Chapter 16 Small Bowel Malignant Tumors
Introduction
Small bowel (SB) malignancies account for only 2% of all gastrointestinal (GI) neoplasms and less than 0.4% of all cancers in the United States.1 Common malignant tumors of the SB include primary adenocarcinoma, carcinoid, lymphoma, GIST (gastrointestinal stromal tumor), and metastases. Recent increases in the incidence of carcinoid tumors have now made carcinoids the most common primary SB tumor: carcinoid 44%, adenocarcinoma 33%, sarcomas 17%, and lymphoma 8%.2 The risk of a specific SB tumor type depends on the exact location in the SB, with adenocarcinomas the most common duodenal tumor, carcinoids the most common ileal tumor, and both sarcoma and lymphoma more equally distributed throughout the entire SB. The clinical presentation of SB tumors is nonspecific with abdominal pain, weight loss, nausea, vomiting, GI bleeding, and SB obstruction the most common symptoms.3 Endoscopic evaluation of the SB has been hampered by the long length of the SB, approximately 5 to 6 m. However, recent advances in endoscopic technologies, such as push enteroscopy, video capsule endoscopy, and double-balloon enteroscopy, have allowed the evaluation of the entire SB.4–6
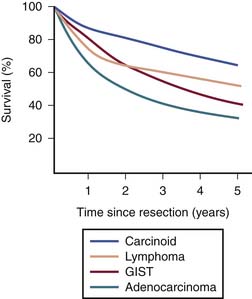
Heterogeneous biology of SB tumors is reflected in survival, being lowest for adenocarcinoma and highest for carcinoid (Figure 16-1). Owing to substantial differences in clinical and imaging features,7–9 the most common malignancies of the SB including adenocarcinoma, carcinoid, GIST, lymphoma, and metastases are discussed in this chapter as separate entities.
I Adenocarcinoma
Introduction
Adenocarcinoma for decades has been the most common malignant tumor of the SB, recently surpassed by carcinoid. Currently, adenocarcinoma is the second most common primary malignancy of the SB (33% of all primary SB tumors). One of the more interesting aspects of small intestine adenocarcinoma is its rarity in comparison with large intestine adenocarcinoma. Despite the small intestine representing approximately 70% to 80% of the length and over 90% of the surface area of the alimentary tract, the incidence of SB adenocarcinoma is 30- to 50-fold less than that of colon adenocarcinoma.10 Theories to explain the small intestine’s relative protection from the devolvement of carcinoma center around two concepts: (1) the rapid turnover time of small intestinal cells results in epithelial cell shedding before the necessary acquisition of multiple genetic defects, and (2) exposure to the carcinogenic components of our diet are limited owing to a rapid SB transit time, lack of bacterial degradation activity, and a relatively dilute, alkaline environment of the SB.
Epidemiology and Risk Factors
Most cases of adenocarcinoma are sporadic, with a male predominance. The incidence of SB adenocarcinoma peaks in the seventh and eighth decades, with an average age of 65 years. An increased incidence is associated with the genetic cancer syndromes of hereditary nonpolyposis colorectal cancer, Peutz-Jeghers, and familial adenomatous polyposis. Inflammatory bowel disease, and in particular Crohn’s disease, is a risk factor with risk correlated with both the extent and the duration of SB involvement. Additional risk factors include personal history of colorectal cancer, celiac disease, and the ingestion of smoked or salt-cured foods.11,12
Anatomy and Pathology
Most frequently, the tumor occurs within the duodenum (49%), particularly around the papilla of Vater, and with decreasing frequency in the jejunum (21%) and ileum (15%).13 In Crohn’s disease–associated cases, 70% of tumors present in the distal ileum.
There are four histologic types of adenocarcinoma: well, moderately, and poorly differentiated, and undifferentiated.7 Prognostic factors consistently associated with poor outcome include the presence of metastatic disease, noncurative surgical resection, poor differentiation, and advanced age.2 In patients who have had surgical resection, the pathologic factors associated with increased risk of relapse include lymph node involvement, positive surgical margins, poor tumor differentiation, T4 tumor stage, and lymphovascular spread.2,13,14 As with colorectal cancer, adenocarcinoma of the SB undergoes a similar phenotypic adenoma-carcinoma transformation. Both increased size of SB adenomas and the presence of villous histology are risk factors for the development of invasive adenocarcinoma. The molecular understanding of SB adenocarcinoma is limited, although mutations in both the K-ras oncogene and the tumor suppressor gene p53 are common.15
Clinical Presentation
Symptoms of SB adenocarcinoma are nonspecific and frequently do not occur until advanced disease is present. A delay in diagnosis is common, with one report demonstrating an average delay from first symptom to diagnosis of 4 months.16 The most commonly reported symptoms are abdominal pain, nausea, vomiting, weight loss, and GI bleeding. The presenting stage distribution is stage I in 12%, stage II in 30%, stage III in 26%, and stage IV in 32%.2 Adenocarcinoma of the proximal duodenum involving the ampulla of Vater may present with obstructive jaundice.
Patterns of Tumor Spread
Adenocarcinomas spread via lymphatics to the regional mesenteric lymph nodes; the most common sites of hematogenous spread are the liver and lung. Owing to the advanced stage of presentation for most patients, peritoneal carcinomatosis is also common. After a curative resection, the pattern of failure for SB adenocarcinoma is predominantly systemic with the most common sites being liver in 67%, lung in 38%, retroperitoneum in 29%, and peritoneal carcinomatosis in 25%.14
Staging Evaluation (Table 16-1 and Figure 16-2)
Key Points Staging of small bowel adenocarcinoma
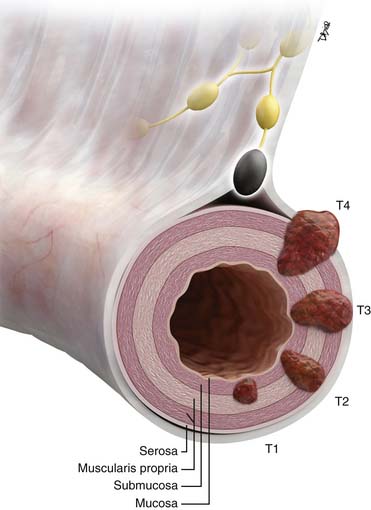
• T staging: based on depth of tumor penetration into and beyond the SB wall. Imaging is contributory for T3 and T4 tumors.
• N staging by imaging: limited by an overlap in appearance of benign reactive and metastatic mesenteric lymph nodes.
• M staging: liver and lung metastases best appreciated on cross-sectional imaging. Peritoneal carcinomatosis is commonly unmeasurable by imaging.
Table 16-2 Proposed Staging System for Small Bowel Carcinoid Tumors
| STAGE | CHARACTERISTICS OF TUMOR-NODE-METASTASIS CLASSIFICATION SYSTEM |
|---|---|
| T T1 T2 T3 |
Primary Tumor <2 cm up to the muscularis propria >2 cm up to the muscularis propria or < 2 cm propria beyond muscularis propria >2 cm beyond muscularis propria |
| N N0 N1 |
Regional Lymph Nodes No regional lymph node metastasis present Regional lymph node metastasis |
| M M0 M1 |
Distant Metastases No distant metastasis present Distant metastasis |
| STAGE | GROUPING |
| I II III IV |
T1, N0-N1, M0 T2, any N, M0 T3, any N, M0 Any T, any N, M1 |
From Landry CS, et al. A proposed staging system for small bowel carcinoid tumors based on an analysis of 6,380 patients. Am J Surg. 2008;196:896-903; discussion 903.
Imaging
Tumor Detection
Enteroclysis has been a standard invasive imaging modality before the introduction of video capsule endoscopy to better evaluate SB loops.17 The sensitivity of enteroclysis is as high as 95% with 90% correct estimation of the actual size of the tumor.18
Conventional cross-sectional imaging with multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) can scan the entire SB, but can be limited by lack of optimal opacification of the entire GI tract. Newer imaging techniques based on MDCT, such as MDCT enterography and MDCT enteroclysis, share advantages and disadvantages of both conventional enteroclysis and cross-sectional imaging. MDCT enterography is a noninvasive study performed without nasojejunal cannulation, which achieves good or excellent SB distention with negative oral contrast such as water or mannitol substituted for positive contrast media such as barium or gastrografin.19 MDCT enteroclysis is a relatively new technique more sensitive than conventional barium studies and less invasive than enteroscopy.20,21 Lesions as small as 5 mm can be identified.20
SB tumors may be demonstrable on routine MRI.22 MRI enteroclysis is an evolving technique capable of demonstrating an SB abnormality,23,24 with a reported sensitivity of 86%, a sensitivity of 98%, and an accuracy of 97%.
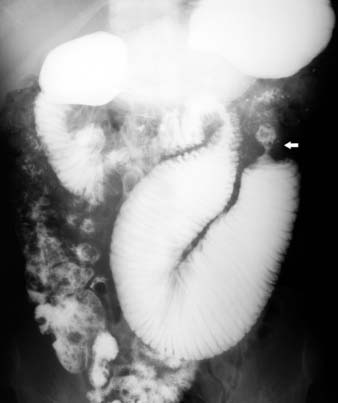
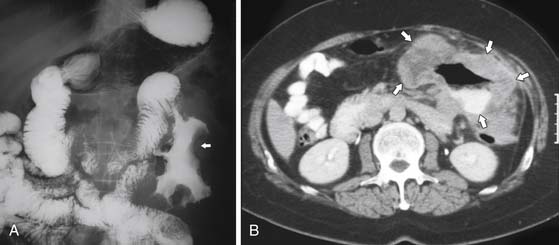
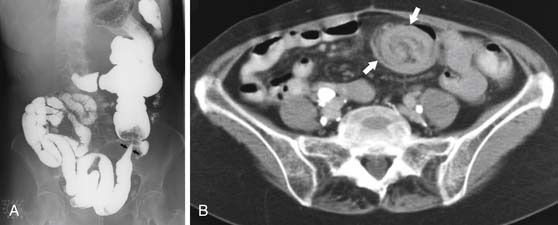
On barium studies such as standard SB series and enteroclysis, the tumor is seen as a short, circumferentially narrowed segment with overhanging borders, an “apple-core” lesion (Figure 16-3).
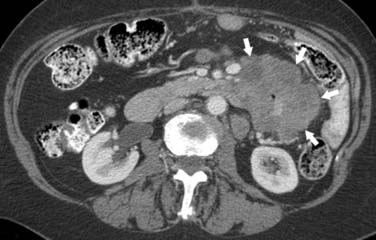
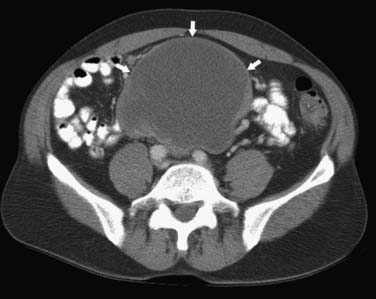
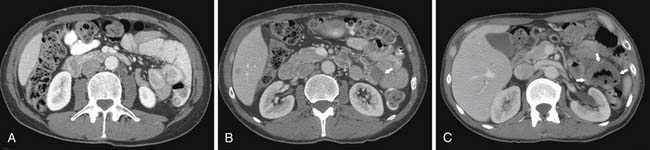
On computed tomography (CT), adenocarcinomas typically appear as a focal area of wall thickening causing luminal narrowing25 (Figure 16-4). These tumors are often rigid and fibrotic and, therefore, result in early obstruction, although infiltrative lesions without narrowing have also been reported (Figure 16-5).
Ulceration, present in 40% of pathologic specimens, is not reliably visualized on CT.
Adenocarcinomas complicating longstanding Crohn’s disease generally arise in the distal ileum. These tumors are difficult to detect because of a preexisting abnormality causing thickening and retraction of the bowel, deforming normal anatomy and masking early diagnosis.
Metastatic Disease (M)
Key Points The imaging report for small bowel adenocarcinoma
• Location and size of the primary tumor, if detectable by imaging.
• Tumorous involvement of adjacent vascular structures, which may preclude resection. This is particularly critical for duodenal adenocarcinoma, potentially involving superior mesenteric vessels and the portal vein.
• Size and number of the regional mesenteric lymph nodes.
• Presence of distant metastases in the liver or lungs. Presence of ascites is suspicious for peritoneal carcinomatosis until proved otherwise and can be confirmed by fluid cytology. Peritoneal spread commonly presents as ascites rather than measurable discrete omental implants.
Treatment
Wide segmental resection with regional mesenteric lymphadenectomy is the standard approach for both treatment and staging purposes. In the case of adenocarcinoma involving the proximal duodenum, pancreaticoduodenectomy may be required.26 Curative radical surgery is the most important prognostic factor.27
The role of adjuvant therapy for SB adenocarcinoma has not been well delineated, with no prospective or retrospective studies having demonstrated a benefit of adjuvant therapy. Despite this lack of data, adjuvant chemotherapy, generally utilizing a combination of 5-fluorouracil (5-FU) and oxaliplatin, is often used in patients at high risk for relapse. In addition, for patients with curatively resected adenocarcinoma of the duodenum, adjuvant 5-FU-based chemoradiation has been utilized to reduce the risk of local failure.27 Locally advanced unresectable tumors and metastatic tumors are treated with chemotherapy, most commonly 5-FU combined with either oxaliplatin or irinotecan. Median survival for patients with metastatic disease is approximately 12 to 18 months.28,29 The overall survival for SB adenocarcinoma remains poor, with 5-year disease-specific survival of 65% for stage I, 48% for stage II, 35% for stage III, and 4% for stage IV.30
Key Points Treatment of small bowel adenocarcinoma
• Radical curative surgery is the most desired approach when not precluded by preoperative proof of distant metastases. Duodenal adenocarcinoma requires pancreaticoduodenectomy (Whipple’s surgery). Limited detection of peritoneal spread and military liver metastases by imaging sometimes leads to the need for diagnostic laparoscopy as the first step before attempted curative resection.
• Adjuvant chemoradiation based on 5-FU is used to decrease the risk of local recurrence.
• 5-FU-based chemotherapy for unresectable disease has a low response rate with a poor 5-year survival of 4% at stage IV.
Anatomy and Pathology
Macroscopically, carcinoid tumors are present as small submucosal nodules, often subcentimeter in size, not causing obstruction of the lumen per se, with intense desmoplastic response within the adjacent mesentery. Thirty percent of SB carcinoids have multicentric disease at diagnosis.31 Although much rarer than carcinoid tumors, intermediate- and high-grade neuroendocrine tumors, characterized by both a higher rate of mitotic activity and tumor necrosis, can also arise in the SB. These tumors have a more aggressive biology, with high-grade neuroendocrine tumors of the SB behaving like small cell carcinomas of the lung.
Key Points Anatomy and pathology of carcinoid
• Carcinoid is a well-differentiated neuroendocrine carcinoma.
• Microscopically, carcinoid is composed of uniform small cells containing neurosecretory granules, with bioactive products such as serotonin, somatostatin, glucagon, histamine, or gastrin.
• Macroscopically, carcinoid tumors are often small submucosal nodules, multifocal in 30% of cases, with intense desmoplastic response within the adjacent mesentery.
Clinical Presentation
Because of their indolent growth, most SB carcinoids are asymptomatic and identified incidentally. Generally, symptoms from SB carcinoids relate to either mass effect from the primary or metastatic tumors or from hypersecretion of bioactive products such as serotonin. One third of midgut carcinoids are symptomatic with abdominal pain or bowel obstruction, and only 10% are associated with carcinoid syndrome. The carcinoid syndrome is primarily seen in the context of liver metastases, in which the release of serotonin gains access to the systemic circulation without undergoing hepatic metabolism.32 Carcinoid syndrome consists of secretory diarrhea, bouts of cutaneous flushing, wheezing, and dyspnea due to bronchospasm. Longstanding carcinoid syndrome may cause fibrotic changes in the cardiac valves predominantly affecting the right heart, typically leading to tricuspid regurgitation and pulmonic stenosis. A 24-hour urinary collection for the serotonin metabolite 5-hydroxyindole acetic acid (5-HIAA) has both good sensitivity and specificity for the diagnosis of the carcinoid syndrome.
Patterns of Tumor Spread
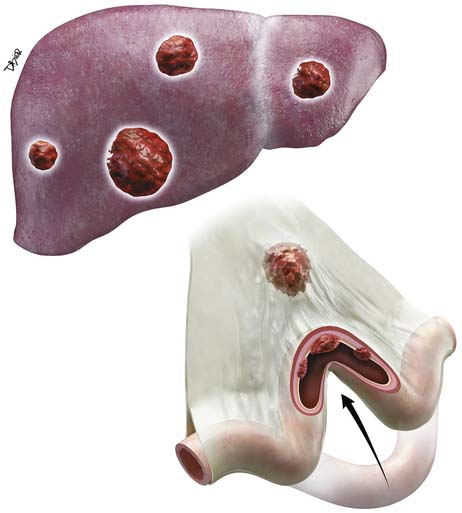
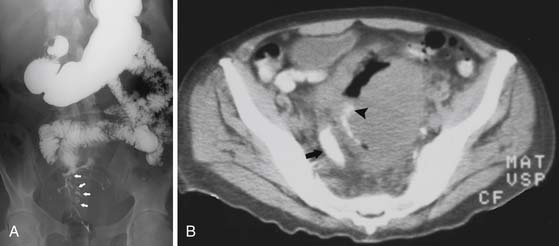
Tumorous spread via lymphatics into the mesenteric lymph nodes induces extensive desmoplastic reaction leading to retraction of the mesentery, kinking and obstruction of the mesenteric veins, and retraction and mechanical obstruction of the surrounding SB loops (Figure 16-6). This mesenteric nodal metastasis produces a typical mesenteric mass detectable by CT, as opposed to the small primary tumor that is commonly too small to detect by imaging.
Key Points Patterns of tumor spread of carcinoid
• Lymphatic spread to mesenteric lymph nodes is associated with desmoplastic reaction causing retraction of the mesentery, potentially leading to bowel obstruction.
• Hematogenous spread to the liver may be associated with the development of carcinoid syndrome.
• Peritoneal metastases are commonly small and rarely cause ascites.
Staging Evaluation
A revised TNM staging for carcinoid tumors, which is slightly different from the TNM staging of SB adenocarcinoma, has recently been proposed33 (Table 16-2).
Table 16-1 American Joint Committee on Cancer Staging for Small Bowel Adenocarcinoma
| STAGE | CHARACTERISTICS OF TUMOR-NODE-METASTASIS CLASSIFICATION SYSTEM |
|---|---|
| T TX T0 Tis T1a T1b T2 T3 T4 |
Primary Tumor Primary tumor cannot be assessed No evidence of primary tumor present Carcinoma in situ Tumor invades the lamina propria Tumor invades the submucosa Tumor invades the muscularis propria. Tumor invades through the muscularis propria into subserosa or into nonperitonealized perimuscular tissue (mesentery or retroperitoneum), with extension of < 2 cm Tumor penetrates the visceral peritoneum or directly invades other organs or structures |
| N NX N0 N1 N2 |
Regional Lymph Nodes Regional lymph nodes cannot be assessed No regional lymph node metastasis Regional lymph node metastasis with one to three lymph nodes involved Regional lymph node metastasis with four or more lymph nodes involved |
| M MX M0 M1 |
Distant Metastases Presence of distant metastasis cannot be assessed No distant metastasis Distant metastasis |
| Stage | Grouping |
| 0 I IIA IIB IIIA IIIB IV |
Tis, N0, M0 T1-T2, N0, M0 T3, N0, M0 T4, N0, M0 Any T, N1, M0 Any T, N2, M0 Any T, any N, M1 |
From Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010:127-129.
Key Point Staging of carcinoid
• The TNM staging of carcinoid is similar to that of adenocarcinoma, with the exception of T staging including not only the depth of invasion into the bowel wall but also a size as a separate criterion. Two centimeters serves as a threshold, with tumors larger than 2 cm upstaged in T status to T3, having a worse prognosis.
Imaging
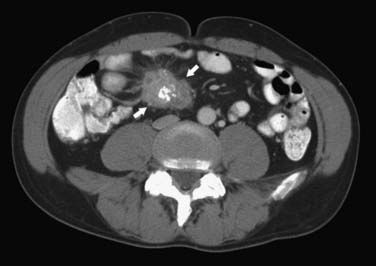
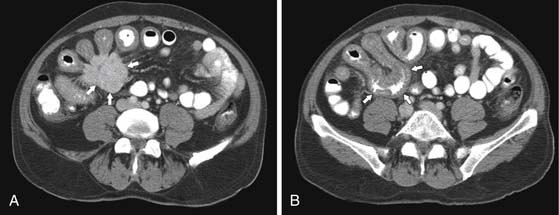
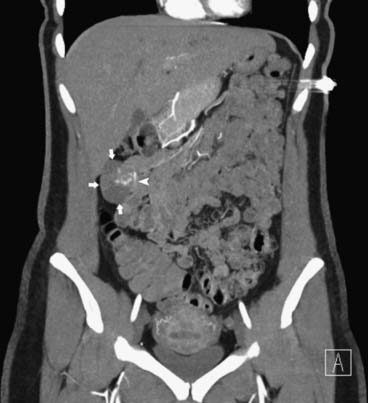
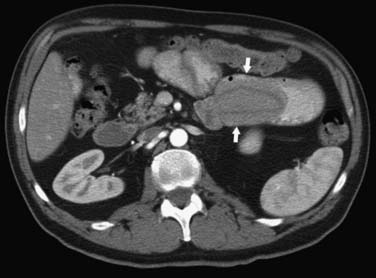
CT may identify the submucosal carcinoid tumor as a small mural mass with early intense contrast enhancement due to hyperemia.34 Hyperenhancing tumor can be best appreciated on the background of negative contrast in the bowel lumen in the arterial phase of contrast injection (Figures 16-7 and 16-8). Classic mesenteric nodal metastasis of carcinoid has a nearly pathognomonic CT pattern as a spiculated soft tissue density mesenteric mass due to desmoplastic reaction34,35 (Figures 16-9 and 16-10). Sometimes, a longer segment of adjacent SB has a thickened edematous wall due to mesenteric venous engorgement (see Figure 16-10). Calcification within the mesenteric extension can be seen in up to 70% of cases (see Figure 16-9). Differential diagnostic considerations for the mesenteric component include mesenteric panniculitis and treated lymphoma. Minority of mesenteric nodal metastases demonstrate a nonspecific pattern of well-circumscribed ovoid or round masses.
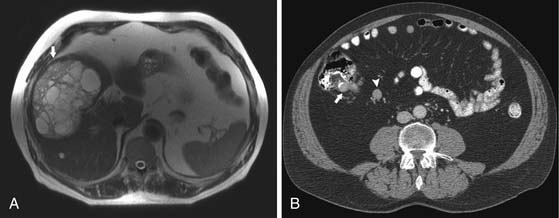
Liver metastases are hypervascular and best detected as hyperenhancing lesions on the late arterial phase of CT (see Figures 16-7 and 16-8) and as hypoenhancing lesions on the delayed venous phase of CT. Lesions may become isodense and least conspicuous in the portal phase. Dual-phase contrast-enhanced CT of the liver should always be performed to detect carcinoid liver metastasis. Noncontrast CT is very helpful for liver metastases detection, unless limited by fatty liver changes. Small carcinoid metastases can be mistaken for benign hypervascular lesions such as hemangiomas or small foci of focal nodular hyperplasia, especially in the absence of a baseline scan. Rarely, liver metastases have a cystic appearance (Figure 16-11).
MRI with gadolinium can demonstrate the primary hyperenhancing carcinoid in the bowel on fast dynamic sequences with T1 contrast (see Figure 16-8).22 Owing to increased sensitivity to gadolinium enhancement, MRI can detect more liver metastases than CT.36 MRI has a higher accuracy in detection of liver metastases and is a preferred modality when there are no extrahepatic metastases. In opposite to CT, MRI evaluation of liver metastases is not limited by fatty liver. Whereas gadolinium is necessary for initial characterization of liver lesions in a patient with carcinoid, follow-up MRI examinations maintain diagnostic quality even in the absence of contrast, serving patients with severely impaired renal function or lack of intravenous access.
Although somatostatic receptor scintigraphy (indium-111 octreotide imaging known as OctreoScan) has a complementary role to CT and MRI in detection of liver metastases and mesenteric nodal metastasis,37 the role of this nuclear study in detection of the occult primary SB carcinoid is limited. Scintigraphy performance in detection of liver metastasis is inferior to CT and MRI owing to decreased spatial resolution of single-photon emission computed tomography (SPECT) and increased scattered background activity of the tracer in a normal liver.36 However, OctreoScan may provide valuable information for predicting the efficacy of somatostatin analogue (octreotide) treatment.
Key Points The imaging report for carcinoid tumor
• Location of primary SB carcinoid if possible (rarely visualized on CT or MRI as a hyperenhancing mural nodule).
• Description of mesenteric nodal metastasis that may have a pathognomonic CT pattern as a spiculated soft tissue density mesenteric mass due to desmoplastic reaction, with 70% calcification rate.
• Evaluation of the liver for presence of hypervascular metastases best seen on the late arterial phase of CT or MRI. Small liver metastasis is difficult to distinguish from hemangioma by either CT of MRI.
Treatment
Localized disease requires treatment with wide en bloc surgical resection that includes the adjacent mesentery and lymph nodes. Resection of a primary tumor should also be considered in patients with liver metastases, in order to prevent the development of fibrosing mesenteritis and possible mechanical obstruction, bleeding, and perforation.38
Liver metastases are the most common site of metastatic disease and can become symptomatic owing to hormone secretion or pain. Owing to the slow-growing nature of carcinoid tumors, local modalities addressing the liver metastases have been investigated and shown to result in improvements in disease-free survival. Therefore, in patients in whom all sites of liver involvement can be surgically addressed, resection should be considered.39 When surgical treatment is either not feasible or incomplete, other local ablative techniques such as chemoembolization, radiofrequency ablation, and cryotherapy should be considered.32
The primary medical therapy for carcinoid tumors is octreotide, an analogue of the potent inhibitory GI hormone somatostatin. The use of octreotide is extremely effective in controlling the symptoms from the carcinoid syndrome, slowing the growth of carcinoid tumors, and inducing biochemical marker responses in approximately 50% of patients. Actually, tumor shrinkage from octreotide treatment is extremely rare, occurring in fewer than 10% of patients.40
New Therapies
Key Points Therapies for carcinoid
• Resection of primary tumor is indicated, regardless of the presence of liver metastases, to prevent bowel obstruction.
• Local modalities such as resection or ablation for the treatment of liver metastases improve disease-free survival.
• The somatostatic analogue octreotide is of value in the symptomatic relief of carcinoid syndrome, although actual tumor responses are extremely rare.
• Systemic cytotoxic chemotherapy does not have a role in the treatment of carcinoid tumors.
Surveillance
MRI is a preferred imaging modality for evaluating carcinoid involvement of the liver and is in particular useful for intrahepatic target lesions. Functional MRI with diffusion-weighted imaging has been explored as a tool to assess response of liver metastases to transhepatic arterial chemoembolization (TACE).41
Two effective markers available for monitoring patients with metastatic carcinoid tumors are
Epidemiology
Non-Hodgkin’s lymphoma (NHL) is the third most common SB malignancy, representing about 10% of all SB malignancies. Involvement of the SB by Hodgkin’s lymphoma, either primary or secondary involvement, is extremely rare.
Anatomy and Pathology
Lymphomas most often involving the GI tract consist of the extranodal marginal zone B-cell lymphoma of MALT type, diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, enteropathy-associated T-cell intestinal lymphoma, and Burkitt’s lymphoma.8 Although the majority of high-grade lymphomas will present with systemic disease at presentation, low-grade MALT lymphoma commonly presents with localized involvement.
The distribution of lymphoma in the SB follows the distribution of lymphoid follicles in the SB, with the lymphoid-rich distal ileum the most common site of SB lymphoma.8
Key Points Anatomy and pathology of lymphoma
• Only NHL involves the SB. The five most common types include MALT (extranodal marginal zone B-cell lymphoma of MALT), DLBCL, mantle cell lymphoma, enteropathy-associated T-cell intestinal lymphoma, and Burkitt’s lymphoma.
• Aggressive types of lymphoma tend to involve SB as part of systemic involvement. Low-grade MALT lymphoma can be isolated to the SB and, more commonly, to the stomach.
• SB lymphoma originates from the submucosal layer and may present with a large mass without obstruction of the lumen.
Staging Evaluation
NHL is traditionally staged by the Ann Arbor staging system (Table 16-3).42 This system, developed in 1971 for Hodgkin’s lymphoma, is less accurate in defining the prognosis of NHL. The Ann Arbor system does not reflect the tumor burden, one of the most important prognostic factors of lymphoma.
| STAGE | CHARACTERISTICS |
|---|---|
| I | Involvement of a single lymphatic site (I) or a single extralymphatic organ or site (IE) |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm (II) or a single extralymphatic organ or site with regional lymph node involvement (IIE) |
| III | Involvement of lymph node region on both sides of the diaphragm (III), with extralymphatic extension (IIIE), involvement of the spleen (IIIS) or both (IIIE, S) |
| IV | Diffuse or disseminated involvement of one or more extralymphatic organs (e.g., liver, bone marrow, lungs) with or without lymph node involvement |
From Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010:607.
The International Prognostic Index (IPI) is an alternative prognostic system based on a patient’s age, serum lactate dehydrogenase (LDH) concentration greater than normal, poor Eastern Cooperative Oncology Group (ECOG) performance status (≥2), Ann Arbor clinical stage III or IV, and the number of involved extranodal disease sites greater than 1. These factors were found to correlate significantly with shorter overall or relapse-free survival. IPI score of 0 to 1 indicates low risk; 2, low-intermediate risk; 3, intermediate-high risk, and 4 to 5, high risk.43
Imaging
Four major patterns of SB lymphoma have been identified on radiographic studies25:
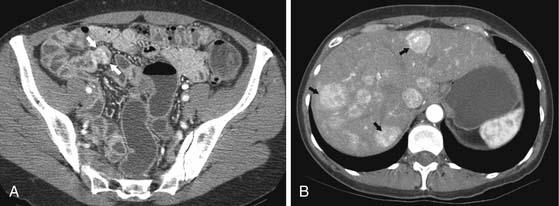
1. Infiltrating pattern that appears as wall thickening with destruction of the normal SB folds. Because the tumor infiltrates the muscular layer of the wall, it can inhibit peristalsis and, therefore, result in aneurysmal dilatation of the affected bowel loop. As a result, the lumen is dilated with focal excentric bulging of the wall. On CT, this mass appears homogeneously hypodense with minimal contrast enhancement (Figure 16-12).
2. Exophytic mass, which can ulcerate. This pattern can simulate an adenocarcinoma or GIST. Ulceration may result in localized perforation.
3. Single mass lesion, which can lead to intussusception (Figure 16-13) but rarely will result in obstruction because the masses are typically pliable and soft.
4. Multifocal submucosal nodules within the SB. This appearance is often better appreciated on an SB series or enteroclysis as “bull’s-eye” or “target” lesions because the nodules can be very small. Small-volume lymphomatous implants in the bowel are not well resolved by CT or PET/CT.
PET/CT is not required as the baseline staging imaging for lymphomas with known FDG-avidity, such as DLBCL, mantle cell lymphoma, or Burkitt’s lymphoma. However, baseline PET/CT is mandatory for NHL with variable FDG avidity such as MALT lymphoma or enteropathy-associated T-cell intestinal lymphoma, if PET is used to assess response to treatment.44 PET/CT evaluation of the bowel lymphoma may be limited by incidental physiologic increased background activity within the bowel, occasionally in the SB, and even more commonly in the colon.
Key Points The imaging report for lymphoma
• Location and extent of SB involvement. When characterizing an undiagnosed SB mass, aneurysmal dilatation of the involved segment of the bowel and lack of bowel obstruction despite the large tumor are features suggestive of lymphoma.
• Presence of lymphadenopathy or other extranodal sites of lymphomatous involvement.
Treatment
As in lymphomas of lymph node origin, chemotherapy is the primary therapeutic modality. In general, patients should be assumed to have systemic disease and be treated with systemic chemotherapy, even if a complete surgical resection has been performed. Responsiveness to chemotherapy is based upon disease histology, with more aggressive histologic subtypes demonstrating more dramatic responses. Aggressive lymphomas such as DLBCL require multiagent chemotherapy with RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).45 More indolent, slow-growing types (mantle cell and follicular lymphomas) are generally not cured with chemotherapy, but the use of chemotherapy will result in a significant prolongation of disease-free survival. With the recent improvements in chemotherapy, radiation therapy for the management of SB lymphoma is rarely utilized. This primarily relates to the poor tolerance of the normal SB to the effects of radiation. H. pylori eradication is an effective primary treatment option for low-grade MALT lymphoma of the stomach, although the applicability to MALT lymphoma of the SB is not known.46 The prognosis of SB lymphomas is overall better than for adenocarcinoma, with 20% to 50% 5-year survival for aggressive advanced-stage lymphoma, and up to 80% to 90% 5-year survival for localized less aggressive subtypes.8
Surveillance
Monitoring of tumor response to chemotherapy is performed by CT and PET/CT. Close monitoring during the initial course of chemotherapy is recommended for aggressive histologic types of lymphoma owing to the risk of perforation with the rapid response to therapy and shrinking of the lymphomatous mass involving the SB wall. PET/CT scan is highly sensitive for detection of extranodal involvement of lymphoma, including within the SB, and plays a crucial role in monitoring response to treatment. A positive FDG-PET scan after the completion of chemotherapy is a strong predictor of relapse and lower disease-free survival.47,48
Epidemiology and Risk Factors
GISTs represent the fourth most common malignant SB tumors (9%). Most cases of GIST are sporadic. No known risk factors have been identified, although there are reports of increased incidence of both benign and malignant GISTs in patients with neurofibromatosis type 1.51
Anatomy and Pathology
By light microscopy alone, the distinction among GISTs and other tumors (leiomyosarcoma, leiomyoma, malignant melanoma, schwannoma, malignant peripheral nerve sheath tumor, or desmoid tumor) can be difficult because histologic findings do not reliably relate to the immunophenotype or the molecular genetics of the lesions. Eighty percent of GIST cells, however, express the CD117 antigen, part of the KIT transmembrane receptor tyrosine kinase (RTK) that is the product of the c-kit (KIT) proto-oncogene and can be diagnosed using this sophisticated technique.9
Staging Evaluation
TNM staging of GIST is similar to adenocarcinoma staging by the American Joint Committee on Cancer (AJCC; see Table 16-2).
Imaging
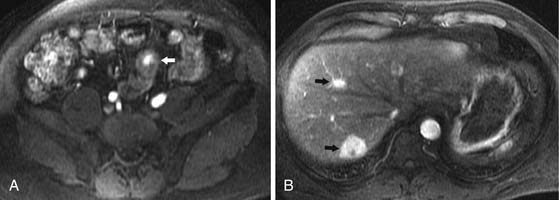
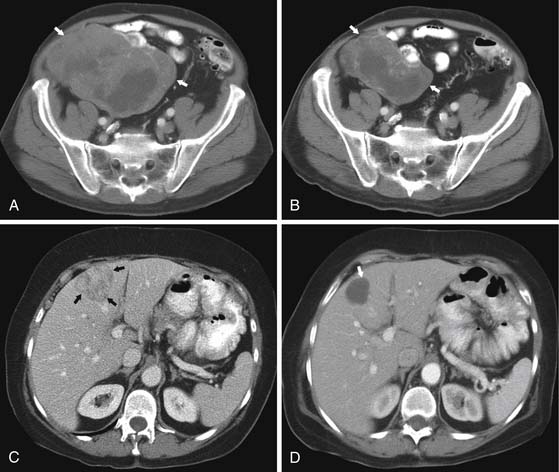
Because of the exophytic growth, CT is the imaging modality of choice. Small tumors tend to appear homogeneous on CT, although larger tumors (>6 cm) frequently show central areas of necrosis or hemorrhage.52 These tumors may be so large that it may be difficult to appreciate the communication of the tumor with the intestinal wall (Figure 16-14). If intraluminal contrast is seen in the tumor, bowel origin is confirmed (Figure 16-15). Large size, significant necrosis, and cavitation are signs suggestive of malignancy. It is difficult to distinguish benign from malignant GIST unless obvious metastases are present. GISTs tend to demonstrate an early contrast enhancement due to the hypervascular nature (Figure 16-16).
Liver metastases appear isodense or hypodense to normal liver parenchyma on noncontrast images and usually are hypodense on the postcontrast images. They can appear cystic and can be difficult to differentiate from other cystic hepatic lesions, but this appearance is more commonly seen post treatment (Figure 16-17). Stable treated cystic-appearing GIST metastases should not be mistaken for benign hepatic cysts. Growing solid nodules within these cystic lesions indicate reactivation of tumor growth. Peritoneal metastasis may be seen as separate nodules (sarcomatous pattern of peritoneal spread), but ascites is rarely seen.
Treatment
Surgical resection with negative margin is the treatment of choice. Unlike adenocarcinoma and carcinoid that spread through lymphatics, GISTs metastasize hematogenously, obviating the need for more complete surgery with removal of the lymphatic drainage in the mesentery. This may be significant in the duodenal tumor, allowing the surgeon to avoid a major procedure such as pancreaticoduodenectomy. Although GISTs may present as very large masses, the “pushing” rather than the invasive nature of tumor growth may sometimes allow for the development of a safe plane of resection. GISTs smaller than 2 cm are generally considered benign with a very low risk of recurrence.
KIT-positive GISTs can be specifically targeted therapeutically with orally active tyrosine kinase inhibitors (TKIs) such as imatinib mesylate (Gleevec) and sunitinib (Sutent). These agents can be used in both the neoadjuvant setting to downstage borderline surgical candidates and the adjuvant setting and have been shown to be highly effective.53 These agents are used for metastatic unresectable disease.
New Therapies
With prolonged treatment, resistance can develop most likely due to secondary KIT mutations. Dose escalation can be attempted initially. Newer TKI can be used for broader target profiles and increased activity. Other strategies like inhibition of downstream signaling molecules, transcriptional repression, combination with chemotherapy, receptor-mediated therapy of highly expressed cell surface receptors or targeting of cancer stem cells may prove to be useful in future.54
Key Points Therapies for gastrointestinal stromal tumor
• Radical resection with curative intent is the treatment of choice.
• Unresectable KIT-positive GIST is successfully treated by TKIs imatinib mesylate and sunitinib. The same agents can be used in the adjuvant and neoadjuvant settings for surgical candidates.
• Resistance to imatinib may develop after prolonged treatment.
Surveillance
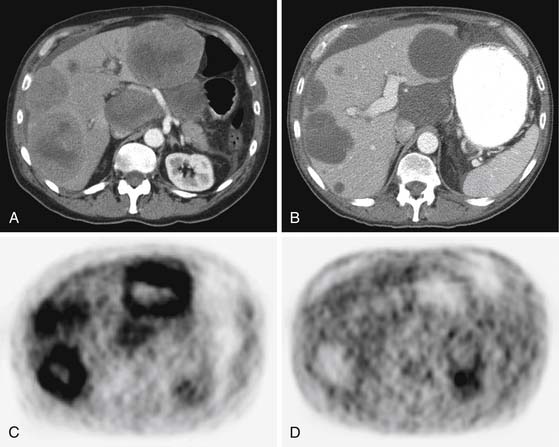
Follow-up of GIST patients treated with imatinib showed that tumor size alone was a poor indicator of a positive response. In 75% of the patients, the tumor was stable by RECIST criteria despite the favorable clinical outcome. Decrease in tumor density after treatment indicated radiographic response due to myxoid degeneration and necrosis within the tumor (Figure 16-18; see also Figure 16-17). Successfully treated GISTs sometimes became so uniformly hypodense that they could mimic benign cysts and vice versa. Tumor progression may be manifested as not only an enlarging mass or new masses but also as a partial to complete filling-in of a previously hypodense lesion or as a hyperdense “nodule-within-a-mass” pattern.55
In order to address the discrepancy between the standard RECIST criteria and GISTs behavior, an alternative set of criteria were developed by Choi and coworkers55,56: 10% decrease in unidimensional tumor size or a 15% decrease in tumor density on contrast-enhanced CT scans.
FDG-PET/CT exceeds CT in monitoring tumor response to chemotherapy.55,57–59 A dramatic decrease in FDG uptake within initially hypermetabolic GIST tumors is noted within a short interval after initiation of imatinib therapy (see Figure 16-18). However, no clear quantitative concordance was demonstrated between Hounsfield units measured on CT as a single criterion and standardized uptake value (SUV) as a measure of FDG uptake because of the differences in measurement methods.
Key Points Detecting recurrence of gastrointestinal stromal tumor
• Clinical response to imatinib does not follow RECIST criteria. Alternative Choi and coworkers’ criteria are based not only on tumor size but also on the degree of enhancement.
• Tumor progression may be manifested as not only an enlarging mass but also as a new hyperdense nodule within a previously uniformly hypodense mass.
Clinical Presentation
As with other SB masses, metastases may cause bowel obstruction and GI bleeding.
Patterns of Tumor Spread
There are three pathways of secondary involvement of the SB:
1. Intraperitoneal spread: Commonly from GI and ovarian-uterine cancers. The areas most prone to involvement are the ileocecal valve and ileal loops in the cul-de-sac. Mesenteric metastases generally involve the mesenteric margin of the bowel. Desmoplastic reaction of these tumors may cause obstruction.
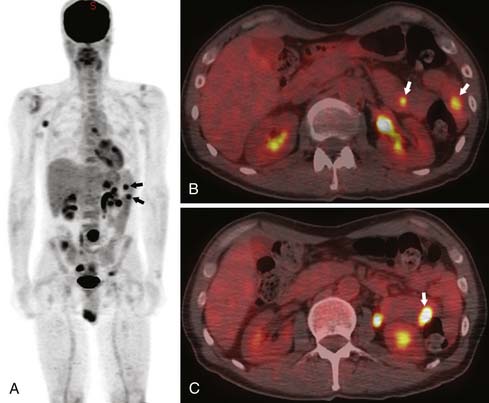
2. Hematogenous dissemination. Melanoma is the most common solid tumor metastatic to the SB, followed by lung cancer and breast cancer.60 Although SB metastases in lung cancer are usually concomitant with overall progressive disease and often clinically obscure, the situation may be different with melanoma (Figure 16-19) in which metastases may appear years after the diagnosis of the primary tumor as an only site of recurrence.61 Hematogenous metastases are usually multiple and commonly involve the antimesenteric border of the bowel.
3. Extension from an adjacent tumor either directly or via lymphatics. The most common primary tumor accounting for this appearance is colon cancer.
Key Points Patterns of tumor spread of small bowel metastases
• Intraperitoneal spread is common from GI and ovarian-uterine cancers. Dependent areas of the peritoneal cavity are most prone to be involved, including the ileocecal valve and ileal loops in the cul-de-sac. The mesenteric margin of the SB is usually involved. Desmoplastic reaction of these tumors may cause obstruction.
• Hematogenous spread is most common from melanoma, lung, and breast cancer, usually with multiple lesions involving the antimesenteric border of the bowel.
• Extension from an adjacent tumor either directly or via lymphatics is most commonly from colon cancer.
Imaging
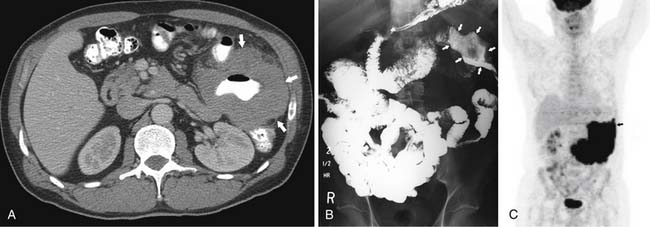
Neither small bowel follow-through (SBFT; 58% sensitivity) nor conventional CT (66% sensitivity) seems to be reliable in demonstrating melanoma metastases to the SB.62 Focal wall thickening and polypoid filling defects are seen with the advanced cases. PET/CT is more sensitive and specific for detection of early SB metastases, among other early soft tissue metastases63 (Figures 16-20 and 16-21).
Key Points The imaging report for small bowel metastases
• Description of the suspicious areas of focal wall thickening, within limitations of suboptimal SB distention or filling with contrast.
• Presence or imminent danger of bowel obstruction.
• Areas of focal highly increased FDG uptake in the SB should be considered suspicious for metastases even when not associated with any detectable abnormality on CT. Capsule endoscopy may be suggested for further evaluation.
Treatment
SB metastases overall indicate poor prognosis with less than 12 months’ survival. Resection of melanoma metastases is indicated when possible for palliation and increase in symptom-free survival.61
1. Hatzaras I., Palesty J.A., Abir F., et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229-235.
2. Bilimoria K.Y., Bentrem D.J., Wayne J.D., et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71.
3. Minardi A.J.Jr., Zibari G.B., Aultman D.F., et al. Small-bowel tumors. J Am Coll Surg. 1998;186:664-668.
4. Eisen G.M., Dominitz J.A., Faigel D.O., et al. Enteroscopy. Gastrointest Endosc. 2001;53:871-873.
5. Eliakim R. Video capsule endoscopy of the small bowel. Curr Opin Gastroenterol. 2008;24:159-163.
6. Yamamoto H., Sekine Y., Sato Y., et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220.
7. Weiss N.S., Yang C.P. Incidence of histologic types of cancer of the small intestine. J Natl Cancer Inst. 1987;78:653-656.
8. Koch P., del Valle F., Berdel W.E., et al. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3861-3873.
9. Miettinen M., Lasota J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12.
10. Jemal A., Siegel R., Ward E., et al. Cancer statistics. CA Cancer J Clin. 2008;2008(58):71-96.
11. Wu A.H., Yu M.C., Mack T.M. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int J Cancer. 1997;70:512-517.
12. Neugut A.I., Jacobson J.S., Suh S., et al. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomarkers Prev. 1998;7:243-251.
13. Abrahams N.A., Halverson A., Fazio V.W., et al. Adenocarcinoma of the small bowel: a study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum. 2002;45:1496-1502.
14. Agrawal S., McCarron E.C., Gibbs J.F., et al. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14:2263-2269.
15. Kariv R., Arber N. Malignant tumors of the small intestine—new insights into a rare disease. Isr Med Assoc J. 2003;5:188-192.
16. Carnazzo S.A., Laurentini G.M., Fasone M.A., et al. Primary small bowel adenocarcinoma: a single centre evaluation of survival. Dig Liver Dis. 2004;36:782-783.
17. Hara A.K., Leighton J.A., Sharma V.K., et al. Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiographics. 2005;25:697-711. discussion 711–718
18. Bessette J.R., Maglinte D.D., Kelvin F.M., et al. Primary malignant tumors in the small bowel: a comparison of the small-bowel enema and conventional follow-through examination. AJR Am J Roentgenol. 1989;153:741-744.
19. Arslan H., Etlik O., Kayan M., et al. Peroral CT enterography with lactulose solution: preliminary observations. AJR Am J Roentgenol. 2005;185:1173-1179.
20. Lappas J., Heitkamp D.E., Maglinte D.D. Current status of CT enteroclysis. Crit Rev Comput Tomogr. 2003;44:145-175.
21. Orjollet-Lecoanet C., Menard Y., Martins A., et al. CT enteroclysis for detection of small bowel tumors [in French]. J Radiol. 2000;81:618-627.
22. Semelka R.C., John G., Kelekis N.L., et al. Small bowel neoplastic disease: demonstration by MRI. J Magn Reson Imaging. 1996;6:855-860.
23. Masselli G., Polettini E., Casciani E., et al. Small-bowel neoplasms: prospective evaluation of MR enteroclysis. Radiology. 2009;251:743-750.
24. Rieber A., Aschoff A., Nussle K., et al. MRI in the diagnosis of small bowel disease: use of positive and negative oral contrast media in combination with enteroclysis. Eur Radiol. 2000;10:1377-1382.
25. Horton K.M., Fishman E.K. Multidetector-row computed tomography and 3-dimensional computed tomography imaging of small bowel neoplasms: current concept in diagnosis. J Comput Assist Tomogr. 2004;28:106-116.
26. Barnes G.Jr., Romero L., Hess K.R., et al. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73-78.
27. Dabaja B.S., Suki D., Pro B., et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526.
28. Fishman P.N., Pond G.R., Moore M.J., et al. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: a retrospective review of 113 cases. Am J Clin Oncol. 2006;29:225-231.
29. Locher C., Malka D., Boige V., et al. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology. 2005;69:290-294.
30. Howe J.R., Kamell L.H., Menck H.R., et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer. 1999;86:2693-2706.
31. Caplin M.E., Buscombe J.R., Hilson A.J., et al. Carcinoid tumour. Lancet. 1998;352:799-805.
32. Modlin I.M., Champaneria M.C., Chan A.K., et al. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464-1473.
33. Landry C.S., Brock G., Scoggins C.R., et al. A proposed staging system for small bowel carcinoid tumors based on an analysis of 6,380 patients. Am J Surg. 2008;196:896-903. discussion 903
34. Horton K.M., Kamel I., Hofmann L., et al. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am J Roentgenol. 2004;182:559-567.
35. Woodard P.K., Feldman J.M., Paine S.S., et al. Midgut carcinoid tumors: CT findings and biochemical profiles. J Comput Assist Tomogr. 1995;19:400-405.
36. Dromain C., de Baere T., Lumbroso J., et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70-78.
37. Kvols L.K., Brown M.L., O’Connor M.K., et al. Evaluation of a radiolabeled somatostatin analog (I-123 octreotide) in the detection and localization of carcinoid and islet cell tumors. Radiology. 1993;187:129-133.
38. Loftus J.P., van Heerden J.A. Surgical management of gastrointestinal carcinoid tumors. Adv Surg. 1995;28:317-336.
39. Chen H., Hardacre J.M., Uzar A., et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88-92. discussion 92–93
40. Leong W.L., Pasieka J.L. Regression of metastatic carcinoid tumors with octreotide therapy: two case reports and a review of the literature. J Surg Oncol. 2002;79:180-187.
41. Liapi E., Geschwind J.F., Vossen J.A., et al. Functional MRI evaluation of tumor response in patients with neuroendocrine hepatic metastasis treated with transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2008;190:67-73.
42. Rosenberg S.A. Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep. 1977;61:1023-1027.
43. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987-994.
44. Delbeke D., Stroobants S., de Kerviler E., et al. Expert opinions on positron emission tomography and computed tomography imaging in lymphoma. Oncologist. 2009;14(Suppl 2):30-40.
45. Daum S., Ullrich R., Heise W., et al. Intestinal non-Hodgkin’s lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin’s Lymphoma. J Clin Oncol. 2003;21:2740-2746.
46. Lee S.K., Lee Y.C., Chung J.B., et al. Low grade gastric mucosa associated lymphoid tissue lymphoma: treatment strategies based on 10 year follow-up. World J Gastroenterol. 2004;10:223-226.
47. Reinhardt M.J., Herkel C., Altehoefer C., et al. Computed tomography and 18F-FDG positron emission tomography for therapy control of Hodgkin’s and non-Hodgkin’s lymphoma patients: when do we really need FDG-PET? Ann Oncol. 2005;16:1524-1529.
48. Yang D.H., Min J.J., Jeong Y.Y., et al. The combined evaluation of interim contrast-enhanced computerized tomography (CT) and FDG-PET/CT predicts the clinical outcomes and may impact on the therapeutic plans in patients with aggressive non-Hodgkin’s lymphoma. Ann Hematol. 2009;88:425-432.
49. Miettinen M., Kopczynski J., Makhlouf H.R., et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641.
50. Tran T., Davila J.A., El-Serag H.B. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162-168.
51. Zoller M.E., Rembeck B., Oden A., et al. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer. 1997;79:2125-2131.
52. Horton K.M., Juluru K., Montgomery E., et al. Computed tomography imaging of gastrointestinal stromal tumors with pathology correlation. J Comput Assist Tomogr. 2004;28:811-817.
53. Blanke C.D., Rankin C., Demetri G.D., et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
54. Nilsson B., Nilsson O., Ahlman H. Treatment of gastrointestinal stromal tumours: imatinib, sunitinib—and then? Expert Opin Investig Drugs. 2009;18:457-468.
55. Choi H., Chamsangavej C., Faria S.C., et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759.
56. Benjamin R.S., Choi H., Macapinlac H.A., et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760-1764.
57. Choi H., Chamsangavej C., de Castro Faria S., et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619-1628.
58. Gayed I., Vu T., lyer R., et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17-21.
59. Goldstein D., Tan B.S., Rossleigh M., et al. Gastrointestinal stromal tumours: correlation of F-FDG gamma camera-based coincidence positron emission tomography with CT for the assessment of treatment response—an AGITG study. Oncology. 2005;69:326-332.
60. Farmer R.G., Hawk W.A. Metastatic tumors of the small bowel. Gastroenterology. 1964;47:496-504.
61. Schuchter L.M., Green R., Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr Opin Oncol. 2000;12:181-185.
62. Bender G.N., Maglinte D.D., McLamey J.H., et al. Malignant melanoma: patterns of metastasis to the small bowel, reliability of imaging studies, and clinical relevance. Am J Gastroenterol. 2001;96:2392-2400.
63. Swetter S.M., Carroll L.A., Johnson D.L., et al. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646-653.