Chapter 2. Soft tissues of the musculoskeletal system
CHAPTER CONTENTS
CONNECTIVE TISSUE
The connective tissues form a large class of tissues responsible for providing tensile strength, substance, elasticity and density to the body, as well as facilitating nourishment and defence. Connective tissue has a major role in repair following trauma and a mechanical role in providing connection and leverage for movement, as well as preventing friction, pressure and shock between mobile structures. Connective tissue is the main focus of treatment procedures in orthopaedic medicine.
Connective tissue consists primarily of cells embedded in an extracellular matrix which is composed of fibres and an interfibrillar component – the amorphous ground substance (Fig. 2.1). The synthesis, degradation and maintenance of the matrix depends on the cells within it (September et al 2007).
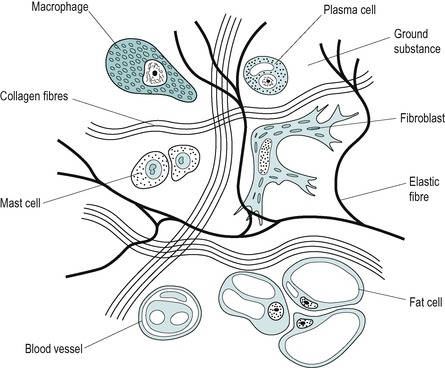 |
| Figure 2.1
Irregular connective tissue, cellular and fibre content.
Adapted from Cormack D ‘Ham’s Histology’ © Lippincott William & Wilkins (1987) with permission.
|
Not all types of connective tissue cell are found in each tissue, and the cell content can alter, with some cells being resident in the tissue and others brought to specific areas at times of need. Generally, the cells make up approximately 20% of the tissue volume and mainly consist of fibroblasts, macrophages and mast cells.
Connective tissue cells
Fibroblasts
Fibroblasts, usually the most abundant of the connective tissue cells, are responsible for producing the contents of the extracellular matrix, namely fibres and amorphous ground substance. They are found lying close to the bundles of fibres they produce and are closely related to chondroblasts and osteoblasts, the cells responsible for producing cartilage and bone matrix. The less active mature fibroblasts are known as fibrocytes.
Since fibroblasts produce the contents of the extra-cellular matrix, they play a key role in the repair process after injury. Stearns (1940b) observed that once fibre formation was initiated the fibroblast was able to produce an extensive network of fibrils in a remarkably short time.
Myofibroblasts are specialized cells which contain contractile filaments producing similar properties to smooth muscle cells. They assist wound closure after injury.
Macrophages (histiocytes or mononuclear cells)
Macrophages may be resident in the connective tissues or circulating as monocytes which migrate to an area of injury and modulate into tissue macrophages (Fowler 1989). They are large cells that have two important roles. The first is phagocytosis and the second is to act as director cells, in which role they have a considerable influence on scar formation (Hardy 1989).
As a phagocyte, the macrophage acts as a housekeeper to the wound, ingesting cellular debris and subjecting it to lysosomal hydrolysis, thus debriding the wound in preparation for the fibroblasts to begin the repair process. Matter such as bacteria and cellular debris is engulfed by the phagocyte on contact.
As a director cell, the macrophage chemically activates the number of fibroblasts required for the repair process. They also play an important role in muscle regeneration leading to increased satellite cell differentiation and muscle fibre proliferation (Grefte et al 2007). A reduced number of macrophages in muscle tissue has been shown to lead to reduced muscle regeneration (Shen et al 2008). Corticosteroids can inhibit the function of the macrophage in the early inflammatory stage, resulting in a delay in fibre production (Dingman 1973, Leibovich & Ross 1974, Fowler 1989). This should be taken into consideration when exploring treatment options in the early stage.
Treatment techniques that agitate tissue fluid increase the chance contact of the macrophage with debris and can be applied during the early stages of inflammation to promote phagocytosis (Evans 1980), e.g. gentle transverse frictions and grade A mobilization (see Ch. 4) as well as heat, ice, ultrasound, pulsed electromagnetic energy, etc.
Mast cells
Mast cells are large, round cells containing secretory granules that manufacture a number of active ingredients including heparin, histamine and possibly serotonin. The contents of the mast cell granules are released in response to mechanical or chemical trauma and they therefore play a role in the early stages of inflammation. Heparin temporarily prevents coagulation of the excess tissue fluid and blood components in the injured area while histamine causes a brief vasodilatation in the neighbouring non-injured area (Wilkerson 1985, Hardy 1989). Serotonins are internal nociceptive substances released during platelet aggregation in response to tissue damage. They cause contraction of blood vessels and activate pain signals (Kapit et al 1987).
Extracellular matrix
The extracellular matrix accounts for about 80% of the total tissue volume, with approximately 30% of its substance being solids and the remaining 70% being water. It consists of fibres and the interfibrillar amorphous ground substance, the substances responsible for supporting and nourishing the cells. The amorphous ground substance also determines the connective tissue’s compliance, mobility and integrity.
The fibrous portion of connective tissue is responsible for determining the tissue’s biomechanical properties. Two major groups of fibres exist: collagen fibres and elastic fibres.
Collagen fibres
Collagen is a protein in the form of fibre and is the body’s ‘glue’. It possesses two major properties, great tensile strength and relative inextensibility, and forms the major fibrous component of connective tissue structures, i.e. tendons, ligaments, fascia, sheaths, bursae, bone and cartilage.
Individual collagen fibres are normally mobile within the amorphous ground substance, producing discrete shear and gliding movement as well as dealing with compression and tension. Collagen is also the main constituent of scar tissue, in which it demonstrates its great versatility by attempting to mimic the structure it replaces.
Collagen fibres are large in diameter and appear to be white in colour. They are arranged in bundles and do not branch or anastomose. They are flexible but inelastic individually. The arrangement and weave of individual collagen fibres and collagen fibre bundles give the connective tissue structure elastic qualities; for example, an individual piece of nylon thread is inelastic, but when woven to produce tights, the weave gives the material elasticity (Peacock 1966). Collagen fibre bundles elongate under tension to their physiological length and recoil when tension is released.
The bundles of fibres are laid down parallel to the lines of the main mechanical stress, often in a wavy, sinusoidal or undulating configuration. This gives the tissue an element of crimp when not under tension. Crimp provides a buffer so that longitudinal elongation can occur without damage, as well as acting as a shock absorber along the length of the tissue, to control tension (Amiel et al 1990).
Collagen fibres are strong under tensile loading but weak under compressive forces, when they have a tendency to buckle. The orientation of the collagen fibres determines the properties that a structure will have. Crimp patterns are dependent upon function and therefore differ in different tissues, i.e. the arrangement of collagen fibres perpendicular to the surface in articular cartilage provides a cushioning force for weight-bearing, while the parallel arrangement in tendons provides great tensile strength for transmitting loads and resisting pull. Crimp patterns may also vary between different ligaments and different tendons.
Production and structure of collagen fibres and collagen cross-linking
Procollagen, the first step in collagen fibre formation, is produced intracellularly by the fibroblast. Amino acids are assembled to form polypeptide chains, which are attracted and held together by weak intramolecular hydrogen bonds known as cross-links (Fig. 2.2). Three polypeptide chains bond to form a procollagen molecule in the form of a triple helix which is exocytosed by the fibroblast into the extracellular space.
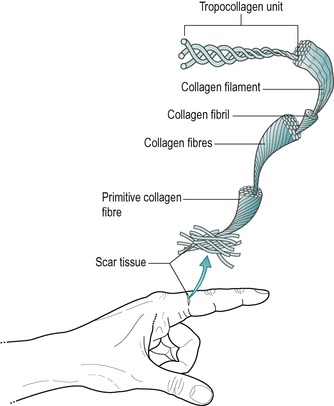 |
| Figure 2.2
Collagen aggregation.
Reprinted from Hardy M The Biology of Scar Formation. Physical Therapy (1989) 69 (12): 1014–1024, with permission.
|
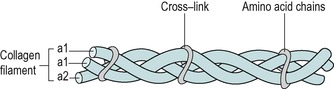
Once outside the cell the chains are known as tropo-collagen molecules and several tropocollagen molecules become bonded by intermolecular cross-links to form a filament or microfibril (Fig. 2.3). With the maturation into tropocollagen, the cross-links are stronger covalent bonds and occur at specific nodal intercept points, making the structure more stable (Nimni 1980, Donatelli & Owens-Burkhart 1981, Hardy 1989). Cross-links exist at every level of organization of collagen acting to weld the units together into a rope-like structure (Fig. 2.4). Intermolecular cross-linking in particular gives collagen its great tensile strength as it matures. The greater the intermolecular cross-linking, the stronger the collagen structure, with bone being considered to be the most highly cross-linked tissue (Hardy 1989).
 |
| Figure 2.3
Intramolecular cross-links.
Reprinted from Hardy M The Biology of Scar Formation. Physical Therapy (1989) 69 (12): 1014–1024, with permission.
|
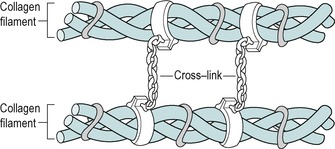 |
| Figure 2.4
Intermolecular cross-links.
Reprinted from Hardy M The Biology of Scar Formation. Physical Therapy (1989) 69 (12): 1014–1024, with permission.
|
Collagen turnover, the dynamic state of the tissue, may be related to the number of cross-links with fibres being continuously and simultaneously produced and broken down. When collagen production exceeds breakdown, more cross-links develop and the structure resists stretching. If collagen breakdown exceeds production there is a reduction in the number of cross-links and the structure stretches more easily (Alter 2000). Immature collagen tissue possesses reducible cross-links and as collagen matures these reducible links stabilize to form stronger non-reducible cross-links. Excessive cross-link formation can be prevented in immature scar tissue by the application of transverse frictions and graded mobilization techniques. Established cross-links in adhesive scar tissue are mobilized by transverse frictions before the longitudinal orientation of the fibres can be encouraged through the application of graded stress. In some instances manipulative rupture of adhesive scar tissue is indicated (see Ch. 3).
Many microfibrils make up a collagen fibril and many collagen fibrils make up a collagen fibre. Collagen fibres continue to aggregate together into larger and larger bundles and the production, aggregation and orientation of collagen are strongly influenced by mechanical tension and stress. Bundles of collagen are arranged in a specific pattern to accommodate to the function of each individual connective tissue structure (Chamberlain 1982).
It has been shown that when fibroblasts grown in tissue culture are subjected to regional tension, the cells exposed to the tensile forces multiply more rapidly and orient themselves in parallel lines in the direction of the tension (Le Gros Clark 1965).
Stearns (1940a, 1940b) identified that internal and external mechanical factors influence fibre orientation. Cell movement and occasional cytoplasmic retraction produced early local orientation of fibres, while a period of secondary orientation of fibres into heavy parallel layers was probably the result of external mechanical factors. This secondary orientation of fibres appeared to take place during the remodelling phase of wound healing and is the way in which soft tissue structures develop in response to intermittent stress and mechanical tension.
The influence of mechanical stress and tension on collagen alignment can be used to advantage in orthopaedic medicine during the repair process, when collagen fibres are initially laid down in the early repair phase and in the later remodelling phase of healing. In order to promote tissue gliding and to regain tissue length, the use of graded mobilization techniques is advocated.
Immobilization produces rapid changes of collagen tissue as it adapts to its new resting length. Collagen which develops in the absence of mechanical stress (i.e. in the absence of movement) has a random orientation, a change in the numbers and thickness of the fibres and loss of ground substance. This reduction in the lubricating interfibular gel allows greater adherence at the fibre–fibre interface (Hardy & Woodall 1998).
Collagen takes on many forms and functions. In tendons it is tough and inelastic, in cartilage it is resilient, while in bone it is hard. This difference in structure is related to the diameter, orientation and concentration of the fibres. Collagen fibres have been classified into groups (Nimni 1980). The most common form is type I collagen consisting of large-diameter fibres, found abundantly in structures subjected to tensile forces. Type II collagen consists of a mixture of large- and narrow-diameter fibres and is abundant in structures subjected to pressure or compressive forces. Reticulin is considered to be a delicate supporting network of fragile type III collagen fibres; it may be present in the earliest stages of soft tissue repair.
Elastic fibres
Elastic fibres, consisting of the protein elastin, are yellow in colour and much thinner and less wavy than collagen fibres. Elastic fibres run singly, never in bundles, and freely branch and anastomose.
Elastic fibres provide the tissue with extensibility so that it can be extended in all directions but if tension is constantly exerted in one direction the elastic fibres may be laid down in sheets known as lamellae, e.g. ligamentum flavum (the ‘yellow ligament’). Elastic fibres make up some of the connective tissue fibres of ligaments, joint capsules, fascia and connective tissue sheaths.
Amorphous ground substance
The connective tissue extracellular matrix comprises the interfibrillar amorphous ground substance, with its fibrous content. As well as maintaining the mobility and integrity of the tissue structure at a macrostructural level, the amorphous ground substance is responsible for nourishing the living cells by facilitating the diffusion of gases, nutrients and waste products between the cells and capillaries.
It contains carbohydrate bound to protein (Standring 2009). The carbohydrate is in the form of polysaccharides, hexuronic acid and amino sugars, alternately linked to form long-chain molecules called glycosaminoglycans (GAGs). The main GAGs in connective tissue matrix are hyaluronic acid, chondroitin-4-sulphate, chondroitin-6-sulphate and dermatan sulphate (Donatelli & Owens-Burkhart 1981).
When GAGs are covalently bonded to proteins, the molecules are called proteoglycans (Cormack 1987). These proteoglycan molecules have the property of attracting and retaining water (Bogduk 2005). This hydration of structures depends on the proportion of proteoglycans and the flow of water into the extracellular matrix. Increased hydration creates rigidity in the extracellular matrix, allowing it to exist as a semisolid substance or gel, which improves the tissue’s ability to resist compressive forces. Therefore tissues which are subjected to high compressive forces, such as bone and articular cartilage, have a high proteoglycan content. The proteoglycans also form a supporting substance for the fibre and cellular components. Decreased hydration allows it to exist as a viscous semisolution or sol, which improves the tissue’s ability to resist tensile forces. Therefore tissues which are subjected to high tensile forces, such as tendons and ligaments, have a low proteoglycan concentration (Levangie & Norkin 2001).
The concentration of GAGs present in tissues is related to their function and gives connective tissue structures viscous properties. More water is associated with a higher GAG concentration and rabbit ligamentous tissue has a significantly greater water content than that in rabbit tendinous tissue (Amiel et al 1982). This increase in GAG and water content alters the viscoelastic properties and may provide the ligament with an additional shock-absorbing feature that is unnecessary in most tendons.
The amorphous ground substance forms a lubricant, filler and spacing buffer system between collagen fibres, fibrils, microfibrils and the intercellular spaces (Akeson et al 1980). It reduces friction and maintains distance between fibres as well as facilitating the discrete shear and gliding movement of individual collagen fibres and fibrils. It is the lubrication and spacing at the fibre–fibre interface that are crucial to the gliding function at nodal intercept points where the fibres cross in the tissue matrices (Amiel et al 1982). If the tissues are allowed to adopt a stationary attitude (i.e. becoming immobile), anomalous cross-links form at the nodal intercept points.
A balance between the cross-link formation relative to the tissue’s tensile strength and mobility is important to normal connective tissue function. Excessive cross-linking and loss of GAGs and water volume result in loss of the critical distance between the fibres. The fibres come into contact with each other and stick together leading to altered tissue function and pain resulting from loss of extensibility and increased stiffness.
The elasticity of connective tissue fibres together with the viscosity of the amorphous ground substance gives connective tissue structures viscoelastic properties which ensure that normal connective tissues are mobile.
The biomechanical properties of connective tissue depend on the number and orientation of collagen fibres and the proportion of amorphous ground substance present. Each connective tissue structure is specifically designed for function but the tissues can be grouped simply into irregular and regular connective tissue.
The aim in orthopaedic medicine is to maintain normal connective tissue mobility through the phases of acute inflammation, repair and remodelling, and to regain mobility in the chronic inflammatory situation. This mobility is essential to function and the bias of orthopaedic medicine treatment techniques is towards preserving the mobility of connective tissue structures.
IRREGULAR CONNECTIVE TISSUE
Irregular connective tissue consists of a mixture of collagen and elastic fibres interwoven to form a loose meshwork that can withstand stress in any direction (Fig. 2.5). Its main function is to support and protect regular connective tissue structures.
 |
| Figure 2.5
Irregular connective tissue. (Paratenon; elastic-Van Gieson stain.)
Provided by Dr. T. Brenn.
|
The following examples of irregular connective tissue are commonly encountered in orthopaedic medicine.
The dura mater is the outermost of three irregular connective tissue sleeves which enclose the brain and the spinal cord. It is extended to form the dural nerve root sleeve that invests the nerve roots within the intervertebral foramen. At or just beyond the intervertebral foramen the dural nerve root sleeve fuses with the epineurium of the nerve root. The dura mater and dural nerve root sleeve extensions are formed of sheets of collagen and elastic fibres providing a tough but loose fibrous tube. The dura mater is separated from the bony margins of the vertebral canal by the epidural space that contains fat, loose connective tissue and a venous plexus. These structures are mobile in a non-pathological state and can accommodate normal movement (Netter 1987, Palastanga et al 2006, Standring 2009). Adhesions may develop in the dura mater and dural nerve root sleeve, compromising this mobility and giving rise to clinical symptoms.
An aponeurosis is a sheet of fibrous tissue that increases the tendinous attachment to bone. It distributes the tendon forces, increasing the tendon’s mechanical advantage, and needs to retain mobility in its attachment to perform its function.
The epimysium is a layer of irregular connective tissue surrounding the whole muscle; the perimysium surrounds the fascicles within the muscle; and the endomysium surrounds each individual muscle fibre.
In a similar arrangement, a fibrous sheath, the epineurium, surrounds each nerve; the perineurium surrounds each fascicle; and each individual nerve fibre is invested in a delicate sheath of vascular loose connective tissue, the endoneurium. Since connective tissue mobility is important to the function of muscle, its arrangement in nerve structure implies that it is also important to the function of nervous tissue.
The paratenon is an irregular connective tissue fibroelastic sheath, adherent to the outer surface of all tendons. It is composed of relatively large amounts of proteoglycans to provide a gliding surface around the tendon, allowing it to move freely among other tissues with a minimum of drag (Merrilees & Flint 1980). True tendon synovial sheaths are found most commonly in the hand and foot where they act to reduce friction between the tendon and surrounding tissues. The synovial sheath consists of two layers, an outer fibrotic sheath and an inner synovial sheath which has parietal and visceral layers. Between these two layers is an enclosed space containing a thin film of synovial fluid. The synovial sheath may also assist in tendon nutrition (Józsa & Kannus 1997).
Like the synovial tendon sheaths, bursae, flat synovial sacs, also prevent friction and pressure and facilitate movement between adjacent connective tissue structures. Bursae can be subcutaneous (e.g. the olecranon bursa), subtendinous (e.g. psoas bursa), sub- or intermuscular (e.g. gluteal bursa), or adventitious – developing in response to trauma or pressure (e.g. subcutaneous Achilles bursa).
Fascia lies in sheets to facilitate movement between the various tissue planes. Deep fascia has a more regular formation as it forms a tight sleeve to retain structures, adds to the contours of the limbs and is extended to form the intermuscular septa. It provides a compressive force which facilitates venous return and may act as a mechanical barrier preventing the spread of infection.
Fascia may develop retinacula which hold tendons in place, preventing a bowstring effect on movement, e.g. the retinacula at the ankle. It may produce thickenings, forming protective layers such as the palmar and plantar aponeuroses, or it may form envelopes to enclose and protect major neurovascular bundles, e.g. the femoral sheath in the femoral triangle.
Tissue injury involves the surrounding and supporting irregular connective tissue as well as the regular connective tissue structure itself. It is therefore important to recognize the extensive nature of irregular connective tissue and its close relationship with the regular connective tissue structures encountered in orthopaedic medicine.
REGULAR CONNECTIVE TISSUE
In contrast to irregular connective tissue, this group of tissues has a highly organized structure with fibres running in the same linear direction in a precise arrangement that is related to function (Fig. 2.6) The main collagen fibre bundles will be aligned parallel to the line of major mechanical stress, which functionally suits such structures as tendons and ligaments that are mainly subjected to unidirectional stress (Donatelli & Owens-Burkhart 1981).
 |
| Figure 2.6
Regular connective tissue. (Tendon; elastic-Van Gieson stain.)
Provided by Dr. T. Brenn.
|
The following examples of regular connective tissue are commonly encountered in orthopaedic medicine.
Tendons
Muscles and tendons are distinct tissues, although they functionally act as one structure, the contractile unit. The tendon cells are derived from the embryonic mesenchyme, the tissue occupying the areas between the embryonic layers, classifying tendon as a connective tissue (Standring 2009). Muscle cells are derived from mesoderm, the intermediate embryonic layer, such that muscle itself belongs to a separate tissue group.
The tendon is an inert structure which does not contract, but as part of the contractile unit it is directly involved in muscle action. Therefore the tendon is assessed by resisted testing via the muscle belly. Active movement does not produce transverse movement within the tendon but its fibrous structure may be moved passively by transverse frictions, to prevent or to mobilize adhesions.
A tendon provides tensile force transmission and storage and release of elastic energy during locomotion – which is particularly important for many sports and activities (Witvrouw et al 2007).
• To attach a muscle to bone.
• To transmit the force of muscle contraction to the bone to produce functional movement.
• To set the muscle belly in the optimal position for functional movement and to affect the direction of muscle pull.
• To be able to glide within the surrounding tissues, accepting stress and tensile forces with minimal drag.
Tendons are exposed to strong, unidirectional forces and to function they require great tensile strength and inelastic properties. They are able to withstand much greater tensile forces than ligaments and are composed of closely packed parallel bundles of collagen micro-fibrils, fibrils and fibres, bound together by irregular connective tissue sheaths into larger bundles (Fig. 2.7). Most fibres are oriented in one direction, parallel to the long axis, which is the direction of normal physiological stress (Alter 2000). Tendons do not usually contain any elastic fibres since their muscle belly acts as an energy damper such that elastic fibres are not required (Akeson 1990). They do have the ability to store and release elastic energy, however, and rehabilitation programmes should aim to increase tendon elasticity. Tendon elasticity has been shown to increase significantly with ballistic stretching (Witvrouw et al 2007).
 |
| Figure 2.7
Structure of a tendon.
|
The fibres are large-diameter type I collagen, well suited to accept tensile forces. The proteoglycans are packed in between the fibres and, because the tendon is so compact, there is little room for the tendon cells or an adequate blood supply. The tendon’s blood vessels lie in the epitenon (adherent to the surface of the tendon) and the endotenon (a division between collagen fibre bundles) (Gelberman et al 1983). The vascularization of tendons is relatively sparse compared with that of muscles (Alfredson et al 2002).
Calcification may occur within the tendon structure that is generated by extracellular organelles known as matrix vesicles. Proteoglycans in the tendon usually suppress the mineralization but ageing and diabetes may alter proteoglycan levels, thus allowing calcification of the normally unmineralized extracellular matrix (Gohr et al 2007).
As mentioned above, tendons are surrounded by a fibroelastic paratenon functioning as an elastic sleeve to facilitate their gliding properties and permitting free movement in the surrounding tissues. Under the paratenon, the entire tendon is surrounded by the thin connective tissue sheath of the epitenon; the two together are sometimes referred to as the peritendon. On its inner surface the epitenon is continuous with the endotenon, which invests each tendon fibre (Józsa & Kannus 1997).
The musculotendinous junction is the area where tension generated in the muscle fibres is transmitted from intra-cellular contractile proteins to extracellular connective tissue proteins. It is a relatively weak area making it susceptible to injury (Józsa & Kannus 1997).
The teno-osseous junction is the point of insertion of the tendon into the bone where the viscoelastic tendon transmits force to the rigid bone. At this point the tendon goes though a transition from tendon to fibrocartilage, mineralized fibrocartilage and finally bone. The mechanism of overuse injuries involving the teno-osseous junction, or enthesis, is not well understood. Benjamin et al (2006) note that bony spurs are well documented at numerous entheses and tend to occur with high levels of physical activity. They are more frequently found in males and occur more commonly with increasing age. They can be compared to osteophytes forming around the articular surfaces in arthritic synovial joints. Some individuals appear to have a greater tendency to form bone than others, both at joint margins and at entheses. Where bony spurs are well documented, e.g. at the attachment of the plantar fascia and the Achilles tendon, osteoarthritis-like degenerative changes have been noted in the enthesis fibrocartilage of the fascia or tendon.
The term tendinopathy is a generic descriptor to indicate clinical conditions affecting tendons. The inflammatory model of tendon pain has been challenged (Khan & Cook 2000, Cook et al 2000) and ‘tendinopathy’ is a more appropriate term since it does not commit to pathology. The terms ‘tendinosis’, ‘paratendinitis’ and ‘tendinitis’ are reserved as histopathological labels. Tendinopathy will be used throughout this text to indicate painful overuse tendon lesions and a discussion on the aetiology and pathological processes associated with tendinopathy is included in Ch. 3.
Tenosynovitis occurs in ensheathed tendons and involves the synovium rather than the tendon itself. Inflammation of the synovium can produce adhesions between the two layers of the sheath which may produce a palpable crepitus (Cyriax 1982). Adhesion formation interferes with the normal function of the tendon sheath, which is to allow controlled movement of the sheath around the tendon and to facilitate nutrition by forcing synovial fluid into the tendon (Barlow & Willoughby 1992).
Tenovaginitis is a term used to describe thickening of the synovial sheath (Cyriax 1982) and is associated with chronic tenosynovitis, e.g. de Quervain’s stenosing tenosynovitis of the sheath containing the abductor pollicis longus and extensor pollicis brevis tendons.
As with all other connective tissue structures, tendons remodel in response to mechanical demand and they are sensitive and responsive to changes in physical load, a property which may be demonstrated by a difference in structure within one tendon unit. Eccentric exercise programmes affect type I collagen production and tend to increase tendon volume over the long term, an important consideration in the rehabilitation of tendinopathies (Alfredson & Cook 2007).
Merrilees & Flint (1980) looked at the macrostructural features of different regions of the flexor digitorum profundus tendon in the rat. This tendon, in common with most tendons, is subjected to longitudinal stress throughout its length, but it also possesses a sesamoid-like area as it passes under the calcaneus and talus. In this area the tendon is subjected to compressive forces, as well as to the normal longitudinal stress.
In the zone subjected to tension, the collagen fibres were arranged in longitudinal bundles, but in the zone subjected to pressure, the sesamoid-like area took on a form similar to that of fibrocartilage, i.e. a loose weave of collagen fibres and chondrocyte-like cells arranged in columns perpendicular to the main fibre axis.
This illustrates the ability of connective tissue structures to adapt to different functions and to changes in physical loads in a normal situation. In abnormal situations, such as a period of immobility when physical demand is reduced, adaptations in collagen turnover occur. When physical demand is increased, collagen production increases. The turnover (synthesis and lysis) of collagen is a continuing process allowing the remodelling and adaptation of structures to suit demand.
Several factors affect the mechanical forces that act on tendons and influence their adaptation in response. The different types of activity and the location of the tendon in the body induce different levels of force on tendons, since different tendons are subject to different levels of mechanical loading. The relative size between the muscle and the tendon and the level of muscle contraction also affects the stress applied to the tendon, as does the position and movement in the adjacent joints and the activity within the antagonist muscles (Wang et al 2006).
With regard to viscoelasticity, Eliasson et al (2007) set out to observe the effect of disuse on the mechanical properties (primarily creep and hysteresis) in the Achilles tendon. Achilles tendon was harvested at 1 and 6 weeks from 78 rats divided into three experimental groups. Two of the groups were immobilized and the other served to provide loaded controls. The result showed that the tendons lost viscoelasticity (i.e. as shown by the effects on creep and hysteresis) which was possibly more influenced by glycosaminoglycan chains than collagen.
The most marked biochemical change in tendon ageing is decreased tensile strength. Collagen cross-linking increases and alters the mechanical properties of the tendon; the ability to withstand load, the modulus of elasticity and tensile strength all decrease and there is an increase in mechanical stiffness (Kannus et al 2005).
Ligaments and joint capsules
The joint capsule and its supporting ligaments are similar in function, both allowing and restraining movement at the joints. Ligaments can be considered to be a reinforcement of the joint capsule in an area of special stress. As inert tissue structures they are assessed by passive movements, which should be applied at the extremes of range to test function.
The ligaments, together with the fibrous capsule, guide and stabilize the articular surfaces. When excessive stresses are applied to a joint, proprioceptive impulses recruit a muscle response so that the passive stabilizing effect of the ligament is reinforced by dynamic muscle stabilization (Akeson et al 1987).
To meet its functional requirements of resisting shear as well as tensile and compressive forces, the structure of a ligament is different from that of a tendon. The main ligamentous fibres are 70–80% collagen laid down in bundles, which assume a wavy configuration providing an element of elongation and recoil to facilitate movement (Fig. 2.8). Interwoven with these main fibre bundles are 3–5% elastic fibres, to enhance extensibility and elasticity (Akeson et al 1987). When ligaments are put under longitudinal tensile stress, the parallel wavy bundles of collagen straighten out to prevent excessive movement and to provide tensile strength.
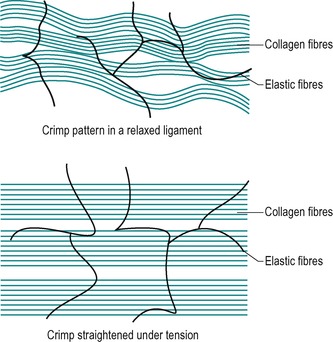 |
| Figure 2.8
Structure of a ligament.
|
Although the joint capsule is similar in function to ligaments, its structure is slightly different. The capsule consists of sheets of collagen fibres which form a fibrous cuff joining opposing bony surfaces. The fibrous structure is predominantly collagen but, rather than a parallel array of fibres, its pattern is more a criss-cross weave, with the fibres becoming more parallel as the capsule is loaded (Fig. 2.9) (Amiel et al 1990, Woo et al 1990). The ability of the fibres to change and straighten depends on them being mobile and able to slide independently of one another. Capsular contractures in the form of disorganized collagen will prevent independent fibre gliding at the nodal intercept points, considerably reducing function and causing pain.
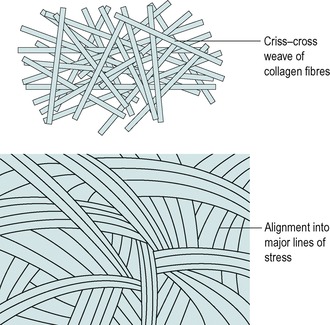 |
| Figure 2.9
Structure of the joint capsule.
|
The joint capsule has two layers: an outer fibrous capsule and an internal synovial membrane. The outer fibrous capsule is strong and flexible but relatively inelastic. It is supported functionally by its ligaments which may be intrinsic, forming an integral part of the joint capsule (e.g. coronary and medial collateral ligaments of the knee), or accessory, being either intracapsular (e.g. the cruciate ligaments) or extracapsular (e.g. lateral collateral ligaments of the knee). The fibrous capsule is perforated by vessels and nerves and contains afferent sensory nerve endings, including mechanoreceptors and nociceptors.
The synovial membrane is mainly a loose connective tissue membrane with a degree of elasticity to prevent its folds and villi becoming nipped during movement. It covers all surfaces within the joint except the articular surfaces themselves and menisci. Adipose fat pads may exist in the joint, acting as shock absorbers, and distinct fringes of synovium may be present, e.g. the plicae of the knee joint. These folds, fringes and villi allow the joints to accommodate to movement.
The synovium is a highly cellular membrane containing synoviocytes, i.e. the synovium-producing cells, and collagen fibres. It has a rich nerve, blood and lymphatic supply. A capillary network is situated on the inner surface of the synovium to produce synovial fluid. Synovial fluid is pale yellow and viscous. It lubricates the ligamentous structures of the joint and nourishes cartilage and menisci through a mechanism of transsynovial flow aided by movement (Akeson et al 1987).
In an arthritis of a joint, the inflammation causes pain and involuntary muscle spasm which prevents full range of movement. The relative immobility causes changes to occur in the connective tissue which lead to capsular contracture, further loss of function and pain. This will be seen clinically as the capsular pattern (see Ch. 1).
Trauma can cause a haemarthrosis and the difference between this and a trauma-induced synovial effusion is indicated clinically by the immediate onset of swelling as a result of bleeding into the joint, as opposed to the more slowly developing synovial effusion, sometimes over several hours.
Cartilage
Cartilage is a weight-bearing connective tissue displaying a combination of rigidity, which is resistant to compression, resilience and some elasticity. It is relatively avascular and relies on tissue fluid for nourishment. There are three main types:
1. Elastic cartilage
2. Fibrocartilage
3. Hyaline cartilage.
Elastic cartilage consists of a matrix of yellow elastic fibres and is very resilient. It is found in the external ear, the epiglottis and the larynx.
Fibrocartilage has a large proportion of type I collagen fibres in its matrix, providing it with great tensile strength. Examples are the annulus fibrosus of the intervertebral discs, the menisci of the knee joint, the acetabular and glenoid labra, the articular disc of the acromioclavicular and wrist joints, the lining of the grooves that house tendons, and as a transitional cartilage at the teno-osseous junction of the tendons (Cormack 1987, Palastanga et al 2006, Standring 2009). The annulus fibrosus in the lumbar spine has a unique geometrical pattern of collagen fibres.
Hyaline cartilage is articular cartilage. Its relatively solid gel-like matrix provides a weight-bearing surface which is elastic and resistant to compression. It moulds to the shape of the bones, presenting a smooth articular surface for movement.
Hyaline articular cartilage is composed of scantily deposited chondroblasts and chondrocytes which produce the gel extracellular matrix consisting of type II collagen fibres and amorphous ground substance. The extracellular matrix contains distinctive large super-molecular proteoglycan aggregates which resemble bottle brushes (Fig. 2.10). These provide a network for trapping and retaining water which significantly contributes to the resilience of cartilage (Cormack 1987). The collagen fibres themselves are relatively weak under compression: therefore the water-enhanced matrix compensates for this by providing a resilient weight-bearing surface.
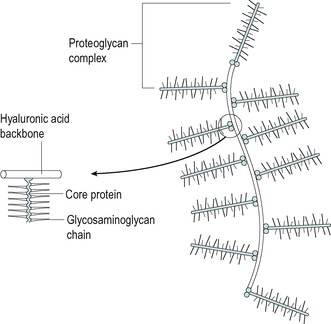 |
| Figure 2.10
Proteoglycan complex of hyaline cartilage.
Adapted from Gray’s Anatomy 40th edn by S. Standring. (2009). By permission of Elsevier Ltd.
|
Three separate structural zones exist in hyaline articular cartilage which contribute to its biomechanical functions (Nordin & Frankel 2001). A superficial zone helps to prevent friction between the joint surfaces and to distribute the compressive forces. It consists of fine, tangential, densely packed fibres lying in a plane parallel to the articular surface (Fig. 2.11). In the middle, vertical zone the cells are arranged in vertical columns, perpendicular to the surface, with scattered collagen fibres. The middle layer allows deformation of the collagen fibres absorbing some of the compressive forces. The deep zone forms a transition between the articular cartilage and the underlying calcified cartilage layer; the fibres are arranged in radial bundles. Hyaline articular cartilage has no blood vessels and depends on fluid flow through compressive forces for nutrition. This fluid flow depends on the magnitude and duration of the compressive force and a balance of weight-bearing and non-weight-bearing is important to its health (Levangie & Norkin 2001).
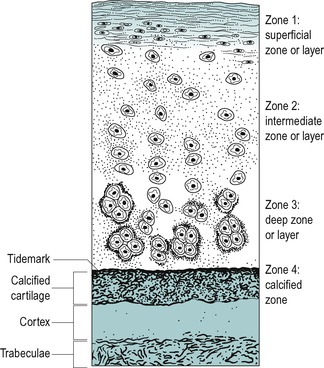 |
| Figure 2.11
Zonal arrangement of articular cartilage.
From Orthopaedic and Sports Physical Therapy by Gould J.A. Reprinted by permission of Elsevier Ltd.
|
The zonal arrangement of cartilage provides a ‘well-sprung mattress’ arrangement to cope with compression forces. The variation in collagen fibre orientation in each zone enables the articular cartilage to vary its material property with direction of the load.
Articular cartilage requires movement for nutrition and to maintain fluid levels within its matrix in order to withstand compressive forces. Prolonged loading reduces fluid levels in the matrix through the action of tissue creep, which may lead to degenerative changes. It is essential, therefore, to maintain mobilization of inflamed or degenerate joints.
The fluid content of articular cartilage is responsible for its nutrition as well as its mechanical properties, allowing diffusion of nutrients and products between the cells and the synovial fluid. When cartilage is loaded, the fluid in the ‘sponge’ moves, which is important for the mechanical properties of the cartilage as well as for joint lubrication. Intermittent loading creates a pumping effect but prolonged loading will eventually press fluid out of the cartilage without allowing new fluid to be taken up, leading to degeneration.
MUSCLE
Muscle tissue is a separate tissue group responsible for contraction and functional movement. It consists of cells known as muscle fibres due to their long, narrow shape.
Muscle has a large connective tissue component which supplies its nutrients for its metabolism and facilitates contraction by providing a continuous connective tissue harness. This continuous harness consists of the epimysium, perimysium and endomysium (Fig. 2.12). Each end of the harness is continuous with strong connective tissue structures which anchor it to its attachments.
Connective tissue mobility is important to normal muscle function. Although skeletal muscles have some regenerating properties, healing of large muscle belly lesions is largely through the formation of scar tissue. Disorganized scar tissue alters function and acts as a physical barrier to regenerating muscle fibres.
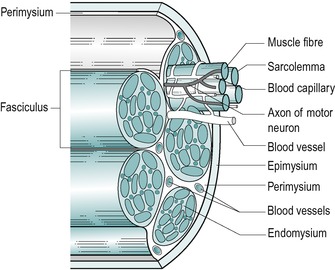 |
| Figure 2.12
Connective tissue component of skeletal muscle.
From Anatomy and Human Movement by Palastanga N, Field D and Soames R. Reprinted by permission of Elsevier Ltd.
|
Skeletal muscle is of obvious concern in orthopaedic medicine and following trauma it is capable of some regenerating properties. During the healing of injured muscle tissue, satellite cells, located next to the muscle fibres, are capable of forming completely new muscle fibres or restoring damaged muscle fibres (Grefte et al 2007). At the proliferation stage the satellite cells become myoblasts that may either fuse to each other to create new myofibres or may fuse to existing damaged myofibres for repair. Macrophages are important in muscle regeneration since their infiltration leads to increased satellite cell proliferation and differentiation. Most muscle injuries heal without dysfunctional scar tissue but large muscle belly lesions resulting from major trauma will be filled with a mixture of disorganized scar tissue and new muscle fibres, which can inhibit regeneration and lead to incomplete functional recovery.
NERVOUS TISSUE
Nervous tissue is designed for the conduction of nerve impulses and initiation of function. The central nervous system is largely devoid of connective tissue, being made up of specialized tissue held together by neuroglia. Three connective tissue meninges (pia mater, arachnoid mater and dura mater) and the cerebrospinal fluid protect the system inside its bony framework.
The peripheral nervous system is not so delicate, with connective tissue constituting part of the nerves, providing strength and resilience. The epineurium is an outer connective tissue sheath enclosing large nerves. The perineurium surrounds each fascicle or bundle of nerve fibres and the endoneurium invests each individual nerve fibre.
BEHAVIOUR OF CONNECTIVE TISSUES TO MECHANICAL STRESS
Excessive mechanical stress is responsible for connective tissue injury and manual techniques utilize mechanical stresses to mobilize, permanently elongate or rupture scar tissue, where the adhesions formed are preventing full painless function. Understanding the mechanical response of connective tissue structures to stress is helpful in interpreting mechanisms of injury and rationalizing treatment programmes. However, it should be appreciated that most experimental evidence has been derived from animal and cadaveric specimens in the laboratory setting, and the physical principles have been adapted in order to explain the mechanical properties demonstrated. Connective tissue can change its structure and function in response to applied forces by altering the composition of the extracellular matrix, demonstrating its dynamic nature and the relationship between form and function (Levangie & Norkin 2001).
The stress, or load, is the mechanical force applied to the tissue. The strain is the resultant deformation produced by the applied stress.
The stress–strain curve is a way of illustrating the reaction of connective tissue structures to loading (Fig. 2.13). Experimentally, a tensile stress is applied to collagen until it ruptures. The applied stress or elongating force is plotted on the y axis and the strain, the extent to which collagen elongates, measured as a percentage of its original length, is plotted along the x axis.
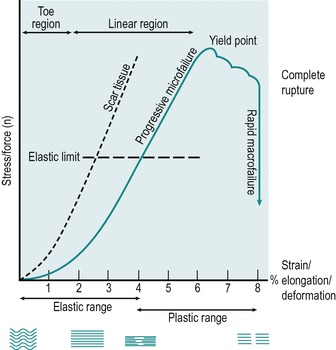 |
| Figure 2.13
Stress–strain curve.
Adapted from Bogduk 2005, Nordin & Frankel 2001, Keir 1991, Kisner & Colby 1990 in Sports Injuries by C. Norris. Reprinted by permission of Elsevier Ltd.
|
Collagen at rest is crimped; as stress is applied, the fibres straighten initially, responding with an elastic type of elongation and the crimp pattern is lost (Akeson et al 1987, Hardy & Woodall 1998, Bogduk 2005,). The straightening out of the fibres is represented by the first part of the curve, known as the toe region. Crimp straightens easily and there is little or no resistance to the applied stress. At the end of the toe region some of the elongation may be due to sliding of the collagen fibres in the interfibrillar gel (Nordin & Frankel 2001). The capacity of the tissues to lengthen is initially determined by their structural weave; the more regularly oriented the collagen fibres, the shorter the toe region, for example a ligament displays a shorter toe region than the more loosely woven joint capsule but a longer toe region than the more regularly arranged tendon (Threlkeld 1992, Hardy & Woodall 1998).
The second part of the curve is known as the linear region. The straightened fibres realign in the linear direction of the applied stress and the structure becomes longer and thinner.
In the first half of the linear region water and proteoglycans are displaced and the chemical cross-links between fibres and fibrils are strained, producing a resistance or stiffness in the tissue, so that a progressively greater stress is required to produce equivalent amounts of elongation. The steeper the stress–strain curve, the stiffer the tissue. If the deforming stress is removed at this point, the elastic properties of the collagen tissue allow the structure to return to its original resting length. In the early part of the linear region a point is reached at which slack is taken up in connective tissue and this represents the end of passive range.
In the second half of the linear region, stress causes some of the strained cross-links to break and microfailure begins to occur in a few overstretched fibres. Microfailure is said to occur somewhere after 4% of elongation has been achieved (Bogduk 2005), at which point the collagen is said to have reached its elastic limit. A traumatic stress applied at this stage produces minor pain and swelling but no clinical laxity (equivalent to a Grade I injury – see Ch. 11 for definitions of the grades of injury as applied to the medial collateral ligament of the knee).
Once the elastic limit of collagen has been exceeded, collagen exhibits plastic properties, the property of the tissue to deform permanently when loaded beyond its elastic limit, and progressive microfailure produces permanent elongation once the deforming stress is removed. A traumatic stress applied within the plastic range produces more pain and swelling, together with some clinical laxity (equivalent to a Grade II injury – see Ch. 11 for definitions of the grades of injury as applied to the medial collateral ligament of the knee).
A further increase in stress causes major collagen fibre failure and the yield point is reached, represented by the peak of the stress–strain curve, where a large number of cross-links are irreversibly broken. The stress–strain curve drops rapidly, indicating macrofailure or complete rupture, where the structure is unable to sustain further stress even though it may remain physically intact. Threlkeld (1992), reporting Noyes et al, stated that the estimated macrofailure of connective tissue occurs at approximately 8% of elongation. Wang et al (2006) propose that this is a conservative figure and state 12% as the percentage value for complete rupture. They suggest that even this value could be an underestimation and that it might be as much as 14% of elongation. A traumatic stress that produces complete rupture causes severe pain initially which is followed by less pain and gross clinical laxity (equivalent to a Grade III injury – see Ch. 11 for definitions of the grades of injury as applied to the medial collateral ligament of the knee).
The stress applied to tissues can be divided into several categories (Norris 2004, Bogduk 2005):
• Tensile stress – a pulling or elongating force applied longitudinally parallel to the long axis of the structure
• Compressive stress – a pushing or squashing force applied perpendicular to the long axis of the structure
• Shear stress – a sliding force applied across the long axis of the structure
• Torsional stress – a twisting force or torque applied in opposite directions about an axis of rotation.
Collagen, however, does not have pure elastic properties and the presence of the amorphous ground substance provides a viscous fluid factor. Therefore the viscoelastic properties of collagen may be affected by the type of stress applied and the speed of application, influencing the outcome of the different mobilization techniques used in orthopaedic medicine, as discussed in Ch. 4.
The stiffness of a structure is its resistance to deformation under the applied stress. A stiff structure displays reduced elastic properties and a shorter toe phase. Scar tissue, which forms within a connective tissue structure, is not as elastic as the surrounding normal tissue (Fig 2.13). Therefore, slack will be taken up sooner in the adherent scar tissue and mobilization techniques can be applied to produce elongation or rupture. Tough scar tissue requires considerable force or stress to deform it, and it does not easily resume its original shape. Once the failure point of scar tissue is reached it ruptures relatively quickly (Norris 2004).
The viscoelastic properties of connective tissue structures cause them to behave differently under different loading rates. If the structure is loaded quickly it behaves more stiffly than the same tissue loaded at a slower rate (Threlkeld 1992). Tendons, for example, are more easily deformed at low strain rates where they absorb more energy but are less effective at transmitting loads. At high strain rates, they become stiffer and are less easily deformed but are more effective at moving large loads (Wang et al 2006). Higher force, short duration stretching at normal or lower temperatures favours recoverable elastic tissue deformation whereas low force, long duration stretching at higher temperatures favours permanent plastic deformation (Warren et al 1971, Leban, cited in Alter 2000).
Warren et al (1971) considered the effect of temperature and load on elongation of the collagen fibre structure of rat tail tendon. A range of loads was applied at selected temperatures of 39, 41, 43 and 45°C. The greatest elongation with least microdamage was achieved with lower loads at the higher therapeutic temperatures. The mechanism of a combined application of temperature and load affected the viscous flow properties of collagen.
This behaviour of the tissue is utilized in tissue mobilization techniques. Adhesions may be ruptured by a quickly applied shear stress, or stretched by a slow sustained tensile stress. Increasing the temperature of a structure allows lower sustained loads to achieve greater elongation (Warren et al 1971, Usuba et al 2007).
Creep is a property of viscous structures which occurs when a prolonged stress is applied in the linear phase. Creep, or elongation of the tissue, is inversely proportional to the velocity of the stress and the slower the applied stress, the greater the lengthening (Hardy & Woodall 1998). Deformation occurs through a gradual rearrangement of the collagen fibres, proteoglycan gel and water and/or through straining and perhaps breaking some of the collagen fibre cross-links (Bogduk 2005). When the stress is released, resumption of the original length of the structure occurs at a slower rate than its deformation and this mechanical behaviour is known as hysteresis – i.e. the loading and unloading stress–strain curves are not identical as would be demonstrated in a purely elastic structure. The original length may not be achieved and the difference between the two lengths is known as set.
Repeated or cyclical loading may achieve an increment of elongation with each loading cycle. This may lead to eventual failure of the structure through accumulated fatigue. A larger load requires fewer repetitions to produce failure, but a certain minimum load (the endurance limit) must be applied to achieve this effect (Norris 2004).
The aim of mobilization is to maintain or regain the gliding function and length of the tissues, allowing or restoring full painless function. Mobilization to maintain tissue function is conducted within the elastic range, while mobilization aimed at elongating or rupturing established scar tissue adhesions occurs by applying appropriate stress at the end of the linear region and within the plastic range of the tissue. Timing of application of the mobilization stresses is important and is determined by the grade of the injury and resultant irritability of the tissues. Young scar tissue is ‘ripe’ for mobilization and can be altered by stress parameters that do not affect older scar tissue. The tensile strength limitations of the healing tissues should be respected, and uncontrolled or overaggressive mobilization avoided (Madden cited in Hardy & Woodall 1998).
REFERENCES
Akeson, W., The response of ligaments to stress modulation and overview of the ligament healing response, In: (Editors: Daniel, D.; Akeson, W.H.; O’Connor, J.J.) Knee Ligaments: Structure, Function, Injury and Repair ( 1990)Lippincott, Williams & Wilkins, Philadelphia, pp. 315–327.
Akeson, W.; Amiel, D.; Woo, S.L.-Y., Immobility effects on synovial joints, the pathomechanics of joint contracture, Biorheology 17 (1980) 95–110.
Akeson, W.; Amiel, D.; Abel, M.F.; et al., Effects of immobilisation on joints, Clin. Orthop. Relat. Res. 219 (1987) 28–37.
Alfredson, H.; Cook, J., A treatment algorithm for managing Achilles tendinopathy: new treatment options, Br. J. Sports Med. 41 (2007) 211–216.
Alfredson, H.; Bjur, D.; Thorsen, K.; et al., High intratendinous lactate levels in painful chronic Achilles tendinosis. An investigation using microdialysis technique, J. Orthop. Res. 20 (5) ( 2002) 934–938.
Alter, M.J., Science of Flexibility. 2nd edn ( 2000)Human Kinetics, Champaign, Illinois.
Amiel, D.; Woo, S.L.-Y.; Harwood, L.; et al., The effect of immobilization on collagen turnover in connective tissue: a biochemical–biomechanical correlation, Acta Orthop. Scand. 53 (1982) 325–332.
Amiel, D.; Billings, E.; Akeson, W., Ligament structure, chemistry and physiology, In: (Editors: Daniel, D.; Akeson, W.H.O.; Connor, J.J.) Knee Ligaments: Structure, Function, Injury and Repair ( 1990)Lippincott, Williams & Wilkins, Philadelphia, pp. 77–90.
Barlow, Y.; Willoughby, J., Pathophysiology of soft tissue repair, Br. Med. Bull. 48 (1992) 698–711.
Benjamin, M.; Toumi, H.; Ralphs, J.R.; et al., Where tendons and ligaments meet bone: attachment sites (entheses) in relation to exercise and/or mechanical load, J. Anat. 208 (4) ( 2006) 471–490.
Bogduk, N., Clinical Anatomy of the Lumbar Spine and Sacrum. 4th edn ( 2005)Churchill Livingstone, Edinburgh.
Chamberlain, G.J., Cyriax’s friction massage: a review, J. Orthop. Sports Phys. Ther. 4 (1982) 16–22.
Cook, J.L.; Khan, K.M.; Maffulli, N.; et al., Overuse tendinosis, not tendinopathy, part 2: applying the new approach to patellar tendinopathy, Phys. Sports Med. 28 (6) ( 2000) 31–41.
Cormack, D.H., Ham’s Histology. 9th edn ( 1987)Lippincott, Williams & Wilkins, Philadelphia.
Cyriax, J., 8th ednTextbook of Orthopaedic Medicine. vol. 1 ( 1982)Baillière Tindall, London.
Dingman, R.O., Factors of clinical significance affecting wound healing, Laryngoscope 83 (9) ( 1973) 1540–1555.
Donatelli, R.; Owens-Burkhart, H., Effects of immobilisation on the extensibility of periarticular connective tissue, J. Orthop. Sports Phys. Ther. 3 (1981) 67–72.
Eliasson, P.; Fahlgren, A.; Pasternak, B.; et al., Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity., J. Appl. Physiol. 103 (2007) 459–463.
Evans, P., The healing process at cellular level: a review., Physiotherapy 66 (1980) 256–259.
Fowler, J.D., Wound healing: an overview., Semin. Vet. Med. Surg. 4 (1989) 256–262.
Gelberman, R.H.; Vande Berg, J.S.; Lundberg, G.N.; et al., Flexor tendon healing and restoration of the gliding surface., J. Bone Joint Surg. 65A (1983) 70–83.
Gohr, C.; Fahey, M.; Rosenthal, A., Calcific tendonitis: a model, Connect. Tissue Res. 48 (2007) 286–291.
Gould, J.A., Orthopaedic and Sports Physical Therapy. 2nd edn ( 1990)Mosby, St. Louis.
Grefte, S.; Kuijpers-Jagtman, A.M.; Torensma, R.; Von den Hoffe, J.W.; et al., Skeletal muscle development and regeneration, Stem Cells Dev. 16 (2007) 857–868.
Hardy, M.A., The biology of scar formation, Phys. Ther. 69 (1989) 1014–1023.
Hardy, M.; Woodall, W., Therapeutic effects of heat, cold and stretch on connective tissue, J. Hand Ther. 11 (2) ( 1998) 148–152.
Józsa, L.; Kannus, P., Human Tendons: Anatomy, Physiology and Pathology. ( 1997)Human Kinetics, Champaign, Illinois.
Kannus, P.; Paavola, M.; Józsa, L., Aging and degeneration of tendons, In: (Editors: Maffulli, N.; Renström, P.; Leadbetter, W.B.) Tendon Injuries: Basic Science and Clinical Medicine ( 2005)Springer-Verlag, London, pp. 25–31.
Kapit, W.; Macey, R.; Meisami, E., The Physiology Coloring Book. ( 1987)HarperCollins, New York.
Khan, K.M.; Cook, J.L., Overuse tendon injuries: where does the pain come from?Sports Med. Arthroscopic Review 8 (2000) 17–31.
Le Gros Clark, W.E., The tissues of the body. 5th edn ( 1965)Oxford University Press, Oxford.
Leibovich, S.J.; Ross, R., The role of the macrophage in wound repair, Am. J. Pathol. 78 (1974) 71–91.
Levangie, P.K.; Norkin, C.C., Joint Structure and Function: a Comprehensive Analysis. 3rd edn ( 2001)F A Davis, Philadelphia.
Merrilees, M.J.; Flint, M.H., Ultrastructural study of tension and pressure zones in a rabbit flexor tendon, Am. J. Anat. 157 (1980) 87–106.
Netter, F.H., Musculoskeletal system, part 1. Ciba Collection of Medical Illustrations. Vol. 8 ( 1987)Ciba-Geigy, Summit, New Jersey.
Nimni, M.E., The molecular organisation of collagen and its role in determining the biophysical properties of the connective tissues, Biorheology 17 (1980) 51–82.
Nordin, M.; Frankel, V.H., Basic Biomechanics of the Musculoskeletal System. 3rd edn ( 2001)Lippincott, Williams & Wilkins, Philadelphia.
Norris, C.M., Sports Injuries: Diagnosis and Management for Physiotherapists. 3rd edn ( 2004)Butterworth-Heinemann, Oxford.
Palastanga, N.; Field, D.; Soames, R., Anatomy and Human Movement. 5th edn ( 2006)Butterworth-Heinemann, Edinburgh.
Peacock, E.E., Some biochemical and biophysical aspects of joint stiffness, Ann. Surg. 164 (1966) 1–12.
September, A.V.; Schwellnus, M.P.; Collins, M., Tendon and ligament injuries: the genetic component [review], Br. J. Sports Med. 41 (4) ( 2007) 241–246.
Shen, W.; Li, Y.; Zhu, J.; et al., Interaction between macrophages. TGF-B1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury, J. Cell. Physiol. 214 (2008) 405–412.
Standring, S., Gray’s Anatomy: the Anatomical Basis of Clinical Practice. fortieth edn ( 2009)Churchill Livingstone, Edinburgh.
Stearns, M.L., Studies on the development of connective tissue in transparent chambers in the rabbit’s ear, I, Am. J. Anat. 66 (1940) 133–176.
Stearns, M.L., Studies on the development of connective tissue in transparent chambers in the rabbit’s ear, II, Am. J. Anat. 67 (1940) 55–97.
Threlkeld, A.J., The effects of manual therapy on connective tissue, Phys. Ther. 72 (1992) 893–902.
Usuba, M.; Akai, M.; Shirasaki, Y.; et al., Experimental joint contracture correction with low torque – long duration repeated stretching, Clin. Orthop. Relat. Res. 456 (2007) 70–78.
Wang, J.; Losifidus, M.; Fu, F., Biomechanical basis for tendinopathy, Clin. Orthop. Relat. Res. 443 (2006) 320–332.
Warren, C.G.; Lehmann, J.F.; Koblanski, J.N., Elongation of the rat tail tendon: effect of load and temperature., Phys. Med. Rehabil. 52 (1971) 465–474.
Wilkerson, G.B., Inflammation in connective tissue: etiology and management, Athl. Train. Winter (1985) 298–301.
Witvrouw, E.; Mahieu, N.; Roosen, P.; et al., The role of stretching in tendon injuries, Br. J. Sports Med. 41 (4) ( 2007) 224–226.
Woo, S.L.-Y.; Wang, C.W.; Newton, P.O.; et al., The response of ligaments to stress deprivation and stress enhancement, In: (Editors: Daniel, D.; Akeson, W.H.; O’Connor, J.J.) Knee Ligaments: Structure, Function, Injury and Repair. ( 1990)Lippincott, Williams & Wilkins, Philadelphia, pp. 337–349.