Chapter 41 Small Cell Lung Cancer
During 2009, lung cancer was diagnosed in an estimated 219,440 patients and caused an estimated 159,390 deaths in the United States.1 About 15% of patients with lung cancer have SCLC, and of these, 30% have L-SCLC.2 Very few SCLC patients have stage I disease. Lung cancer is a disease of the elderly, with a median age of 71 years at diagnosis.3
The natural history of untreated SCLC included rapid tumor progression, with a median survival time of only 2 to 4 months.4 Until the late 1960s, physicians did not differentiate the management of SCLC from that of NSCLC, and clinical trials in the 1970s continued to include both major histologic types. Investigators recognized that most patients with SCLC had poor survival times following resection and/or thoracic radiotherapy, with little apparent survival benefit from either treatment. A major change in management occurred in the late 1960s and was linked to the recognition that SCLC was far more responsive to chemotherapy than NSCLC.5
Pathologic Findings And Pathways Of Spread
The 2004 World Health Organization (WHO) classification defined SCLC as “a malignant epithelial tumor consisting of small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli.” The cells are round, oval, and spindle shaped. Nuclear molding is prominent. Necrosis is extensive, and the mitotic count is high. SCLC occurs in two variant forms: small cell carcinoma and combined small cell carcinoma. The latter type includes SCLC cells and any of the histologic types of NSCLC.6
Biologic And Molecular Biologic Characteristics
Cigarette smoke is a powerful mutagen that is strongly associated with the development of SCLC. Acquired hypermethylation of the promoter region of key genes has become a common mechanism that tumors use to inactivate tumor suppressor genes. A number of genetic mutations are frequently observed in SCLC tumors, which frequently involve tumor suppressor genes. The mutations lead to dysfunction of these suppressor molecules and then to unrestricted tumor growth. The most common mutations are deletions in the short arm of chromosome 3 in the 3p14-23 region (>80% of SCLC cases), inactivation of the retinoblastoma (RB) gene on chromosome 13 (90%), and mutations of the TP53 tumor suppressor gene on the short arm of chromosome 17 (>80%) (Table 41-1). There has been substantial progress in understanding the relationship of the molecular abnormalities that cause normal bronchial epithelium to become carcinoma. However, there has not yet been much progress in preventing these events or reversing them once they have taken place.
| Molecular Abnormality | SCLC (%) | NSCLC (%) |
|---|---|---|
| RAS mutation | <1 | 30-40 |
| MYC amplification | 30 | 10 |
| EGFR expression | NR | 40-80 |
| ERBB2 (HER2) overexpression | 10 | 30 |
| KIT (SCFR) coexpression | 70 | 15 |
| BCL2 expression | 95 | 35 |
| TP53 mutation | 75-100 | 50 |
| RB1 deletion (loss of RB1 protein) | 90 | 20 |
| CDKN2A (pl6) inactivation | <1 | 70 |
| COX-2 expression | NR | 70 |
| 3p deletion | 90 | 50 |
| VEGF expression | >100-fold variation | |
| Matrix metalloproteinase (gelatinase) | 50 | 65 |
| Neuropeptides | 90 | NR |
BCL2, B-cell lymphoma 2; CDKN2A, cyclin-dependent kinase inhibitor 2A gene (formerly designated pl6); cKit, tyrosine-protein kinase Kit; COX-2, cyclooxygenase-2; EGFR, epidermal growth factor receptor; ERBB (HER2), human epidermal growth factor receptor 2; NR, not reported; NSCLC, non–small cell lung cancer; RAS, rat sarcoma; RB1, retinoblastoma gene; SCFR, stem cell factor receptor; SCLC, small cell lung cancer; TP53, tumor protein 53; VEGF, vascular endothelial growth factor; 3p deletion, deletion in the short arm of chromosome 3 in the 3p14-23 region.
Adapted from Dye GK, Adjei AA: Novel targets for lung cancer therapy. Part I. J Clin Oncol 20:2881-2894, 2002.
The chromosome 3 genetic deletions have been observed in both dysplastic and preneoplastic changes and have been demonstrated as the genetic mutations associated with the transformation of precancerous lesions into carcinoma. Recently emerged evidence has shown that the critical deletion in SCLC and many other malignant diseases may be in a fragile portion of chromosome 3 known as the fragile histidine triad gene (FHIT gene).7,8 Mutations in the FHIT gene are found in many different cancers, suggesting that defects in these genes may have a role in tumor development. Inactivation of the RB gene most likely results in a loss of control of cell growth. It is thought that a functional RB gene keeps the G1/S cell cycle boundary in check and that its inactivation will result in uncontrolled growth.9 The TP53 mutations specific to SCLC have also been observed in preneoplastic lesions and appear to most closely resemble TP53 mutations observed in other malignant diseases for which tobacco is a known carcinogen. It is likely that the TP53 mutations in SCLC impair the ability of tumor cells to undergo apoptosis in response to various therapies.10 Another growth regulator, which is overexpressed in 95% of SCLC tumors, is BCL2. This overexpression may prevent the tumor’s apoptotic response to therapy.11
Amplification or overexpression of the myc family of oncogenes is often observed, most notably, c-myc, N-myc, and L-myc. It appears that abnormalities are more often observed in recurrent tumors, tumors with variant rather than classic SCLC, or tumors with a more aggressive and unfavorable prognosis. This has led to the concept that the overexpression of the myc oncogenes is a relatively late event in the pathogenesis of SCLC.12
Another biologic feature that distinguishes SCLC from NSCLC is the more common expression of neuroendocrine markers in SCLC. These neuroendocrine markers include enzymes such as neuron-specific enolase and L-dopa decarboxylase, peptide hormones such as gastrin-releasing peptide and arginine vasopressin, and surface markers such as neural cell adhesion molecule. The two peptide hormones meet the criteria of autocrine growth factors, which require the production of a growth-promoting protein for which the producing cell has functional receptors. In the case of gastrin-releasing peptide, there is clear evidence that it is produced and secreted by many SCLC cells and then attaches to its cellular membrane receptors, stimulating tumor growth.13
Clinical Manifestations, Patient Evaluation, And Staging
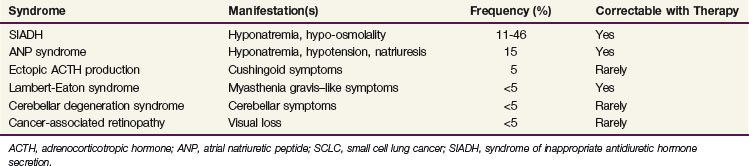
SCLC is the most common solid tumor to have a number of associated paraneoplastic syndromes. Several of these are endocrinologic and neurologic syndromes. The most common endocrinologic abnormality is the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). This condition results from the excessive secretion of antidiuretic hormone from tumor tissue, leading to severe hyponatremia with resultant hypo-osmolality. SIADH occurs in 11% to 46% of SCLC patients and typically resolves after response to anticancer therapy. Restriction of free water intake is critical to maintaining proper sodium concentrations before SIADH improves secondary to cancer therapy.14 Table 41-2 lists paraneoplastic syndromes associated with SCLC.15
The neurologic syndromes associated with SCLC include Lambert-Eaton syndrome, cerebellar degeneration syndrome, encephalomyelitis, sensory neuropathy, and cancer-associated retinopathy. Each of these is observed in well under 5% of SCLC patients. Lambert-Eaton syndrome is an autoimmune disorder that affects calcium channels of the neuromuscular junction. Antibodies are directed against the calcium channels responsible for the presynaptic release of acetylcholine. These antibodies prevent the opening of calcium channels and, therefore, the release of acetylcholine. Patients with Lambert-Eaton syndrome present with myasthenia gravis–like symptoms of proximal myopathy, autonomic dysfunction, and hyporeflexia. Like many paraneoplastic syndromes, this condition generally improves with response to anticancer therapy, although there can also be symptomatic responses to antimyasthenia therapies.16 The other neurologic syndromes are thought to be primarily autoimmune phenomena and usually respond poorly to cancer therapy.17,18
The Veterans Administration Lung Cancer Study Group staging system, which distinguishes between L-SCLC and E-SCLC is most commonly used.19 The initial definition of limited disease was an extent of intrathoracic disease that could be encompassed within a “reasonable” radiation field. Investigators in recent years have recognized the dangers of variable interpretations, and recent cooperative group trials require the staging of SCLC patients with the standard TNM system. However, the categorization of SCLC lesions as limited-stage versus extensive-stage disease has been extremely helpful for making rational treatment choices and designing trials.
Approximately two thirds of SCLC patients have extensive-stage, or stage IV, disease at presentation. Staging evaluation would traditionally include a history and physical examination, complete blood cell count, chemistry panel, contrast-enhanced CT scanning of the thorax and upper abdomen, pulmonary function testing, and CT or magnetic resonance imaging (MRI) scanning of the brain. Positron emission tomography (PET) scans are quite accurate for the staging of this SCLC. PET scans can aid with the choice of appropriate therapy and with radiation therapy planning by better identifying the tumor.20 PET/CT scanning can replace the traditional radiographic staging studies for SCLC with the exception of CT or MRI of the brain because uptake of the tracer is high in the brain and metastases are difficult to distinguish from normal brain tissue.
There have been several efforts to identify prognostic factors other than staging as a means of better selecting patients for specific therapies. As with many malignant diseases, good performance status, young age, and female gender are associated with a better prognosis, and these factors have been verified in large multivariate analyses.21,22,23,24 The Mayo Clinic/North Central Cancer Treatment Group (NCCTG) recently evaluated 1598 patients with SCLC to determine prognostic factors. Multivariate analysis revealed that performance status, age, gender, number of metastatic sites, and baseline creatinine levels were all associated with survival rates of E-SCLC patients. Among L-SCLC patients, only age and gender were associated with survival rates.24 One critical reason for determining prognostic factors is to help in selecting and stratifying patients for well-designed trials, so that investigators can decrease the possibility that uncontrolled biases will cloud the results.
Treatment
Thoracic Radiotherapy
After the demonstration in the late 1960s of the activity of several chemotherapeutic agents and the poor prognosis of patients treated with surgery and/or radiation alone, chemotherapy became the primary therapy for SCLC.25,26 Unfortunately, recurrence inevitably followed the response to chemotherapy, and the relapses were most frequently in areas of previous disease. This pattern of failure led investigators to reexamine the use of thoracic radiation therapy for L-SCLC. Today, both radiation therapy and chemotherapy have central roles in the treatment of SCLC.27,28 A series of randomized trials were performed comparing chemotherapy alone with chemotherapy with thoracic radiation therapy.29–31,32 In 1992, two meta-analyses were published regarding the role of thoracic radiation therapy in addition to chemotherapy.2,32 Pignon and colleagues32 reported a 3-year survival rate of 14.3% with combined-modality therapy compared with 8.9% with chemotherapy alone (p = .001). This 5.4% absolute difference in 3-year survival rates was identical to the 5.4% difference in 2-year survival rates (p <.001) reported by Warde and Payne.2 Although this 5.4% difference appears rather small, it represented a 61% increase in the 3-year survival rate of 8.9% achieved with chemotherapy alone.32 In addition, the intrathoracic tumor control rate was improved by 25% with thoracic radiation therapy.2
Sequencing and Timing of Thoracic Radiation Therapy and Chemotherapy
Chemotherapy and thoracic radiation therapy can be delivered concurrently, sequentially, or in an alternating manner. Potential advantages of concurrent delivery include the shorter overall treatment time, an increase in treatment intensity, and potential anticancer synergism between the various therapies. Disadvantages include the heightened toxicity and the inability to assess the antitumor response rate of the chemotherapy alone. The Japanese Clinical Oncology Group performed a phase III trial in which L-SCLC patients were randomized to sequential or concurrent therapy. All 231 patients received four cycles of etoposide-cisplatin therapy every 3 weeks (sequential arm) or 4 weeks (concurrent arm) and were randomized to receive thoracic radiation therapy during the first cycle of chemotherapy in the concurrent arm or after the fourth cycle in the sequential arm. Thoracic radiation therapy included 45 Gy (1.5 Gy twice daily) over 3 weeks. Concurrent therapy yielded a trend toward better survival times than sequential therapy (p = .097). The median survival time was 19.7 months in the sequential arm versus 27.2 months in the concurrent arm. The 5-year survival rate for patients treated sequentially was 18.3% versus 23.7% for those treated concurrently. Hematologic toxicity was more severe in the concurrent arm, as was esophagitis, which occurred in 9% in the concurrent arm and 4% in the sequential arm. The authors concluded that the findings strongly suggested that concurrent therapy was more effective for L-SCLC.33
There have been conflicting results from the randomized trials that have addressed the issue of timing of thoracic radiation therapy during chemotherapy. However, recent meta-analyses help make sense of the contradictory data. One study analyzed randomized trials published after 1985 addressing the timing of thoracic radiotherapy relative to chemotherapy in L-SCLC. Early thoracic radiation therapy was defined as radiation therapy initiated less than 9 weeks after the start of chemotherapy, and late thoracic radiation therapy as radiation therapy initiated ≥9 weeks or longer after the start of chemotherapy. Seven trials (n = 1524 patients) met the inclusion criteria and were included in the analysis. The relative risk of survival for early thoracic radiation therapy compared with late thoracic radiation therapy for all studies was 1.17 (p = .03), indicating an increased 2-year survival rate for early-therapy patients. This translated to a 5.2% (p = .03) improvement in the 2-year survival rate for early-therapy patients.34 A subsequent meta-analysis investigated the influence of the time interval between the start of any treatment until the end of thoracic radiation therapy (SER) on rates of local tumor control, survival, and esophagitis. There was a significantly higher 5-year survival rate in the shorter SER arms (relative risk [RR] = 0.62; p = .0003), which was more than 20% when the SER was less than 30 days. A low SER was also associated with a higher incidence of severe esophagitis (RR = 0.55; p <.0001). Each week of extension of the SER beyond that of the study arm with the shortest SER resulted in an overall absolute decrease in the 5-year survival rate of 1.83%. Therefore, earlier, shorter, more intense thoracic radiation therapy programs improved survival rates.35
Thoracic Radiation Therapy Doses
SCLC is considered a radioresponsive malignant tumor because low doses of thoracic radiation therapy, previously used, produced encouraging responses. Total thoracic radiation therapy doses for L-SCLC have ranged from 25 to 30 Gy in 10 fractions in the 1970s to up to 70 Gy in 35 fractions in recent years. Doses in the lower end of this range were acceptable when chemotherapy was less effective and disseminated disease occurred earlier in the disease course. Improvements in systemic therapy have increased the need for aggressive thoracic radiation therapy regimens that produce more durable responses. Choi and Carey36 estimated that the risk of intrathoracic tumor failure at total doses of 40 Gy or less was 80%, and this was confirmed in a National Cancer Institute of Canada (NCIC) L-SCLC trial in which patients were randomized between 25 Gy in 10 fractions versus 37.5 Gy in 15 fractions.37 The 2-year actuarial rates of local failure were 80% and 69%, respectively.
The most commonly administered doses of thoracic radiation therapy range from 45 to 70 Gy in 1.8- to 2-Gy daily fractions. Most trials estimated the local control rates in this dose range to be between 58% and 85%.38 The Cancer and Leukemia Group B (CALGB) found a dose of 70 Gy in 35 daily fractions to be both tolerable and effective. Eligible patients received two cycles of induction paclitaxel and topotecan with granulocyte colony-stimulating factor support, followed by three cycles of carboplatin and etoposide. Thoracic radiation therapy was initiated with the first cycle of carboplatin and etoposide. Prophylactic cranial irradiation was offered to patients achieving a favorable response. Nonhematologic grade 3 to 4 toxicities affecting more than 10% of patients, during or after thoracic radiation therapy, were dysphagia (16% and 5%) and febrile neutropenia (12% and 4%), respectively. The median overall survival time was 22 months. The researchers concluded that 70 Gy daily of thoracic radiation therapy could be delivered safely. They hypothesized that high-dose once-daily therapy resulted in comparable or improved survival rates compared with twice-daily therapy and that the theory warranted testing in a phase III trial.39
Altered Fractionation in Thoracic Radiation Therapy
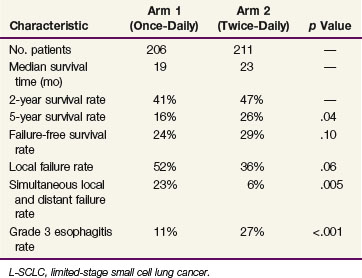
Several encouraging pilot studies led to the development of a phase III trial led by the Eastern Cooperative Group (ECOG) testing the concept that accelerated hyperfractionation would improve the outcome for L-SCLC patients. The experimental regimen was 45 Gy in 1.5-Gy twice-daily fractions beginning on day 1 of a four-cycle regimen of etoposide plus cisplatin. The interfraction interval was 6 to 8 hours, and the elapsed treatment time was 19 to 21 days. A total of 419 patients were randomized between the thoracic radiation therapy regimens of 45 Gy in 1.8-Gy once-daily fractions versus 45 Gy in 30 1.5-Gy twice-daily fractions. Of these, eight treatments were often given with oblique fields off the spinal cord to limit the dose to the spinal cord to 36 Gy. In both arms, the thoracic radiation therapy began on day 1 of a four-cycle course of etoposide plus cisplatin. There was a significant survival advantage for the patients who received twice-daily therapy as compared with those who received once-daily therapy, with 5-year survival rates of 26% versus 16%, respectively (p = .04). The median survival time was 19 months for the once-daily therapy group and 23 months for the twice-daily therapy group. The intrathoracic tumor failure rate was 36% for the twice-daily therapy arm and 52% for the once-daily therapy arm (p = .06). The principal difference in toxicity was a higher rate of grade 3 esophagitis in the twice-daily therapy arm (27% vs. 11%; p <.001).40 This study confirms the principle that intensification of thoracic radiation therapy beyond the relatively low dose once-daily regimen can improve rates of both local control and survival. This trial has altered the standard of care of L-SCLC patients because the twice-daily fractionation program is now considered to be the standard against which all other programs are measured.
The North Central Cancer Treatment Group (NCCTG) performed a trial (89-20-52) that has been misinterpreted as contradicting the findings of Intergroup Trial 0096. This trial included 310 patients with L-SCLC initially treated with three cycles of etoposide-cisplatin therapy.41 Subsequently, the 261 patients without progression were randomized to two cycles of etoposide-cisplatin plus either once-daily thoracic radiation therapy (50.4 Gy in 28 fractions) or split-course, twice-daily thoracic radiation therapy (24 Gy in 16 fractions followed by a 2.5-week break and then 24 Gy in 16 fractions). Patients then received a sixth cycle of etoposide-cisplatin therapy followed by prophylactic cranial irradiation. The median time and 5-year survival rate were 20.6 months and 21% with once-daily therapy versus 20.6 months and 22% for twice-daily therapy (p = .68). The findings of these two phase III studies comparing once-daily to twice-daily thoracic radiation therapy for L-SCLC lead to the conclusion that continuous-course, twice-daily thoracic radiation therapy is better than once-daily thoracic radiation therapy but split-course twice-daily radiation therapy is not.
Higher-dose altered fractionation regimens have been investigated in an effort to further improve outcomes. NCCTG 95-20-53 was a phase II trial that included six cycles of etoposide-cisplatin therapy.42 Prophylactic cranial irradiation (25 Gy in 10 fractions) was delivered during cycle 3. Cycles 4 and 5 included concurrent etoposide-cisplatin therapy and thoracic radiation therapy (30 Gy in 20 twice-daily fractions followed by a 2-week break and then 30 Gy in 20 twice-daily fractions). This high-dose twice-daily thoracic radiation therapy regimen resulted in a favorable 5-year survival rate of 24%, despite it being a split-course regimen. The Radiation Therapy Oncology Group (RTOG) studied concomitant boost thoracic radiation therapy initiated with the first of four cycles of etoposide-cisplatin therapy. The maximum tolerated dose was 61.2 Gy in 34 fractions of 1.8 Gy per fraction in 5 weeks, with twice-daily thoracic radiation therapy given during the final nine treatment days.43 The overall survival time for the cohort receiving 61.2 Gy was 82% at 18 months, and a follow-up phase II study testing 61.2-Gy concomitant boost thoracic radiation therapy has now been completed. The CALGB and RTOG are leading a national trial (CALGB 30601/RTOG 0538) comparing the standard arm of 45 Gy in 30 fractions twice daily with 70 Gy in 35 fractions once daily and the 61.2-Gy concomitant boost regimen. The hypothesis behind this trial is that the two higher biologic equivalent dose (BED) regimens will provide better tumor control and survival rates than the standard twice-daily thoracic radiation therapy regimen.
Treatment Volumes and Normal Tissue Considerations
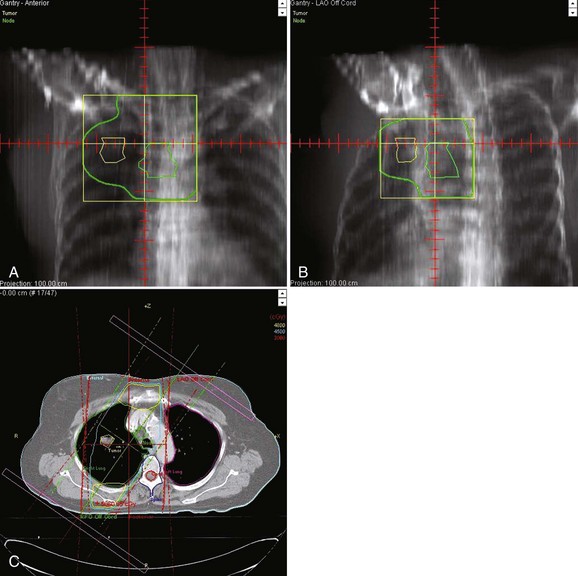
The ability of an L-SCLC patient to tolerate increasingly aggressive radiotherapy regimens and higher total doses during concurrent chemotherapy is in part related to the thoracic radiation therapy target volume selection.38 Evidence emerged in the 1980s and 1990s that the use of smaller radiation fields did not adversely influence tumor control rates in patients receiving concurrent cisplatin-based chemotherapy. The fields employed in Intergroup Trial 0096 confined the high-dose volume to the tumor with a 1.5-cm margin, the ipsilateral hilum, and the mediastinum from the thoracic inlet to the subcarinal region. The contralateral hilum and supraclavicular regions were excluded. These treatment volumes have been widely adopted in clinical trials.
Smaller treatment volumes have the advantage of further sparing of the surrounding normal tissues: lung, esophagus, heart, spinal cord, and bone marrow. We compiled the toxicity statistics for patients treated in NCCTG 89-20-52, which included concurrent chemotherapy plus either twice-daily or once-daily thoracic radiation therapy. The most common toxicity was hematologic; 90% of patients had grade 3 or 3+ hematologic toxicity, and 43% had grade 4+ hematologic toxicity. Forty-seven percent of patients had grade 3+ nonhematologic toxicity, and 11% had grade 4+ nonhematologic toxicity. Nausea, vomiting, and esophagitis were the most common nonhematologic toxicities. Fatal toxicity occurred in four patients (2%).41 Therefore the data suggest that toxicity is substantial with combined-modality therapy, and more precise fields may allow safer treatment delivery.
Irradiation-induced toxicity is related to dose-volume parameters. In a classic study, Graham and associates44 reported that the risk of grade 2+ pneumonitis was 0% when the V20 (total lung volume that received ≥20 Gy) was less than 22%, 7% when the V20 was 22% to 31%, 13% when the V20 was 32% to 40%, and 36% when the V20 was more than 40%. It is generally accepted that the spinal cord can safely receive 45 to 50 Gy in 1.8 to 2-Gy fractions. In most twice-daily thoracic radiation therapy regimens employing 1.5-Gy fractions, the spinal cord is limited to 36 to 37 Gy without incident.41 When using the Intergroup 0096 regimen, the oblique fields off the spinal cord are used for the second daily treatment for the last 8 to 10 treatments (Fig. 41-1). Esophagitis is related to the dose of radiation, the volume of esophagus irradiated, the fractionation scheme, and the timing of chemotherapy. Of the patients in Intergroup Trial 0096 who received twice-daily thoracic radiation therapy, 32% had grade 3+ esophagitis compared with 16% for those who received once-daily thoracic radiation therapy (p = .001)40 (Table 41-3). Watkins45 described the factors associated with the development of severe acute esophagitis during twice-daily thoracic radiation therapy with concurrent chemotherapy in patients with L-SCLC. Twice-daily chemoradiotherapy included 45 Gy at 1.5 Gy with concurrent platinum-based chemotherapy. The most strongly associated dosimetric variable was the V15 (grade 3 esophagitis rates of 15% vs. 64% for a V15 <60% vs. >60%, respectively).45 Although esophagitis is uncomfortable and can lead to dehydration and the need for intravenous hydration or hospitalization, this should not be used as a reason to deny fit patients twice-daily thoracic radiation therapy.
Chemotherapy
The selection of chemotherapeutic agents to combine with thoracic radiation therapy for patients with L-SCLC is largely based on trials of multiagent regimens used for E-SCLC. The currently accepted standard chemotherapy regimen for both E-SCLC and L-SCLC in the United States is the two-drug regimen of etoposide plus cisplatin. SWOG was the first group to report a completed trial of concurrent thoracic radiation therapy with etoposide-cisplatin therapy for L-SCLC. They reported a median survival time of 17.5 months and a 4-year survival rate of 30%; these findings appeared to be superior to the previously reported results with thoracic radiation therapy combined with cytoxan, adriamycin, and vincristine (CAV) and other non–platinum-containing regimens.46 The pulmonary, cardiac, and cutaneous risks of combining etoposide-cisplatin with thoracic radiation therapy were thought to be lower than with CAV or other anthracycline-containing regimens. Additionally, promising results were subsequently reported with thoracic radiation therapy and etoposide-cisplatin therapy and were confirmed in Intergroup Trial 0096.40,47,48 No randomized trial has demonstrated the superiority of thoracic radiation therapy and etoposide-cisplatin therapy over any other regimen for L-SCLC.
New Chemotherapeutic Agents
Although encouraging response rates may be seen with several novel agents in SCLC, no regimen has consistently resulted in improved overall survival rates. Great enthusiasm built for studying the topoisomerase I inhibitor irinotecan after a Japanese trial demonstrated better survival rates with irinotecan plus cisplatin compared with etoposide plus cisplatin for E-SCLC. The median survival time was 12.8 months with irinotecan plus cisplatin versus 9.4 months with etoposide plus cisplatin (p = .002).49 A similarly designed SWOG trial (S0124) was unable to confirm a survival advantage obtained by irinotecan and cisplatin.50 Hanna and associates51 reported similarly negative findings from a third similar phase III trial. Therefore, the standard of care remains etoposide plus cisplatin for the treatment of SCLC in the Western hemisphere.
Role of Surgery
The use of resection as primary management of SCLC was largely abandoned by the 1970s when poor survival rates were reported and few complete resections were achieved.52 In a report by the Veterans Administration Surgical Oncology Group published in 1982, 5-year survival rates for patients with resected T1N0, T1N1, and T2N0 SCLC were 60%, 31%, and 28%, respectively.53 All of these patients received chemotherapy. A SWOG protocol enrolled 15 L-SCLC patients who underwent resection followed by chemoirradiation and found this group to have a better 2-year survival rate than a cohort of matched patients treated nonoperatively in other SWOG trials (45% vs. 14%).54 Ichinose and colleagues55 reported on 112 SCLC patients who underwent surgical resection and were then randomized between two chemotherapy regimens. Although the chemotherapy regimens produced comparable outcomes, the 3-year survival rates were encouraging: 65% for N0 disease, 52% for N1 disease, and 29% for N2 disease. Chandra and colleagues56 also found a favorable 5-year survival rate of 38% in patients with stage I and II SCLC who underwent resection. Each of these three reports suggests that for the rare N0 or N1 L-SCLC patient, resection followed by chemotherapy is a reasonable consideration, although it is not clear if such patients would have fared just as well with combined chemotherapy and thoracic radiation therapy. The role of thoracic radiation therapy for surgically treated patients is uncertain. A pattern-of-failure study of patients with resected SCLC receiving postoperative chemotherapy alone would help clarify this issue.
A randomized trial was conducted by the Lung Cancer Study Group in which L-SCLC patients achieving a partial response or complete response to chemotherapy either underwent resection or did not.57 There was no survival difference between the arms, with a 2-year survival rate of 20% in both arms (p = .55). Based on this trial and on the proven survival benefit of concurrent chemotherapy and thoracic radiation therapy, adjuvant surgery following chemotherapy is not recommended.
There are limited data regarding the role of resection of intrathoracic recurrences. Shepherd and associates58 reported on 28 L-SCLC patients who underwent salvage surgery following either a partial response to chemotherapy or subsequent progressive disease. Ten of these 28 patients had NSCLC elements in their specimen. Their 5-year survival rate was 23%. There is only anecdotal information regarding surgical salvage of patients initially treated with both chemotherapy and thoracic radiation therapy. Although it appears that some patients may be salvaged after a partial response or local failure, resection has not been widely employed. This may be due in part to the propensity of SCLC to metastasize.
Prophylactic Cranial Irradiation for L-SCLC and E-SCLC
Prophylactic cranial irradiation was initially introduced into practice in the 1960s for patients with acute lymphoblastic leukemia who had a high risk of failure in the central nervous system (CNS).59 It was first tested for patients with SCLC in the 1970s following the recognition that brain metastases were frequent. The blood-brain barrier prevents the penetration of most chemotherapeutic agents leaving the brain as a sanctuary site for relapse.
Prophylactic cranial irradiation has been shown to improve survival rates of patients who achieve a complete response. Auperin and associates60 published a meta-analysis that included data from seven phase III studies comparing prophylactic cranial irradiation with no prophylactic cranial irradiation after a complete response was achieved. As in the thoracic radiation therapy meta-analyses, the 3-year survival rate was 5.4% better for those who received prophylactic cranial irradiation, at 20.7% versus 15.3% for those who did not receive prophylactic cranial irradiation (p = .01). Although an improved rate of 5.4% appears small, it does reflect a 35% increase in 3-year survivors.
Prophylactic cranial irradiation may also substantially affect patients with E-SCLC. Slotman and associates61 conducted a randomized trial (EORTC 08993-22993) of prophylactic cranial irradiation in patients with E-SCLC who had any degree of response to chemotherapy. Brain imaging was not required either before protocol entry or before randomization. Patients received either prophylactic cranial irradiation or no further therapy (control group). The prophylactic cranial irradiation included 20 Gy in 5 to 8 fractions, 24 Gy in 12 fractions, 25 Gy in 10 fractions, or 30 Gy in 10 or 12 fractions. The cumulative risk of brain metastases within 1 year was 14.6% in the prophylactic cranial irradiation group and 40.4% in the control group (hazard ratio [HR] = .27; p <.001). Prophylactic cranial irradiation was associated with an increase in median survival time from 5.4 months to 6.7 months. The 1-year survival rate was 27.1% in the prophylactic cranial irradiation group and 13.3% in the control group (p = .003). The largest mean difference in health-related quality of life between the two arms was the degree of fatigue and hair loss, which were greater with prophylactic cranial irradiation.62 Given the overall survival benefit in this trial, prophylactic cranial irradiation should be considered for all patients with SCLC who have a response to initial chemotherapy. This trial did not evaluate which dose-fractionation regimen was best.
The optimal prophylactic cranial irradiation regimen would include the following desirable characteristics: decreases brain metastases, increases survival rates, takes the least time from the patient’s remaining life, decreases cost, and causes the least toxicity. An international consortium including the EORTC and RTOG studied dose-fractionation regimens in a phase III trial. This trial included 720 patients with L-SCLC in complete remission after chemotherapy and thoracic radiation therapy. Patients were assigned to either a standard dose (n = 360; 25 Gy in 10 fractions) or a high dose of prophylactic cranial irradiation (n = 360; 36 Gy). The high-dose regimen was either a conventional regimen of 18 once-daily fractions of 2 Gy or an accelerated hyperfractionated regimen of 24 twice-daily fractions of 1.5 Gy. There was no significant difference in the 2-year incidence of brain metastases between the standard group and the higher-dose group (29% and 23%, respectively; p = .18). The 2-year overall survival rate was 42% in the standard-dose group and 37% in the higher-dose group (p = .05). The most common acute toxic events were fatigue (30% in the standard-dose group vs. 34% in the higher-dose group), headache (24% vs. 28%), and nausea or vomiting (23% vs. 28%). The authors concluded that higher-dose prophylactic cranial irradiation did not reduce brain metastases but did significantly increase mortality rates. Therefore prophylactic cranial irradiation at 25 Gy in 10 fractions should be the standard of care for SCLC.63 Although this trial did not address the effects of dose fractionation for E-SCLC patients, it still presents the most compelling data available regarding the regimen used for prophylactic cranial irradiation for SCLC patients. Although the regimen of 25 Gy in 10 fractions did not meet all the characteristics for the optimal regimen, it was associated with better survival rates, decreased patient time required, and decreased health care costs.63
Management Of Metastatic And Recurrent Disease
Approximately two thirds of SCLC patients have extensive-stage disease.2 The most common sites of extrathoracic metastases are the liver, bone, brain, and adrenal glands.
Initial management of most patients with newly diagnosed E-SCLC is four or more cycles of etoposide plus cisplatin. Novel targeted agents are the focus of much research, and it is hoped that newer agents will improve outcomes. Second-line chemotherapy often includes topotecan because this was proven superior to the best supportive care in patients failing initial therapy.64
Role of Definitive Radiotherapy for E-SCLC
Although the role of thoracic radiation therapy is less clear in patients with E-SCLC, there appears to be a distinct survival benefit to delivering thoracic radiation therapy to carefully selected patients. Jeremic and colleagues65 performed a phase III study that evaluated chemotherapy with or without thoracic radiation therapy in patients with E-SCLC. Patients initially received three cycles of carboplatin and etoposide. Those patients with a complete response at distant sites and a partial response or better in the chest were randomly assigned to receive either (1) twice-daily thoracic radiation therapy (54 Gy in 36 fractions) plus concurrent carboplatin and etoposide, followed by two cycles of the same chemotherapy or (2) four cycles of the same chemotherapy without thoracic radiation therapy. All patients with a complete response at the distant sites also received prophylactic cranial irradiation. Patients who received thoracic radiation therapy had significantly better survival times than those who did not (median survival time, 17 months vs. 11 months; 5-year survival rate, 9.1% vs. 3.7%; p = .041).65 This study suggests a role for thoracic radiation therapy in E-SCLC patients if systemic disease has been controlled with chemotherapy.
Bonner and associates66 performed a trial that explored the use of radiation therapy for both thoracic and metastatic lesions in patients with E-SCLC. This study included an aggressive regimen of seven cycles of an alternating six-drug combination and split-course prophylactic cranial irradiation during the first week of chemotherapy cycles 2 and 3 (17 Gy in 5 fractions each week), and split-course thoracic radiation therapy during the first week of chemotherapy cycles 5 and 6 (20 Gy in 5 fractions each week). After completing chemotherapy, patients received 6 Gy in one dose of upper-hemibody radiation therapy followed by 8 Gy in one dose of lower-hemibody radiation therapy. Toxicity was substantial, including fatal hematologic complications in 10% of patients, severe peripheral neurologic toxicity in 15%, severe CNS toxicity in 10%, and severe cardiac toxicity in 5%.67,68 CNS toxicity may have been related to concurrent administration of large-fraction prophylactic cranial irradiation with chemotherapy, which would not be considered appropriate by current standards. In spite of the toxicity issues, the overall 5-year survival rate was 16%, much better than expected for E-SCLC patients, suggesting that irradiation to gross disease may have efficacy in E-SCLC.
One particular subgroup of E-SCLC patients includes those with disease limited to the chest and brain only. This group may benefit from the use of chemotherapy, whole brain radiation therapy, and thoracic radiation therapy. Kochhar and associates69 identified 30 such patients who initially received cisplatin-based chemotherapy and concomitant whole brain radiation therapy consisting of 36 to 48 Gy. Subsequently, 22 patients also received thoracic radiation therapy. The median survival time was 14 months. The outcome of E-SCLC patients with the brain as the sole site of metastases at diagnosis was similar to that of L-SCLC patients. As with studies from Jeremic and colleagues65 and Bonner and associates,66 this result suggests possible benefit from targeting gross disease in the chest and/or distantly with radiation therapy in E-SCLC. Currently, the RTOG is planning a trial exploring this concept.
Treatment of Metastases
Patients with a solitary primary or recurrent brain metastasis or only a few such metastases can be considered for other more aggressive treatment options such as radiosurgery. There is little data addressing specialized therapies such as radiosurgery for SCLC patients. Sheehan and associates68 performed a retrospective review of 27 patients with 47 recurrent SCLC brain metastases who underwent radiosurgery. The overall median survival time was 18 months after the diagnosis of brain metastases. They concluded that radiosurgery for recurrent SCLC metastases provided effective local tumor control in Most patients.
Superior vena cava syndrome is more common with SCLC than with other histologic types because of a propensity of SCLC patients to develop large bulky mediastinal adenopathies. Chan and colleagues70 reported on 76 consecutive SCLC patients with superior vena cava syndrome; 93% had improvement in superior vena cava syndrome symptoms after chemotherapy versus 94% after thoracic radiation therapy. Additionally, a favorable response was obtained with a wide range of radiation therapy fractionation patterns and total doses administered. Those who received chemotherapy and thoracic radiation therapy had a longer time to recurrence of the superior vena cava syndrome (p = .018) versus those who received chemotherapy alone. The early mortality rate from superior vena cava syndrome was 2%. The researchers concluded that there is no obvious need to use large radiation fraction sizes for the first few treatments. Thoracic radiation therapy resulted in more durable palliation than was achieved with chemotherapy alone. This study debunked the myth that radiation therapy needs to begin emergently within hours, particularly if chemotherapy can be initiated in a timely manner.
Treatment Algorithm, Controversies, And Clinical Trials
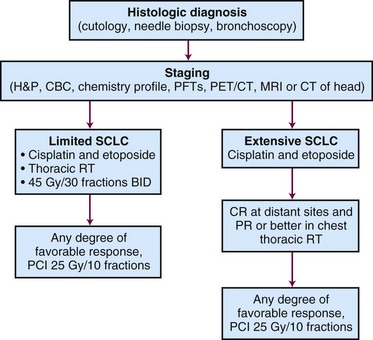
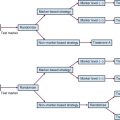
A proposed treatment algorithm for patients with SCLC is shown in Figure 41-2.
Thoracic Radiation Therapy for L-SCLC Patients with Good Performance Status
The management of SCLC is dependent on stage and medical fitness. For patients with good performance status and L-SCLC, the recommended therapy would include concurrent thoracic radiation therapy with etoposide plus cisplatin chemotherapy. Evidence shows that the best treatment is four cycles of etoposide-cisplatin chemotherapy with twice-daily thoracic radiation therapy beginning early.34,35 The twice-daily radiation therapy is delivered in 1.5-Gy fractions, with an inter–fraction interval of 6 hours or longer, to a total dose of 45 Gy (see Figure 41-1). This recommendation is based on Intergroup Trial 0096.40 The CALGB/RTOG trial (CALGB 30601/RTOG 0538) will help determine which of three thoracic radiation therapy regimens is best. For patients with huge tumors, postobstructive pneumonia, or atelectasis, there may be value in delaying initiation of thoracic radiation therapy until a later cycle of chemotherapy. This may be necessary in some cases if the initial tumor volume would require an excessive volume of normal lung to be irradiated. It is not known whether adding thoracic radiation therapy improves the outcome of the rare patient with resected L-SCLC. However, chemotherapy is generally administered after resection.
Management of L-SCLC in Elderly Patients and Patients with Lower Performance Status
Treatment in fit elderly patients (>70 years of age) can be carried out in a manner similar to that for fit younger individuals. NCCTG 89-20-52 included 263 patients with L-SCLC and an ECOG performance status of less than 2, who were randomized to once-daily thoracic radiation therapy or split-course, twice-daily radiation therapy. The outcomes of the younger patients (<70 years old) were compared with those of the elderly patients (>70 years old). The overall incidence of grade 3+ or 4+ toxicity was not significantly greater in elderly patients. One specific toxicity, grade 4+ pneumonitis, occurred in none of the patients who were younger than 70 years versus 6% of older patients (p = .008). Grade 5 toxicity occurred in 3 of 54 patients (5.6%) who were more than 70 years old versus 1 of 209 younger patients (0.5%) (p = .03). Survival rates were not significantly worse in older individuals; the 5-year survival rate was 22% in younger patients versus 17% in older patients (p = .14). Fit elderly patients with L-SCLC can receive combined-modality therapy, if carefully monitored, with a reasonable expectation of surviving for 5 years.71
E-SCLC and Thoracic Radiation Therapy
Initial management of E-SCLC involves primary chemotherapy (etoposide plus cisplatin), with radiation therapy being reserved for brain metastases, bulky and symptomatic intrathoracic disease, and symptomatic bony or visceral metastatic sites. In addition, patients with a good performance status, a complete remission of metastatic disease, and chest disease that is, at least, stable following chemotherapy benefit from the addition of thoracic radiation therapy.65
Prophylactic Cranial Irradiation for E-SCLC and L-SCLC
Prophylactic cranial irradiation should be considered for all SCLC patients who have achieved any degree of favorable response following initial management with chemotherapy or chemoirradiation. A dose of 25 Gy in 10 fractions is considered to be the standard regimen.63
2 Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890-895.
6 Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90-97.
8 Sozzi G, Veronese ML, Negrini M, et al. The FHIT gene 3p14.2 is abnormal in lung cancer. Cell. 1996;85:17-26.
9 Harbour JW, Lai SL, Whang-Peng J, et al. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988;241:353-357.
10 Gazdar AF. Molecular markers for the diagnosis and prognosis of lung cancer. Cancer. 1992;69:1592-1599.
11 Ben-Ezra JM, Kornstein MJ, Grimes MM, Krystal G. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol. 1994;145:1036-1040.
12 Gazdar AF. The molecular and cellular basis of human lung cancer. Anticancer Res. 1994;14:261-267.
13 Kelly M, Linnoila R, Avis I. Antitumor activity of a monoclonal antibody directed against gastrin-releasing peptide in patients with small cell lung cancer. Chest. 1997;112:256-261.
15 Dimopoulos MA, Fernandez JF, Samaan NA, et al. Paraneoplastic Cushing’s syndrome as an adverse prognostic factor in patients who die early with small cell lung cancer. Cancer. 1992;69:66-71.
16 Patel AM, Davila DG, Peters SG. Paraneoplastic syndromes associated with lung cancer. Mayo Clin Proc. 1993;68:278-287.
19 Stahel R, Ginsberg R, Havermann K. Staging and prognostic factors in small cell lung cancer: A consensus. Lung Cancer. 1989;5:119-126.
20 Kamel EM, Zwahlen D, Wyss MT, et al. Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med. 2003;44:1911-1917.
21 Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer. An analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol. 1990;8:1563-1574.
24 Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer. A pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115:2721-2732.
26 Lowenbraun S, Bartolucci A, Smalley RV, et al. The superiority of combination chemotherapy over single agent chemotherapy in small cell lung carcinoma. Cancer. 1979;44:406-413.
28 Byhardt RW, Cox JD, Holoye PY, et al. The role of consolidation irradiation in combined modality therapy of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1982;8:1271-1276.
32 Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618-1624.
33 Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer. Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054-3060.
34 Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837-4845.
35 De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057-1063.
36 Choi NC, Carey RW. Importance of radiation dose in achieving improved loco-regional tumor control in limited stage small-cell lung carcinoma. An update. Int J Radiat Oncol Biol Phys. 1989;17:307-310.
37 Coy P, Hodson I, Payne DG, et al. The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys. 1988;14:219-226.
38 Lichter AS, Turrisi AT3rd. Small cell lung cancer. The influence of dose and treatment volume on outcome. Semin Radiat Oncol. 1995;5:44-49.
39 Bogart JA, Herndon JE2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer. Analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460-468.
40 Turrisi AT3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265-271.
41 Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943-951.
42 Schild SE, Bonner JA, Hillman S, et al. Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95-20-53). J Clin Oncol. 2007;25:3124-3129.
43 Komaki R, Swann S, Ettinger D, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:342-350.
44 Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329.
45 Watkins JM, Wahlquist AE, Shirai K, et al. Factors associated with severe acute esophagitis from hyperfractionated radiotherapy with concurrent chemotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:1108-1113.
46 McCracken JD, Janaki LM, Crowley JJ, et al. Concurrent chemotherapy/radiotherapy for limited small-cell lung carcinoma. A Southwest Oncology Group Study. J Clin Oncol. 1990;8:892-898.
49 Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85-91.
50 Lara PNJr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer. Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530-2535.
51 Hanna N, Bunn PAJr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038-2043.
53 Shields TW, Higgins GAJr, Matthews MJ, Keehn RJ. Surgical resection in the management of small cell carcinoma of the lung. J Thorac Cardiovasc Surg. 1982;84:481-488.
54 Friess GG, McCracken JD, Troxell ML, et al. Effect of initial resection of small-cell carcinoma of the lung. A review of Southwest Oncology Group Study 7628. J Clin Oncol. 1985;3:964-968.
55 Ichinose Y, Hara N, Ohta M, et al. Comparison between resected and irradiated small cell lung cancer in patients in stages I through IIIa. Ann Thorac Surg. 1992;53:95-100.
56 Chandra V, Allen MS, Nichols FC3rd, et al. The role of pulmonary resection in small cell lung cancer. Mayo Clin Proc. 2006;81:619-624.
57 Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106:320S-323S.
58 Shepherd FA, Ginsberg R, Patterson GA, et al. Is there ever a role for salvage operations in limited small-cell lung cancer? J Thorac Cardiovasc Surg. 1991;101:196-200.
60 Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
61 Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-672.
63 Le Pechoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01). A randomised clinical trial. Lancet Oncol. 2009;10:467-474.
64 O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441-5447.
65 Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer. A randomized study. J Clin Oncol. 1999;17:2092-2099.
66 Bonner JA, Eagan RT, Liengswangwong V, et al. Long term results of a phase I/II study of aggressive chemotherapy and sequential upper and lower hemibody radiation for patients with extensive stage small cell lung cancer. Cancer. 1995;76:406-412.
68 Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain. Outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247-254.
69 Kochhar R, Frytak S, Shaw EG. Survival of patients with extensive small-cell lung cancer who have only brain metastases at initial diagnosis. Am J Clin Oncol. 1997;20:125-127.
70 Chan RH, Dar AR, Yu E, et al. Superior vena cava obstruction in small-cell lung cancer. Int J Radiat Oncol Biol Phys. 1997;38:513-520.
71 Schild SE, Stella PJ, Brooks BJ, et al. Results of combined-modality therapy for limited-stage small cell lung carcinoma in the elderly. Cancer. 2005;103:2349-2354.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
2 Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890-895.
3 Available at http://seer.cancer.gov/statfacts/html/lungb.html#incidence-mortality
4 National Cancer Institute. Small cell lung cancer treatment. Available at www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional
5 Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med. 1969;46:516-525.
6 Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90-97.
7 Hibi K, Takahashi T, Yamakawa K, et al. Three distinct regions involved in 3p deletion in human lung cancer. Oncogene. 1992;7:445-449.
8 Sozzi G, Veronese ML, Negrini M, et al. The FHIT gene 3p14.2 is abnormal in lung cancer. Cell. 1996;85:17-26.
9 Harbour JW, Lai SL, Whang-Peng J, et al. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988;241:353-357.
10 Gazdar AF. Molecular markers for the diagnosis and prognosis of lung cancer. Cancer. 1992;69:1592-1599.
11 Ben-Ezra JM, Kornstein MJ, Grimes MM, Krystal G. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol. 1994;145:1036-1040.
12 Gazdar AF. The molecular and cellular basis of human lung cancer. Anticancer Res. 1994;14:261-267.
13 Kelly M, Linnoila R, Avis I. Antitumor activity of a monoclonal antibody directed against gastrin-releasing peptide in patients with small cell lung cancer. Chest. 1997;112:256-261.
14 List AF, Hainsworth JD, Davis BW, et al. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4:1191-1198.
15 Dimopoulos MA, Fernandez JF, Samaan NA, et al. Paraneoplastic Cushing’s syndrome as an adverse prognostic factor in patients who die early with small cell lung cancer. Cancer. 1992;69:66-71.
16 Patel AM, Davila DG, Peters SG. Paraneoplastic syndromes associated with lung cancer. Mayo Clin Proc. 1993;68:278-287.
17 de la Monte SM, Hutchins GM, Moore GW. Paraneoplastic syndromes and constitutional symptoms in prediction of metastatic behavior of small cell carcinoma of the lung. Am J Med. 1984;77:851-857.
18 Marchioli CC, Graziano SL. Paraneoplastic syndromes associated with small cell lung cancer. Chest Surg Clin North Am. 1997;7:65-80.
19 Stahel R, Ginsberg R, Havermann K. Staging and prognostic factors in small cell lung cancer: A consensus. Lung Cancer. 1989;5:119-126.
20 Kamel EM, Zwahlen D, Wyss MT, et al. Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med. 2003;44:1911-1917.
21 Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer. An analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol. 1990;8:1563-1574.
22 Souhami RL, Bradbury I, Geddes DM, et al. Prognostic significance of laboratory parameters measured at diagnosis in small cell carcinoma of the lung. Cancer Res. 1985;45:2878-2882.
23 Cerny T, Blair V, Anderson H, et al. Pretreatment prognostic factors and scoring system in 407 small-cell lung cancer patients. Int J Cancer. 1987;39:146-149.
24 Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer. A pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115:2721-2732.
25 Edmondson J, Lagakos S, Selawry O. Cyclophosphamide and CCNU in the treatment of inoperable small cell carcinoma and adenocarcinoma of the lung. Cancer Treat Rep. 1976;60:925-932.
26 Lowenbraun S, Bartolucci A, Smalley RV, et al. The superiority of combination chemotherapy over single agent chemotherapy in small cell lung carcinoma. Cancer. 1979;44:406-413.
27 Souhami RL, Geddes DM, Spiro SG, et al. Radiotherapy in small cell cancer of the lung treated with combination chemotherapy. A controlled trial. Br Med J(Clin Res Ed). 1984;288:1643-1646.
28 Byhardt RW, Cox JD, Holoye PY, et al. The role of consolidation irradiation in combined modality therapy of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1982;8:1271-1276.
29 Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med. 1987;316:912-918.
30 Bunn PAJr, Lichter AS, Makuch RW, et al. Chemotherapy alone or chemotherapy with chest radiation therapy in limited stage small cell lung cancer. A prospective, randomized trial. Ann Intern Med. 1987;106:655-662.
31 Perez CA, Krauss S, Bartolucci AA, et al. Thoracic and elective brain irradiation with concomitant or delayed multiagent chemotherapy in the treatment of localized small cell carcinoma of the lung. A randomized prospective study by the Southeastern Cancer Study Group. Cancer. 1981;47:2407-2413.
32 Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618-1624.
33 Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer. Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054-3060.
34 Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837-4845.
35 De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057-1063.
36 Choi NC, Carey RW. Importance of radiation dose in achieving improved loco-regional tumor control in limited stage small-cell lung carcinoma. An update. Int J Radiat Oncol Biol Phys. 1989;17:307-310.
37 Coy P, Hodson I, Payne DG, et al. The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys. 1988;14:219-226.
38 Lichter AS, Turrisi AT3rd. Small cell lung cancer. The influence of dose and treatment volume on outcome. Semin Radiat Oncol. 1995;5:44-49.
39 Bogart JA, Herndon JE2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer. Analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460-468.
40 Turrisi AT3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265-271.
41 Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943-951.
42 Schild SE, Bonner JA, Hillman S, et al. Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95-20-53). J Clin Oncol. 2007;25:3124-3129.
43 Komaki R, Swann S, Ettinger D, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:342-350.
44 Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329.
45 Watkins JM, Wahlquist AE, Shirai K, et al. Factors associated with severe acute esophagitis from hyperfractionated radiotherapy with concurrent chemotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:1108-1113.
46 McCracken JD, Janaki LM, Crowley JJ, et al. Concurrent chemotherapy/radiotherapy for limited small-cell lung carcinoma. A Southwest Oncology Group Study. J Clin Oncol. 1990;8:892-898.
47 Johnson DH, Turrisi AT, Chang AY, et al. Alternating chemotherapy and twice-daily thoracic radiotherapy in limited-stage small-cell lung cancer. A pilot study of the Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:879-884.
48 Turrisi AT3rd, Glover DJ, Mason BA. A preliminary report. Concurrent twice-daily radiotherapy plus platinum-etoposide chemotherapy for limited small cell lung cancer. Int J Radiat Oncol Biol Phys. 1988;15:183-187.
49 Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85-91.
50 Lara PNJr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer. Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530-2535.
51 Hanna N, Bunn PAJr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038-2043.
52 Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63-65.
53 Shields TW, Higgins GAJr, Matthews MJ, Keehn RJ. Surgical resection in the management of small cell carcinoma of the lung. J Thorac Cardiovasc Surg. 1982;84:481-488.
54 Friess GG, McCracken JD, Troxell ML, et al. Effect of initial resection of small-cell carcinoma of the lung. A review of Southwest Oncology Group Study 7628. J Clin Oncol. 1985;3:964-968.
55 Ichinose Y, Hara N, Ohta M, et al. Comparison between resected and irradiated small cell lung cancer in patients in stages I through IIIa. Ann Thorac Surg. 1992;53:95-100.
56 Chandra V, Allen MS, Nichols FC3rd, et al. The role of pulmonary resection in small cell lung cancer. Mayo Clin Proc. 2006;81:619-624.
57 Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106:320S-323S.
58 Shepherd FA, Ginsberg R, Patterson GA, et al. Is there ever a role for salvage operations in limited small-cell lung cancer? J Thorac Cardiovasc Surg. 1991;101:196-200.
59 Bleyer WA, Poplack DG. Prophylaxis and treatment of leukemia in the central nervous system and other sanctuaries. Semin Oncol. 1985;12:131-148.
60 Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
61 Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-672.
62 Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms. Results of an international phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol. 2009;27:78-84.
63 Le Pechoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01). A randomised clinical trial. Lancet Oncol. 2009;10:467-474.
64 O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441-5447.
65 Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer. A randomized study. J Clin Oncol. 1999;17:2092-2099.
66 Bonner JA, Eagan RT, Liengswangwong V, et al. Long term results of a phase I/II study of aggressive chemotherapy and sequential upper and lower hemibody radiation for patients with extensive stage small cell lung cancer. Cancer. 1995;76:406-412.
67 Frytak S, Shaw JN, O’Neill BP, et al. Leukoencephalopathy in small cell lung cancer patients receiving prophylactic cranial irradiation. Am J Clin Oncol. 1989;12:27-33.
68 Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain. Outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247-254.
69 Kochhar R, Frytak S, Shaw EG. Survival of patients with extensive small-cell lung cancer who have only brain metastases at initial diagnosis. Am J Clin Oncol. 1997;20:125-127.
70 Chan RH, Dar AR, Yu E, et al. Superior vena cava obstruction in small-cell lung cancer. Int J Radiat Oncol Biol Phys. 1997;38:513-520.
71 Schild SE, Stella PJ, Brooks BJ, et al. Results of combined-modality therapy for limited-stage small cell lung carcinoma in the elderly. Cancer. 2005;103:2349-2354.