Skin of the Premature Infant
Erin F. Mathes, Mary L. Williams
Introduction
The premature infant assumes the challenge of postnatal life, despite the immaturity of essential functions. Skin functions are primarily protective, and immaturity of the skin contributes to the vulnerability of the preterm infant. The main function of the skin is to provide a permeability barrier that both protects the aqueous interior of the infant from desiccation in the xeric atmosphere and prevents massive influx of water when immersed in hypotonic solutions.1 Other important functions of skin include barriers to percutaneous absorption of exogenous xenobiotics, to injury from mechanical trauma, to colonization and penetration by microorganisms, and to injury from ultraviolet light. In addition to its barrier functions, skin also participates in the thermoregulatory, neurosensory, and immunologic systems.
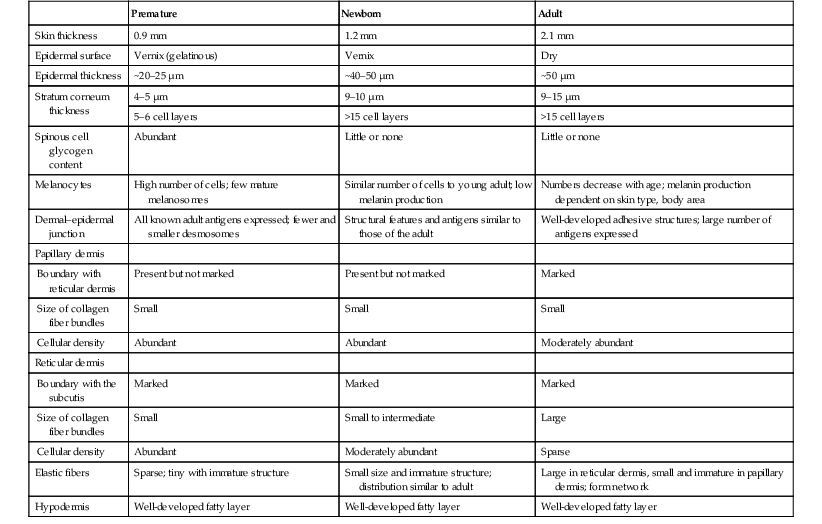
The consequences of skin immaturity for the premature infant depend on the infant’s position on the maturational timetable for each cutaneous function, which is in turn dependent on the infant’s gestational and postnatal ages. All skin layers (i.e. epidermis, dermis, and subcutaneous fat) are thinner in the preterm infant than at term (Table 4.1).2 Because the outermost layers of the epidermis (i.e. the stratum corneum) are the primary effectors of most of the barrier properties of skin, the timetable for maturation of the stratum corneum predicts the competence of many skin functions. Stratum corneum begins to form around hair follicles at about 14 weeks’ gestational age and spreads to include the epidermis between hair follicles by 22–24 weeks’ gestational age (see Table 1.2). During the ensuing weeks, the thickness of the stratum corneum increases from only a few to several cell layers,2 such that by term, it is actually thicker than adult stratum corneum. The ‘excess’ outermost layers of stratum corneum are then shed during the first days of life; this process of physiologic desquamation is accentuated in postmature babies. Another component of fetal skin, the vernix caseosa (a complex proteolipid material) is formed in part by sebaceous gland secretions beginning at about 28 weeks’ gestational age. The percentage surface area covered with vernix peaks at 33–37 weeks’ gestational age, then decreases in full-term and post-term infants. Its functions may include roles in temperature regulation, permeability barrier, and innate immunity.3
TABLE 4.1
Comparative features of premature, newborn, and adult skin
| Premature | Newborn | Adult | |
| Skin thickness | 0.9 mm | 1.2 mm | 2.1 mm |
| Epidermal surface | Vernix (gelatinous) | Vernix | Dry |
| Epidermal thickness | ~20–25 µm | ~40–50 µm | ~50 µm |
| Stratum corneum thickness | 4–5 µm | 9–10 µm | 9–15 µm |
| 5–6 cell layers | >15 cell layers | >15 cell layers | |
| Spinous cell glycogen content | Abundant | Little or none | Little or none |
| Melanocytes | High number of cells; few mature melanosomes | Similar number of cells to young adult; low melanin production | Numbers decrease with age; melanin production dependent on skin type, body area |
| Dermal–epidermal junction | All known adult antigens expressed; fewer and smaller desmosomes | Structural features and antigens similar to those of the adult | Well-developed adhesive structures; large number of antigens expressed |
| Papillary dermis | |||
| Boundary with reticular dermis | Present but not marked | Present but not marked | Marked |
| Size of collagen fiber bundles | Small | Small | Small |
| Cellular density | Abundant | Abundant | Moderately abundant |
| Reticular dermis | |||
| Boundary with the subcutis | Marked | Marked | Marked |
| Size of collagen fiber bundles | Small | Small to intermediate | Large |
| Cellular density | Abundant | Moderately abundant | Sparse |
| Elastic fibers | Sparse; tiny with immature structure | Small size and immature structure; distribution similar to adult | Large in reticular dermis, small and immature in papillary dermis; form network |
| Hypodermis | Well-developed fatty layer | Well-developed fatty layer | Well-developed fatty layer |
Reproduced with permission from Schachner LA, Hansen RC, eds. Pediatric dermatology, 4th edn. London: Mosby; 2010.
The histologic features described above underlie the clinical characteristics of skin maturation embodied in the Ballard scale (see Table 1.5) widely used for assessing gestational age.4 In the extremely premature infant (<24 weeks), the skin is sticky, friable, and transparent (Fig. 4.1); lanugo hairs are absent. As gestation progresses, the skin becomes less transparent, and peeling and surface cracking are increasingly seen, indicative of a thickening stratum corneum, and lanugo hair density peaks and then regresses. Despite definition of these milestones of gross and microscopic skin development, with the exception of the permeability barrier, little is known about the competency or developmental timetable of most skin functions in premature infants.
The permeability barrier in the preterm infant
The permeability barrier resides in the stratum corneum through its provision of a hydrophobic lipid shield over the underlying nucleated cell layers.1 Because of their plasma and intracellular membranes, most cells are hydrophobic relative to the vascular and extracellular compartments; however, in the stratum corneum this pattern is reversed. Instead, the extracellular compartment of the stratum corneum is filled with a highly organized series of hydrophobic lipid membranes in the extracellular spaces, whereas the anucleate corneocytes form an aqueous compartment as a result of loss of their plasma and organelle membranes. This interposition of hydrophobic lipid membranes in the extracellular compartment retards the movement of water inward or outward across the stratum corneum. The stacking of multiple layers of cornified cells surrounded by extracellular lipid bilayers further enhances this barrier to water movement, through the generation of a tortuous intercellular pathway for water movement.
As a multilayered stratum corneum develops in the third trimester,2 the barrier to transepidermal water loss (TEWL) also matures, such that by 34 weeks’ gestation TEWL rates approximate adult values.5,6 Barrier competence can be measured non-invasively by a variety of direct and indirect means.7–9 By all of these measures, the extent of permeability barrier immaturity parallels the degree of prematurity. In addition to increasing stratum corneum thickness, the development of a competent permeability barrier in fetal rat skin is accompanied by (1) deposition of neutral lipid in the intercellular domains of stratum corneum; (2) increasing stratum corneum cholesterol and ceramide content; and (3) the organization of these lipids into mature lamellar membrane structures, as viewed by electron microscopy.10,11 Whether these same lipid biochemical and ultrastructural changes also underlie barrier formation during human skin development has not yet been determined, but a large body of evidence from other sources predicts that this will be the case.
Permeability barrier maturation accelerates after birth, such that by 2–3 weeks’ postnatal age, most premature infants, regardless of gestational age, have competent barriers.6 Thus, maturation that may require approximately 10 weeks to complete in utero is accelerated following premature delivery. However, as the limits of viability have been lowered to include survivors of 25 weeks’ (<750 g) to even 22 weeks’ gestational age, barrier function may take as long as 8 weeks following birth, to mature.12,13 The gestational ages of these very immature infants directly coincide with the timetable for stratum corneum formation (see above). It may not be surprising, therefore, that extremely premature infants do not respond as rapidly to maturational signals initiated by birth. In fetal rat skin, it is air exposure with evaporation of water from the skin surface that stimulates accelerated barrier formation, because this acceleration can be prevented by covering the skin surface with a vapor-impermeable membrane.14 The accelerated postnatal development of human preterm skin may also be prevented if it is covered with occlusive materials or exposed to a humid environment.15,16
Permeability barrier ontogenesis is developmentally regulated; hence, small-for-dates infants exhibit a barrier function that is appropriate for their gestational age.17 In fetal rats, barrier maturation is regulated by glucocorticoids,18 thyroid hormone, and sex hormones, as well as by activators and ligands of the PPARα and LXR nuclear hormone receptors (Table 4.2).11 Some of these agents also regulate lung development; and glucocorticoids often are administered prepartum to mothers to accelerate fetal lung maturation when premature delivery is imminent.19 Whether barrier maturation is also stimulated by these interventions in humans is presently uncertain. One group demonstrated that preterm infants of mothers treated with glucocorticoids have reduced insensible water losses and lower serum sodium concentrations in the first 4 days of life, consistent with a maturational effect on the skin barrier.20 In contrast, Jain and colleagues21 reported that epidermal maturation and barrier function did not appear to be influenced by antenatal steroids.
TABLE 4.2
Regulatory signals for skin barrier formation in fetal rats
| Activator | Effect | Nuclear receptor | Class |
| Dexamethasone | Accelerate | Glucocorticoid | I |
| Thyroid hormone | Accelerate | Thyroid hormone | II |
| Diethylstilbestrol | Accelerate | Estrogen | I |
| Testosterone | Retard | Androgen | I |
| Linoleic acid | Accelerate | PPARα | II |
| Clofibric acid | Accelerate | PPARα | II |
| Farnesol | Accelerate | ?FXR or PPARα | II |
| 25-hydroxycholesterol | Accelerate | LXR | II |
| Ciglitazone prostaglandin J2 | No effect | PPARγ | II |
| GW-1514 (free fatty acids) | Accelerate | PPAR-β/Δ | II |
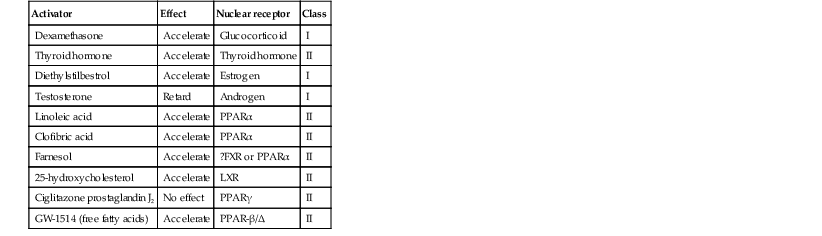
Class I: Steroid hormone receptors. Ligand binds in cytosol, translocates to nucleus after ligand binding; regulates gene transcription as homodimer.
Class II: RXR-interacting subfamily of receptors. Ligand binds to receptor in nucleus; regulates gene transcription as heterodimer with the RXR receptor and its ligand, 9-cis retinoic acid (e.g., RXR-T3R; RXR-Vitamin D3R).
Another aspect of the permeability barrier that undergoes postnatal maturation is the ‘acid mantle’. An acidic pH within the stratum corneum is required for normal permeability barrier homeostasis and for stratum corneum integrity and cohesion. At birth, human stratum corneum has a near-neutral surface pH. This pH declines over the ensuing days to weeks to become acidic, comparable with that of adults. Complete acidification may not occur until 3–6 months of age in term infants.22 Whether this maturation is further delayed in preterm infants is unknown.23–25
Consequences of permeability barrier immaturity
Fluid and electrolyte imbalance and evaporative energy loss
The primary consequences of permeability barrier immaturity are: (1) increased evaporative loss of free water from the skin surface, placing the infant at risk for volume depletion, particularly hypernatremic dehydration; and (2) energy loss through heat of evaporation; that is, 0.58 kilocalories (kcal) are expended for each milliliter (mL) of water that evaporates.26 Therefore, optimal care of the premature infant requires both accurate compensation for cutaneous water losses to preserve fluid and electrolyte balance, and maintenance of the infant in a thermally neutral environment, such that caloric intake can be directed toward growth and not heat production.26 Although concurrent cutaneous water losses can be measured directly (i.e. by measuring TEWL), this procedure is not standard practice in most nurseries. Instead, cutaneous water losses and respiratory fluid losses are together considered insensible (i.e. not directly measured). In term infants, TEWL accounts for approximately two-thirds of insensible losses; but cutaneous water losses are much higher in preterm infants, whereas respiratory fluid losses remain relatively constant.27,28 Neonatal fluid requirements are estimated using complex formulas that take into account the following:
• Measured losses in urine and feces
• Estimates of insensible losses
• Requirements to support growth (increasing with post-gestational age).
Neonatal fluid requirements must be modified by postnatal age to compensate for fluid redistribution (i.e. requirements on the first extrauterine day are decreased as a result of contraction of the extracellular compartment). There is also considerable variability in TEWL among preterm infants of the same gestational and post-gestational ages; moreover, barrier maturation is often quite precipitous.7,12 Fluid replacements are also adjusted as needed to compensate for excessive weight loss or gain and/or for serologic parameters of fluid or electrolyte imbalance. This inevitably results in ‘chasing fluids’. While the foregoing describes usual nursery practices, it is important to bear in mind that cutaneous fluid losses are not inherently insensible (i.e. unmeasurable). Indeed, it has been shown that measurement of TEWL using rapid and noninvasive instrumentation from as few as three body sites permits accurate estimation of total cutaneous losses in preterm infants.29 Despite this, measurement of TEWL has not been adopted widely by intensive-care nurseries in the approach to fluid management.
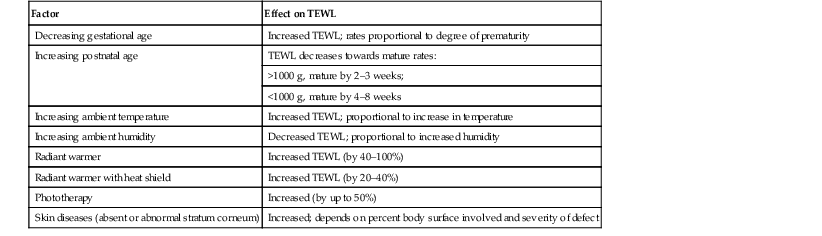
Fluid replacements must be adjusted for a number of environmental conditions (Table 4.3), because cutaneous losses are not merely a function of stratum corneum maturity. They are also modified by the ambient temperature and humidity, since both affect the vapor pressure of water at the skin surface.17 Phototherapy for hyperbilirubinemia also increases TEWL30 and fluid requirements, particularly when white light systems are used.31–33 Many skin disorders also adversely affect the competence of the permeability barrier (see below and Table 4.3).
TABLE 4.3
Factors modifying cutaneous water losses in preterm infants
| Factor | Effect on TEWL |
| Decreasing gestational age | Increased TEWL; rates proportional to degree of prematurity |
| Increasing postnatal age | TEWL decreases towards mature rates: |
| >1000 g, mature by 2–3 weeks; | |
| <1000 g, mature by 4–8 weeks | |
| Increasing ambient temperature | Increased TEWL; proportional to increase in temperature |
| Increasing ambient humidity | Decreased TEWL; proportional to increased humidity |
| Radiant warmer | Increased TEWL (by 40–100%) |
| Radiant warmer with heat shield | Increased TEWL (by 20–40%) |
| Phototherapy | Increased (by up to 50%) |
| Skin diseases (absent or abnormal stratum corneum) | Increased; depends on percent body surface involved and severity of defect |
Strategies for minimizing fluid and evaporative energy losses
A humidified incubator (Fig. 4.2) can provide a thermally neutral environment with low rates of evaporative water loss, because at a relative humidity of 80% or more, skin surface evaporation effectively ceases.16,34,35 Scrupulous antisepsis, however, is required to prevent bacterial colonization of this environment, particularly with water-loving organisms such as Pseudomonas spp. Moreover, these devices obstruct access to extremely ill or unstable infants. Therefore, these infants are commonly cared for on an open bed, where a radiant warmer provides a thermally neutral environment at the expense of greatly increased rates of TEWL.34,36,37 Infants requiring care under radiant warmers are typically the youngest and most premature; that is, the population with the poorest skin barriers. Newer hybrid beds with a radiant warmer and humidifier allow for easy access to and observation of infants.35 Use of a plastic cover or plastic bubble blanket may increase the humidity and mitigate to some extent, the adverse effects of the radiant heating on TEWL.38–42 Although these plastic shields are widely employed, standards for thermal stability and transmission have not been established.43
Other strategies to reduce TEWL in the preterm infant include the use of protective skin dressings or ointments. Semipermeable dressings (e.g., Bioclusive®, Omniderm®, Opsite®, Tegaderm®) that permit some passage of water vapor and other gases, but are impervious to water and microorganisms, can reduce TEWL rates and also are protective against the trauma caused by adhesives from monitors.40,44–47 Moreover, barrier maturation is not inhibited by these dressings, and neither have increased rates of infection nor colonization by microorganisms been observed. Nonetheless, increased bacterial colonization under such dressings is observed in other clinical settings48 and remains a serious consideration with their use on preterm infants. In addition, many of these dressings contain adhesive materials, and even those without adhesives can cling to the moist skin surface of the preterm newborn and injure the epidermis, unless they are either removed carefully or allowed to detach spontaneously. Furthermore, partial body applications (i.e. trunk and abdomen) to very immature infants (<1000 g), may not be sufficient to reduce total fluid requirements.44 These limitations hinder the widespread adoption of artificial dressings in routine skin care. Because the benefits of these agents have only been shown in studies with small numbers of subjects, confirmation in larger cohorts is required before their routine use can be generally recommended.42
Topical ointments, such as petrolatum49 or Aquaphor®50,51 reduce TEWL, although the effect from a single application lasts only 4–8 h.50–52 Less frequent applications do not decrease fluid requirements, but they appear to improve the overall condition of the skin and protect against skin trauma.50 Although largely composed of nonphysiologic lipids (e.g., long-chain hydrocarbons and wax esters), these emollients have a long history of dermatologic use without associated toxicity or evidence of significant percutaneous absorption. Nonetheless, internal hydrocarbon accumulations (paraffinomas) are reported, albeit rarely.53,54 Hence, the possibility remains that these lipids may not be entirely innocuous when applied to the skin of very premature infants (see also Chapter 5). A systematic review of randomized controlled trials comparing prophylactic application of topical ointment in preterm infants to routine skin care55 reported that daily application of topical ointment increases the risk for coagulase-negative staphylococcal and nosocomial infections in these patients. Therefore, routine application of topical ointments is no longer recommended for premature infants. However, this should not necessarily be extrapolated to developing countries. Circumstances may be very different in these settings: survival of preterm infants is much lower, care practices differ, and the morbid risks divergent. Indeed, sunflower seed oil and Aquaphor® have been shown to reduce the incidence of nosocomial infections in Bangladesh and Egypt.56–58 An alternative approach to barrier fortification in these infants would be use of mixtures of lipids physiologic to the skin. The extracellular membranes of the stratum corneum that provide the barrier to TEWL comprise an approximately equimolar mixture of ceramides, long-chain free fatty acids, and cholesterol.59 The effect of various mixtures of these physiologic lipids on permeability barrier function has been examined in mature skin in experimental systems, as well as in aged skin and in some skin disorders.59–61 Recent studies in animal and adult human models suggest that in addition to improving epidermal hydration, vernix-based creams may improve the rate of barrier recovery from injury.3,62 Yet, despite the theoretic advantages of employing either physiologic lipid mixtures, or vernix-based creams, these formulations have not been examined for efficacy in treating the barrier immaturity of the preterm infant.
Increased percutaneous absorption of xenobiotics
Another direct consequence of skin barrier immaturity is the increased absorption of topically applied substances (Table 4.4), sometimes with tragic consequences (see Chapter 5).43,63–65 This vulnerability was first recognized historically when preterm infants developed methemoglobinemia through the absorption of aniline dyes in the laundry marks placed on diapers.66 Subsequently, the demonstration of neurotoxicity due to percutaneous absorption of hexachlorophene, a commonly used antibacterial cleanser,66 led to wider recognition of the vulnerability of the preterm infant to toxicity from topically applied agents.
TABLE 4.4
Hazardous or potentially hazardous compounds that may be absorbed across the skin of preterm infants (see also Chapter 5).63–87
| Compound | Toxicity | Sources |
| Alcohol (methylated spirits) | Skin necrosis, neurotoxic | Topical antiseptic, vehicle for topical medications/products |
| Aluminuma | Neurotoxicity | Metal containers for topical ointments |
| Aniline dyes | Methemoglobinemia | Laundry marks (historical) |
| Boric acid, borax | Shock, renal failure | Antifungals, talc powders |
| Benzocaine | Methemoglobinemia | Topical analgesics; teething products |
| Benzethonium chloridea | Carcinogen | Antiseptic soap |
| Benzyl benzoatea | Neurotoxicity | Scabicide |
| Bicarbonate | Metabolic alkalosis | Baking soda for diaper dermatitis |
| Camphora | Gastrointestinal toxin, neurotoxicity | Topical antipruritic; camphorated oils (VapoRub®; Campho-Phenique®) |
| Coal tarsa | Carcinogen | Topical anti-inflammatory products |
| Chlorhexidine gluconate | Skin necrosis | Topical antiseptic |
| Corticosteroid | Adrenal suppression, hyperadrenocorticism | Topical corticosteroids |
| Diphenhydramine | Neurotoxicity | Topical analgesics (Caladryl®) |
| Epinephrine | High output failure | Topical vasoconstriction |
| Gentian violet | Possibly carcinogenic | Antimicrobial |
| Glycerina | Hyperosmolarity | Emollients; cleansers (Aquanil®) |
| Hexachlorophene | Neurotoxicity | Antiseptic soaps (pHisoHex®) (historical) |
| Iodochlorhydroxyquin | Optic neuritis | Topical antibiotic (Vioform®) |
| Imidazoles | Drug interactions secondary to p450 inhibition | Topical antifungal medications: ketoconazole, miconazole, clotrimazole (Lotrimin®) |
| Isopropyl alcohol | Skin necrosis; neurotoxicity | Topical antiseptics |
| Lactic acida | Metabolic acidosis | Topical keratolytics (Lac-Hydrin®) |
| Lindane | Neurotoxicity | Scabicide (Kwell®) |
| Mercury | Neurotoxicity; acrodynia; nephrotic syndrome | Disinfectants; teething powder (historical) |
| Methylene blue | Methemoglobinemia | Vital stain (historical) |
| Neomycin | Ototoxicity | Topical antibiotic (Neosporin®) |
| Nystatina | Nephrotoxicity | Topical antifungal (Mycostatin®) |
| Phenol | Cardiac and neurotoxicity | Disinfectants (e.g., commercial laundries); local anesthetic/antimicrobials (e.g., Castellani’s paint) |
| Propylene glycola | Hyperosmolarity; neurotoxicity | Topical vehicles; emollients, cleansers (Cetaphil®) |
| Povidone–iodine | Skin necrosis; hypothyroidism | Topical antiseptic (Betadine®) |
| Prilocaine | Methemoglobinemia | Topical anesthetic (EMLA®) |
| Resorcinol | Methemoglobinemia | Topical antiseptic |
| Salicylic acid | Salicylism | Topical keratolytics |
| Silver sulfadiazine | Kernicterus; argyria | Topical antibiotic (Silvadene®) |
| Sulfura | Paralysis; death | Scabicide ointment |
| Triclosana | Neurotoxicity | Topical antiseptic |
| Urea | Elevated BUN | Topical keratolytics/emollients |
a Potentially hazardous compounds.
The same factors that determine the movement of water from inside out also regulate the movement of low molecular weight substances from outside in.65 In mature skin, small (<800 Da) hydrophilic molecules are effectively excluded by the extracellular membrane system from penetration across a mature stratum corneum, whereas small hydrophobic molecules or those with amphipathic properties (containing both hydrophilic and hydrophobic parts) are able to penetrate.1,67 In the preterm infant, the thinner stratum corneum results in a reduction in the length (tortuosity) of the intercellular pathway, which would enhance percutaneous absorption of hydrophilic molecules. It is likely that there are also qualitative changes in the lipid composition and/or structural integrity of the lipid bilayers of immature stratum corneum that may further alter its permeability function.
In addition to immaturity of the permeability barrier, several other factors in premature infants may contribute to toxicity from topical xenobiotics.64,66 First, the surface area/volume ratio is increased in all infants, but even more so in premature infants; this effectively increases the absorptive surface while reducing the volume of distribution for the absorbed drug. Once absorbed, reduced levels of serum-binding proteins, such as albumin, may increase the proportion of free drug. Similarly, the relative lack of an adipose reservoir to buffer against the redistribution of fat-soluble drugs, such as lindane, to lipid-enriched neural tissues, may make the premature infant particularly vulnerable to central nervous system (CNS) toxicity from such agents. Immaturity of detoxification mechanisms, such as hepatic conjugation and renal function, also alters drug pharmacodynamics and can increase toxicity.
The increased permeability of premature skin to small hydrophilic molecules has also been exploited to enhance the percutaneous delivery of medications such as theophylline.64,88 A confounding factor in developing transdermal delivery systems for the premature infant is their variability of barrier competence, particularly during postnatal maturation.
Clinical implications of increased percutaneous absorption of xenobiotics
In the care of the preterm infant, it is safest to assume that any medication applied to the skin may be absorbed systemically. As a corollary, the ideal topical medications for preterm infants are those with low systemic toxicity. It is also necessary to consider the composition of the vehicle used for topical drug delivery, because some components may also be absorbed across the immature skin barrier (see Chapter 5).43 The provision of a safe skin antiseptic is a particularly problematic issue, especially in the care of the most immature infants, who both have the most immature skin and are the most unstable, and therefore need repeated applications of antiseptics for intravenous access.68 Topical iodine-containing antiseptics (10% povidone–iodine) have been largely replaced by chlorhexidine-containing antiseptics (0.5% chlorhexidine gluconate solutions) in the neonatal intensive-care nursery because of their potential for transient hypothyroxinemia and hypothyroidism due to systemic iodine absorption.69,89 Yet chlorhexidine-induced skin injury is also reported,70–72,90 with both aqueous and alcoholic vehicles. While chlorhexidine in alcohol provides better antisepsis than either ingredient alone,91 alcohol itself poses a risk for percutaneous absorption and skin necrosis in the premature neonate, especially when used under occlusion.71,73,74,90 Avoiding pooling of the antiseptic on the skin and wiping it off with normal saline after the procedure may help prevent chemical burns.90
Impact of permeability barrier immaturity on other organ systems
Excluding congenital malformations and genetic diseases, the major causes of morbidity and mortality in premature infants are: respiratory distress syndrome leading to bronchopulmonary dysplasia (BPD); patent ductus arteriosus (PDA); necrotizing enterocolitis (NEC); periventricular/intraventricular hemorrhage (IVH); and overwhelming infection. Maintenance of normal blood pressure and ‘optimal’ blood volume protects against these major causes of morbidity and mortality in the preterm infant, and cutaneous fluid losses are perhaps the most important destabilizing factor in fluid homeostasis of the premature infant. For example, overhydration contributes to the development of symptomatic PDA,92 as larger blood volumes increase shunting through the ductus arteriosus. Conversely, systemic hypotension may increase the likelihood of an intracranial hemorrhage. Alterations in cerebral blood pressure induce hemorrhage into the periventricular germinal matrix, a gelatinous and highly vascular fetal structure, which is present up to 34 weeks’ gestational age.93 The period of greatest risk for IVH is in the first week of life, particularly the first 2 days, which coincides not only with the time of greatest permeability barrier incompetence but also with the time when tissue fluids undergo redistribution with contraction of the extracellular fluid compartment.94 Similarly, bronchopulmonary dysplasia is associated with increased total fluid intake in the first few days of postnatal life and increased time of ventilator use.95 Kim and colleagues recently found that extremely low birthweight infants cared for in humidified incubators had decreased fluid intake, improved electrolyte imbalance and a lower rate of severe bronchopulmonary dysplasia.35 Thus, to the extent that BPD, IVH, and PDA are precipitated by fluctuations in systemic blood pressure, overcompensated or uncompensated cutaneous water losses may be an exacerbating factor.
The pathogenesis of NEC is attributed to a triad of ischemia, oral feeding, and infection;96,97 each of these pathogenic factors may be exacerbated by skin immaturity. As in PDA, IVH, and BPD, fluid imbalance resulting from skin immaturity could be a cofactor, as both overcorrection of fluids98 and hypotensive ischemia–reperfusion injury99 are implicated in NEC. Early initiation of oral feeds100 is undertaken to reverse a negative energy balance in the preterm infant. Caloric losses caused by increased evaporative water loss from the skin surface in all likelihood contribute to this caloric drain.
It seems likely, therefore, that efforts to closely monitor cutaneous losses through direct measurements of TEWL, with the goal of tight control of replacement fluids, would decrease the incidence and severity of these major complications of prematurity and improve the outcome of these infants.101
Immaturity of other skin barriers
Cutaneous barrier to mechanical injury
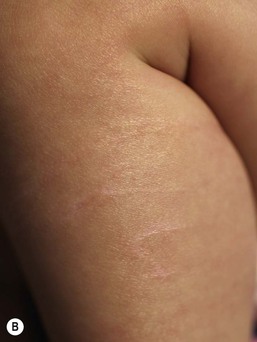
The skin of the premature infant is much more vulnerable to mechanical injury than that of term infants. In addition to a thinner stratum corneum, the epidermal–dermal interface lacks interdigitations (i.e. rete ridges or dermal papillary projections),102 resulting in a decreased resistance to shear forces (Table 4.1). The dermis is also thinner, less collagenized, and more gelatinous. Although the major structural proteins that underlie the mechanical strength of skin are expressed by mid-gestation, they may not be as abundant or organized into functionally mature units as in adult or even term infants’ skin.102 Skin fragility is a major problem in the care of the preterm infant. They are vulnerable to abrasions and deeper wounds from the use of adhesive tapes to secure monitors, airways, and intravenous lines (see Fig. 4.3B; see also Chapter 8). Similarly, the threshold for irritant contact dermatitis from fecal contact (diaper dermatitis), for chemical burns from prolonged contact with antiseptics,90 or for thermal burns is much reduced.103 Gentle handling, minimal use of adhesives coupled with substitution of hydrophilic gel104 or pectin barrier105 adhesives only when required, and the use of adhesive removers,106 can minimize these injuries. A regimen of emollient lubrication or use of nonadherent, semipermeable dressings may also help protect against mechanical injuries (see above).
Cutaneous barrier to infection
Although mature skin is colonized by a variety of bacteria and other microorganisms, these organisms are effectively excluded from the interior. The basis for the barrier to transcutaneous infection includes both provision of a mechanical shield against invading microorganisms and specific components, such as certain lipids107,108 and antimicrobial peptides (defensins and cathelicidins) in the stratum corneum, that inhibit the growth of microorganisms and modulate immune responses.109 The thinner, easily abraded stratum corneum of the preterm infant constitutes an impaired mechanical shield against the ingress of microorganisms. In addition, specific biochemical components of the cutaneous barrier to infection may also be immature in preterm infants. Extremely preterm infants also lack the initial protection of antimicrobial peptides in vernix caseosa.3,110 The contribution of the vernix to the antimicrobial barrier is unknown.
Preterm infants’ skin is colonized soon after birth with coagulase-negative staphylococci, predominantly S. epidermidis. Colonization with Malassezia and Propionibacterium occurs later, after 3 weeks,111 coincident with the maturation of the permeability barrier. S. epidermidis has become the most frequent cause of postnatally acquired systemic infections in these infants.112–115 Malassezia, as well as the opportunistic fungi Aspergillus, Candida, and Rhizopus, are also systemic pathogens in this group.116–119 Direct invasion across the immature epidermis by fungi of low pathogenicity in very preterm infants has been documented,120 although exploitation of a portal of entry, such as site of skin injury or along transcutaneous catheter lines, is probably a more common means of entry. Regardless of the route of transcutaneous entry, the immaturity of the immune system, particularly opsonic mechanisms, then permits organisms of low pathogenic potential to mature hosts to establish disease in the preterm infant.121 The use of intravenous lipid supplements also favors the establishment of a nidus of infection once entry into the circulation has been obtained.113
Cutaneous barrier to light injury
Energy absorbed from ultraviolet (UV) light passing through the skin may damage critical cellular functions through the generation of free radicals, principally singlet oxygen, as well as through inflammatory responses initiated by cytokine release and the generation of eicosanoids.
For example, free radical damage to deoxyribonucleic acid (DNA) may either result in cell death or initiate carcinogenic mutations. Although shorter wavelengths are more energetic and hence more damaging, they do not penetrate as deeply. In mature skin, ultraviolet B (UVB) (290–320 nm) does not penetrate into the dermis, but ultraviolet A (UVA) (320–400 nm) does, and visible wavelengths (400–800 nm) reach even deeper levels.122 Hence, in considering the effects of light on the skin, one must consider not only the cumulative energy of the light absorbed but also the depth of penetration. Cutaneous defenses against UV light injury include:
Rules about depth of penetration of different wavelengths based on studies of mature skin may not hold for premature skin. For example, the stratum corneum is the first-line of defense, filtering out approximately 80% of incident UVB light.122 Therefore, the much thinner stratum corneum of the premature infant is one factor that would likely result in increased vulnerability to UV penetration. In addition, although melanocytes are present in the basal epidermis by the end of the first trimester, melanin granule formation is not fully mature, even at term (Table 4.1).102 Thus, inherent skin color is lighter in neonates. Moreover, the ability of melanocytes in the skin of preterm infants to increase melanin synthesis in response to UV stress is unknown. Other antioxidant defenses may be immature in the epidermis as they are in the lungs of preterm infants.123 Taken together, it is apparent that the premature infant is particularly vulnerable to UV injury.
UVB is filtered out by window glass, but UVA is not. Thus, the preterm infant in the nursery may be exposed to solar UVA, as well as longer wavelengths from artificial light sources. UVA is implicated in certain phototoxic and photoallergic responses.124 Chronic UVA exposure is linked to photoaging and is potentially carcinogenic.125 Additionally, visible light in the near infrared range (760 nm – 1 mm) is now recognized to contribute to skin photoaging.126
Portions of the visible light spectrum (420–500 nm) induce isomerization of bilirubin to compounds that can be excreted without hepatic conjugation. Hence, phototherapy with a variety of light sources is standard therapy for neonatal unconjugated hyperbilirubinemia. Because the commonly used fluorescent daylight bulbs emit UVA, the interposition of a Plexiglas® shield is essential to avoid burns in preterm infants.127 Although long considered without risk of long-term complications, recent evidence points to increased numbers of melanocytic nevi (an important independent phenotypic risk factor for melanoma) in children who were exposed to neonatal phototherapy.128,129 However, several retrospective studies have not found an association with neonatal phototherapy in patients with non-melanoma skin cancer and melanoma, although these studies may not have sufficient follow-up time to capture this association, nor were these sequelae specifically studied in preterm infants. Severe phototoxicity is reported in a preterm infant following use of intravenous fluorescein with prolonged dye retention due to renal immaturity.130 Moreover, even internal organs are potentially affected by phototherapy, because light may penetrate more deeply through the skin of the preterm infant. For example, phototherapy has been linked to an increased incidence of symptomatic PDA in preterm infants.131,132 One study found that shielding the chest wall overlying the heart can mitigate this complication, suggesting a direct photo-effect on ductal tissue; however a follow-up study did not confirm this finding.132,133
In summary, it should be assumed that the cutaneous barrier to the penetration of light is immature in the preterm infant, hence preterm infants should be physically shielded from exposure to sunlight from exterior windows and other sources of ultraviolet light. Limitation on the intensity of ambient nursery lighting is also recommended out of concern for a contribution to the retinopathy of prematurity.134 It would seem prudent also to consider the potential deleterious cutaneous and transcutaneous effects of nursery lighting.
Cutaneous contribution to immaturity of thermal homeostasis
Body heat may escape through a number of mechanisms, as listed below:
• Evaporative heat loss (0.58 kcal/mL of evaporated water)
• Conduction: direct gain or loss of heat to objects in direct contact with the infant’s body
Maintenance of a thermally neutral environment, minimizing unwanted heat loss or heat gain, is a major challenge to those who care for premature infants. The survival of very low birthweight infants has been shown to be affected by alterations in thermoregulation, particularly in the ‘golden-hour’ immediately after birth.135 The evaporation of amniotic fluid from the skin surface following birth constitutes a major thermal stressor to these infants. Interventions such as plastic wraps or bags, skin-to-skin care and transwarmer mattresses used immediately after delivery keep preterm infants warmer, leading to higher temperatures on admission to neonatal units and less hypothermia.42
The skin participates in thermal homeostasis through several mechanisms. Water loss occurs both through passage of water across the stratum corneum as a function of barrier competency (see above) and from secreted water delivered to the skin surface by ducts (eccrine sweating) in response to neural and other stimuli.23 Caloric losses caused by an immature skin barrier are a major contributor to the preterm infant’s heat loss, particularly in the first week of life.135,136
Conversely, because eccrine function is immature, the premature infant is unable to compensate for heat stress by sweating.137 Even in term infants, sweating is not functionally mature because the term infant’s set-point is higher than in mature individuals. Sweating in preterm infants matures rapidly following birth; however, the efficiency remains poor, with fewer body sites sweating in response to thermal stimuli and maintenance of a high set-point.137 Vasomotor control of cutaneous blood flow is a third component of the skin’s contribution to thermal homeostasis. Both vasoconstriction and vasodilation in response to thermal stimuli are attenuated in the skin of preterm infants, although these responses appear to mature within 2–3 weeks.23,138 Finally, the subcutaneous adipose reservoir is deficient in preterm infants, reducing both their insulation against heat loss and their energy reserves for thermal conversion.
Neurocutaneous development in the premature infant
Responsiveness to touch is present at a very early age: that is, by the end of the first trimester, the fetus withdraws in response to skin stroking.139 In the preterm infant, neurocutaneous responses may be immature and ‘globalized’. Thus, in the unstable premature infant, handling is minimized to avoid adverse effects of skin stimulation, such as changes in heart rate, hypoxia, and apneic episodes.140,141 However, older and more stable preterm infants may benefit from skin contact in the form of skin-to-skin contact and massage.142
Originally, skin-to-skin contact was explored as a mechanism to facilitate maternal bonding with the term infant. A modification of this principle, designated ‘kangaroo care,’ has been advocated for preterm infants in developing countries as a safe and effective mode of care for stable premature infants143,144 in which the infant is continually housed against the mother’s skin and permitted ad libitum breast-feeding. In low-income countries, kangaroo care can reduce both morbidity and mortality from inadequate thermal protection and infections associated with overcrowding in nurseries and formula-feedings.143 Briefer periods of maternal skin-to-skin contact have also been advocated in the care of the preterm infant in high-income countries as a means to enhance maternal confidence and to humanize the nursery experience.143–146 When preterm infants as early as 22 weeks’ gestational age are carefully selected to avoid the inclusion of unstable ones, the practice does not appear to be deleterious, and may be beneficial (e.g., reduce periods of ‘purposeless’ activity).147,148 Recent studies suggest that autonomic and psychomotor pain responses are improved in preterm infants who have skin-to-skin contact during heel-sticks, compared with those receiving conventional incubator care.149,150 Long-term benefits to mother and child of skin-to-skin contact are difficult to distinguish from the benefits of more conventional mother–child contact, but may include prolonged maternal lactation and less infant crying.143
Infant massage has been advocated by some as a way to decrease stress and promote growth and development of preterm infants. The mechanisms by which massage improves weight gain are not completely understood, but may involve increased vagal tone, which leads to increased gastric motility, and levels of insulin and insulin-like growth factor-1.151 If larger studies confirm its utility, this relatively simple intervention has the potential to significantly improve weight gain and developmental outcomes, thus decreasing length of hospital stay.152
Although it may seem intuitively obvious that both parents and infants would benefit psychologically from the humanizing effects of skin-to-skin contact, the neurocutaneous pathways that are likely to underlie these responses are only beginning to be delineated in mature skin.153 It will be important, as this work proceeds, to examine the maturation of these pathways in the preterm infant, so as to both better understand the capacity of the maturing infant to respond to these external stimuli and to develop systems of care that are both rational and ‘humanistic’.
Skin diseases in the premature infant
Barrier function of abnormal skin
The consequences of barrier immaturity in premature infants may be compounded if the infant also has a primary skin disorder. Infants with severe genetic skin disorders are often born prematurely. Skin diseases that result in hyperkeratosis (scaling) or a thickened stratum corneum, such as the ichthyoses, are commonly associated with impaired barrier function.154 These infants are at-risk for hypernatremia as a result of increased evaporative loss of free water from the skin surface (see Chapter 19).155,156 Similarly, infants with widespread blistering diseases, whether resulting from underlying infection, as in staphylococcal scalded skin syndrome, or from one of the genetic mechanobullous diseases, e.g., epidermolysis bullosa, have increased fluid requirements as a result of loss of barrier integrity. Skin disease also increases the risk of systemic absorption of topical medications, both because of a further compromise in barrier competence and because of increased exposure to topical medications. Instances of this include prolonged use of topical steroids to dermatitic skin, resulting in adrenal suppression and other signs of hypercortisolism,157,158 and use of salicylate-containing ointments to remove excessive scale, resulting in salicylism.159 Skin diseases can also increase the risk of systemic infection by providing portals of entry through breaks in skin integrity.
Scars of prematurity
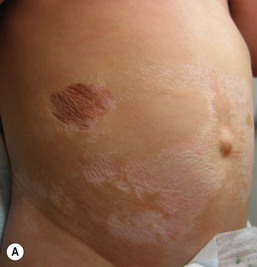
A number of nursery procedures may lead to scars; the number of scars nursery graduates bear is correlated with their degree of prematurity and the duration of intensive care (see also Chapter 8).160 Although most of these scars are not of great concern, more severe scarring sequelae can occur, particularly those resulting from wounds from chest tubes, skin stripping from adhesive tapes, and extravasated intravenous fluids. In addition, very premature infants (<29 weeks) may develop atrophic scars or so-called ‘anetoderma of prematurity’.161 Although the term ‘anetoderma’ implies a loss of elastic tissue and clinically manifests as an ‘outpouching’ of the skin, scars of prematurity can also be flat. These scars often only become evident at a few weeks to months of life. They often develop at sites of previous application of monitors and adhesives, but may also develop in areas that do not correspond to known areas of injury (Fig. 4.3).
Hemangiomas
Infantile hemangiomas are more common in preterm infants and their frequency is related to the degree of prematurity.162 In contrast to term infants, there is no significant female predominance in preterm infants with hemangiomas. Premature infants also have higher risk of having multiple hemangiomas, but the exact basis for this increase is not known.162 Hypotheses include the possibility that preterm infants have higher numbers of endothelial progenitor cells than term infants, and/or are more frequently exposed to medications that may predispose them to hemangiomas such as beta-2-sympathomimetic medications for tocolysis in preterm labor, or erythropoietin (see Chapter 21).162–165
Summary
Neonatologists have long been cognizant of the importance of fluid balance and thermal homeostasis in the care of the premature infant. Now, through advances in the care of fragile premature infants, and particularly through the advent of surfactant replacement therapy, neonates of ever-greater prematurity survive, relentlessly pushing back the age of viability. In this context, the consequences of skin barrier immaturity have emerged as a critical frontier in their management. Barrier failure contributes to the morbidity and mortality of the preterm neonate through fluid and electrolyte instability and the effects of fluid imbalance on blood volume and blood pressure, as well as an increased susceptibility to transcutaneous infections and toxicity from transcutaneous absorption of xenobiotics. As a result of the interlocking interdependence of organ systems, all of which are immature, the magnitude of the skin’s contribution to neonatal morbidity cannot currently be estimated.
At present, it is only possible to outline broad principles for the care of the premature infant’s skin. Most of these are self-evident, such as the need to avoid exposure to topical agents of potential systemic toxicity, or for gentle handling to prevent abrogation of the skin’s integrity. However, the optimal application of these principles in practice is often less evident. For example, all ingredients in topical medicaments, emollients, or cleansers need to be identified and their potential for toxicity considered, yet this information may not be easily obtained.43 In most instances, skin care practices have not been systematically studied to determine optimal regimens. Just as the term infant’s skin is more resilient and protective than the skin of a premature infant of 30 weeks’ gestation, procedures that may not be hazardous to an infant of this gestational age may be toxic to the extremely immature ‘micropreemie.’ Therefore, in designing future studies, it will be important to consider the differences in skin function in babies of varying gestational and postnatal ages. Greater awareness of skin functions and their physiologic bases, as well as the infant’s position on the maturational timetable of these functions, will be needed for the development of rational regimens of skin care in the future.
Acknowledgment
The authors would like to thank Amy E. Gilliam for her substantial contributions to the prior edition of this chapter.
Access the full reference list at ExpertConsult.com ![]()