Soft capsules
Keith G. Hutchison and Josephine Ferdinando
Chapter contents
Description of the soft gelatin capsule dosage form (softgels)
Rationale for the selection of softgels as a dosage form
Improved drug absorption characteristics
Patient compliance and consumer preference
Safety for potent and cytotoxic drugs
Oils and low melting point drugs
Dose uniformity of low-dose drugs
Properties of soft gelatin shells
Formulation of softgel fill materials
Key points
• Soft gelatin capsules (softgels) comprise a liquid or semi-solid preparation in a capsule that is formed in a single-step encapsulation process.
• They can be used as a formulation approach with the potential to:
• increase the rate of drug absorption, the extent of bioavailability and reduce drug variability in plasma
• improve patient compliance and consumer preference
• improve manufacturing safety for potent and cytotoxic drugs
• improve manufacturability of low melting point and low dose drugs.
• Careful consideration should be given to any migration of drug or other formulation components when formulating a softgel in order to achieve satisfactory product stability and shelf-life.
• There are a number of fill formulation approaches which can be used including suspensions and solutions, using hydrophilic or lipophilic excipients or a mixture of these to produce emulsions or self-emulsifying micro- or nano-emulsions.
Introduction
When pharmaceutical formulation scientists are designing a solid oral dosage form for drug compounds, they have a number of choices which can be influenced by consumer preference/compliance, economics and technical feasibility. Over recent years, new drug molecules tend to be less soluble in aqueous systems and if intended for oral administration, this can present a considerable formulation challenge for delivering drug for absorption at the desired rate and extent. One approach is to make a liquid formulation containing the drug either in solution or suspended in a matrix more readily dissolved on contact with gastrointestinal media. In order to convert a liquid formula into a solid dosage form, it may be encapsulated into soft gelatin capsules, also known as softgels.
This chapter explains:
Description of the soft gelatin capsule dosage form (softgels)
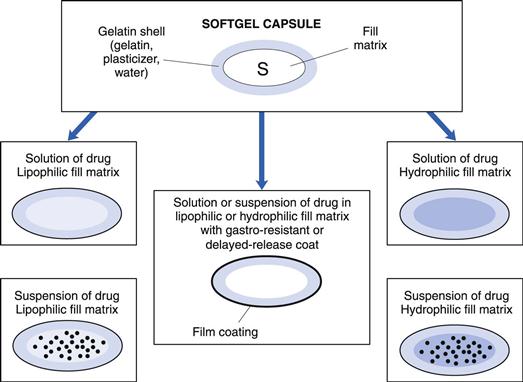
Softgels consist of a liquid or a semi-solid matrix inside a one-piece outer gelatin shell (Fig. 34.1). Ingredients that are solid at room temperature can also be encapsulated into softgels providing they are at least semi-solid below approximately 40 °C. The drug compound itself may be either in solution or in suspension in the capsule-fill matrix. The characteristics of the fill matrix may be hydrophilic (for example, polyethylene glycols), lipophilic (such as triglyceride vegetable oils), or a combination of hydrophilic and lipophilic ingredients (see also Fig. 34.1).
Significant advances have been made in recent years regarding the formulation of softgel fill matrices (Gullapalli 2010). These include self-emulsifying micro-emulsions and nano-emulsions encapsulated as preconcentrates in softgels. The term ‘preconcentrate’ means that the softgel fill matrix which is a combination of lipophilic and hydrophilic liquids as well as surfactant components disperses after oral administration to form an emulsion, with a droplet size either in the micro- or nanometre size range.
The softgel capsule shell consists of gelatin, water and a plasticizer. The shell may be transparent or opaque and can be coloured and flavoured if desired. Preservatives are not normally required owing to the low water activity in the finished product. The softgel can be coated with enteric-resistant or delayed-release coating materials. Although virtually any shape of softgel can be made, oval or oblong shapes are usually selected for oral administration.
Softgels can be formulated and manufactured to produce a number of different drug delivery systems:
• Orally administered softgels containing solutions or suspensions that release their contents in the stomach in an easy-to-swallow, convenient unit dose form (Fig. 34.2)
• Chewable softgels, where a highly flavoured shell is chewed to release the drug liquid fill matrix. The drug(s) may be present in both the shell and fill matrix
• Suckable softgels, which consist of a gelatin shell containing the flavoured medicament to be sucked and a liquid matrix or just air inside the capsule
• Twist-off softgels, which are designed with a tag to be twisted or snipped off, thereby allowing access to the fill material. This type of softgel can be used for unit dosing of topical medication, inhalations or for oral dosing of a paediatric product (Fig. 34.3)
• Meltable softgels designed for use as pessaries or suppositories.
Rationale for the selection of softgels as a dosage form
Some of the reasons why softgels may be selected as the preferred formulation approach are summarized in Table 34.1 and a more detailed description follows. Whilst softgels can solve various technical formulation challenges not possible with tablets, consideration should be given to the fact that they can be more costly than tablet formulations and require specialized manufacturing equipment.
Table 34.1
Summary of the key features and advantages of the softgel dose form
| Features | Advantages |
| Improved drug absorption | Improved rate and extent of absorption and/or reduced variability, mainly for poorly water-soluble drugs |
| Patient compliance and consumer preference | Easy to swallow. Absence of poor taste or other sensory problem. Convenient administration of a liquid-drug dosage form |
| Safety – potent and cytotoxic drugs | Avoids dust-handling problems during dosage form manufacture; better operator safety and environmental controls |
| Oils and low melting point drugs | Overcomes problems with manufacture as compressed tablet or hard-shell capsules |
| Dose uniformity for low-dose drugs | Liquid flow during dosage form manufacture is more precise than powder flow. Drug solutions provide better homogeneity than powder or granule mixtures |
| Product stability | Drugs are protected against oxidative degradation by lipid vehicles and softgel capsule shells |
Improved drug absorption characteristics
Increased rate of absorption
Major advances have been made in the area of developing softgel formulations to address drug absorption issues (Ferdinando 2000, Perlman et al 2008, Aboul-Einien 2009). For poorly water-soluble drugs, ideally the dosage form would present the drug to the gastrointestinal tract in solution form, from which the drug can be rapidly absorbed. This can be achieved using a drug-solution matrix in a softgel formulation and such formulations can provide faster absorption than from other solid oral dosage forms, such as compressed tablets (Lissy et al 2010). This is probably because absorption of a poorly soluble drug from a tablet formulation requires time for disintegration of the tablet into granules, then drug dissolution into gastrointestinal fluid. With the solution-softgel approach, the shell ruptures within minutes to release the drug solution, which can be in a hydrophilic or highly dispersing vehicle that aids the rate of drug absorption. This may be beneficial for (a) therapeutic reasons, such as the treatment of migraine or acute pain, or (b) where there is a limited absorptive region or ‘absorption window’ high in the gastrointestinal tract. Figure 34.4 shows the faster absorption that can be achieved using a solution-softgel formulation of ibuprofen compared to a tablet (Saano et al 1991).
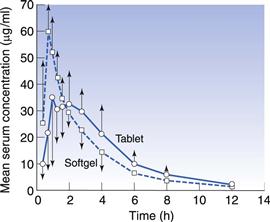
Fig. 34.4 Pharmacokinetic evaluation of softgels and tablets containing 400 mg ibuprofen (in 12 volunteers). (Courtesy of Saano et al 1991, with permission.)
Increased bioavailability
As well as increasing the rate of absorption, softgels may improve the extent of absorption (Aboul-Einien 2009). This can be particularly effective for drugs with poor aqueous solubility and a relatively high molecular weight. An example of such a product is the protease inhibitor saquinavir, which was formulated as a solution-softgel product (Perry & Noble 1988). The solution-softgel formulation provided around three times greater bioavailability than a saquinavir hard-shell capsule formulation as measured by the area under the plasma – time curve (AUC).
In some cases a drug may be solubilized in vehicles that are capable of spontaneously dispersing into an emulsion on contact with gastrointestinal fluid. This is known as a self-emulsifying drug delivery system (SEDDS) (Gao et al 2006). Drug may be dissolved in an oil/surfactant vehicle that produces a micro-emulsion or a nano-emulsion on contact with gastrointestinal fluids. A nano-emulsion of progesterone has been developed whereby the vehicle consists of oils and surfactants in appropriate proportions. On contact with aqueous fluids, it produces an emulsion with an average droplet size less than 100 nm. The solubility of the drug is maintained as long as possible, delivering solubilized drug directly to the enterocyte membrane. This can increase bioavailability compared to formulations in which the drug is dosed in the solid state. Figure 34.5 shows the plasma concentration – time profile for progesterone absorbed from the nano-emulsion formulation (Ferdinando 2000).
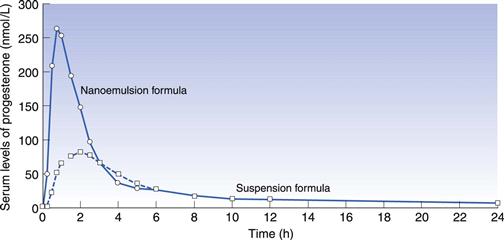
Fig. 34.5 Pharmacokinetic evaluation of progesterone comparing a softgel nano-emulsion solution of progesterone with a softgel containing a suspension of the drug in an oil following single dose administration in 12 healthy human volunteers. Reproduced from Ferdinando 2000.
Softgel formulations may contain excipients, for example one or more surfactants that can aid stability, wettability or even enhance permeability of the drug (Aungst 2000).
Decreased plasma variability
High variability in drug plasma levels is a common characteristic of drugs with limited bioavailability. By dosing drug optimally in solution, the plasma level variability of such drugs can be significantly reduced, particularly if absorption is limited by drug solubility. SEDDS have been shown to reduce variability of exposure to the lipophilic drug torcetrapib compared to a formulation in oil (Perlman et al 2008). The cyclic polypeptide drug ciclosporin (Sandimmune Neoral®) benefits from such an approach by using a micro-emulsion preconcentrate in a softgel (Drewe et al 1992, Meinzer 1993).
Patient compliance and consumer preference
A number of self-medicating consumer preference studies have been carried out to gauge the user’s perception of softgels relative to hard-shell capsules and tablets. The results of the studies showed that consumers expressed their preference for softgels in terms of (a) ease of swallowing, (b) absence of taste and (c) convenience in use.
This expressed appeal of the softgel dosage form may have a positive impact on patient compliance. Compliance may be further enhanced if the softgel formulation enables dosing of smaller or fewer dosage units, as a result of increased bioavailability.
Safety for potent and cytotoxic drugs
The mixing, granulation and compression/filling processes used in preparing tablets and hard-shell capsules can generate a significant quantity of airborne powders. This can be a cause of concern for the manufacture of highly potent or cytotoxic compounds because of safety considerations for the operator and environment.
By preparing a solution or suspension of drug, where the active component is essentially protected from the environment by the liquid, these safety concerns can be reduced.
Oils and low melting point drugs
When the pharmaceutical active is an oily liquid, has a melting point lower than about 75 °C or proves difficult to compress, liquid filling of softgels (with or without other diluents) can provide a successful approach to presenting it in a solid oral dosage form.
Dose uniformity of low-dose drugs
Presentation of low-dose drugs in a solution form can overcome the challenges of achieving dosage unit homogeneity compared to other solid oral dosage forms. Where the dose is in the order of micrograms, it can be difficult mixing it with other powders sufficiently well to ensure an even distribution in the bulk materials prior to compression of tablets or filling of hard shell capsules. This can result in variation in assays due to the inhomogeneity of content. By dissolving the drug in a liquid and encapsulating it in a softgel, such inhomogeneity concerns can be avoided.
Product stability
If a drug is subject to oxidative or hydrolytic degradation, the preparation of a liquid-filled softgel may prove beneficial. The liquid is prepared and encapsulated in a protective nitrogen atmosphere and the subsequent dried shell has very low oxygen permeability. By formulating in a lipophilic vehicle and packaging in well-designed blister packs using materials of low moisture transmission, the drug can be protected from moisture. As for all dosage forms, thorough stability evaluation is required, including excipient compatibility studies, to check against negative drug stability effects, for example caused by component migration between fill formula and capsule shell, exposure to moisture during manufacture or interaction between the drug and the fill excipients. This may result in the requirement for refrigerated storage (Klein et al 2007).
Manufacture of softgels
Softgels were used in the 19th century as a means of administering bitter-tasting or liquid medicines. These were manufactured individually by preparing a small sack of gelatin and allowing it to set. Each sack, or gelatin shell, was then filled with the medication and heat sealed. This method of manufacture was improved using a process that involved sealing two sheets of gelatin film between a pair of matching flat brass dies. Each die contained pockets into which the gelatin sheet was pressed and into which the medication was filled. The pressure between the two plates enabled individual capsules to be cut out from the die mould and these capsules were subsequently dried.
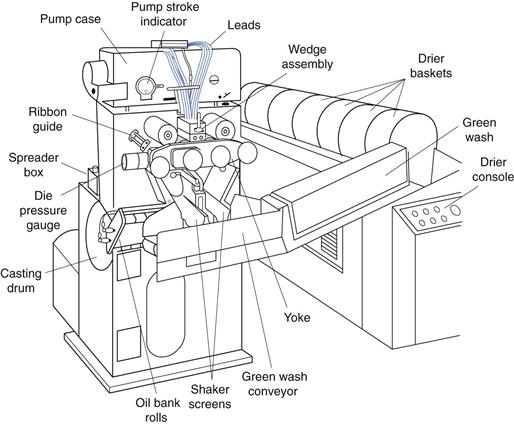
However, it was not until the invention of the rotary die encapsulation machine by Robert Pauli Scherer in 1933 that liquid fill capsules could be manufactured on a production scale. The rotary die process involves continuous formation of a heat seal between two ribbons of gelatin simultaneous with dosing of the fill liquid into each capsule. Although the speed and efficiency of the manufacturing process have improved greatly in recent years, the basic manufacturing principle remains essentially unchanged. The overall layout of a soft gelatin encapsulation machine is shown in Figure 34.6.
Before the encapsulation process takes place, two sub-processes are often carried out simultaneously, yielding the two components of a softgel. These are (a) the gel mass which will provide the softgel shell and (b) the fill matrix for the softgel contents.
The gel mass is prepared by dissolving the gelatin in water at approximately 80 °C under vacuum, followed by the addition of the plasticizer, for example, glycerol. Once the gelatin is fully dissolved then other components such as colours, opacifier and flavours may be added. The hot gel mass is then supplied to the encapsulation machine through heated transfer pipes by a casting method that forms two separate gelatin ribbons, each with a width of approximately 150 mm. During the casting process, the gelatin passes through the sol-gel transition and the thickness of each gel ribbon is controlled to ±0.1 mm in the range 0.5–1.5 mm. The thickness of the gel ribbons is checked regularly during the manufacturing process.
The two gel ribbons are then carried through rollers (at which a small quantity of vegetable oil lubricant is applied) and onwards to the rotary die encapsulation tooling (shown in Fig. 34.7). Each gel ribbon provides one half of the softgel. It is possible to make bicoloured softgels using gel ribbons of two different colours.
The liquid fill matrix containing the active drug substance is manufactured separately from preparation of the molten gel. Manufacture of the active fill matrix involves dispersing or dissolving the drug substance in the non-aqueous liquid vehicle using conventional mixer-homogenizers.
A number of different parameters are controlled during preparation of the active fill matrix, depending on the properties of the drug substance. For example, oxygen-sensitive drugs are protected by mixing under vacuum and/or inert gas; in some cases an antioxidant component may be added to the formulation. Also, if the drug substance is present as a suspension in the liquid fill matrix, then it is important to ensure that particle size of the drug does not exceed approximately 200 µm. By doing this, it is possible to ensure that drug particles do not become entrapped within the capsule seal, potentially leading to loss of integrity of the softgel.
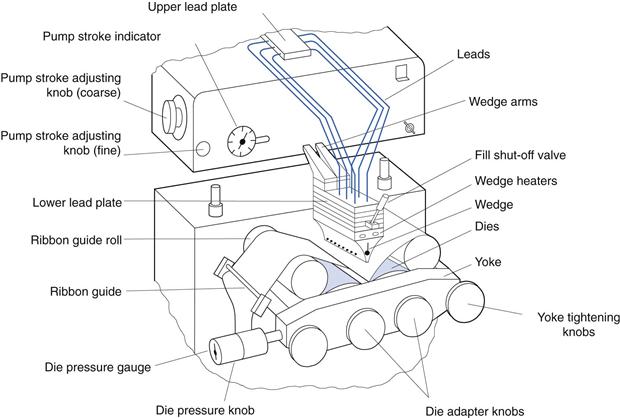
In the rotary die encapsulation process, the gel ribbon and the unit dose of liquid fill matrix are combined to form the softgel. The process involves careful control of three parameters:
• Temperature – this controls the heat available for capsule seal formation
• Timing – the timing of the dosing of unit quantities of liquid fill matrix into the softgel during its formation is critical
• Pressure – the pressure exerted between the two rotary dies controls the softgel shape and the final cut-out from the gel ribbon.
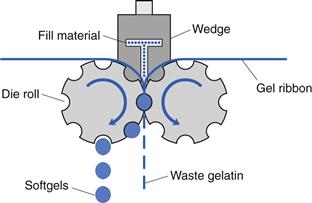
Figure 34.8 is a simplified diagram representing the mechanism of softgel formation using contra-rotating dies and the wedge-shaped fill matrix injection system.
Accurately metered volumes of the liquid fill matrix are injected from the wedge device into the space between the gelatin ribbons as they pass between the die rolls. The wedge-shaped injection system is itself heated to approximately 40 °C. The injection of liquid between the gel ribbons forces the gel to expand into the pockets of the dies, which govern the size and shape of the softgels. The ribbon continues to flow past the heated wedge injection system and is then pressed between the die rolls. Here, the two softgel capsule halves are sealed together by the application of heat and pressure. The softgel capsules are cut automatically from the gel ribbon by raised rims around each die on the rollers.
After manufacture, the capsules are passed through a tumble dryer and then, to complete the drying process, they are spread onto trays and stacked in a tunnel dryer that supplies air at 20% relative humidity. The tunnel drying process may take 2–3 days, or possibly as long as 2 weeks, depending on the specific softgel formulation. Finally, the softgels are inspected and packed into bulk containers in order to prevent further drying, and for storage.
Formulation of softgels
Gelatin shell formulation
Typical softgel shells are made up of gelatin, plasticizer and materials that impart the desired appearance (colourants and/or opacifiers) and sometimes flavours. The following sections describe each of these materials, their functions, types and the amounts most frequently used in manufacturing softgel shells.
Gelatin
A large number of different gelatin shell formulations are available depending on the nature of the liquid fill matrix. Most commonly, the gelatin is alkali- (or base-) processed (type B) gelatin and it normally constitutes 40% of the wet molten gel mass. Type A acid-processed gelatin can also be used.
Plasticizers
Plasticizers are used to make the softgel shell elastic and pliable. They usually account for 20–30% of the wet gel formulation. The most common plasticizer used in softgels is glycerol, although sorbitol and propylene glycol are also frequently used, often in combination with glycerol. The amount and choice of the plasticizer contribute to the hardness of the final product and may even affect the final product’s dissolution or disintegration characteristics, as well as physical and chemical stability. Plasticizers are selected on the basis of their compatibility with the fill formulation, ease of processing and desired properties of the final softgels, including hardness, appearance, handling characteristics and physical stability.
One of the most important aspects of softgel formulation is to ensure that there is minimum interaction or migration between the liquid fill matrix and the softgel shell. The choice of plasticizer type and concentration is important in ensuring optimum compatibility of the shell with the liquid fill matrix.
Water
The other essential component of the softgel shell is water. Water usually accounts for 30–40% of the wet gel formulation and its presence is important to ensure proper processing during gel preparation and softgel encapsulation. Following encapsulation, excess water is removed from the softgels through controlled drying. In dry softgels, the equilibrium water content is typically in the range of 5–8% w/w which represents the proportion of water that is bound to the gelatin in the softgel shell. This level of water is important for good physical stability of softgels because in harsh storage conditions, softgels will become either too soft and fuse together or too hard and brittle.
Colourants/opacifiers
Colourants (soluble dyes or insoluble pigments or lakes) and opacifiers are typically used at low concentrations in the wet gel formulation. Colourants can be either synthetic or natural and are used to impart desired shell colour for product identification. An opacifier, usually titanium dioxide, may be added to produce an opaque shell when the fill formulation is a suspension or to prevent photodegradation of light-sensitive fill ingredients. Titanium dioxide can either be used alone to produce a white opaque shell, or in combination with pigments to produce a coloured opaque shell.
Properties of soft gelatin shells
Oxygen permeability
The gelatin shell of a soft gelatin capsule provides a good barrier against the diffusion of oxygen into the contents of the product. The quantity of oxygen (q) that passes through the gelatin is governed by the permeability coefficient (P), the area (A), thickness (h) of the shell, the pressure difference (p1 – p2) and the time of diffusion (t) by the following equation:
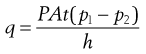 (34.1)
(34.1)
The permeability coefficient (P) is related to the diffusion coefficient (D) and the solubility coefficient (S) by the equation P = DS. This relationship, described by Henry’s Law, assumes no interaction between the gas and the polymeric film, but P is clearly affected by the formulation of the gelatin shell as shown in Figure 34.9.
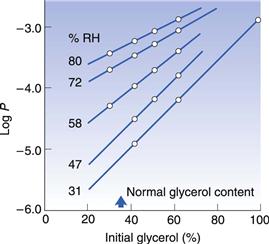
Fig. 34.9 Relationship between oxygen permeability coefficient and the glycerol concentration in the shell of softgels at room temperature and a range of relative humidity values. (Reproduced from Hom et al 1975.)
Figure 34.9 shows the relationship between oxygen permeability coefficient and the glycerol concentration in the gelatin shell of softgels at room temperature and relative humidity values from 31% to 80%. The oxygen permeability decreases with the % RH and the glycerol content in the gelatin shell formulation (Hom et al 1975). For maximum protection against the ingress of oxygen, the gelatin shell should be dry and formulated to contain about 30–40% glycerol.
Residual water content
Softgels contain little residual water and compounds which are susceptible to hydrolysis may be protected if dissolved or dispersed in an oily liquid fill material and encapsulated as a soft gelatin capsule. Figure 34.10 shows the relationship between the equilibrium water content and the concentration of glycerol in the gelatin shell of a softgel, stored at room temperature and environmental relative humidities of between 31% and 80%. The data show that the minimum water values are found at glycerol levels in the shell of between 30% and 40%. Such a formulation dried at 31% RH has a water content in the shell of about 7% (Hom et al 1975), and a water content in the fill in equilibrium with the atmosphere. The residual water content of most pharmaceutical compounds stored at 20% RH (the drying condition for softgels) is low and the water levels in the fills of softgels therefore are very small.
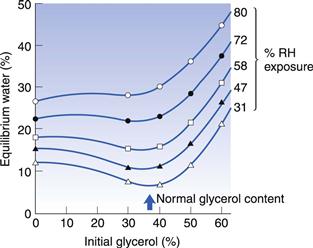
Fig. 34.10 The relationship between equilibrium water content and the concentration of glycerol in the shell of soft gelatin capsules at room temperature and a range of relative humidity values. (Reproduced from Hom et al 1975.)
Formulation of softgel fill materials
In terms of formulation requirements, the softgel should be considered as a biphasic dosage form: a solid-phase capsule shell and a liquid-phase capsule fill matrix. Although it is possible to incorporate a drug in the shell of a softgel, the overwhelming majority of products have the active ingredient(s) within the fill matrix. The liquid-phase fill matrix is selected from components with a wide range of different physicochemical properties. The choice of components is made according to one or more of a number of criteria, including the following:
• capacity to dissolve the drug (if a solution fill is required)
• rate of dispersion in the gastrointestinal tract after the softgel shell ruptures and releases the fill matrix
• capacity to retain the drug in solution in the gastrointestinal fluid
• compatibility with the softgel shell
• ability to optimize the rate, extent and consistency of drug absorbed.
Types of softgel fill matrices
Lipophilic liquids/oils.
Trigylceride oils, such as soya bean oil, are commonly used in softgels. When used alone, however, their capacity to dissolve drugs is limited. Nevertheless, active ingredients such as hydroxycholecalciferol and other vitamin D analogues, and steroids such as oestradiol, can be formulated into simple oily solutions for encapsulation in softgels. Drug may also be suspended in oils with appropriate excipients to ensure homogeneity during the manufacturing process.
Hydrophilic liquids.
Polar liquids with a sufficiently high molecular weight are commonly used in softgel formulation either to dissolve or suspend the drug. Polyethylene glycol (PEG) is the most frequently used, for example PEG 400 which has an average molecular weight of approximately 400 Da. Smaller hydrophilic molecules, such as ethanol or indeed water, can be incorporated in the softgel fill matrix in low levels, typically below 10% by weight. If included at higher levels, they may cause physical instability as they can migrate into the shell. Additional excipients may be included with hydrophilic liquids to increase the drug solubility in the matrix such as polyvinylpyrrolidone (PVP) or using a counter ion approach as developed for the ESS (Enhanced Solubility System) for drugs such as ibuprofen (Seager 1993).
Self-emulsifying drug delivery systems (SEDDS).
A combination of a pharmaceutical oil and a surfactant such as polyoxyethylene sorbitan monooleate can provide a formulation which emulsifies and disperses rapidly in the gastrointestinal fluid. The resulting droplets enable rapid transfer of the drug to the mucosa and subsequent drug absorption. If the droplets formed on contact with aqueous media are in the micrometre size range then it is known as a micro-emulsion, if they are in the nanometre range then they are known as a nano-emulsion.
In order to produce a micro-emulsion or nano-emulsion in the gastrointestinal tract, a ‘preconcentrate’ is formulated in the softgel fill matrix. The preconcentrate fill matrix contains a lipid component and one or more surfactants, which spontaneously form a micro-emulsion or a nano-emulsion on dilution in an aqueous environment such as in gastrointestinal fluid (Fig. 34.11).
Micro-emulsion and nano-emulsion systems have the advantage of a high capacity to solubilize drug compounds, and can retain the drug in solution even after dilution in gastrointestinal fluids. In addition, the micro-emulsion droplets have a high surface area, and are essentially surfactant micelles swollen with solubilized oil and drug. This high surface area facilitates the rapid diffusion of drug from the dispersed oil phase into the aqueous intestinal fluids, until an equilibrium distribution is established. Thereafter, as drug is removed from the intestinal fluids through absorption, it is quickly replenished by the flow of fresh material from the micro-emulsion droplets. Improved pharmacokinetic characteristics may be achieved using this formulation approach.
Lipolysis systems.
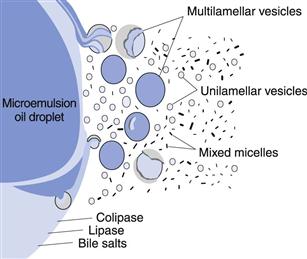
In addition to promoting the solubility of drug compounds, lipid formulations can also facilitate dissolution by taking advantage of the natural process of lipolysis. Lipid components of a softgel fill matrix, which comprise triglycerides or a partial (mono-/di-) glyceride, are often subject to intestinal fat digestion or lipolysis. Lipolysis is the action of the enzyme pancreatic lipase on triglycerides and partial glycerides, to form 2-monoglycerides and fatty acids. These 2-monoglycerides and fatty acids, known as lipolytic products, then interact with bile salts to form small droplets or vesicles. These vesicles are broken down into smaller and smaller vesicles, ultimately resulting in the formation of mixed micelles that are approximately 3–10 nm in size.
If a drug substance possesses higher solubility in lipolytic products than in triglyceride oils, then it is advantageous for lipolysis to occur in the intestinal lumen. In this way, the process of lipolysis promotes the formation of an excellent dissolution medium for the drug, namely lipolytic products. On the other hand, the absorption of a drug compound may be adversely affected by the presence of bile salts, and in such a case it may be advantageous for lipolysis to be reduced or blocked completely. It has been found that certain hydrophilic and lipophilic surfactants have the ability to block or promote lipolysis (MacGregor et al 1997). These hydrophilic and lipophilic surfactants are often used in softgel fill matrix formulations.
Measurement of the rate and extent of lipolysis for a softgel fill matrix formulation can be achieved by an in vitro pH stat measurement technique. In this, lipolysis is quantified by the amount of free fatty acids liberated by enzymatic digestion of the lipids in the softgel fill matrix. The quantity of a 1.0M sodium hydroxide titrant is directly proportional to the extent of lipolysis.
The mixed intestinal micelles produced as a result of this lipolysis process are of physiological importance because these structures can transport high concentrations of hydrophobic molecules across the aqueous boundary layer which separates the absorptive membrane from the intestinal lumen. Thus, lipolytic products (i.e. fatty acids and monoglycerides), and hydrophobic drug, if present, reside in the hydrophobic core regions of mixed intestinal micelles. In contrast, the surface of the micelles remains hydrophilic and this facilitates rapid micellar diffusion across the aqueous boundary layer to the intestinal membrane. In the microclimate adjacent to the intestinal membrane, the pH is lower than in the intestinal lumen. This promotes demicellization, leading to the formation of a supersaturated solution of lipolytic products (and hydrophobic drug, if present) in close proximity to the enterocyte surface. These materials are then readily absorbed across the cell membrane by passive diffusion.
Mixed intestinal micelles comprising bile salts and lipolytic products can enhance the bioavailability of hydrophobic drugs whose absorption is normally dissolution rate limited. This is because mixed intestinal micelles can be very potent solubilising agents for a wide range of hydrophobic drugs, much more so than simple bile salt micelles formed in the absence of lipolytic products. For example, under simulated physiological conditions, the aqueous solubility of cinnarizine in simple bile salt micelles is 4 µg/mL, compared to 0.5 µg/mL in aqueous buffer. However, in the presence of mixed micelles, the solubility of cinnarizine is further enhanced to approximately 44 µg/mL (Embleton et al 1995).
Taking cinnarizine as an example, it would be advantageous to formulate a softgel fill matrix that allows lipolysis to occur in the intestinal lumen because of the high drug solubility in lipolytic products. If the inhibition of lipolysis by a hydrophobic surfactant were allowed to occur, then it is highly likely that cinnarizine absorption would be impaired because of the reduced flow of drug into mixed micelles. However, if certain lipophilic surfactants with an HLB less than 10 are added to the formulation, then the inhibitory effects of hydrophilic surfactants on lipolysis can be reduced or eliminated.
Two formulations containing cinnarizine, a hydrophobic drug whose absorption is normally dissolution rate limited, have been compared (Embleton et al 1995). Formulation [A] was prepared as a lipolysing formulation and [B] as a non-lipolysing formulation, as demonstrated by the in vitro model. Formulation [A] was composed of a digestible triglyceride oil, a hydrophilic surfactant and a lipophilic surfactant which was chosen on the basis of its ability to overcome the inhibitory effects of the hydrophilic surfactant on the in vitro triglyceride lipolysis. In vitro, this formulation exhibited 79% lipolysis after 60 minutes compared to the digestible oil alone. In contrast, the non-lipolysing formulation contained a lipophilic surfactant that did not overcome the inhibitory effects of the hydrophilic surfactant on the lipolysis of the triglyceride oil and was shown to lipolyse to an extent of only 3%. It is proposed that the oil in Formulation [A], which forms a fine oil-in-water emulsion on aqueous dilution, is rapidly digested, forming mixed intestinal micelles with endogenous bile. These micelles transport the drug to the intestinal membrane where the pH of the microclimate promotes micellar breakdown, facilitating enterocyte transport to the systemic circulation. In contrast, on dilution with aqueous fluids, Formulation [B] forms a translucent micro-emulsion (as indicated by a blue tinge resulting from the Tyndall effect). As a result of this formulation failing to lipolyse and thereby remaining unaffected by enzymic activity, the drug is maintained within the oil phase, inhibiting the production of mixed intestinal micelles, hence restricting absorption of the drug.
The significance of the lipolysis process in enhancing the bioavailability of hydrophobic drugs was investigated further with an in vivo study. This study compared the bioavailability of cinnarizine (30mg) orally administered as the lipolysing formulation [A] and non-lipolysing formulation [B] with a commercially available tablet, Formulation [C], to six beagle dogs. The AUC(0–24 h) for Formulation [A] was significantly increased by 64% compared to the tablet preparation and by 48% compared to Formulation [B]. The Cmax of Formulation [A] was approximately 75% higher than both Formulations [B] and [C] (see Fig. 34.12).
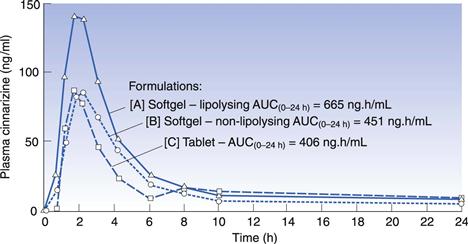
Fig. 34.12 Plasma concentration versus time curves for three formulations of cinnarizine in the dog (n = 6). (Reproduced from Embleton et al 1995.)
The results of this study have given a valuable insight into the effect of the micro-emulsion formulation on absorption of a hydrophobic drug in the gastrointestinal tract, and new information as to how the lipolysis process may influence bioavailability (Lacy et al 2000). More recently several studies have been carried out to improve the understanding of drug disposition from lipid-based formulation systems (Porter et al, 2008). A lipid formulation classification system has been devised to organize drug-lipid compositions according to the type of excipients used: Type I: Oils (triglycerides or mixed mono- and di-glycerides); Type II: Water-insoluble surfactants HLB < 12; Type III: Water-soluble surfactants HLB > 12 and Type IV: Hydrophilic cosolvents such as PEG (Pouton 2006). The tendency for drugs to precipitate from lipid formulas in gastrointestinal fluid can be mitigated by the presence of polymeric precipitation inhibitors such as cellulosic excipients (Warren et al 2010).
Product quality considerations
Ingredient specifications
All the ingredients of a softgel dosage form are controlled and tested to ensure compliance with pharmacopoeial specifications. Additional specification tests may be added for certain excipients in order to ensure manufacture of a high-quality softgel product. For example, it is important to limit certain trace impurities such as aldehydes and peroxides that may be present in polyethylene glycol. The presence of high levels of these impurities gives rise to crosslinking of the gelatin polymer, leading to non-solubilization through further polymerization. On prolonged storage, this can lead to slow dissolution of the capsule shell and subsequent retarded drug release.
Gelatin also requires careful control of quality to ensure a manufacturable and stable product. The quality of gelatin is controlled using parameters such as viscosity of a hot solution and bloom strength of the gel. The bloom strength is a measure of gel rigidity (see also Chapter 33).
In-process testing
During the encapsulation process the following four tests are carried out:
• softgel seal thickness at the time of encapsulation
• fill matrix weight and capsule shell weight
• softgel shell moisture level and softgel hardness at the end of the drying stage.
Appropriate control levels for these parameters are established during process development for each softgel product, and are applied in routine production-scale manufacture.
Finished product testing
Finished softgels are subjected to a number of tests in accordance with compendial requirements for unit dose capsule products. These normally include capsule appearance, active ingredient assay and related substances assay as well as fill weight, content uniformity, microbiological and dissolution testing. Development of a dissolution test using traditional media can be a challenge for certain softgel formulations including those with oily fills or those which rely on physiological conditions to release drug. Some have argued that disintegration testing may be more suitable for certain softgels (Han & Gallery 2006) whilst others use surfactant or enzyme-based media to achieve full dissolution in vitro.
References
1. Aboul-Einien MH. Formulation and evaluation of felodipine in softgels with a solubilized core. Asian Journal of Pharmaceutical Sciences. 2009;4:144–160.
2. Aungst BJ. Mini review: intestinal permeation enhancers. Journal of Pharmaceutical Sciences. 2000;89:429–442.
3. Drewe J, Meier R, Vonderscherer J, et al. Enhancement of the oral absorption of cyclosporin in man. British Journal of Clinical Pharmacology. 1992;34:60–64.
4. Embleton J, Hutchison KG, Lacy J. The effect of in-vivo lipolysis in improving the bioavailability of orally administered cinnarizine in self-emulsifying oily vehicles. Miami Beach: American Association of Pharmaceutical Sciences Conference; 1995.
5. Ferdinando JC. Formulation solutions – softgels. Pharmaceutical Manufacturing and Packaging Sourcer 2000;(Spring Issue):69–73.
6. Gao P, Charton M, Morozowich W. Speeding development of poorly soluble/poorly permeable drugs by SEDDS/S-SEDDS formulations and prodrugs (part II). American Pharmaceutical Review. 2006;9:16–23.
7. Gullapalli RP. Review: Soft gelatin capsules (softgels). Journal of Pharmaceutical Sciences. 2010;99:4107–4148.
8. Han J-H, Gallery J. A risk based approach to in vitro performance testing: a case study on the use of dissolution vs disintegration for liquid filled soft gelatine capsules. American Pharmaceutical Review. 2006;9:152–157.
9. Hom FS, Veresh SA, Ebert WR. Soft gelatin capsules II: Oxygen permeability study of capsule shells. Journal of Pharmaceutical Sciences. 1975;64:851–857.
10. Klein CE, Chiu Y-L, Awani W, et al. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. Journal of Acquired Immune Deficiency Syndromes. 2007;44:401–410.
11. Lacy JE, Embleton JK, Perry EA. Delivery systems for hydrophobic drugs. US Patent 2000; 6 096 338.
12. Lissy M, Scallion R, Stiff DD, Moore K. Pharmacokinetic comparison of an oral diclofenac potassium liquid-filled soft gelatin capsule with a diclofenac potassium tablet. Expert Opinion on Pharmacotherapy. 2010;11:701–708.
13. MacGregor KJ, Embleton JK, Lacy JE. Influence of lipolysis on drug absorption from the gastrointestinal tract. Advanced Drug Delivery Reviews. 1997;25:33–46.
14. Meinzer A. Sandimmun® Neoral® Soft Gelatin Capsules. Wisconsin, USA: International Industrial Pharmaceutical Research Conference; 1993.
15. Perlman ME, Murdande SB, Gumkowski MJ, et al. Development of a self-emulsifying formulation that reduces the food effect for torcetrapib. International Journal of Pharmaceutics. 2008;351:15–22.
16. Perry CM, Noble S. Saquinavir softgel capsule formulation. Drugs. 1988;55:461–486.
17. Porter CJ, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilization using lipid-based delivery systems. Advanced Drug Delivery Reviews. 2008;60:673–691.
18. Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. European Journal of Pharmaceutical Sciences. 2006;29:278–287.
19. Saano V, Paronen P, Peura P. Relative pharmacokinetics of three oral 400 mg ibuprofen dosage forms in healthy volunteers. International Journal of Clinical Pharmacology, Therapy and Toxicology. 1991;29:381–385.
20. Seager H. Soft gelatin capsule technology – a route to improved drug delivery. Pharmaceutical Manufacturing Review. 1993;5:9–10.
21. Warren DB, Benameur H, Porter CJ, Pouton CW. Using polymeric precipitation inhibitors to improve the absorption of poorly water soluble drugs: a mechanistic basis for utility. Journal of Drug Targeting. 2010;18:704–731.
Bibliography
1. Ferdinando JC. Formulation solutions – softgels. Pharmaceutical Manufacturing and Packaging Sourcer 2000;(Spring Issue):69–73.
2. Gao P, Charton M, Morozowich W. Speeding development of poorly soluble/poorly permeable drugs by SEDDS/S-SEDDS formulations and prodrugs (part II). American Pharmaceutical Review. 2006;9:16–23.
3. Gullapalli RP. Review: Soft gelatin capsules (softgels). Journal of Pharmaceutical Sciences. 2010;99:4107–4148.
4. Meinzer A. Sandimmun® Neoral® Soft Gelatin Capsules. Wisconsin, USA: International Industrial Pharmaceutical Research Conference; 1993.
[/level-membership-for-basic-science-category]
Soft capsules
Keith G. Hutchison and Josephine Ferdinando
Chapter contents
Description of the soft gelatin capsule dosage form (softgels)
Rationale for the selection of softgels as a dosage form
Improved drug absorption characteristics
Patient compliance and consumer preference
Safety for potent and cytotoxic drugs
Oils and low melting point drugs
Dose uniformity of low-dose drugs
Properties of soft gelatin shells
Formulation of softgel fill materials
Key points
• Soft gelatin capsules (softgels) comprise a liquid or semi-solid preparation in a capsule that is formed in a single-step encapsulation process.
• They can be used as a formulation approach with the potential to:
• increase the rate of drug absorption, the extent of bioavailability and reduce drug variability in plasma
• improve patient compliance and consumer preference
• improve manufacturing safety for potent and cytotoxic drugs
• improve manufacturability of low melting point and low dose drugs.
• Careful consideration should be given to any migration of drug or other formulation components when formulating a softgel in order to achieve satisfactory product stability and shelf-life.
• There are a number of fill formulation approaches which can be used including suspensions and solutions, using hydrophilic or lipophilic excipients or a mixture of these to produce emulsions or self-emulsifying micro- or nano-emulsions.
Introduction
When pharmaceutical formulation scientists are designing a solid oral dosage form for drug compounds, they have a number of choices which can be influenced by consumer preference/compliance, economics and technical feasibility. Over recent years, new drug molecules tend to be less soluble in aqueous systems and if intended for oral administration, this can present a considerable formulation challenge for delivering drug for absorption at the desired rate and extent. One approach is to make a liquid formulation containing the drug either in solution or suspended in a matrix more readily dissolved on contact with gastrointestinal media. In order to convert a liquid formula into a solid dosage form, it may be encapsulated into soft gelatin capsules, also known as softgels.
This chapter explains:
Description of the soft gelatin capsule dosage form (softgels)
Softgels consist of a liquid or a semi-solid matrix inside a one-piece outer gelatin shell (Fig. 34.1). Ingredients that are solid at room temperature can also be encapsulated into softgels providing they are at least semi-solid below approximately 40 °C. The drug compound itself may be either in solution or in suspension in the capsule-fill matrix. The characteristics of the fill matrix may be hydrophilic (for example, polyethylene glycols), lipophilic (such as triglyceride vegetable oils), or a combination of hydrophilic and lipophilic ingredients (see also Fig. 34.1).
Significant advances have been made in recent years regarding the formulation of softgel fill matrices (Gullapalli 2010). These include self-emulsifying micro-emulsions and nano-emulsions encapsulated as preconcentrates in softgels. The term ‘preconcentrate’ means that the softgel fill matrix which is a combination of lipophilic and hydrophilic liquids as well as surfactant components disperses after oral administration to form an emulsion, with a droplet size either in the micro- or nanometre size range.
The softgel capsule shell consists of gelatin, water and a plasticizer. The shell may be transparent or opaque and can be coloured and flavoured if desired. Preservatives are not normally required owing to the low water activity in the finished product. The softgel can be coated with enteric-resistant or delayed-release coating materials. Although virtually any shape of softgel can be made, oval or oblong shapes are usually selected for oral administration.
Softgels can be formulated and manufactured to produce a number of different drug delivery systems:
• Orally administered softgels containing solutions or suspensions that release their contents in the stomach in an easy-to-swallow, convenient unit dose form (Fig. 34.2)
• Chewable softgels, where a highly flavoured shell is chewed to release the drug liquid fill matrix. The drug(s) may be present in both the shell and fill matrix
• Suckable softgels, which consist of a gelatin shell containing the flavoured medicament to be sucked and a liquid matrix or just air inside the capsule
• Twist-off softgels, which are designed with a tag to be twisted or snipped off, thereby allowing access to the fill material. This type of softgel can be used for unit dosing of topical medication, inhalations or for oral dosing of a paediatric product (Fig. 34.3)
• Meltable softgels designed for use as pessaries or suppositories.
Rationale for the selection of softgels as a dosage form
Some of the reasons why softgels may be selected as the preferred formulation approach are summarized in Table 34.1 and a more detailed description follows. Whilst softgels can solve various technical formulation challenges not possible with tablets, consideration should be given to the fact that they can be more costly than tablet formulations and require specialized manufacturing equipment.
Table 34.1
Summary of the key features and advantages of the softgel dose form
| Features | Advantages |
| Improved drug absorption | Improved rate and extent of absorption and/or reduced variability, mainly for poorly water-soluble drugs |
| Patient compliance and consumer preference | Easy to swallow. Absence of poor taste or other sensory problem. Convenient administration of a liquid-drug dosage form |
| Safety – potent and cytotoxic drugs | Avoids dust-handling problems during dosage form manufacture; better operator safety and environmental controls |
| Oils and low melting point drugs | Overcomes problems with manufacture as compressed tablet or hard-shell capsules |
| Dose uniformity for low-dose drugs | Liquid flow during dosage form manufacture is more precise than powder flow. Drug solutions provide better homogeneity than powder or granule mixtures |
| Product stability | Drugs are protected against oxidative degradation by lipid vehicles and softgel capsule shells |
Improved drug absorption characteristics
Increased rate of absorption
Major advances have been made in the area of developing softgel formulations to address drug absorption issues (Ferdinando 2000, Perlman et al 2008, Aboul-Einien 2009). For poorly water-soluble drugs, ideally the dosage form would present the drug to the gastrointestinal tract in solution form, from which the drug can be rapidly absorbed. This can be achieved using a drug-solution matrix in a softgel formulation and such formulations can provide faster absorption than from other solid oral dosage forms, such as compressed tablets (Lissy et al 2010). This is probably because absorption of a poorly soluble drug from a tablet formulation requires time for disintegration of the tablet into granules, then drug dissolution into gastrointestinal fluid. With the solution-softgel approach, the shell ruptures within minutes to release the drug solution, which can be in a hydrophilic or highly dispersing vehicle that aids the rate of drug absorption. This may be beneficial for (a) therapeutic reasons, such as the treatment of migraine or acute pain, or (b) where there is a limited absorptive region or ‘absorption window’ high in the gastrointestinal tract. Figure 34.4 shows the faster absorption that can be achieved using a solution-softgel formulation of ibuprofen compared to a tablet (Saano et al 1991).

Fig. 34.4 Pharmacokinetic evaluation of softgels and tablets containing 400 mg ibuprofen (in 12 volunteers). (Courtesy of Saano et al 1991, with permission.)
Increased bioavailability
As well as increasing the rate of absorption, softgels may improve the extent of absorption (Aboul-Einien 2009). This can be particularly effective for drugs with poor aqueous solubility and a relatively high molecular weight. An example of such a product is the protease inhibitor saquinavir, which was formulated as a solution-softgel product (Perry & Noble 1988). The solution-softgel formulation provided around three times greater bioavailability than a saquinavir hard-shell capsule formulation as measured by the area under the plasma – time curve (AUC).
In some cases a drug may be solubilized in vehicles that are capable of spontaneously dispersing into an emulsion on contact with gastrointestinal fluid. This is known as a self-emulsifying drug delivery system (SEDDS) (Gao et al 2006). Drug may be dissolved in an oil/surfactant vehicle that produces a micro-emulsion or a nano-emulsion on contact with gastrointestinal fluids. A nano-emulsion of progesterone has been developed whereby the vehicle consists of oils and surfactants in appropriate proportions. On contact with aqueous fluids, it produces an emulsion with an average droplet size less than 100 nm. The solubility of the drug is maintained as long as possible, delivering solubilized drug directly to the enterocyte membrane. This can increase bioavailability compared to formulations in which the drug is dosed in the solid state. Figure 34.5 shows the plasma concentration – time profile for progesterone absorbed from the nano-emulsion formulation (Ferdinando 2000).

Fig. 34.5 Pharmacokinetic evaluation of progesterone comparing a softgel nano-emulsion solution of progesterone with a softgel containing a suspension of the drug in an oil following single dose administration in 12 healthy human volunteers. Reproduced from Ferdinando 2000.
Softgel formulations may contain excipients, for example one or more surfactants that can aid stability, wettability or even enhance permeability of the drug (Aungst 2000).
Decreased plasma variability
High variability in drug plasma levels is a common characteristic of drugs with limited bioavailability. By dosing drug optimally in solution, the plasma level variability of such drugs can be significantly reduced, particularly if absorption is limited by drug solubility. SEDDS have been shown to reduce variability of exposure to the lipophilic drug torcetrapib compared to a formulation in oil (Perlman et al 2008). The cyclic polypeptide drug ciclosporin (Sandimmune Neoral®) benefits from such an approach by using a micro-emulsion preconcentrate in a softgel (Drewe et al 1992, Meinzer 1993).
Patient compliance and consumer preference
A number of self-medicating consumer preference studies have been carried out to gauge the user’s perception of softgels relative to hard-shell capsules and tablets. The results of the studies showed that consumers expressed their preference for softgels in terms of (a) ease of swallowing, (b) absence of taste and (c) convenience in use.
This expressed appeal of the softgel dosage form may have a positive impact on patient compliance. Compliance may be further enhanced if the softgel formulation enables dosing of smaller or fewer dosage units, as a result of increased bioavailability.
Safety for potent and cytotoxic drugs
The mixing, granulation and compression/filling processes used in preparing tablets and hard-shell capsules can generate a significant quantity of airborne powders. This can be a cause of concern for the manufacture of highly potent or cytotoxic compounds because of safety considerations for the operator and environment.
By preparing a solution or suspension of drug, where the active component is essentially protected from the environment by the liquid, these safety concerns can be reduced.
Oils and low melting point drugs
When the pharmaceutical active is an oily liquid, has a melting point lower than about 75 °C or proves difficult to compress, liquid filling of softgels (with or without other diluents) can provide a successful approach to presenting it in a solid oral dosage form.