Chapter 124 Soy Isoflavones and Other Constituents
 Introduction
Introduction
The health effects of soy and its constituent isoflavones are one of the most thoroughly researched fields in nutritional science. Over 2000 soy-related papers are published annually and more than one half are related to isoflavones.1 Overall, soy foods are considered a heart-healthy source of protein because of their high content of polyunsaturated fats, fiber, vitamins, and minerals and low content of saturated fat.2 The data related to specific health benefits of isoflavones—such as supporting bone health, decreasing menopausal hot flashes, improving cognitive function, and reducing the risk of certain types of cancers—are inconsistent. Several factors have been identified that contributed significantly to inconsistent results from early studies. These factors were rarely accounted for during the first 20 years of soy research (Box 124-1) and include individual variability of the human response to soy, differences in the isoflavone composition of different soy preparations, and the lack of analytic methods to assess soy constituents in foods, supplements, and biological fluids and tissues.3 The design and evaluation of future soy-food and isoflavone research will be guided by these realizations, and will produce higher-quality data to help answer remaining questions about the health benefits and safety considerations of consuming soy and its bioactive constituents.
BOX 124-1 Historic Overview of Soy and Isoflavone Research
Potential Benefits Explored
• 1990s: Research evaluates soy for hypocholesterolemic and mixed estrogen receptor properties, including potential benefits for postmenopausal hot flashes, bone health, and influence on hormone-sensitive cancers
• 1996: First isoflavone supplement becomes available
• 1999: FDA approves health claim for soy protein and coronary heart disease
Data from Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr 2010;140:1350S-1354S and Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol 2010;8:89-98.
 Chemical Composition of Soy
Chemical Composition of Soy
Interest in the constituents of soybeans, particularly soy protein and isoflavones, has catapulted soy to the status of a promising nutrient. Isoflavones remain in soy protein and other soy foods unless the preparation is extracted with alcohol.4 Commonly consumed soy foods—including soy milk, protein powders, and soy-based infant formula—are considered rich sources of dietary isoflavones.5 These are believed to be the most biologically active compounds in soy, but this has not been determined with certainty.
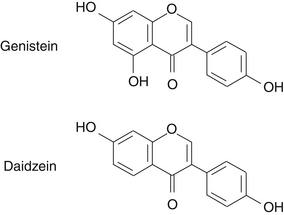
The principal isoflavones and most researched soy constituents in soy are genistein (4’,5,7-trihydroxyisoflavone) and daidzein (4’,7-dihydroxyisoflavone) (Figure 124-1). Glycetein is another isoflavone found in soy, but relatively little is published on its biological activity. In addition to isoflavones, soybeans contain lignans, coumestans, saponins, plant sterols, phytates, and protease inhibitors. The chemical composition, including the isoflavone content, of different soy-food preparations are variable and dependent on soybean strain, growing conditions, harvest time, and processing method (Table 124-1).
TABLE 124-1 Percentage and Ratio of Principle Isoflavones in Common Soy Products
| Soy Product | Isoflavone Content | Genistein:Daidzein |
|---|---|---|
| Whole soybeans | 0.1%-0.2% | 1:1 |
| Soy flour (defatted) | 0.07%-0.3% | 1:1 |
| Soy germ | 5% | 1:4 |
| Synthetic genistein | 100% | 1:0 |
| Soy protein isolate | 0.01%-0.3% | 1:1 |
| Tofu | 0.02%-0.07% | 1:0.7 |
| Soy milk | 0.001%-0.02% | 1:1 |
| Miso (dry) | 0.02%-0.09% | 1:0.9 |
Data from Empie MW. Compositional specificity in soy isoflavone supplements. soy/protein/isoflavone research: challenges in designing and evaluating intervention studies. NIH Workshop; Bethesda, Maryland: July 28-29, 2009.
Isoflavones
Classification
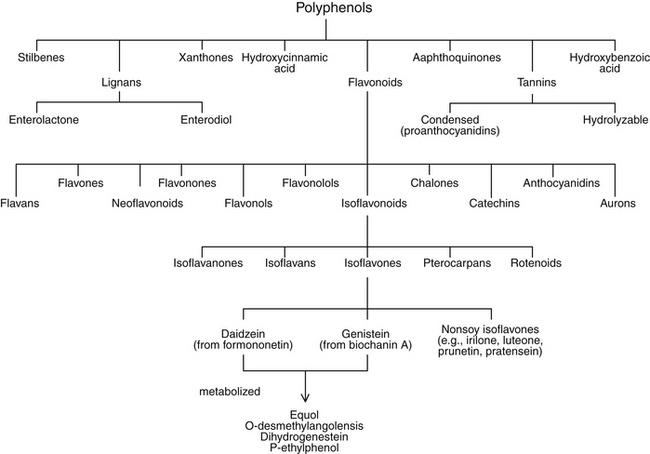
Flavonoids are a subgroup of a larger group of plant constituents, the polyphenols. Flavonoids are further differentiated into isoflavonoids, of which isoflavones are a subcategory (Figure 124-2). Isoflavonoids are not as ubiquitous in nature as some of the other flavonoids, such as flavones and flavonols. Approximately 600 isoflavonoids have been identified and further divided into subclasses according to the oxidation level of the central pyran ring. Isoflavones are the most abundant of the subclasses of isoflavonoids.
Absorption, Metabolism, and Excretion
The primary human exposure to isoflavones comes from the diet. Evidence suggests that the absorption and metabolism of isoflavones vary considerably among individuals.6 Factors that influence the bioavailability and possibly the bioactivity of isoflavones include absorption, metabolism by intestinal bacteria, liver metabolism, and enterohepatic circulation.
The native isoflavones in soy and unfermented soy foods—genistein and daidzein—occur conjugated to sugar moieties as glycosides and are not readily absorbed. After ingestion, isoflavone glycosides are hydrolyzed by intestinal brush border and bacterial glucosidases and released as the absorbable aglycone isoflavones genistein and daidzein. After absorption, the bioactive isoflavones are attached to glucuronic acid and made more water-soluble. It is predominantly the glucuronidated metabolites of isoflavones that circulate in the blood and are excreted in urine.7 In adults, genistein and daidzein can be further metabolized by bacteria to specific metabolites: equol, O-desmethylangolensis, dihydrogenistein, and p-ethylphenol. Emerging evidence suggests that the biological activity of isoflavones may be dependent, at least in part, on the bacterial conversion of isoflavones to corresponding metabolites such as equol, which is highly variable among individuals. It has been reported that 20% to 30% of Westerners have the intestinal bacteria necessary to metabolize daidzein to equol, whereas in Asian countries the frequency of equol producers is 50% to 60%.8 The hypothesis that individuals who possess the necessary bacteria to produce equol are more likely to achieve health benefits from soy-food consumption than those who do not is currently being investigated with great interest. Isoflavones attain maximal plasma levels 4 to 8 hours after ingestion and are excreted from the body within 24 hours, mainly in urine and to a lesser extent in feces.7
Isoflavone aglycones are thought to be more absorbable than their glucoside counterparts because aglycones have greater hydrophobicity and lower molecular weights. However, the relative bioavailability of isoflavone aglycones versus glucosides is debated. One study examined plasma and urinary concentrations of isoflavones and daidzein metabolites in healthy volunteers after ingesting soy milk, glucosidase-treated soy milk, and fermented soy milk. The researchers concluded that in humans, isoflavone aglycones, which are higher in fermented soy foods, were absorbed faster and in greater amounts than glucosides.9 Another study conducted on healthy male subjects (aged 20 to 40 years) found that urinary excretion of genistein and daidzein was greater after consumption of 112 g of tempeh, a fermented soy product, than after 125 g of unfermented soy pieces.10 These and other findings11 seem to indicate that fermentation of soy products increases the bioavailability of the isoflavones. Other studies have reported conflicting results. One study reported the greater bioavailability of glycoside isoflavones, as measured from the area under the curve of plasma appearance and disappearance,7 whereas other studies have reported no significant difference in absorption between aglycone and glycoside isoflavones.12,13
Isoflavone Content of Soy Products
A 2009 National Institutes of Health (NIH) workshop on designing, implementing, and reporting clinical studies of soy interventions has identified product composition and integrity as key challenges to accurately interpreting soy clinical studies.3 The type and proportion of isoflavones will vary depending on plant genetics, growing and harvesting conditions, storage before and after processing, plant parts used, and processing and extraction method. The typical isoflavone content and genistein:daidzein ratios from different soy foods are listed in Table 124-1. Differences in the isoflavone content of soy products may account for different biological effects and consequently contribute to the heterogeneous results observed among clinical and epidemiologic studies of soy foods.
Soy foods are the primary dietary source of isoflavones and the only foods that provide isoflavones in physiologically relevant amounts. Other plants, such as red clover (Trifolium pratense) and kudzu (Pueraria lobata), contain isoflavones, but these are generally not consumed via the diet. Traditional Asian diets provide a different isoflavone profile when compared with Western diets. Traditionally, Asians consumed minimally processed and fermented soy foods. Fermented soy foods account for approximately 30% of total soy-food consumption among Asians,14 which correlates with a higher consumption of isoflavone aglycones. Forms of soy consumed in Asia include boiled soybeans, miso soup with tofu, natto, and soy milk.15 Americans consume much less soy overall but also more processed soy foods, such as soy flour, textured vegetable protein, and isolated soy protein. Soy foods, including protein powders and soy-based infant formula, are considered rich sources of dietary isoflavones and contain 1 to 4.2 mg isoflavones per gram.5 Approximately 25% of formula-fed infants consume soy formula, and these infants have isoflavone concentrations that are significantly higher on a body-weight basis than do adults with high soy intake.16 The impact of early exposure to higher levels of isoflavones remains unclear. However, Asian populations whose isoflavone exposure begins earlier in life and continues throughout life have been the source of epidemiologic data demonstrating a relationship between soy consumption and health benefits.
Individuals from Western cultures consume approximately 1 to 2 mg of isoflavones daily,17 whereas total mean intake of isoflavones among Asian populations ranges from 25 to 50 mg daily.3 In 1999, the U.S. Food and Drug Administration (FDA) approved a food-labeling health claim for soy protein in the prevention of coronary heart disease,18 which subsequently led to a significant increase in retail soy-food consumption. Sales of U.S. soy foods increased from $2 billion in 1999 to $4.3 billion in 2005, and the number of soy-food products increased from hundreds to thousands.5 Soy is also increasingly being used in food processing. Soy flour and soy protein isolates are added to processed meats, meat substitutes, breads, and other processed foods, making these items additional hidden sources of dietary isoflavones. For a comprehensive list of foods and their isoflavones content, the reader is referred to extensive published lists.19,20 Overall, the combined effect of increased availability of frank soy-food products and hidden isoflavones in processed foods suggests an overall net increase in isoflavone intake among Western populations.
Dietary supplements are an additional source of isoflavones that contribute to an individual’s total isoflavone intake. Soy, red clover, and kudzu root are the primary materials used to manufacture isoflavone supplements. These three materials provide differing amounts and types of isoflavones. For example, red clover contains formononetin and biochanin A, which are methoxylated forms of genistein and daidzein. Kudzu contains high levels of daidzein and puerarin, another methoxylated derivative of daidzein (Table 124-2).
Other Soy Constituents
For a summary of soy constituents and their functions, see Table 124-3; for a detailed review, see Kang et al.21
| Constituent | Function |
|---|---|
| Protease inhibitors (Bowman-Birk inhibitor, Kunitz trypsin inhibitor) | Inhibit oncogene expression Inhibit chemically induced carcinogenesis Implicated in pancreatic hypertrophy (animal studies) |
| Lignans (enterolactone, enterodiol) | Phytoestrogen effects (agonistic/antagonistic) Antitumor Antiviral |
| Phytosterols, beta-sitosterol | Binds cholesterol in the gut |
| Coumestans (coumestrol) | Phytoestrogen effects (agonistic/antagonistic) |
| Saponins | Antioxidant Bind cholesterol and bile acids in the gut Antiviral (human immunodeficiency virus) |
| Phytates | Antioxidant Chelate metal ions (e.g., iron) Enhance natural killer cell activity |
| Isoflavones (genistein, daidzein, and their metabolites) | Phytoestrogen effects (agonistic/antagonistic) Antimutagenic Antioxidant Antiinflammatory Antiproliferative Antihypertensive Angiogenesis inhibition |
Protease Inhibitors
Researchers have looked with interest at protease inhibitors (PIs) and their potential anticancer and antiinflammatory effects. Soybeans contain several possibly active PIs, including Bowman-Birk inhibitor (BBI), lunasin, and Kunitz trypsin inhibitor (KTI). BBI is particularly effective in suppressing carcinogenesis, and concentrated BBI, known as BBIC, was approved by the FDA as an Investigational New Drug in 1998.22
Researchers have questioned the concept that PIs contribute significantly to the anticancer effects of soy. This is partly because raw and cooked soy products are equally effective in reducing cancer incidence even though heating destroys virtually all PI activity.23 Another point to consider is that PIs are peptides, and ingested PIs (such as BBIC) may not survive digestion and reach target tissues intact. Data suggest that lunasin may be the actual bioactive cancer-preventive agent in BBIC, and that BBI simply protects lunasin when soybeans and other PI foods are eaten by humans.24
Lignans
Lignans are capable of exerting a phytoestrogenic effect in humans.25,26 In addition, they exhibit antitumor and antiviral activity.27 The presence of lignans in soybeans has been hypothesized to support beneficial health effects of soy foods while negating potential adverse effects in postmenopausal women by opposing the potential stimulatory effects of isoflavones, particularly genistein, on human breast tissue.28
Phytosterols
Phytosterols, such as beta-sitosterol, are found in soy products, although it has not been established that the amounts present in soy foods are sufficient to derive the health benefits associated with phytosterol consumption. Although poorly absorbed, phytosterols bind cholesterol in the gut and, based on this activity, phytosterols are the subject of an FDA-approved health claim for reducing the risk of heart disease.29 An analysis of 22 randomized trials on isolated soy protein showed an average 3% reduction in low-density-lipoprotein (LDL) cholesterol.2 The phytosterol content of isolated soy protein is significantly reduced compared with soy food, which suggests that the LDL-lowering activity of soy protein is not related to its phytosterol content.
Coumestans
The phytoestrogen coumestrol and other coumestan isoflavonoids have been found by some researchers in significant quantities in soy foods of all types.19 On the other hand, Adlercreutz and Mazur25 report the presence of coumestans only in soy sprouts.
Saponins
Saponins are found in many plants, including soybeans. They appear to have anticancer properties by virtue of their antioxidant and antimutagenic properties.30 They are also known to reduce plasma and LDL cholesterol levels by affecting intestinal cholesterol absorption.31 It has been suggested that saponins found in soybeans have important synergistic functions and may contribute to the LDL-lowering effect of soy protein.32
Phytates
Although phytic acid (inositol hexaphosphate) has been implicated in blocking the absorption of minerals, the phytate content of plants, including soy, seems to be responsible for some of the anticancer properties of vegetable-based foods. Phytic acid is a highly charged antioxidant, capable of scavenging hydroxyl radicals and chelating metal ions such as the prooxidant iron. Graf and Eaton33 reported the iron-chelating ability of phytate to be more important than the activity of fiber in the dietary prevention of colon cancer. Vucenik et al34 reported antitumor effects of phytic acid both in vitro and in animal models.
 Pharmacology
Pharmacology
Soy constituents have been shown to have estrogenic,4 antiestrogenic,4 antiviral,35 anticarcinogenic,36–38 bacteriocidal, and antifungal39 effects. Isoflavones have demonstrated selective estrogen-receptor modulating,40 antimutagenic,37 antioxidant,41,42 mild antiinflammatory,43 antihypertensive,43 and antiproliferative effects.38,44
Hormonal Effects in Infants
Soy formula is an infant food made from soy protein and other soy components; it is fed to millions of infants worldwide as a supplement or replacement for human milk or cow’s milk. Soy formula has been shown to be comparable with milk-based formulas in supporting infant growth and development.45 The potential for estrogenic effects to occur in infants fed with soy formula has raised the possibility of long-term safety considerations.
In the United States and Canada, approximately 25% of formula-fed infants are fed soy-based formulas.5 The range of total isoflavone content in soy formula has been reported to be 10 to 47 mg/L.46,47 Genistein is the predominant isoflavone found in soy formula (about 58% to 67%), followed by daidzein (about 29% to 34%) and glycetein (about 5% to 8%).48 Infants fed soy formula also have higher blood levels of genistein and daidzein compared with other populations, with relatively high levels among vegan and Asian infants also consuming a traditional diet high in soy foods.48 Data from the United States show that the concentrations of total genistein in blood samples from infants fed soy formula were higher than the maximum total genistein concentrations available for any other population. The average blood levels of total genistein in the soy formula-fed infants were approximately 160 times higher than the mean levels of total genistein in omnivorous adults in the United States, and a similar pattern was observed for urinary concentrations of genistein and daidzein.49
Adverse effects of phytoestrogens on the development and reproductive capacity of livestock, wildlife, and experimental animals have been reported.50 Historically, there have been relatively few studies of children fed soy formula, and data are insufficient to judge if soy feeding results in adverse health consequences. The Arkansas Children’s Nutrition Center is conducting a prospective longitudinal study comparing the growth, development, and health of breastfed children with those of children fed soy- and milk-based formulas from birth through age 6. After the first 5 years of the study, all of the children were found to be growing and developing within normal limits; there were no indications of adverse effects in the soy-fed children.45 In 2009, the National Toxicology Program Center for the Evaluation of Risks to Human Reproduction convened an expert panel to evaluate soy formula. This panel reviewed and evaluated all available data on soy formula and concluded that there is minimal concern for adverse developmental effects in infants fed soy infant formula.48
Millions of Asians have consumed large quantities of soy foods for hundreds of years without any apparent health risk and seemingly with health benefits. Long-term human studies of children fed soy formula and conclusions from the 2009 National Toxicology Program expert review panel collectively suggest that soy formula supports normal growth and is not associated with any adverse health effects.45,48 Healthy dietary choices during the early phases of life positively influence long-term health. Therefore, additional studies are needed to affirm the safety of soy-based formulas and to assess the potential beneficial effects of consuming phytoestrogens in the form of soy isoflavones early in life.
Hormonal Effects in Adults
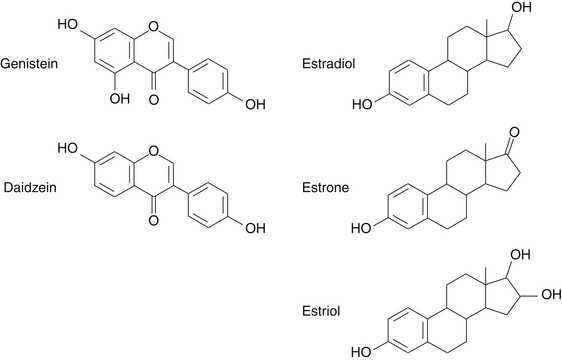
Much of the interest in the biological activity of isoflavones relates to their structural similarity to estrogens. In the 1960s it was discovered that isoflavones had a weak binding affinity for estrogen receptor alpha. It was later determined that isoflavones had mixed estrogen receptor agonist-antagonist properties and that isoflavones preferentially bind to estrogen receptor beta,51 which has led some researchers to classify isoflavones as natural selective estrogen receptor modulators.40 The binding of isoflavones to mammalian estrogen receptors is approximately 100 times weaker than the binding affinity of physiologic estrogens. However, this can be offset by higher circulating levels of isoflavones.52
After ingestion, isoflavone glycosides are converted by gut bacteria in the intestines to compounds with molecular weights and structures similar to those of steroid hormones (Figure 124-3). The pattern of isoflavone and lignan excretion in the urine is similar to that seen with endogenous estrogens.53 Hormonal effects of isoflavones have been evaluated in several human clinical trials with equivocal results. Overall, isoflavones appear to have a modest hormonal effect in women, but the clinical implications of these modest hormonal changes remain to be determined.
A study of premenopausal women found that 60 g of soy protein, providing 45 mg of isoflavones daily, resulted in the suppression of midcycle surges of follicle-stimulating hormone (FSH) and luteinizing hormone (LH).54 Plasma concentrations of estradiol rose during the follicular phase in the soy group, and cholesterol values decreased by 9.6%. The researchers noted that a similar effect occurs in women given tamoxifen (Nolvadex). There were no significant differences in estradiol levels between the soy and control groups at midcycle or during the luteal phase.54 Studies have also reported soy protein isolate to have a stimulatory effect on breast tissue in premenopausal women, which is characterized by increased breast secretions, epithelial cell hyperplasia, and elevations of serum estradiol.55 A meta-analysis of data from 8 randomized controlled trials comprising 1287 women compared isoflavones with placebo for 6 months to 3 years; it suggested no overall effect of isoflavones on breast density in all women combined or in postmenopausal women. There was a modest increase in mammographic breast density in premenopausal women, but it remains to be determined if these small effects are clinically relevant.56 A study in which postmenopausal women received soy supplementation found a small estrogenic effect on vaginal cytology. However, no difference between soy-supplemented subjects and controls with regard to serum levels of FSH, LH, sex hormone–binding globulin (SHBG), endogenous estradiol, or body weight was observed.57
A systematic review and meta-analysis of 47 studies that investigated the effects of soy protein or isoflavones on circulating hormone concentrations in pre- and postmenopausal women concluded that isoflavone-rich soy products decrease FSH and LH in premenopausal women, and may increase estradiol in postmenopausal women. In premenopausal women, the meta-analysis demonstrated that isoflavone consumption did not affect estradiol, estrone, or SHBG concentrations, but did reduce FSH and LH by approximately 20%. Menstrual cycle was increased by 1.05 days. In postmenopausal women, there were no statistically significant effects on estradiol, estrone, SHBG, FSH, or LH. However, there was a small nonsignificant increase in estradiol.58
Mechanisms of Action of Soy Isoflavones
There are many proposed mechanisms for the therapeutic effects of isoflavones. These include:
• Binding of estrogen receptors59
• Inhibition of protein tyrosine kinase60
• Interaction with peroxisome proliferator–activated receptors (PPAR alpha and gamma)62
Several other mechanisms have also been proposed, including modulation of steroid synthesis and metabolism,64 induction of DNA strand breakage resulting in apoptosis or cell death,65 inhibition of angiogenesis,66 modulation of SHBG,67 inhibition of thrombin formation and platelet activation,68 increase of LDL receptor activity,69 and increase of membrane fluidity.70
 Clinical Applications
Clinical Applications
Cancer
Consumption of soy foods may contribute to lower incidences of certain types of cancers, such as breast and prostate cancers. The first clues that soy diets might provide protection from cancer came from epidemiologic studies in which people from Asian cultures eating a diet high in soy foods demonstrated lower rates of several types of cancers. Messina et al71 reviewed 21 epidemiologic studies that evaluated the effect of soy diets on 26 different cancer sites. An evaluation of the effect of nonfermented soy products in these studies found that 10 showed decreased risks for rectal, stomach, breast, prostate, colon, and lung cancers, and 15 showed no significant effect. Only one, in which fried bean curd was evaluated, showed an increased risk for esophageal cancer. On the other hand, the effects of fermented soy products—miso soup and soybean paste—were much less consistent. In 21 studies evaluating fermented soy products involving 25 cancer sites, a higher cancer risk was found in 4, mixed results were obtained in 4, no significant effects were found in 14, and a decreased risk was found in 3. The higher risks of cancer from consumption of fermented soy products appear to involve primarily the gastrointestinal tract—esophageal, stomach, colorectal, and pancreatic cancers.
A community-based prospective study of more than 30,000 Japanese adults (35 years and older) was conducted over 7 years. Data on soy and isoflavone intake from food frequency questionnaires determined that men in the highest tertile of soy intake had a significant inverse risk of death from stomach cancer. The same was seen in women, although the inverse association was of marginal significance. These results suggest that soy intake may reduce the risk of death from stomach cancer.72 A 2010 systematic review of epidemiologic studies on the relationship between soy consumption and colorectal cancer risk identified a 21% reduction in colorectal cancer risk in women but not in men,73 whereas a 2009 meta-analysis of epidemiologic studies investigating the associations between soy intake and the risk of endocrine-related gynecologic cancers (endometrial and ovarian) showed protective effects.74
Studies have shown that in addition to higher consumption of soy foods, Asian communities have demonstrated higher levels of biomarkers of isoflavone intake. Adlercreutz et al75 found high urinary excretion of the soy isoflavones equol, daidzein, and O-desmethylangolensin in both men and women living in rural Japan. Mean plasma concentrations of the isoflavones genistein and daidzein in Japanese men were 492.7 and 282.5 nmol/L, respectively; men consuming a Western diet had 33.2 nmol/L for genistein and 17.9 nmol/L for daidzein.76 An unanswered question related to the study of isoflavones in the treatment of cancer is whether the concentration achieved by dietary consumption of soy products is enough to influence tumor growth. Studies on human volunteers consuming soy beverages, which provided 42 mg genistein and 27 mg daidzein daily, resulted in peripheral blood concentrations of 0.5 to 1.0 mM,4 concentrations much lower than necessary to inhibit growth of cultured cancer cells.77 However, the same researchers found nontransformed mammary epithelial cell cultures to be much more sensitive to genistein, with inhibition of growth stimulation occurring in the range of 1 to 2 mM. This finding suggests a role for isoflavones in chemoprevention rather than as chemotherapeutic agents.
Breast Cancer
The breast cancer rate among Chinese and other Asian women is one-third to one-half that of Caucasian women, and evidence suggests that this variation is due to environmental rather than genetic differences.78 Case-controlled, epidemiologic, in vitro, and animal studies point to the effectiveness of isoflavones in the prevention of breast cancer. The largest of the case-controlled studies, a prospective study of over 35,000 Singapore Chinese women, demonstrated that breast cancer risk was reduced significantly among women with high soy intake relative to women with lower soy intake. The study found a significant 18% reduction in breast cancer risk in association with the above median isoflavone intake. The association was more apparent in postmenopausal women, but this may be because of the relatively few premenopausal women included in the study. The authors concluded that these prospective findings suggest that approximately 10 mg of isoflavones per day, obtained in a standard serving of soy food, may have lasting beneficial effects against breast cancer development.79 Another epidemiologic study showed that higher soy intake during adolescence is associated with a lower risk of breast cancer in adulthood. During adolescence, breast tissue is most sensitive to environmental stimuli, and exposure to soy isoflavones during this life stage may have an effect on the subsequent risk of developing breast cancer. The Shanghai Breast Cancer Study analyzed 1459 breast cancer cases and 1556 age-matched controls. Adolescent soy-food intake was inversely associated with the risk of developing breast cancer in a dose-dependent fashion for both pre- and postmenopausal women.80
Several other case-controlled studies have investigated the association between soy intake and breast cancer risk. Of these, several were conducted in Western populations with very low soy intake (average 1 to 2 mg/day) and not considered relevant.81 Some studies conducted in populations with moderate to high soy intake have also demonstrated an inverse association between soy intake and breast cancer risk,82 whereas others have demonstrated no relationship.83,84 Emerging evidence suggests that the timing of soy exposure is an important codeterminant of risk, with stronger effects resulting from exposures at earlier ages.80 Overall, there is a strong case for the cancer-inhibitory effects of soy on breast cancer that is underpinned by biological plausibility; it is supported by animal and in vitro evidence, as well as compelling results from several epidemiologic studies demonstrating an inverse relationship between soy intake and the incidence of breast cancer.
Prostate Cancer
The incidence of prostate cancer is lower in Japan than in Western nations. However, when Japanese men immigrate to regions where a Western diet is consumed, the prostate cancer rates are six times higher than those among native Japanese.85 Environmental factors such as diet have been implicated in the development of prostate cancer. Isoflavones in the plasma of Japanese men were between 7 to 110 times the levels in Finnish men, with genistein present in the highest concentrations.86 Similarly, low plasma isoflavone levels have been observed in men from other Western regions.76
Epidemiologic evidence points to the benefits of soy constituents in the prevention of prostate cancer.85,87,88 For example, a case-controlled study of Japanese men examined associations between nutritional and other lifestyle factors and the prevalence of prostate cancer. In this study of 200 men and 200 age-matched controls from three geographic areas of Japan, BMI, physical activity, occupation, family history of prostate cancer, and medical history were not associated with prostate cancer risk. Intake of isoflavones and the aglycones genistein and daidzein were significantly associated with decreased risk.85 In other experimental studies, soy protein or isoflavones have shown anticancer effects on biomarkers of cancer development and progression in cell lines89,90 and in patients with prostate cancer.91–93 However, in one study, supplementation with soy protein providing 116 mg of isoflavones daily did not lower serum total or free prostate-specific antigen in healthy men.94 Overall, evidence suggests that consuming soy foods and the associated isoflavones may affect levels of prostate-specific antigen in cancer patients and high-risk men but not in healthy men. Additional studies are necessary to further elucidate the relationship between isoflavone intake and the prevention of prostate cancer development or progression.
Cardiovascular Disease
There has been great interest surrounding soy protein and isoflavones and their potential role in reducing risk factors associated with cardiovascular disease. The majority of this interest focuses on the health claim approved by the FDA in 1999 for soy foods and coronary heart disease.18 This claim was based on data showing the cholesterol-lowering effects of soy protein, not isoflavones. Conflicting data have emerged since the FDA-approved health claim for soy protein, and the FDA has announced its intent to reevaluate the evidence in support of the health claim. The American Heart Association (AHA) has acknowledged that soy foods are beneficial to cardiovascular health and overall health because of their high content of polyunsaturated fats, fiber, vitamins, and minerals and low saturated fat content, but concluded that the direct cholesterol-lowering effects of soy protein were of relatively little significance.2 The beneficial cardiovascular health effects of consuming soy foods are likely magnified when these foods are used to replace other dietary proteins that are higher in saturated fat and common in Western diets.
Epidemiologic studies have shown that a higher intake of soy foods is inversely associated with coronary events, such as nonfatal myocardial infarction95,96 and mortality from heart disease.97 Animal studies have also demonstrated the cardioprotective effects of soy. In monkeys, soy protein diets compared with casein diets resulted in significant improvements in lipid profiles and insulin sensitivity and a decrease in arterial lipid peroxidation.98 To date, however, human studies have not confirmed these findings. The cardiovascular benefits of soy may not be limited to reducing elevations of LDL cholesterol. Soy has been investigated for its impact on other cardiovascular disease risk factors with mixed results. Other possible cardiovascular benefits of soy may include its ability to modestly raise high-density lipoprotein (HDL) and lower triglycerides,2,99 improve endothelial function,100,101 and elicit mild antihypertensive effects, which are discussed below.
Lipid-Lowering Effects
In 1995, Anderson et al published a large meta-analysis of 38 controlled studies comparing soy- and animal-protein diets that found a statistically significant 12.9% decrease in serum lipids in the soy group. These changes were most significant in subjects with hypercholesterolemia.102 The intakes of energy, total fat, saturated fat, and cholesterol were similar in the two groups. Subsequent to this meta-analysis, several additional well-controlled studies on the LDL-lowering effect of soy protein have emerged. Meta-analyses that included the newer data show a consistent LDL lowering effect of soy protein; however, the magnitude of the reduction is more modest, averaging 3%.2,99 The AHA science advisory council has concluded that the cholesterol-lowering effect of soy protein is very small relative to the large amount of soy protein intake required to achieve this effect, which averages 50 grams daily. This is about half of the total daily protein intake for an adult. Several studies have tested the effects of soy protein and isoflavones separately, and a meta-analysis of existing data does not support a significant role of isoflavones in the cholesterol-lowering effects of soy protein.103
Hypotensive Effects
Dietary soy may have modest effects on blood pressure (BP). Several studies have evaluated the effect of a variety of isoflavone preparations on BP. One double-blind randomized trial of 40 men and women with mild to moderate hypertension examined the effect of soy milk consumption (isoflavone content unknown) on hypertension. Three months of soy milk consumption resulted in modest decreases in diastolic, systolic, and mean BP values compared with the same values in those subjects who consumed cow’s milk (placebo). Urinary genistein measurements showed a significant association with the decrease in BP, which suggests a hypotensive effect of isoflavones.104 In another study, 213 healthy subjects (108 men and 105 postmenopausal women) aged 50 to 75 years received either soy protein isolate (40 g soy protein, 118 mg isoflavones) or casein placebo for 3 months. A statistically significant decrease in BP in the soy protein group was observed.105 However, several other studies have demonstrated less clinically significant results. In five other studies, reductions in BP were insignificant, with a weighted average reduction of only 1 mm Hg systolic pressure.2 In 2005, the Agency for Health Research and Quality made a careful evaluation of 22 studies and concluded that neither soy protein nor isoflavones had a hypotensive effect,106 and this conclusion has been echoed by the AHA.2 However, a more specific meta-analysis reported the effect of the different soy preparations on BP with varied results. Soy protein isolate significantly reduced diastolic BP (by 1.99 mm Hg), although the effect on systolic BP was not significant. Soy foods or isoflavone supplements did not show a significant reduction in systolic or diastolic BP, but soy foods’ reduction of both systolic and diastolic BP was nearly significant. The data for isoflavone extracts suggest a reduction in systolic BP that was close to significant but showed no effect on diastolic BP.107 Additional research is needed to better understand the relationship between soy and BP, particularly work that focuses on comparing the effects of different soy preparations.
Other Cardiovascular Risk Factors
Limited evidence suggests that soy protein modestly raises serum HDL2,99 and lowers serum triglyceride levels,99 but a comprehensive review concluded that the effect was minimal to nil.2 Several clinical studies have evaluated the effects of different soy preparations on endothelial function and have yielded mixed results. Overall, the evidence suggests a possible improvement in endothelial function with the consumption of soy products,106 with some evidence suggesting that improvements may be more prominent in patients with initially impaired endothelial function108 and higher levels of serum isoflavones.109
Menopause
Isoflavones are classified as phytoestrogens, which has led to much speculation about their ability to act as a natural alternative to hormonal therapy in postmenopausal women. Observational data indicate that Japanese women, who consume 50 to 100 times greater amounts of isoflavones than women consuming Western diets, have a nearly ten-fold lower incidence of vasomotor symptoms, such as hot flashes.110 Over 50 studies have been conducted on various soy and isoflavone preparations (including those derived from non-soy sources) for their ability to reduce symptoms of menopause, primarily hot flashes. Many of these studies demonstrate the ability of isoflavones to diminish the severity and frequency of hot flashes in postmenopausal women, whereas others do not. Systematic reviews of existing data have concluded that isoflavones are efficacious,111–113 whereas other reviews have concluded that they are no better than placebo.114,115 Reviews that have failed to show an effect of isoflavones on hot flashes have not accounted for the potential for the type of soy preparation and relative isoflavone content to influence results. Critical reviews of studies that used interventions with well characterized isoflavone content have concluded that the consumption of genistein, rather than total isoflavones, is responsible for reducing menopausal hot flashes and that 15 mg of genistein is minimally required to achieve benefit.113
Conflicting results from a large number of clinical trials that investigated the potential influence of isoflavones on menopausal hot flashes make it difficult to draw firm conclusions. The heterogeneity among trial results may be related to baseline frequency of hot flashes,111,116 individually varying metabolism of isoflavones, and the genistein content of the intervention.117 Overall, it appears that daily consumption of as little as 30 mg isoflavones (containing at least 15 mg genistein), in soy protein or as a dietary supplement may reduce hot flashes by as much as 50%. A 50% reduction in hot flashes accounts for a 30% placebo effect and a 20% benefit of isoflavones over placebo, which results in clinically relevant improvements in quality of life. Efficacy increases with increasing dose, up to 100 mg isoflavone daily; the benefit is greatest in women with the highest number of baseline hot flashes and when isoflavones are consumed in divided doses throughout the day.112 In considering reports suggesting that hormone replacement therapy may be associated with negative side effects, health professionals are justified in recommending 50 to 100 mg isoflavones (with a minimum of 15 mg genistein) to women for relief of menopausal hot flashes. The efficacy of the treatment can be individually determined, and the benefits should be evident within 4 weeks.118
Osteoporosis
A large body of research has investigated the impact of isoflavones on various markers of bone health, including bone mineral density (BMD) and markers of bone formation and resorption. A meta-analysis of randomized controlled trials concluded that there is evidence demonstrating that isoflavone intervention attenuates bone loss of the spine and decreases markers of bone resorption while increasing markers of bone formation in menopausal women.119 Similar results have been shown in postmenopausal women.120,121 Importantly, evidence indicates that soy protein and isoflavone-poor soy protein do not improve markers of bone health, suggesting that isoflavones are the constituents responsible for the beneficial effects and that soy protein may negate the improvements on the relevant markers.5
A 2010 meta-analysis conducted to explore the effect of ingesting soy isoflavone extracts on BMD in menopausal women included 11, 7, 5, and 5 randomized controlled trials for estimating the effects on spine, femoral neck, hip total, and trochanter BMD respectively. The analysis included data from 1240 menopausal women and showed that an average intake of 82 mg (47 to 150 mg) isoflavones for 6 to 12 months significantly increased spine BMD by 22.25 mg/cm2 (2.38%) compared with controls. Varying effects among all the studies appeared to be dependent on intervention duration, region of participation (Asian versus Western) and baseline BMD. No significant effects were found for areas of the hip. Overall, the data suggest a positive role for isoflavones in the prevention of bone loss, but more human trials are needed to address factors affecting the magnitude of this effect, and to determine why results have been generally more favorable in the spine and not in the hip.122
Cognitive Function
Epidemiologic studies have associated decreased brain size and memory with tofu consumption.123,124 On the other hand, epidemiologic studies also point to lower rates of dementia in Asian populations.125
Human studies investigating the effect of isoflavones on cognitive function have reported beneficial effects.126 Long-term administration of soy or isolated isoflavones for menopausal support has also been found to improve learning, logical thinking, and planning ability in postmenopausal women.127,128 Other studies have demonstrated that isoflavone consumption enhances spatial working memory in men129 and immediate and long-term episodic memory and mental flexibility in men and women.130 Although the association between tofu consumption and reduced brain size in men raises interesting questions, clinical studies suggest that soy foods and their constituents may enhance cognitive function and have not been shown to be detrimental to central nervous system function.
Safety Considerations
The estrogen-like properties of isoflavones proposed to be responsible for several health benefits have also raised questions about potential adverse health effects at specific stages of life. The primary concern is the potential risk of isoflavone exposure to estrogen-sensitive breast cancer patients and women at high risk of developing the disease. In vitro and animal studies have shown growth-promoting effects of genistein on mammary tumors.131 However, there are many limitations to drawing conclusions from these experimental models.132 The available human research, including both clinical and epidemiologic evidence, is supportive of safety. In fact, a large epidemiologic study in China showed that, among women with breast cancer, soy-food consumption was significantly associated with decreased risk of recurrence and death. This population-based cohort study of 5033 breast cancer patients followed for over 3.9 years showed that women in the fourth quartile of soy intake experienced a reduced risk of tumor recurrence and lower total mortality.133 Previous epidemiologic studies have demonstrated similar results,134,135 and clinicians with expertise in nutrition have concluded that in light of the in vitro experiments together with the epidemiologic observations and clinical experience, isoflavones can be considered to be safe compounds and their consumption as whole soy foods to be safe for breast cancer patients.132,136 Additional data on the impact of soy-food and isoflavone consumption on breast cancer prognosis are necessary before these nutrients should be recommended in the treatment of breast cancer.
Like the breast, the endometrium is a hormone-dependent tissue that has estrogen receptors. Estrogen-only therapy is known to increase endometrial cancer risk.137 The cumulative data providing evidence related to isoflavone intake on risk markers for endometrial cancer have not demonstrated clinically significant changes in endometrial histology that would support the hypothesis of risk associated with isoflavone intake.74,115
Isoflavones act as weak competitive inhibitors of thyroid peroxidase, which converts triiodothyronine (T3) to thyroxine (T4).138 This mechanism has led to concerns that isoflavone consumption may exacerbate thyroid dysfunction in subjects with subclinical hypothyroid disease. All polyphenols from fruits and vegetables are inhibitors of thyroid peroxidase, many with more potent inhibition than soy isoflavones.139 It is possible that in individuals with very low iodine intake, polyphenol-induced reductions in thyroid peroxidase could result in a low production of thyroid hormones; however, attempts to demonstrate thyroid deficiency in consumers of soy or isoflavones have failed. A comprehensive review concluded on the basis of 14 clinical trials that in euthyroid individuals consuming sufficient iodine, there is no evidence that isoflavones exposure affects thyroid function.140 Iodine-deficient individuals should be advised to consume the recommended amount of iodine and not to avoid fruit and vegetable polyphenols such as isoflavones.
 Drug-Nutrient Interactions
Drug-Nutrient Interactions
Dietary supplementation of soy protein concurrent with oral levothyroxine therapy appears to decrease absorption of levothyroxine.141 This effect can be mitigated by separated dosing.
In a model of estrogen receptor–positive postmenopausal breast cancer, lower doses of genistein have been shown to inhibit the therapeutic effects of tamoxifen therapy.142 An animal study demonstrated that tamoxifen suppression of breast tumor growth in ovariectomized athymic mice was negated by the administration of 1000 ppm dietary genistein.143 Therefore, it has been proposed that postmenopausal women undergoing tamoxifen therapy for estrogen-responsive breast cancer should use caution in consuming dietary genistein. In contrast, other in vitro studies showed that isolated genistein, as well as an isoflavone-rich soy concentrate, markedly inhibits tumor growth and enhances rather than inhibits the efficacy of tamoxifen.144,145 In addition, the largest epidemiologic study of breast cancer survivors specifically designed to evaluate the influence of soy-food intake on breast cancer outcomes found no evidence that isoflavones inhibited the efficacy of tamoxifen.133 Despite conflicting evidence, the totality of the data suggest that isoflavones may have a synergistic or additive effect to that of tamoxifen in patients with breast cancer. However, until more clinical data become available, patients should be advised that a moderate intake of soy foods is safe and potentially beneficial, whereas the therapeutic use of isolated isoflavones is not advised for women being treated for breast cancer with tamoxifen.
1. Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140:1350S–1354S.
2. Sacks F.M., Lichtenstein A., Van Horn L., et al. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044.
3. Klein M.A., Nahin R.L., Messina M.J., et al. Guidance from an NIH workshop on designing, implementing, and reporting clinical studies of soy interventions. J Nutr. 2010;140:1192S–1204S.
4. Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8:89–98.
5. Xiao C.W. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S–1249S.
6. Xu X., Harris K.S., Wang H.J., et al. Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995;125:2307–2315.
7. Setchell K.D., Brown N.M., Desai P., et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S.
8. Setchell K.D., Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–1362S.
9. Kano M., Takayanagi T., Harada K., et al. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–2296.
10. Hutchins A.M., Slavin J.L., Lampe J.W. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. J Am Diet Assoc. 1995;95:545–551.
11. Izumi T., Piskula M.K., Osawa S., et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–1699.
12. Tsangalis D., Wilcox G., Shah N.P., et al. Bioavailability of isoflavone phytoestrogens in postmenopausal women consuming soya milk fermented with probiotic bifidobacteria. Br J Nutr. 2005;93:867–877.
13. Zubik L., Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003;77:1459–1465.
14. Somekawa Y., Chiguchi M., Ishibashi T., et al. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97:109–115.
15. Wakai K., Egami I., Kato K., et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33:139–145.
16. Setchell K.D., Zimmer-Nechemias L., Cai J., et al. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27.
17. Chun O.K., Chung S.J., Song W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–1252.
18. 21 CFR Part 101. Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease. Federal Register. 1999;64:57699–57733.
19. Reinli K., Block G. Phytoestrogen content of foods: a compendium of literature values. Nutr Cancer. 1996;26:123–148.
20. Horn-Ross P.L., Barnes S., Lee M., et al. Assessing phytoestrogen exposure in epidemiologic studies: development of a database (United States). Cancer Causes Control. 2000;11:289–298.
21. Kang J., Badger T.M., Ronis M.J., et al. Non-isoflavone Phytochemicals in Soy and Their Health Effects. J Agric Food Chem. 2010;58:8119–8133.
22. Kennedy A.R. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am J Clin Nutr. 1998;68:1406S–1412S.
23. Yavelow J., Finlay T.H., Kennedy A.R., et al. Bowman-Birk soybean protease inhibitor as an anticarcinogen. Cancer Res. 1983;43:S2454–S2459.
24. Hsieh C.C., Hernández-Ledesma B., Jeong H.J., et al. Complementary roles in cancer prevention: protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLos One. 2010;5:e8890.
25. Adlercreutz H., Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120.
26. Knight D.C., Eden J.A. A review of the clinical effects of phytoestrogens. Obstet Gynecol. 1996;87:897–904.
27. Harbone J.B., Baxter H. Phytochemical dictionary. Basingstoke, England: Burgess Science Press, 1995.
28. Power K.A., Thompson L.U. Can the combination of flaxseed and its lignans with soy and its isoflavones reduce the growth stimulatory effect of soy and its isoflavones on established breast cancer? Mol Nutr Food Res. 2007;51:845–856.
29. 21 CFR Part 101. Food Labeling: Health Claims; Plant Sterol/Stanol Esters and Coronary Heart Disease Federal Register. 2000;65:54685–54739.
30. Elias R., De Meo M., Vidal-Ollivier E., et al. Antimutagenic activity of some saponins isolated from Calendula officinalis L., C. arvensis L. and Hedera helix L. Mutagenesis. 1990;5:327–331.
31. Cohn J.S., Kamili A., Wat E., et al. Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atheroscler Suppl. 2010;11:45–48.
32. Oakenfull D. Soy protein, saponins and plasma cholesterol. J Nutr. 2001;131:2971–2972.
33. Graf E., Eaton J.W. Dietary suppression of colonic cancer: fiber or phytate? Cancer. 1985;56:717–718.
34. Vucenik I., Tomazic V.J., Fabian D., et al. Antitumor activity of phytic acid (inositol hexaphosphate) in murine transplanted and metastatic fibrosarcoma, a pilot study. Cancer Lett. 1992;65:9–13.
35. MacRae W.D., Hudson J.B., Towers G.H. The antiviral action of lignans. Planta Med. 1989;55:531–535.
36. Hirano T., Fukuoka K., Oka K., et al. Antiproliferative activity of mammalian lignan derivatives against the human breast carcinoma cell line, ZR-75-1. Cancer Invest. 1990;8:595–602.
37. Hartman P.E., Shankel D.M. Antimutagens and anticarcinogens: a survey of putative interceptor molecules. Environ Mol Mutagen. 1990;15:145–182.
38. Hirano T., Gotoh M., Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55:1061–1069.
39. Naim M., Gestetner B., Zilkah S., et al. Soybean isoflavones: characterization, determination, and antifungal activity. J Agric Food Chem. 1974;22:806–810.
40. Brzezinski A., Debi A. Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol. 1999;85:47–51.
41. Jha H.C., von Recklinghausen G., Zilliken F. Inhibition of in vitro microsomal lipid peroxidation by isoflavonoids. Biochem Pharmacol. 1985;34:1367–1369.
42. Wei H., Wei L., Frenkel K., et al. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20:1–12.
43. Wu E.S., Loch J.T., 3rd., Toder B.H., et al. Synthesis, biological activities, and conformational analysis of isoflavone derivatives and related compounds. J Med Chem. 1992;18:3519–3525.
44. Hirano T., Oka K., Akiba M. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75-1. Res Commun Chem Pathol Pharmacol. 1989;64:69–78.
45. Badger T.M., Gilchrist J.M., Pivik, et al. The health implications of soy infant formula. Am J Clin Nutr. 2009;89:1668S–1672S.
46. Setchell K.D., Brown N.M., Desai P., et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 1998;131:1362S–1375S.
47. Genovese M.I., Lajolo F.M. Isoflavones in soy-based foods consumed in Brazil: levels, distribution, and estimated intake. J Agric Food Chem. 2002;50:5987–5993.
48. Draft NTP brief on soy infant formula. National Toxicology Program. Available at http://cerhr.niehs.nih.gov/evals/genistein-soy/SoyFormulaUpdt/DraftNTPBriefSoyFormula16Mar2010_508.pdf
49. Cao Y.A., Calafat A.M., Doerge, et al. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol. 2009;19:223–234.
50. Sheehan D.M. Isoflavone content of breast milk and soy formulas: benefits and risks. Clin Chem. 1997;43:850.
51. Kuiper G.G., Lemmen J.G., Carlsson B., et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263.
52. Kuiper G.G., Carlsson B., Grandien K., et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870.
53. Adlercreutz H., van der Wildt J., Kinzel J., et al. Lignan and isoflavonoid conjugates in human urine. J Steroid Biochem Mol Biol. 1995;52:97–103.
54. Cassidy A., Bingham S., Setchell K.D. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–340.
55. Petrakis N.L., Barnes S., King E.B., et al. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–794.
56. Hooper L., Madhavan G., Tice J.A., et al. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745–760.
57. Baird D.D., Umbach D.M., Lansdell L., et al. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab. 1995;80:1685–1690.
58. Hooper L., Ryder J.J., Kurzer M.S., et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440.
59. Reiter E., Beck V., Medjakovic S., et al. Isoflavones are safe compounds for therapeutical applications: evaluation of in vitro data. Gynecol Endocrinol. 2009;25:554–580.
60. Akiyama T., Ishida J., Nakagawa S., et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5599.
61. Patel R.P., Boersma B.J., Crawford J.H., et al. Antioxidant mechanisms of isoflavones in lipid systems: paradoxical effects of peroxyl radical scavenging. Free Radic Biol Med. 2001;31:1570–1581.
62. Kim S., Shin H.J., Kim S.Y., et al. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol cell Endocrinol. 2004;220:51–58.
63. Divi R.L., Chang H.C., Doerge D.R. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54:1087–1096.
64. Brooks J.D., Thompson L.U. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94:461–467.
65. Barnes S., Peterson T.G., Coward L. Rationale for the use of genistein-containing soy matrices in chemoprevention trials for breast and prostate cancer. J Cell Biochem. 1995;22:S181–S187.
66. Fotsis T., Pepper M., Adlercreutz H., et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–2694.
67. Martin M.E., Haourigui M., Pelissero C., et al. Interactions between phytoestrogens and human sex steroid binding protein. Life Sci. 1996;58:429–436.
68. Wilcox J.N., Blumenthal B.F. Thrombotic mechanisms in atherosclerosis: potential impact of soy proteins. J Nutr. 1995;125:S631–S638.
69. Potter S.M. Soy protein and serum lipids. Curr Opin Lipidol. 1996;7:260–264.
70. Ajdzanović V., Spasojević I., Filipović B., et al. Effects of genistein and daidzein on erythrocyte membrane fluidity: an electron paramagnetic resonance study. Can J Physiol Pharmacol. 2010;88:497–500.
71. Messina M.J., Persky V., Setchell K.D., et al. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131.
72. Nagata C., Takatsuka N., Kawakami N., et al. A prospective cohort study of soy product intake and stomach cancer deaths. Br J Cancer. 2002;87:31–36.
73. Yan L., Spitznagel E.L., Bosland M.C. Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:148–158.
74. Myung S.K., Ju W., Choi H.J., et al. Soy intake and risk of endocrine-related gynaecological cancer: a meta-analysis. BJOG. 2009;116:1697–1705.
75. Adlercreutz H., Honjo H., Higashi A., et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–1100.
76. Morton M.S., Arisaka O., Miyake N., et al. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–3171.
77. Barnes S., Peterson T.G. Biochemical targets of the isoflavone genistein in tumor cell lines. Proc Soc Exp Biol Med. 1995;208:103–108.
78. Shu X.O., Jin F., Dai Q., et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–488.
79. Wu A.H., Koh W.P., Wang R., et al. Soy intake and breast cancer risk in Singapore Chinese Health Study. Bri J Can. 2008;99:196–200.
80. Shu X.O., Jin F., Dai Q., et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers and Prev. 2001;10:483–488.
81. Wu A.H., Yu M.C., Tseng C.C., et al. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14.
82. Yamamoto S., Sobue T., Kobayashi M., et al. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906–913.
83. Travis R.C., Allen N.E., Appleby P.N., et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705–710.
84. Nishio K., Niwa Y., Toyoshima H., et al. Consumption of soy foods and the risk of breast cancer: findings from the Japan Collaborative Cohort (JACC) Study. Cancer Causes Control. 2007;18:801–808.
85. Nagata Y., Sonoda T., Mori M., et al. Dietary isoflavones may protect against prostate cancer in Japanese men. Nagata Journal of Nutr. 2007;137:1974–1979.
86. Adlercreutz H., Markkanen H., Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210.
87. Sonoda T., Nagata Y., Mori M., et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95:238–242.
88. Allen N.E., Sauvaget C., Roddam A.W., et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Controls. 2004;15:911–920.
89. Xiang H., Schevzov G., Gunning P., et al. A comparative study of growth-inhibitory effects of isoflavones and their metabolites on human breast and prostate cancer cell lines. Nutr Cancer. 2002;42:224–232.
90. Shen J.C., Klein R.D., Wei Q., et al. Low-dose genistein induces cyclin-dependent kinase inhibitors and G(1) cell-cycle arrest in human prostate cancer cells. Mol Carcinog. 2000;29:92–102.
91. Kwan W., Duncan G., Van Patten C., et al. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. 2010;62:198–207.
92. Dalais F.S., Meliala A., Wattanapenpaiboon N., et al. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64:510–515.
93. Kumar N.B., Cantor A., Allen K., et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59:141–147.
94. Jenkins D.J., Kendall C.W., D’Costa M.A., et al. Soy consumption and phytoestrogens: effect on serum prostate specific antigen when blood lipids and oxidized low-density lipoprotein are reduced in hyperlipidemic men. J Urol. 2003;169:507–511.
95. Zhang X., Shu X.O., Gao Y.T., et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–2878.
96. Sasazuki S. ., Fukuoka Heart Study Group. Case-control study of nonfatal myocardial infarction in relation to selected foods in Japanese men and women. Jpn Circ J. 2001;65:200–206.
97. Nagata C. Ecological study of the association between soy product intake and mortality from cancer and heart disease in Japan. Int J Epidemiol. 2000;29:832–836.
98. Wagner J.D., Cefalu W.T., Anthony M.S., et al. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesteryl ester content in ovariectomized cynomolgus monkeys. Metabolism. 1997;46:698–705.
99. Zhan S., Ho S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81:397–408.
100. Squadrito F., Altavilla D., Crisafulli A., et al. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med. 2003;114:470–476.
101. Squadrito F., Altavilla D., Morabito N., et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–347.
102. Anderson J.W., Johnstone B.M., Cook-Newell M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–282.
103. Weggemans R.M., Trautwein E.A. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr. 2003;57:940–946.
104. Rivas M., Garay R.P., Escanero J.F., et al. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr. 2002;132:1900–1902.
105. Teede H.J., Dalais F.S., Kotsopoulos D., et al. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060.
106. Balk E., Chung M., Chew et al., et al Effects of Soy on Health Outcomes . Evidence Report/Technology Assessment No. 126. (Prepared by Tufts-New England Medical Center Evidence-based Practice Center under Contract No. 290-02-0022.) AHRQ Publication No. 05-E024-2. Rockville, MD: Agency for Healthcare Research and Quality; August 2005.
107. Hooper L., Kroon P.A., Rimm E.B., et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50.
108. Chan Y.H., Lau K.K., Yiu K.H., et al. Reduction of C-reactive protein with isoflavone supplement reverses endothelial dysfunction in patients with ischaemic stroke. Eur Heart J. 2008;29:2800–2807.
109. Hall W.L., Formanuik N.L., Harnpanich D., et al. A meal enriched with soy isoflavones increases nitric oxide-mediated vasodilation in healthy postmenopausal women. J Nutr. 2008;138:1288–1292.
110. Brzezinski A., Adlercreutz H., Shaoul R., et al. Short-term effects of phytoestrogens-rich diet on postmenopausal women. Menopause. 1997;4:89–94.
111. Howes L.G., Howes J.B., Knight D.C. Isoflavone therapy for menopausal flushes: a systematic review and meta-analysis. Maturitas. 2006;55:203–211.
112. Kurzer M.S. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology. 2008;16:227–229.
113. Williamson-Hughes P.S., Flickinger B.D., Messina M.J., et al. Isoflavone supplements containing predominantly genistein reduce hot flash symptoms: a critical review of published studies. Menopause. 2006;13:831–839.
114. Nelson H.D., Vesco K.K., Haney E., et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071.
115. Lethaby A., Marjoribanks J., Kronenberg F., et al. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database of Systematic Reviews. (Issue 4):2007. Art. No.: CD001395 http://dx.doi.org/10.1002/14651858.CD001395.pub3
116. Messina M., Hughes C. Efficacy of soyfoods and soybean isoflavone supplements for alleviating menopausal symptoms is positively related to initial hot flush frequency. J Med Food. 2003;6:1–11.
117. Messina M. Investigating the optimal soy protein and isoflavone intakes for women: a perspective. Women’s Health (Lond Engl). 2008;4:337–356.
118. Messina M. Isoflavones and Hot Flush Relief. Historical overview and Conclusions from Published Analysis. Symposium on Evaluating the Efficacy and Safety of Isoflavones for Postmenopausal Women. Presented on May 14, 2009; Milan, Italy.
119. Ma D.F., Qin L.Q., Wang P.Y., et al. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr. 2008;27:57–64.
120. Marini H., Minutoli L., Polito F., et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–847.
121. Morabito N., Crisafulli A., Vergara C., et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–1912.
122. Taku K., Melby M.K., Takebayashi J., et al. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2010;19:33–42.
123. Hogervorst E., Sadjimim T., Yesufu A., et al. High tofu intake is associated with worse memory in elderly Indonesian men and women. Dement Geriatr Cogn Disord. 2008;26:50–57.
124. White L.R., Petrowitch H., Ross G.W., et al. Brain aging and midlife tofu consumption. J Am Coll Nutr. 2000;19:242–255.
125. White L., Petrovitch H., Ross G.W., et al. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia Aging Study. JAMA. 1996;276:955–960.
126. Karvaj M., Beno P., Fedor-Freybergh P.G. Positive effect of flavonoids to cardiovascular and central nervous system. Neuro Endocrinol Lett. 2007;28:1–3.
127. Duffy R., Wiseman H., File S.E. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. 2003;75:721–729.
128. Kritz-Silverstein D., Von Mühlen D., Barrett-Connor E., et al. Isoflavones and cognitive function in older women: the SOy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause. 2003;10:196–202.
129. Thorp A.A., Sinn N., Buckley J.D., et al. Soya isoflavone supplementation enhances spatial working memory in men. Brit J Nutr. 2009;102:1348–1354.
130. File S.E., Jarrett N., Fluck E., et al. Eating soya improves human memory. Psychopharmacology (Berl). 2001;157:430–436.
131. Helferich W.G., Andrade J.E., Hoagland M.S. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–226.
132. Messina M., Abrams D.I., Hardy M. Can clinicians now assure their breast cancer patients that soyfoods are safe? Women’s Health (Lond Engl). 2010;6:335–338.
133. Shu X.O., Zheng Y., Cai H., et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443.
134. Boyapati S.M., Shu X.O., Ruan Z.X., et al. Soyfood intake and breast cancer survival: a follow up. Breast Cancer Res Treat. 2005;92:11–17.
135. Guha N., Kwan M.L., Quesenberry C.P., Jr., et al. Soy isoflavones and risk of cancer of the Shanghai Breast Cancer Study. Recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395–405.
136. Reiter E., Reiter E., Beck V., et al. Isoflavones are safe compounds for therapeutical applications: evaluation of in vitro data. Gynecol Endocrinol. 2009;25:554–580.
137. Gennari L., Merlotti D., Valleggi F., et al. Selective estrogen receptor modulators for postmenopausal osteoporosis: current state of development. Drugs Aging. 2007;24:361–379.
138. Divi R.L., Chang H.C., Doerge D.R. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54:1087–1096.
139. Divi R.L., Doerge D.R. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9:16–23.
140. Messina M., Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–258.
141. Bell D.S., Ovalle F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 2001;7:193–194.
142. Jones J.L., Daley B.J., Enderson B.L., et al. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am Surg. 2002;68:575–577.
143. Ju Y.H., Doerge D.R., Allred K.F., et al. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477.
144. Mai Z., Blackburn G.L., Zhou J.R. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis. 2007;28:1217–1223.
145. Tanos V., Brzezinski A., Drize O., et al. Synergistic inhibitory effects of genistein and tamoxifen on human dysplastic and malignant epithelial breast cells in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;102:188–194.