chapter 43 Sleep disorders
INTRODUCTION AND OVERVIEW
In the first part of this chapter we provide a brief introduction to the sleep process. In the second part, we outline sleep disorders such as obstructive sleep apnoea, narcolepsy and sleep-related movement disorders, bruxism and the parasomnias, which require referral to a specialised sleep centre. In the final part we describe the diagnosis and management of the more common but heterogenous sleep disorders of insomnia and sleep–wake schedule disorders. More detailed accounts of all these areas are available.1
THE NATURE OF SLEEP
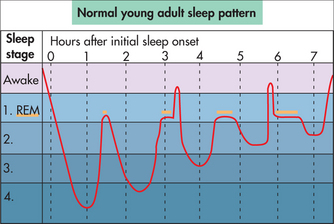
Sleep research over the past 50 years has confirmed some interesting and very important facts about the nature of the sleep process. A state of sleep was discovered during which the sympathetic nervous system (‘fight-or-flight’ mechanism) was activated. It has variously been called activated, paradoxical and dreaming sleep, but now officially is termed rapid eye movement or REM sleep. One of its interesting characteristics is that it occurs in about four or five discrete episodes (only a few minutes long initially, up to 30–40 minutes in duration later) spaced about 90 minutes apart during a normal sleep period. But most of our sleep (80%) occurs between these REM sleep episodes. This non-REM state of sleep has been subdivided into different stages based on the variety of EEG cortical activity, from light (easily awoken, sleep not perceived) Stage 1 sleep to the deepest or behaviourally non-responsive Stage 4 sleep. The 90-minute non-REM/REM sleep cycles repeat four or five times during the nocturnal sleep period in a roller-coaster type pattern, as illustrated in Fig 43.1.
An intriguing aspect of this typical sleep pattern is the spontaneous lightening of sleep at the end of each deep sleep phase. As a result of this 90-minute cycle of lighter sleep throughout the sleep period, awakenings out of this lighter sleep are normal events. Even children and adolescents who normally sleep ‘soundly’ have these awakenings but they are usually brief and not recalled. Normal ageing results in sleep becoming lighter, with more awakenings that are more likely to be remembered. Therefore, awakenings are a normal part of the sleep period, particularly in more mature individuals, but need not have any detrimental impact on daytime functioning.
Another curious aspect of sleep is that we cannot directly perceive our own sleep. Therefore, judgments about time spent sleeping are often incorrect. Our minds are usually active, with some fleeting images and thoughts, during sleep. When we awaken we may be aware of only the last vestiges of this activity because earlier thoughts are not stored in long-term memory. People with chronic insomnia are more likely to attribute these thoughts, incorrectly, to the state of being awake.2 Therefore two awakenings—for example, an hour apart—may seem to be a continuous hour of wakefulness rather than two brief, separate awakenings, thus leading to an underestimation of total sleep time. Because multiple awakenings become more common with age, there is an increased vulnerability to underestimating sleep time, concern about sleep and the likelihood of developing insomnia.
BIOLOGICAL DETERMINERS OF SLEEP PROPENSITY
Circadian rhythms
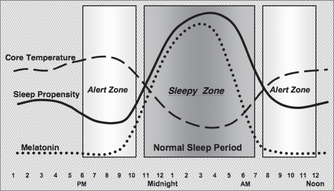
Independent of the sleep homeostasis mechanism, another major influence on sleepiness/alertness arises from our circadian (circa = about, dia = a day) or 24-hour rhythms.3 Virtually all our physiological, biochemical and hormonal measures show circadian variation—that is, variation from peak to trough (minimum) and back to peak, taking about 24 hours to complete a full cycle. To some extent they are influenced directly by the 24-hour external environment (night/day) and our behaviour. However, free of all these influences they are shown to be endogenous. The circadian timing of these rhythms is controlled by a small nucleus in the hypothalamus of the brain, the suprachiasmatic nucleus (SCN). For example, the SCN signals the peripheral vasculature to vasodilate at about 8 pm, starting the process of decreasing core body temperature to its trough at about 4–5 am. The SCN signals the pineal gland to start manufacturing and secreting the hormone melatonin at about 9 pm and to stop its activity at about 4 am. Likewise, many other biological rhythms are kept in synchrony, a main function of which is to maximise sleepiness at night and alertness during the day, for us diurnal-adapted humans.
Maximum circadian alertness occurs at about the time of core temperature peak (6–9 pm for most individuals) and maximum circadian sleepiness is at the trough of core temperature (about 4–5 am). A few hours later (9–11 am), alertness increases again. Thus most individuals would have a circadian sleep-conducive zone from about 11 pm to about 7 am. The variation of circadian temperature, melatonin and sleepiness for a normal sleeper is illustrated in Fig 43.2.
However, this ‘sleepy’ zone is bracketed by ‘alert’ zones (one normally in the early evening and one in the later morning), which can be problematic if the timing of the circadian system comes adrift. For example, if the circadian timing is delayed by 2–3 hours, the evening alert zone may span the time when sleep is intended (e.g. 11 pm) and thus inhibit sleep. Alternatively, an early-timed circadian rhythm may wake an individual prematurely before sufficient sleep is obtained.4
The SCN, serving as the central body clock, receives its main sensory input from the optic nerve, arising from retinal ganglion cells. Retinal light stimulation, particularly at the blue end of the spectrum, is capable of re-timing the SCN clock. Considerable research has shown that light stimulation before the core body temperature minimum produces a delay of the circadian timing and, conversely, light stimulation after the core temperature minimum results in a shift of the clock to earlier times. Therefore, a sleep difficulty resulting from circadian timing gone astray may be treated with appropriately timed light stimulation. For example, sleep-onset insomnia arising from a circadian delay can be treated with morning bright light, and the opposite timing problem, early morning awakening insomnia arising from an abnormally early timed circadian rhythm, can be treated with evening bright light.
THE IMPACT OF SLEEP UPON HEALTH
Chronic sleep problems can have a negative impact on health but, equally, chronic health problems can affect sleep. For example, factors predicting the later development of insomnia include being overweight (35% more likely than average), physical inactivity (42%), alcohol dependence (75%) and having a joint or lower back disorder (195%).5 Having a major psychiatric disorder increases the risk eight-fold. Managing the sleep problem in such situations therefore requires attention to the underlying health problem.
The mismanagement of sleep problems and the overuse of sedatives can also have a negative impact upon health.6,7 Only a small proportion of patients taking sleeping pills regularly will note improvement in their insomnia in the long term. A higher proportion will report that their insomnia is worsened by them, despite the fact that the person soon finds themselves unable to sleep without them. Sleep medications can also be associated with a worsening of depression and reduced energy. Using pharmacological methods alone for management of long-term insomnia is an inadequate and incomplete solution.8 Sleeping tablets also accumulate in the body, particularly in the elderly for whom the half-life of the drugs is far longer, and are associated with other problems such as falls, drowsiness and lowered life expectancy.
LIFESTYLE FACTORS AND SLEEP
Education
As the problems associated with poorer sleep become more recognised, educating people from an early age as to what constitutes healthy sleep (as illustrated in Fig 43.1), and helping a person with sleep problems to understand the ways in which they can improve sleep, is an increasingly important role for the general practitioner.
Stress management
The two-way links between poor sleep and poor mental health are now well established. Depression and anxiety produce effects on various stress hormones including cortisol and catechols, which also have a negative impact on sleep patterns.9 The vicious circle of stress leading to poor sleep, lowered mood and more stress is a common one in our community.
It has been a common assumption in medical circles that sleep disturbance is secondary to depression, which is true, but evidence also suggests that it goes the other way as well. If a detailed chronological history is taken from a patient with depression, it is often found that sleep disturbance precedes the onset of lowered mood.10–12 Because a patient may present with concerns about the mood, the underlying role of sleep may be undervalued. Chronic insomnia nearly trebles the risk of depression, and in one study was found to be second only to recent bereavement as a risk factor and was a more significant risk factor than having had a previous episode of depression.13 Another study put the risk for depression at four times greater for women and twice as great for men if they suffered from long-term insomnia.14
Some studies suggest that, if people with depression undertake effective behavioural strategies for improving sleep, the depression will resolve in 57% of people and be improved by more than 40% in another 13%.15 These kinds of findings have been replicated in other studies on other behavioural interventions for insomnia in those with depression.16
Exercise
Being active during the day and exercising regularly will tend to improve sleep at night and wakefulness in the day.17,18 Some people find that vigorous exercise close to bedtime has a negative effect on sleep, because of sympathetic nervous system (SNS) activation. A solution can be to exercise earlier or to have a period of quiet activity between exercising and going to bed.
SLEEP DISORDERS
SLEEP DISORDERS REQUIRING REFERRAL
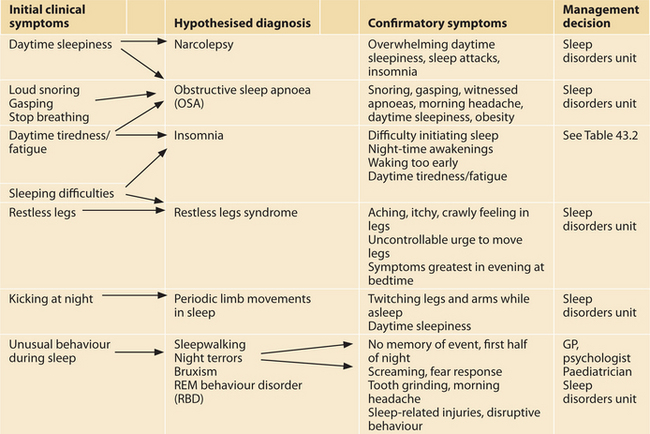
In this section we outline a range of sleep disorders that are best referred to a specialist unit for confirmation of diagnosis and initial treatment. Table 43.1 summarises the most common clinical symptoms and how they relate to the different sleep disorders outlined below.
TABLE 43.1 Clinical symptoms, possible diagnosis and clinical management decisions
 |
Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is characterised by repetitive episodes of upper airway collapse and obstruction of airflow during sleep. These events can result in reductions in blood oxygen saturation and are usually terminated by brief arousals from sleep.21
Prevalence
OSA can occur in any age group, but in adults the prevalence rate for mild OSA has been estimated at 24% in men and 9% in women, and for moderate to severe OSA, 4% in men and 2% in women.21,22 The prevalence rate increases with age, with a higher rate in overweight middle-aged males and in women after menopause.
Narcolepsy
Narcolepsy, a disorder of excessive daytime sleepiness, is a rare, chronic, neurological disorder with prevalence rates in the United States and Europe of approximately 1 in 2000 people.23 Age of onset is typically adolescence or young adulthood.
Diagnosis
Narcolepsy is characterised by excessive and overwhelming daytime sleepiness temporarily relieved by a brief sleep. An adequate bed period at night but somewhat disrupted sleep is usually reported. Narcolepsy may be accompanied by cataplexy that is characterised by sudden weakening of large skeletal muscles provoked by emotional response, for example, elation, laughter, surprise or anger.21 The loss of muscle tone may range from a mild sensation of weakness to complete paralysis and collapse. Hypnagogic hallucinations and sleep paralysis are also associated with ‘narcolepsy with cataplexy’. An overnight PSG and a daytime multiple sleep latency test are commonly used in the diagnosis of this disorder.
Sleep-related movement disorders
Restless legs syndrome
Restless legs syndrome (RLS) is a sensorimotor disorder characterised by uncomfortable sensations in the legs, such as aching or internal itchy or burning feelings, that causes an irresistible urge to move the legs. These unpleasant feelings are most noticeable and problematic in the evening or whenever the person is trying to relax, but then often decline during the early morning period, suggesting a circadian influence. RLS can therefore prolong sleep onset and contribute to insomnia. PSG findings have demonstrated fragmented non-REM and REM sleep in individuals with RLS.24
Prevalence
RLS is reported by 5–10% of the population and occurs up to two times more commonly in women than in men.21 A recent survey found that clinically significant RLS, occurring at least twice a week and causing moderate distress, occurs in almost 3% of the population.25 RLS is generally idiopathic, and in 40–60% of cases there is a familial association. RLS may also be symptomatic of such associated conditions as peripheral neuropathies, uraemia, iron deficiency, diabetes, Parkinson’s disease and pregnancy.26
Management
Treatment options include both behavioural and pharmacological approaches. Lifestyle changes of possible benefit include a balanced diet and avoiding caffeine, nicotine and alcohol in the evening. RLS appears to respond to dopaminergic agonists as short-term therapy.27 Because RLS is strongly associated with periodic limb movements in sleep (see below), the diagnoses and treatment of both would best rely on PSG and sleep physician respectively at a sleep disorders unit.
Periodic limb movements in sleep
Periodic limb movements in sleep (PLMS) are characterised by periodic episodes of repetitive (every 20–40 seconds) short bursts (0.5–5 seconds) of limb movements that occur during sleep.21 They arise from dorsiflexion of the ankle and toes and a partial flexion of the knee. PLMS may sometimes occur in the arms. Although typically the individual is unaware of these movements, they can be associated with a cortical arousal or an awakening and hence lead to sleep onset and/or sleep maintenance problems and concomitant daytime sleepiness and fatigue.21
Prevalence
The prevalence of PLMS is estimated to be 4–11% in adults, with PLMS occurring in 80–90% of people with RLS.21,29 A large cross-sectional European study found that factors associated with PLMS are female gender, shift work, caffeine intake and stress.30
Diagnosis
Although clinical symptoms may be acutely evident to a bed partner, who may suffer from sleep disturbance from the frequent movements, diagnosis should be confirmed with PSG measured as the number of PLMS per hour of total sleep time.21
Management
Lifestyle measures that target the associated factors include reducing caffeine and nicotine intake, and stress management. One study has shown that magnesium supplementation can help. Most treatments use the newer dopaminergic medications that have been found to fully suppress PLMS. However, these potent drugs should be used cautiously as they are not free of side effects, including daytime augmentation of RLS symptoms.21
Sleep-related bruxism
Sleep bruxism (SB) is characterised by grinding or clenching of the teeth during sleep and is usually associated with cortical arousals but rarely full awakenings.21 It is associated with temporomandibular pain, headaches and tooth wear.
Prevalence
Prevalence is highest in childhood (14–17%) and then decreases over the lifespan to about 3% in older adults.21 A large cross-sectional survey found that risk factors for SB include OSA, loud snoring, heavy alcohol consumption, caffeine consumption (> 6 cups), smoking, anxiety and stress.31
Management
The management of SB includes pharmacological (clonazepam), psychological and occlusal therapeutic approaches.32 Occlusal splints, fitted by a dentist, appear to be a more symptomatic treatment option. Psychological therapies have used biofeedback, relaxation, hypnosis, counselling, sleep hygiene and lifestyle changes. A recent study compared the effectiveness of an occlusal splint to cognitive–behavioural treatment (CBT) for sleep bruxism.32 The CBT comprised progressive muscle relaxation, nocturnal feedback and stress-management training. Both groups experienced significant reductions in SB activity and associated symptoms as well as improved psychological measures.
Parasomnias
Parasomnias are undesirable physical events or experiences that occur during entry into sleep, within sleep or during arousal from sleep. They involve sleep-related behaviours and experiences over which there is no conscious, deliberate control.21 These events can be injurious to the patient and others and can produce a significant disruption of the sleep–wake pattern. This section discusses the more common parasomnias including NREM disorders such as sleep terrors and sleepwalking, and parasomnias associated with REM sleep such as nightmares and REM sleep behaviour disorder (RBD).
According to the International Classification of Sleep Disorders21 only RBD requires PSG for a diagnosis, although an overnight sleep study can confirm a clinical diagnosis of other parasomnias and rule out other disorders, such as epilepsy. Diagnosis of other parasomnias requires a clinical interview with the individual and a family member to elucidate the description, timing, frequency and duration of the behavioural event.
Non-REM: sleep terrors and sleepwalking
Sleep terrors and sleepwalking are disorders caused by partial arousal from slow-wave sleep and therefore typically occur 1 to 3 hours after sleep onset, when slow-wave sleep is usually most prominent.33 Apart from diminished alertness, both are associated with amnesia for the event. They are common in childhood and decrease in incidence during early adolescence, when slow-wave sleep declines.
Sleep terrors are accompanied by a cry or piercing scream and behavioural manifestations of intense fear such as dilated pupils and pallor of the skin.21 Sleepwalking (somnambulism) consists of a series of complex behaviours such as walking about in a lowered state of consciousness, and impaired judgment.21
As both sleep terrors and sleep walking are associated with irregular sleep schedules, sleep deprivation and psychosocial stress, helpful actions include increasing time in bed and a regular sleep pattern.33 Adults experiencing sleepwalking and sleep terrors may also have a past or current history of depression or anxiety; however, control of these psychiatric disorders does not control the parasomnias.21 A recent study has found that sleep-disordered breathing is associated with parasomnias, with respiratory effort leading to a number of arousal reactions occurring in slow-wave sleep.34 Referral to a sleep disorders unit and PSG may be indicated in this instance.
REM: nightmares
Nightmares are characterised by awakening primarily from REM sleep, experienced as disturbing mentations.35
Prevalence and diagnosis
Up to 50% of children between 3 and 5 years of age experience nightmares severe enough to disturb their parents.21 In adults, 50–85% report experiencing at least an occasional nightmare.21 Because nightmares typically arise during REM sleep, they usually occur in the latter half of the night when REM propensity is high. Numerous studies have indicated that nightmares are associated with increased life stress, anxiety and other psychopathologies. Nightmares can also be associated with alcohol abuse and some medications that initially suppress REM sleep, and when these are stopped, REM rebound may occur, with often bizarre and frightening nightmares.
INSOMNIA
Insomnia is defined as chronic difficulty with initiation, consolidation or duration of sleep, resulting in daytime impairment.21 It is the most common sleep disturbance in the general population and is said to be the third-highest complaint seen by the GP. Epidemiological surveys have yielded prevalence rates of 20–40% in the adult population, with 10–20% experiencing severe or frequent insomnia.37 Insomnia is often comorbid with other disorders (e.g. chronic pain, depression, anxiety) and therefore the GP needs to be alert for sleep problems when treating other physical and psychological disorders. Although insomnia may have been originally secondary to other health problems, if of long duration the insomnia is likely to be self-sustaining and require its own treatment.
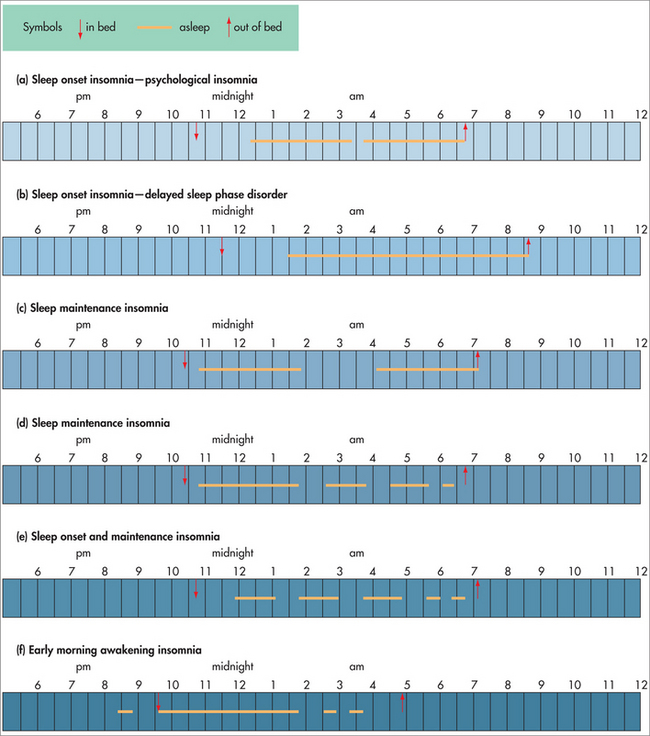
Table 43.2 describes the three main types of insomnia complaints seen by the GP—that is, sleep onset, sleep maintenance and/or early morning awakening insomnia. These are illustrated in Fig 43.3, with examples of different sleep patterns from a typical sleep diary. Some patients may have a combination of insomnia diagnoses—for example, they may present with sleep onset insomnia but have a combination of psychophysiological insomnia as well as a delayed circadian rhythm, or they may have sleep maintenance insomnia as well as sleep onset insomnia.
TABLE 43.2 Clinical symptoms of insomnia subtypes and suggested treatment options
| Clinical symptoms of insomnia type | Treatment suggestions |
|---|---|
| Sleep onset insomnia | |
| Psychophysiological insomnia (PI) (Fig 43.2a) | |
Management
Still the most common medical treatment for insomnia is hypnotics and, more recently, antidepressants. However, these have negative consequences such as tolerance, dependence, withdrawal rebound insomnia and altered sleep physiology, as well as side effects such as amnesia, psychomotor slowing, cognitive impairment and unusual night-time behaviours. Particularly in the older population, hypnotics tend to exacerbate all the normal cognitive/behavioural impairment of ageing as well as potentially interacting with a patient’s other medications. Medication may provide temporary symptomatic relief only and does not address the causes of insomnia. In recent studies, cognitive behaviour therapy for chronic insomnia has been shown to be comparable to pharmacotherapy in the short term and more effective in the long term.38,39 Cognitive/behaviour and light therapies aimed at correcting the underlying aetiologies are suggested to produce greater long-term effectiveness. However, it is important to correctly identify the subtype of insomnia and its likely aetiology in order to provide the most appropriate treatment. Figure 43.3 and Table 43.2 can be used as a guide to this process. Identify the pattern of insomnia in Figure 43.3, then check the treatment suggestions in Table 43.2, which are elaborated in detail below.
Non-drug, evidence-based insomnia treatments
Good sleep practices (sleep hygiene)
A good bedtime routine includes the following measures:
Stimulus control therapy
To extinguish these responses:
Bedtime restriction
Relaxation techniques
Morning bright light
Morning bright light will advance a delayed circadian rhythm and lead to an earlier sleep pattern.4 For example, if a patient wishes to sleep between 11 pm and 7 am but is unable to fall asleep until 3 am and has difficulty waking earlier than 11 am, they may have delayed sleep phase disorder.21 To advance the sleep period back to the preferred earlier time, we suggest the following protocol:
Evening bright light
The abnormally early timed circadian rhythm and sleep pattern can be delayed by exposure to bright evening light.4 The patient with an early timed circadian rhythm (advanced sleep phase21) will struggle to stay awake until the desired or conventional bedtime and may experience frequently unintentional naps in the evening before retiring to bed. They will also experience early morning awakening. For example, the patient may have overwhelming sleepiness at 8 pm, prolong bedtime until 10 pm but despite this, still wake at 4 am and be unable to fall back to sleep. We suggest the following:
Herbal treatments
Common herbal treatments for insomnia include:
American Academy of Sleep Medicine. http://www.aasmnet.org.
Australasian Sleep Association. http://www.sleep.org.au.
Australasian Sleep Association, information on sleep disorders. http://www.sleepaus.on.net/factsheets.html.
Australasian Sleep Association, insomnia treatment services (in each Australian state). http://www.sleepaus.on.net/servicesinsomniatreatments.php.
British Sleep Society. http://www.sleeping.org.uk/.
European Sleep Research Society. http://www.esrs.eu/cms/front_content.php.
Lack L, Wright H, Bearpark H. Insomnia: how to sleep easy. Sydney: Media 21; 2003.
Morin CM, Espie CA. Insomnia: a clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum, 2003.
Poceta JS, Mitler MM. Sleep disorders: diagnosis and treatment. New Jersey: Humana Press, 1998.
1 Poceta JS, Mitler MM. Sleep disorders: diagnosis and treatment. New Jersey: Humana Press, 1998.
2 Mercer JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25:564-571.
3 Borbely AA. A two-process model of sleep regulation. Human Neurob. 1982;1:195-204.
4 Lack LC, Wright HR. Treating chronobiological components of chronic insomnia. Sleep Med. 2007;8:637-644.
5 Janson C, Lindberg E, Gislason T, et al. Insomnia in men—a 10-year prospective population-based study. Sleep. 2001;24(4):425-430.
6 Mant A, Mattick RP, de Burgh S, et al. Benzodiazepine prescribing in general practice: dispelling some myths. Fam Pract. 1995;12(1):37-43.
7 Mant A, de Burgh S, Mattick RP, et al. Insomnia in general practice. Results from NSW General Practice Survey 1991–1992. Aust Fam Physician. 1996;Suppl 1:S15-S18.
8 Hohagen F, Rink K, Kappler C, et al. Prevalence and treatment of insomnia in general practice. A longitudinal study. Eur Arch Psychiatry Clin Neurosci. 1993;242(6):329-336.
9 Steiger A. Sleep and endocrinology. J Intern Med. 2003;254(1):13-22.
10 Holsboer-Trachsler E, Seifritz E. Sleep in depression and sleep deprivation: a brief conceptual review. World J Biol Psychiatry. 2000;1(4):180-186.
11 Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59(2):47-51. quiz 52.
12 Riemann D, Voderholzer U. Primary insomnia: a risk factor for depression? J Affect Disord. 2003;76(1–3):255-259.
13 Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
14 Mallon L, Broman J, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12(3):295-306.
15 Morawetz D. Insomnia and depression: which comes first? Sleep Res Online. 2003;5(2):77-81.
16 Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2(4):403-406.
17 Merrill RM, Aldana SG, Greenlaw RL, et al. The effects of an intensive lifestyle modification program on sleep and stress disorders. J Nutr Health Aging. 2007;11(3):242-248.
18 Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355-365. xi
19 Tooley GA, Armstrong SM, Norman TR, et al. Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol. 2000;53(1):69-78.
20 Massion AO, Teas J, Hebert JR, et al. Meditation, melatonin and breast/prostate cancer: hypothesis and preliminary data. Med Hypotheses. 1995;44(1):39-46.
21 American Academy of Sleep Medicine. International classification of sleep disorders: Diagnostic and coding manual. 2nd edn. Westchester, Illinois: AASM, 2005.
22 Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906-1914.
23 Ohayon MM, Zulley J, Smirne S, et al. Prevalence of narcolepsy, symptomatology and diagnosis in the European general population. Neurology. 2002;58:1826-1833.
24 Hornyak M, Feige B, Voderholzer U, et al. Polysomnography findings in patients with restless legs syndrome and in healthy controls: a comparative observational study. Sleep. 2007;30:861-865.
25 Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact. Arch Intern Med. 2005;165:1286-1292.
26 Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5:293-299.
27 Oertel WH, Benes H, Bedenschatz R, et al. Efficacy of cabergoline in restless legs syndrome: a placebo-controlled study with polysomnography (CATOR). Neurology. 2006;67:1040-1046.
28 Hornyak M, Voderholzer U, Hohagen F, et al. Magnesium therapy for periodic leg movements-related insomnia and restless legs syndrome: an open pilot study. Sleep. 1998;21(5):501-505.
29 Hornyak M, Feige B, Riemann D, et al. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169-177.
30 Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547-554.
31 Ohayon MM, Li KL, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119:53-61.
32 Ommerborn MA, Schneider C, Giraki M, et al. Effects of an occlusal splint compared with cognitive–behavioral treatment on sleep bruxism activity. Eur J Oral Sci. 2007;115:7-14.
33 D’Cruz OF, Vaughn BV. Parasomnias—an update. Sem Pediatr Neurol. 2001;8:251-257.
34 Espa F, Dauvilliers Y, Ondze B, et al. Arousal reactions in sleepwalking and night terrors in adults: the role of respiratory events. Sleep. 2002;25:32-36.
35 Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11:295-310.
36 Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in sleep. Sleep. 2002;25:120-138.
37 Ford DE, Kamerow DB. Epidemiology of sleep disturbance and psychiatric disorders: an opportunity for prevention. JAMA. 1989;262:1479-1484.
38 Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
39 Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: randomised controlled trial. JAMA. 1999;281:991-999.
40 Donath F, Quispe S, Diefenback K, et al. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry. 2000;33(2):1333-1336.
41 Goel N, Kim H, Lao R. An olfactory stimulus modifies night time sleep in young men and women. Chronobiol Int. 2005;22(5):889-904.
42 Cerny A, Schmidt K. Tolerability and efficacy of valerian/lemon balm in healthy volunteers: a double blind, placebo-controlled multicentre study. Fitoterapia. 1999;70(3):221-228.