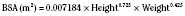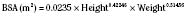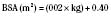CHAPTER 1 Special Characteristics of Pediatric Anesthesia
Perioperative monitoring
In the 1940s and 1950s, the techniques of pediatric anesthesia, as well as the skills of those using and teaching them, evolved more as an art than as a science, as †Dr. Robert Smith vividly and eloquently recollects through his firsthand experiences in his chapter on the history of pediatric anesthesia (see Chapter 41, History of Pediatric Anesthesia, as updated by Mark Rockoff). The anesthetic agents and methods available were limited, as was the scientific knowledge of developmental differences in organ-system function and anesthetic effect in infants and children. Monitoring pediatric patients was limited to inspection of chest movement and occasional palpation of the pulse until the late 1940s, when Smith introduced the first physiologic monitoring to pediatric anesthesia by using the precordial stethoscope for continuous auscultation of heartbeat and breath sounds (Smith, 1953; 1968.). Until the mid-1960s, many anesthesiologists monitored only the heart rate in infants and small children during anesthesia and surgery. Electrocardiographic and blood-pressure measurements were either too difficult or too extravagant and were thought to provide little or no useful information. Measurements of central venous pressure were thought to be inaccurate and too invasive even in major surgical procedures. The insertion of an indwelling urinary (Foley) catheter in infants was considered invasive and was resisted by surgeons.
Smith also added an additional physiologic monitoring: soft, latex blood-pressure cuffs suitable for newborn and older infants, which encouraged the use of blood pressure monitoring in children (Smith, 1968). The “Smith cuff ” (see Chapter 41, History of Pediatric Anesthesia, Fig. 41-4) remained the standard monitoring device in infants and children until the late 1970s, when it began to be replaced by automated blood pressure devices.
The introduction of pulse oximetry for routine clinical use in the early 1990s has been the single most important development in monitoring and patient safety, especially related to pediatric anesthesia, since the advent of the precordial stethoscope in the 1950s (see Chapters 10, Equipment; 11, Monitoring; and 40, Safety and Outcome) (Smith, 1956). Pulse oximetry is superior to clinical observation and other means of monitoring, such as capnography, for the detection of intraoperative hypoxemia (Coté et al., 1988, 1991). In addition, Spears and colleagues (1991) have indicated that experienced pediatric anesthesiologists may not have an “educated hand” or a “feel” adequate to detect changes in pulmonary compliance in infants. Pulse oximetry has revealed that postoperative hypoxemia occurs commonly among otherwise healthy infants and children undergoing simple surgical procedures, presumably as a result of significant reductions in functional residual capacity (FRC) and resultant airway closure and atelectasis (Motoyama and Glazener, 1986). Consequently, the use of supplemental oxygen in the postanesthesia care unit (PACU) has become a part of routine postanesthetic care (see Chapter 3, Respiratory Physiology).
Although pulse oximetry greatly improved patient monitoring, there were some limitations, namely motion artifact and inaccuracy in low-flow states, and in children with levels of low oxygen saturation (e.g., cyanotic congenital heart disease). Advances have been made in the new generation of pulse oximetry, most notably through the use of Masimo Signal Extraction Technology (SET). This device minimizes the effect of motion artifact, improves accuracy, and has been shown to have advantages over the existing system in low-flow states, mild hypothermia, and moving patients (Malviya et al., 2000; Hay et al., 2002; Irita et al., 2003).
Anesthetic agents
More than a decade after the release of isoflurane for clinical use, two volatile anesthetics, desflurane and sevoflurane, became available in the 1990s in most industrialized countries. Although these two agents are dissimilar in many ways, they share common physiochemical and pharmacologic characteristics: very low blood-gas partition coefficients (0.4 and 0.6, respectively), which are close to those of nitrous oxide and are only fractions of those of halothane and isoflurane; rapid induction of and emergence from surgical anesthesia; and hemodynamic stability (see Chapters 7, Pharmacology; 13, Induction Maintenance and Recovery; and 34, Same-Day Surgical Procedures). In animal models, the use of inhaled anesthetic agents has been shown to attenuate the adverse effects of ischemia in the brain, heart, and kidneys.
Although these newer, less-soluble, inhaled agents allow for faster emergence from anesthesia, emergence excitation or delirium associated with their use has become a major concern to pediatric anesthesiologists (Davis et al., 1994; Sarner et al., 1995; Lerman et al., 1996; Welborn et al., 1996; Cravero et al., 2000; Kuratani and Oi, 2008). Adjuncts, such as opioids, analgesics, serotonin antagonists, and α1-adrenergic agonists, have been found to decrease the incidence of emergence agitation (Aono et al., 1999; Davis et al., 1999; Galinkin et al., 2000; Cohen et al., 2001; Ko et al., 2001; Kulka et al., 2001; Voepel-Lewis et al., 2003; Lankinen et al., 2006; Aouad et al., 2007; Tazeroualti et al., 2007; Erdil et al., 2009; Bryan et al., 2009; Kim et al., 2009).
Propofol has increasingly been used in pediatric anesthesia as an induction agent, for intravenous sedation, or as the primary agent of a total intravenous technique (Martin et al., 1992). Propofol has the advantage of aiding rapid emergence and causes less nausea and vomiting during the postoperative period, particularly in children with a high risk of vomiting. When administered as a single dose (1 mg/kg) at the end of surgery, propofol has also been shown to decrease the incidence of sevoflurane-associated emergence agitation (Aouad et al., 2007).
Remifentanil, a μ-receptor agonist, is metabolized by nonspecific plasma and tissue esterases. The organ-independent elimination of remifentanil, coupled with its clearance rate (highest in neonates and infants compared with older children), makes its kinetic profile different from that of any other opioids (Davis et al., 1999; Ross et al., 2001). In addition, its ability to provide hemodynamic stability, coupled with its kinetic profile of rapid elimination and nonaccumulation, makes it an attractive anesthetic option for infants and children. Numerous clinical studies have described its use for pediatric anesthesia (Wee et al., 1999; Chiaretti et al., 2000; Davis et al., 2000, 2001; German et al., 2000; Dönmez et al., 2001; Galinkin et al., 2001; Keidan et al., 2001; Chambers et al., 2002; Friesen et al., 2003). When combined, intravenous hypnotic agents (remifentanil and propofol) have been shown to be as effective and of similar duration as propofol and succinylcholine for tracheal intubation.
The development of more predictable, shorter-acting anesthetic agents (see Chapter 7, Pharmacology) has increased the opportunities for pediatric anesthesiologists to provide safe and stable anesthesia with less dependence on the use of neuromuscular blocking agents.
Airway devices and adjuncts
The development and refinement of airway visualization equipment such as the Glidescope, Shikani Seeing Stylet, and the Bullard laryngoscope have added more options to the management of the pediatric airway and literally give the laryngoscopist the ability to see around corners (see Chapters 10, Equipment; and 12, Airway Management).
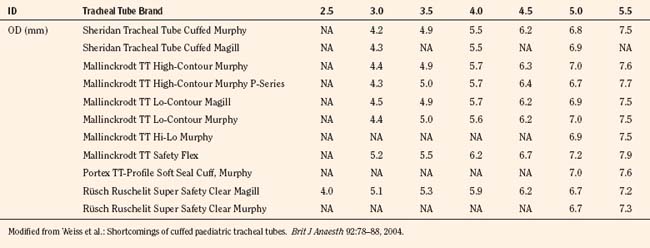
The variety of pediatric endotracheal tubes (ETTs) has focused on improved materials and designs. ETTs are sized according to the internal diameter; however, the outer diameter (the parameter most likely involved with airway complications) varies according to the manufacturer (Table 1-1). Tube tips are both flat and beveled, and a Murphy eye may or may not be present. The position of the cuff varies with the manufacturer. The use of cuffed endotracheal tubes in pediatrics continues to be controversial. In a multicenter, randomized prospective study of 2246 children from birth to 5 years of age undergoing general anesthesia, Weiss and colleagues (2009) noted that cuffed ETTs compared with uncuffed ETTs did not increase the risk of postextubation stridor (4.4% vs. 4.7%) but did reduce the need for ETT exchanges (2.1% vs. 30.8%). However, the role of cuffed ETTs in neonates and infants who require prolonged ventilation has yet to be determined.
Intraoperative and postoperative analgesia in neonates
It has long been thought that newborn infants do not feel pain the way older children and adults do and therefore do not require anesthetic or analgesic agents (Lippman et al., 1976). Thus, in the past, neonates undergoing surgery were often not afforded the benefits of anesthesia. Later studies, however, indicated that pain experienced by neonates can affect behavioral development (Dixon et al., 1984; Taddio et al., 1995, 2005). Rats exposed to chronic pain without the benefit of anesthesia or analgesia showed varying degrees of neuroapoptosis (Anand et al., 2007). However, to add further controversy to the issue of adequate anesthesia for infants, concerns regarding the neurotoxic effects of both intravenous and inhalational anesthetic agents (GABAminergic and NMDA antagonists) have been raised. Postoperative cognitive dysfunction (POCD) has been noted in adult surgical patients (Johnson et al., 2002; Monk et al., 2008). In adults, POCD may also be a marker for 1-year survival after surgery. Although POCD is an adult phenomenon, animal studies by multiple investigators have raised concerns about anesthetic agents being toxic to the developing brains of infants and small children (Jevtovic-Todorovic et al., 2003, 2008; Mellon et al., 2007; Wang and Slikker, 2008). Early work by Uemura and others (1985) noted that synaptic density was decreased in rats exposed to halothane in utero. Further work with rodents, by multiple investigators, has shown evidence of apoptosis in multiple areas of the central nervous system during the rapid synaptogenesis period. This window of vulnerability appears to be a function of time, dose, and duration of anesthetic exposure. In addition to the histochemical changes of apoptosis, the exposed animals also demonstrated learning and behavioral deficits later in life.
In addition to apoptotic changes that occurred in rodents, Slikker and colleagues have demonstrated neuroapoptotic changes in nonhuman primates (rhesus monkeys) exposed to ketamine (an NMDA antagonist). As with the rodents, ketamine exposure in monkeys resulted in long-lasting deficits in brain function (Dr. Merle Poule, personal communication on the Safety of Key Inhaled and Intravenous Drugs in Pediatric Anesthesia [SAFEKIDS] Scientific Workshop, November 2009, White Oaks Campus Symposium). How these animal studies relate to human findings is unclear to date. However, three clinical studies have been reported, and all three studies are retrospective. Wilder et al. (2009) studied a cohort group of children from Rochester, Minnesota, and noted that children exposed to two or more anesthetics in the first 4 years of life were more likely to have learning disabilities, compared with children exposed to one anesthetic or none at all. Kalkman and others (2009) studied a group of children undergoing urologic surgery before age 6 years and reported that there was a tendency for parents to report more behavioral disturbances than those operated on at a later age. However, in a twin cohort study from the Netherlands, Bartels and coworkers (2009) reported no causal relationship between anesthesia and learning deficits in 1,143 monozygotic twin pairs.
Regional analgesia in infants and children
Although conduction analgesia has been used in infants and children since the beginning of the twentieth century, the controversy about whether anesthetic agents can be neurotoxic has caused a resurgence of interest in regional anesthesia (Abajian et al., 1984; Williams et al., 2006).
As newer local anesthetic agents with less systemic toxicity become available, their role in the anesthetic/analgesic management of children is increasing. Studies of levobupivacaine and ropivacaine have demonstrated safety and efficacy in children that is greater than that of bupivacaine, the standard regional anesthetic used in the 1990s (Ivani et al., 1998, 2002, 2003; Hansen et al., 2000, 2001; Lönnqvist et al., 2000; McCann et al., 2001; Karmakar et al., 2002). A single dose of local anesthetics through the caudal and epidural spaces is most often used for a variety of surgical procedures as part of general anesthesia and for postoperative analgesia. Insertion of an epidural catheter for continuous or repeated bolus injections of local anesthetics (often with opioids and other adjunct drugs) for postoperative analgesia has become a common practice in pediatric anesthesia. The addition of adjunct drugs, such as midazolam, neostigmine, tramadol, ketamine, and clonidine, to prolong the neuroaxial blockade from local anesthetic agents has become more popular, even though the safety of these agents on the neuroaxis has not been determined (see Chapters 15, Pain Management; and 16, Regional Anesthesia) (Ansermino et al., 2003; de Beer and Thomas, 2003).
In addition to neuroaxial blockade, specific nerve blocks that are performed with or without ultrasound guidance have become an integral part of pediatric anesthesia (see Chapter 16, Regional Anesthesia). The use of ultrasound has allowed for the administration of smaller volumes of local anesthetic and for more accurate placement of the local anesthetic (Ganesh et al., 2009; Gurnaney et al., 2007; Willschke et al., 2006). The use of catheters in peripheral nerve blocks has also changed the perioperative management for a number of pediatric surgical patients. Continuous peripheral nerve catheters with infusions are being used by pediatric patients at home after they have been discharged from the hospital (Ganesh et al., 2007). The use of these at-home catheters has allowed for shorter hospital stays. In addition, the use of regional techniques with ultrasound guidance, coupled with the natural interest in pain management, has allowed for pediatric anesthesiologists to spearhead pediatric acute and chronic pain management programs.
In addition to advances in anesthetic pharmacology and equipment, advances in the area of pediatric minimal invasive surgery have improved patient morbidity, shortened the length of hospital stays, and improved surgical outcomes (Fujimoto et al., 1999).
Although minimally invasive surgery (MIS) imposes physiologic challenges in the neonate and small infant, numerous neonatal surgical procedures can nevertheless be successfully approached with such methods, even in infants with single ventricle physiology (Georgeson, 2003; Ponsky and Rothenberg, 2008). The success of MIS has allowed for the evolution of robotic techniques, stealth surgery (scarless surgery), and Natural Orifice Transluminal Endoscopic Surgery (NOTES) (Dutta and Albanese, 2008; Dutta et al., 2008; Isaza et al., 2008).
Fundamental differences in infants and children
Psychological Differences
For a child’s normal psychological development, continuous support of a nurturing family is indispensable at all stages of development; serious social and emotional deprivation (including separation from the parents during hospitalization), especially during the first 2 years of development, may cause temporary or even lasting damage to psychosocial development (Forman et al., 1987). A young child who is hospitalized for surgery is forced to cope with separation from parents, to adapt to a new environment and strange people, and to experience the pain and discomfort associated with anesthesia and surgery (see Chapters 2, Behavioral Development; and 8, Psychological Aspects).
The most intense fear of an infant or a young child is created by separation from the parents, and it is often conceived as loss of love or abandonment. The sequence of reactions observed is often as follows: angry protest with panicky anxiety, depression and despair, and eventually apathy and detachment (Bowlby, 1973). Older children may be more concerned with painful procedures and the loss of self-control that is implicit with general anesthesia (Forman et al., 1987). Repeated hospitalizations for anesthesia and surgery may be associated with psychosocial disturbances in later childhood (Dombro, 1970). In children who are old enough to experience fear and apprehension during anesthesia and surgery, the emotional factor may be of greater concern than the physical condition; in fact, it may represent the greatest problem of the perioperative course (see Chapter 8, Psychological Aspects) (Smith, 1980).
All of these responses can and should be reduced or abolished through preventive measures to ease the child’s adaptation to the hospitalization, anesthesia, and surgery. The anesthesiologist’s role in this process, as well as having a basic understanding of neurobehavioral development, are important (Table 1-2).
TABLE 1-2 Aspects of Developmental Assessment and Common Developmental Milestones
| Follows dangling object from midline through a range of 90° | 1 mo |
| Follows dangling object from midline through a range of 180° | 3 mo |
| Consistent conjugate gaze (binocular vision) | 4 mo |
| Alerts or quiets to sound | 0-2 mo |
| Head up 45° | 2 mo |
| Head up 90° | 3-4 mo |
| Weight on forearms | 3-5 mo |
| Weight on hands with arms extended | 5-6 mo |
| Complete head lag, back uniformly rounded | Newborn |
| Slight head lag | 3 mo |
| Rolls front to back | 4-5 mo |
| Rolls back to front | 5-6 mo |
| Sits with no support | 7 mo |
| Hands predominantly closed | 1 mo |
| Hands predominantly open | 3 mo |
| Foot play | 5 mo |
| Transfers objects from hand to hand | 6 mo |
| Index finger approach to small objects and finger-thumb opposition | 10 mo |
| Plays pat-a-cake | 9-10 mo |
| Pulls to stand | 9 mo |
| Walks with one hand held | 12 mo |
| Runs well | 2 y |
| Social smile | 1-2 mo |
| Smiles at image in mirror | 5 mo |
| Separation anxiety/stranger awareness | 6-12 mo |
| Interactive games: peek-a-boo and pat-a-cake | 9-12 mo |
| Waves “bye-bye” | 10 mo |
| Cooing | 2-4 mo |
| Babbles with labial consonants (“ba, ma, ga”) | 5-8 mo |
| Imitates sounds made by others | 9-12 mo |
| First words (≈︀4-6, including “mama,” “dada”) | 9-12 mo |
| Understands one-step command (with gesture) | 15 mo |
Modified from Illingworth RS: The development of the infant and young child: normal and abnormal, New York, 1987, Churchill Livingstone; ages are averages based primarily on data from Arnold Gesell.
Differences in Response to Pharmacologic Agents
The extent of the differences among infants, children, and adults in response to the administration of drugs is not just a size conversion. During the first several months after birth, rapid development and growth of organ systems take place, altering the factors involved in uptake, distribution, metabolism, and elimination of anesthetics and related drugs. Interindividual variability of a response to a given drug may be determined by a variety of genetic factors. Genetic influences in biotransformation, metabolism, transport, and receptor site all affect an individual’s response to a drug. These changes appear to be responsible for developmental differences in drug response and can be further modified by age-related and environmental-related factors. The pharmacology of anesthetics and adjuvant drugs and their different effects in neonates, infants, and children are discussed in detail in Chapter 7, Pharmacology.
Anatomic and Physiologic Differences
Body Size
As stated, the most striking difference between children and adults is size, but the degree of difference and the variation even within the pediatric age group are hard to appreciate. The contrast between an infant weighing 1 kg and an overgrown and obese adolescent weighing more than 100 kg who appear in succession in the same operating room is overwhelming. It makes considerable difference whether body weight, height, or body surface area is used as the basis for size comparison. As pointed out by Harris (1957), a normal newborn infant who weighs 3 kg is one third the size of an adult in length but 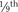 the adult size in body surface area and
the adult size in body surface area and 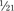 of adult size in weight (Fig. 1-1). Of these body measurements, body surface area (BSA) is probably the most important, because it closely parallels variations in basal metabolic rate measured in kilocalories per hour per square meter. For this reason, BSA is believed to be a better criterion than age or weight in judging basal fluid and nutritional requirements. For clinical use, however, BSA proves somewhat difficult to determine, although a nomogram such as that of Talbot and associates (1952) facilitates the procedure considerably (Fig. 1-2). For the anesthesiologist who carries a pocket calculator, the following formulas may be useful to calculate BSA:
of adult size in weight (Fig. 1-1). Of these body measurements, body surface area (BSA) is probably the most important, because it closely parallels variations in basal metabolic rate measured in kilocalories per hour per square meter. For this reason, BSA is believed to be a better criterion than age or weight in judging basal fluid and nutritional requirements. For clinical use, however, BSA proves somewhat difficult to determine, although a nomogram such as that of Talbot and associates (1952) facilitates the procedure considerably (Fig. 1-2). For the anesthesiologist who carries a pocket calculator, the following formulas may be useful to calculate BSA:
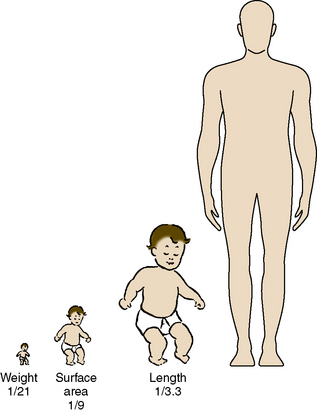
FIGURE 1-1 Proportions of newborn to adult with respect to weight, surface area, and length.
(From Crawford JD, Terry ME, Rourke GM: Simplification of drug dosage calculation by application of the surface area principle, Pediatrics 5:785, 1950.)
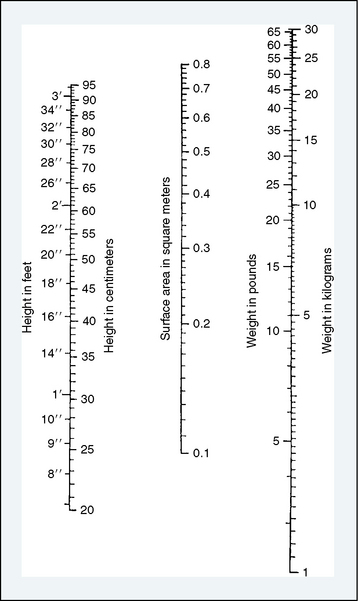
FIGURE 1-2 Body surface area nomogram for infants and young children.
(From Talbot NB, Sobel FH, McArthur JW, et al.: Functional endocrinology from birth through adolescence, Cambridge, 1952, Harvard University Press.)
Formula of DuBois and DuBois (1916)
Formula of Gehan and George (1970)
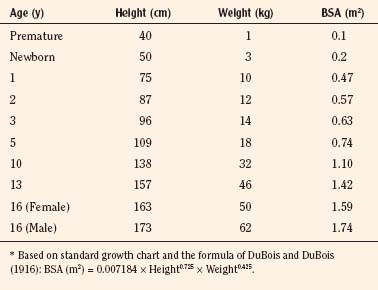
At full-term birth, BSA averages 0.2 m2, whereas in the adult it averages 1.75 m2. A table of average height, weight, and BSA is given for reference in Table 1-3. A simpler, crude estimate of BSA for children of average height and weight is given in Table 1-4. The formula:
TABLE 1-4 Approximation of Body Surface Area (BSA) Based on Weight
| Weight (kg) | Approximate BSA (m2) |
| 1-5 | 0.05 × kg + 0.05 |
| 6-10 | 0.04 × kg + 0.10 |
| 11-20 | 0.03 × kg + 0.20 |
| 21-40 | 0.02 × kg + 0.40 |
Modified from Vaughan VC III, Litt IF: Assessment of growth and development. In Behrman RE, Vaughn VC III, editors: Nelson’s textbook of pediatrics, ed 13, Philadelphia, 1987, WB Saunders.
is also reasonably accurate in children of normal physique weighing 21 to 40 kg (Vaughan and Litt, 1987).
Relative Size or Proportion
Less obvious than the difference in overall size is the difference in relative size of body structure in infants and children. This is particularly true with the head, which is large at birth (35 cm in circumference)—in fact, larger than chest circumference. Head circumference increases by 10 cm during the first year and an additional 2 to 3 cm during the second year, when it reaches three-fourths of the adult size (Box 1-1).
At full-term birth, the infant has a short neck and a chin that often meets the chest at the level of the second rib; these infants are prone to upper airway obstruction during sleep. In infants with tracheostomy, the orifice is often buried under the chin unless the head is extended with a roll under the neck. The chest is relatively small in relation to the abdomen, which is protuberant with weak abdominal muscles (Fig. 1-3). Furthermore, the rib cage is cartilaginous and the thorax is too compliant to resist inward recoil of the lungs. In the awake state, the chest wall is maintained relatively rigid with sustained inspiratory muscle tension, which maintains the end-expiratory lung volume functional residual capacity (FRC). Under general anesthesia, however, the muscle tension is abolished and FRC collapses, resulting in airway closure, atelectasis, and venous admixture unless continuous positive airway pressure (CPAP) or positive end-expiratory pressure (PEEP) is maintained.
Central and Autonomic Nervous Systems
At birth the spinal cord extends to the third lumbar vertebra. By the time the infant is 1 year old, the cord has assumed its permanent position, ending at the first lumbar vertebra (Gray, 1973).
In contrast to the central nervous system, the autonomic nervous system is relatively well developed in the newborn. The parasympathetic components of the cardiovascular system are fully functional at birth. The sympathetic components, however, are not fully developed until 4 to 6 months of age (Friedman, 1973). Baroreflexes to maintain blood pressure and heart rate, which involve medullary vasomotor centers (pressor and depressor areas), are functional at birth in awake newborn infants (Moss et al., 1968; Gootman, 1983). In anesthetized newborn animals, however, both pressor and depressor reflexes are diminished (Wear et al., 1982; Gallagher et al., 1987).
The laryngeal reflex is activated by the stimulation of receptors on the face, nose, and upper airways of the newborn. Reflex apnea, bradycardia, or laryngospasm may occur. Various mechanical and chemical stimuli, including water, foreign bodies, and noxious gases, can trigger this response. This protective response is so potent that it can cause death in the newborn (see Chapters 3, Respiratory Physiology; and 4, Cardiovascular Physiology).
Respiratory System
At full-term birth, the lungs are still in the stage of active development. The formation of adult-type alveoli begins at 36 weeks post conception but represents only a fraction of the terminal air sacs with thick septa at full-term birth. It takes more than several years for functional and morphologic development to be completed, with a 10-fold increase in the number of terminal air sacs to 400 to 500 million by 18 months of age, along with the development of rich capillary networks surrounding the alveoli. Similarly, control of breathing during the first several weeks of extrauterine life differs notably from control in older children and adults. Of particular importance is the fact that hypoxemia depresses, rather than stimulates, respiration. Anatomic differences in the airway occur with growth and development. Recently, the concept of the child having a funnel-shaped airway with the cricoid as the narrowest portion of the airway has been challenged. Based on bronchoscopic images, Dalal and colleagues (2009) suggest for infants and children the glottis, not the cricoid, may be the narrowest portion. The development of the respiratory system and its physiology are detailed in Chapter 3, Respiratory Physiology.
Cardiovascular System
During the first minutes after birth, the newborn infant must change his or her circulatory pattern dramatically from fetal to adult types of circulation to survive in the extrauterine environment. Even for several months after initial adaptation, the pulmonary vascular bed remains exceptionally reactive to hypoxia and acidosis. The heart remains extremely sensitive to volatile anesthetics during early infancy, whereas the central nervous system is relatively insensitive to these anesthetics. Cardiovascular physiology in infants and children is discussed in Chapter 4.
Fluid and Electrolyte Metabolism
Like the lungs, the kidneys are not fully mature at birth, although the formation of nephrons is complete by 36 weeks’ gestation. Maturation continues for about 6 months after full-term birth. The glomerular filtration rate (GFR) is lower in the neonate because of the high renal vascular resistance associated with the relatively small surface area for filtration. Despite a low GFR and limited tubular function, the full-term newborn can conserve sodium. Premature infants, however, experience prolonged glomerulotubular imbalance, resulting in sodium wastage and hyponatremia (Spitzer, 1982). On the other hand, both full-term and premature infants are limited in their ability to handle excessive sodium loads. Even after water deprivation, concentrating ability is limited at birth, especially in premature infants. After several days, neonates can produce diluted urine; however, diluting capacity does not mature fully until after 3 to 5 weeks of life (Spitzer, 1978). The premature infant is prone to hyponatremia when sodium supplementation is inadequate or with overhydration. Furthermore, dehydration is detrimental to the neonate regardless of gestational age. The physiology of fluid and electrolyte balance is detailed in Chapter 5, Regulation of Fluids and Electrolytes.
Temperature Regulation
Temperature regulation is of particular interest and importance in pediatric anesthesia. There is a better understanding of the physiology of temperature regulation and the effect of anesthesia on the control mechanisms. General anesthesia is associated with mild to moderate hypothermia, resulting from environmental exposure, anesthesia-induced central thermoregulatory inhibition, redistribution of body heat, and up to 30% reduction in metabolic heat production (Bissonette, 1991). Small infants have disproportionately large BSAs, and heat loss is exaggerated during anesthesia, particularly during the induction of anesthesia, unless the heat loss is actively prevented. General anesthesia decreases but does not completely abolish thermoregulatory threshold temperature to hypothermia. Mild hypothermia can sometimes be beneficial intraoperatively, and profound hypothermia is effectively used during open heart surgery in infants to reduce oxygen consumption. Postoperative hypothermia, however, is detrimental because of marked increases in oxygen consumption, oxygen debt (dysoxia), and resultant metabolic acidosis. Regulation of body temperature is discussed in detail in Chapter 6, Thermoregulation.
Abajian J.C., Mellish R.W., Browne A.F., et al. Spinal anesthesia for surgery in the high-risk infant. Anesth Analg. 1984;63:359-362.
American Society of Anesthesiologists. Standards for basic intraoperative monitoring. ASA Newsl. 1986;50:12.
Anand K.J., Sippell W.G., Aynsley-Green A. Pain, anaesthesia, and babies. Lancet. 1987;2(8569):1210.
Anand K.J.S., Hickey P.R. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1.
Anand K.J.S., Sippell W.G., Aynsley-Green A. Randomized trial of fentanyl anesthesia in preterm babies undergoing surgery. Lancet. 1987;1:243.
Anand K.J.S., Garg S., Rovnaghi C.R., Narsinghani U., et al. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283-290.
Ansermino M., Basu R., Vandebeek C., Montgomery C. Nonopioid additives to local anaesthetics for caudal blockade in children: a systematic review. Paediatr Anaesth. 2003;13:561-573.
Aono J., Mimiya K., Manube M. Preoperative anxiety is associated with high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol Scand. 1999;43:542-544.
Aono J., Ueda W., Mamiya K., et al. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology. 1997;87:1298-1300.
Aouad M.T., Yazbeck-Karam V.G., Nasr V.G., et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. 2007;107(5):733-738.
Bainbridge W.S. Report of 12 operations on infants and young children under spinal anesthesia. Arch Pediatr. 1901;18:510.
Bannister C.F., Brosius K.K., Sigl J.C., Meyer B.J., Sebel P.S. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92:877-881.
Barker P., Langton J.A., Murphy P.J., Rowbotham D.J. Regurgitation of gastric contents during general anesthesia using the laryngeal mask airway. Br J Anaesth. 1992;69:314.
Bartels M., Althoff R.R., Boomsma D.I. Anesthesia and cognitive performance in children: No evidence for a causal relationship. Twin Res Hum Genet. 2009;12(3):246-253.
Bevan J.C., Johnston C., Haig M.J., et al. Preoperative parental anxiety predicts behavioural and emotional responses to induction of anesthesia. Can J Anaesth. 1990;37:177.
Bissonette B. Body temperature and anesthesia. Anesth Clin N Am. 1991;9:849.
Booker P.D. Management of postoperative pain in infants and children. Curr Opin Anesthesiol. 1988;1:17.
Bowlby J. Attachment and loss. New York: Basic Books, 1973.
Brain A.I.J. The laryngeal mask airway: a new concept in airway management. Br J Anaesth. 1983;55:801.
Bryan Y.F., Hoke L.K., Taghon T.A., et al. A randomized trial comparing sevoflurane and propofol in children undergoing MRI scans. Paediatr Anaesth. 2009;19(7):672-681.
Chambers N., Lopez T., Thomas J., James M.F.M. Remifentanil and the tunneling phase of paediatric ventriculoperitoneal shunt insertion: a double-blind, randomized, prospective study. Anaesthesia. 2002;57:133-139.
Chiaretti A., Pietrini D., Piastra M., et al. Safety and efficacy of remifentanil in craniosynostosis repair in children less than 1 year old. Pediatr Neurosurg. 2000;33:83-88.
Choudhry D.K., Brenn B.R. Bispectral index monitoring: a comparison between normal children and children with quadriplegic cerebral palsy. Anesth Analg. 2002;95:1582-1585.
Cohen I.T., Hannallah R.S., Hummer K.A. The incidence of emergence agitation associated with desflurane anesthesia in children is reduced by fentanyl. Anesth Analg. 2001;93:88-91.
Coté C.J., Goldstein E.A., Coté M.A., et al. A single-blind study of pulse oximetry in children. Anesthesiology. 1988;68:184.
Coté C.J., Rolf N., Liu L.M.P., Gousouzian N.G. A single-blind study of pulse oximetry and capnography in children. Anesthesiology. 1991;74:984.
Cravero J., Surgenor S., Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth. 2000;10:419-424.
Dalal P.G., Murray D., Messner H., et al. Pediatric laryngeal dimensions: an age-based analysis. Anesth Analg. 2009;108(5):1475-1479.
Davis P.J., Cohen I.T., McGowan F.X.Jr, Latta K. Recovery characteristics of desflurane vs. halothane for maintenance of anesthesia in pediatric ambulatory patients. Anesthesiology. 1994;80:293-302.
Davis P.J., Finkel J., Orr R., et al. A randomized, double-blinded study of remifentanil versus fentanyl for tonsillectomy and adenoidectomy surgery in pediatric ambulatory surgical patients. Anesth Analg. 2000;90:863-871.
Davis P.J., Galinkin J., McGowan F.X., et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. I. Emergence and recovery profiles. Anesth Analg. 2001;93:1380-1386.
Davis P.J., Greenberg J.A., Gendelman M., Fertal K. Recovery characteristics of sevoflurane and halothane in preschool aged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesth Analg. 1999;88:34-38.
Davis P.J., Wilson A.S., Siewers R.D., et al. The effects of cardiopulmonary bypass on remifentanil kinetics in children undergoing atrial septal defect repair. Anesth Analg. 1999;89:904-908.
de Beer D.A., Thomas M.L. Caudal additives in children: solutions or problems? Br J Anaesth. 2003;90:487-498.
Denman W.T., Swanson E.L., Rosow D., et al. Pediatric evaluation of the bispectral index (BIS) monitor and correlation of BIS with end-tidal sevoflurane concentration in infants and children. Anesth Analg. 2000;90:872-877.
Devitt J.H., Wenstone R., Noel A.G., O’Donnell P.O. The laryngeal mask airway and positive pressure ventilation. Anesthesiology. 1994;80:550.
Dixon S., Snyder J., Holve R., Bromberger P. Behavioral effects of circumcision with and without anesthesia. J Dev Behav Pediatr. 1984;5:246.
Dombro R.H. The surgically ill child and his family. Surg Clin North Am. 1970;50:759.
Dönmez A., Kizilkan A., Berksun H., et al. One center’s experience with remifentanil infusions for pediatric cardiac catheterization. J Cardiothoracic Vasc Anaesth. 2001;15:736-739.
DuBois D., DuBois E.F. A height-weight formula to estimate the surface area of man. Proc Soc Exp Biol Med. 1916;13:77.
Dutta S., Albanese C.T. Transaxillary subcutaneous endoscopic release of the sternocleidomastoid muscle for treatment of persistent torticollis. J Pediatr Surg. 2008;43:447-450.
Dutta S., Slater B., Butler M., Albanese C.T. “Stealth surgery:” transaxillary subcutaneous endoscopic excision of benign neck lesions. J Pediatr Surg. 2008;43:2070-2074.
Eichhorn S.M., Cooper J.B., Cullen D.J., et al. Standards for patient monitoring during anesthesia at Harvard Medical School. JAMA. 1986;256:1017.
Erdil F., Demirbilek S., Begec Z., et al. The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth Intensive Care. 2009;37(4):571-576.
Fisher D.M., Zwass M.S. MAC of desflurane in 60% nitrous oxide in infants and children. Anesthesiology. 1992;76:354.
Forman M.C., Kerschbaum W.E., Hetznecker W.H., Dunn J.M.. Psychosocial dimensions. Behrman R.E., Vaughan V.C.III, editors. Nelson’s textbook of pediatrics. Philadelphia: WB Saunders; 1987.
Friedman W.F.. The intrinsic physiologic properties of the developing heart. Friedman W.F., Lesch M., Sonnenblick E.H., editors. Neonatal heart disease. New York: Grune & Stratton; 1973.
Friesen R.H., Veit A.S., Archibald D.J., Campanini R.S. A comparison of remifentanil and fentanyl for fast track paediatric cardiac anaesthesia. Paediatr Anaesth. 2003;13:122-125.
Fujimoto T., Segawa O., Lane G.J., et al. Laparoscopic surgery for newborn infants. Surg Endosc. 1999;13:773-777.
Galinkin J.L., Davis P.J., McGowan F.X., et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy: II. Perioperative breathing patterns in neonates and infants with pyloric stenosis. Anesth Analg. 2001;93:1387-1392.
Galinkin J.L., Fazi L.M., Cuy R.M., et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93:1378-1383.
Gallagher T., Lerman J., Volgyesi G.A., et al. Effects of halothane and isoflurane on the baroreceptor response in newborn swine. Anesth Analg. 1987;66:564.
Ganesh A., Gurnaney H.G. Ultrasound guidance for pediatric peripheral nerve blockade. Anesthesiol Clin. 2009;27(2):197-212.
Ganesh A., Rose J.B., Wells L., et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105(5):1234-1242.
Gehan E.A., George S.L. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54(Pt 1):225.
Georgeson K. Minimally invasive surgery in neonates. Semin Neonatol. 2003;8:243-248.
German J.W., Aneja R., Heard C., Dias M. Continuous remifentanil for pediatric neurosurgery patients. Pediatr Neurosurg. 2000;33:227-229.
Gootman P.M.. Neural regulation of cardiovascular function in the perinatal period. Gootman N., Gootman P.M., editors. Perinatal cardiovascular function. New York: Marcel Dekker; 1983.
Gray H. Anatomy of the human body, ed 29. Philadelphia: Lea & Febiger, 1973.
Grevenik C.R., Ferguson C., White A. The laryngeal mask airway in pediatric radiology. Anesthesiology. 1990;72:474.
Gurnaney H., Ganesh A., Cucchiaro G. The relationship between current intensity for nerve stimulation and success of peripheral nerve blocks performed in pediatric patients under general anesthesia. Anesth Analg. 105(6), 2007. 160-5-9
Hannallah R.S., Rosales J.K. Experience with parents’ presence during anaesthesia induction in children. Can Anaesth Soc J. 1983;30:286.
Hansen T.G., Ilett K.F., Lim S.I., et al. Pharmacokinetics and clinical efficacy of long-term epidural ropivacaine infusion in children. Br J Anaesth. 2000;85:347-353.
Hansen T.G., Ilett K.F., Reid C., et al. Caudal ropivacaine in infants. Anesthesiology. 2001;94:579-584.
Harris J.S. Special pediatric problems in fluid and electrolyte therapy in surgery. Ann N Y Acad Sci. 1957;66:966.
Hay W.W.Jr, Rodden D.J., Collins S.M., et al. Reliability of conventional and new pulse oximetry in neonatal patients. J Perinatol. 2002;22:360-366.
Irita K., Kai Y., Akiyoshi K., et al. Performance evaluation of a new pulse oximeter during mild hypothermic cardiopulmonary bypass. Anesth Analg. 2003;96:11-14.
Isaza N., Garcia P., Dutta S. Advances in pediatric minimal access therapy: a cautious journey from therapeutic endoscopy to transluminal surgery based on the adult experience. J Pediatr Gastroenterol Nutr. 2008;46:359-369.
Ivani G., DeNegri P., Conio A., et al. Comparison of racemic bupivacaine, ropivacaine, and levobupivacaine for pediatric caudal anesthesia: effects on postoperative analgesia and motor block. Reg Anesth Pain Med. 2002;27:157-161.
Ivani G., Lampugnani E., Torre M., et al. Comparison of ropivacaine with bupivacaine for paediatric caudal block. Br J Anaesth. 1998;81:247-248.
Ivani G., DeNegri P., Lönnqvist P., et al. A comparison of three different concentrations of levobupivacaine for caudal block in children. Anesth Analg. 2003;97:368-371.
Jevtovic-Todorovic V., Hartman R.E., Izumi Y., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876-882.
Jovtovic-Todorovic V., Olney J.W. PRO: Anesthesia-induced developmental neuroapoptosis: status of the evidence. Anesth Analg. 2008;106:1659-1663.
Johnson T., Monk T., Rasmussen L.S., et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351-1357.
Johnston D.F., Wrigley S.R., Robb P.J., Jones H.E. The laryngeal mask airway in paediatric anaesthesia. Anaesthesia. 1990;45:924.
Jusko W.J. Pharmacokinetic principles in pediatric problems. Pediatr Clin North Am. 1972;19:81.
Kain Z., Mayes L., Wang S., et al. Parental presence during induction of anesthesia vs. sedative premedication: which intervention is more effective? Anesthesiology. 1998;89:1147-1156.
Kain Z., Mayes L., Wang S., et al. Parental presence and a sedative premedicant for children undergoing surgery: a hierarchical study. Anesthesiology. 2000;92:939-946.
Kain Z., Mayes L.C., Bell C., et al. Premedication in the United States: a status report. Anesth Analg. 1997;84:427-432.
Kain Z.N., Caldwell-Andrews A., Wang S-M., et al. Parental intervention choices for children undergoing repeated surgeries. Anesth Analg. 2003;96:970-975.
Kain Z.N., Caldwell-Andrews A.A., Mayes L.C., et al. Parental presence during induction of anesthesia: physiological effects on parents. Anesthesiology. 2003;98:58-64.
Kain Z.N., Caldwell-Andrews A.A., Krivutza D., et al. Trends in the practice of parental presence during induction of anesthesia and the use of preoperative sedative premedication in the United States, 1995–2002: Results of a follow-up national survey. Anesth Analg. 2004.
Kain Z.N., Mayes L.C., Caramico L.A., et al. Parental presence during induction of anesthesia: a randomized controlled trial. Anesthesiology. 1996;84:1060-1067.
Kalkman C.J., Peelen L., Moons K.G., et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805-812.
Karmakar M.K., Aun C.S.T., Wong E.L.Y., et al. Ropivacaine undergoes slower systemic absorption from the caudal epidural space in children than bupivacaine. Anesth Analg. 2002;94:259-265.
Keidan I., Berkenstadt H., Segal E., Perel A. Pressure versus volume-controlled ventilation with a laryngeal mask airway in paediatric patients. Paediatr Anaesth. 2001;11:691-694.
Keidan I., Berkenstadt H., Sidi A., Perel A. Propofol/remifentanil versus propofol alone for bone marrow aspiration in paediatric haemato-oncological patients. Paediatr Anaesth. 2001;11:297-301.
Kim J.Y., Chang Y.J., Lee J.Y., et al. Post-induction alfentanil reduces sevoflurane-associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol Scand. 2009;53(5):678-681.
Ko Y.-P., Huang C-J., Hung Y-C., et al. Premedication with low-dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia. Acta Anaesthesiol Sin. 2001;36:169-177.
Kulka P.J., Bressem M., Tryba M. Clonidine prevents sevoflurane-induced agitation in children. Anesth Analg. 2001;93:335-338.
Kuratani N., Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane. Anesthesiology. 2008;109:225-232.
Lankinen U., Avela R., Tarkkila P. The prevention of emergence agitation with tropisetron or clonidine after sevoflurane anesthesia in small children undergoing adenoidectomy. Anesth Analg. 2006;102(5):1383-1386.
Lerman J., Davis P.J., Welborn L.G., et al. Induction, recovery, and safety characteristics of sevoflurane I children undergoing ambulatory surgery: a comparison with halothane. Anesthesiology. 1996;84:1332-1340.
Lippmann M., Nelson J., Emmanouilides G.C., et al. Ligation of patent ductus arteriosus in premature infants. Br J Anaesth. 1976;48:365.
Loepke A.W., Soriano S.G. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681-1707.
Lönnqvist P.A., Westrin P., Laarsson B.A., et al. Ropivacaine pharmacokinetics after caudal block in 1- to 8-year-old children. Br J Anaesth. 2000;85:506-511.
Malviya S., Reynolds P.I., Voepel-Lewis T., et al. False alarms and sensitivity of conventional pulse oximetry versus the Masimo SET technology in the pediatric postanesthesia care unit. Anesth Analg. 2000;90:1336-1340.
Martin L.D., Pasternak L.R., Pudimar M.A. Total intravenous anesthesia with propofol in pediatric patients outside the operating room. Anesth Analg. 1992;74:609.
McCann M.E., Bacsik J., Davidson A., et al. The correlation of bispectral index with end tidal sevoflurane concentration and haemodynamic parameters in preschoolers. Paediatr Anaesth. 2002;12:519-525.
McCann M.E., Sethna N.F., Mazoit J.X., et al. The pharmacokinetics of epidural ropivacaine in infants and young children. Anesth Analg. 2001;93:893-897.
Mellon R.D., Simone A.F., Rappaport B.A. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509-520.
Mizushima A., Wardall G.J., Simpson D.L. The laryngeal mask airway in infants. Anaesthesia. 1992;47:849.
Monk T.G., Weldon B.C., Garvan C.W., et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18-30.
Moss A.J., Emmanouilides G.C., Monset-Couchard M., Marcan B. Vascular responses to postural changes in normal newborn infants. Pediatrics. 1968;42:250.
Motoyama E.K., Glazener C.H. Hypoxemia after general anesthesia in children. Anesth Analg. 1986;65:267.
Murthy B.V.S., Pandya K.S., Booker P.D., et al. Pharmacokinetics of tramadol in children after IV or caudal epidural administration. Br J Anaesth. 2000;84:346-349.
New York State Hospital Code. 1988. Section 405.13
Ochiai R., Guthrie R.D., Motoyama E.K. Effects of varying concentrations of halothane on the activity of the genioglossus, intercostals, and diaphragm in cats: an electromyographic study. Anesthesiology. 1989;70:812-816.
Ponsky T.A., Rothenberg S.S. Minimally invasive surgery in infants less than 5 kg: Experience of 649 cases. Surg Endosc. 2008;22:2214-2219.
Ross A.K., Davis P.J., Dear G., et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg. 2001;93:1393-1401.
Sarner J.B., Levine M., David P.J., et al. Clinical characteristics of sevoflurane in children: a comparison with halothane. Anesthesiology. 1995;82:38-46.
Sebel P.S., Lang E., Rampil I.J., et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesth Analg. 1997;84:891.
Smith R.M. Anesthesia for infants and children, ed 3. St. Louis: The CV Mosby Co, 1968.
Smith R.M. Anesthesia for infants and children, ed 4. St. Louis: The CV Mosby Co, 1980.
Smith R.M. Anesthesia for pediatric surgery. In: Gross R.E., editor. Surgery of infants and children. Philadelphia: WB Saunders, 1953.
Smith R.M. Some reasons for the high mortality in pediatric anesthesia. N Y J Med. 1956;56:2212.
Soliman I.E., Broadman L.M., Hannalah R.S., McGill W.A. Comparison of the analgesic effects of EMLA (eutectic mixture of local anesthetics) to intradermal lidocaine infiltration prior to venous cannulation in unpremedicated children. Anesthesiology. 1988;68:804.
Spears R.S., Yeh A., Fisher D.M., Zwass M.S. The “educated hand:” can anesthesiologists assess changes in neonatal pulmonary compliance manually? Anesthesiology. 1991;75:693.
Spitzer A. Renal physiology and functional development. In: Edelman C.M.Jr, editor. Pediatric kidney disease. Boston: Little, Brown and Co; 1978:25.
Spitzer A. The role of the kidney in sodium homeostasis during maturation. Kidney Int. 1982;21:539.
Taddio A., Goldbach M., Ipp M., et al. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet. 1995;345:291-292.
Taddio A., Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr Drugs. 2005;7:245-257.
Talbot N.G., Sobel E.H., McArthur J.W., Crawford J.D. Functional endocrinology from birth through adolescence. Cambridge, MA: Harvard University Press, 1952.
Tazeroualti N., De Groote F., De Hert S., et al. Oral clonidine vs midazolam in the prevention of sevoflurane-induced agitation in children: a prospective, randomized, controlled trial. Br J Anaesth. 2007;98(5):667-671.
Uemura E., Levin Ed., Bowman R.E. Effects of halothane on synaptogenesis and learning behavior in rats. Exp Neurol. 1985;89:520-529.
Vaughan V.C.III, Litt I.F. Assessment of growth and development. In Behrman B.E., Vaughan V.C.ID, editors: Nelson’s textbook of pediatrics, ed 13, Philadelphia: WB Saunders, 1987.
Voepel-Lewis T., Malviya S., Tait A.R. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96:1625-1630.
Wacha M.F., Simeon R.M., White P.F., Stevens J.L. Effect of propofol on the incidence of postoperative vomiting after strabismus surgery in pediatric outpatients. Anesthesiology. 1991;75:204.
Wang C., Slikker W.Jr. Strategies and experimental models for evaluating anesthetics: Effects on the developing nervous system. Anesth Analg. 2008;106:1643-1658.
Wear R., Robinson S., Gregory G.A. The effect of halothane on the baroresponse of adult and baby rabbits. Anesthesiology. 1982;56:188.
Wee L.H., Moriarty A., Cranston A., Bagshaw O. Remifentanil infusion for major abdominal surgery in small infants. Paediatr Anaesth. 1999;9:415-418.
Weiss M., Dullenkopf A., Fischer J.E., Anaesth Br J., et al, editors. Prospective randomized controlled multicentre trial of cuffed or uncuffed endotracheal tubes in small children. 2009;103:867-873.
Weiss M., Dullenkopf A., Gysini C., et al. Shortcomings of cuffed paediatric tracheal tubes. BJA. 2004;92:78-88.
Welborn L.G., Hannallah R.S., Norden J.M., et al. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg. 1996;83:917-920.
Wilder R.T., Flick R.P., Sprung J., et al. Early exposure to anesthesia and learning disablities in a population-based birth cohort. Anesthesiology. 2009;110:796-804.
Williams R.K., Adams D.C., Aladjem E.V., et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont Infant Spinal Registry. Anesth Analg. 2006;102(1):67-71.
Willschke H., Bosenberg A., Marhofer P., et al. Ultrasonographic-guided ilioinguinal/iliohypogastric nerve block in pediatric anesthesia: what is the optimal volume? Anesth Analg. 2006;102(6):1680-1684.
Wilson I.G. The laryngeal mask airway in paediatric practice. Br J Anaesth. 1993;70:124.
Yelderman M.F., New W. Evaluation of pulse oximetry. Anesthesiology. 1983;59:349.