CHAPTER 4 Spacer Devices—Old and New
 Bone and joint preservation are the main goals of treatment of unicompartmental osteoarthritis, especially in younger patients.
Bone and joint preservation are the main goals of treatment of unicompartmental osteoarthritis, especially in younger patients. Knee osteotomies provide the adjustment of leg axis malalignment, but do not address rebuilding the worn cartilage surface of the compartment.
Knee osteotomies provide the adjustment of leg axis malalignment, but do not address rebuilding the worn cartilage surface of the compartment. Unilateral knee arthroplasty calls for bone resection and has shown shorter durability than total knee arthroplasty.
Unilateral knee arthroplasty calls for bone resection and has shown shorter durability than total knee arthroplasty. Early unilateral implants such as the McKeever tibial hemiarthroplasty have addressed this problem without bone resection, with single-surface tibial resurfacing, and with reasonable long-term results. Later the UniSpacer, a self-centering mobile unilateral interpositional metal implant, was introduced, but failed because of inadequate alignment and its tendency for dislocation.
Early unilateral implants such as the McKeever tibial hemiarthroplasty have addressed this problem without bone resection, with single-surface tibial resurfacing, and with reasonable long-term results. Later the UniSpacer, a self-centering mobile unilateral interpositional metal implant, was introduced, but failed because of inadequate alignment and its tendency for dislocation. Recently, based on the principles of the nonfixed implants, the iForma, an individual metal interpositional device, was developed using patients’ individual magnetic resonance imaging data, mimicking the shape of the affected joint compartment. The iForma device can provide improvement in knee function and reduction in pain within a narrow indication of patients with unicompartmental knee arthritis, but with a significantly higher risk of early revision compared to traditional unicompartmental arthroplasty.
Recently, based on the principles of the nonfixed implants, the iForma, an individual metal interpositional device, was developed using patients’ individual magnetic resonance imaging data, mimicking the shape of the affected joint compartment. The iForma device can provide improvement in knee function and reduction in pain within a narrow indication of patients with unicompartmental knee arthritis, but with a significantly higher risk of early revision compared to traditional unicompartmental arthroplasty.Early Development of Hemiarthroplasty
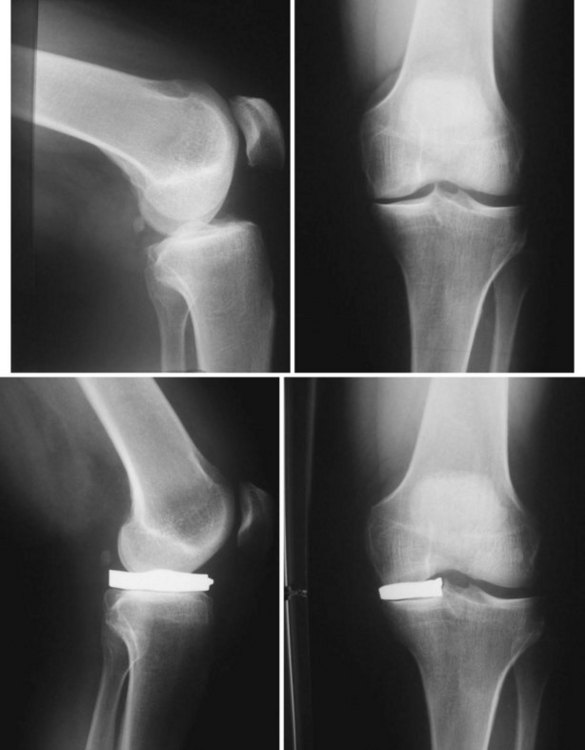
For patients with osteoarthritis limited to a single femoral-tibial compartment, the concept of a metallic hemiarthroplasty has a long history. The genesis of the concept of using an iPD, in which a material is placed between the condyles within the joint to reduce wear or prevent adhesion, goes back almost 150 years, when it was first suggested by Verneuil.1 Over the ensuing years, a wide variety of materials were tried, ranging from chromicized pig bladder in 1918 to vitallium in 1940. In 1960, McKeever2 described a metallic prosthesis that was designed to be placed in the femorotibial compartment and fixed to the tibial condyle (Howmedica, Rutherford, NJ) (Fig. 4–1). This design was subsequently modified by MacIntosh. Metallic hemiarthroplasty was introduced into orthopaedic practice in the 1950s and 1960s by McKeever2 and MacIntosh.3 MacIntosh and Hunter described the hemiarthroplasty approach as follows: “The aims of hemiarthroplasty are to correct the varus or valgus deformity by inserting a tibial plateau prosthesis of appropriate diameter and thickness to build up the worn side of the joint and thus to restore normal stability of the knee, to relieve pain and to improve function and gait. The collateral ligaments usually maintain their own length in spite of long-standing varus and valgus deformity, and stability is maintained by a prosthesis that is thick enough to correct the deformity and to take up the slack of the collateral ligaments.”4
Reports of early experience with both of these devices were generally encouraging. The two approaches differed primarily in the method of fixation. The McKeever implant had a keel that was inserted into the tibial condyle to provide mechanical fixation. In contrast, the MacIntosh implant was “held in position by the anatomy of the knee joint, and stability depends upon the taut collateral ligaments. No additional fixation is necessary. The top of the prosthesis has a contoured surface with rounded edges to provide the condyle with a permanent low-friction area. The undersurface is flat with multiple serrations to ensure a snug fit and stability.”4 In a series reporting 10-year follow-up for 75 MacIntosh implants, Wordsworth et al. found that 11 (14.7%) had been revised to arthroplasty and concluded that, although “greater angular deformities pre-operatively reduced the chance of success in the medium term, late failure of the arthroplasty after five years was very rare.”5 In a more recent clinical report on 44 McKeever implants followed for an average of 8 years, Scott et al. noted that, at final follow-up, 70% of the knees were rated as good or excellent.6 Similarly, Emerson and Potter also reported good results in 61 McKeever implants followed for up to 13 years (average, 5 years), in which 72% were rated as having good to excellent results.7 The most recent report of long-term results, published by Springer et al. in 2006, continued to show excellent long-term results with tibial hemiarthroplasty using the McKeever device.8 In spite of reports of early experience with these prostheses that were generally encouraging, the approach gained only limited use within the orthopaedic community due to its invasive nature and the subsequent development of total knee arthroplasty.
Recent Developments
UniSpacer
Decades after being set aside, the fundamental concept of hemiarthroplasty reemerged several years ago. The first such iPD to come to market was the UniSpacer (Zimmer, Warsaw, IN). The UniSpacer (Fig. 4–2) is a mobile iPD that does not achieve fixation to the tibial plateau using a keel, as did the McKeever device, or by means of a roughened undersurface, as did the MacIntosh prosthesis. The UniSpacer is designed to move freely on the tibial plateau as determined by the conforming articulation of its top surface with the femoral condyle. The UniSpacer is intended for use only in the medial compartment, primarily because, in the lateral compartment, roll-back could cause prosthetic dislocation, soft tissue impingement, or both. The design of the UniSpacer permits insertion using a minimally invasive approach.9 In a peer-reviewed article, Sisto and Mitchell reported on a series of 37 UniSpacer cases followed for an average of 26 months.10 In this series, the mean Knee Society Function Score was 69 and the Knee Score was 72. There were 12 revisions (35.4%), 6 as a result of device dislocation (17.6%) and 6 for pain or other reasons (17.6%). Friedman reported on an initial series of 23 cases in which there was an overall revision rate of 34% with an 8% dislocation rate.11 The results of these surgeons are consistent with early reports from the implant’s developers.
OrthoGlide
Another recently introduced hemiarthroplasty iPD is the OrthoGlide (Advanced Bio-Surfaces, Inc., Minnetonka, MN). The OrthoGlide implant (Fig. 4–3) is available only for use in the medial compartment, where it is placed between the tibial plateau and the femoral condyle by means of a minimally invasive surgical approach. The device is intended “to improve the alignment of the knee, with the aim of returning the joint to a more valgus position. Realignment of the knee tends to distribute the weight-bearing forces across the joint and thus helps restore the normal relationships of the articular surfaces and the surrounding capsular, ligamentous and muscular structures. The device is designed to relieve pain by providing an articulating surface with a low coefficient of friction and high durability.”12 The device geometry and ligament tension combine to keep the implant in place along with a posterior “lip” or overhang designed to prevent excessive movement of the device.
iForma
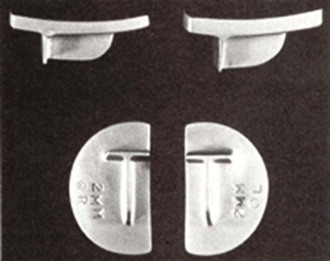
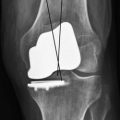
The iForma (ConforMIS, Burlington, MA) is a patient-specific iPD that replicates the tibial articular surface and uses functional fixation to maintain implant stability with minimal implant motion (Fig. 4–4). Each implant is unique, matching the patient’s articular anatomy and developed from a standard magnetic resonance imaging scan using a novel technology that converts the topography of the patient’s articular cartilage and subchondral bone to a patient-specific implant. The top surface of the implant conforms to the shape of the femoral condyle while the bottom surface conforms to the shape of the tibial plateau. Because the iForma is designed to replicate the patient’s unique anatomy, it can be designed for either the medial or the lateral condyle (Fig. 4–5).

Figure 4–4 iForma medial and lateral implants. Note: product is no longer offered by the manufacturer.
(Courtesy of ConforMIS, Burlington, MA.)
One of the stated aims of treatment using interpositional or hemiarthroplasty prostheses is the restoration or improvement of alignment. The ability of such a device to actually achieve improvement in alignment has been evaluated in a formal study of the iForma. Koeck et al.13 evaluated changes in leg axis in a clinical study of 27 patients who received a patient-specific iForma implant. All patients had early to moderate-stage osteoarthritis of the knee (Kellgren-Lawrence grade 3 or less). A single surgeon implanted a total of 27 iForma prostheses (23 medial, 4 lateral) in sequential cases. The average age of the patients (15 women and 12 men) was 55.3 years (range, 38–67 years). Standardized preoperative and postoperative standing long-leg radiographs were obtained and the deviation from the load axis of the surgically treated knee joint under stress was determined twice by two independent evaluators. The preoperative objective was to correct the leg axis to 0° and/or to a slight undercorrection of up to 2°. This was achieved in 23 of 27 cases (85.2%). The correlation coefficient between the implant offset as determined by the design algorithm and the extent of the axis correction was 0.838.
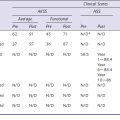
A multicenter study to report safety and efficacy of the iForma patient–specific interpositional device was performed from June 2005 to June 2008.14 Seventy-eight subjects (42 men, 36 women) received an iForma implant. The mean age was 53 years, the mean body mass index 29.0. The WOMAC scores, the visual analog pain scale and the Knee Society Scores were surveyed. The mean follow-up was 16.4 months. The mean WOMAC knee scores increased from 48.3 before surgery to 71.3 after 24 months. A reduction in pain was achieved for all five pain measures using a standard visual analog scale (VAS). Knee Society Knee Score improved from 39.2 before to 61.9 24 months after surgery. The Knee Society Function Scores improved from preoperative 64.5 to 82.5 2 years postoperative. The preoperative range of motion could be restored. The overall revision rate was 24%. Fifteen implants were removed early, 4 knees were revised without implant removal. Within a narrow indication of patients with unicompartmental disease, the iForma device can provide improvement in knee function and reduction in pain, however, with a significantly higher risk of early revision compared to traditional arthroplasty. Respecting this limitation, it may be an alternative option for arthritic patients with unicompartmental disease who have contraindications to high tibial osteotomy or are too young for knee replacement; the iForma device further has the distinct advantage of time and cost-saving compared to those procedures.
Discussion
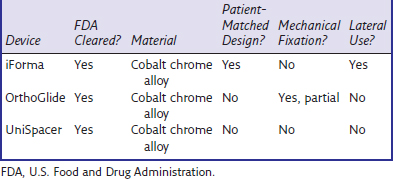
Hemiarthroplasty has undergone a renaissance, and the insights of McKeever and MacIntosh have evolved into several new alternatives for the treatment of unicompartmental femorotibial osteoarthritis. Mobile interpositional implants have shown a lack of adjustment and a tendency for roll-back and dislocation. The introduction of an individualized mobile interpositional implant has provided better functional fixation because of an accurate anatomic fit, even improving the anatomic axis. Therefore, individual mobile implants provide a minimally invasive alternative treatment option for a range of patients with unilateral osteoarthritis (![]() see Video 4-1). Against the backdrop of high early revision rates, biomechanical testings on implant stability and pressure distribution within the treated compartment are required as well. Longer follow-ups must be investigated to evaluate clear indications and the relevance of the latest generation of spacers. Table 4–1 presents a comparison of some of the features of the currently available devices.
see Video 4-1). Against the backdrop of high early revision rates, biomechanical testings on implant stability and pressure distribution within the treated compartment are required as well. Longer follow-ups must be investigated to evaluate clear indications and the relevance of the latest generation of spacers. Table 4–1 presents a comparison of some of the features of the currently available devices.
1 Verneuil AS. De la création d’une fausse articulation par section ou resection partielle de l’os maxillaire inférieur, comme moyen de rémedier a l’ankylose vraie ou fausse de la machoire inférieure. Arch Gen Med. 1860;15:174-179.
2 McKeever DC. Tibial plateau prosthesis. Clin Orthop Relat Res. 1960;18:86-95.
3 MacIntosh DL. Hemi-arthroplasty of the knee using a space occupying prosthesis for painful varus and valgus deformities. Proceedings of the Joint Meeting of Orthopaedic Associations of the English Speaking World. J Bone Joint Surg [Am]. 1958;40:1431.
4 MacIntosh DL, Hunter GA. The use of the hemiarthroplasty prosthesis for advanced osteoarthritis and rheumatoid arthritis of the knee. J Bone Joint Surg [Br]. 1972;54:244-255.
5 Wordsworth BP, Shakespeare DT, Mowat AG. MacIntosh arthroplasty for the rheumatoid knee: a 10-year follow up. Ann Rheum Dis. 1985;44:738-741.
6 Scott RD, Joyce MS, Ewald FC, Thomas WH. McKeever metallic hemiarthroplasty of the knee in unicompartmental degenerative arthritis. J Bone Joint Surg [Am]. 1985;67:203-207.
7 Emerson R, Potter T. The use of the McKeever metallic hemiarthroplasty for unicompartmental arthritis. J Bone Joint Surg [Am]. 1985;67:208-212.
8 Springer BD, Scott RD, Sah AP, Carrington R. McKeever hemiarthroplasty of the knee in subjects less than sixty years old. J Bone Joint Surg [Am]. 2006;88:366-371.
9 Scott RD. The UniSpacer: insufficient data to support its widespread use. Clin Orthop Relat Res. 2003;416:164-166.
10 Sisto DJ, Mitchell IL. UniSpacer arthroplasty of the knee. J Bone Joint Surg [Am]. 2005;87:1706-1711.
11 Friedman MJ. Unispacer. Arthrosc. 2003;19(Suppl):120-121.
12 Pre-Market Notification. 510(k) Summary, Advanced Bio-Surfaces, Inc. OrthoGlide® (Medial Knee Implant, K053094). www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=19941, Feb. 6, 2006. Available at
13 Koeck FX, Perlick L, Luring C, et al. Leg axis correction with ConforMIS iForma (interpositional device) in unicompartmental arthritis of the knee. Int Orthop. 2009;33:955-960.
14 Koeck FX, Luring C, Goetz J, et al. Prospective single-arm, multi-center trial of a patient-specific interpositional knee implant: Early clinical results. Open Orthop J. 2011;5:37-43.