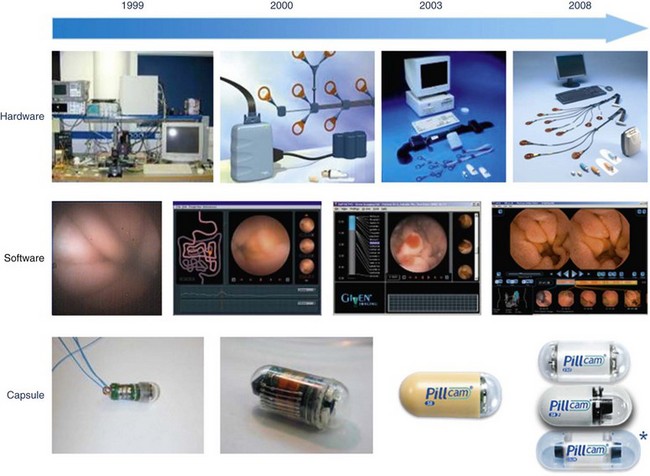
CHAPTER 5 Small bowel endoscopy
Indications and technique
5.1 Video capsule endoscopy
Key Points
1 Technical principles
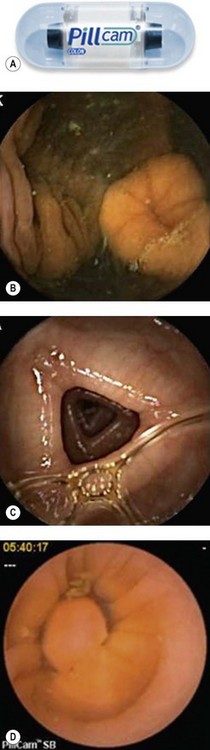
Development of the first capsule (Fig. 1) was based on three scientific advances: a CMOS (complementary metal oxide silicone) microchip capable of producing an image comparable with that obtained with a CCD camera; an ASIC (application-specific integrated circuit) system, which allows integration of a small, low-energy video transmitter; and miniature high-power lighting such as an LED (light-emitting diode). These three components are placed in a capsule measuring 1.1 cm × 2.6 cm that can be swallowed. The field of vision obtained is 140°. It is weighted so that it retains its longitudinal orientation for approximately 80% of its intestinal journey, and it passes through the body naturally. The system also comprises a series of sensors which are placed on the surface of the patient’s abdomen and detect signals emitted by the capsule. These are transmitted to a high-frequency tape recorder, contained in a case and worn on a belt by the patient, before transfer to a workstation. The capsule is eliminated in the stools and is a single-use device. This description corresponds to the system developed by Given Imaging Ltd. Since then, the capsule has been improved and is available under the name ‘PillCam SB’, characterized by a wider angle of vision (156°), better resolution (65 536 pixels), and an increased depth of field. The ‘EndoCapsule’, ‘MiRoCam’ and ‘OMOM’ capsules differ in using a CCD rather than CMOS sensor (Fig. 2) to capture images. Studies comparing the ‘Pillcam’ and ‘Endoscapsule’ have not demonstrated any differences in diagnostic yield in patients with obscure gastrointestinal bleeding. The ‘MiroCam’ capsule (Fig. 3) has longer-life batteries (11 h), is smaller in size (11 × 24 mm), has a larger number of pixels (102,400), takes three images per second, and transmits data using conduction through body tissues. This requires constant contact with the mucosa, which may be a limiting factor. Comparative studies with the other two systems are currently underway. The ‘OMOM’ capsule (27.9 mm long, 13 mm diameter, and 6 g weight) is significantly bigger than the others (24–26 × 11 mm and 3.4–3.6 g weight).
3 Safety of VCE and contraindications
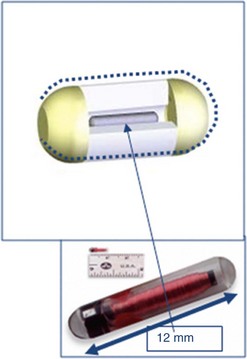
To solve the problem of retention, Given Imaging Ltd has developed a calibration capsule, called the ‘M2A Patency Capsule’. If it has not been expelled after 2–3 days, this breaks down spontaneously into small fragments, which easily pass through a narrowed segment. Latest modifications have incorporated two openings at each end of the capsule (the Agile Patency Capsule, Fig. 4), to enhance capsule breakdown. It is important to remember that neither small intestine barium studies nor CT or MR enteroclysis can detect all strictures. It is therefore essential to enquire about the patient’s medical history (complex surgery, use of NSAIDs, radiotherapy of the abdomen, and recent episodes of obstructive symptoms) before carrying out an examination by VCE. The risk of obstruction should be explained clearly before VCE, along with the possibility that a retained capsule may have to be removed endoscopically or surgically. CT or MR enteroclysis or the use of an Agile Patency Capsule is recommended before performing VCE in patients felt to be at risk of small bowel strictures.
4 Indications and results
VCE has already changed the management of patients in the following disorders:
4.1 Chronic obscure gastrointestinal bleeding
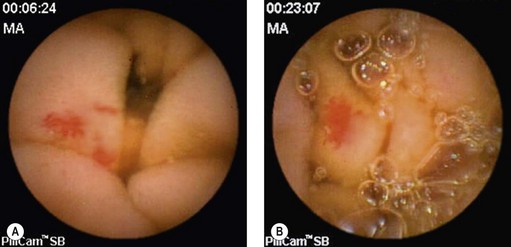
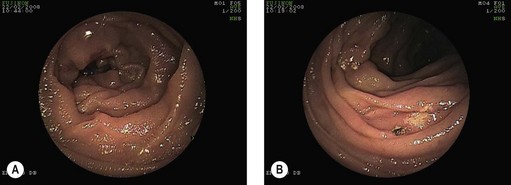
This is defined as isolated or recurrent melena, rectal bleeding or iron deficiency anemia with evidence of gastrointestinal bleeding. Patients should have undergone negative upper endoscopy and total colonoscopy before VCE is considered. A positive diagnosis may be found in 55–81% of patients, the yield being higher in those with overt as opposed to obscure bleeding. VCE is superior in detecting lesions responsible for bleeding compared with push enteroscopy (PE), particularly in patients with overt bleeding. Studies have also emphasized the need for examination by VCE as soon as possible after the bleeding episode, the diagnostic yield dropping as time elapses. Finally, the use of a repeat study in patients in whom VCE had initially been negative will yield a diagnosis in a significant number of patients. Two meta-analyses of 14 and 17 studies, respectively, demonstrate that VCE yields a positive diagnosis in 63% of patients compared with 28% for push-enteroscopy. The lesions detected are usually, in decreasing frequency, arteriovenous malformations (Fig. 5), ulceration secondary to NSAIDs, and tumors. When compared with double balloon enteroscopy (DBE), both methods have similar diagnostic yields in obscure gastrointestinal bleeding: 43–60% for DBE and 59–80% for VCE.
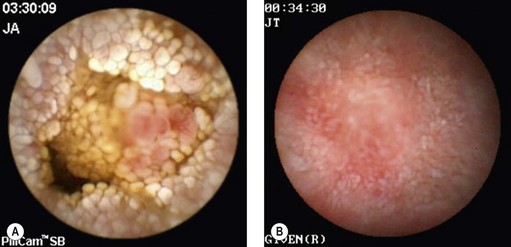
4.3 Crohn’s disease
VCE detects more intestinal lesions in patients with Crohn’s disease (Fig. 6) than conventional radiological imaging. The lesions usually detected are mucosal: erosions, purpuric lesions, ulceration, aphthoid lesions, and strictures. Some practical conclusions are shown in Box 1.
Box 1 The role of VCE in Crohn’s disease
4.4 Celiac disease
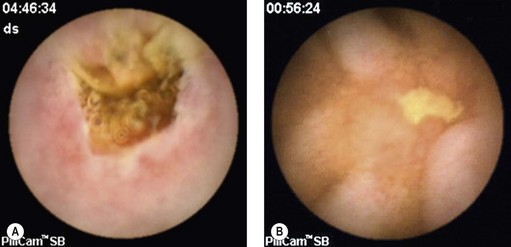
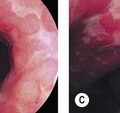
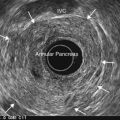
Some authors have suggested that VCE could be an alternative to endoscopic duodenal biopsies obtained at OGD, particularly in patients unwilling to undergo the procedure, and could be carried out for: chronic iron-deficiency anemia, children with clinical evidence and laboratory results suggesting celiac disease, patients with anti-transglutaminase antibodies, and atypical symptoms in elderly patients (Fig. 7A,B).
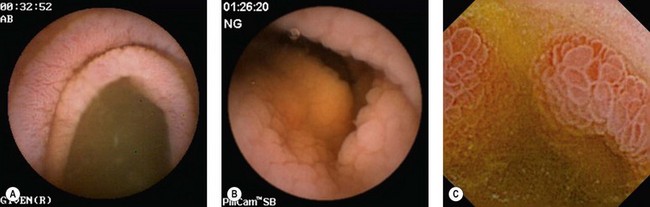
Figure 7 (A,B) Celiac disease. (C) Enteropathy associated T-cell lymphoma (EATL) complicating celiac disease.
VCE in combination with DBE is, moreover, the best way of examining patients with celiac disease who have warning symptoms (weight loss, anemia and abdominal pain), while adhering closely a gluten-free diet. VCE is a useful tool for monitoring patients with celiac disease to detect malignant lesions, i.e. adenocarcinoma or lymphoma (Fig. 7C), particularly if ulcerative jejunitis is present.
4.5 NSAID enteropathy
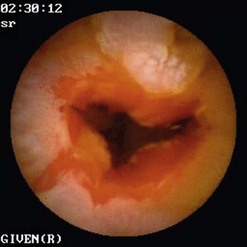
Ulcers, erosions and stenotic diaphragms or webs are usually found (Fig. 8). The clinical significance of minor lesions accompanying the use of NSAIDs is uncertain, as they are also detected in up to 22% of healthy volunteers participating in the control group in studies of NSAID toxicity.
4.6 Detection of intestinal tumors
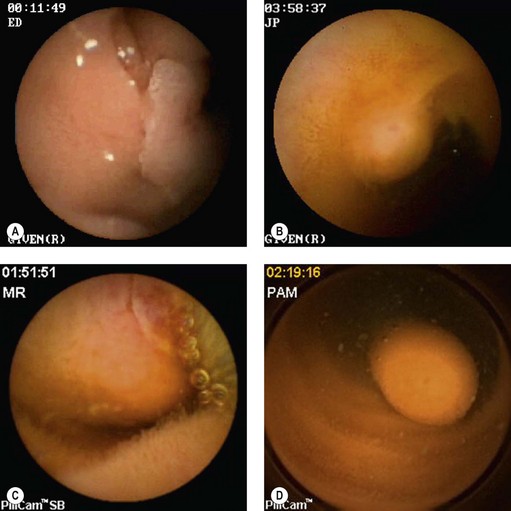
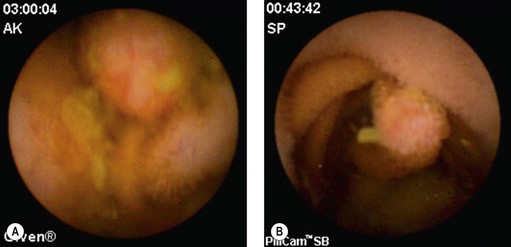
The frequency of these tumors (Fig. 9) in patients examined by VCE for chronic obscure gastrointestinal bleeding is approximately 6–12%, and 60% of these are malignant. Since the introduction of VCE, it has been noted that the most frequent presentation of these intestinal tumors is chronic obscure bleeding rather than abdominal pain, weight loss or obstruction. This means that VCE has the potential to detect these tumors at an earlier stage.
4.7 Surveillance of familial polyposis
VCE is capable of demonstrating the existence of polyposis (Fig. 10) along the small intestine. Although it may miss duodenal lesions in comparison with PE and DBE, it performs better in the jejunum and ileum. Its use is even more impressive in Peutz–Jeghers syndrome in which it can detect lesions capable of causing intussusception, and demonstrate ulcerated polyps responsible for chronic anemia. Its use is now widely accepted in the surveillance of familial adenomatous polyposis (FAP) associated with duodenal polyps. The same applies in juvenile polyposis. If VCE is used to monitor patients with FAP, it should be kept in mind that it does not detect all lesions in the duodenum, particularly the periampullary region. The duodenum must be investigated by a side-viewing endoscope in these patients. Finally, VCE cannot accurately assess the size of tumors in patients with familial polyposis, and often overestimates this. MR-enteroclysis appears to be better for assessing the size of these lesions.
Box 2 summarizes the role of VCE in small intestinal disorders.
6 Technical aids to reading images obtained by VCE
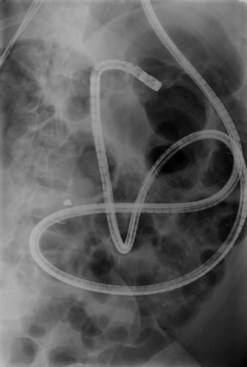
Precise anatomic localization of the capsule (Figs. 12, 13) remains too inaccurate to be used in practice, regardless of the electronic means of detection used. The capsule is, in fact, located based on differentiation between the appearance of the jejunum and the ileum and the time elapsed in relation to passage through the pylorus and the caecum. The Real-Time Viewer, which is available from Olympus, Given Imaging Ltd and MiroCam, allows the images observed by the capsule to be read directly in real-time. These three systems are similar in principle and can be used at the patient’s bedside. It is useful for determining whether the capsule has passed through the pylorus and for deciding to administer an erythromycin infusion to make sure the small intestinal examination is completed within the battery’s life. It may be particularly useful for locating the site of active gastrointestinal bleeding, thereby allowing targeted therapeutic endoscopy.
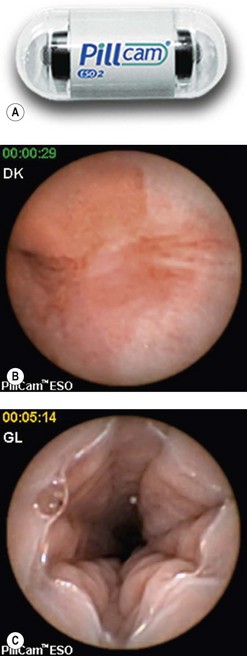
8 Recent developments in VCE: esophagus and colon
8.2 The colon
This is a recent development proposed by Given Imaging Ltd. The PillCam Colon (Fig. 15) is a capsule measuring 11 mm by 32 mm with two domes and two cameras. Images are acquired at a rate of 4/s. The batteries have a life of approximately 10 h. This capsule first examines the esophagus, stomach and duodenum, then switches off. It is programmed to switch on again when it reaches the terminal ileum. Patients require intensive preparation before the introduction of the colon capsule since exquisite cleanliness is required for a complete and careful examination of the mucosa, and passage of the capsule must also be facilitated. Stimulant agents (sodium phosphate) and laxatives (bisacodyl) are therefore used. This type of preparation, which is therefore slightly harsher than typical colonic preparation, can be used to obtain a clean colon.
American Gastroenterological Association. Medical position statement: evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:197-200.
Cellier C, Green PH, Collin P, et al. ICCE consensus for celiac disease. Endoscopy. 2005;37:1055-1059.
Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure gastrointestinal bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36:1067-1073.
Delvaux M, Gay G. Capsule endoscopy: technique and indications. Best Pract Res Clin Gastroenterol. 2008;20:813-837.
Galmiche JP, Coron E, Sacher-Huvelin S. Recent developments in capsule endoscopy. Gut. 2008;57:695-703.
Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49-58.
Hartmann D, Eickhoff A, Damian U, et al. Diagnosis of small-bowel pathology using paired capsule endoscopy with two different devices: a randomized study. Endoscopy. 2007;39:1041-1045.
Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature. 2000;405:417.
Jones BH, Fleischer DE, Scharma VK, et al. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:1058-1064.
Ladas SD, Triantafyllou K, Spada C, et al. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. ESGE Clinical Guidelines Committee. Endoscopy. 2010;42(3):220-227.
Solem CA, Loftus JR, Fletcher JG, et al. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255-266.
5.2 Enteroscopy
Introduction
Box 1 Overtube-assisted enteroscopy
1 Indications and contraindications
2 General enterosocpy technique
3 Double balloon enteroscopy
3.2 Equipment
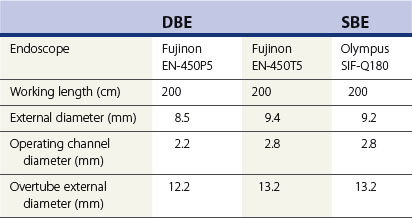
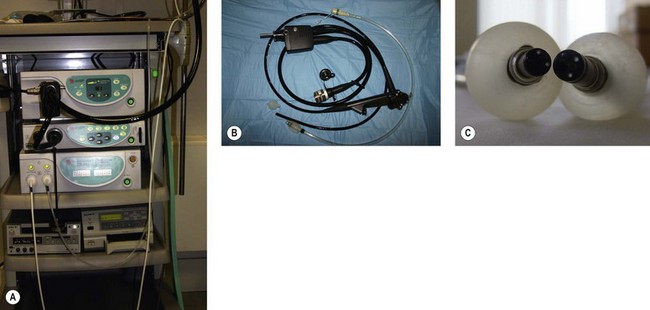
The DBE (see Table 1) consists of a thin endoscope 8.5 mm in diameter and 2300 mm long, with a flexible overtube measuring 1450 mm with an external diameter of 12.2 mm. The two latex balloons attach to the end of the overtube and the endoscope, respectively, and are inflated and deflated by a pump, which maintains constant pressure in the balloons (Fig. 3).
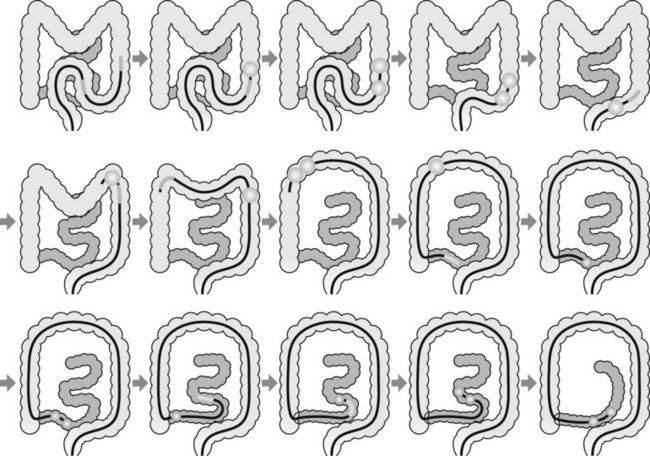
3.3 Technique (Fig. 4)
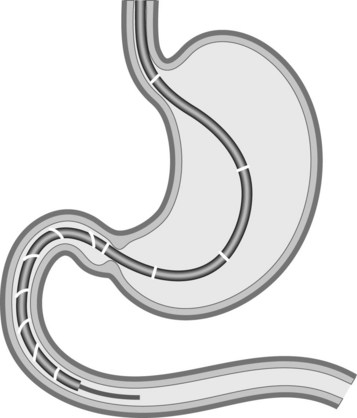
DBE is performed with two endoscopists (at least one of whom is experienced). Anterograde (Fig. 5) and retrograde (Fig. 6) approaches are usually required to visualize the entire small bowel.
Anterograde DBE enteroscopy is performed as follows:
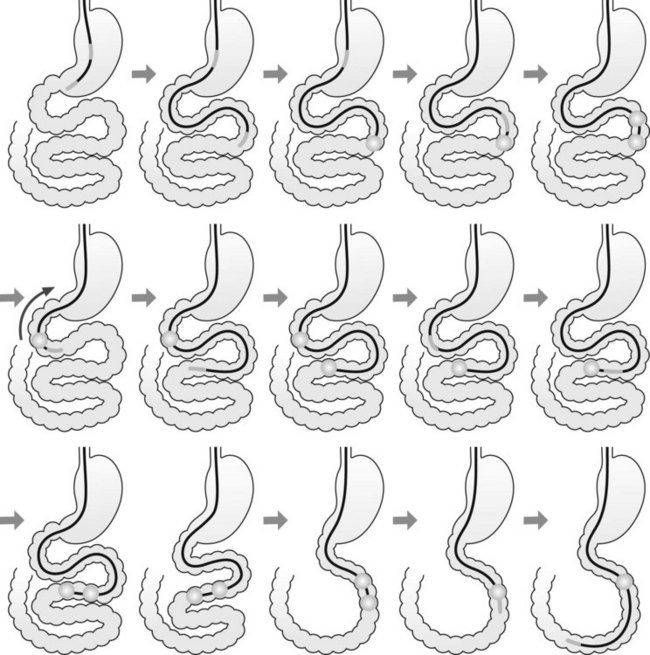
The enteroscope is advanced using the following series of maneuvers (Fig. 5). The enteroscope is advanced. The scope balloon is inflated. The overtube is advanced. The overtube balloon is inflated. A ‘short endoscope’ position is achieved by pulling back on the endoscope and overtube together. The scope balloon is then deflated and the enteroscope advanced. The process is then repeated.
Retrograde DBE enteroscopy is performed as follows:
The enteroscope is advanced using a series of maneuvers similar to those described above. The enteroscope is inserted as far as the descending colon (Fig. 6). The scope balloon is inflated. The overtube is advanced and the overtube balloon inflated. The enteroscope and overtube are withdrawn, straightening the sigmoid colon. The enteroscope is then advanced by repeating these maneuvers.
3.6 Obscure GI bleeding
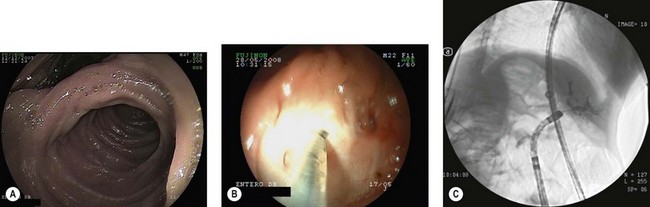
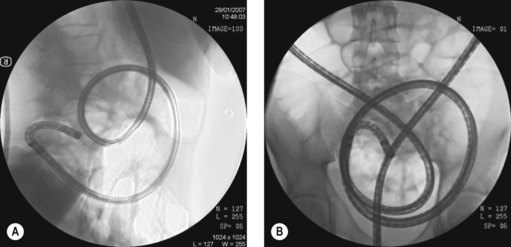
The diagnostic superiority of DBE over push enteroscopy in obscure digestive bleeding was demonstrated in a series of 52 patients with obscure GI bleeding in whom anterograde DBE found lesions in 38 patients, compared with 23 patients who underwent push enteroscopy (Fig. 7).
3.7 Crohn’s disease
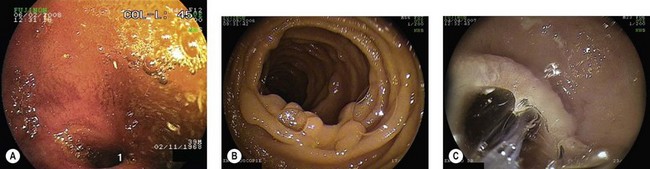
DBE is the method of choice for obtaining endoscopic and histological confirmation of Crohn’s disease affecting the small intestine (Fig. 8A,B). DBE should be performed only if it alters patient management either by assessing the severity of mucosal lesions and their extent, or to determine whether they are fibrostenotic or inflammatory in nature. DBE should be used if a short fibrous stenotic stricture requires dilation. DBE should never be performed in active Crohn’s disease (Fig. 8C). Some 50% of dilated patients retain the benefit of this endoscopic procedure at 6 months.
3.8 Tumors
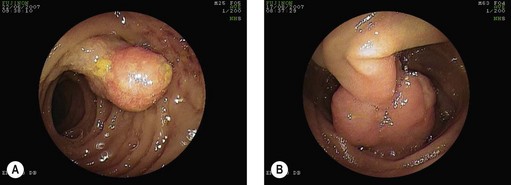
DBE and VCE are comparable for detecting tumors. DBE is useful for the detection and removal of polyps in both familial adenomatous polyposis (FAP) and Peutz–Jeghers syndrome (PJS) (Fig. 9A). DBE should be used as the first procedure if polypectomy is envisaged. Use caution when performing polypectomies in this group of patients, particularly for large polyps or those with a broad stalk (Fig. 9B). Submucosal injection with dilute adrenaline (epinephrine) should be used to decrease the risk of bleeding or perforation.
4 Single balloon enteroscopy
The single balloon enteroscopy (SBE) system (Fig. 10) was developed by Olympus. It can be used to perform both anterograde and retrograde examinations. Total enteroscopy rates appear to be lower in SBE (15%) compared with DBE (40%). The overtube is coated with silicone and measures 140 cm (ST-SB1 Olympus) with an external diameter of 13 mm (see Table 1). The balloon is made of silicone and is attached to the end of the overtube.
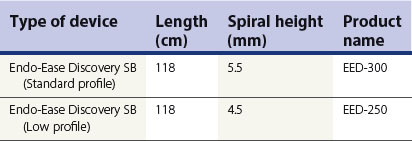
5 Spiral enteroscopy
The Spirus system (Fig. 11), developed by Endo-Ease Discovery SB (Table 2), is based on a different principle from the previous two systems. The small intestine is concertinaed by rotating the overtube.
Table 2 Spiral enteroscopy overtubes.Two spiral overtube diameters are available, with the standard profile used in the majority of patients
5.1 Technique
![]() Clinical Tips
Clinical Tips
Technical tips for spiral enteroscopy
6 Intraoperative enteroscopy
Ell C, May A, Nachbar L, et al. Push and pull enteroscopy in the small bowel using the double balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613-616.
Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49-58.
Lo SK. Technical matters in double balloon enteroscopy. Gastro Intest Endosc. 2007;66:S15-S18.
May A, Farber M, Aschmoneit I, et al. Prospective multicenter trial comparing push-and-pull enteroscopy with the single- and double-balloon techniques in patients with small-bowel disorders. Am J Gastroenterol. 2010;105:575-581.
May A, Nachbar L, Pohl J, et al. Endoscopic interventions in the small bowel using double-balloon enteroscopy: feasibility and limitations. Am J Gastroenterol. 2007;102:527-535.
Mensink P, Haringsma J, Kucharzik TF, et al. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613-615.
Monkemuller K, Bellutti M, Neumann H, et al. Therapeutic ERCP with the double-balloon enteroscope in patients with Roux-en-Y anastomosis. Gastrointest Endosc. 2008;67:992-996.
Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136.
Teshima CW, Kuipers EJ, Van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2010 Oct 18. doi: 10.1111/j.1440-1746.2010.06530.x. [Epub ahead of print]
Tsujikawa T, Saitoh Y, Andoh A, et al. Novel single-balloon enteroscopy for diagnosis and treatment of the small intestine: preliminary experiences. Endoscopy. 2008;40:11-15.
Yamamoto H, Kita H, Sunada K, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016.