Chapter 7 Small Vessel Disease
Small vessel disease is the less understood of the major mechanisms of cerebral ischemia. This is the case despite its high prevalence in the elderly population, in whom it can present in the form of lacunar strokes, intracerebral hemorrhage, and cognitive decline.1
The seminal work of Dr. C. M. Fisher based on his clinicopathological observations led to the definition of lacunar strokes as small subcortical infarcts measuring between 3 mm and 2 cm and caused by the occlusion of penetrating branches of cerebral arteries.2 He described lipohyalinosis, a hypertensive microvasculopathy, as the main anatomical substrate for the development of lacunar infarctions and also deep cerebral hemorrhages.3 However, he recognized that plaques of atheroma in the parent vessel occluding the origin of the penetrating branch4 and probably microembolism (in patients with structurally normal penetrating branch corresponding to the area of small infarction and a systemic cause of embolism)4 could also produce lacunar strokes. He characterized the classic lacunar syndromes but noted that a number of atypical clinical presentations could also occur. He was then well aware of the fact that small vessel disease does not constitute a uniform disorder but rather may result from a complex spectrum of conditions with various clinical manifestations.
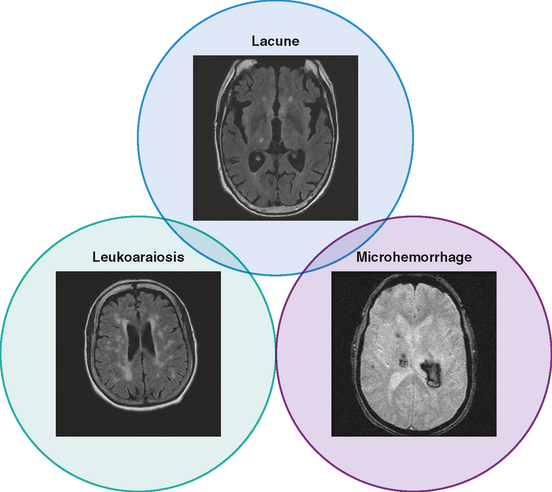
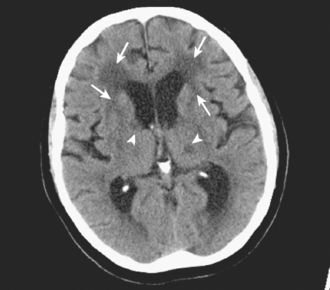
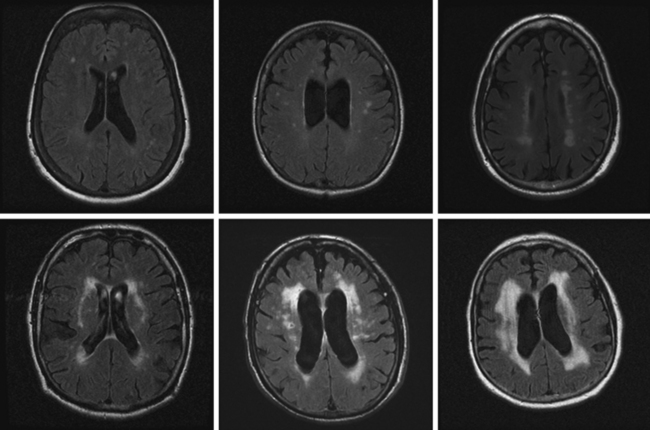
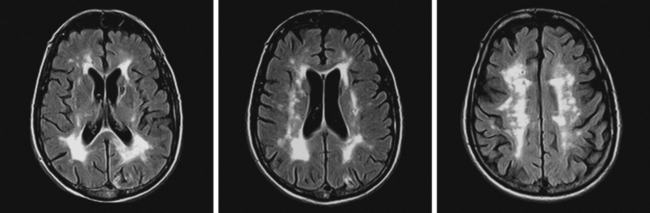
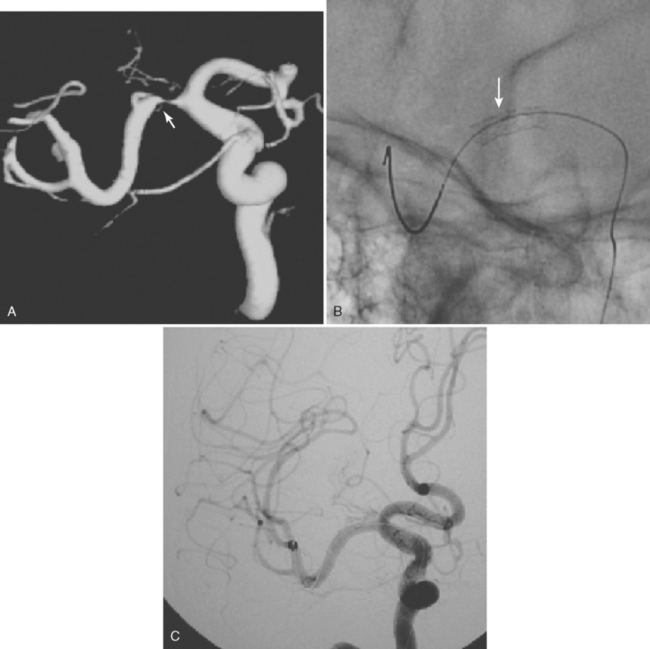
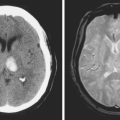
Unfortunately, the conceptualization of lacunar strokes became quite simplified and dogmatic (and therefore untrue) over time. Lacunar infarctions had to manifest with one of the traditional “lacunar syndromes,” they had to be less than 15 mm in size, they only happened in hypertensive patients, and they were always caused by “small vessel disease” (typically understood as lipohyalinosis according to what became known as the “lacunar hypothesis”). However, the advent of brain imaging techniques came to prove that these general assumptions cannot be always applied. First, computed tomography (CT) scan and most recently and especially magnetic resonance imaging (MRI) have reactivated research on this topic by uncovering the real dimension of the intricate disorder we now call small vessel disease to include small subcortical infarctions, microhemorrhages, and white matter changes (also known as leukoaraiosis for the rarefaction of the white matter seen on pathological specimens) (Figure 7-1).
Longitudinal studies using serial MRI scans are shedding light on the incidence, progression, and severity of cognitive decline in patients with white matter disease. As a consequence, vascular dementia is emerging as a major public health concern.5 Neuroimaging is replacing pathology in the study of the pathophysiology of small vessel strokes, a remarkable indication of progress because small subcortical strokes are not fatal and therefore pathological examinations only allow the examination of chronic lesions. In fact, new theories defying the preeminence of lipohyalinosis have been postulated to explain the genesis of small subcortical strokes.6,7 Additionally, hemosiderin-sensitive sequences (such as gradient recall echo) allow visualization of areas of microhemorrhage, which were previously impossible to diagnose in vivo.8,9
SMALL SUBCORTICAL INFARCTIONS
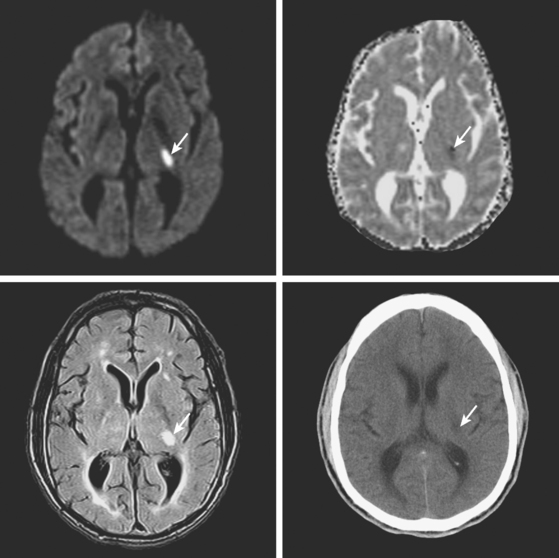
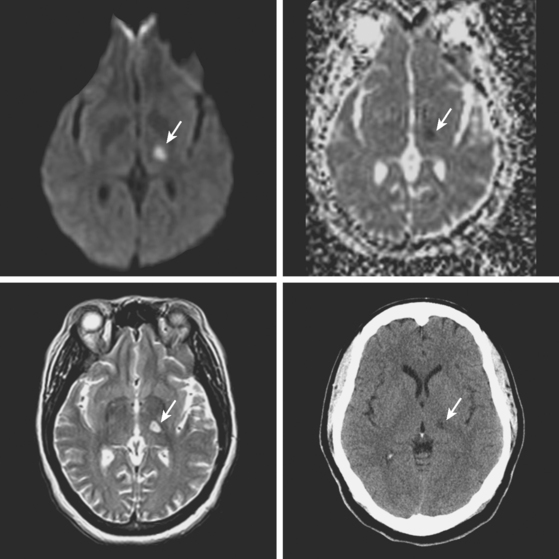
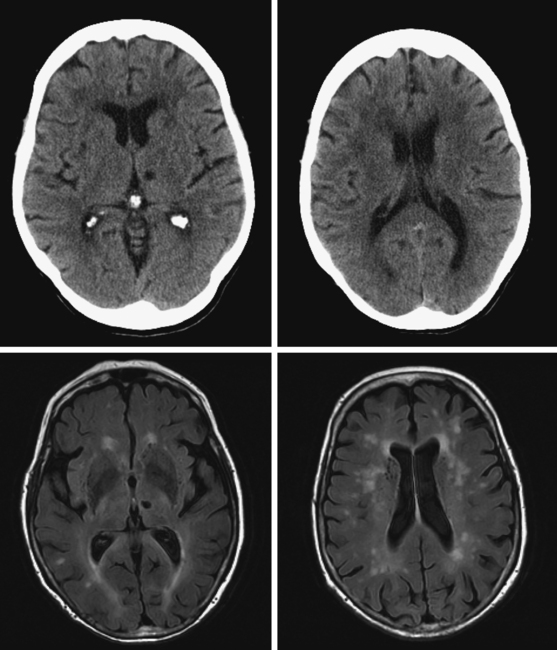
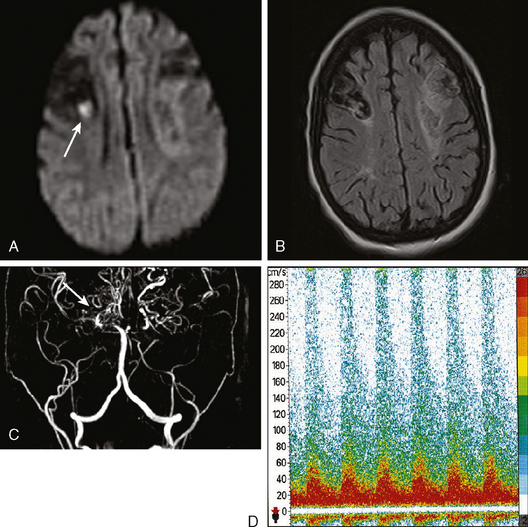
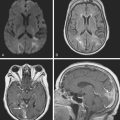
A 78-year-old man with history of hypertension and type 2 diabetes awoke with inability to move his right side. On examination, his blood pressure was 180/98, his pulse was regular, and he had no carotid bruits. He had a dense right hemiparesis with no other associated neurological deficits. Initial CT of scan of the head did not reveal any acute intracranial abnormalities. Brain MRI (Figure 7-2) showed an acute small subcortical stroke in the posterior limb of the left internal capsule. Carotid ultrasound and cardiac workup were unremarkable. The patient was discharged on antiplatelet therapy, antihypertensives, an adjusted regimen for his diabetes, and a statin. He evolved favorably over the subsequent few months.
LACUNAR SYNDROMES
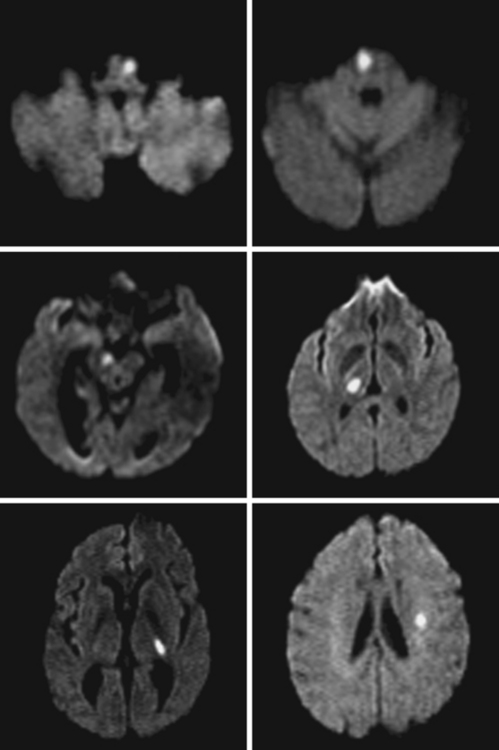
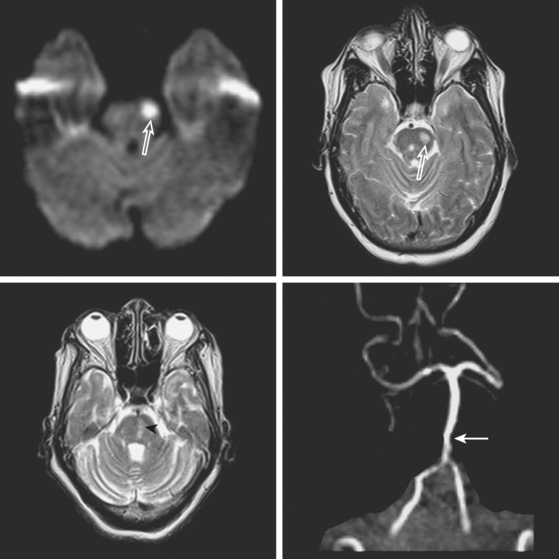
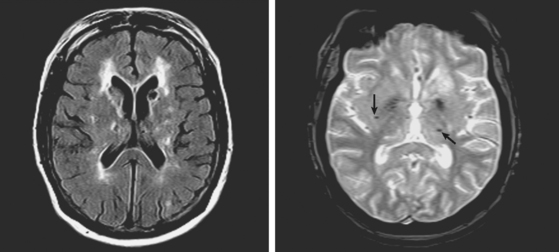
A 72-year-old obese woman with history of hypertension and metabolic syndrome presented with acute onset of right-sided numbness and weakness. Symptoms had started the day before, but she had not come to the emergency department because they had fluctuated in severity to the point that a few times she felt almost back to normal. However, over the previous 6 to 8 hours before her arrival to the emergency department, her deficits had become constant. Examination demonstrated right hemiparesis and hypoesthesia without associated cortical signs. Brain imaging confirmed the clinical suspicion that the patient had a small subcortical stroke (Figure 7-5) that had presented with an initially stuttering course. Her deficits improved over the following days, and she had nondisabling residual motor and sensory sequelae 3 months later.
STRIATOCAPSULAR INFARCTIONS
CAPSULAR WARNING SYNDROME
MULTIPLE LACUNAR INFARCTIONS
SILENT INFARCTIONS, WHITE MATTER DISEASE, AND DEMENTIA
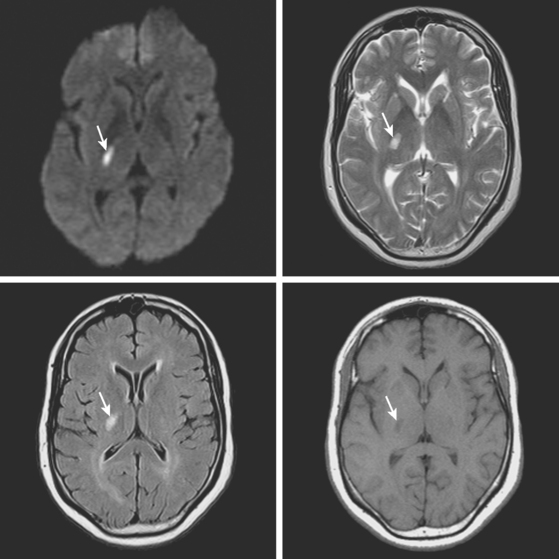
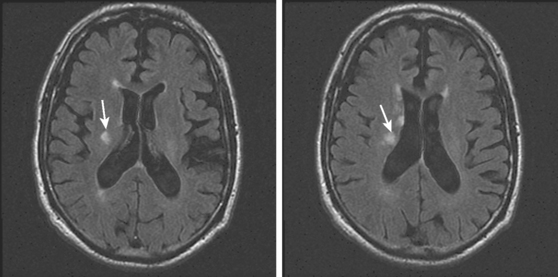
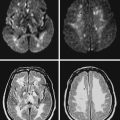
A 78-year-old woman with treated hypertension was brought to the neurological consultation by the family because of concerns about cognitive decline. The patient was a retired teacher who had been excellent at multitasking, but over the previous couple of years, she had been noticed to have increasing difficulties performing regular chores at her house. She only acknowledged some difficulties with concentration. In addition, both she and her family reported worsening gait balance. On examination, the patient had fairly pronounced cognitive problems, particularly on executive functions. Her gait was frankly apraxic. Although she denied ever having had a stroke, MRI of her brain revealed extensive white matter changes indicative of subcortical ischemia (Figure 7-10).
MICROHEMORRHAGES
1 Benavente O, White CL, Roldan AM. Small vessel strokes. Curr Cardiol Rep. 2005;7:23-28.
2 Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871-876.
3 Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol (Berl). 1968;12:1-15.
4 Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979;36:65-73.
5 Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215-1222.
6 Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005;76:617-619.
7 Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806-812.
8 Wardlaw JM, Lewis SC, Keir SL, Dennis MS, Shenkin S. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006;37:2633-2636.
9 Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536-1540.
10 Ay H, Oliveira-Filho J, Buonanno FS, Ezzeddine M, Schaefer PW, Rordorf G, et al. Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke. 1999;30:2644-2650.
11 Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. Severe ipsilateral carotid stenosis and middle cerebral artery disease in lacunar ischaemic stroke: innocent bystanders? J Neurol. 2002;249:266-271.
12 Tejada J, Diez-Tejedor E, Hernandez-Echebarria L, Balboa O. Does a relationship exist between carotid stenosis and lacunar infarction? Stroke. 2003;34:1404-1409.
13 Arboix A, Padilla I, Massons J, Garcia-Eroles L, Comes E, Targa C. Clinical study of 222 patients with pure motor stroke. J Neurol Neurosurg Psychiatry. 2001;71:239-242.
14 Hommel M, Besson G, Le Bas JF, Gaio JM, Pollak P, Borgel F, et al. Prospective study of lacunar infarction using magnetic resonance imaging. Stroke. 1990;21:546-554.
15 Rajajee V, Kidwell C, Starkman S, Ovbiagele B, Alger J, Villablanca P, et al. Diagnosis of lacunar infarcts within 6 hours of onset by clinical and CT criteria versus MRI. J Neuroimaging. 2008;18:66-72.
16 Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. Should computed tomography appearance of lacunar stroke influence patient management? J Neurol Neurosurg Psychiatry. 1999;67:682-684.
17 Rabinstein AA, Chirinos JA, Fernandez FR, Zambrano JP. Is TEE useful in patients with small subcortical strokes? Eur J Neurol. 2006;13:522-527.
18 Lindgren A, Staaf G, Geijer B, Brockstedt S, Stahlberg F, Holtas S, et al. Clinical lacunar syndromes as predictors of lacunar infarcts. A comparison of acute clinical lacunar syndromes and findings on diffusion-weighted MRI. Acta Neurol Scand. 2000;101:128-134.
19 Schonewille WJ, Tuhrim S, Singer MB, Atlas SW. Diffusion-weighted MRI in acute lacunar syndromes. A clinical-radiological correlation study. Stroke. 1999;30:2066-2069.
20 Jung S, Hwang SH, Kwon SB, Yu KH, Lee BC. The clinico-radiologic properties of deep small basal ganglia infarction: lacune or small striatocapsular infarction? J Neurol Sci. 2005;238:47-52.
21 Wessels T, Rottger C, Jauss M, Kaps M, Traupe H, Stolz E. Identification of embolic stroke patterns by diffusion-weighted MRI in clinically defined lacunar stroke syndromes. Stroke. 2005;36:757-761.
22 Gerraty RP, Parsons MW, Barber PA, Darby DG, Desmond PM, Tress BM, et al. Examining the lacunar hypothesis with diffusion and perfusion magnetic resonance imaging. Stroke. 2002;33:2019-2024.
23 Oliveira-Filho J, Ay H, Schaefer PW, Buonanno FS, Chang Y, Gonzalez RG, et al. Diffusion-weighted magnetic resonance imaging identifies the “clinically relevant” small-penetrator infarcts. Arch Neurol. 2000;57:1009-1014.
24 Arboix A, Lopez-Grau M, Casasnovas C, Garcia-Eroles L, Massons J, Balcells M. Clinical study of 39 patients with atypical lacunar syndrome. J Neurol Neurosurg Psychiatry. 2006;77:381-384.
25 Cho AH, Kang DW, Kwon SU, Kim JS. Is 15 mm size criterion for lacunar infarction still valid? A study on strictly subcortical middle cerebral artery territory infarction using diffusion-weighted MRI. Cerebrovasc Dis. 2006;23:14-19.
26 Steinke W, Ley SC. Lacunar stroke is the major cause of progressive motor deficits. Stroke. 2002;33:1510-1516.
27 Donnan GA, O’Malley HM, Quang L, Hurley S, Bladin PF. The capsular warning syndrome: pathogenesis and clinical features. Neurology. 1993;43:957-962.
28 Benito-Leon J, Alvarez-Linera J, Porta-Etessam J. Detection of acute pontine infarction by diffusion-weighted MRI in capsular warning syndrome. Cerebrovasc Dis. 2001;11:350-351.
29 Staaf G, Geijer B, Lindgren A, Norrving B. Diffusion-weighted MRI findings in patients with capsular warning syndrome. Cerebrovasc Dis. 2004;17:1-8.
30 Lammie GA, Brannan F, Wardlaw JM. Incomplete lacunar infarction (Type Ib lacunes). Acta Neuropathol (Berl). 1998;96:163-171.
31 Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214-222.
32 Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period. The Leukoaraiosis and Disability Study. Stroke. 2008.
33 Roman GC. On the history of lacunes, etat criblé, and the white matter lesions of vascular dementia. Cerebrovasc Dis. 2002;13(2 Suppl):1-6.
34 Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23-30.
35 Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184-1188.
36 Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426-436.
37 Longstreth WTJr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217-1225.
38 Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246-250.
39 Wen HM, Mok VC, Fan YH, Lam WW, Tang WK, Wong A, et al. Effect of white matter changes on cognitive impairment in patients with lacunar infarcts. Stroke. 2004;35:1826-1830.
40 Jokinen H, Kalska H, Mantyla R, Ylikoski R, Hietanen M, Pohjasvaara T, et al. White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. J Neurol Neurosurg Psychiatry. 2005;76:1229-1233.
41 Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, et al. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870-1876.
42 Smith CD, Snowdon DA, Wang H, Markesbery WR. White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology. 2000;54:838-842.
43 Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126-1129.
44 Kuller LH, Longstreth WTJr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJJr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821-1825.
45 Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536-1540.
46 Wardlaw JM, Lewis SC, Keir SL, Dennis MS, Shenkin S. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006;37:2633-2636.