24 Skin Care Products
Skin care is an essential component of aesthetic medicine. Topical skin care products are used for treatment of photoaging, such as fine lines, rough texture, and dyschromia, and are particularly beneficial in patients with darker Fitzpatrick skin types, where more aggressive treatments may be contraindicated. Visible changes with most topical products are subtle and slow, and typically require regular use over a 3- to 6-month period. Another key role for topical products in aesthetic practice is supporting and enhancing the results of minimally invasive aesthetic procedures. They are used preprocedure to condition and prepare the skin, postprocedure to promote healing and soothe skin, and are helpful in the management of complications.1,2
The three main categories of topical products, based on their regulatory status, are:
Dry Skin (Xerosis)
Xerosis, scaling, winter itch, and flaking are all terms used to describe dry skin. To understand treatment options for dry skin, one must first consider how the skin naturally maintains moisture. Skin is comprised of the epidermis, dermis, and subcutaneous layers (see Figure 19-3 in Chapter 19, Aesthetic Principles and Consultation). The outermost layer of the epidermis, the stratum corneum, forms the epidermal barrier. It functions as an evaporative barrier, maintaining skin hydration and suppleness, and as a protective physical barrier against microbes, trauma, and chemical irritants, and it reduces the harmful effects of ultraviolet (UV) light.3
Photoaged skin has sluggish, disorganized keratinocyte maturation, which leads to corneocyte retention and disruption of the epidermal barrier. Water escapes more freely from the skin causing dehydration and dryness, which can be measured as increased transepidermal water loss.4 Environmental insults such as harsh topical chemicals, temperature and humidity extremes, and genetic abnormalities (e.g., ichthyosis) can also damage the integrity of the skin barrier contributing to dryness.
Treatment of Dry Skin
Moisturizers used to treat dry skin increase hydration by binding and trapping water in the epidermis, and by enhancing epidermal barrier function to reduce evaporative water loss. Skin is rendered more supple and healthy, and clinically demonstrates improvements in smoothness and wrinkles.5 These effects can be temporary, such that once the moisturizer is removed the effects are gone. Effects can also be long-lasting when the integrity of the skin barrier is strengthened following repeated use.
Moisturizers contain three main types of ingredients, each of which serves a unique function6–8:
Most moisturizers are composed of all three ingredient types (occlusives, humectants, and emollients) to create a product that is both effective and cosmetically elegant and does not feel oily to the touch. Although the term emollient is sometimes used interchangeably with moisturizer, it is truly one of three components that can be found in moisturizers. Table 24-1 lists common ingredients found in moisturizers.
TABLE 24-1 Common Moisturizing Agents and Their Function4,12
|
Moisturizer Types |
Functions |
Ingredients |
|---|---|---|
| Occlusive | Trap water on the skin surface and enhance lipid component of epidermal barrier. | Oils (petrolatum, mineral oil) Cholesterol, squalene Waxes (beeswax, carnauba wax, liquid wax) Silicones (dimethicone, cyclomethicone) Stearic acid, cetyl alcohol, stearyl alcohol Lanolin, lanolin alcohol |
| Humectant | Attract water from the dermis. | Glycerin Urea Hyaluronic acid Sorbitol Propylene glycol |
| Emollient | Smooth roughened epidermis. | C12-15 alkyl benzoate Cetyl stearate Glyceryl stearate Octyl octanoate Decyl oleate Isostearyl alcohol |
Barrier repair creams are a newer class of moisturizer that contain occlusive and humectant ingredients at ratios that closely mimic the content of normal, healthy stratum corneum: ceramides (50%), fatty acids (30%), and cholesterol (30%). They enhance barrier function, reduce transepidermal water loss, and increase the skin’s resistance to environmental irritants.9 These products may be used by patients with compromised barriers, such as those exposed to cleansers and chemicals, and conditions such as atopic dermatitis. Barrier repair products include nonprescription CeraVe® and TriCeram®, and prescription Mimyx®, Atopiclair®, and EpiCeram®.
Dry skin may also be associated with hyperkeratosis as seen with ichthyosis, keratosis pilaris, and callouses. Topical hydroxy acids such as lactic acid 5% to 12% (e.g., generic ammonium lactate available in the brand names of Lac-Hydrin® and AmLactin®) and urea 10% to 40% (e.g., Carmol® and Vanamide®) are keratolytic and function to reduce stratum corneum thickness and increase hydration.10
Exfoliation treatments, which superficially resurface the skin by removing the topmost layers of the epidermis, can also be used to treat dry skin. Exfoliation stimulates the keratinocyte maturation process with regeneration of a healthier epidermis. This results in a compacted stratum corneum and increased production of ceramides, which improves epidermal barrier function and skin hydration.11,12 Exfoliation, where the outermost layers of skin are removed, can be achieved using mechanical methods (e.g., microdermabrasion) with gentle abrasion, or using chemical methods (e.g., chemical peels) with application of topical products such as acids (see Chapter 23, Microdermabrasion, and Chapter 22, Chemical Peels, respectively).
Photoaging
Most of the changes seen with skin aging are due to years of cumulative UV exposure, referred to as photoaging.13,14 Skin is affected by ultraviolet A radiation (UVA, 320 to 400 nm), long-wavelength solar radiation that penetrates into the dermis, and ultraviolet B (UVB, 290 to 320 nm), shorter wavelength radiation that is absorbed by the epidermis.
Skin Rejuvenation Products
Products used for the treatment of photoaging must address several aspects of the aging process, including increasing skin exfoliation and stimulating epidermal renewal, increasing synthesis of collagen and other dermal matrix components, reducing hyperpigmentation, and increasing hydration. Products used for skin protection are aimed at preventing damage from UV exposure and free-radical oxidation. Skin care regimes usually consist of multiple topical products, including prescription medications and cosmeceuticals, to address these various aspects of skin aging. Table 24-2 lists topical products commonly used for skin rejuvenation and their functions.15
TABLE 24-2 Skin Rejuvenation Products and Their Functions16–19
| CLEANSE | |
| Cleanser selected based on skin oiliness/dryness | |
| TREAT | |
| Textural changes Fines lines Rough skin |
Retinoids, hydroxy acids, N-acetyl glucosamine, growth factors, kinetin, peptides, moisturizers |
| Dyschromia Solar lentigines Postinflammatory hyperpigmentation |
Retinoids, hydroquinone, kojic acid, azelaic acid, arbutin, and others (Tables 24-3 and 24-4) |
| Vascular ectasias Telangiectasias Rosacea |
Bisoprolol, allantoin, aloe, algae extract, borage and evening primrose oil, vitamin C, chamomile, green tea extracts |
| PROTECT | |
| Antioxidants | Vitamin C, vitamin E, ferulic acid, coffee berry, idebenone, gluconolactone, polyhydroxy acids, resveratrol, tea and red wine extracts |
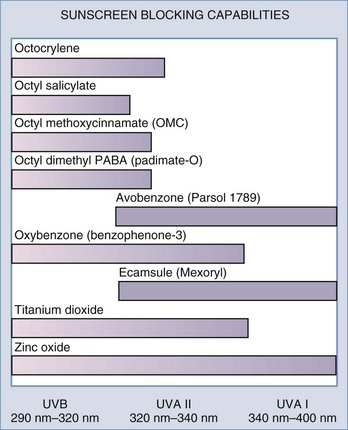
| Sunscreens | Zinc oxide, titanium dioxide, and others (Figure 24-1) |
Prevention of Photoaging
Antioxidants
UV light generates free radicals in skin which oxidize nucleic acids, proteins, and lipids, leading to the development of skin cancers and signs of photoaging.13 The skin protects itself by preventing free-radical damage with endogenous antioxidants consisting primarily of vitamin C in the aqueous compartment, and vitamin E in the lipid compartment such as cell membranes and stratum corneum. Vitamins C and E can be applied topically to enhance antioxidant protection.16,17 These two antioxidants act synergistically. Ferulic acid is another antioxidant that can improve the protective effects of vitamins E and C.17 In addition to antioxidant properties, vitamin C also directly increases collagen synthesis and clinically reduces fine lines.20 The greatest percutaneous absorption of topical vitamin C is obtained with L-ascorbic acid in acidic formulations at pH 3.5 or less, with concentrations of 10% to 20%.17
Sunscreens
Sunscreens protect the skin by reducing UV exposure. There are two main types of sunscreen ingredients each of which has a different mechanism of action. Physical sunscreens, composed of inorganic compounds, reflect and scatter UV light, whereas chemical sunscreens, composed of organic sunscreens, absorb UV light.18 To achieve broad-spectrum UVA and UVB coverage, multiple sunscreens are often combined. Figure 24-1 shows common sunscreen ingredients and their UV blocking capabilities. Certain sunscreen combinations offer greater photostability. For example, avobenzone alone has poor photostability; however, the addition of oxybenzone (HelioPlex®) renders it highly photostable. Sunscreens with broad-spectrum UVA and UVB coverage with an SPF 30 are generally recommended for daily use. Because sunscreens do not block all UV radiation, maximal protection from UV damage is provided by using an antioxidant in addition to a sunscreen.17
Treatment of Photoaging
Retinoids
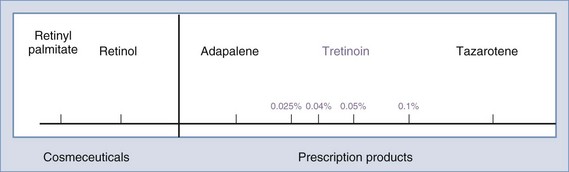
Topical retinoids are used to treat virtually all cosmetic aspects of photoaged skin, including: fine lines, dry and rough skin, hyperpigmentation, as well as acne.19,21,22 Retinoids are vitamin A derivatives, which range from potent prescription products such as tretinoin and synthetic tretinoin derivatives such as tazarotene, to less active cosmeceutical products such as retinol (Figure 24-2). Two topical retinoid products are FDA approved to treat photoaging: Renova® (tretinoin) and Avage® (tazarotene). Several other topical prescription retinoids approved to treat acne are also used off-label to treat photoaging such as Retin-A® (tretinoin), Retin-A® Micro (a less irritating formulation of tretinoin), Differin® (adapalene), and other generic versions of these.
Cosmeceutical retinoids include retinol (vitamin A), retinal (vitamin A aldehyde), and retinol storage forms (retinyl acetate and retinyl palmitate).23 They are less irritating than prescription retinoids and, when used in recommended doses, do not cause a retinoid dermatitis. However, they are much less effective than prescription retinoids in reducing the signs of photoaging.
Matrix Metalloproteinase Inhibitors
Matrix metalloproteinases (MMPs) are naturally occurring enzymes that degrade the skin’s extracellular matrix to facilitate recycling of collagen, elastin, and glycoaminoglycans.24 In young, healthy skin, production of matrix components exceeds destruction caused by MMPs. In photoaged skin, matrix degradation is accelerated because UV light upregulates MMPs. Enhanced matrix degradation combined with age-related decline in collagen synthesis, decreases the structural integrity of the skin’s matrix and contributes to the formation of wrinkles, skin laxity, and telangiectasias.25 Products that inhibit MMPs, such as zinc, calcium, idebenone, and lactobionic acid (polyhydroxy acid) reduce destruction of dermal matrix, increase dermal collagen and elastin, and clinically improve textural changes in photoaged skin.26,27
Human-Derived Growth Factors
Endogenous growth factors are regulatory proteins that initiate wound healing by mediating signaling pathways within and between cells.28 Multiple growth factors migrate to wounds and interact synergistically to promote wound healing and tissue regeneration including synthesis of new dermal matrix components. Topical application of growth factor products, such as Nouricel-MD®, which is derived from human fibroblast cultures, twice daily for 3 months has been shown to improve skin hydration and reduce roughness, hyperpigmentation, and wrinkles in photoaged skin.29,30 Growth factors are large protein molecules and their size may limit penetration through an intact epidermis. Combining them with skin resurfacing procedures that thin or disrupt the skin’s barrier, such as laser resurfacing, chemical peels, and microdermabrasion, or topical exfoliants such as hydroxy acids, may improve penetration of these active proteins.31
Peptides
Peptides are short-chain amino acids that act as protein mimics to stimulate repair processes in photoaged skin.32 Peptides, rather than their parent proteins, are used topically because these smaller compounds have greater skin penetration. Palmitoyl pentapeptide-3 (e.g., Matrixyl® by Sederma, StriVectin® by Klein Becker) is a sequence from procollagen that has been found to stimulate the production of collagens. Palmitoyl oligopeptide (by Sederma) is derived from elastin and stimulates growth of fibroblasts and angiogenesis.33
Another peptide, acetyl hexapeptide-8 (e.g., Argireline® by Lipotec), is derived from the SNAP-25 protein, which is designed to reduce the effects of acetylcholine in producing muscle contraction and purported to have “botulinum toxin–like effects.” One report suggests that 10% acetyl hexapeptide-8 used for 30 days reduces wrinkle depth by 30%.33 Additional studies are necessary to support this finding and its proposed mechanism of action. Novel peptide research is a growing area of cosmeceutical development. Significant effects with many peptides can be demonstrated in vitro, where target receptors are easily accessed, and the challenge for peptides, as with all topical products, is to demonstrate penetration through the skin barrier and good clinical efficacy in vivo, when the biologic target is less accessible.
Plant Hormones
Kinetin (N6-furfuryladenine) is a plant cytokinin that functions as a natural antioxidant and is used as an ingredient in rejuvenation skin care products.34,35 It has reported rejuvenation effects on cultured human fibroblast cells (in vitro),36 but no studies are currently available in vivo to assess for clinical efficacy.
Hydroxy Acids
Alpha hydroxy acids (AHAs) are part of almost any skin rejuvenation topical product regime, and they include glycolic, lactic, citric, and mandelic acids. AHAs stimulate epidermal cell production, exfoliation, and even out the distribution of melanin in photodamaged skin.10,37,38 AHA histologic effects include increased deposition of glycosaminoglycans, improved elastin fiber quality, and increased collagen synthesis.37–39 When formulated in more acidic preparations with higher concentrations, AHAs are commonly used as chemical peel agents. Polyhydroxy acids (such as gluconolactone) and bionic acids (such as lactobionic acid and maltobionic acid) are second-generation hydroxyacids that have AHA-like effects but are less irritating. They also function as humectant moisturizers and antioxidants.40–42
Amino Sugars
N-acetylglucosamine is a naturally occurring amino sugar compound that is a building block of hyaluronic acid (a glycosaminoglycan). Topical application stimulates synthesis of hyaluronic acid in fibroblasts and keratinocytes, increases skin thickness,43,44 and enhances exfoliation.45 Clinically, N-acetylglucosamine has been found to reduce wrinkles, increase hydration, and improve hyperpigmentation.46–48
In summary, prescription retinoid products can address many aspects of skin aging and offer significant improvements for photodamaged skin. For patients intolerant of prescription retinoids, alternative cosmeceutical therapies are available. These rejuvenation skin care regimes typically contain a combination of three to four cosmeceutical products that address fine lines and rough skin texture, improve skin brightness, and offer protection from UV damage. Box 24-1 lists topical products that can be used for a rejuvenation skin care regime (or as a preprocedure skin care regime).
Hyperpigmentation
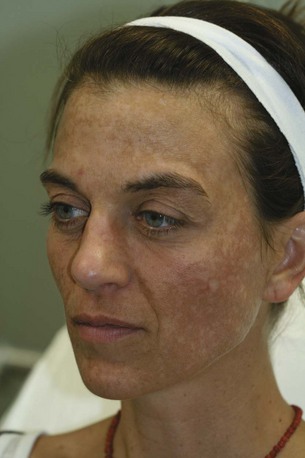
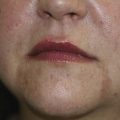
Hyperpigmentation is a common aesthetic complaint and typically presents as solar lentigines, postinflammatory hyperpigmentaion or melasma. Solar lentigines are apparent in photoaged skin as small brown macules that increase in size and number with chronic sun exposure. Postinflammatory hyperpigmentation can be seen in susceptible individuals, especially darker Fitzpatrick skin types (IV through VI), as brown discoloration commonly arising at sites of previously inflamed acne lesions or sites of wound healing. Melasma presents as hyperpigmented reticular patches and brown macules, typically on the cheekbones, upper lip, forehead, and/or chin (Figure 24-3). Melasma is frequently observed following a change in female hormonal status such as during pregnancy (chloasma) or with use of oral contraceptives.49
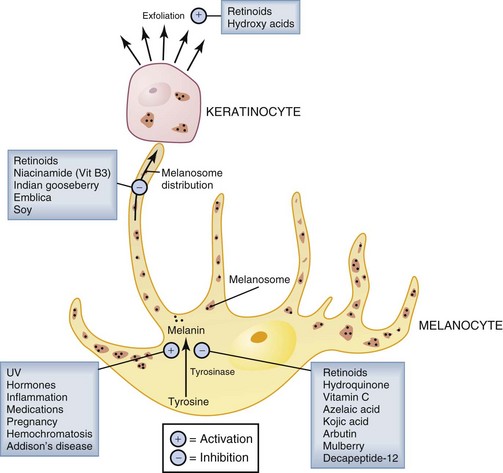
Hyperpigmentation results from increased melanin synthesis and deposition in the epidermis and dermis. An understanding of the melanin synthesis and distribution pathways is helpful in selecting treatments for hyperpigmentation. The key regulatory step in melanin synthesis is the enzymatic conversion of tyrosine to melanin by tyrosinase in melanocytes. Melanin is packaged into melanosomes in the melanocytes and then distributed to surrounding epidermal keratinocytes (Figure 24-4). One of the physiologic roles of melanin in the skin is to shield keratinocyte nuclei by absorbing harmful UV light. Upregulation of melanin synthesis is thus a physiologic protective mechanism against UV damage. Many other factors, however, can also upregulate melanin synthesis contributing to unwanted hyperpigmentation (see Figure 24-4).50
Treatment of Hyperpigmentation
Treatment of hyperpigmentation with topical products is aimed at suppressing melanin synthesis and deposition in the skin and at removing pigment that is already present. Topical products for hyperpigmentation, commonly called lightening, brightening, or bleaching agents, include prescription products and cosmeceuticals. Most topical lightening agents act by inhibiting tyrosinase, the key enzymatic step in melanin production (Figure 24-4).
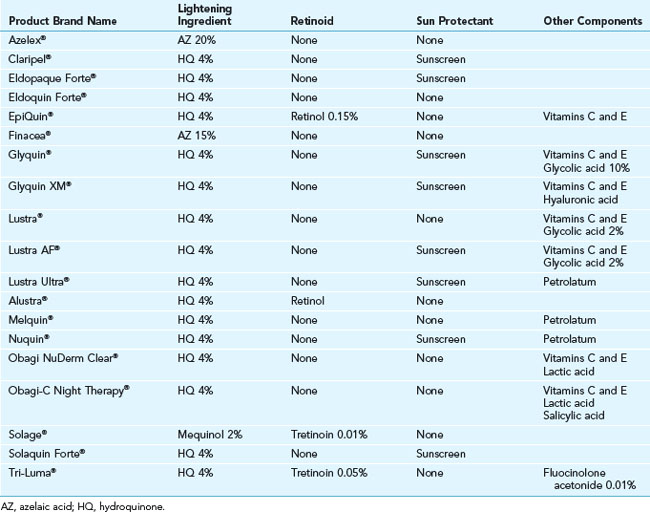
Hydroquinone, a tyrosinase inhibitor, is the most potent topical lightening agent. It is available as an OTC drug in 2% formulations and may be prescribed as a 4% cream or formulated by compounding pharmacies in strengths of 6% to 8%. Combination products containing hydroquinone and retinoids are commonly used, and some of these often have steroids added to reduce the irritation associated with their use. Tri-Luma® is one such combination product that is FDA approved for treatment of melasma. Other prescription topical products commonly used for hyperpigmentation are summarized in Table 24-3.51
Cosmeceutical skin lightening agents, such as kojic acid and arbutin, are also tyrosinase inhibitors. Although not as effective as hydroquinone, their efficacy can be improved by combining them with products that enhance penetration into the skin such as hydroxy acid exfoliants or retinoids, such as retinol. Copper metal chelators can also be used to help reduce pigment production, because copper is an essential cofactor necessary for synthesis of melanin. Common cosmeceuticals used for treatment of hyperpigmentation are summarized in Table 24-4.
TABLE 24-4 Cosmeceutical Topical Products for Hyperpigmentation16,37,52–55
|
Product |
Occurrence |
|---|---|
| Acetyl glucosamine | Chitin |
| Arbutin, methyl arbutin | Bearberry extract, certain herbs, pear trees |
| Decapeptide-12 (Lumixyl®) | Synthetic |
| Emblica | Indian gooseberry |
| Glycolic acid | Sugar cane |
| Kojic acid | Fungi |
| Licorice extract (glabridin) | Licorice root |
| Mulberry extract | Roots of the broussonetia papyrifera tree |
| Niacinamide | Amide form of vitamin B3 |
| Retinoids (retinol, retinal, retinyl acetate, retinyl palmitate) | Vitamin A |
| Soy isoflavones | Soybean |
| Vitamin C | Citrus fruits |
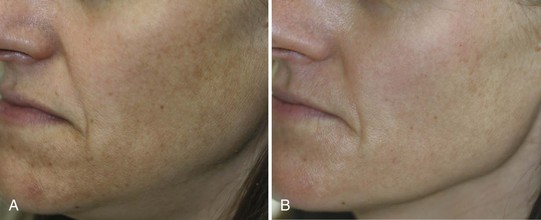
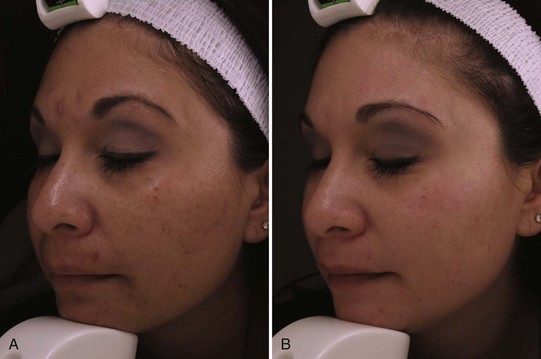
Reduction of hyperpigmentation with topical products typically requires 3 months of daily application. Figure 24-5 shows a patient with lentigines and mottled hyperpigmentation of the lower face (A) prior to treatment and (B) after 3 months with daily use of 0.05% tretinoin, 4% hydroquinone, hydroxy acid, 20% vitamin C, and broad spectrum sunscreen. Figure 24-6 shows a patient with melasma and postinflammatory hyperpigmentation (A) before and (B) after 3 months of twice daily use of four main products containing 0.5% retinol, an exfoliant (lactic acid), brightening agents (2% hydroquinone, 3% kojic acid and 5% azelaic acid, licorice extract, and arbutin), anti-inflammatory ingredients (borage and evening primrose oils), and a broad-spectrum sunscreen.
Preprocedure and Postprocedure Products
Preprocedure topical regimens often contain the same products as rejuvenation skin care regimes and usually consist of retinoids, hydroxy acids, antioxidants, sunscreens, and skin lightening agents (see Box 24-1).56 The goal for using these products is to condition the skin and create a healthier baseline epidermis and dermis. This allows for more even penetration of products, as with chemical peels, and promotes more rapid postprocedure healing. Preprocedure skin care regimens are ideally started 1 month prior to cosmetic procedures and discontinued 1 week prior to the procedure, to ensure a fully intact epidermis at the time of treatment. In persons prone to hyperpigmentation, a topical lightening agent is also used, such as hydroquinone 4% to 8%, which may reduce the risk of postinflammatory hyperpigmentation postprocedure.
Selection of postprocedure products depends on the type of cosmetic procedure performed. Ablative procedures, which disrupt the epidermis, require use of an occlusive ointment moisturizer without active ingredients immediately postprocedure. Common occlusive postprocedure products are shown in Table 24-5. This product is used for approximately 4 to 7 days postprocedure, until re-epithelialization occurs. There is some evidence that certain nonocclusive products, such as Biafine® (see Table 24-6), may be used as alternatives to occlusive topical products during this phase of wound healing.57 Because sunscreen is not used during this time, sun avoidance is critical.
|
Product Brand Name (Manufacturer) |
Hydrating Ingredients |
Other Ingredients |
|---|---|---|
| Aquaphor® (Beiersdorf) | Mineral oil Lanolin |
Panthenol (vitamin B5) Bisoprolol (chamomile) |
| Catrix 10® (Lescarden) | Petrolatum Beeswax Paraffin |
10% Bovine mucopolysaccharide cartilage |
| Primacy® (SkinCeuticals) | Petrolatum Squalene Oat kernel oil Rose oil |
Bisoprolol (chamomile) Aloe Vitamin E |
| Protective Recovery Balm® (BiO2 Cosmeceuticals) | Mineral oil Petrolatum Paraffin Dimethicone |
|
| Puralube® (Nycomede) | Light mineral oil White petrolatum |
TABLE 24-6 Nonocclusive Postprocedure Products57,59,60
|
Product Brand Name (manufacturer) |
Reparative Ingredients (intended effect) |
|---|---|
| Epidermal Repair® (SkinCeuticals) | Beta glucan Centella asiatica (collagen synthesis) |
| TNS Ceramide Treatment Cream® (SkinMedica) | Hydroxypropyl bispalmitamide MEA (ceramide for barrier enhancement) NouriCel-MD® (growth factors) Palmitoyl oligopeptide and palmitoyl tetrapeptide-7 (matrix synthesis) |
| Biafine® (Ortho-McNeil) | Trolamine and sodium alginate (wound healing) |
Once the skin has fully re-epithelialized, postprocedure occlusive products may be discontinued and products that are soothing and reparative used. Some common non-occlusive reparative products are listed in Table 24-6. A broad-spectrum sunscreen (SPF 30 or greater) containing zinc and/or titanium is used daily. At 1 month postprocedure, when skin is no longer sensitive, patients’ preprocedural rejuvenation products may be resumed. If postinflammatory hyperpigmentation is a concern or if the procedure is targeting pigment reduction, skin-lightening products are also resumed.
1. Small R. Aesthetic procedures introduction. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams and Wilkins; 2009:195-199.
2. Small R. Aesthetic procedures in office practice. Am Fam Physician. 2009;80(11):1231-1237.
3. Proksch E, Folster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43(3):156-169.
4. Del Rosso JQ. Moisturizers: function, formulation and clinical applications. In: Draelos Z, Dover JS, Alam M, editors. Procedures in Cosmetic Dermatology: Cosmeceuticals. Philadelphia: Saunders/Elsevier; 2009:97-101.
5. Loden M. The clinical benefit of moisturizers. J Eur Dermatol Venereol. 2005;19(6):672-688.
6. Draelos ZD. Therapeutic moisturizers. Dermatol Clin. 2000;18(4):597-607.
7. Flynn TC, Petros J, Clark RE, et al. Dry skin and moisturizers. Clin Dermatol. 2001;19(4):387-392.
8. Del Rosso JQ. Moisturizers: function, formulation and clinical applications. In: Draelos Z, Dover JS, Alam M, editors. Procedures in Cosmetic Dermatology: Cosmeceuticals. Philadelphia: Saunders/Elsevier; 2009:97-101.
9. Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079-1081.
10. Van Scott EJ, Yu RJ. Hyperkeratinization, corneocyte cohesion and alpha hydroxy acids. J Am Acad Dermatol. 1984;11:867-879.
11. Rawlings AV, Davies A, Carlomusto M. Effect of lactic acid isomers on keratinocyte ceramide synthesis, stratum corneum lipid levels and stratum corneum barrier function. Arch Dermatol. 1996;288:383-390.
12. Draelos Z. Dry skin. In: Draelos Z, Dover JS, Alam M, editors. Procedures in Cosmetic Dermatology: Cosmeceuticals. Philadelphia: Saunders/Elsevier; 2009:174.
13. Haywood R, Wardman P, Saunders R, et al. Sunscreens inadequately protect against ultraviolet-A-induced free radicals in skin: implications for skin aging and melanoma? J Invest Dermatol. 2003;121:862-868.
14. Lowe NJ, Meyers DP, Wieder JM. Low doses of repetitive ultraviolet A induce morphologic changes in human skin. J Invest Dermatol. 1995;105:739-743.
15. Draelos Z. The latest cosmeceutical approaches for anti-aging. J Cosm Derm. 2007;6:2-6.
16. Placzek M, Gaube S, Kerkmann U, et al. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-α-tocopherol. J Invest Dermatol. 2005;124:304-307.
17. Lin FH, Lin JY, Gupta RD, et al. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005;125:826-832.
18. Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
19. Kligman A, Grove GL, Hirose E, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15:836-859.
20. Farris PK. Cosmeceutical vitamins: vitamin C. In: Draelos Z, Dover JS, Alam M, editors. Procedures in Cosmetic Dermatology: Cosmeceuticals. Philadelphia: Saunders/Elsevier; 2009:51-56.
21. Singh M, Griffiths CE. The use of retinoids in the treatment of photoaging. Dermatol Ther. 2006;19(5):297-305.
22. Del Rosso JQ. Topical retinoids in the management of acne: the best path to clear results. Cutis. 2004;74(4 Suppl):2-3.
23. Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol. 2007;143(5):606-612.
24. Ratzinger G, Stoitzner P, Ebner S, et al. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol. 2002;168:4361-4371.
25. Thibodeau A. Metalloproteinase inhibitors. Cosmet Toil. 2000;115(11):75-76.
26. Ryan ME, Ramamurthy S, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. J Current Opin Periodont. 1996;3(1):85-96.
27. Upadhya GA, Strasberg SM. Glutathione, lactobionate, and histidine: cryptic inhibitors of matrix metalloproteinases contained in the University of Wisconsin and histidine/tryptophan/ketoglutarate liver preservation solutions. Hepatology. 2000;31(5):1115-1122.
28. Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003;5(1):25-34.
29. Fitzpatrick RE. Endogenous growth factors as cosmeceuticals. Derm Surg. 2005;31:827-831.
30. Mehta RC, Smith SR, Grove GL, et al. Reduction in facial photodamage by a topical growth factor product. J Drugs Dermatol. 2008;7(9):864-871.
31. Small R. Microdermabrasion. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams and Wilkins; 2009:265-277.
32. Lupo MP. Cosmeceutical peptides. Derm Surg. 2005;31:832-836.
33. Fields K, Falla TJ, Rodan K, et al. Bioactive peptides: signaling the future. J Cosm Derm. 2009;8:8-13.
34. Rattan SI. N6-furfuryladenine (Kinetin) as a potential anti-aging molecule. J Anti-Aging Medicine. 2002;5(1):113-116.
35. Rattan SI, Sodagam L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res. 2005;8(1):46-57.
36. Minorsky PV. Kinetin: the elixir of life? Plant Physiol. 2003;132(3):1135-1136.
37. Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha hydroxyacids on photoaged skin: a pilot clinical, histological and ultrastructural study. J Am Acad Dermatol. 1996;34:187-195.
38. Bernstein EF, Underhill CB, Lakkakorpi J, et al. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun-damaged skin. Derm Surg. 1997;123(8):689-694.
39. Bernstein EF, Lee J, Brown DB, et al. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Derm Surg. 2001;27(5):429-433.
40. Berardesca E, Distante F, Vignoli GP, et al. Alpha hydroxyacids modulate stratum corneum barrier function. Br J Dermatol. 1997;137(6):934-938.
41. Bernstein EF, Green BA, Edison BL, et al. Poly hydroxy acids (PHAs): clinical uses for the next generation of hydroxy acids. Skin and Aging. 2001;9(Suppl):4-11.
42. Green BA, Briden ME. PHAs and bionic acids: next generation hydroxy acids. In: Draelos Z, Dover JS, Alam M, editors. Procedures in Cosmetic Dermatology: Cosmeceuticals. Philadelphia: Saunders/Elsevier; 2009:209-215.
43. Sayo T, Sakai S, Inoue S. Synergistic effect of N-acetylglucosamine and retinoids on hyaluronan production in human keratinocytes. Skin Pharmacol Physiol. 2004;17:77-83.
44. Breborowicz A, Kuzlan-Pawlaczyk M, Wieczorowska-Tobis K, et al. The effect of N-acetylglucosamine as a substrate for in vitro synthesis of glycosaminoglycans by human peritoneal mesothelial cells and fibroblasts. Adv Perit Dial. 1998;14:31-35.
45. Mammone T, Gan D, Fthenakis C, et al. The effect of N-acetyl-glucosamine on stratum corneum desquamation and water content in human skin. J Cosmet Sci. 2009;60:423-428.
46. Briden ME. Alpha-hydroxyacid chemical peeling agents: case studies and rationale for safe and effective use. Cutis. 2004;73(2 Suppl):18-24.
47. Bissett DL, Robinson LR, Raleigh PS, et al. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J Cosm Derm. 2007;6:20-26.
48. Bissett DL. Glucosamine: An ingredient with skin and other benefits. J Cosm Derm. 2006;5:309-315.
49. Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282(38):27557-27561.
50. Pugliese PT. Physiology of the skin: pigmentation revisited. Skin Inc. 2009;21(3):68-76.
51. Rendon MI, Gaviria JI. Review of skin-lightening agents. Derm Surg. 2005;31:886-889.
52. Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462-1470.
53. Rabe JH, Mamelak AJ, McElgunn PJS, et al. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55:1-19.
54. Zhai H, Cordoba-Diaz M, Wa C, et al. Determination of the antioxidant capacity of an antioxidant complex and idebenone: an in vitro rapid and sensitive method. J Cosm Derm. 2008;7:96-100.
55. Thornfeldt CR. Cosmeceutical botanicals: part 2. In: Draelos Z, Dover JS, Alam M, editors. Procedures in Cosmetic Dermatology: Cosmeceuticals. Philadelphia: Saunders/Elsevier; 2009:77-85.
56. Weinstein GD, Nigra TP, Pochi PE, et al. Topical tretinoin for treatment of photodamaged skin: a multicenter study. Arch Dermatol. 1991;127:659-665.
57. Rendon MI, Cardona L, Benitez A. The safety and efficacy of trolamine/sodium alginate topical emulsion in postlaser resurfacing wounds. J Drugs Dermatol. 2008;7(5):S23-S28.
58. Tanzi EL, Perez M. The effect of a mucopolysaccharide-cartilage complex healing ointment on Er:YAG laser resurfaced facial skin. Dermatol Surg. 2002;28:305-308.
59. Maquart FX, Chastang F, Simeon A, et al. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol. 1999;9(4):289-296.
60. Atkin DH, Trookman NS, Rizer RL, et al. Combination of physiologically balanced growth factors with antioxidants for reversal of facial photodamage. J Cosmet Laser Ther. 2010;12(1):14-20.
Small R, Linder J. Skin care products. In: Small R, Linder J, editors. A Practical Guide to Skin Care Procedures and Products. Philadelphia: Lippincott Williams and Wilkins, 2011.
Smeltzer W. Cosmeceutical skin care. In Pfenninger JL, Fowler GC, editors: Pfenninger and Fowler’s Procedures for Primary Care, 3rd ed, Philadelphia: Mosby/Elsevier, 2011.

 tsp for the face and 1 oz for the body of cream sunscreens.
tsp for the face and 1 oz for the body of cream sunscreens.