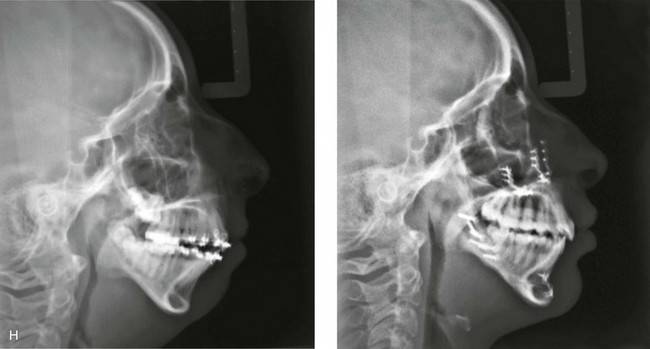
Figure 8-10 A 16-year-old girl arrived with her parents for the surgical evaluation of a developmental jaw deformity with malocclusion. She was diagnosed with a primary mandibular deficiency, a maxillary arch constriction, and a Class II excess overjet growth pattern. Attempts at orthodontic growth modification and camouflage in the past were unsuccessful. There was a lifelong history of obstructed nasal breathing, heavy snoring, and the suggestion of sleep apnea. The patient experienced difficulty with chewing, swallowing, speech articulation, breathing, and lip closure/posture. A formal evaluation by a speech–language pathologist was carried out.
Chewing/swallowing:
• Difficulty with chewing solid, textured food
• Only able to chew on the back teeth
• Frequently swallows foods partially whole, especially raw vegetables and meat
• Normal to mild labial weakness during repeated puckering and retraction tasks
• Assessed via the Fisher-Logemann Test of Articulation and informally during speech and conversation
• Misarticulations were identified, including /s/, /sh/, /st/, /z/, /dz/, and /sk/
Recommendations:
The patient’s presenting jaw deformity and malocclusion interfere with her ability to communicate information precisely to a listener and to be understood. Orthodontic treatment and orthognathic surgery are recommended to correct the maxillofacial deformities. If successful, then improvements in the dysfunctions described should be expected. It is highly doubtful that speech therapy alone could solve the extent of the deficits.
Treatment
The patient agreed to a combined orthodontic and surgical approach. Mandibular first bicuspid extractions provided space to orthodontically relieve dental compensation. Surgery included a maxillary Le Fort I osteotomy (arch expansion and horizontal advancement); bilateral sagittal split ramus osteotomies (horizontal advancement); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction.
A, Frontal views in repose before and after treatment. B, Frontal views with smile before and after treatment. C, Oblique facial views before and after treatment. D, Profile views before and after treatment. E, Occlusal views with orthodontics in progress (mandibular bicuspid extractions) and then after treatment. F, Articulated dental casts that indicate analytic model planning. G, Computed tomography scans prior to reconstruction confirming non-progressive glenoid fossa and condyle malformations. H, Lateral cephalometric radiographs before and after surgery.
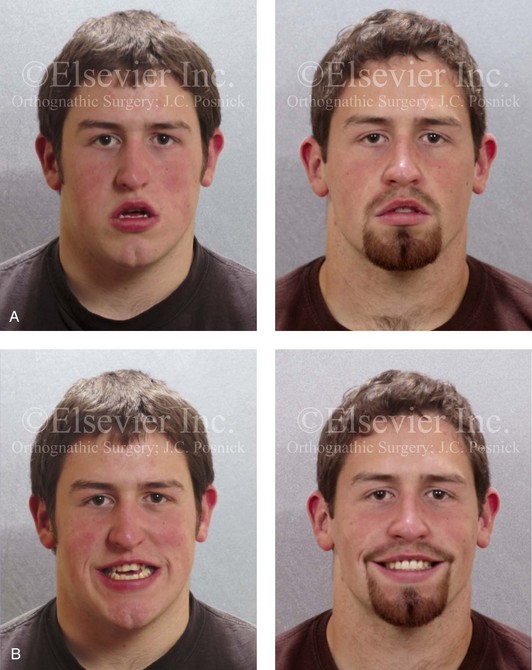
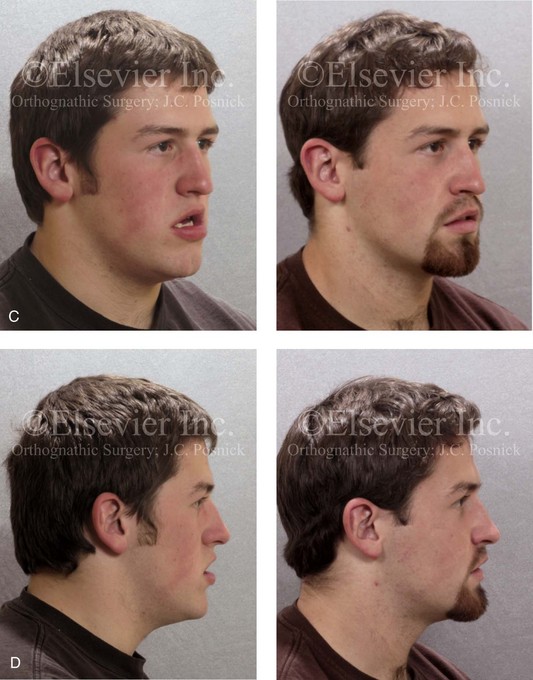
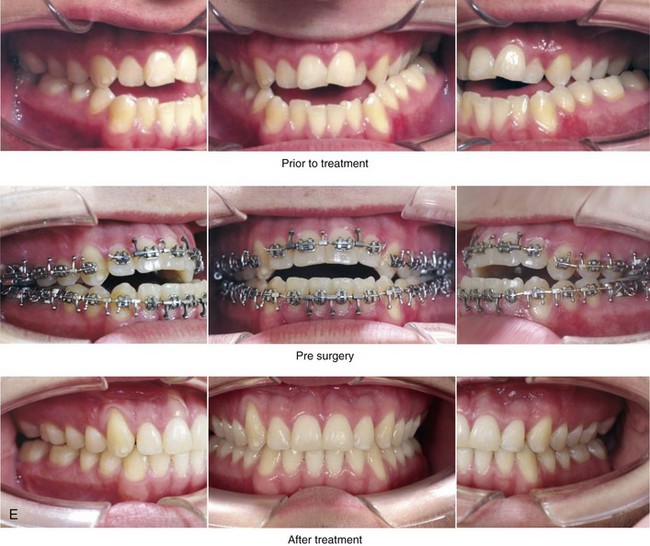
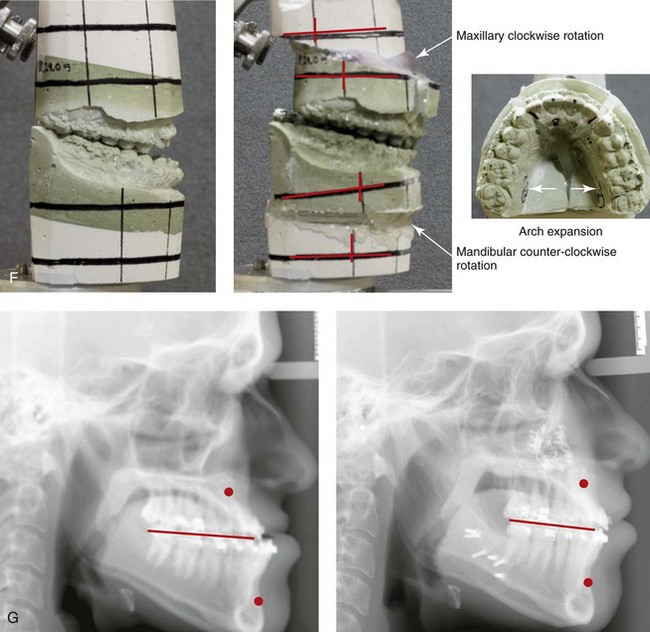
Figure 8-11 An 18-year-old man arrived with his parents for the surgical evaluation of a developmental jaw deformity with malocclusion. He was diagnosed with a long face Class III anterior open-bite negative overjet growth pattern. This was due to both a hereditary Class III tendency and a lifelong obstructed nasal breathing open-mouth posture. The patient described difficulty with chewing, swallowing, speech articulation, breathing, and lip closure/posture. A formal speech–language pathologist’s evaluation was carried out.
Chewing/swallowing:
• Frequent need to tear food apart before chewing
• Unable to bite into solid, textured food
• Only able to chew on the posterior teeth
• Labial motion and strength greatly reduced, especially with puckering and retraction of the tongue
• Assessed via the Fisher-Logemann Test of Articulation and informally during spontaneous conversation
• Misarticulations were identified, including /s/, /sh/, /z/, /dz/, /sk/, /ch/, and /s/ blends
• Production of sounds required anterior tongue thrusting and lateral lisping
• Exhibited imprecision in coarticulation, which often interfered with speech intelligibility
• Speech rate is rapid, with mumbling used as a compensatory feature for communication
• States that friends, teachers, and parents often comment that they cannot understand his speech
• Unintelligible speech interferes with daily performance at school and in social situations
• Avoids telephone conversations and hesitates to leave voice messages as a method of communicating
Recommendations:
The presenting jaw anomalies and malocclusion are responsible for the described findings. Orthodontics and orthognathic surgery are recommended to correct the maxillofacial anatomy. If successful, it is highly probable that the dysfunction described will resolve.
Treatment
The patient agreed to a combined orthodontic and surgical approach. With the removal of dental compensation, surgical procedures included a maxillary Le Fort I osteotomy (arch expansion, arch form correction, horizontal advancement, clockwise rotation, and vertical shortening); bilateral sagittal split ramus osteotomies (horizontal advancement and counterclockwise rotation); osseous genioplasty (horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring.
A, Frontal views in repose before and after treatment. B, Frontal views with smile before and after treatment. C, Oblique facial views before and after treatment. D, Profile views before and after treatment. E, Occlusal views before treatment, with orthodontics in progress, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after treatment.
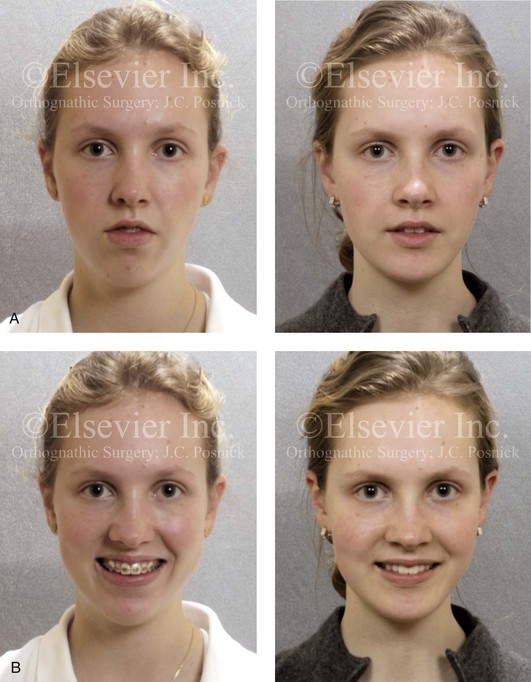
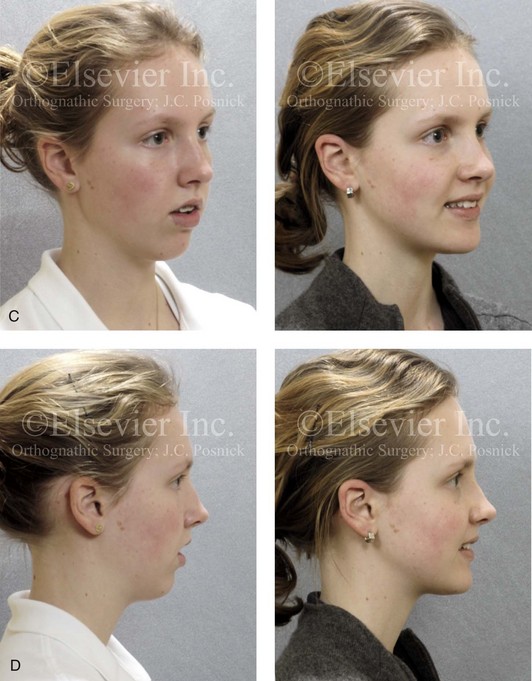
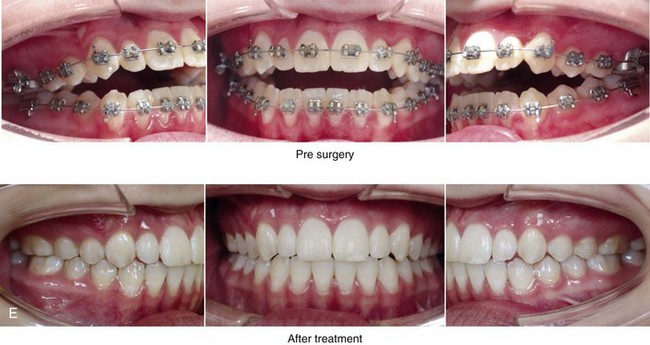
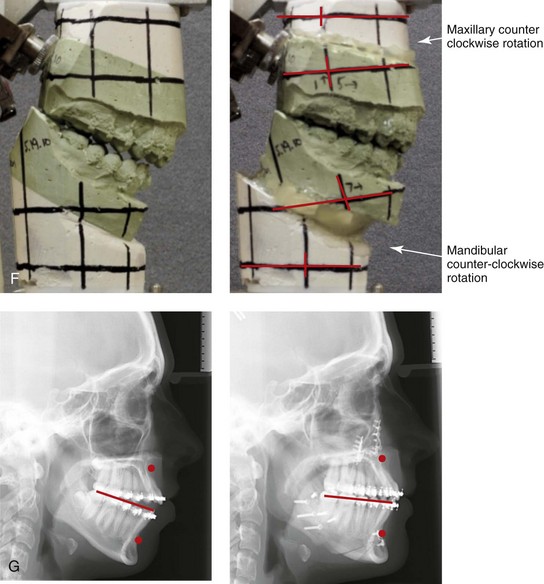
Figure 8-12 A 16-year-old girl arrived with her parents for the evaluation of a developmental jaw deformity with malocclusion. She was diagnosed with a long face Class II anterior open-bite growth pattern. She had undergone years of orthodontic growth modification and camouflage treatment, without success. Her dentofacial deformity was found to affect speech, chewing, swallowing, breathing, and lip closure/posture. A formal speech–language pathologist’s evaluation was carried out.
Chewing/swallowing:
• Only able to chew on the posterior teeth
• Malocclusion results in frequently swallowing food whole
• Noted to frequently have food falling out of her mouth secondary to malocclusion
• Demonstrates anterior carriage of the tongue at rest
• Tends to hold oral cavity in a closed posture during speech
• Assessed via the Fisher-Logemann Test of Articulation and informally during conversation
• Misarticulations were identified, including /s/, /sh/, /z/, /dz/, /z/, /sk/, and /st/
• Sound production is with anterior tongue thrusting and lateral lisping during contextual speech
• Exhibits imprecise coarticulation, which interferes with speech intelligibility
Recommendations:
The correction of the presenting abnormal jaw morphology and dental malposition is recommended, if feasible, through orthodontics and orthognathic surgery.
Treatment
Further orthodontic (dental) decompensation in combination with orthognathic surgery was planned. The procedures included a maxillary Le Fort I osteotomy (vertical intrusion, horizontal advancement, and counterclockwise rotation); bilateral sagittal split ramus osteotomies (horizontal advancement and counterclockwise rotation); osseous genioplasty (vertical shortening and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring.
A, Frontal views in repose before and after treatment. B, Frontal views with smile before and after treatment. C, Oblique facial views before and after treatment. D, Profile views before and after treatment. E, Occlusal views before surgery and then after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery.
The jaw deformity patterns that are most frequently associated with significant articulation errors include those with 1) Angle Class II open-bite malocclusion in association with vertical maxillary excess and mandibular deficiency (i.e., the long face growth pattern; see Chapter 21) and 2) Angle Class III negative overjet malocclusion in association with maxillary deficiency and relative mandibular excess (see Chapter 20).46,61,157 There were less articulation errors found in patients with Angle Class II primary mandibular deficiency malocclusions (see Chapter 19) and in patients with conditions that involve jaw asymmetry without significant malocclusion (i.e., mild hemifacial microsomia; see Chapter 28).67 Voice disorders were more frequently seen with closed-bite malocclusion as compared with open-bite malocclusion. Closed-bite jaw deformities are generally associated with adequate overjet and often with a deep overbite (i.e., the short face growth pattern; see Chapter 23).50
Patients with Class III skeletal patterns show distinct differences with regard to the consonant fricatives (/f/ and /s/) as compared with normal controls.73 Distortion type speech errors are also frequently associated with this type of malocclusion. This is especially true for patients with combined acoustic and visual errors. The speech sounds that are most commonly affected in the presence of malocclusion are sibilant sounds and bilabial (i.e., upper lip to lower lip) sounds. Errors in bilabial sounds are generally found in individuals with wide lip separation, such as those with maxillary deficiency in combination with mandibular excess or long face growth patterns (i.e., vertical maxillary excess and mandibular deficiency).87
Class II mandibular retrusion with excess overjet is likely to produce significant bilabial errors (see Fig. 8-10). Patients with Class II mandibular retrognathia also frequently show misarticulation errors (e.g., with the sound /r/). It is known that individuals with mild to moderate Class II mandibular retrusion are frequently able to adapt by posturing their lower jaw forward (i.e., by having a dual bite) to maintain control of their speech. This compensatory jaw position mechanism tends to break down with rapid speech or when the individual is otherwise fatigued.130 As a result of the inherent anatomic barriers, it is not possible for patients with maxillary deficiencies or mandibular excesses with significant negative overjet to posture their lower jaw posteriorly as a method of speech compensation (see Fig. 8-11).147 This makes the production of the /s/ sound difficult for most individuals with Class III malocclusions.133
Published reports indicate that, for a majority of the individuals studied, the orthodontic and surgical correction of the baseline jaw deformity and malocclusion either eliminates or dramatically reduces articulation errors.95,97,106,107,113,127,146 A minority of the study patients report little change and occasionally speech deterioration. This lack of success in some patients is most likely attributable to 1) initial errors in dental or jaw diagnosis; 2) the incomplete initial correction of the dental or jaw deformity 3) or long-term skeletal or dental relapse with a return of the abnormal dental or jaw position. The inability of an individual with normal neuromotor ability to favorably adapt to the corrected dental and jaw relationships is not the most common reason for a lack of speech improvement. Nevertheless, some individuals will be in need of speech articulation retraining after corrective surgery and orthodontics.
Unfortunately, many of the published studies that set out to evaluate speech aspects after the orthodontic and surgical correction of the initial jaw deformity or malocclusion include too few subjects, have inadequate controls, mix varied patterns of dental and jaw deformities, and lack a standardization of the sampling times before and after treatment.* Some of the published studies seem to lack an understanding of the expected postoperative convalescence, during which short-term speech disability is to be expected. Definitive speech evaluation should occur after adequate healing (i.e., 3 to 6 months) and with documentation of the level of success of the correction of the jaw deformity and malocclusion. As a result of these study shortcomings, there is a need for the further investigation of the speech effects of jaw and dental corrections with 1) larger samples of homogenous patterns of jaw deformity and malocclusion 2) standardized treatment protocols 3) the confirmation of the actual correction of jaw dysmorphology and malocclusion 4) the setting of agreed-upon speech sampling parameters 5) interobserver and intraobserver error testing of speech evaluations and 6) appropriate statistical analysis.
Cause and Effect Relationships
If the upper and lower lips cannot easily close together—as is frequently observed in patients with Angle Class III negative overjet skeletal patterns (e.g., maxillary deficiency with relative mandibular excess; see Fig. 8-11) or in those with classic long face growth patterns with Class II anterior open-bite malocclusion (e.g., maxillary vertical excess with mandibular deficiency; see Fig. 8-12)—then bilabial sounds (e.g., words such as pill, baby, and man) cannot be normally formed.55,95,97,104,136 These sounds will then be attempted through labiodental articulation. If the lower lip cannot contact the upper incisors (e.g., long face growth pattern with Class II anterior open-bite malocclusion or maxillary deficiency with mandibular excess negative overjet), then labiodental sounds (e.g., words such as five and vein) will be affected. Compensation will be attempted with bilabial sounds. If the tongue has difficulty reaching the lingual alveolar ridge of the maxilla (e.g., with moderate to severe Class II mandibular retrognathia), if the airstream cannot be correctly directed to the edges of the incisors (e.g., with an anterior open bite), or if the tongue is too far from the anterior aspect of the lower jaw (e.g., with Class III mandibular excess), then sibilant sounds (e.g., words such as sun, zoo, ship, chair, judge, and measure) will be affected (see Fig. 8-10).
Interestingly, from a speech perspective, some individuals are able to adapt to their dental or jaw defects more effectively than others. The individual’s ability to alter the airflow over the incisors or through other articulation points is necessary to effectively compensate for anatomic variations in the teeth and jaws. Marked deformities such as severe Class III maxillary deficiency (see Fig. 8-11), severe long face growth pattern (i.e., anterior open bite with mentalis strain; see Fig. 8-12), and severe Class II mandibular retrognathia with the inability to posture the jaw forward (see Fig. 8-10) are examples of situations in which compensation is not possible and speech articulation is noticeably affected. With successful orthodontics and jaw surgery, improved positioning of the teeth, jaws, lips, tongue, and soft palate results in ease of articulation to accomplish effective speech.28,74,126,131,137
It is not surprising that vowel sounds are less frequently disturbed by a jaw disproportion or malocclusion. Physiologically, the major difference between vowel sounds and consonant sounds is that consonant sounds require significant constriction of the airflow within the vocal tract, whereas vowel sounds are formed by the tongue being positioned to only partially constrict the airflow.143 Therefore, consonant sounds are more dependent on an exact positioning of the teeth with respect to each other and the lips with respect to each other (i.e., complete valve closure). Consonant sounds are more affected by jaw deformities such as Class III maxillary deficiency; long face anterior open bite; and severe Class II mandibular retrusion. These abnormal skeletal and occlusal patterns significantly interfere with the production of speech sounds. However, each individual’s speech system has variable adaptive ability. The adaptability primarily comes from the stretching of the lips (i.e., mentalis strain); the stretching of the tongue to extended positions; the posturing of the lower jaw forward (i.e., centric relation to the centric occlusal slide); and the ability to alter the airflow force across the valves and sphincters. The ability of an individual to control these factors is also dependent on the speed of speech and the amount and length of continuous speech required.
Effects of Successful Orthodontics and Orthognathic Surgery on Speech
Effects on Speech Articulation: Review of the Literature
A study by Witzel and colleagues examined the articulation of 41 individuals before and after orthognathic surgery that was carried out to improve facial aesthetics and to correct the occlusion.150 The investigators found a direct relationship between the pretreatment degree of mandibular retrognathia and the confirmed speech articulation errors. Twenty-two of 29 (76%) of the study patients had documented articulation errors before surgery; these included errors in sibilant production, labiodental sounds, and bilabial sounds. After surgery, significant improvement in the mean total articulation score for each patient was documented. There was also significant improvement in the sibilant sounds for all groups and in the mean bilabial scores of patients with mandibular retrognathia. Individuals with a Class III skeletal pattern and significant negative overjet (e.g., maxillary deficiency with relative mandibular excess) achieved the maximum improvement in labiodental scores after successful orthodontic and surgical correction.
Ruscello and colleagues studied the speech characteristics of 20 individuals who were scheduled to undergo orthognathic surgery for the correction of a variety of dentofacial deformities.106,107 They underwent speech evaluation before and at intervals for up to 6 months after surgery. Speech assessment at each interval included the Templin-Darley Screening Test of Articulation, a 60-item nonsense syllable task, the Deep Test of Articulation, and the Rainbow Passage. Sixty percent of the patients with jaw deformities demonstrated preoperative articulation errors. The majority of those who were exhibiting preoperative articulation errors had documented improvements after surgery. None of the patients experienced a deterioration in their articulation after surgery at the close of the study.
Kummer and colleagues studied 16 skeletal Class III patients before and after Le Fort I maxillary advancement.71 Seven of the 16 study patients were adolescents with repaired cleft lip and palate. Each patient underwent preoperative and postoperative speech evaluations and a multi-view videofluoroscopic speech study. Patients were evaluated before surgery as well as 3 and 6 months after surgery. The Templin-Darley Screening Test of Articulation was given, and articulation, resonance, and nasal emission were judged at each time interval. Eleven of 16 patients (69%) demonstrated articulation errors before surgery. After surgery, 7 of these 11 (64%) patients (4 without clefts and 3 with clefts) showed a decrease in the number of misarticulation speech sounds. None of the patients showed deteriorations in articulation after surgery.
Vallino studied the articulation, voice, resonance, hearing sensitivity, and middle-ear function of 34 individuals before and at intervals (i.e., at 3, 6, 9, and 12 months) after they underwent orthognathic surgery.129 Thirty of the 34 patients (88%) exhibited articulation errors before surgery. The most frequent sibilant errors were /s/ and /z/, followed by /j/, /zh/, /ch/, and /sh/. The errors were predominantly distortions of both the visual and acoustic types. After surgery, articulation generally improved spontaneously, without the need for speech training or intervention. Most of the preoperative articulation errors were eliminated by 3 months after surgery. In a minority of the study patients, a gradual decline in some of these improvements was measured at 12 months after surgery. This could be explained by either a relapse of the initial satisfactory occlusion and jaw correction or a limited sustained adaptation of speech ability in some individuals. Interestingly, voice, resonance, and hearing sensitivity were not altered by the surgeries that were carried out.
Xue and colleagues carried out a study to compare vocal tract configuration in males with skeletal Class III malocclusion (n = 8) with that of their normally developed Class I counterparts (n = 8).157 The goal of these researchers was to investigate the concomitant acoustic changes caused by any alterations in the vocal tract configuration that were identified. The findings of the study confirmed that young adolescents with Class III malocclusion had different vocal tract configurations than their counterparts with normal occlusion. The differences occurred in the oral cavity only. The individuals with Class III malocclusion had significantly longer oral length and larger oral volume than their counterparts in the control group. They surmised that the Class III dentofacial deformity restricted lip protrusion during the production of /u/, thereby contributing to the significantly higher resonating frequency (first formant or F1) of /u/ as compared with that of normal controls.
Effects on Velopharyngeal Function: Review of the Literature
Velopharyngeal insufficiency (VPI) is the hallmark sign of the negative speech effects among individuals who are born with cleft palates.7,12,22,52,76,91 The basic etiology of VPI in the repaired or unrepaired cleft palate is dysfunction of the soft palate (Fig. 8-13).13,19,20,51,101,111 The muscular anatomy that is affected by clefting includes both the elevators of the soft palate (i.e., the musculus uvulus and the levator veli palatini) and the antagonists to the elevators (i.e., the palatoglossus and the palatopharyngeus).56,69,70,116–118,145,160 VPI involves the inadequate closure of the velum to the pharyngeal walls while speaking.78,108,141,142 VPI results in increased nasal air escape with increased nasal resonance that can be heard.72,88,159 Resonance disorders include both hypernasality and hyponasality.77 When there is improper control of airflow across the velopharyngeal (VP) sphincter, hypernasality can occur, with increased nasal resonance occurring during the production of vowel sounds.84,153,154 Hyponasality is defined as a reduction in normal nasal resonance as a result of the blockage of airflow within the nasal cavity.92,104 A review of the literature is helpful to better understand the effects of Le Fort I osteotomy with advancement on VP function.*
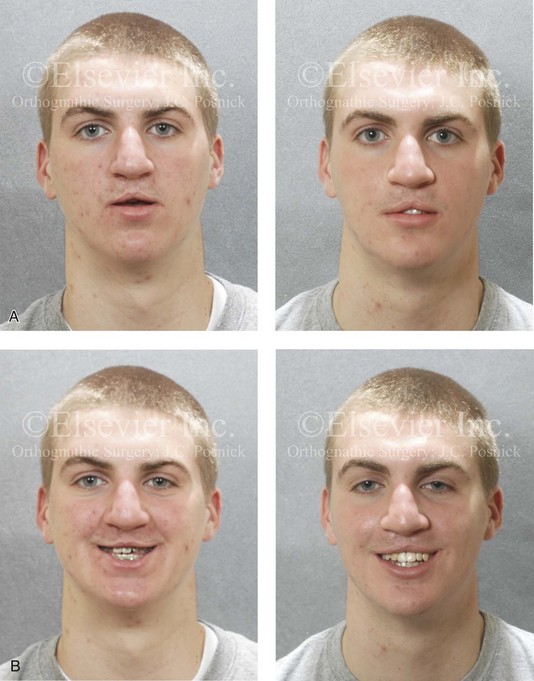
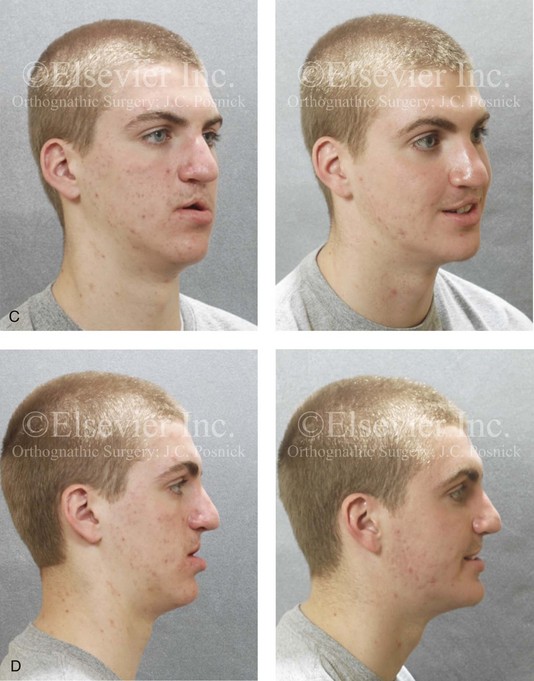
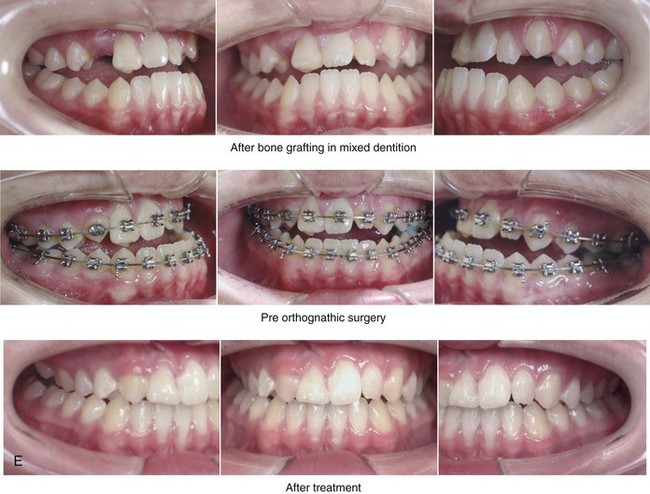
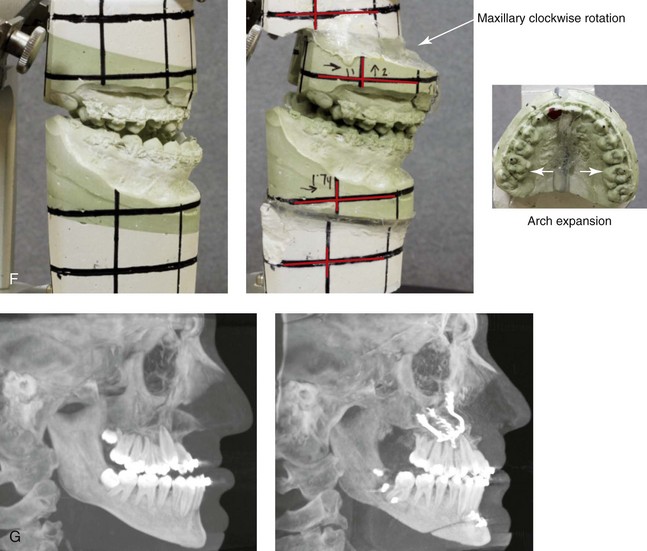
Figure 8-13 A 15-year-old boy was born with bilateral cleft lip and palate. Previous cleft surgery included bilateral cleft lip repair (3 months of age); cleft palate repair (12 months of age); and alveolar bone grafting (8 years of age). The patient has maxillary deficiency and secondary deformities of the mandible, and there is chronic nasal obstruction (i.e., septal deviation, inferior turbinate hypertrophy, and nasal floor irregularities). There is a severe Class III negative overjet malocclusion. Orthodontic (dental) decompensation has been carried out in preparation for jaw and intranasal surgery. The patient’s current maxillofacial findings affect speech, chewing, swallowing, breathing, and lip closure/posture. A formal speech–language pathologist’s evaluation was carried out.
Chewing/swallowing:
• Able to chew on posterior teeth only
• Occasional nasopharyngeal leakage (into the nose) when drinking milk
• Maxillary hypoplasia with Class III malocclusion
• Exhibits mild lip weakness with protrusion and retraction
• Assessed via the Fisher-Logemann Test of Articulation and during informal conversation
• Misarticulations were identified, including /s/, /sh/, /z/, /dz/, /sk/, and /st/
• Nasal emission under right nares observed during non-nasal speech sounds
• Shortened palate during non-nasal speech production of phrases and single words
• Exhibits sound misarticulations associated with jaw anomaly and malocclusion
• Misarticulations affect speech function during routine conversation
• Rated as having inadequate velopharyngeal closure before jaw reconstruction
• Reassess 3 to 6 months after jaw surgery, with anticipated need for a pharyngeal flap
Treatment
Further orthodontic (dental) decompensation in combination with orthognathic surgery was planned. The procedures included maxillary Le Fort I osteotomy (vertical intrusion, horizontal advancement, and counterclockwise rotation); bilateral sagittal split ramus osteotomies (horizontal advancement and counterclockwise rotation); osseous genioplasty (vertical shortening and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring.
A, Frontal views in repose before and after treatment. B, Frontal views with smile before and after treatment. C, Oblique facial views before and after treatment. D, Profile views before and after treatment. E, Occlusal views before surgery and then after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after surgery. Six months after successful jaw and intranasal reconstruction; a nasoendoscopic speech assessment confirmed the need for a tailor-designed pharyngeal flap.
In 1969, Jabaley and Edgerton were the first to report about the potential for changes in the spatial relationship of the soft palate to the pharyngeal wall as a result of maxillary Le Fort I advancement.63 They described an 18-year-old patient without clefting who underwent a Le Fort I osteotomy with advancement. They stated that there was “no adverse alteration of velopharyngeal function accompanying the maxillary advancement.”63
In 1976, Schwarz and Gruner reported about 40 patients who were undergoing Le Fort I osteotomy with repositioning (31 out of 40 with repaired cleft palate).113 The authors concluded that the “subjective evaluation of velopharyngeal function and nasality in the cleft palate patients showed no relationship between the degree of hypernasality before or after the Le Fort I advancement.”113
Witzel and Munro discussed a 16-year-old patient with a repaired unilateral cleft lip and palate who presented to them with maxillary hypoplasia and malocclusion.149 Before surgery, this patient’s VP valving mechanism showed only touch closure of the soft palate against the posterior pharyngeal wall. Two months after Le Fort I osteotomy (10 mm of advancement and 4 mm of vertical lengthening), his speech evaluation revealed marked hypernasality throughout connected discourse. The authors concluded that “maxillary advancement may have a disastrous effect on the velopharyngeal valving mechanism.”149 They recommended that “all patients undergoing this procedure should have detailed clinical investigation of speech and velopharyngeal function pre- and postoperatively.”149
Epker and Wolford observed that adolescent patients with repaired clefts, maxillary deficiency, and preoperative VPI became worse after Le Fort I advancement.29 The authors stated that individuals with only borderline VP closure may demonstrate notable VPI after maxillary osteotomy, particularly if the advancement exceeds 10 mm. Bralley and Schoeny reported about a 19-year-old patient with a submucous cleft palate and maxillary deficiency who showed no change in nasality after Le Fort I advancement.9 Schendel and colleagues evaluated 21 patients without clefts before and after maxillary Le Fort I osteotomy to assess VP closure.110 They did this through speech evaluation, lateral cephalometric radiography, and nasopharyngoscopy, and they found no change in VP competence after Le Fort I advancement.
Witzel described a sample of 41 patients without cleft palate and 50 patients with repaired cleft palate (bilateral, unilateral, or isolated) who underwent Le Fort I maxillary advancement.152 The results indicated that patients without cleft palate have a very low risk of deterioration of VP function. Patients with a repaired cleft palate who had adequate VP function before Le Fort I advancement were also at lower risk. However, 11 of 15 of the study patients with a repaired cleft palate and a preoperative rating of borderline VP closure acquired surgically induced symptomatic VPI after Le Fort I advancement. Patients who were considered to have inadequate VP closure before surgery remained so after surgery. For most patients, the extent of preoperative competence of the VP sphincter before Le Fort I advancement predicted the competence of the VP sphincter after surgery (as documented by videofluoroscopy and nasoendoscopy).
Kummer and colleagues reported on VP function in two different patient groups: those with repaired clefts and those without clefts who underwent maxillary Le Fort I advancement with or without vertical change.71 The measured change in VP function was not clinically significant. They concluded that “Le Fort I maxillary osteotomy has a negligible effect on velopharyngeal function in both cleft and non-cleft cases.”71
Vallino discussed 34 patients without clefts before and at intervals after orthognathic surgery that included Le Fort I osteotomy.129 The VP port area was measured in the study patients before and at intervals (i.e., 3, 6, 9, and 12 months) after Le Fort I osteotomy. The estimation of the size of the VP port area was obtained with the pressure-flow technique described by Warren and Dubois.139 All study patients demonstrated VP port areas that measured between 0 and 0.49 cm2 both before and after surgery across all speech tasks, which indicated adequate VP competence. The author concluded that the Le Fort I osteotomies carried out in patients without clefts did not alter the VP valve or cause hypernasal speech.
Watzke and colleagues evaluated VP function with the use of aerodynamic testing before and at least 1 year after Le Fort I maxillary advancement in 24 adolescents with repaired cleft palates.144 Five of these patients (23%) demonstrated VP deterioration, whereas another five (23%) showed improvement after Le Fort I advancement. Interestingly, those patients with a pharyngeal flap in place had a higher incidence of improved VP function after surgery. Of the five (23%) that showed VP deterioration, four had adequate VP function and one had borderline VP function preoperatively. Fourteen (46%) of the patients with clefts showed no significant change in VP function as a result of Le Fort I advancement.
Janulewicz and colleagues completed a retrospective study that evaluated VP function in patients with repaired cleft lips and palates (N = 54) who underwent maxillary Le Fort I advancement.64 The authors documented a significant deterioration of VP function in many of the patients with clefts after Le Fort I advancement. There was also an increase in hypernasality, which further supported the findings of VP deterioration in a subgroup of the patients with repaired clefts after Le Fort I advancement. The authors also found improvements in hyponasality as compared with in preoperative values, which was attributed to a decrease in nasal airflow obstruction after maxillary advancement.
Velopharyngeal Evaluation Among Individuals with Repaired Cleft Palates and Maxillary Deficiencies Undergoing Le Fort I Advancement
A variety of methods for the assessment of VP function have been described.57,62,86,89,90,93,94,99,100,105,115,119,140,152 A protocol for the evaluation of patients undergoing orthognathic surgery has been advanced by the American Cleft Palate-Craniofacial Association in Parameters for Evaluation and Treatment of Patients with Cleft Lip/Palate or Other Craniofacial Anomalies (American Cleft Palate-Craniofacial Association, Revised edition 2009 www.acpa-cpf.org).
Witzel also developed a protocol for the evaluation of VP function in adolescents and adults after palate repair to anticipate alterations in the VP sphincter after maxillary Le Fort I osteotomy.154 She recommends a routine speech assessment that includes instrumentation evaluation of VP function, preferably with nasoendoscopy or, as a second choice, videofluoroscopy. After surgery and sufficient time for healing (i.e., 6 to 12 months), a final assessment of articulation and VP function can be carried out. At that time, if VP function is inadequate, definitive management can go forward (e.g., pharyngeal flap surgery or the revision of a flap that is already in place).
• Adequate function is defined as normal nasal resonance, normal nasal air emission, and adequate VP closure during speech.
• Borderline function is defined as normal nasal resonance or clinically insignificant hypernasality, inaudible nasal air emission (via mirror test), and borderline or marginal VP closure during speech. Patients with this level of functioning usually have small pinhole gaps in the VP valve through which bubbles, barium, or mucus is observed during videofluoroscopy, nasoendoscopy, or both.
• Inadequate function is defined as clinically significant hypernasal resonance, nasal air emission, and VP insufficiency during speech.
References
1. Ahlgren, J. Mechanism of mastication. Acta Odontol Scand. 1966; 24:44–45.
2. Anderson, DJ, Picton, DCA. Tooth contact during chewing. J Dent Res. 1957; 36:21–26.
3. Anderson, DM. Dorland’s illustrated medical dictionary, ed 28. Philadelphia: Saunders; 1988.
4. Aronson, AE. Nasal resonatory disorders. In: Aronson AE, ed. Clinical voice disorders. New York: Thieme, 1990.
5. Beyron, HL. Occlusal changes in the adult dentition. J Am Dent Assoc. 1954; 48:674–686.
6. Beyron, HL. Occlusal relations and mastication in Australian aborigines. Acta Odontol Scand. 1964; 22:597–678.
7. Blakeley, RW. The rationale for a temporary speech prosthesis in palatal insufficiency. Br J Disord Commun. 1969; 4:134–139.
8. Bowers, J, Tobey, EA, Shaye, R. An acoustic-speech study of patients who received orthognathic surgery. Am J Orthod. 1985; 88:373–379.
9. Brally, RC, Schoeny, ZG. Effect of maxillary advancement on the speech of a sub-mucosal cleft palate patient. Cleft Palate J. 1977; 14:98.
10. Brekhus, PH. Stimulation of the muscles of mastication. J Dent Res. 1941; 20:87–92.
11. Bruce, FA, Hanson, ML. Speech and swallowing changes associated with sagittal osteotomy: a report of four subjects. Int J Orofac Myol. 1987; 13:1.
12. Brunner, M, Stellzig-Eisenhauer, A, Proschel, U, et al. The effect of nasopharyngoscopic biofeedback in patients with cleft palate and velopharyngeal dysfunction. Cleft Palate Craniofac J. 2005; 42:649–657.
13. Cable, BB, Canady, JW, Karnell, MP, et al. Pharyngeal flap surgery: long-term outcomes at the University of Iowa. Plast Reconstr Surg. 2004; 113:475.
14. Chanchareonsook, N, Samman, N, Whitehill, TL. The effect of cranio-maxillofacial osteotomies and distraction osteogenesis on speech and velopharyngeal status: a critical review. Cleft Palate Craniofac J. 2006; 43:477–487.
15. Chanchareonsook, N, Whitehill, TL, Samman, N. Speech outcome and velopharyngeal function in cleft palate: comparison of Le Fort I maxillary osteotomy and distraction osteogenesis–early results. Cleft Palate Craniofac J. 2007; 44:23–32.
16. Clark, HM. Neuromuscular treatments for speech and swallowing: a tutorial. Am J Speech Lang Pathol. 2003; 12:400–415.
17. Clark, HM. The role of strength training in speech sound disorders. Semin Speech Lang. 2008; 29:276–283.
18. Cleall, JF. Deglutition: a study of form and function. Am J Orthod. 1965; 51:161–182.
19. Costello, BJ, Ruiz, RL, Turvey, T. Surgical management of velopharyngeal insufficiency in the cleft patient. Oral Maxillofac Surg Clin North Am. 2002; 14:539.
20. Croft, CB, Shprintzen, RJ, Daniller, A, et al. The occult submucous cleft palate and the musculus uvulae. Cleft Palate J. 1978; 15:150.
21. Dalston, RM, Vig, PS. Effect of orthognathic surgery on speech: a prospective study. Am J Orthod. 1984; 86:291–298.
22. Dalston, RM. Velopharyngeal impairment in the orthodontic population. Semin Orthodon. 1996; 2:220–227.
23. Daniloff, RG, Wilcox, K, Stephens, MI. An acoustic-articulatory description of children’s defective /s/ productions. J Commun Dis. 1980; 13:347.
24. De Bodt, M, Wuyts, F, van de Heyning, P, Croux, C. Test-retest study of the GRBAS Scale: the influence of experience and professional background on perceptual rating of voice quality. J Voice. 1997; 11:74–80.
25. Dellow, PG, Lund, JP. Evidence for central timing of rhythmical mastication. J Physiol. 1971; 215(1):1–13.
26. Denes, PB, Pinson, EN. The speech chain: the physics and biology of spoken language. Garden City, NY: Anchor Books, Doubleday & Co; 1973.
27. DeRuyter, F, Diefendorf, AO. Hearing sensitivity and measurements of middle ear and eustachian tube function after maxillary osteotomy with advancement surgery. J Oral Surg. 1980; 38:343.
28. Epker, BN, Wolford, LM. Middle-third facial osteotomies: their use in the correction of acquired and developmental dentofacial and craniofacial deformities. J Oral Surg. 1975; 33:491.
29. Epker, BN, Wolford, LM. Middle-third facial osteotomies: their use in the correction of congenital dentofacial and craniofacial deformities. J Oral Surg. 1976; 34:324–342.
30. Fairbanks, G, Lintner, MVH. A study of minor organic deviations in ‘functional’ disorders of articulation. 4. The teeth and hard palate. J Speech Hearing Dis. 1951; 16:273.
31. Fant, G. Acoustic theory of speech production, ed 2. The Hague: Mouton & Co NV; 1970.
32. Fisher, MA, Logemann, JA. The Fisher-Logemann test of articulation competence. Boston: Houghton-Mifflin; 1971.
33. Flanagan, JB. The 24-hour pattern of swallowing in man. J Dent Res. 1956; 35:109–114.
34. Flanagan, JL. Speech analysis synthesis and perception, ed 2. Berlin, Germany: Springer-Verlag; 1972.
35. Fletcher, SG, Casteel, RL, Bradley, DP. Tongue-thrust swallow, speech articulation and age. J Speech Hear Dis. 1961; 26:201.
36. Fletcher, SG. Contingencies for bioelectronic modification of nasality. J Speech Hear Disord. 1972; 37:329–346.
37. Forssell, H, Finne, K, Forssell, K, et al. Expectations and perceptions regarding treatment: a prospective study of patients undergoing orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1998; 13:107–113.
38. Garber, SR, Speidel, TM, Marse, G. The effects on speech of surgical premaxillary osteotomy. Am J Orthod. 1981; 79:54.
39. Gardner, AF. Dental, oral, and general causes of speech pathology. Oral Surg Oral Med Oral Pathol. 1949; 2:742.
40. Geffen, D. The effects of mandibular osteotomy on articulation and resonance. South African J Comm Disord. 1978; 25:54.
41. Gibbs, CH, Messerman, T, Reswick, JB, Derda, HJ. Functional movements of the mandible. J Prosthet Dent. 1971; 26:604–620.
42. Glass, L, Knapp, J, Bloomer, HH. Speech and lingual behavior before and after mandibular osteotomy. J Oral Surg. 1977; 35:104–109.
43. Glickman, I, Pameijer, JH, Roeber, FW, Brian, MA. Functional occlusion as revealed by miniaturized radio transmitters. Dent Clin North Am. 1969; 13:667–679.
44. Goodstein, DB, Cooper, D, Wallaca, L. The effect on speech of surgery for correction of mandibular prognathism. Oral Surg. 1974; 37:846.
45. Gotzfried, HF, Thumfart, WE. Pre- and postoperative middle ear function and muscle activity of the soft palate after total maxillary osteotomy in cleft patients. J Craniomaxillofac Surg. 1988; 16:64.
46. Guay, AH, Maxwell, DL, Beecher, R. Radiographic study of tongue posture at rest and during the phonation of /s/ in class III malocclusion. Angle Orthod. 1978; 48:10.
47. Guenther, TA, Sather, AH, Kern, EB. The effect of Le Fort I maxillary impaction on nasal airway resistance. Am J Orthod. 1984; 85:388.
48. Guyette, TW, Polley, JW, Figueroa, AA, Cohen, M. Mandibular distraction osteogenesis: effects on articulation and velopharyngeal function. J Craniofac Surg. 1996; 7:186.
49. Guyette, TW, Polley, JW, Figueroa, A, Smith, BE. Changes in speech following maxillary distraction osteogenesis. Cleft Palate Craniofac J. 2001; 38:199–205.
50. Hamlet, S, Cullison, BL, Stone, ML. Physiological control of sibilant duration: insights afforded by speech compensation to dental prosthesis. J Acoust Soc Am. 1979; 65:1276.
51. Hardin, MA, Van Demark, DR, Morris, HL, Payne, MM. Correspondence between nasalance scores and listener judgments of hypernasality and hyponasality. Cleft Palate Craniofac J. 1992; 29:346–351.
52. Hardin-Jones, M, Chapman, K. The impact of early intervention on speech and lexical development for toddlers with cleft palate: a retrospective look at outcome. Lang Speech Hear Serv Sch. 2008; 39:89–96.
53. Harvold, EP, Vargervik, K, Chierici, G. Primate experiments on oral sensation and dental malocclusion. Am J Orthod Dentofacial Orthop. 1973; 63:494.
54. Haryett, RD, Hansen, FC, Davidson, PO. Chronic thumb sucking: the psychological effects and relative effectiveness of various methods of treatment. Am J Orthod Dentofacial Orthop. 1967; 53:569.
55. Hassan, T, Naini, F, Gill, D. The effects of orthognathic surgery on speech: a review. J Oral Maxillofac Surg. 2007; 65:8.
56. Henningsson, GE, Isberg, AM. Velopharyngeal movements in patients alternating between oral and glottal articulation: a clinical and cineradiographical study. Cleft Palate J. 1986; 23:1–9.
57. Hirschberg, J, Van Demark, DR. A proposal for standardization of speech and hearing evaluations to assess velopharyngeal function. Folia Phoniatr Logop. 1997; 49:158–167.
58. Horio, T, Kawamura, Y. Effects of texture of food on chewing patterns in the human subject. J Oral Rehabil. 1989; 16:177–183.
59. Houde, JF, Jordan, MI. Sensorimotor adaptation in speech production. Science. 1998; 279:1213.
60. Howell, AH, Brudevold, F. Vertical forces used during chewing of food. J Dent Res. 1950; 29:133–136.
61. Hu, W, Zhou, Y, Fu, M. Effect of skeletal class III malocclusion on speech articulation. Zhonghua Kou Qiang Yi Xue Za Zhi. 1997; 32:3.
62. Iglesias, A, Kuehn, DP, Morris, HL. Simultaneous assessment of pharyngeal wall and velar displacement for selected speech sounds. J Speech Hear Res. 1980; 23:429–446.
63. Jabaley, MF, Edgerton, MT. Surgical correction of congenital midface retrusion in the presence of mandibular prognathism. Plast Reconstr Surg. 1969; 44:1–8.
64. Janulewicz, J, Costello, BJ, Buckley, MJ, et al. The effects of Le Fort I osteotomies on velopharyngeal and speech functions in cleft patients. J Oral Maxillofac Surg. 2004; 62:308–314.
65. Jenkins, GN. The physiology and biochemistry of the mouth, ed 4. Oxford, UK: Blackwell Scientific Publications; 1974.
66. Kavanagh, M, Fee, E, Kalinowski, J, et al. Nasometric values for three dialectical groups within the Atlantic provinces of Canada. J Speech Language Pathol Audiol. 1994; 18:7–13.
67. Kent, K. The effects of dental abnormalities on speech production. Quintessc Int Dent Dig. 1982; 12:1353.
68. Ko, EW, Figueroa, AA, Guyette, TW, et al. Velopharyngeal changes after maxillary advancement in cleft patients with distraction osteogenesis using a rigid external distraction device: 1-year cephalometric follow-up. J Craniofac Surg. 1999; 10:312–320.
69. Kuehn, DP. Velopharyngeal anatomy and physiology. Ear Nose Throat J. 1979; 58:59–136.
70. Kuehn, DP, Moon, JB. Levator veli palatini muscle activity in relation to intraoral air pressure variation. J Speech Hear Res. 1994; 37:1260–1270.
71. Kummer, AW, Strife, JL, Grau, WH, et al. The effects of Le Fort I osteotomy with maxillary movement on articulation, resonance, and velopharyngeal function. Cleft Palate J. 1989; 26:193–199.
72. Kummer, AW, Briggs, M, Lee, L. The relationship between the characteristics of speech and velopharyngeal gap size. Cleft Palate Craniofac J. 2003; 40:590–596.
73. Laine, T. Malocclusion traits and articulatory components of speech. Eur J Orthod. 1992; 14:302.
74. Lee, SY, Whitehill, TL, Ciocca, V, et al. Acoustic and perceptual analysis of the sibilant sound /s/ before and after orthognathic surgery. J Oral Maxillofac Surg. 2002; 60:364.
75. Lof, GL, Watson, MM. A nationwide survey of nonspeech oral motor exercise use: implications for evidence-based practice. Lang Speech Hear Serv Sch. 2008; 39:392–407.
76. Lohmander, A, Olsson, M. Methodology for perceptual assessment of speech in patients with cleft palate: a critical review of the literature. Cleft Palate Craniofac J. 2004; 41:64.
77. Lubker, JF. Normal velopharyngeal function in speech. Clin Plast Surg. 1975; 2:249.
78. Luce, EA, McGibbon, B, Hoopes, JE. Velopharyngeal insufficiency in hemifacial microsomia. Plast Reconstr Surg. 1977; 30(4):602–606.
79. Lund, JP, Dellow, PG. The influence of interactive stimuli on rhythmical masticatory movements in rabbits. Arch Oral Biol. 1971; 16(2):215–223.
80. Lund, JP. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. 1991; 2(1):33–64.
81. Lundeen, HC, Gibbs, CH. Advances in occlusion. Boston: John Weight; 1982.
82. Maegawa, J, Sells, RK, David, DJ. Speech changes after maxillary advancement in 40 cleft lip and palate patients. J Craniofac Surg. 1998; 9:177–182.
83. Mason, RM, Turvey, TA, Warren, DW. Speech considerations with maxillary advancement procedures. J Oral Surg. 1980; 38:752–758.
84. Massengill, R, Quinn, GW. Adenoidal atrophy, velopharyngeal incompetence and sucking exercises: a 2-year follow-up case report. Cleft Palate J. 1974; 11:196–199.
85. McCarthy, JG, Coccaro, JP, Schwartz, MD. Velopharyngeal function following maxillary advancement. Plast Reconstr Surg. 1979; 64:180.
86. McComb, R, Marrinan, E, Nuss, RC, et al. Predictors of velopharyngeal insufficiency after Le Fort I maxillary advancement in patients with cleft palate. J Oral Maxillofac Surg. 2011; 69:2226–2232.
87. McFarland, DH, Baum, SR. Incomplete compensation to articulatory perturbation. J Acoust Soc Am. 1995; 97:1865.
88. Marrinan, EM, LaBrie, RA, Mulliken, JB. Velopharyngeal function in nonsyndromic cleft palate: relevance of surgical technique, age at repair, and cleft type. Cleft Palate Craniofac J. 1998; 35:95–100.
89. Miyazaki, T, Matsuya, T, Yamaoka, M. Fiberscopic methods for assessment of velopharyngeal closure during various activities. Cleft Palate J. 1975; 12:107–114.
90. Moll, KL. A cinefluorographic study of velopharyngeal function in normals during various activities. Cleft Palate J. 1965; 2:112–122.
91. Moon, JB, Kuehn, DP, Chan, G, Zhao, L. Induced velopharyngeal fatigue effects in speakers with repaired palatal clefts. Cleft Palate Craniofac J. 2007; 44:251–260.
92. Morris, HL, Spreistersbach, DC, Darley, FL. An articulation for assessing competency of velopharyngeal closure. J Speech Hearing Res. 1961; 4:48.
93. Morris, HL. Some questions and answers about velopharyngeal dysfunction during speech. Am J Speech Lang Pathol. 1992; 1:26–28.
94. Niemi, M, Laaksonen, J, Peltomaki, T, et al. Acoustic comparison of vowel sounds produced before and after orthognathic surgery for mandibular advancement. J Oral Maxillofac Surg. 2006; 64:910.
95. Niemi, M, Laaksonen, J, Peltomaki, T, Aaltonen, O. Acoustic comparison of vowel sounds produced before and after orthognathic surgery for mandibular advancement. J Oral Maxillofac Surg. 2006; 64:7.
96. Nozaki, S, Iriki, A, Nakamura, Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. Neurophysiology. 1986; 55:806–825.
97. O’Gara, M, Wilson, K. The effects of maxillofacial surgery on speech and velopharyngeal function. Clin Plast Surg. 2007; 34:8.
98. Okazaki, K, Satoh, K, Kato, M, et al. Speech and velopharyngeal function following maxillary advancement in patients with cleft lip and palate. Ann Plast Surg. 1993; 30:304–311.
99. Pannbacker, M, Lass, NJ, Stout, BM. Speech language pathologists: opinions on the management of velopharyngeal insufficiency. Cleft Palate J. 1990; 27:68–71.
100. Pannbacker, M. Velopharyngeal incompetence: the need for speech standards. Am J Speech Lang Pathol. 2004; 13:195–201.
101. Peterson-Falzone, SJ, Hardin-Jones, MA, Karnell, MP. Cleft palate speech. St. Louis: Mosby; 2001.
102. Phillips, JH, Klaiman, P, Delorey, R, MacDonald, DB. Predictors of velopharyngeal insufficiency in cleft palate orthognathic surgery. Plast Reconstr Surg. 2005; 115:681–686.
103. Pond, LH, Barghi, N, Barnwell, GM. Occlusion and chewing side preference. J Prosthet Dent. 1986; 55(4):498–500.
104. Proffit, WR, Mason, RM. Myofunctional therapy for tongue thrusting: background and recommendations. J Am Dent Assoc. 1975; 90:403.
105. Roll, DJ. Modification of nasal resonance in cleft palate children by informative feedback. J Appl Behav Anal. 1973; 6:397–403.
106. Ruscello, DM, Tekieli, ME, Van Sickels, JE. Speech production before and after orthognathic surgery: a review. Oral Surg. 1985; 59:10.
107. Ruscello, DM, Tekieli, ME, Jakomis, T, et al. The effects of orthognathic surgery on speech production. Am J Orthod. 1986; 89:237–241.
108. Ruscello, DM. Treatment of velopharyngeal closure for speech: discussion and implications for management. J Speech Lang Pathol Appl Behav Anal. 2007; 1:62–82.
109. Ruscello, DM. Oral motor treatment issues related to children with developmental speech sound disorders. Lang Speech Hear Serv Sch. 2008; 39:380–391.
110. Schendel, SA, Oeschlaeger, M, Wolford, LM, Epker, BN. Velopharyngeal anatomy and maxillary advancement. J Maxillofac Surg. 1979; 7:116–124.
111. Schneider, E, Shprintzen, RJ. A survey of speech pathologists: current trends in the diagnosis and management of velopharyngeal insufficiency. Cleft Palate J. 1980; 17:249–253.
112. Schneyer, LH, Pigman, W, Hanahan, L, Gilmore, RW. Rate of flow of human parotid, sublingual and submaxillary secretions during sleep. J Dent Res. 1956; 35:109–114.
113. Schwarz, C, Gruner, E. Logopaedic findings following advancement of the maxilla. J Maxillofac Surg. 1976; 4:40–55.
114. Schweitzer, JM. Masticatory function in man. J Prosthet Dent. 1961; 11:625–647.
115. Seaver, E, Dalston, R, Leeper, H, Adams, L. A study of nasometric values for normal nasal resonance. J Speech Hearing Res. 1991; 34:715–721.
116. Shelton, RL, Lindquist, AF, Arndt, WB, et al. Effect of speech bulb reduction on movement of the posterior wall of the pharynx and posture of the tongue. Cleft Palate J. 1971; 8:10–17.
117. Shelton, RL, Paesani, A, McClelland, KD, Bradfield, SS. Panendoscopic feedback in the study of voluntary velopharyngeal movements. J Speech Hear Disord. 1975; 40:232–244.
118. Shprintzen, RJ, McCall, GN, Skolnick, ML. The effect of pharyngeal flap surgery on the movements of the lateral pharyngeal walls. Plast Reconstr Surg. 1980; 66:570.
119. Shprintzen, RJ, Golding-Kushner, KJ. Evaluation of velopharyngeal insufficiency. Otolaryngol Clin North Am. 1989; 22:519.
120. Stevens, K. On the quantal nature of speech. J Phon. 1989; 17:3.
121. Subtelny, JD, Mestre, JC, Subtelny, JD. Comparative study of normal and defective articulation of /s/ as related to malocclusion and deglutition. J Speech Hearing Dis. 1964; 29:264.
122. Thompson, AE, Hixon, TJ. Nasal airflow during normal speech production. Cleft Palate J. 1979; 16:412–420.
123. Thompson, EC, Murdoch, BE, Stokes, PD. Tongue function in subjects with upper motor neuron type dysarthria following cerebrovascular accident. J Med Speech Lang Pathol. 1995; 3:27–40.
124. Thompson, EC, Murdoch, BE, Stokes, PD. Interlabial contact pressures during performance of speech and nonspeech tasks in young adults. J Med Speech Lang Pathol. 1997; 5:191–199.
125. Trindale, IE, Yamashita, RP, Suguimoto, RM, et al. Effects of orthognathic surgery on speech and breathing of subjects with cleft lip and palate: acoustic and aerodynamic assessment. Cleft Palate Craniofac J. 2003; 40:54–64.
126. Turvey, TA, Joumot, V, Epker, BN. Correction of anterior open bite deformity: a study of tongue function, speech changes, and stability. J Maxillofac Surg. 1976; 4:93.
127. Turvey, TA, Hall, DJ, Warren, DW. Alterations in nasal airway resistance following superior repositioning of the maxilla. Am J Orthod. 1984; 85:109.
128. Vallino, L. The effects of orthognathic surgery on speech, velopharyngeal function, and hearing. University of Pittsburgh; 1987.
129. Vallino, L. Speech, velopharyngeal function, and hearing before and after orthognathic surgery. J Oral Maxillofac Surg. 1990; 48:1274–1281.
130. Vallino, LD, Tompson, B. Perceptual characteristics of consonant errors associated with malocclusion. J Oral Maxillofac Surg. 1993; 51:850–856.
131. Van de Weijer, J, Slis, I. Nasaliteitsmeting met de nasometer. Logopedie en Foniatrie. 1991; 63:97–101.
132. Van Lierde, KM, Wuyts, FL, De Bodt, M, Van Cauwenberge, P. Nasometric values for normal nasal resonance in the speech of young Flemish adults. Cleft Palate Craniofac J. 2001; 38:112–118.
133. Van Lierde, KM, De Bodt, M, Baetens, I, et al. Outcome of treatment regarding articulation, resonance and voice in Flemish adults with unilateral and bilateral cleft palate. Folia Phoniatrica et Logopaedica. 2003; 55:80–90.
134. Van Lierde, KM, Schepers, S, Timmermans, L, et al. The impact of mandibular advancement on articulation, resonance, and voice characteristics in Flemish speaking adults: a pilot study. Int J Oral Maxillofac Surg. 2006; 35:1.
135. Wakumoto, M, Isaacson, KG, Friel, S, et al. Preliminary study of articulatory reorganization of fricative consonants following osteotomy. Folia Phoniatr Logop. 1996; 48:275.
136. Wang, LL, Yang, JF, Chen, J, et al. Correlation analysis on the malocclusion and articulation of skeletal angle III malocclusion in mixed dentition. Hua Xi Kou Qiang Yi Xue Za Zhi. 2006; 24:3.
137. Ward, EC, McAuliffe, M, Holmes, SK, et al. Impact of malocclusion and orthognathic reconstruction surgery on resonance and articulatory function: an examination of variability of five cases. Br J Oral Maxillofac Surg. 2002; 40:410.
138. Warren, DW. Velopharyngeal orifice size and upper pharyngeal pressure flow patterns in normal speech. Plast Reconstr Surg. 1964; 2:148.
139. Warren, DW, DuBois, AB. A pressure-flow technique for measuring velopharyngeal orifice area during continuous speech. Cleft Palate J. 1964; 1:52.
140. Warren, DW. Perci: a method for rating palatal efficiency. Cleft Palate J. 1979; 16:279.
141. Warren, DW. Compensatory speech behaviors in cleft palate: a regulation/control phenomenon. Cleft Palate J. 1986; 23:251.
142. Warren, DW, Dalston, RM, Mayo, R. Hypernasality in the presence of “adequate velopharyngeal closure. ”. Cleft Palate Craniofac J. 1993; 30:150–154.
143. Warren, DW. Perceptual characteristics of consonant errors associated with malocclusion [discussion]. J Oral Maxillofac Surg. 1993; 51:856.
144. Watzke, I, Turvey, TA, Warren, DW, Dalston, R. Alterations in velopharyngeal function after maxillary advancement in cleft palate patients. J Oral Maxillofac Surg. 1990; 48:685–689.
145. Weber, J, Jobe, RP, Chase, RA. Evaluation of muscle stimulation in the rehabilitation of patients with hypernasal speech. Plast Reconstr Surg. 1970; 46:173–174.
146. Weimer, K, Astrand, P. Effect on speech of mandibular prognathism before and after surgical treatment. Swed Dental J. 1977; 1:173–176.
147. Whitehill, TL, Samman, N, Wong, LLN, et al. Speech errors associated with dentofacial abnormalities in Cantonese speakers. J Med Speech-Lang Pathol. 2001; 9:177.
148. Wickwire, NA, White, RP, Proffit, WR. The effect of mandibular osteotomy on tongue position. J Oral Surg. 1972; 30:184–190.
149. Witzel, MA, Munro, IR. Velopharyngeal insufficiency after maxillary advancement. Cleft Palate J. 1977; 14:176–180.
150. Witzel, MA, Ross, RB, Munro, IR. Articulation before and after facial osteotomy. J Maxillofac Surg. 1980; 8:195–202.
151. Witzel, MA, Ross, RB, Munro, K. Articulation before and after facial osteotomy. J Maxillofac Surg. 1980; 8:8.
152. Witzel, MA. Orthognathic defects and surgical correction: The effects on speech and velopharyngeal function. University of Pittsburgh; 1981.
153. Witzel, MA, Tobe, J, Sayler, KE. The use of nasopharyngoscopy biofeedback therapy in the correction of inconsistent velopharyngeal closure. Int J Pediatr Otorhinolaryngol. 1988; 15:137–142.
154. Witzel, MA, Tobe, J, Sayler, KE. The use of videonasopharyngoscopy after pharyngeal flap surgery. Cleft Palate J. 1989; 26:129–135.
155. Witzel, MA. The effects of Le Fort I osteotomy with maxillary movement on articulation, resonance, and velopharyngeal function [commentary]. Cleft Palate J. 1989; 26:199–200.
156. Worner, HK, Anderson, MN. Biting force measurements in children. Aust Dent J. 1944; 48:1–5.
157. Xue, SA, Lam, CW, Whitehill, TL, Samman, N. Effects of Class III malocclusion on young male adults’ vocal tract development: a pilot study. J Oral Maxillofac Surg. 2011; 69:845–852.
158. Yamashita, S, Hatch, JP, Rugh, JD. Does chewing performance depend upon a specific masticatory pattern? J Oral Rehabil. 1999; 26(7):547–553.
159. Ysunza, A, Pamplona, C, Toledo, E. Change in velopharyngeal valving after speech therapy in cleft palate patients: a videonasopharyngoscopic and multi-view videofluoroscopic study. Int J Pediatr Otorhinolaryngol. 1992; 24:45–54.
160. Ysunza, A, Pamplona, C, Ramirez, E, et al. Velopharyngeal surgery: a prospective randomized study of pharyngeal flaps and sphincter pharyngoplasties. Plast Reconstr Surg. 2000; 110:1401.
161. Yules, RB, Chase, RA. A training method for reduction of hypernasality in speech. Plast Reconstr Surg. 1969; 43:180–185.