7 Special Topics
Running injuries: etiology and recovery-based treatment
Allan Besselink, PT, Dip MDT, and Bridget Clark, PT, MSPT, DPT
However, running also displays a trend toward a significant rate of injury. The current literature indicates various injury rates, depending on the study. Koplan et al. (1982) reported that 60% of all runners will sustain an injury within any given year that is severe enough to force them to alter their training. It has also been reported that the yearly incidence injury rate for runners training for a marathon is as high as 90%. Given that the average runner will have 800 to 2000 footstrikes per mile, the opportunity for injury to occur is significant. Running injuries are not limited to any one joint or anatomic region (Table 7-1), although a large percentage of injuries tend to occur at the knee.
| Anatomic Region | Percentage of Injuries |
|---|---|
| Knee | 7.2–50.0 |
| Shin, Achilles tendon, calf, heel | 9–32.0 |
| Foot and toes | 5.7–39.0 |
| Hamstring, quadriceps | 3.4–38.0 |
Data from van Gent RN, Siem D, van Middelkoop M, van Os AG, Bierma-Zeinstra SM, Koes BW. Incidence and determinants of lower extremity running injuries in long distance runners: A systematic review. Br J Sports Med 2007;41:469–480.
Gait: Walking and Running
The gait cycle has been defined by Thordarson (1997) as the period from initial contact of one foot until the initial contact of that same foot. A brief review of the gait cycle will provide some background on the nature of mechanical loading and the neuromuscular requirements of both walking and running.
Running Mechanics
The running gait cycle (Fig. 7-1) is also divided into a stance phase and a swing phase. The stance phase may involve an initial foot contact which takes place as a heel strike, midfoot strike, or forefoot strike. Initial foot contact exists on a continuum with increasing gait speed, progressing from heel strike in walking to forefoot strike in sprinting. The percentage of the gait cycle spent in the stance phase varies depending on gait speed—60% with walking, 40% with running, and just 22% with world class sprinters. The walking gait cycle is distinct in that it involves a period of double limb support in which both of the feet are on the ground. The running gait cycle is distinct in that it involves a period of double float in which both of the feet are off the ground. The progression from walking gait to running gait is a continuum—from double limb support in walking to double float period in running.
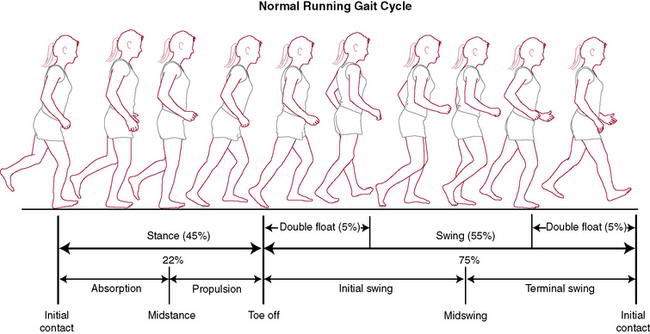
Figure 7-1 Normal running gait cycle.
(Redrawn from Mann RA, Coughlin MJ. Surgery of the Foot and Ankle, 6th ed. St. Louis: Mosby, 1993.)
At a certain walking speed, there is a transition from walking to running gait which occurs in order to maintain biomechanical, metabolic, and aerobic efficiency (Fig. 7-2). The speed at which this transition occurs varies between individuals, although it tends to be at or near a velocity of 12:00 per mile (5.0 mph) for most. This becomes an important issue when 70% of the running population runs at a pace of 10:00 per mile or slower. Though fast walking and slow jogging have a similar cardiovascular response, slow jogging creates ground reaction forces and loading rates as much as 65% greater than fast walking (Table 7-2). The progression from walking to running involves certain requirements from the body including the ability to tolerate increased mechanical loads (i.e., ground reaction forces) and the strength not only to progress the body forward concentrically, but also to eccentrically control the stance leg. Running and sprinting require more power and range of motion at the hip, knee, and ankle as speed is increased.
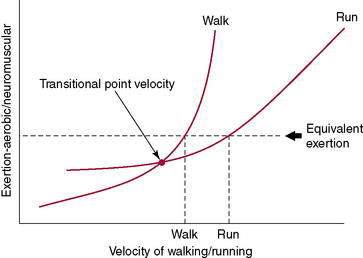
Figure 7-2 Transition from walking to running.
(Redrawn from Besselink A. RunSmart: A Comprehensive Approach to Injury-Free Running, Morrisville, 2008, Lulu Press.)
Table 7-2 Ground Reaction Forces Associated with Walking and Running at Various Speeds
| Running Speed | Pace (Per Mile) | Vertical Ground Reaction Force (Body Weight) |
|---|---|---|
| 1.5 m/s–1 (3.4 mph) (walk) | 17:53/mile | 1.1–1.5 |
| 2.5 to 3.0 m/s–1 (5.6–6.7 mph) (slow jog) | 8:56–10:44/mile | 2.5 |
| 5.0 to 8 m/s–1 (11.2–17.9 mph) (run) | 3:21–5:22/mile, or 0:50–1:20/quarter | 2.5–2.88 |
Data adapted from Keller TS, Weisberger AM, Ray JL, Hasan SS, Shiavi RG, Spengler DM. Relationship between vertical ground reaction force and speed during walking, slow jogging, and running. Clin Biomech 1996;11: 253–259 and Munro CF, Miller DI, Fuglevand AJ. Ground reaction forces in running: A reexamination. J Biomech 1987;20:147–155.
Ground reaction forces appear to increase linearly up to a gait speed of 60% of maximum speed (average of 4.0m/s−1), but at higher speeds, ground reaction forces appear to stay at approximately 2.5–2.8 times body weight (Table 7-2). It is also noteworthy that during running, athletes that heel strike upon initial contact have a higher initial peak in vertical ground reaction force than midfoot strikers. There is a strong relationship between impact peak and loading rate. The loading rate associated with running has been found to be positively correlated with running velocity, finding an average rate of 77 BW/s−1 (body weight) at slower speeds of 3.0m/s−1, increasing to 113 BW/s−1 at faster speeds of 5.0 m/s−1.
The anterior and posterior calf muscles, quadriceps, hip extensors, and hamstrings all work eccentrically during the stance phase. Of note is the function of the quadriceps, which is the primary shock absorber, absorbing 3.5 times as much energy as it produces. After the initial ground reaction forces are attenuated, the foot then supinates during the propulsion phase to provide a more rigid lever for push off. Winter (1983) noted that the gastrocneminus generates the primary propulsive force during the propulsion phase of running and produces forces between 800–1500 W, compared to 150 W for slow walking and 500 W for fast walking.
Causes of Running Injuries
Review of the current scientific research does in fact yield a definitive answer. One primary factor has been directly associated with the onset of running injury—training or errors in training. James et al. (1978) noted that the primary etiology in two thirds of all causes of injury can be directly related to “training error.” Lysholm and Wiklander (1987) reported that training errors alone, or in combination with other factors, were implicated in injuries in 72% of runners. Simply stated, training error is most often an issue of “too much, too soon,” the importance of which is explained later.
Contrary to the commonly held beliefs of the medical and running communities, there is not any specific correlation between anatomic malalignment or variations in the lower extremity and any specific pathologic entities or predisposition to any “overuse” syndromes. In fact, Reid (1992) noted that “normal variations in the human body abound, and only a few percent of the population are actually good examples of ‘normal.’… Furthermore, all of these variations are found in world class athletes and seem to produce little adverse effect on their ability to perform their sports.… [T]he corollary of this enormous variation of body build among enthusiastic amateur and the professional athletes is that there is a poor correlation of specific malalignments with specific conditions.” Table 7-3 summarizes the sport sciences literature regarding the factors that have been noted to have a direct association with running injury and those that either have no direct association or do not presently have scientific evidence to support an association with running injuries.
Table 7-3 Evidence-Based Factors Associated and Not Associated with Running Injuries
| Factors Having a Direct Association with Injury | Factors That Do Not Have Evidence for Association with Injury | Factors Known to Not Have a Direct Association with Injury |
|---|---|---|
| “Training error” (most often too much, too soon) Running distance History of prior injury Previous competition in running events |
Warmup and stretching exercises Body height Malalignment Muscular imbalance Decreased range of motion Running frequency Level of performance (current skill level) Stability of running shoes Running on one side of the road Orthotics |
Gender Age Body mass index Running on hard surfaces Running hills Participation in other sports Time of year Time of day |
Data from van Mechelen W. Running injuries. A review of the epidemiological literature. Sports Med 1992;14:320–335.
Training error is the only factor that consistently displays a cause–effect relationship with running injuries. Reid (1992) has gone so far as to state that “every running injury should be viewed as a failure of training technique, even if other contributing factors are subsequently identified.” In addition, running distance of more than 25 to 40 miles per week, previous competition in running events, and a history of prior injury have been found to be strongly associated with running injuries.
Under-Recovery Not Overuse
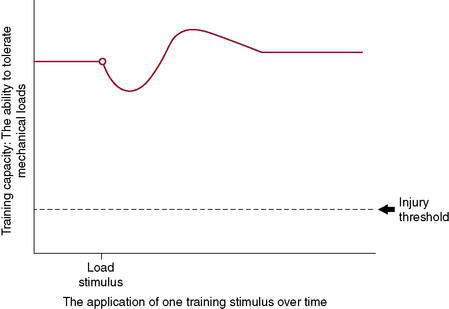
For years, the health care community has pointed to the “overuse” running injury, but if “overuse” were the problem, then there would be a preset threshold at which point all runners would get injured—and this simply is not the case. Physiologic causes of running injuries can be explained by Wolfe’s law. The body aims to attain homeostasis at the cellular level. As a stimulus is applied to tissues (including bone, tendon, muscle, ligament, and collagen-based tissues), a cellular response is triggered and, over time and with sufficient recovery, an adaptation occurs. This adaptation could be greater tissue integrity, strength, or similar mechanical response. Tissues adapt to mechanical loading if given an environment in which to do so and sufficient metabolic capacity to allow this to occur (Fig. 7-3). This has been shown repeatedly with studies on astronauts and deep sea divers, two populations that face altered repeated and/or sustained mechanical loads. There is a precise balance between stimulus and response—or, for the athlete, the application of a training stimulus and the recovery and adaptation to this stimulus. With this in mind, “overuse” injuries should be more accurately described as “under–recovery” injuries because, given appropriate time for recovery, adaptation to the stimulus will take place successfully.
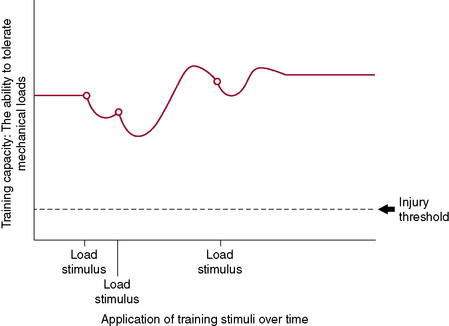
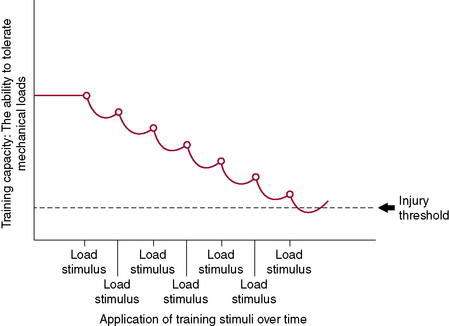
Figure 7-3 illustrates the body’s ability to recover from and adapt to a single training stimulus. Figures 7-4 and 7-5 display the effect of several training stimuli: Figure 7-4 with appropriate and sufficient recovery and Figure 7-5 with insufficient recovery and poor training adaptation. Injuries occur when the rate of application of training stimulus exceeds the rate of recovery and adaptation.
Mechanical Assessment
Subjective
A thorough examination should begin with a review of the patient’s prior running program. We have compiled a list of characteristic traits of the run training program that typically contribute to factors related to overuse/under-recovery (Table 7-4). This assists the clinician’s understanding of the athlete’s current capacity to tolerate mechanical loading. The intent and rationale for each question has also been provided.
| Running Experience | Intent/Rationale of Question |
| 1. Have you been involved in any other sport or fitness activities, and if so, for how long? | General level of conditioning and tissue “health” and current loading capacity. |
| 2. How long have you been a runner? | More experienced runners tend toward lower injury risk. |
| 3. Have you had any previous running injuries? If so, where and when? | Injury risk increases if history of a prior running injury. |
| Current Training Program | Intent/Rationale of Question |
| 1. How many days per week do you run? | Number of recovery days per week. |
| 2. How many miles do you run per week? | Most programs emphasize “more is better”; injury risk tends to increase at 25–40 miles per week |
| 3. What is your average running pace (minutes/mile)? | Running mechanics change with running pace. |
| 4. What was your longest run in the month prior to injury? | The rate of progression of the total volume of training and loading capacity. |
| 5. Do you recall any change in your running program that occurred just prior to the onset of your injury? | Injured runners most typically have some type of sudden change in the volume of their training; the rate of application of training stimuli exceeds the rate of adaptation to training. |
| 6. Are you training with a group or individually? Are you using a published program or a coach? | Access to the program itself can be valuable for further analysis by the clinician (see #5). |
| 7. What is the longest run that you have done since you noted the injury? How long ago was this done? | Allows the clinician to better understand where to resume running when the athlete is ready (i.e., longer break = more gradual resumption of training). |
| 8. Do you compete in races? If so, what distance(s)? Are you currently training for a particular event? | Injury risk is higher in those who have competed in the past. If they are currently training for an event, it may affect their rate of progression and return to running, along with their overall goal setting. |
| 9. Do you do interval training (speed work) in your training program? If so, what and how often? | Is the athlete doing any run training activities that are building power and loading capacity? |
| 10. Do you do strength and/or plyometric training as part of your training program? If so, what exercises are you doing? Typical number of sets and repetitions? Light, moderate, or heavy resistance? Number of days per week? | Strength and plyometric training (high load, low repetitions) build greater loading capacity and power output. |
| 11. Is there anything else you would like to tell me about your running program? | It is common that the athlete will have an inherent “sense” of the factors that contributed to the injury. Ask them! |
Objective
The McKenzie method of Mechanical Diagnosis and Therapy, or MDT™ (The McKenzie Institute, Syracuse, NY), forms the basis of the mechanical assessment and is presented here because it is a comprehensive classification and treatment system that has scientific research to support not only its assessment process, but also its classification algorithm. Although MDT™ initially gained widespread international acceptance for the treatment of spinal pain, its principles also are readily applied to the extremities. Three primary aspects are unique to the McKenzie method™—mechanical assessment, self-treatment, and prevention (Table 7-5). Although a complete description of the McKenzie method™ is not within the scope of this chapter, further resources can be found in the reference list at the end of this chapter.
Table 7-5 Basic Concepts of Mechanical Diagnosis and Therapy™
| Mechanical Assessment |
1. Establish a relationship between symptom response and mechanical loading (typically via repeated test movements).
. |
.
.
Education
Education of the patient is a critical element in the effective treatment of the injured runner.
As the patient recovers from injury and returns to running, the physical therapist thoroughly reviews the progression back to running to prevent reinjury (see Table 7-4). Runners, like most athletes, are eager to return to athletic training and competition. Because running injuries are generally training related, it is imperative that athletes understand how to modify their training to foster injury recovery and tissue repair, how to prepare their body to accept the increasing mechanical loads with running, and how to optimize their performance. Most runners are under the mindset that “more is better.” Because research clearly dictates otherwise for runners, it is imperative to educate the patient.
Building Capacity
Principles of Optimal Run Training
The training plan is essential to review, discuss, and modify if necessary as an integral part of the treatment plan. The following training principles should assist the clinician in making good recommendations for the running athlete (Table 7-6).
| Principles | Intent/Rationale |
|---|---|
| 1. A runner requires at least 2 days of recovery per week. | The time is required to foster training adaptations. |
| 2. Incorporate at least 1 day of strength and plyometric training per week (high load, low repetition, e.g., 1 set of 10 reps). | To foster training adaptations and increase loading capacity |
| 3. 1–2 days of interval training per week, depending on the total number of run days per week. | Interval training provides a small dosage of quality work, which has favorable effects on running mechanics, loading capacity, and power output. |
| 4. Plan of progression should be on a biweekly basis. | It takes about 10 to 14 days for the body to adapt to the current level of training load. At this time, training volume and load can be progressed. |
| 5. Progress the longest run according to the following guidelines: If running less than 30 minutes, increase longest run by no more than 5 minutes every other week. If running 30–60 minutes, increase longest run by no more than 10 minutes every other week. If running > 60 minutes, increase longest run by no more than 20 minutes every other week. |
This accommodates the normal time factor for rate of adaptation to training. |
Adapted from Besselink A. RunSmart: A Comprehensive Approach to Injury-Free Running. Raleigh, NC: Lulu Publishing, 2008.
Evidence suggests that an arbitrary 10% increase in weekly mileage is not effective at reducing running-related injuries because 7 days may not be long enough for the body to adapt to increased repeated loading. Because of this and evidence to support that recovery from an increased run distance takes 10 to 14 days, we recommend a progression of loading based on the current level of training adaptations (Table 7-6). Table 7-6 is not a comprehensive list, but it does include the primary elements of an optimal and effective training program.
Clinical Case Study
Based on the history, some hallmark issues in the training program can be addressed. Although Bob is an experienced runner, he was unable to adapt to the increased load and increased load frequency placed on his body, thus resulting in an injury (see Fig. 7-5).
Running injuries: shoes, orthotics, and return-to-running program
Scott T. Miller, PT, MS, SCS, CSCS, and Janice K. Loudon, PT, PhD
Biomechanical and Anatomic Factors
No specific anatomic or biomechanical variation necessarily correlates with a specific condition or injury, but lower quarter biomechanics do play an important role (Table 7-7). The most important aspect of the examination is to evaluate the entire lower extremity and not just concentrate on the area of injury (Table 7-8). The lower extremity functions as a kinetic chain and disruption at any given area can affect function throughout.
| Biomechanical Fault | Contributing Factor(s) |
|---|---|
| Increase vertical excursion | Overstriding; weak core muscles |
| Horizontal sway/tilt | Scoliosis; leg-length difference; pelvic obliquity; weak gluteus medius |
| Forward trunk lean | Tight hip flexors; SI joint pain |
| Arm swing crosses midline | Excessive pelvic rotation; scoliosis; leg-length difference; weak abdominals |
| Asymmetric pelvic rotation | Hypomobile SI joint; leg-length difference, lumbar spine dysfunction |
| Excessive lateral pelvic tilt | Contralateral drops: Weak hip abductors on reference limb Ipsilateral drops: Compensation for shortened limb |
| Increase AP pelvic tilt between foot contact and midstance | Weak gluteal and abdominal muscles |
| Increase AP pelvic tilt during propulsion | Tight hip flexors; lack of hip extension |
| Increase lumbar extension | Tight hip flexors; weak abdominal muscles |
| Decreased hip flexion | Weak hip flexors; tight hamstrings; hip dysfunction (OA, labrum) |
| Excessive hip internal rotation | Weak hip ER; femoral anteversion; excessive lumbar rotation |
| Excessive hip external rotation (ER) | Femoral retroversion; tight ER; limited dorsiflexors |
| Genu valgum | Weak gluteus medius; excessive pronation; excessive lumbar motion |
| Genu varum | Tight iliotibial band; rigid foot |
| Forefoot striker | Tight Achilles tendon/calf; hallux rigidus |
| Heel whip | Tibial torsion; tight lateral hamstring; genu valgum |
| Foot abduction | Limited dorsiflexion; tight hip; tight foot evertors |
AP = anteroposterior; SI = sacroiliac; OA = osteoarthritis; ER = external rotation
Table 7-8 Objective Examination of the Running Athlete
| Standing |
The running stride is divided into an active and passive absorption phase and a generation phase (see Fig. 7-1). The purpose of the active absorption phase is initially to decelerate the rapidly forward-swinging recovery leg with eccentric hamstring activity, first absorbing and then transferring the energy to the extending hip, placing the hamstrings under considerable stress. Passive absorption begins at footstrike with absorption of the shock of ground reaction force resulting in a force 2.5 to 3 times body weight (BW) and up to 10 times BW running downhill. This initial shock is attenuated by the surface, the shoe, and the heel pad but not to a great extent. Subsequently, the ground reaction force is actively absorbed by muscles and tendons as it increases to midsupport with a relative shortening of the extremity. This is accomplished by hip and knee flexion, ankle dorsiflexion, and subtalar pronation accompanied by eccentric contraction of the hip abductors, quadriceps, and gastroc–soleus muscles along with stretching of the quadriceps and patellar tendon, Achilles tendon, and plantar fascia. At this point, the ground reaction force with running may be as much as five times BW. The stretched tendons absorb energy, store it as potential energy, and then return 90% of it later in the generation or propulsive phase as kinetic energy, with the remaining 10% creating heat in the tendon.
During the generation phase in the second half of support, there is a relative lengthening of the extremity with concentric muscle contraction and joint extension, with return of stored potential energy as kinetic energy from the tendons significantly assisting the now concentrically contracting muscles. Peak forces maximize at the sites of chronic injury (Scott and Winter 1990). Forces in the patellofemoral joint estimated at 7 to 11.1 times BW, 4.7 to 6.9 times BW in the patellar tendon, 6 to 8 times BW in the Achilles tendon, and 1.3 to 2.9 times BW in the plantar fascia predispose the tissues to potential injury from repetitive overuse—particularly if combined with even a minor anatomic or functional variation.
Examination of the entire lower extremity thus becomes essential (Fig. 7-6) when the extremity is viewed as a kinetic chain whose normal function is dependent on the proper sequential function of each segment. Therefore, concentrating on only the area of complaint may overlook the underlying cause of the problem (e.g., anterior knee pain related to compensatory foot pronation and imbalances in proximal stabilizers).
Shoes
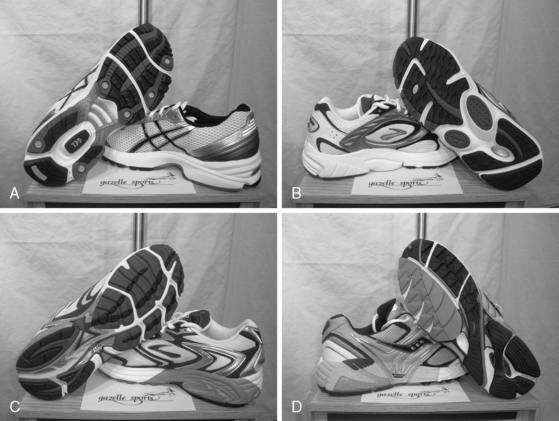
To provide appropriate recommendations on running footwear, having a basic understanding of how the shoe is constructed is important. The key features of a running shoe include the outsole, midsole, and upper. The outsole is the bottom of the shoe and is generally made from carbon or blown rubber. The midsole is the shock-absorbing layer between the outsole and the “upper” part of the shoe. This midsole is the most important part of a running shoe because the construction and materials used will affect the levels of both cushioning and stability in the shoe. The amount of cushioning in the shoe is generally proportionate to the shoe’s heel height. The two types of cushioning generally found in running shoes are ethylene vinyl acetate (EVA) and polyurethane (PU). Increased stability in a shoe is accomplished through the incorporation of a heavier density EVA or PU in combination with the existing cushioning materials. This type of construction is referred to as a dual-density midsole. Finally, the “upper” is the soft body of the shoe that encloses the foot and is usually made of a combination of materials, from lightweight, durable synthetic mesh to heavier materials such as leather. The materials and construction of the upper provide stability, comfort, and a snug fit. Features to consider in the upper include the last (the shape of the shoe), the toe box (the front of the shoe), the heel counter (the part holding the heel, which can vary in stiffness for increased stability), and the Achilles notch (a groove in the heel piece to protect the tendon from irritation). Running footwear can be divided into four primary categories related to their overall cushioning and stability properties (Table 7-9): (1) light cushion, (2) straight last cushion, (3) stability, and (4) motion control.
Table 7-9 Classifications and Characteristics of Running Shoe Types
| Light Cushion Shoe |
* Board last construction primarily used with older running shoes and basketball shoes. Combination last primarily used now in newer running shoes.
Source: Gazelle Sports, Grand Rapids, MI, and Agility Physical Therapy & Sports Performance, Portage, MI.
A light cushion running shoe (Fig. 7-8A) is best for a true supinatory foot or for someone who is an underpronator. This foot type is generally fairly rigid in nature with pes cavus presentation; thus it does not absorb shock during the initial contact phase of running. A light cushion running shoe is not a very substantial shoe and is constructed of single-density material for the midsole with minimal arch support. This shoe is extremely flexible through the arch to allow the foot as much motion as possible. In general, a light cushion shoe will break down quickly (typically less than 400 miles/643 km).
A straight last cushion running shoe (Fig. 7-8B) is a newer category shoe, described as a hybrid shoe that is a transition between a light cushion and stability (described next) shoe. This type of shoe is best for someone who is an underpronator but still presents with some of the forefoot and/or rearfoot alignment concerns (e.g., forefoot varus or calcaneal varus). This foot type is generally somewhat rigid but more accurately does not have the necessary motion available at the subtalar joint to accommodate for the positional faults (e.g., uncompensated forefoot varus). This unique shoe still uses the single-density cushioning material for the midsole, while providing more inherent stability based on the geometry of the shoe (straight last construction) versus implementing a dual-density midsole or stability system commonly seen in the stability shoes. Clinically, this shoe provides a more stable platform for the foot and/or foot orthosis to function without the extrinsic influence of the shoe, which may or may not be desirable.
A stability running shoe (Fig. 7-8C) is best for someone who is a mild to moderate overpronator. This type of shoe generally has enough mobility in the subtalar joint to assist in shock absorption during stance phase. This shoe encompasses some additional stability through the midsole with some type of added stability feature like a dual-density material found in most brands or the Graphite Rollbar system found exclusively in the New Balance shoes. A stability shoe does allow for some flexibility through the midfoot, but it has enough rigidity to provide pronation control.
Finally, a motion control shoe (Fig. 7-8D) is designed for the moderate to severe overpronator. This foot type generally has the same forefoot and rearfoot alignment concerns, but by stark contrast to the more rigid foot, it has an excessive amount of subtalar and/or midtarsal joint motion available. A foot type that can compensate for a forefoot or calcaneal varus can present dynamically as an overpronator (at midsupport) or as a late pronator (at take-off). This causes the foot to roll inward, placing excessive stress on soft tissue structures proximal to the foot, including the lower leg, knee, hip, and back. Motion control shoes are straight-lasted, have a very broad base for support, and are constructed of either a dual-density midsole or a Graphite Rollbar system. This shoe is very rigid through the midsole, much more than the stability shoe, to provide maximum pronation control.
Other factors to take into consideration include
Orthotics
When specifically dealing with runners, a semi-flexible, full-length device using extrinsic posting on a neutral shell (Fig. 7-9C) is recommended for several clinical reasons. First, the functions of the foot during the gait cycle are adaptation, shock absorption, rigid support for leverage, and torque conversion. More specifically, at footstrike, the foot acts as a shock absorber to the impact forces and then adapts to the uneven surfaces. If the prescribed device is rigid (e.g., carbon fiber), this rigidity creates the potential for decreased shock absorption by the device attenuated through the soft tissue structures and less ability for the foot to adapt to the surface. Furthermore, at take-off, the foot has to return to a rigid lever to transmit the explosive force from the lower extremity to the running surface. If primary abnormalities of the foot are related to the forefoot (e.g., forefoot varus), consideration needs to be given to correcting this alignment issue with a full-length device to assist in the transition back to a rigid foot from a supple foot. Finally, most researchers will concur that the use of orthotic therapy is both a “science and an art.” There are advantages to using extrinsically posted, neutral module devices (versus intrinsically designed modules) such as ease of modifications or adjustments. With extrinsically posted devices, different types and density of materials can be selected for support and posting. For example, felt, cork, and EVA are common supportive or posting materials used for this type of orthosis. There is also variability in the stiffness (durometer) rating of such materials as EVA depending on the desired function of the material or the weight of the patient.
Physical Therapy and Rehabilitation
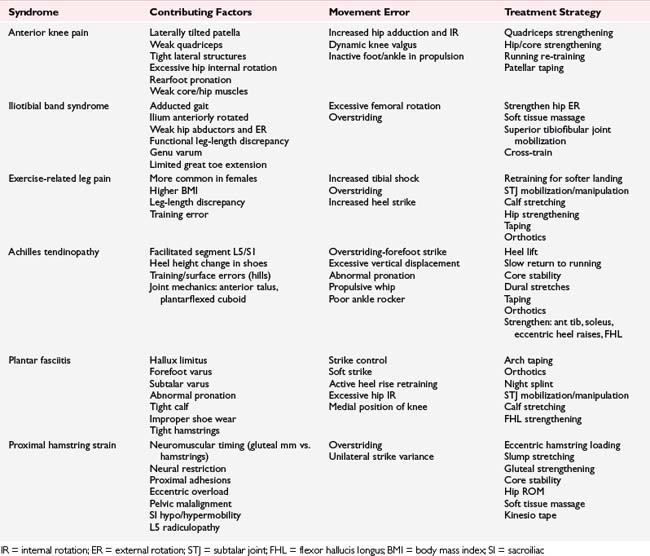
As a general rule, closed chain exercise including concentric and eccentric muscle activity is preferable for runners. Although a good starting point in some cases, isolated, concentric, open chain exercises may induce strength changes in ROM not present during running and create the potential for muscle imbalance. Specific rehabilitation regimens for a given condition are covered in several different sections in this book specific to the condition. Overall, the goal is to develop a functionally based exercise program that will correct any imbalances in the neuromusculoskeletal system. See Table 7-10 for an overview of running injuries and corresponding treatment strategies.
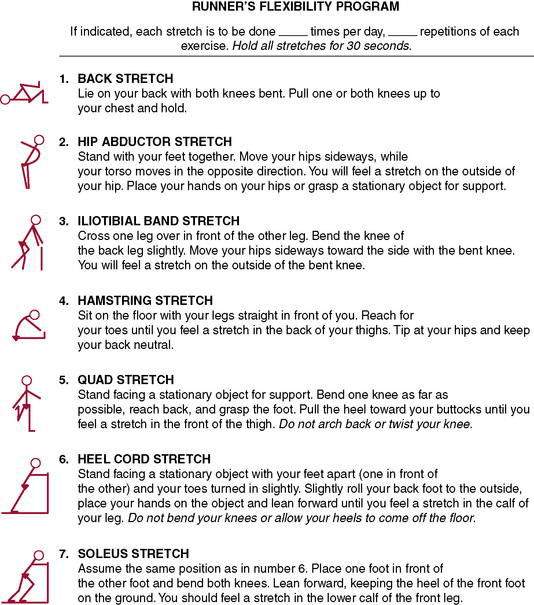
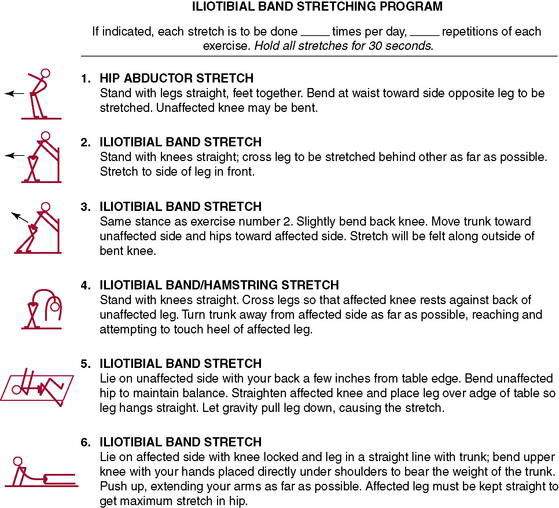
Stretching for flexibility (Figs. 7-10 and 7-11) should be an integral part not only of a rehabilitation program, but also of the daily training program (see each section). Although important for all runners regardless of age, stretching becomes even more significant with aging as tendons become less extensible and joints tend to lose flexibility. Furthermore, isolated tightness can cause muscle inhibition, as described by Janda (1983, 1985). One example is the concept of lower cross syndrome, which is the reciprocal inhibition of the gluteus maximus resulting from iliopsoas tightness. This is a common presentation with runners who have recalcitrant hamstring strains or chronic low back pain. If the iliopsoas tightness is not corrected, the likelihood of retraining the proper gluteus maximus firing pattern is reduced.
Return to Running Algorithm 2 (Miller’s Recommendations)
REHABILITATION PROTOCOL 7-1 Runner’s Guide for Return to Running After Absence from Training of 4 Weeks or More (Nonsurgical)
From James SL, Bates BT, Oslering LR. Injuries to runners. Am J Sports Med 1978;6:40.
REHABILITATION PROTOCOL 7-2 Return to Running Program: Postsurgical
Used with permission from Scott Miller, PT, MS, SCS, CSCS, from Agility Physical Therapy & Sports Performance, LLC. Portage, MI.
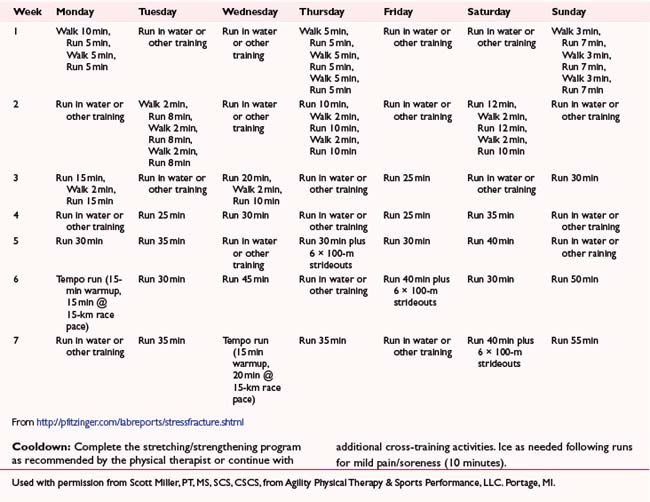
REHABILITATION PROTOCOL 7-3 Return to Running Program: Poststress Fracture
From http://pfitzinger.com/labreports/stressfracture.shtml Used with permission from Scott Miller, PT, MS, SCS, CSCS, from Agility Physical Therapy & Sports Performance, LLC. Portage, MI.
Summary
It is important to incorporate general strength training, specific prescribed rehabilitation exercises (e.g., neuromuscular re-education), and/or stretching program with the return-to-running program. A comprehensive evaluation of the individual plays a vital role in the appropriate management and successful outcomes. This requires looking proximal and distal to the affected area or joint. Performing some type of videotaped gait analysis (Table 7-11) is critical in being able to accurately determine running form aberrances (e.g., heavy slapping asymmetric heel strike) and prescribe the necessary footwear changes or the need for a customized foot orthotic. Finally, a functional exercise program and appropriate return-to-running progression will provide the individual with the greatest opportunity for a successful return and to accomplish their personal goals.
| Sagittal |
Groin pain
Michael P. Reiman, PT, DPT, OCS, SCS, ATC, FAAOMPT, CSCS, and S. Brent Brotzman, MD
Background
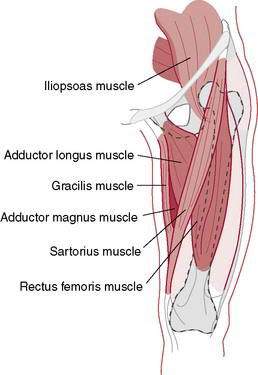
Initially it is important to establish accurately whether this is an acute injury (usually musculoskeletal) or a chronic symptom (often nonmusculoskeletal in origin). Second, the correct anatomic area being described should be identified (e.g., hip adductors [medial], hip, testicle, lower abdominal wall). The commonly accepted definition of a groin strain focuses on injury to the hip adductors and includes the iliopsoas, rectus femoris, and sartorius musculotendinous units (Fig. 7-12). An accurate area of anatomic pain must be delineated by the examiner (e.g., adductor origin or testicular pain with radiation).
In a study of 207 athletes with groin pain (Hölmich 2007), adductor-related dysfunction was the primary clinical entity (58%), followed by iliopsoas-related dysfunction (36%) and rectus abdominus–related dysfunction (6%). Multiple clinical entities were found in 33%.
History
Chronic Injuries or Those with No Clearcut Traumatic, Musculoskeletal Mechanism
Examination
Examination should include the groin, hip area, back, genitourinary, and lower abdominal wall (Tables 7-12 and 7-13). If the patient’s complaint is anatomically hip pain rather than groin pain, differential diagnosis can include a number of possible causes of hip pain in athletes (Table 7-14)
| Patient’s Position | Procedure | Details |
|---|---|---|
| Standing | Observe posture, gait, limb alignment, muscle wasting, ability to sit and stand up, swelling. | Have the patient point to the area of pain and the pattern of radiation. |
| Have the patient reproduce painful movements. | ||
| Examine the low back: active ROM. | Forward flexion, side bending, extension. | |
| Examine the hip: active ROM. | Trendelenburg’s sign (hip adductor strength), ability to squat and duck walk. | |
| Examine the hernia. | Palpate the inguinal region (have the patient cough or strain down). | |
| Supine | Examine the abdomen. | Palpate for abdominal aortic aneurysm, pain, rebound, guarding, hernia, pulses, nodes. |
| Test for costovertebral angle tenderness (renal area). | ||
| When appropriate, perform a rectal examination to palpate the prostate and rule out occult blood. | ||
| Examine male genitalia. | Palpate for a testicular mass, varicocele, or tender epididymis. | |
| Pelvic examination in women, if appropriate. | Look for purulent vaginal discharge of pelvic inflammatory disease and bluish cervix of pregnancy (ectopic). | |
| Palpate for tender cervix or adnexa, ovarian mass. | ||
| Examine low back, sciatic nerve roots. | Perform SLR, test for Lasègue sign and Bragard sign (dorsiflexion of ankle). | |
| Examine hip motion. | Evaluate flexion, external rotation, internal rotation, abduction, adduction, joint play, quadrant tests, any groin pain with internal rotation. | |
| Perform passive SLR, Thomas, and rectus femoris stretch tests. | ||
| Palpate pelvic structures. | Palpate symphysis, pubic rami, iliac crests, adductor insertions, ASIS, PSIS, ischial tuberosities. | |
| Examine sacroiliac joints. | Perform Patrick (flexion, abduction, external rotation, extension [FABERE]). | |
| Look for leg-length discrepancy. | Verify grossly and determine true length by measuring from ASIS to lateral malleoli. | |
| Prone | Examine hip motion. | Evaluate extension and internal and external rotation. |
| Perform Ely and femoral nerve stretch tests. | ||
| Side-lying | Examine iliotibial band. | Perform Ober test. |
| Sitting | Evaluate muscle strength. | Test hip flexion (L2, L3), hip extension (L5, S1, S2), abduction (L4, L5, S1), adduction (L3, L4). |
| Test reflexes. | Assess patellar tendon (L4). | |
| Test sensation. | Assess lower abdomen (T12), groin (L1), medial thigh (L2), anterior quadriceps (L3). |
ASIS = anterior superior iliac spine; PSIS = posterior superior iliac spine; ROM = range of motion; SLR = straight-leg raises.
From Lacroix VJ. A complete approach to groin pain. Phys Sportsmed 2000;28(1):66.
Table 7-13 Potential Causes of Groin Pain: Key Features and Treatments
| Causes | Key Features | Treatment Options |
|---|---|---|
| Musculoskeletal | ||
| Abdominal muscle tear | Localized tenderness to palpation; pain with activation of rectus abdominis | Relative rest, analgesics |
| Adductor tendinitis | Tenderness over involved tendon, pain with resisted adduction of lower extremity | NSAIDs, rest, physical therapy |
| Avascular necrosis of the femoral head | Radiation of pain into the groin with internal rotation of hip; decreased hip ROM | Recommend MRI |
| Mild: conservative measures, possible core decompression; Severe: total hip replacement, needs orthopaedic hip specialty consult | ||
| Avulsion fracture | Pain on palpation of injury site; pain with stretch of involved muscle, x-ray positive, felt a pop when “turning on speed” | Relative rest; ice; NSAIDs; possibly crutches; evaluate for ORIF of fragment if >1 cm displacement |
| Bursitis | Pain over site of bursa | Injection of cortisone, anesthetic, or both; avoid injections around nerves (e.g., sciatic) |
| Conjoined tendon dehiscence | Pain with Valsalva maneuver | Surgical referral (general surgeon) |
| Herniated nucleus pulposus | Positive dural or sciatic tension signs | Physical therapy or appropriate referral (spine specialist) |
| Legg-Calvé-Perthes disease | Irritable hip with pain on rotation, positive x-rays, pediatric (usually ages 5–8 yr) | Pediatric orthopaedic surgeon referral |
| Muscle strain | Acute pain over proximal muscles of medial thigh region; swelling; occasional bruising | Rest; avoidance of aggravating activities; initial ice, with heat after 48 hr; hip spica wrap; NSAIDs for 7–10 days; see section on treatment |
| Myositis ossificans | Pain and decreased ROM in involved muscle; palpable mass within substance of muscle, x-ray shows calcification, often history of blow (helmet) to area | Moderately aggressive active or passive ROM exercises; wrap thigh with knee in maximum flexion for first 24 hr; NSAIDs used sparingly for 2 days after trauma |
| Nerve entrapment | Burning or shooting pain in distribution of nerve; altered light-touch sensation in medial groin; pain exacerbated by hyperextension at hip joint, possibly radiating; tenderness near superior iliac spine | Possible infiltration of site with local anesthetic; topical cream (e.g., capsaicin) |
| Osteitis pubis | Pain around abdomen, groin, hip, or thigh, increased by resisted adduction of thigh; tender on palpation of pubis symphysis; x-ray positive for sclerosis irregularity; osteolysis at the pubis symphysis; bone scan positive | Relative rest; initial ice and NSAIDs; possibly crutches; later, stretching exercises |
| Osteoarthritis | Groin pain with hip motion, especially internal rotation | Non-narcotic analgesics or NSAIDs; hip replacement for intractable pain; see Chapter 6 |
| Public instability | Excess motion at public symphysis; pain in pubis, groin, or lower abdomen | Physical therapy, NSAIDs; compression shorts |
| Referred pain from knee or spine | Hip ROM and palpation response normal | Identify true source of referred pain |
| Seronegative spondyloarthropathy | Signs of systemic illness, other joint involvement | Refer to rheumatologist |
| Slipped capita femoral epiphysis* | Inguinal pain with hip movement; insidious development in ages 8–15 yr; walking with limp, holding leg in external rotation | Discontinue athletic activity; refer to orthopaedic surgeon for probable pinning, crutches; TDWB |
| Stress Fracture | ||
| Pubic ramus | Chronic ache or pain in groin, buttock, and thighs | Relative rest; avoid aggravating activities, crutches PWB |
| Femoral neck* | Chronic ache or pain in groin, buttock, and thighs, or pain with decreased hip ROM (internal rotation in flexion) | Refer to orthopaedist if radiographs or bone scan show lesion; TDWB crutches and cessation of all weightbearing activities until orthopaedic consult |
| Nonmusculoskeletal | ||
| Genital swelling or inflammation | ||
| Epididymitis | Tenderness over superior aspect of testes | Antibiotics if appropriate, or refer to urologist |
| Hydrocele | Pain in lower spermatic cord region | Refer to urologist |
| Varicocele | Rubbery, elongated mass around spermatic cord | Refer to urologist |
| Hernia | Recurrent episodes of pain; palpable mass made more prominent with coughing or straining; discomfort elicited by abdominal wall tension | Refer for surgical evaluation and treatment (general surgeon) |
| Lymphadenopathy | Palpable lymph nodes just below inguinal ligaments; fever, chills, discharge | Antibiotics, work-up, also rule out underlying sexually transmitted disease |
| Ovarian cyst | Groin or perineal pain | Refer to gynecologist |
| PID | Fever, chills, purulent discharge + chandelier sign, “PID shuffle” | Refer to gynecologist |
| Postpartum symphysis separation | Recent vaginal delivery with no prior history of groin pain | Physical therapy, relative rest, analgesics |
| Prostatitis | Dysuria, purulent discharge | Antibiotics, NSAIDs |
| Renal lithiasis | Intense pain that radiates to scrotum | Pain control, increased fluids until stone passes; hospitalization sometimes necessary |
| Testicular neoplasm | Hard mass palpated on the testicle; may not be tender | Refer to urologist |
| Testicular torsion or rupture† | Severe pain in the scrotum; nausea, vomiting; testes hard on palpation or not palpable | Refer immediately to urologist |
| Urinary tract infection | Burning with urination; itching; frequent urination | Short course of antibiotics |
MRI = magnetic resonance imaging; NSAIDs = nonsteroidal anti-inflammatory drugs; ORIF = open reduction and internal fixation; PID = pelvic inflammatory disease; PWB = partial weightbearing; ROM = range of motion; TDWB, touch-down weightbearing.
* Nonweightbearing until orthopaedic evaluation to avoid fracture.
† Emergent immediate referral.
Modified from Ruane JJ, Rossi TA. When groin pain is more than just a strain. Phys Sportsmed 26(4):78.
Table 7-14 Differential Diagnosis of Hip Pain in Athletes
From Lacroix VJ. A complete approach to groin pain. Phys Sportsmed 2000;28(1):66–86.
Treatment
A systematic review of the available literature concerning exercise therapy for groin pain (Machotka et al. 2009) found that exercise, particularly strengthening of the hip and abdominal musculature, can be an effective treatment for athletes with groin pain. The evidence suggested that strengthening exercises may need to be progressed, from static contractions to functional positions, and performed through a ROM. Duration of therapy of 4 to 16 weeks was generally recommended. In a group of 19 National Football League (NFL) players (Schlegel et al. 2009), 14 who were treated nonoperatively returned to play at an average of 6 weeks, whereas 5 treated operatively returned to play at an average of 12 weeks.
Although some have suggested that an exercise program may help prevent groin injuries, a study of 977 soccer players randomized to an exercise program targeting groin injury prevention (strengthening [concentric and eccentric], coordination, and core stability exercises for the muscles related to the pelvis) or their usual training regimen found that the risk of a groin injury was reduced by 31%, but this reduction was not significant. A univariate analysis showed that having had a previous groin injury almost doubles the risk of developing a new groin injury and playing at a higher level almost triples the risk of developing a groin injury (Hölmich et al. 2010).
Schilders et al. (2007, 2009) reported that a single injection of a local anesthetic and corticosteroid into the adductor enthesis was effective in 28 recreational athletes and 24 competitive athletes. Five minutes after the injection all patients reported resolution of their groin pain, but pain relief was lasting only in those with normal findings on MRI; 16 of 17 competitive athletes with enthesopathy on MRI had recurrence within an average of 5 weeks, whereas none of 7 with normal MRI findings had recurrence. Most recreational athletes (75%) had pain relief at 1 year regardless of MRI findings.
For chronic adductor-related groin pain, adductor release has been reported to be successful in about 70% of patients (Atkinson et al. 2009). If a sports hernia is identified, operative treatment usually is required (Garvey et al. 2010), with return to preinjury level of activity at approximately 3 months postoperatively.