CHAPTER 97 Small Intestinal Motor and Sensory Function and Dysfunction
ANATOMY
The small intestine is approximately 3 to 7 meters long and extends from the duodenal side of the pylorus to the ileocecal valve. It is divided into three regions—duodenum, jejunum, and ileum—based on structural and functional considerations. Although some structural and functional differences exist among these three regions, they exhibit similar motor characteristics. At each end of the small intestine, however, physiologic sphincters—the pylorus and the ileocecal valve—have distinctly different motor patterns that give them the ability to act as controllers of flow between the antrum and duodenum and between the ileum and colon, respectively. The motor function of the pylorus is discussed in Chapter 48, the ileocecal region is discussed in Chapter 98, and general anatomy is discussed in Chapter 96. The duodenum is a fixed, largely retroperitoneal structure located in the upper abdomen, and the distal ileum generally is anchored in the right iliac fossa by its attachment to the cecum. Except for these regions, the small intestine is mobile within the peritoneal cavity.
KEY ELEMENTS IN NORMAL SMALL INTESTINAL MOTOR AND SENSORY FUNCTION
SMOOTH MUSCLE
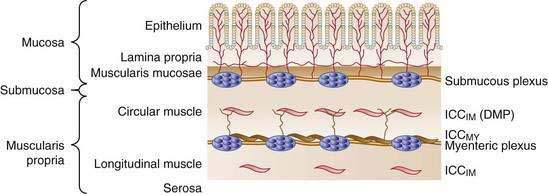
The wall of the small intestine comprises the mucosa, consisting of the epithelium and lamina propria; submucosa; muscular layer (muscularis); and serosa (Fig. 97-1). The muscularis is composed of inner circular and outer longitudinal layers of smooth muscle, which are present in continuity along the length of the small intestine. Contractions within these layers are responsible for gross small intestinal motility. A much smaller additional muscular layer, the muscularis mucosae, is present between the mucosa and the submucosa and plays a role in mucosal or villus motility1 but does not contribute to gross motility and is not considered further in this chapter.
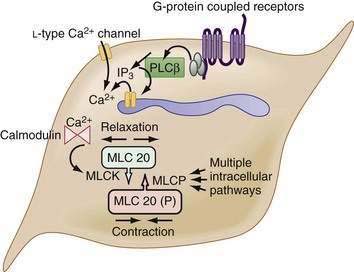
The myocytes themselves are spindle-shaped cells that derive their contractile properties from specialized cytoplasmic filaments and from the attachment of these filaments to cytoskeletal elements. On electron microscopy, condensations of electron-dense, amorphous material are noted around the inner aspect of the cell membrane (dense bands) and throughout the cytoplasm (dense bodies). The contractile filaments—actin and myosin—are arranged in a fashion similar to that in skeletal muscle and insert onto the dense bands and bodies approximately in parallel with the long axis of the cell. Thus, when the contractile filaments are activated to slide over each other, cell shortening results. Most of the Ca2+ required for activating the contractile apparatus enters the cells via L-type Ca2+ channels (Fig. 97-2). Ca2+ entry also can be supplemented to a varying extent by release of Ca2+ from the sarcoplasmic reticulum membrane via IP3 receptor–operated Ca2+ channels. IP3 is generated by phospholipase C, which in turn is activated by G-proteins, coupled to receptors for excitatory transmitters (G-protein–coupled receptors).
The increased cytoplasmic Ca2+ binds to the Ca2+ binding protein calmodulin, enabling it to activate myosin light chain kinase, which phosphorylates the 20 kD light chain of myosin (MLC20). Phosphorylation of MLC20 facilitates actin binding to myosin and initiates cross-bridge cycling and development of mechanical force. Phosphorylation of MLC20 is reduced by MLC phosphatase. Dephosphorylation of MLC20 reduces cross-bridge cycling and leads to muscle relaxation. The dephosphorylation process is under a complex system of hierarchical control, which is important in setting the gain of smooth muscle contractility.2
INTERSTITIAL CELLS OF CAJAL
Interstitial cells of Cajal (ICC) are specialized cells within the smooth muscle layer that are vital for normal small intestinal motor function. ICC are pleomorphic mesenchymal cells that form an interconnecting network via long, tapering cytoplasmic processes. ICC lie in close proximity to both nerve axons and myocytes, with which they form electrical gap junctions.3 ICC serve two roles in control of small intestinal motility: first, they act as pacemakers generating the electrical slow wave that determines the basic rhythmicity of small intestinal contractions4; second, they transduce both inhibitory and excitatory neural signals to the myocytes5 and thus can vary the myocyte membrane potential and, in turn, contractile activity. This transduction occurs because ICC are interposed functionally between nerve terminals and the smooth muscle that the nerves supply. The neuroeffector junctions of the small intestine are not just simple contacts between nerve terminals and smooth muscle cells; they are contacts between enteric nerve terminals and ICC, and from there with myocytes by means of electrical gap junctions. Thus, effective neurotransmission results from the activation of specific sets of receptors on ICC, rather than by direct action on smooth muscle cells.
Cells of the ICCMY population form a dense, electrically coupled network within the intermuscular space at the level of the myenteric plexus between the circular and longitudinal muscle layers. ICCMY are the pacemaker cells in the small intestine that trigger generation of slow waves in the smooth muscle. These cells possess a specialized mechanism that uses their oxidative metabolism to generate an inward (pacemaker) current, resulting from the flow of cations through nonselective cation channels in the plasma membrane. A primary pacemaker initiates slow waves. This depolarization from the primary event then entrains the spontaneous activity of other ICC within the network. This sequence results in a propagation-like phenomenon by which slow waves spread, without decrement, through the ICC network by means of gap junctions. A specialized type of ICCMY line the septa (ICCSEP) between circular muscle bundles; these cells form a crucial conduction pathway for spreading excitation deep into muscle bundles of the human jejunum, which is necessary for the motor patterns underlying mixing.6
ICC, in general, play broadly similar roles in the small intestine and colon, and the reader is referred to Chapter 98 for a discussion of their roles in the large bowel (see also Fig. 98-2), as well as recent reviews by Sanders, Ward, and their colleagues.4,5 Absence or inactivity of ICC has been implicated in a number of clinical disorders that manifest as disturbed intestinal motility (see Chapter 20).
NEURONS
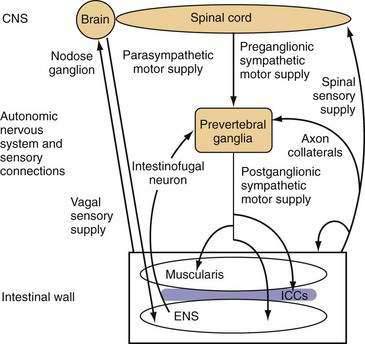
The small intestine is richly innervated with both extrinsic and intrinsic neurons. Intrinsic neurons have their cell bodies within the wall of the small intestine and constitute the ENS. These intrinsic neurons greatly outnumber the neurons of the extrinsic supply, which have their cell bodies outside the gut wall, but they have projections that end within the intestinal wall. Extrinsic neurons can be classified anatomically according to the location of their cell bodies and the route along which their projections travel. Extrinsic motor neurons belong to the ANS and connect the central nervous system (CNS) with the ENS and, from there, the small intestinal smooth muscle through the ICC. Some extrinsic motor neurons terminate directly in the muscle layers. Extrinsic sensory neurons from the small intestine do not belong to the ANS and are classified as spinal or vagal, depending on the route they follow to the CNS (Fig. 97-3).
Neurons supplying the intestine are designated either afferent or efferent, depending on the direction in which they conduct information. By convention, information is conducted centrally by afferent neurons and peripherally by efferent neurons. Thus, the term afferent in regard to neural supply is used to describe pathways conducting information that is detected in the intestine; in most texts “afferent” is interchangeable with the “sensory,” although most sensory information from the small intestine is not perceived at a conscious level. The terms efferent and motor in regard to neural supply are used to describe pathways conducting signals toward the effector small intestinal smooth muscle. Although the importance of motor innervation for motility is self-evident, the pivotal role of afferent function in determining motor responses has been less well appreciated. The importance of the extrinsic afferent innervation is emphasized by the observation that at least 80% of vagal fibers are afferent.7
Intrinsic Neurons
ENS elements of the small intestine can be subdivided into three major functional groups: primary sensory (afferent) neurons, motor (efferent) neurons, and interneurons. Other categories of neurons, including secretomotor and vasomotor neurons and motor neurons to endocrine cells, are recognized, but they are not considered further in this chapter. Many distinct groups of enteric neurons are now well characterized both structurally and functionally and are reviewed in detail elsewhere.8,9
Afferent Supply
The primary afferent neurons of the ENS morphologically are Dogiel type II neurons (neurons with numerous processes).10 Intrinsic primary afferent neurons that respond to mucosal chemical stimuli have their cell bodies in the myenteric plexus, and they project axons toward the mucosa. The myenteric plexus also contains the cell bodies of intrinsic afferent neurons that discharge in response to mechanical stimulation of the muscle layer induced by muscle activity or stretch. Intrinsic afferent neurons that respond to mechanical stimulation of the mucosa also are believed to exist, based on enteric reflexes seen in extrinsically denervated preparations. The cell bodies and processes of these neurons have not yet been identified definitively, although available evidence is consistent with the presence of their cell bodies in the submucosal ganglia.10
Intrinsic sensory neurons synapse in the intramural plexuses with intrinsic motor neurons and interneurons, which they excite mainly by release of acetylcholine and substance P. A more detailed account of the function and role of intrinsic afferent neurons can be found in a review by Furness and coworkers.10
Observations indicate that other classes of enteric neurons also respond to mechanosensory stimuli, suggesting that the ENS behaves as a sensorimotor network rather than as separate components.11
Efferent Supply
The axons of the intrinsic motor neurons that supply small intestinal smooth muscle exit the intramural ganglia and enter either the circular or the longitudinal muscle layer, where they pass in close proximity to both the myocytes and ICC. No specific neuromuscular junctions are present in small intestinal smooth muscle as in skeletal muscle, although the multiple varicosities along the motor axons probably represent specialized areas of neurotransmission. The motor axons discharge along their length, potentially activating large numbers of myocytes through ICC but possibly also directly activating them. The lack of exclusive, specific neuromuscular junctions, the electrical gap junctions among myocytes, and the overlap of innervation of myocytes from more than one motor axon mean that functionally discrete motor units in the intestinal smooth muscle do not appear to exist, in contrast with skeletal muscle. The ENS motor supply itself is both inhibitory and excitatory, and intrinsic motor neurons generally contain both a fast and a slow neurotransmitter. The predominant excitatory transmitters are acetylcholine (fast) and substance P (slow), and the predominant inhibitory transmitters are nitric oxide (fast), vasoactive intestinal polypeptide (VIP) (slow), adenosine triphosphate (ATP) (fast), and the nucleotide β-nicotinamide adenine dinucleotide (β-NAD).12
Interneurons
Interneurons connect ENS neurons of the same class or of different classes with one another. They permit local communication within limited lengths of intestinal wall (measured in millimeters or centimeters) and are implicated in simple local responses by means of release of acetylcholine or nitric oxide, depending on their oral or aboral direction of projection. Some evidence also suggests the presence of connections within the intestinal wall along greater distances, but these neural pathways are not well defined. These connections may be provided anatomically by the ENS or by connections between the ENS and ANS. Interneurons that play an additional sensory role have been identified.11
A special type of interneuron, the intestinofugal neuron, may be important for controlling local reflexes. Intestinofugal neurons have cell bodies within the myenteric plexus. These cell bodies receive input from several local enteric neurons and project to the prevertebral ganglia, where they synapse with sympathetic motor neurons (see Fig. 97-3).13
Extrinsic Neurons
Afferent Supply
The small intestine is innervated by vagal and spinal extrinsic afferents. The pathway of small intestinal vagal afferent innervation is relatively straightforward. The vagal afferent neurons have endings in the intestinal wall and cell bodies within the nodose and jugular ganglia, which deliver input directly to the brainstem. Spinal afferent fibers travel along perivascular nerves to the prevertebral ganglia, where neurons do not end but might give off axon collaterals that synapse on postganglionic sympathetic motor neurons; these fibers then pass into the thoracic spinal cord along the splanchnic nerves. Spinal afferent neurons have their cell bodies throughout the thoracic dorsal root ganglia and enter the spinal cord through the dorsal roots; they synapse mainly on neurons of the superficial laminae of the spinal gray matter. These neurons, in turn, can send projections to numerous areas of the brain involved in sensation and pain control. Spinal afferent neurons also can give off axon collaterals closer to the intestinal wall, which synapse on components of the ENS, blood vessels, smooth muscle, or secretory elements (see Fig. 97-3). The different stimulus response profiles of vagal and splanchnic mechanoreceptors are generally interpreted as evidence that vagal afferents subserve physiologic regulation, and splanchnic afferents mediate pain.14–16
Functionally, three distinct and characteristic patterns of terminal distribution can be identified within the intestinal wall. Extraluminal afferent fibers have responsive endings on blood vessels in the outer, serosal layer and in the mesenteric connections. Muscular afferents form endings either in the muscle layers or in the myenteric plexus.17 Mucosal afferents form endings in the lamina propria, where they are positioned to detect substances absorbed across the mucosal epithelium or released from epithelial and subepithelial cells, including enterochromaffin and immunocompetent cells.17
These three different populations of afferent endings have different sensory modalities, responding to both mechanical and chemical stimulation.14,18 Serosal and mesenteric afferents are found mainly in the splanchnic innervation and are activated by distortion of the intestine and its attachments; they do not normally signal distention or contraction of the bowel wall unless it is strong enough to cause distortion of the outer layers. Serosal and mesenteric receptors also commonly show evidence of chemosensitivity. This observation hints at potential responsiveness to circulating or locally released factors, especially in view of the localization of these receptors on or near blood vessels.19
Muscular afferents respond to distention and contraction with lower thresholds for activation, and they reach maximal responses within levels of distention that are encountered normally during digestion. Muscular afferents show maintained responses to distention of the small intestine and signal each contractile event, giving rise to the term in-series tension receptors. Nerve tracing studies have identified vagal afferent terminals in the longitudinal and circular muscle layers described as intramuscular arrays (IMAs), consisting of several long (up to a few millimeters) and rather straight axons running parallel to the respective muscle layer and connected by oblique or right-angled short connecting branches.17,20 IMAs were proposed to be the in-series tension receptor endings, possibly responding to both passive stretch and active contraction of the muscle, although direct evidence for this proposal is currently lacking.
Vagal afferent terminals surrounding the myenteric plexus throughout the gastrointestinal tract have been described as intraganglionic laminar endings (IGLEs). These endings are in intimate contact with the connective tissue capsule and enteric glial cells surrounding the myenteric ganglia, and they have been hypothesized to detect mechanical shearing forces between the orthogonal muscle layers. Evidence for such a mechanosensory function of IGLEs has been elaborated by mapping the receptive field of vagal afferent endings in the esophagus, stomach, and large intestine, showing morphologically that individual hot spots of mechanosensitivity correspond with single IGLEs.21 Functional evidence exists for muscular afferents in both the vagal and the spinal innervation, but the appearance of spinal distention-sensitive afferents in the small intestine is yet to be determined. It is likely to be distinct from that of vagal afferents due to their higher thresholds for distention.22
Small intestinal mucosal afferents have been found in the vagal supply, but their existence in the spinal supply can be inferred only from the fact that they exist in the colon.14,19 Mucosal afferents do not respond to distention or contraction but are exquisitely sensitive to mechanical deformation of the mucosa, as might occur with particulate material within the lumen.16,23 In the rat duodenum and jejunum, vagal afferent fibers penetrate the circular muscle layer and submucosa to form networks of multiply branching axons within the lamina propria of both crypts and villi.17 Terminal axons are in close contact with, but do not seem to penetrate, the basal lamina and thus are in an ideal position to detect substances including absorbed nutrients and mediators that are released from epithelial cells and other structures within the lamina propria.
Central Connections of Neural Control Elements
The central connections of the spinal and sympathetic supply to the gut are less well described. The spinal sensory neurons enter the spinal cord, where they synapse ipsilaterally on second-order sensory neurons and also provide direct feedback to sympathetic preganglionic motor neurons through axon collaterals. The second-order sensory neurons then ascend the spinal cord either contralaterally or ipsilaterally, after which they terminate in numerous areas,15 including the raphe nuclei and periaqueductal gray matter in the brainstem and the thalamus. The thalamus has extensive ramifications throughout the CNS. The central influence on sympathetic motor output to the small intestine is complex and not well understood, but stress and arousal level play a role. These influences have their output through the brainstem and descending tracts to the sympathetic preganglionic motor neurons in the intermediolateral horn of the spinal cord, which send their axons to the prevertebral ganglia, whereupon they synapse with sympathetic postganglionic adrenergic nerves.13
GASTROINTESTINAL HORMONES
Gastrointestinal hormones are dealt with in detail in Chapter 1, but it is important to emphasize here their vital role in modulation of small intestinal motor and sensory function. Gastrointestinal hormones relevant to small intestinal function can act in either a humoral or paracrine fashion on both enteric neurons and myocytes, and generally they are released in response to the presence (or anticipation) of enteral nutrition. The best known of these hormones include CCK, somatostatin, VIP, glucagon-like peptide-1 (GLP-1), gastric inhibitory peptide (GIP), ghrelin, and motilin. Most of the hormones released in response to the presence of food in the lumen lead to slowing of small intestinal transit, signals of satiety, and increased mixing or segmenting contractions (see later). For a detailed description of these hormones and their effects, the reader is referred to Chapter 1.
INTEGRATED CONTROL OF MOTILITY
INTERDIGESTIVE MOTOR CYCLE
Although the ENS has this regulatory capacity, normal function is modulated by ANS efferent output, which in turn may be influenced by locally or centrally processed information gathered from primary spinal or vagal afferents. In particular, synapses outside the CNS in the prevertebral ganglia are capable of subserving inhibitory intestino-intestinal reflexes that are potentially important in the minute-to-minute regulatory control of motility.13 Small intestinal neuromuscular function is also influenced by a number of hormones acting in either endocrine or paracrine fashion.
Little direct information is available on the precise contribution of each extrinsic pathway to motor function of the small intestine in humans. Vagal reflexes generally are thought to make an important contribution in the integration of major homeostatic functions, such as motility, secretion, blood flow, and the control of food and water intake.14–16 The role of sympathetic reflexes is thought to be concerned primarily with inhibition of motility and other functions in response to noxious stimuli, rather than in digestive small intestinal functions.
MECHANISMS UNDERLYING ABNORMAL MOTOR AND SENSORY FUNCTION
Infection and inflammation of the intestine can result in long-term changes in all elements, including myocytes, ICC, and intrinsic and extrinsic neurons. Symptoms in functional gastrointestinal diseases such as functional dyspepsia and irritable bowel syndrome (IBS) may be attributable partly to specific sensorimotor abnormalities occurring locally in the intestine, but they also are attributable to alterations in the extrinsic neural control system of the intestine and possibly to alterations in central perception, processing of afferent information, or both (see Chapters 13 and 118). Abnormalities in pain control systems in the brain and disordered processing of affective components of visceral sensations also have been described in these conditions24 and can produce symptoms through the central connections described in the preceding sections. Some clinical scenarios in which discrete abnormalities have been identified or hypothesized in small intestinal motility are outlined in Table 97-1.
SMOOTH MUSCLE DYSFUNCTION
It is often difficult to separate pathologic changes in the function of smooth muscle from those in neural control mechanisms; however, a number of changes can be attributed directly to alterations in smooth muscle. Cytokines play an important role in the abnormal smooth muscle function associated with gastrointestinal inflammation and infection. Different insults induce different patterns of cytokines, which in turn determine the type of infiltrating immune or inflammatory cells, which in turn release specific mediators. Thus, the resultant effect on smooth muscle function depends on the origin of disease. For example, nematode infection induces mastocytosis and eosinophilia, which lead to activation of intracellular signaling pathways in smooth muscle by IL-4 and IL-13, ultimately resulting in hypercontractility of smooth muscle.25 By contrast, chemically induced inflammation is characterized by the presence of neutrophils and macrophages among other cells. Inflammation and infection can lead to changes at sites in the small intestine distant from the affected site, and the functional effects of inflammation in smooth muscle can persist following recovery from the acute insult as is seen with post-infection IBS. Smooth muscle hyper-responsiveness may be characterized by enhanced responses to cholinergic and noncholinergic excitation and are observable in human inflammatory bowel disease.26
INTRINSIC NEURAL DYSFUNCTION
Several abnormalities of small intestinal intrinsic control are attributable to developmental dysfunction and are dealt with separately in Chapter 96. Changes in the ENS also can occur after a bout of intestinal infection or inflammation. Many of these changes are centered on the intrinsic primary afferent neurons. These neurons become more excitable because of changes in the expression of ion channels that initiate generation of action potentials and those that determine recovery of membrane potential after an action potential. Thus, the long after-hyperpolarization that characterizes intrinsic primary afferent neurons from other classes is shortened, and they are able to fire in longer trains. This ability directly affects the responses of other interneurons and motor neurons that receive inputs from these afferent neurons and that therefore are involved in intrinsic (ENS) reflexes. Changes in excitability may be observed during an acute phase of infection or inflammation,26 or for several weeks afterward,27 at least in the large intestine. These longer-term changes are referred to as plasticity and might partly explain the occurrence of exaggerated motor responses to a given stimulus in the acute phase and after recovery of mucosal lesions. Changes can result from alterations in gene expression in enteric neurons that persist beyond the initial insult, from persisting increases in locally released mediators following alterations in mucosal cell types, or from both types of responses.28
In animal models of insulin-dependent diabetes mellitus, altered levels of neuropeptides may be seen, which might explain the disordered motility noted clinically in diabetes mellitus. The only reported neuroanatomic human study in a patient with type 1 diabetes mellitus showed that ICC were markedly decreased throughout the entire thickness of the jejunum. A decrease in neuronal nitric oxide synthase, VIP, pituitary adenyl cyclase–activating peptide (PACAP), and tyrosine hydroxylase–immunopositive nerve fibers was observed in the circular muscle layer, and substance P immunoreactivity was increased.29 Although patients with type 1 diabetes mellitus and sympathetic denervation have abnormally slow gastric emptying (see Chapter 48), their transit of a liquid meal through the distal small intestine is more rapid, which might play a part in the production of diarrhea. Diabetic patients also show abnormal duodenal motility patterns such as early recurrence of phase III after a meal (see later). No consistent correlation, however, has been found between changes in manometric parameters and the degree of cardiac autonomic neuropathy, nor has any correlation yet been established between changes in enteric neurotransmitters and ICC and manometric and transit observations.
EXTRINSIC AFFERENT DYSFUNCTION
Some evidence supports the involvement of algesic mediators, including prostaglandins and purines, in changes leading to peripheral sensitization.30 Other endogenous chemical mediators, including somatostatin, can down-regulate small intestinal afferent sensitivity such that an imbalance in prosensitizing and antisensitizing mechanisms leads to a disordered sensory signal. Such mechanisms are likely clinically relevant to functional bowel disorders, such as IBS, in which increased perception of mechanical and chemical stimulation is apparent. Moreover, because these afferents also serve to trigger reflex mechanisms that control and coordinate intestinal motor function, their sensitization can contribute to chronic dysmotility, resulting in a cycle of disordered sensory and motor function.
MEASUREMENT OF SMALL INTESTINAL MOTILITY
BASIC PRINCIPLES
Spatiotemporal Measurements
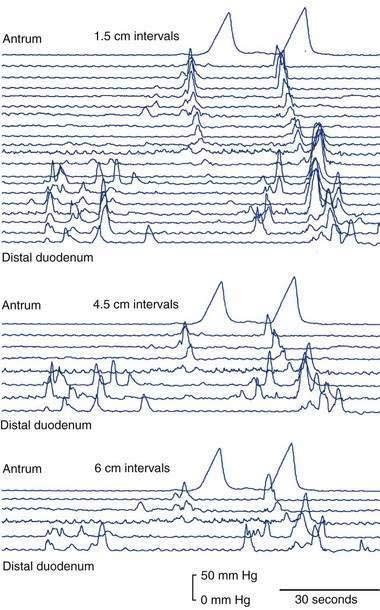
To understand the relationship between individual motor events and transport in the small intestine, the temporal resolution of the measurement technique must be greater than the duration of each discrete motor event. Based on similar principles, the spatial resolution of measurements is also an important parameter to consider if relationships between motor events and intraluminal flow(s) are to be defined. The importance of spatiotemporal resolution can be appreciated by considering Figure 97-4. Direct evaluation of small intestinal motility requires methods of measurement with a time resolution of at least two seconds, because in humans, the intrinsic frequency of duodenal contractions is up to 12 per minute. The optimal spatial resolution for studies of small intestinal motor function has not been determined, but the spatial patterning of pressures is known to vary over relatively small distances,31 with most propagating pressure wave sequences traveling less than 6 cm. Because of practical limitations of data handling and the number of sensors one can place in the small intestine, measurement techniques usually either achieve high temporospatial resolution over a short distance or low temporospatial resolution over a far greater distance. Realistically this means that data gained from different studies are usually interpreted alongside one another to provide more complete information.
Recording of Muscle Contractions
Increased muscle tension generally is directly recorded with strain gauges; these can be used in muscle strips, isolated loops of intestine, and whole-organ preparations or even chronically implanted in animals. Strain gauges are capable of excellent temporal resolution of motor events, but spatial resolution is limited by the size and number of strain gauges that are used concurrently. Over short lengths of intestine, a spatial resolution of approximately 1 cm is possible. Unfortunately, strain gauges are not suitable for use in human subjects, although they have provided much valuable information on the organization of motor events in animals.32
Muscle contractions also can be measured by surrogate measurement techniques that record associated phenomena. One such approach is fluorescence measurement of calcium transients (rapid increases in free intracellular calcium) in smooth muscle.33 Over short sections of intestine (1-2 mm), such measurements provide excellent temporospatial resolution and are helpful in elucidating neurophysiologic control rather than describing whole-organ function. Other measurement techniques that record phenomena resulting from contractions of smooth muscle include luminal manometry (reflecting intraluminal pressure increases), fluoroscopy (showing wall movement and movement of intraluminal contrast), and transit studies performed by a number of approaches.
Luminal manometry measures the change in intraluminal pressure that results mainly from lumen-occlusive or near–lumen-occlusive contractions. Fortunately, because the small intestine is tubular, with a relatively small diameter, a large portion of motor events are recognized as pressure rises. Researchers have hypothesized that contractions not resulting in a detectable change in intraluminal pressure are less important in determining flow, and therefore little mechanical information is lost by failure to detect them, but small changes in intraluminal pressure can be pivotal in producing flows in some regions of the small intestine.34 Manometry can be applied in several settings, ranging from short isolated intestinal segments in the laboratory to clinical use in humans. Modern computer-based recording systems allow excellent temporal resolution (~10 Hz), and spatial resolution can be tailored to give either close spatial resolution (intervals of 1-2 cm) over 20 to 40 cm, or wider resolution, while still covering a longer segment of small intestine. Manometric assemblies are either of the perfused side-hole or the solid-state sensor design and are capable of routinely recording at up to 22 sites.
Wall Motion and Transit Studies
Multichannel intraluminal impedance (MII) is a technique for assessing intraluminal bolus transit rather than motility. The technique is based on the different conductivities of intraluminal air and liquids compared with those of opposed sections of bowel wall. Voltage is applied to a recording assembly along which several electrodes are sited. The current recorded between electrode pairs depends on the conductivity and thickness of any air or fluid bolus straddling the electrode pair. In this fashion, MII sequentially measures the transit of a conducting bolus between electrode pairs. Recordings in the small intestine can, therefore, depend on the state of its filling,35 and motility in an empty bowel might not be assessed accurately.
In vitro techniques for detailed assessment of small intestinal wall movements reveal subtle motility patterns that cannot be detected with manometry or in vivo wall motion studies. For example, one technique using digitized video recording can measure changes in diameter and length of an immobilized segment of intestine36 and has the unique capacity to appreciate discrete changes in the longitudinal and circular muscle layers.
CLINICAL APPROACH
Multiple Intraluminal Impedance
Recording assemblies can be used to measure impedance in humans in much the same fashion as manometry. Multiple intraluminal impedance (MII) can measure episodes of bolus transit in any tubular section of the upper intestine. MII gives good spatiotemporal resolution, but owing to technical limitations of how far apart the sensors can be spaced, it cannot give continuous cover in measuring transit over long lengths of the intestine. MII is increasingly used for the clinical evaluation of esophageal motility, and in recent times it has been applied to the proximal small intestine with success.35 In combination with manometry, MII has the potential to show real-time pressure-flow relationships.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is capable of excellent spatiotemporal resolution of small intestinal wall motion and movement of intraluminal contents. Because the small intestine is not all within one plane, however, it cannot, at present, be viewed routinely all at once; it does not involve a radiation dose and thus is not time limited on this basis. Because it is also an anatomic imaging technique, it has a substantial advantage over other techniques of being able to offer additional information in the assessment of patients with suspected small intestinal motility problems. Wall thickening, fibrosis, inflammatory changes, and stenoses all can be revealed, and this information can help with directing diagnosis and even therapy.37
At present, its use in assessing small intestinal motility is restricted to units with a research interest in functional gastrointestinal MRI.38 Its use is increasing in clinical gastroenterology, however, especially for small intestinal Crohn’s disease,37 and it also has the potential to encompass clinical motility assessments.39
NORMAL IN VIVO SMALL INTESTINAL MOTILITY PATTERNS
CONTRACTIONS AT A FIXED POINT
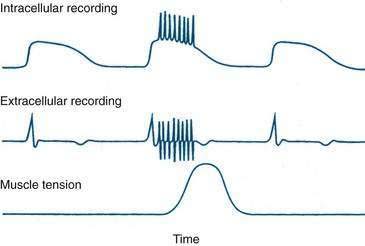
Small intestinal electrical recordings reveal continuous cyclical oscillations in electrical potential, referred to as the slow wave, basic electrical rhythm, or pacesetter potential. This slow wave is generated by the ICC (see earlier). In humans, the slow-wave frequency decreases from a peak of 12 per minute in the duodenum to approximately 7 per minute in the distal ileum. A small intestinal contraction arises when an electrical action potential, or spike burst, is superimposed on the slow wave (Fig. 97-5). Spike bursts may be caused by the intrinsic motor output from the ENS to the ICC and are likely also to be modulated by the extrinsic motor supply. Except during phase III of the IDMC (interdigestive migrating motor complex), not every slow wave leads to a phasic contraction. The region-specific frequency of the slow wave thus controls small intestinal rhythmicity by determining the timing and maximal frequency of contractions.
The rapid increases in free intracellular calcium, or calcium transients, that underlie smooth muscle contraction can be visualized with fluorescence techniques and appear to spread in a coordinated fashion over an area of smooth muscle and to extend over variable distances of the bowel wall. These calcium transients are extinguished by collision with each other or by encountering locally refractory regions.33
CONTRACTIONS THAT TRAVEL ALONG THE SMALL INTESTINE
The electrical slow wave migrates along the small intestine in an aboral direction so that each subsequent site along the intestine is depolarized sequentially. When a slow wave results in contraction, the propagation of the slow wave along the small intestine also leads to the contraction propagating along the small intestine. The propagation velocity of the slow wave thus determines the maximal rate at which contractions can travel along the small intestine. Because not every slow wave leads to a contraction, however, contractions do not always travel at this maximal rate. The distance over which muscular excitation or inhibition spreads appears to be determined by ENS influences mediated through local inhibitory and excitatory circuits.33
Contraction sequences travel aborad (in an antegrade direction) or orad (in a retrograde direction). From animal data and some human studies at high spatial resolution, it is known that a large portion of contractions travel along the small intestine, rather than remaining static, although most contractions are limited to only a few centimeters in extent.40,41 Further data are needed to determine the contribution that these short contraction sequences make to overall transit compared with the less-frequent longer sequences.
PATTERNED MOTILITY
Recordings of human small intestinal motility show isolated (stationary) phasic contractions, but often, spatial patterns are more complex. The limited spatial resolution of many recording techniques can lead to over-reporting of the fraction of stationary contractions. Commonly, phasic motor activity consists of a recognizable group of contractions associated along the small intestine in space and time; phase III activity of the IDMC (see later) is a good example of this association. Several other types of grouped small intestinal contractions have been described and include contractions associated with emesis42 and discrete clustered contractions, which are said to be common in IBS (see Chapter 118).43 The most commonly observed motor patterns in the healthy small intestine, however, are described simply as the fed or postprandial pattern and the fasting (interdigestive) pattern, or IDMC (Fig. 97-6).
Within 10 to 20 minutes of consumption of a meal, the IDMC that is in progress at the time of eating is interrupted.44 The presence of intraluminal nutrients is sensed by mucosal nutrient contact, as evidenced by the fact that portally administered or intravenous nutrients do not have the same effects as those consumed orally.45 Several neural and humoral signals result from mucosal nutrient contact and are implicated in the induction of the fed motor pattern, including vagal afferent signals, cholecystokinin, and GLP-1. Moreover, the sensing of intraluminal nutrients is relatively complex, because different types of nutrients, or variable amounts of the same nutrient, generate recognizably different motor responses.31,32,46,47 In general, the presence of unabsorbed small intestinal nutrients slows small intestine transit by decreasing the frequency and length of travel of phasic contractions, so that the rate at which a substance is absorbed limits its transit rate. In the absence of sufficient proximal small intestinal nutrient stimulation, the fasting motor pattern re-emerges four to six hours after a meal. In the absence of its interruption by intraluminal nutrients, the IDMC repeats continuously.
The small intestine also exerts negative feedback control on the rate of gastric emptying through neural and humoral means. This negative feedback is achieved by the release of neural signals and intestinal hormones that suppress phasic gastric motor activity, relax the gastric fundus, and increase tonic and phasic pyloric pressures subsequent to mucosal sensing of small intestinal nutrients.48 This process indirectly also prolongs whole-meal small intestine transit time by slowing the input of small intestinal chyme. The small intestine, in particular the duodenum, also is thought to offer direct mechanical resistance to gastric emptying by acting as a capacitance resistor49 and by reaugmenting gastric contents as a result of duodenogastric reflux.50
Fed Motor Pattern
Radiologic Observations
Early radiologic observations of the small intestine in animals described several different patterns of wall motion and transit of intestinal contents. Walter Cannon42,51 observed both localized contractions over short segments of intestine in association with to-and-fro movement of contents and intermittent episodes of propulsion of contents over greater distances caused by aborally traveling waves of peristalsis. In the fed state, the most common pattern of wall motion consisted of localized circular contractions that recurrently divided and formed short columns of chyme into new aliquots by temporary local occlusion of the lumen over distances of less than 1 to 2 cm, this pattern being labeled rhythmic segmentation.42,51 These contractions did not travel along the small intestine and did not result in much, if any, net oral movement of contents.42,51
Peristalsis also was commonly observed, often in combination with segmentation. During small intestinal nutrient loading, peristalsis was noted to have two forms: One was a slow advance of chyme over short distances in association with segmentation and the other was a rapid transit of chyme over longer distances, sometimes several loops, of the small intestine. The “fast peristalsis” was often seen in the cat duodenum.51 Similar observations have been made in other animal species32,42 and correlate with some of the motor patterns seen during clinical radiologic studies in humans (although these studies usually are performed when the subject is fasting and show the rapid peristaltic pattern more than the segmenting postprandial activity).
Transit Time Observations
The small intestinal transit time for a meal varies greatly according to the amount and nature of what is consumed, because both caloric content and physical form of a meal determine the gastric emptying rate and the rate of transport along the intestine.41,52–54 Depending on the test and parameter used, postprandial orocecal transit time usually is less than six hours. As assessed by lactulose breath testing, however, orocecal transit time can be as rapid as about 70 minutes with low nutrient loads. A systematic evaluation of the optimal conditions for nutrient loading is much needed to reveal abnormal small intestinal motor function, using transit studies.
Manometric Observations
Postprandial small intestinal motility is characterized by irregular phasic pressure waves without a discernible cyclical pattern. Most small intestinal motility data are quite limited in spatial resolution because of the length of the small intestine. Nevertheless, most phasic pressures (pressure wave sequences) are thought to travel only a short distance31,32 and probably represent the mixing and segmenting contractions noted in earlier radiologic studies.42,51 In animal studies, postprandial small intestinal motility is more segmenting than is fasting phase II activity, and phasic pressures occur less frequently and travel shorter distances along the bowel, resulting in slower transit of the contents.32 A similar suppression in the frequency of pressure wave sequences now has been found in the human duodenum.31 This segmenting motor pattern is thought to assist in mixing food with digestive enzymes and in maximizing the exposure of food to the mucosa to optimize absorption.
Fasting Motor Pattern
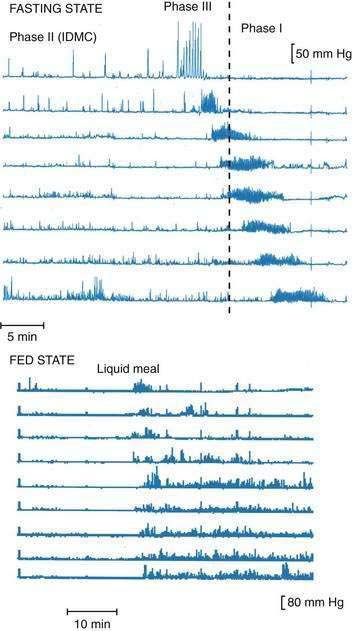
During fasting, small intestinal motor activity adopts a repetitive cyclic motor pattern, the IDMC. The IDMC is absent in a number of disease states, presumably because of a primary neuropathic process. This absence is associated clinically with stasis of small intestinal contents, malabsorption, and small intestinal bacterial overgrowth. For detailed reviews, see the articles by Husebye43 and Sarna.44
Manometric Observations
The IDMC is defined manometrically and comprises three main phases. Phase I is defined as motor quiescence (less than three pressure waves per 10 minutes at any one site); phase II is characterized by random pressure waves at less than the maximal rate; and phase III is characterized by pressure waves at the maximal rate (for the region) for longer than two minutes and, ideally, extending over a segment longer than 40 cm. Some authors also include a fourth phase (phase IV) as a transitional period between phases III and I, although this approach is not universal. Phases I and III are quite distinctive and easily recognized, whereas phase II can be recognized reliably only when sandwiched between phases I and III, because it superficially resembles the fed pattern. The phases of the IDMC start proximally and migrate distally over variable distances; few phase IIIs reaching the ileum.40 Phase III of each IDMC can start at any of a variety of locations; approximately one third of IDMCs have a gastroduodenal component, and most onsets of phase III occur near the proximal jejunum.40 Because of the length of the small intestine and the velocity of travel of the IDMC, one part of the small intestine can be in phase I while other parts are in phase II or III (see Fig. 97-6). The normal periodicity of the IDMC varies greatly both within and between subjects; however, its median duration is 90 to 120 minutes.
CLINICAL CONSEQUENCES OF DISORDERED SMALL INTESTINAL MOTILITY
The most important diseases and clinical settings associated with abnormal small intestinal motility are listed in Table 97-1. Because these disorders are covered elsewhere in this book, they are mentioned here only with regard to the associated small intestinal motor disturbances.
In IBS, a number of abnormalities of visceral sensation have been documented. These sensory abnormalities probably also lead to disordered motility; however, whereas motor abnormalities have been documented in some patients with IBS, they are absent in others (see Chapter 118). Because it appears likely that IBS is an as-yet-undefined generalized enteric neuropathy or low-grade neuroinflammatory disorder,55,56 failure to detect specific motor abnormalities might simply reflect our current poor understanding of normal small intestinal motor physiology and the relatively gross measures by which motility has been assessed in patients with IBS.
Small intestinal motility is severely disrupted in acutely ill persons and is increasingly recognized as an important factor to consider in postoperative and intensive care unit patients. Such motility disturbances likely result from several factors, including sepsis and drugs, which disrupt the slow wave rhythm; abdominal trauma and surgery, which stimulate reflex motor responses; and inflammatory mediators and cytokines, which affect neurotransmission within the CNS, ANS, and ENS. For a more detailed review, see the articles by Ritz and colleagues,57 and Chapman and colleagues.58
Pregnancy is known to alter the function of the lower esophageal sphincter, delay gastric emptying and disturb the frequency of gastric slow waves, and it is often associated with constipation. In view of these widespread findings related to altered intestinal motility, it is likely that small intestinal motor function also is altered. In guinea pigs, the strength of the contraction of intestinal circular smooth muscle has been shown to be impaired during pregnancy by down-regulation of Gαq/11 proteins (which mediate contraction) and up-regulation of Gs alpha protein (which mediates relaxation).59 It is intriguing that G protein associations now are also being reported in functional gastrointestinal disorders, suggesting a final common pathway for sensorimotor intestinal disturbances.60
APPROACH TO PATIENTS WITH POSSIBLE SMALL INTESTINAL MOTOR DYSFUNCTION
Special investigations may be indicated to answer particular questions. No standard approach has been recognized, however, and local interest and expertise often determine which investigations are available. Fluoroscopy is widely available and can help exclude medically or surgically treatable problems. Endoscopy with small intestinal biopsy or aspiration is useful if celiac sprue, small intestinal bacterial overgrowth, or intestinal infection is considered likely. Analysis of stool may be necessary to exclude malabsorptive or secretory causes of small intestinal diarrhea. Small intestinal manometry, if available, can help distinguish neuropathic from myopathic forms of disordered motility, although in many settings, the abnormalities associated with these two forms overlap (see Table 97-1). Manometry can show features typical of intestinal obstruction, although abdominal imaging by a variety of radiologic techniques is a better tool to identify an obstruction. In selected cases, full-thickness biopsy of the small intestine is necessary, but such biopsy should be performed only in centers with expertise in immunohistochemistry of intestinal neurons, because standard histologic approaches often yield little useful information.
Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28-33. (Ref 17.)
Blackshaw LA, Gebhart GF. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol. 2002;2:642-649. (Ref 14.)
Cannon WB. The Mechanical Factors of Digestion. London: Edward Arnold; 1911. (Ref 42.)
Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87-96. (Ref 8.)
Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517(Pt 2):575-90. (Ref 36.)
Hunt JN, Smith JL, Jiang CL. Effect of meal volume and energy density on the gastric emptying of carbohydrates. Gastroenterology. 1985;89:1326-30. (Ref 53.)
Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil. 1999;11:141-61. (Ref 43.)
Krauter EM, Strong DS, Brooks EM, et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990-1000. (Ref 27.)
Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20(Suppl 1):39-53. (Ref 2.)
Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307-43. (Ref 4.)
Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985;89:894-913. (Ref 44.)
Schwizer W, Steingoetter A, Fox M. Magnetic resonance imaging for the assessment of gastrointestinal function. Scand J Gastroenterol. 2006;41:1245-60. (Ref 38.)
Vermillion DL, Huizinga JD, Riddell RH, Collins SM. Altered small intestinal smooth muscle function in Crohn’s disease. Gastroenterology. 1993;104:1692-9. (Ref 26.)
Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675-82. (Ref 5.)
Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149-58. (Ref 9.)
1. Lee JS. Relationship between intestinal motility, tone, water absorption and lymph flow in the rat. J Physiol. 1983;345:489-99.
2. Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20(Suppl 1):39-53.
3. Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653-8.
4. Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307-43.
5. Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675-82.
6. Lee HT, Hennig GW, Fleming NW, et al. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007;133:907-17.
7. Agostoni E, Chinnock JE, De Burgh-Daly M, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182-205.
8. Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87-96.
9. Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149-58.
10. Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143-64.
11. Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007;19:869-78.
12. Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359-64.
13. Furness JB. Intestinofugal neurons and sympathetic reflexes that bypass the central nervous system. J Comp Neurol. 2003;455:281-4.
14. Blackshaw LA, Gebhart GF. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol. 2002;2:642-649.
15. Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95-138.
16. Grundy D, Scratcherd T. Sensory afferents from the gastrointestinal tract. In: Wood JD, editor. Handbook of Physiology: The Gastrointestinal System, Motility, and Circulation, Vol. I. Washington, DC: American Physiological Society; 1989:593-620.
17. Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28-33.
18. Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2-5.
19. Hicks GA, Coldwell JR, Schindler M, et al. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J Physiol. 2002;544:861-9.
20. Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1-26.
21. Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249-55.
22. Booth CE, Kirkup AJ, Hicks GA, et al. Somatostatin sst2 receptor–mediated inhibition of mesenteric afferent nerves of the jejunum in the anesthetized rat. Gastroenterology. 2001;121:358-69.
23. Cottrell DF, Iggo A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. J Physiol. 1984;354:497-522.
24. Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-24.
25. Zhao A, McDermott J, Urban JFJr, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948-54.
26. Vermillion DL, Huizinga JD, Riddell RH, Collins SM. Altered small intestinal smooth muscle function in Crohn’s disease. Gastroenterology. 1993;104:1692-9.
27. Krauter EM, Strong DS, Brooks EM, et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990-1000.
28. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-64.
29. He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427-34.
30. Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280:G787-94.
31. Andrews JM, Doran SM, Hebbard GS, et al. Nutrient-induced spatial patterning of human duodenal motor function. Am J Physiol Gastrointest Liver Physiol. 2001;280:G501-9.
32. Huge A, Weber E, Ehrlein HJ. Effects of enteral feedback inhibition on motility, luminal flow, and absorption of nutrients in proximal gut of minipigs. Dig Dis Sci. 1995;40:1024-34.
33. Stevens RJ, Publicover NG, Smith TK. Induction and organization of Ca2+ waves by enteric neural reflexes. Nature. 1999;399:62-6.
34. Hausken T, Mundt M, Samsom M. Low antroduodenal pressure gradients are responsible for gastric emptying of a low-caloric liquid meal in humans. Neurogastroenterol Motil. 2002;14:97-105.
35. Chaikomin R, Wu KL, Doran S, et al. Concurrent duodenal manometric and impedance recording to evaluate the effects of hyoscine on motility and flow events, glucose absorption, and incretin release. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1099-104.
36. Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517(Pt 2):575-90.
37. Girometti R, Zuiani C, Toso F, et al. MRI scoring system including dynamic motility evaluation in assessing the activity of Crohn’s disease of the terminal ileum. Acad Radiol. 2008;15:153-64.
38. Schwizer W, Steingoetter A, Fox M. Magnetic resonance imaging for the assessment of gastrointestinal function. Scand J Gastroenterol. 2006;41:1245-60.
39. Patak MA, Froehlich JM, von Weymarn C, et al. Non-invasive measurement of small-bowel motility by MRI after abdominal surgery. Gut. 2007;56:1023-5.
40. Kellow JE, Borody TJ, Phillips SF, et al. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386-95.
41. Lin HC, Zhao XT, Wang L. Jejunal brake: inhibition of intestinal transit by fat in the proximal small intestine. Dig Dis Sci. 1996;41:326-9.
42. Cannon WB. The Mechanical Factors of Digestion. London: Edward Arnold; 1911.
43. Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil. 1999;11:141-61.
44. Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985;89:894-913.
45. Gielkens HA, van den Biggelaar A, Vecht J, et al. Effect of intravenous amino acids on interdigestive antroduodenal motility and small bowel transit time. Gut. 1999;44:240-5.
46. Rao SS, Lu C, Schulze-Delrieu K. Duodenum as a immediate brake to gastric outflow: a videofluoroscopic and manometric assessment. Gastroenterology. 1996;110:740-7.
47. Rao SS, Safadi R, Lu C, Schulze-Delrieu K. Manometric responses of human duodenum during infusion of HCl, hyperosmolar saline, bile and oleic acid. Neurogastroenterol Motil. 1996;8:35-43.
48. Horowitz M, Dent J. The study of gastric mechanics and flow: a Mad Hatter’s tea party starting to make sense? Gastroenterology. 1994;107:302-6.
49. Shirazi S, Schulze-Delrieu K, Brown CK. Duodenal resistance to the emptying of various solutions from the isolated cat stomach. J Lab Clin Med. 1988;111:654-60.
50. Hausken T, Odegaard S, Matre K, Berstad A. Antroduodenal motility and movements of luminal contents studied by duplex sonography. Gastroenterology. 1992;102:1583-90.
51. Cannon WB. The movements of the intestines studied by means of the rontgen rays. Am J Physiol. 1902;6:251.
52. Benini L, Castellani G, Brighenti F, et al. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut. 1995;36:825-30.
53. Hunt JN, Smith JL, Jiang CL. Effect of meal volume and energy density on the gastric emptying of carbohydrates. Gastroenterology. 1985;89:1326-30.
54. Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol. 1990;259:G1031-6.
55. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702.
56. Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-20.
57. Ritz MA, Fraser R, Tam W, Dent J. Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol. 2000;95:3044-52.
58. Chapman MJ, Nguyen NQ, Fraser RJ. Gastrointestinal motility and prokinetics in the critically ill. Curr Opin Crit Care. 2007;13:187-94.
59. Chen Q, Xiao ZL, Biancani P, Behar J. Downregulation of Gαq-11 protein expression in guinea pig antral and colonic circular muscle during pregnancy. Am J Physiol. 1999;276:G895-900.
60. Holtmann G, Siffert W, Haag S, et al. G-protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126:971-9.