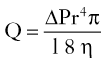Skin Flap Physiology
Physiologic Characteristics of Skin
The blood supply to the skin serves two important functions. It provides nutritional support and a thermoregulatory mechanism for the body. Primarily because of its thermoregulatory function, the rate of blood flow through the skin is one of the most variable in the body. Under ordinary skin temperatures, the amount of blood flowing through the skin (0.25 L/m2 of body surface area) is approximately 10 times the flow required for nutritional support.1 Blood flow can increase up to seven times this value with maximal vasodilation. When the body is exposed to extreme cold, blood flow can be reduced to levels that are marginal for cutaneous nutrition.
The nutrient capillary network in the reticular dermis and the arteriovenous shunts in the more superficial papillary dermis2 perform the two functions of the cutaneous circulation. The amount of blood flow to the skin depends ultimately on arteriolar pressure and flow. Under conditions of adequate systemic vascular pressure, however, arterioles act as preshunt and precapillary sphincters that regulate the flow through each vascular network.3
The sphincters in the two vascular systems respond to different stimuli (Fig. 2-1). The precapillary sphincter, which controls the amount of nutritive4 blood flow to the skin, responds to local hypoxemia and increased metabolic byproducts by dilation.5 Under these conditions, the blood flow is increased (reactive hyperemia being an example). The preshunt sphincters are involved in regulating the changes in blood flow that affect thermoregulation and systemic blood pressure.6 Release of norepinephrine by the postganglionic sympathetic fibers results in contraction of the preshunt sphincters. This diverts blood away from the skin surface, where heat loss can occur. With increased body temperature, the sympathetic vasoconstrictor impulses decrease, allowing increased blood flow to the skin.4
Neurovascular Supply to Local Skin Flaps
Blood vessels travel by one of two main routes to terminate in the cutaneous circulation. Musculocutaneous arteries pass through the overlying muscle to which they provide nutrition; septocutaneous arteries (also referred to as direct cutaneous arteries) travel through fascial septa, which divide the muscle segments (Fig. 2-2).
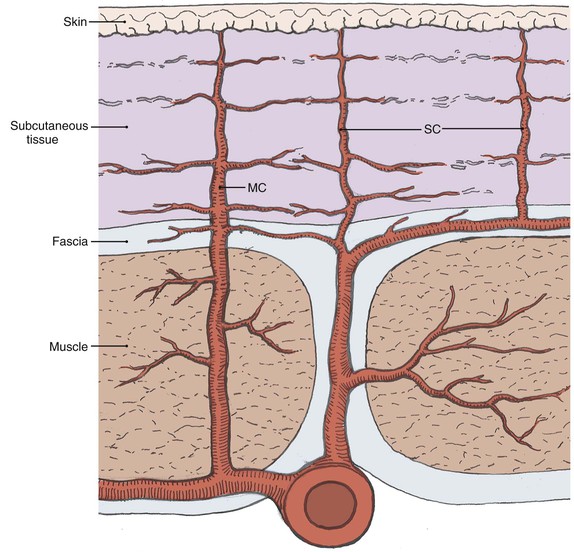
FIGURE 2-2 Depiction of varying pathways to skin that defines musculocutaneous (MC) and septocutaneous (SC) arteries.
The neural supply to the skin originates from both sensory nerves and sympathetic nerves. The sensory nerves are distributed in segmental fashion, forming dermatomes, and participate in the skin’s protective function. The postganglionic terminals of cutaneous sympathetic nerves contain the neurotransmitter norepinephrine and are found in the area of cutaneous arterioles.4
Vascular Design of Common Local Skin Flaps in the Head and Neck
The vascular supply to a flap is critical to its survival and is the basis of one classification system of flaps (Fig. 2-3). Local flaps in the head and neck are primarily random or arterial cutaneous.
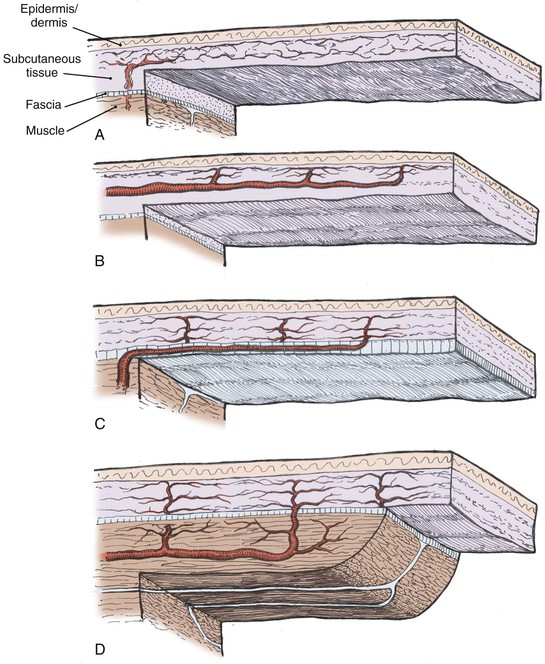
FIGURE 2-3 Classification of skin flaps based on vascular supply. A, Random. B, Arterial cutaneous. C, Fasciocutaneous. D, Musculocutaneous.
Random Cutaneous Flaps
Length-to-width ratios of random cutaneous flaps have been recommended for various areas of the body. These differences reflect a regional variation of the neurovascular supply to the skin. Such a description can serve as a guide in designing random cutaneous flaps but should not imply that a wider flap would extend survival length.7
Arterial Cutaneous Flaps
Arterial cutaneous flaps (also called axial pattern flaps and perforator flaps) typically have an improved survival length relative to random cutaneous flaps.8 This advantage results from the incorporation of a septocutaneous artery within the longitudinal axis. An island flap is an arterial flap with a pedicle consisting of nutrient vessels without the overlying skin. Island flaps can be useful to increase flexibility and to reduce pedicle bulk in certain reconstructive procedures.
The surviving length of arterial flaps is related to length of the included septocutaneous artery. Survival beyond the arterial portion of the flap is based on the subdermal plexus and is essentially a random cutaneous extension of the flap. Flap necrosis secondary to ischemia can be said to occur only in the random portion of the flap (destruction of the arterial pedicle making the entire flap random). Preoperative imaging with computed tomographic angiography to evaluate perforator distribution9 as well as the subcutaneous arterial pattern10 can further increase the usefulness of these flaps.
Physiologic Changes after Skin Flap Elevation
Impairment of Vascular Supply
Partial interruption of the vascular supply to the skin is the most obvious and critical change that occurs with elevation of a cutaneous flap. This interruption results in a local decrease in perfusion pressure to the skin. In arterial or myocutaneous flaps, the blood supply to the skin overlying the vascular pedicle is usually adequate. In random flaps or random extensions of flaps, the decrease in perfusion pressure becomes more pronounced with increasing distance from the base of the flap.11 When perfusion is reduced in one area of a random flap, the adjacent vascular territories supplied by a separate perforating vessel can provide a low-pressure blood supply through the subdermal plexus (Fig. 2-4). Because the nutritional requirements of skin are relatively low, a number of vascular territories can be compromised before necrosis will result.
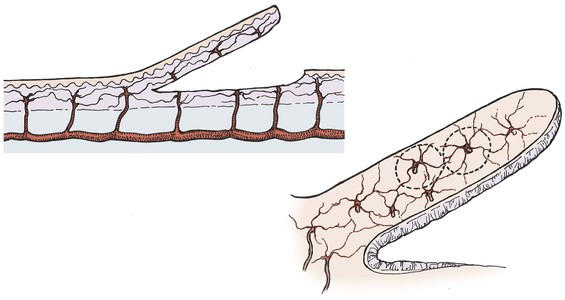
FIGURE 2-4 Vascular territories in skin flap. Multiple perforating vessels exist and are interconnected at the periphery of their vascular territory. When vessels are cut, blood supply can be replaced from nearby perforating vessels and tissue necrosis does not occur.
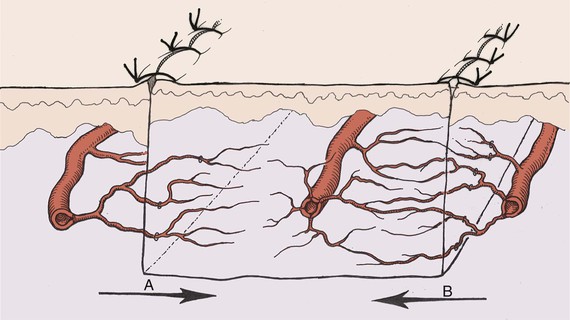
The survival length of the random portion of the flap depends on the physical properties of the supplying vessels (intravascular resistance) and the perfusion pressure.11 When the perfusion pressure drops below the critical closing pressure of the arterioles in the subdermal plexus, nutritional blood flow ceases and flap necrosis occurs. In the past, random cutaneous flaps were often designed relative to a desired length-to-width ratio, a wider base being needed to successfully transfer a longer flap. The wider random flap includes only additional vessels with the same perfusion pressure. The relationship between perfusion pressure and critical closing pressure is not altered, and no change in survival length occurs7 (Fig. 2-5).
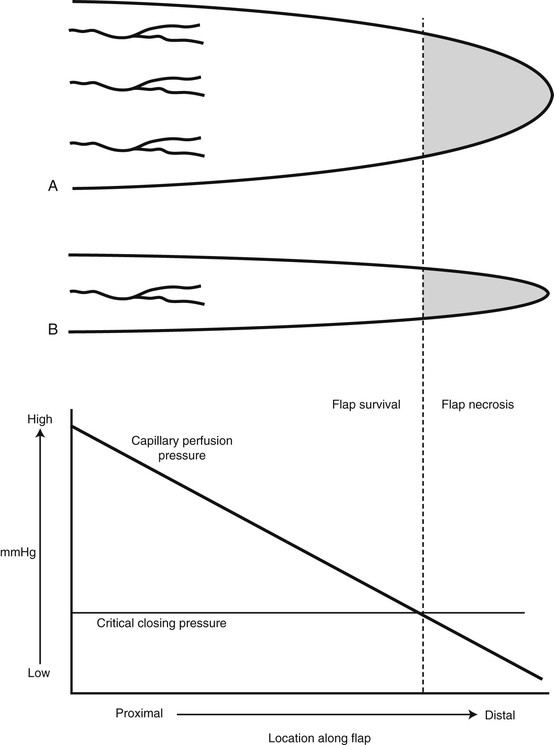
FIGURE 2-5 Fallacy of length-to-width ratio. Slope of decreasing perfusion pressure versus flap length does not change with incorporation of additional vessels (flap A versus flap B) with same perfusion pressure. Flap necrosis occurs when perfusion pressure falls below critical closing pressure of capillary bed.
Myers has emphasized that “fresh flaps are always both viable and ischemic.”12 Depending on the degree of ischemia and the amount of time before recovery of nutrient blood flow, the flap will either die or recover. In the pig model, arterial and random flaps can tolerate an average of 13 hours of total avascularity and remain viable.13 In flaps with reduced perfusion, this time is probably much longer.
In surviving flaps, blood flow gradually increases. If the flap is in a favorable recipient site, a fibrin layer forms within the first 2 days. Neovascularization of the flap begins 3 to 7 days after flap transposition. Early neovascularization has been detected at 4 days in the pig and rabbit models14 and at 3 days in the rat model.15 Revascularization adequate for division of the flap pedicle has been demonstrated by 7 days in animal models and humans.14,16
Beginning with an angiogenic stimulus, the angiogenic process involves a number of discrete yet overlying steps (Fig. 2-6). Initially, the vessels become dilated and permeable with retraction of the endothelial cells and a decrease in endothelial junctions. The basement membrane is then dissolved by proteases, and the endothelial cells migrate from the vascular wall toward the angiogenic stimulus. Behind the leading front of migrating endothelial cells, endothelial cell replication begins, forming a capillary sprout that elongates toward the angiogenic source. The nearby capillary sprouts then anastomose to each other, forming capillary loops. As capillary loops and sprouts continue, the loops become patent, forming newly formed blood vessels. These blood vessels differentiate and lay down basement membrane consisting of type IV collagen, laminin, and proteoglycans. Pericytes and fibroblasts then migrate to the capillary loop sites.17
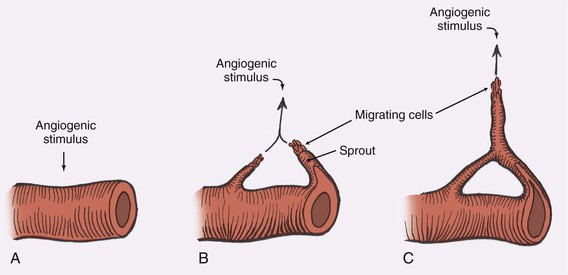
FIGURE 2-6 Steps in angiogenesis. A, Initial stimulus with retraction of endothelial cells and thinning of basement membrane. B, Migration of endothelial cells and formation of capillary sprout. C, Formation of capillary loops, which become patent to form new blood vessels.
With the continued presence or absence of the angiogenic stimulus, substantial remodeling, regression, and rearrangement of the new capillaries occur.17 Some capillaries join preexisting flap vessels (inosculation), but the majority of revascularization appears to involve direct ingrowth of recipient vessels into the flap18 (Fig. 2-7). New capillaries can grow toward an angiogenic source at a mean rate of 0.2 mm/day. When the angiogenic stimulus is discontinued, the capillary vessels regress and eventually disappear during a period of weeks. Angiogenic growth factors can stimulate capillary growth over distances of 2 to 5 mm.19
To prevent an uncontrollable cascade of neovascularization, mechanisms to inhibit angiogenesis are believed to exist. Evidence suggests that pericytes can suppress endothelial growth by direct contact.20 Thus, the physiologic response of angiogenesis may be analogous to the blood coagulation pathway that must be maintained at a constant steady state of control. In soft tissue wound repair, macrophages, lymphocytes, mast cells, and platelets are involved in releasing various factors that modulate angiogenesis.
The venous outflow from the skin is also impaired with flap elevation. Venous flow can occur through the subdermal plexus or by venous channels that accompany the feeding artery in the pedicle. Complete venous occlusion in the early period after elevation may be more damaging to flap survival than inadequate arterial supply.21 Fortunately, the subdermal plexus alone is often adequate to provide sufficient venous outflow. Care must be taken, however, to preserve venous outflow in flaps pedicled solely on the feeding vessels.
Nerve Section
Both cutaneous and sympathetic nerves are severed in the process of flap elevation. Denervation of a skin flap postpones neovascularization of the skin flap. Whereas loss of sensation may limit the usefulness of the flap after transfer, adrenergic denervation has implications for flap survival. When a sympathetic nerve is divided, catecholamines are released from the nerve terminal and the mechanism for catecholamine reuptake is eliminated.22 A local “hyperadrenergic state” develops that produces vasoconstriction mediated by alpha-adrenergic receptors in the cutaneous vasculature. In addition, severe sympathetic denervation contributes to the production of oxygen free radicals, which may exert their inhibitory effects on neovascularization.23
The vasoconstricting effect of sympathectomy further reduces the total flap blood flow24 that has already been diminished by division of supplying vessels. This negatively affects the ratio of perfusion pressure to the critical closing pressure of the arterioles in the subdermal plexus. A greater proportion of the distal flap is excluded from the blood supply and necrosis becomes more likely. The stored transmitter is depleted within 24 to 48 hours,25 and blood flow increases as the concentration of norepinephrine declines. In critical areas of the flap, however, the time to recovery of nutrient blood flow may be delayed sufficiently to produce additional necrosis.
Inflammation and Prostaglandins
The surgical trauma associated with an acutely raised flap results in an inflammatory response. Histamine, serotonin, and kinins are released into the extracellular compartment after flap elevation, increasing the permeability of the microcirculation. The result is an increase in the concentration of proteins and cells within the extracellular space. The presence of nonbacterial inflammation beginning a few days before flap elevation has been shown to improve flap survival.26,27 This is presumably the result of an increase in local blood flow. The inflammation created during flap elevation, however, may have deleterious effects due to the resultant edema formation.
Prostaglandins are derived from essential fatty acids that are incorporated in membrane phospholipids (Fig. 2-8). Activation of phospholipases results in the production of prostaglandin H2 (PGH2) by cyclooxygenase. Prostaglandin E1 (PGE1) and prostaglandin E2 (PGE2) can be synthesized from PGH2 by isomerases in the vascular endothelium. Both PGE1 and PGE2 produce vasodilation. Prostaglandin D2 (PGD2) is also formed by an isomerase reaction and is the principal cyclooxygenase product of the mast cell. Its effects on the cutaneous microvasculature are similar to those of PGE1. Prostacyclin (PGI2) is a vasodilating agent and inhibitor of platelet aggregation that is derived from PGH2 through the action of prostacyclin synthase. In the skin, PGI2 is primarily produced in the endothelial cells of blood vessels.1,28
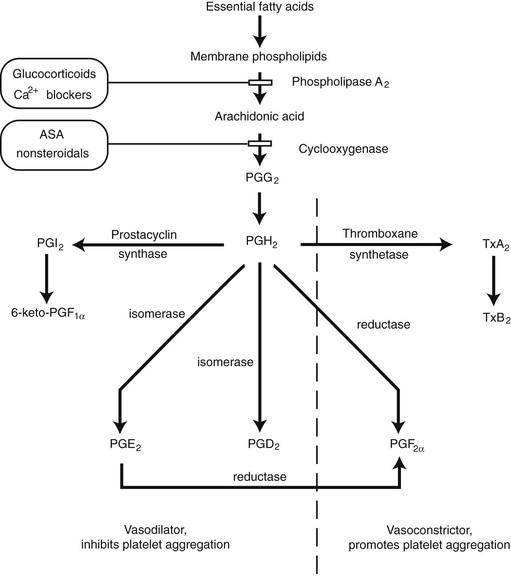
FIGURE 2-8 Synthesis of prostaglandins and thromboxanes and their general effects in cutaneous circulation.
Thromboxane synthetase converts PGH2 into thromboxane A2 (TxA2) and is primarily located in the platelets. Its effects include vessel constriction and promotion of platelet aggregation. Prostaglandin F2α (PGF2α) is derived from PGH2 by a reductase reaction. A marked increase in resistance is seen in cutaneous arteries, arterioles, and venules in the presence of PGF2α.29
The synthesis of prostaglandins and thromboxane can be altered by pharmacologic manipulation. The action of phospholipase A2 can be inhibited by drugs that reduce the availability of calcium. Glucocorticoids also affect phospholipase A2 activity by inducing the synthesis of a protein that inhibits the enzyme.30 Aspirin and other nonsteroidal anti-inflammatory medications interfere with the cyclooxygenase enzyme, inhibiting the synthesis of PGH2.
Prostacyclin levels were found to peak 7 days after elevation of a porcine flank flap.1 Elevation of a bipedicled rat dorsal flap resulted in elevated levels of PGE2, PGF2α, and TxB2, with a return to nearly normal levels by day 7. Conversion to a single pedicle flap (“delay”) resulted in a blunted production of thromboxane and an elevated PGE2 level. Elevation of an acute flap in the same model showed an elevation of PGE2, PGF2α, and TxB2 that was greater and more prolonged than with surgical delay.31 Prostaglandins clearly play a role in the inflammatory response after flap surgery. Whether these changes in prostaglandin levels represent a cause or a side effect of the observed phenomenon remains to be demonstrated.
Reperfusion (Free Radicals)
Return of blood flow to a flap that is ischemic as a result of excessive release of norepinephrine occurs in approximately 12 hours. With norepinephrine depletion and continued inflammatory response, blood flow can reach a maximum at 24 hours.24 When oxygen becomes available with reperfusion, an additional menace to flap survival is produced, the free radical. This byproduct of reperfusion can cause damage at both the cellular and subcellular levels,32 contributing to postischemic tissue necrosis.
Free radicals are extremely reactive compounds because of an unpaired electron in their outer orbitals. Oxygen free radicals are formed by the sequential univalent reduction of molecular oxygen. The superoxide anion radical (O2−) is formed by the addition of a single electron to molecular oxygen. Superoxide is a byproduct of adenosine triphosphate production in the mitochondria and other oxidation reduction reactions.32 Polymorphonuclear cells are a second source of superoxide radicals that are released in response to bacterial inflammation.
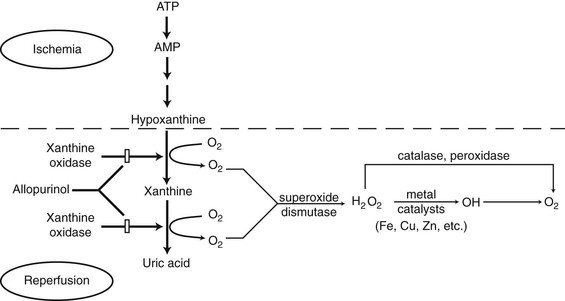
A major source of free radicals in ischemic tissue is the enzyme xanthine oxidase33 (Fig. 2-9). With ischemia, high-energy phosphate compounds are converted to hypoxanthine that accumulates in the tissues. When oxygen becomes available with reperfusion, xanthine oxidase catalyzes the conversion of hypoxanthine into uric acid, producing superoxide in the process. This reaction is an important mechanism in ischemic tissue injury in skin flaps.34 The administration of exogenous vascular endothelial growth factor (VEGF) could protect flaps from ischemia-reperfusion injury through the regulation of proinflammatory cytokines and the inhibition of cytotoxic nitric oxide production.35
Wound Healing
The acute physiologic consequences of flap creation affecting blood flow are of primary importance in determining early flap viability. Other mechanisms, however, play an equally important role in proper flap wound healing and determine the ultimate fate of the flap. These additional mechanisms include the four cascades (clotting, complement, kinin, plasminogen), collagen formation, and collagen degradation. Optimal flap wound healing comprises a well-orchestrated interplay of all of these processes.
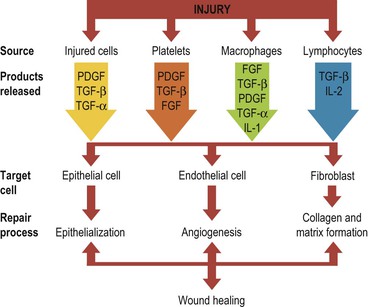
During the last decade, the role of peptide growth factors in soft tissue wound healing has been gaining more attention. These signal proteins contribute to flap wound healing by controlling the recruitment and proliferation of cells (endothelial, epithelial, fibroblast) that modulate the formation of new vessels, collagen, epithelium, and matrix (Fig. 2-10).
Attempts to Alter Skin Flap Viability
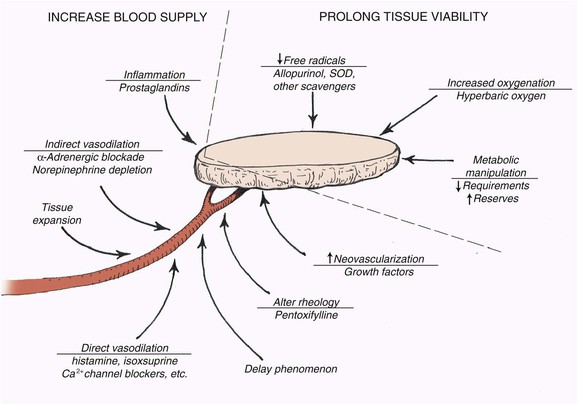
Kerrigan36 outlined extrinsic and intrinsic causes of skin flap failure. Extrinsic reasons for flap necrosis are those not resulting from the design of the raised flap. Examples include systemic hypotension, infection, and pedicle compression. These factors can often be overcome in the clinical situation. The primary intrinsic factor affecting flap survival is inadequate blood flow. Numerous experimental attempts have been made to influence flap microcirculation or to decrease the deleterious effects of inadequate flap blood flow (Fig. 2-11). The most successful has been flap delay.
Research Methods
A large amount of literature is available on skin flap physiology. Several studies give conflicting results. Experimental results are often difficult to interpret because of variation in choice of animal model, timing of treatment, route of drug administration, method of data collection, and repeatability of the study.37 Some standardization of flap research methods would help resolve some of these difficulties.
Skin Flap Delay
Four concepts are accepted concerning the delay phenomenon. First, it requires surgical trauma. Second, a large percentage of the neurovascular supply to the flap must be eliminated. Third, delay results in increased flap survival at the time of tissue transfer. Fourth, the beneficial effects can last up to 6 weeks in the human.38 To explain this phenomenon, three theories regarding the mechanism of delay have been developed: (1) delay improves blood flow, (2) delay conditions tissue to ischemia,39 and (3) delay closes arteriovenous shunts.40 Most recent papers support a mechanism resulting in increased circulation to the flap.
Sympathetic denervation causing depletion of norepinephrine within the flap is one mechanism thought to play a role in increasing blood flow to a delayed flap. Degeneration release of norepinephrine occurs soon after flap elevation, and norepinephrine stores are largely depleted in the first 24 to 48 hours.25 After catecholamine depletion, a relative state of sympathectomy develops, but the vasculature to the flap has an increased sensitivity to the effects of adrenergic drugs (a hyperadrenergic state).22 Pang et al24 theorized that a change in the vasoactivity of the small arteries allowed delivery of more blood to the distal portion of the flap. During elevation of the bipedicled flap, there is a release of vasoconstrictive substances (norepinephrine, thromboxane, and serotonin). Necrosis is not seen during the first stage of delay because the bipedicled flap has an ample blood supply. During the period of delay, catecholamine depletion occurs and there is a recovery from the hyperadrenergic state. Conversion of the delayed flap to a single pedicle is not accompanied by the same degree of vasoconstriction, resulting in improved blood flow and increased survival.41
Another mechanism to increase blood flow to the distal portion of the single pedicle flap is development of vascular collaterals and reorientation of the major vascular channels.42 Longitudinal flow is also enhanced by vasodilating substances released by inflammation and mild ischemia.43 Pang et al43 thought that the depletion of vasoconstricting substances played a role in the early stage of delay, whereas locally released vasodilating substances were involved in the later stages (Table 2-1).
TABLE 2-1
Mechanisms of Improving Blood Flow by the Delay Phenomenon
Depletion of vasoconstricting substances
Formation of vascular collaterals and reorientation of vascular channels
Stimulation of an inflammatory response
Release of vasodilating substances
When a flap is delayed, dilation of existing vessels within the flap occurs. The ingrowth of new vessels is not an important mechanism. The maximal anatomic effect on the arterial vessel appears to occur at the level of “choke” vessels during the delay phenomenon. It is believed that the choke vessel dilation during the delay period is a permanent and irreversible event. Specifically, it is an active process associated with both an increase (hyperplasia) and an enlargement (hypertrophy) of the cells in the choke artery wall that increases the caliber of these vessels.44
Cytokines may be a mechanism by which surgical delay can increase flap survival. Basic fibroblast growth factor (bFGF) and VEGF expressions increased significantly after delay. In the rat flap model, surgical delay resulted in increased VEGF expression and increased skin paddle survival. These results correlate with previous studies showing that the preoperative injection of VEGF increases skin paddle survival.45
Increase of Blood Supply
Indirect Vasodilators
Several anesthetic agents have vasoactive properties and may influence flap survival. Because general anesthetics are often used during the creation of larger flaps, any potential effects on flap survival are important. Isoflurane (a sympatholytic vasodilator) was found to significantly improve flap survival compared with nitrous oxide, which induces vasoconstriction.46
Direct Vasodilators
Direct vasodilators such as histamine, hydralazine, and topical dimethyl sulfoxide have shown both a beneficial effect and no effect on skin flap survival.37 Isoxsuprine is a phenylethylamine derivative of epinephrine having alpha-adrenergic receptor antagonistic and beta-adrenergic receptor agonistic properties, resulting in relaxation of vascular smooth muscle. Isoxsuprine was found to increase blood flow in the area of the dominant artery in porcine myocutaneous and arterial flaps. Unfortunately, no improvement in blood flow was seen in the distal random portion of the flaps or in flap survival.47 The smaller vessels in the distal random portion of a flap were theorized to have a sensitivity to vasodilator drugs different from that of muscular or axial arteries. Manipulation of these distal vascular channels appears to be critical in increasing flap survival.
Experiments investigating the effects of other direct vasodilators either have given mixed results or have yet to be confirmed. Studies have attempted to increase skin flap survival with calcitonin gene–related peptide,48 calcium channel blockers,49 topical nitroglycerin,50 and topical dimethyl sulfoxide.51 The failure of direct vasodilators to reproducibly increase flap survival indicates that mechanisms other than direct arterial dilation are important in survival of the ischemic flap.
Neovascularization
Neovascularization can be potentially accelerated with angiogenic growth factors to improve flap viability. Increased flap survival and vascularity were seen when an endothelial cell growth factor was applied in a sustained-release fashion to accelerate peripheral neovascularization in compromised flaps.52,53
Flap Prefabrication
Prefabrication of flaps into desired skin paddle dimensions before flap transposition has recently gained more attention. Use of angiogenic agents such as bFGF, VEGF, and hyperbaric oxygen to increase vascularity of the skin over the pedicle to enable a larger flap has shown promise. With this concept, it would be possible to tailor the size of a skin paddle of a flap by priming it with angiogenic agents.54–57 One study showed that a prefabricated skin flap can be created 8 weeks after arteriovenous pedicle implantation underneath the planned tailored skin in a rodent model.58
Alteration of Rheology
where ΔP equals pressure gradient, r4 is the fourth power of the vessel radius, l is vessel length, and η is viscosity.4 Although blood is a non-newtonian fluid, the qualitative relationships in the equation remain. In larger vessels, the radius is a dominant factor; but in the capillary microcirculation, viscosity becomes more important. By decreasing the viscosity of blood, it may be possible to increase flow to the distal random portion of the acutely raised flap and beneficially affect flap survival. Viscosity is influenced by the hematocrit, serum proteins, temperature, red blood cell deformability, and aggregation as well as other factors.59 Each of these factors can be potentially manipulated with a resultant change in viscosity.
Pentoxifylline and low-viscosity whole blood substitutes (Fluosol-DA) will also lower viscosity. Pentoxifylline is a hemorrheologic agent that results in increased red blood cell deformability and decreased platelet aggregability.59 When it is given 7 to 10 days before flap elevation, pentoxifylline has increased flap survival in porcine dorsal flank flaps60 and the rat dorsal flap.59 Beneficial effects with limited preoperative dosing of pentoxifylline, however, have not been uniform.61 Fluosol-DA administration has also failed to consistently increase flap survival.62
Inflammation and Prostaglandins
The surgical trauma associated with an acutely raised or delayed flap results in an inflammatory response. This response results in a local increase in blood flow that could benefit flap survival. Improved flap survival has been shown with different methods of creating an inflammatory response before flap creation.26,63 These studies demonstrate that the inflammatory response can be a stimulus for “delay” without sympathectomy or vascular division.
The mechanism by which inflammation produces a beneficial effect appears to involve the products of cyclooxygenase metabolism of arachidonic acid. On the other hand, cyclooxygenase inhibitors such as indomethacin and ibuprofen have been shown to increase skin flap viability.64,65 It has been proposed that the improvement in flap survival with low-level laser energy may be related to a decreased expression of cyclooxygenase 2.66 Glucocorticoids, which inhibit phospholipase A2 activity, have also increased flap survival in some studies.67,68 Administration of prostaglandins that cause vasodilation and decrease platelet aggregation tends to increase survival of experimental flaps.69,70 Blocking of TxA2 synthesis has had mixed results.71,72
Tissue Expansion
Tissue expansion has been demonstrated to increase the size of a transferred flap in experimental animals and humans. Examination of expanded skin in the guinea pig has shown an increase in the thickness73 and mitotic activity74 of the epidermal layer, indicating epidermal proliferation. Blood flow in expanded tissue is greater than in skin overlying a noninflated expander 1 hour after creation of a pedicled flap in the porcine model.75 The increased blood flow to expanded skin compared with delay seems to be short-lived.76 Apart from the acute changes seen with expander manipulation, flap viability and blood flow appear to be similar in expanded skin and in delayed flaps.77 Irradiation to skin reduces the amount of skin that can safely be expanded. It also reduces the effectiveness of surgical delay and skin flap viability.78
Prolonged Viability
Protection Against Harmful Agents
Administration of allopurinol (a xanthine oxidase inhibitor) preoperatively prevents the increased xanthine oxidase activity seen with acute flap elevation.79 Improved survival of dorsal rat flaps has been accomplished with allopurinol when it is given at high doses,80 with lower doses having no effect. The high doses required have led to concern about the use of allopurinol to increase flap survival in the clinical setting.
A number of free radical scavengers are available to protect the tissues from destruction by free radicals. Superoxide dismutase (SOD), an intracellular free radical scavenger, catalyzes the conversion of superoxide to hydrogen peroxide (H2O2) and molecular oxygen. When it is given systemically, SOD is an effective scavenger of the superoxide radical regardless of its source. SOD treatment has resulted in improved flap survival81,82 and increased tolerance to ischemia of rat abdominal flaps.48 Improved flap survival has also been demonstrated with a number of other naturally occurring compounds with free radical scavenging properties. These include deferoxamine83; vitamin E, vitamin A, vitamin C, and glutathione84; various amino acids85; and amino acid derivatives.86
The hydrogen peroxide formed by the dismutation of superoxide is not particularly harmful. In the presence of chelated metal complexes, however, hydrogen peroxide can be converted into a hydroxyl radical (OH.) by the Fenton-type or Haber-Weiss reaction. The hydroxyl radical is much more reactive and may be responsible for much of the damage inflicted by oxygen free radicals.32 The presence of a hematoma under a flap may decrease flap survival by increasing the available iron that acts as a catalyst in the formation of free radicals.87
Nitric Oxide
Nitric oxide synthesized by the enzyme nitric oxide synthase has a major physiologic importance in skin flap survival. Nitric oxide is a radical with properties that induce vasodilation and can protect tissues from neutrophil-mediated ischemia-reperfusion injury. By the administration of a nitric oxide precursor, L-arginine, myocutaneous flaps in porcine skin were protected from ischemia-reperfusion injury with reduced flap necrosis, neutrophil accumulation, and edema.88 It has been proposed that after reperfusion injury, endothelial cell dysfunction leads to disruption of nitric oxide synthase–mediated nitric oxide production and in turn causes the deleterious effects of ischemia-reperfusion injury on flap survival and blood reflow.89 The role of nitric oxide synthase in promoting skin flap angiogenesis in its early stages after flap transposition has shown promise.90 Nitric oxide is also believed to be an important contributor to the phenomenon of skin flap delay.91
Increased Tolerance of Ischemia
Increased Oxygenation.
Improvement in flap survival with hyperbaric oxygen treatment has been documented in the rat model.92 Hyperbaric oxygen treatment, however, increases blood oxygen-carrying capacity by at most 20%.93 A greater effect of hyperbaric oxygen treatment may be increasing oxygen diffusion from surrounding perfused tissue to the ischemic portion of the flap.94 Increased flap survival occurs with treatments using 21% hyperbaric oxygen, implying that increased oxygen could be delivered with an increase in pressure alone.95 A treatment delay of 24 hours or more after elevation of rat dorsal flaps results in little benefit from hyperbaric oxygen.96 An experiment using a porcine model was less successful in improving flap survival.97
Metabolic Manipulation.
Decreasing the metabolic requirements and increasing the metabolic reserves of skin are additional strategies for increasing flap survival. These approaches are based on the idea that flap necrosis occurs when tissue metabolic demand is greater than what the blood supply can deliver. Decreasing temperature is an effective way to reduce metabolic activity and to delay necrotic changes, but improvement in flap survival is not seen.98
Impaired Flaps
Smoking tobacco is associated with an increased chance of flap necrosis in facelift operations.99 Exposure to tobacco smoke resulted in increased flap necrosis of dorsal flaps in rodents.100 The deleterious effects of nicotine appear to increase with prolonged exposure.101 The mechanism whereby tobacco or nicotine lowers flap survival is believed to be direct endothelial damage or vasoconstriction secondary to catecholamine release or local concentrations of prostaglandins. Smoking impairs flap healing by causing vasoconstriction from nicotine and increased levels of carboxyhemoglobin, which limits oxygen delivery. In addition, smoking decreases neutrophilic function and decreases collagen synthesis.102 Smoking cessation for at least 4 to 6 weeks is recommended before skin flap surgery. Medications such as varenicline (Chantix) and the electronic cigarette (E-cigarette) may assist in cessation.
Some animal studies show that hyperbaric oxygen can improve skin flap survival.103 Hyperbaric oxygen therapy delivers oxygen concentrations at pressures higher than atmospheric pressures. With a “dive” of 2.4 ATA, tissue wound partial pressure of oxygen will be 800 to 1100 mm Hg. This exposure induces vasoconstriction with increased partial pressures of oxygen in the blood, which subsequently stimulates angiogenesis and fibroblast proliferation.104 Patient side effects may include barotrauma and exacerbation of congestive heart failure. More clinical studies are required.
VEGF and nitric oxide cause vasodilation, increase angiogenesis, and improve flap survival. One study showed that a single intra-arterial dose of VEGF or L-arginine, a substrate for nitric oxide production, reduced flap regional necrosis.105
Irradiation has been shown to be deleterious to flap survival in some studies.106,107 Discrepancies in these studies are probably due to differences in the radiation regimen administered (i.e., radiation dosage, fractionation, and surgical timing in respect to the irradiation). Radiation treatment results in endarteritis obliterans and altered wound healing. In spite of prior irradiation, the delay phenomenon does improve flap survival.108 Flap neovascularization is delayed but not eliminated after irradiation.109
The use of angiogenic growth factors has the potential to increase the viability of irradiated skin flaps by means of accelerating revascularization.53 Use of bFGF has been shown to increase random skin flap survival of irradiated skin flaps.110 When growth factors are administered to influence wound healing and flap survival, it is difficult to determine the best time and mode of delivery to maximize their therapeutic effects. One major factor is that the half-life of growth factors is short. One article described the feasibility of using hollow-fiber catheters to deliver VEGF to porcine random skin flaps.111 Other modalities for growth factor delivery continue to be investigated.
Preliminary studies using autologous platelet-rich plasma, which contains a number of growth factors released from platelets, have shown increased skin flap survival.112 When platelet-rich plasma is administered by subcutaneous injection, platelet-rich skin flaps have an increased blood vessel density and fewer inflammatory cells compared with platelet-poor plasma and control flaps. Furthermore, platelet-rich plasma flaps appeared to have an upregulation of genes involved in angiogenesis (elevated mRNA levels of VEGF and platelet-derived growth factor).
Tissue Engineering
Tissue engineering to improve the survival of skin flaps remains in its infancy. In using gene therapy to improve flap survival, various methods have employed plasmids or viruses to transplant genes that code for and produce growth factors in ischemic tissue.113
One study showed that liposome-mediated gene transfer could result in useful biologic effects by priming the flap before it is raised. Specifically, cDNA encoding VEGF complexed to commercially available liposomes was injected into rat skin 1 week before raising of a random flap. Flap survival was enhanced 14%.114
Another study investigated the use of adenovirus-mediated gene therapy with VEGF (Ad-VEGF) delivered into the subdermal space to treat compromised skin flaps. Compared with the control, both local and midline subdermal injections of Ad-VEGF showed improved overall flap survival.115 In ischemic tissues, flap survival was enhanced by administration of a cDNA encoding VEGF116 as well as by incorporation of the VEGF gene into mesenchymal stem cells.117 The use of plasmid DNA as a carrier for platelet-derived growth factor B delivered subcutaneously 7 days before flap elevation may also give an effective nonsurgical approach to increase flap survival by increasing vascularity.118
Summary
The blood flow required for nutritional support of the skin is dramatically less than what is available to carry out its thermoregulatory function. This allows skin to survive a compromise of its blood supply during creation of local flaps. The surviving length of a particular flap depends on the relationship between the intravascular perfusion pressure and the critical closing pressure of the arterioles in the subdermal plexus. Appropriate flap design is the most critical factor in maintaining adequate intravascular perfusion pressure and avoiding tissue necrosis.
References
1. Hauben, D, Zijlstra, FJ. Prostacyclin formation in delayed pig flank flaps. Ann Plast Surg. 1984; 13:304.
2. Sherman, J. Normal arteriovenous anastomoses. Medicine (Baltimore). 1963; 42:247.
3. Greene, N. Physiology of sympathetic denervation. Annu Rev Med. 1962; 13:87.
4. Guyton, A. Textbook of medical physiology. Philadelphia: WB Saunders; 1976.
5. Granger, HJ, Goodman, AH, Granger, DN. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res. 1976; 38:379.
6. Folkow, B. Role of the nervous system in the control of vascular tone. Circulation. 1960; 21:760.
7. Daniel, RK. The anatomy and hemodynamics of the cutaneous circulation and their influence on skin flap design. Boston: Little, Brown; 1975.
8. Sinna, R, Boloorchi, A, Mahajan, A, et al. What should define a “perforator flap”? Plast Reconstr Surg. 2010; 126:2258.
9. Ono, S, Chung, K, Hayashi, H, et al. Application of multidetector-row computed tomography in propeller flap planning. Plast Reconstr Surg. 2011; 127:703.
10. Rozen, W, Paddle, A, Chubb, D, et al. Guiding local perforator flaps with preoperative imaging: revealing perforator anatomy to improve flap design. Plast Reconstr Surg. 2012; 130:130.
11. Cutting, C. Critical closing and perfusion pressure in flap survival. Ann Plast Surg. 1982; 9:524.
12. Myers, B. Understanding flap necrosis. Plast Reconstr Surg. 1986; 78:813.
13. Kerrigan, CL, Daniel, RK. Critical ischemia time and the failing skin flap. Plast Reconstr Surg. 1982; 69:986.
14. Tsur, H, Daniller, A, Strauch, B. Neovascularization of skin flaps: route and timing. Plast Reconstr Surg. 1980; 66:85.
15. Gatti, J, LaRossa, D, Brousseau, D, et al. Assessment of neovascularization and timing of flap division. Plast Reconstr Surg. 1984; 73:396.
16. Cummings, C, Trachy, RE. Measurement of alternative blood flow in the porcine panniculus carnosus myocutaneous flap. Arch Otolaryngol. 1985; 111:598.
17. Furcht, L. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986; 55:505.
18. Smahel, J. The healing of skin grafts. Philadelphia: WB Saunders; 1977.
19. Folkman, J. How is blood vessel growth regulated in normal and neoplastic tissue? Cancer. 1986; 46:467.
20. D’Amore, P. The role of growth factors and cell-cell communication in the control of angiogenesis. Princton, NJ: Excerpta Medica; 1987.
21. Su, CT, Im, MJ, Hoopes, JE. Tissue glucose and lactate following vascular occlusion in island skin flaps. Plast Reconstr Surg. 1982; 70:202.
22. Pearl, R. A unifying theory of the delay phenomenon: recovery from the hyperadrenergic state. Ann Plast Surg. 1981; 7:102.
23. Mounsey, RA, Brown, DH, O’Dwyer, TP, et al. Role of hyperbaric oxygen therapy in the management of mandibular osteoradionecrosis. Laryngoscope. 1993; 103:605.
24. Pang, C, Neligan, PC, Forrest, C, et al. Hemodynamics and vascular sensitivity to circulating norepinephrine in normal skin and delayed and acute random skin flaps in the pig. Plast Reconstr Surg. 1986; 78:75.
25. Jurell, G. Adrenergic nerves and the delay phenomenon. Ann Plast Surg. 1986; 17:497.
26. Liston, S. Nonbacterial inflammation as a means of enhancing skin flap survival. Laryngoscope. 1984; 94:1075.
27. Macht, S, Frazier, WH. The role of endogenous bacterial flora in skin flap survival. Plast Reconstr Surg. 1980; 65:50.
28. Kaley, G, Hintze, TH, Panzenbeck, M, et al. Role of prostaglandins in microcirculatory function. New York: Raven Press; 1985.
29. Nakano, J. General pharmacology of prostaglandins. Philadelphia: JB Lippincott; 1973.
30. Campbell, W. Lipid-derived autacoids: eicosanoids and platelet-activating factor. New York: Pergamon Press; 1990.
31. Murphy, R, Lawrence, WT, Robson, M, et al. Surgical delay and arachidonic acid metabolites: evidence for an inflammatory mechanism: an experimental study in rats. Br J Plast Surg. 1985; 38:272.
32. Southorn, P, Powis, G. Free radicals in medicine. I. Chemical nature and biologic reactions. Mayo Clin Proc. 1988; 63:381.
33. McCord, J. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985; 312:159.
34. McCord, J. Improved survival of island flaps after prolonged ischemia by perfusion with superoxide dismutase. Plast Reconstr Surg. 1986; 77:643.
35. Pang, Y, Lineaweaver, WC, Lei, MP, et al. Evaluation of the mechanism of vascular endothelial growth factor improvement of ischemic flap survival in rats. Plast Reconstr Surg. 2003; 112:556.
36. Kerrigan, C. Skin flap failure: pathophysiology. Plast Reconstr Surg. 1983; 72:766.
37. Kerrigan, C, Daniel, RK. Pharmacologic treatment of the failing skin flap. Plast Reconstr Surg. 1982; 70:541.
38. Pearl, R. The delay phenomenon: why the fuss? Ann Plast Surg. 1984; 13:307.
39. McFarlane, RM, Heagy, FC, Radin, S, et al. A study of the delay phenomenon in experimental pedicle flaps. Plast Reconstr Surg. 1965; 35:245.
40. Reinisch, J. The pathophysiology of skin flap circulation: the delay phenomenon. Plast Reconstr Surg. 1974; 54:585.
41. Pang, C, Forrest, CR, Neligan, P, et al. Augmentation of blood flow in delayed random skin flaps in the pig: effect of length of delay period and angiogenesis. Plast Reconstr Surg. 1986; 78:68.
42. Suzuki, S, Isshiki, N, Ohtsuka, M, et al. Experimental study on “delay” phenomenon in relation to flap width and ischaemia. Br J Plast Surg. 1988; 41:389.
43. Pang, C, Neligan, PC, Nakatsuka, T, et al. Pharmacologic manipulation of the microcirculation in cutaneous and myocutaneous flaps in pigs. Clin Plast Surg. 1985; 12:273.
44. Dhar, SC, Taylor, GI. The delay phenomenon: the story unfolds. Plast Reconstr Surg. 1999; 104:2079.
45. Lineaweaver, WC, Lei, MP, Mustain, W, et al. Vascular endothelium growth factor, surgical delay, and skin flap survival. Ann Surg. 2004; 239:866–873.
46. Dohar, JGG, Azarshin, K. The effects of inhalation anesthetic agents on survival in a pig random skin flap model. Arch Otolaryngol. 1992; 118:37.
47. Neligan, P, Pang, CY, Nakatsuka, T, et al. Pharmacologic action of isoxsuprine in cutaneous and myocutaneous flaps. Plast Reconstr Surg. 1985; 75:363.
48. Knight, K, Martin, TJ, Coe, S, et al. Effect of the vasodilator peptides calcitonin gene-related peptide and atriopeptin on rabbit microvascular blood flow: preliminary communication. Br J Plast Surg. 1988; 41:147.
49. Hira, M, Tajima, S, Sano, S. Increased survival length of experimental flap by calcium antagonist nifedipine. Ann Plast Surg. 1990; 24:45.
50. Nichter, L, Sobieski, MW, Edgerton, M. Efficacy of topical nitroglycerin for random-pattern skin-flap salvage. Plast Reconstr Surg. 1985; 75:847.
51. Myers, M, Donovan, W. Augmentation of tissue oxygen by dimethyl sulfoxide and hydrogen peroxide. In: Bruley C, Dicher H, eds. Oxygen transport to tissue: instrumentation, methods and psychology. New York: Plenum Press, 1973.
52. Hom, D, Assefa, G. The effects of endothelial cell growth factor on vascular compromised skin flaps. Arch Otolaryngol. 1992; 118:624.
53. Hom, D, Assefa, G, Song, CW. Endothelial cell growth factor (ECGF) application to irradiated soft tissue. Laryngoscope. 1993; 103:165.
54. Bayati, S, Russell, RC, Roth, AC. Stimulation of angiogenesis to improve the viability of prefabricated flaps. Plast Reconstr Surg. 1998; 101:1290.
55. Haws, MJ, Erdman, D, Bayati, S, et al. Basic fibroblast growth factor induced angiogenesis and prefabricated flap survival. J Reconstr Microsurg. 2001; 17:39–42.
56. Hickey, MJ, Wilson, Y, Hurley, JV, et al. Mode of vascularization of control and basic fibroblast growth factor–stimulated prefabricated skin flaps. Plast Reconstr Surg. 1998; 101:1296–1304.
57. Li, QF, Reis, ED, Zhang, WX, et al. Accelerated flap prefabrication with vascular endothelial growth factor. J Reconstr Microsurg. 2000; 16:45.
58. Kostakoglu, N, Manek, S, Green, CJ. The development of neovascularisation in flap prefabrication with vascular implantation: an experimental study. Br J Plast Surg. 1997; 50:428.
59. Roth, A, Briggs, PC, Jones, E, et al. Augmentation of skin flap survival by parenteral pentoxifylline. Br J Plast Surg. 1988; 41:525.
60. Yessenow, R, Maves, MD. The effects of pentoxifylline on random skin flap survival. Arch Otolaryngol Head Neck Surg. 1989; 115:179.
61. Chu, B, Deshmukh, N. The lack of effect of pentoxifylline on random skin flap survival. Plast Reconstr Surg. 1989; 83:315.
62. Chowdary, R, Berkower, AS, Moss, M, et al. Fluorocarbon enhancement of skin flap survival in rats. Plast Reconstr Surg. 1987; 79:98.
63. Kami, T, Yoshimura, Y, Nakajima, T, et al. Effects of low-power diode lasers on flap survival. Ann Plast Surg. 1985; 14:278.
64. Sasaki, G, Pang, CY. Experimental evidence for involvement of prostaglandins in viability of acute skin flaps: effects on viability and mode of action. Plast Reconstr Surg. 1981; 67:335.
65. Robson, M, Del Beccaro, EJ, Heggers, J. The effects of prostaglandins on the dermal microcirculation after burning and the inhibition of the effect by specific pharmacological agents. Plast Reconstr Surg. 1979; 63:781.
66. Junior, I, Masson, I, Oshima, C, et al. Low-level laser irradiation, cyclooxygenase-2 (COX-2) expression and necrosis of random skin flaps in rats. Lasers Med Sci. 2012; 27:655.
67. Mendelson, B, Woods, JE. Effect of corticosteroids on the surviving length of skin flaps in pigs. Br J Plast Surg. 1978; 31:293.
68. Mes, L. Improving flap survival by sustained cell metabolism within ischemic cells: a study using rabbits. Plast Reconstr Surg. 1980; 65:56.
69. Suzuki, S, Isshiki, N, Ogawa, Y, et al. Effect of intravenous prostaglandin E1 on experimental flaps. Ann Plast Surg. 1987; 19:49.
70. Silverman, DB, Brousseau, DA, Norton, KJ, et al. The effect of a topical PGE2 analogue on global flap ischemia in rats. Plast Reconstr Surg. 1989; 84:794.
71. Ono, I, Ohura, T, Murazumi, M, et al. A study on the effectiveness of a thromboxane synthetase inhibitor (OKY-046) in increasing survival length of skin flaps. Plast Reconstr Surg. 1990; 86:1164.
72. Kay, S, Green, C. The effect of a novel thromboxane synthetase inhibitor dazmegrel (UK38485) on random pattern skin flaps in the rat. Br J Plast Surg. 1986; 39:361.
73. Austad, E, Pasyk, KA, McClatchey, K, et al. Histomorphologic evaluation of guinea pig skin and soft tissue after controlled tissue expansion. Plast Reconstr Surg. 1982; 70:704.
74. Francis, A, Marks, R. Skin stretching and epidermopoiesis. Br J Exp Pathol. 1977; 58:35.
75. Marks, M, Burney, RE, Mackenzie, J, et al. Enhanced capillary blood flow in rapidly expanded random pattern flaps. J Trauma. 1986; 26:913.
76. Ricciardeli, E, Goding, GS, Bright, D, et al. Acute blood flow changes in rapidly expanded and adjacent skin. Arch Otolaryngol. 1989; 115:182.
77. Sasaki, G, Pang, CY. Pathophysiology of skin flaps raised on expanded pig skin. Plast Reconstr Surg. 1984; 74:59.
78. Dvali, LT, Dagum, AB, Pang, CY, et al. Effect of radiation on skin expansion and skin flap viability in pigs. Plast Reconstr Surg. 2000; 106:624.
79. Im, M, Shen, WH, Pak, C, et al. Effect of allopurinol on the survival of hyperemic island skin flaps. Plast Reconstr Surg. 1984; 73:276.
80. Pokorny, A, Bright, DA, Cummings, C. The effects of allopurinol and superoxide dismutase in a rat model of skin flap necrosis. Arch Otolaryngol Head Neck Surg. 1989; 115:207.
81. Zimmerman, T, Sasaki, GH, Khattab, S. Improved ischemic island skin flap survival with continuous intraarterial infusion of adenosine triphosphate–magnesium chloride and SOD: a rat model. Ann Plast Surg. 1987; 18:218.
82. Freeman, TJ, Maisel, RH, Goding, GS, et al. Inhibition of endogenous superoxide dismutase with diethyldithiocarbamate in acute island skin flaps. Otolaryngol Head Neck Surg. 1990; 103:938.
83. Angel, M, Narayanan, K, Swartz, W, et al. Deferoxamine increases skin flap survival: additional evidence of free radical involvement in ischaemic flap surgery. Br J Plast Surg. 1986; 39:469.
84. Hayden, R, Paniello, RC, Yeung, C, et al. The effect of glutathione and vitamins A, C, and E on acute skin flap survival. Laryngoscope. 1987; 97:1176.
85. Paniello, R, Hayden, RE, Bello, S. Improved survival of acute skin flaps with amino acids as free radical scavengers. Arch Otolaryngol. 1988; 114:1400.
86. Kim, YS, Im, MJ, Hoopes, JE. The effect of a free-radical scavenger, N-2-mercaptopropionylglycine, on the survival of skin flaps. Ann Plast Surg. 1990; 25:18.
87. Angel, M, Narayanan, K, Swartz, W, et al. The etiologic role of free radicals in hematoma-induced flap necrosis. Plast Reconstr Surg. 1986; 77:795.
88. Cordeiro, PG, Santamaria, E, Hu, QY. Use of a nitric oxide precursor to protect pig myocutaneous flaps from ischemia-reperfusion injury. Plast Reconstr Surg. 1998; 102:2040–2048.
89. Khiabani, KT, Kerrigan, CL. Presence and activity of nitric oxide synthase isoforms in ischemia-reperfusion–injured flaps. Plast Reconstr Surg. 2002; 109:1638.
90. Furuta, S, Vadiveloo, P, Romeo-Meeuw, R, et al. Early inducible nitric oxide synthase 2 (NOS 2) activity enhances ischaemic skin flap survival. Angiogenesis. 2004; 7:33.
91. McDonald, WS, Lo, TP, Jr., Thurmond, M, et al. Role of nitric oxide in skin flap delay. Plast Reconstr Surg. 2004; 113:927.
92. Perrins, D. The effect of hyperbaric oxygen on ischemic skin flaps. Boston: Little, Brown; 1975.
93. Kernahan, D, Zingg, W, Kay, C. The effects of hyperbaric oxygen on the survival of experimental skin flaps. Plast Reconstr Surg. 1965; 36:19.
94. Cutting, C. Skin flap physiology. St. Louis: Mosby–Year Book; 1986.
95. Tan, C, Im, MJ, Myers, R, et al. Effect of hyperbaric oxygen and hyperbaric air on survival of island skin flaps. Plast Reconstr Surg. 1984; 73:27.
96. Nemiroff, P, Merwin, GE, Brant, T, et al. Effects of hyperbaric oxygen and irradiation on experimental skin flaps in rats. Otolaryngol Head Neck Surg. 1985; 93:485.
97. Caffee, HH, Gallagher, TJ. Experiments on the effects of hyperbaric oxygen on flap survival in the pig. Plast Reconstr Surg. 1988; 81:954.
98. Goding, G, Cummings, C, Bright, D. Effects of local hypothermia on the porcine myocutaneous flap. Philadelphia: BD Decker; 1991.
99. Rees, T, Liverett, DM, Guy, C. The effect of cigarette smoking on skin flap survival in the face lift patient. Plast Reconstr Surg. 1984; 73:911.
100. Craig, S, Rees, TD. The effects of smoking on experimental skin flaps in hamsters. Plast Reconstr Surg. 1985; 75:842.
101. Forrest, C, Pang, CY, Lindsay, W. Dose and time effects of nicotine treatment on the capillary blood flow and viability of random pattern skin flaps in the rat. Br J Plast Surg. 1987; 40:295.
102. Sorensen, LT, Karlsmark, T, Gottrup, F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003; 238:1.
103. Friedman, HI, Fitzmaurice, M, Lefaivre, JF, et al. An evidence-based appraisal of the use of hyperbaric oxygen on flaps and grafts. Plast Reconstr Surg. 2006; 117:175S–190S.
104. Tibbles, PM, Edelsberg, JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996; 334:1642.
105. Komorowska-Timek, E, Timek, TA, Brevetti, LS, et al. The effect of single administration of vascular endothelial growth factor or L-arginine on necrosis and vasculature of the epigastric flap in the rat model. Br J Plast Surg. 2004; 57:317.
106. Patterson, T, Berry, R, Hopewell, J, et al. The effect of radiation in survival of experimental skin flaps. In: Grabb WC, Myers MB, eds. Skin flaps. Boston: Little, Brown, 1975.
107. Young, CM, Hopewell, JW. The effects of preoperative x-irradiation on the survival and blood flow of pedicle skin flaps in the pig. Int J Radiat Oncol Biol Phys. 1983; 9:865.
108. Fisher, J, Hurn, I, Rudolph, R, et al. The effect of delay on flap survival in an irradiated field. Plast Reconstr Surg. 1984; 73:99.
109. Clarke, H, Howard, CR, Pynn, B, et al. Delayed neovascularization in free skin flap transfer to irradiated beds in rats. Plast Reconstr Surg. 1985; 75:560.
110. Hom, D, Unger, G, Pernell, K, et al. Improving surgical wound healing with basic fibroblast growth factor after radiation. Laryngoscope. 2005; 115:412.
111. Anderson, TA, Yu, V, Hom, DB, et al. Interstitial delivery of vascular endothelial growth factor to skin flaps. Arch Facial Plast Surg. 2010; 12:326.
112. Li, W, Enomoto, M, Ukegawa, M, et al. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg. 2012; 129:858.
113. Waller, W, Lee, J, Zhang, F, Lineaweaver, WC. Gene therapy in flap survival. Microsurgery. 2004; 24:168.
114. Liu, PY, Tong, W, Liu, K, et al. Liposome-mediated transfer of vascular endothelial growth factor cDNA augments survival of random-pattern skin flaps in the rat. Wound Repair Regen. 2004; 12:80.
115. Lubiatowski, P, Goldman, CK, Gurunluoglu, R, et al. Enhancement of epigastric skin flap survival by adenovirus-mediated VEGF gene therapy. Plast Reconstr Surg. 2002; 109:1986.
116. Taub, PJ, Marmur, JD, Zhang, WX, et al. Locally administered vascular endothelial growth factor cDNA increases survival of ischemic experimental skin flaps. Plast Reconstr Surg. 1998; 102:2033.
117. Zheng, Y, Yi, C, Xia, W, et al. Mesenchymal stem cells transduced by vascular endothelial growth factor gene for ischemic random skin flaps. Plast Reconstr Surg. 2008; 121:59.
118. Hijjawi, J, Mogford, JE, Chandler, LA, et al. Platelet-derived growth factor B, but not fibroblast growth factor 2, plasmid DNA improves survival of ischemic myocutaneous flaps. Arch Surg. 2004; 139:142.

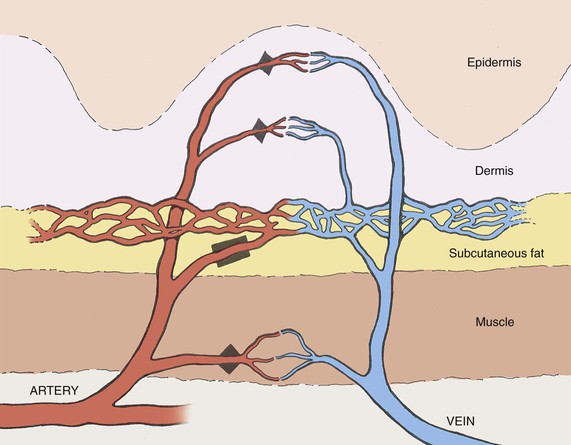
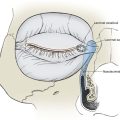
 ) and preshunt (
) and preshunt ( ) sphincters in skin. The precapillary sphincter regulates nutritive blood flow to skin and responds to locally produced stimuli. The preshunt sphincter is involved in thermoregulation and is affected by sympathetic stimuli from the central nervous system.
) sphincters in skin. The precapillary sphincter regulates nutritive blood flow to skin and responds to locally produced stimuli. The preshunt sphincter is involved in thermoregulation and is affected by sympathetic stimuli from the central nervous system.