Chapter 2 Skin Care Including Chemical Peeling
Summary
Introduction
The skin is composed of three layers: the stratum corneum, epidermis, and dermis.
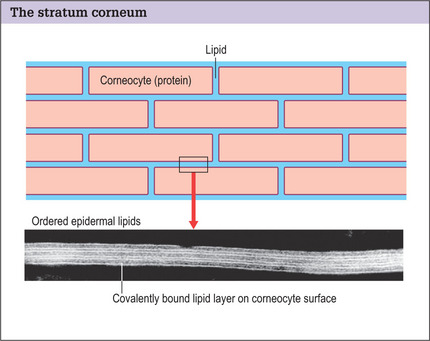
The stratum corneum forms the skin barrier and is composed of two distinct anatomic units:
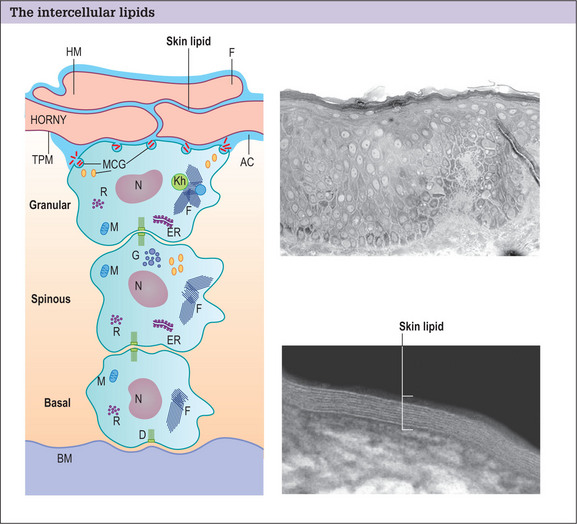
Structural damage to the corneocytes or to the lipids results in a defective barrier. With the aid of an electron microscope, it is possible to see the covalently bound lipid layer between the corneocytes forming an organized watertight seal over the body (Fig. 2.2).
Indications
Skin peeling is a controlled removal and renewal of the various layers of the skin, depending on the depth of the wound:
A chemical peel can be performed for a variety of indications:
Operative Approach
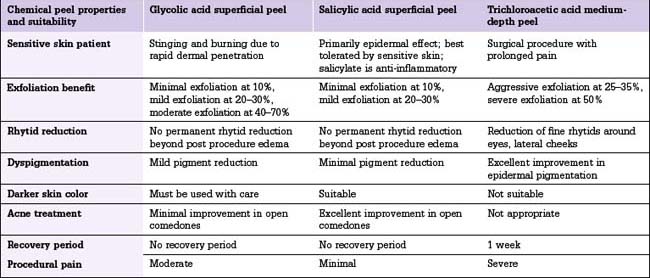
The peeling procedure for superficial, medium, and deep peels is somewhat similar, each deeper peel building on a more superficial skin wounding (Table 2.1).
Superficial peels
Glycolic acid peels
Glycolic acid peels in concentrations of 10% or less may be applied by unsupervised aestheticians in a spa setting, but concentrations of 20% or higher should be applied by a physician or in a carefully supervised setting.
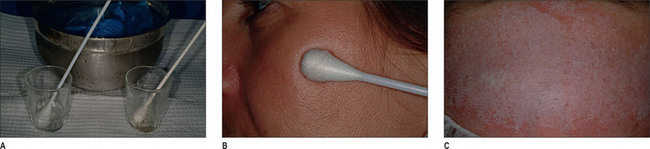
Peeling procedure
Superficial peel
Medium-depth peel
Deep chemical peels
Optimizing a medium-depth peel result
Complications and Side Effects
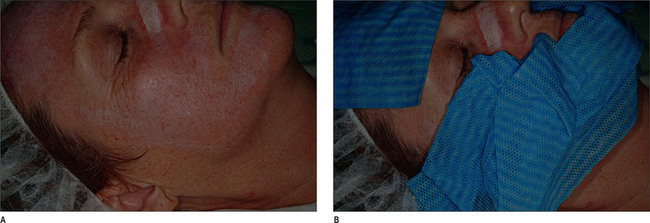
Chemical peeling is a relatively safe procedure, but a few complications should be avoided. Proper patient selection to avoid using the procedure in dark complected patients and those with healing disorders is key to avoiding complications of scarring, hyperpigmentation, and hypopigmentation. In addition, patients who have a history of frequent herpes simplex infections of the peeled body area should be treated with preventive acyclovir, famciclovir, or valacyclovir on the day of the procedure to prevent dissemination of the herpes simplex virus in the wounded area.
Postoperative Care
Skin care
Postpeel skin care is important and includes the use of cleansers and moisturizers.
Cleansers
There are a variety of skin cleansers,1–3 including soaps, syndets, and combars (Table 2.2), which can be placed on a variety of cleansing implements from the hands to a washcloth to a disposable face cloth.
| Type of cleanser (commercial examples) | Advantages | Disadvantages |
|---|---|---|
| Soap | Excellent cleansing thorough sebum removal | Can dry skin; not recommended for sensitive, healing or diseased skin |
| Syndet (synthetic detergent) Combar |
More mild cleansing; recommended post-surgery; may be used for sensitive or diseased skin | Not as thorough sebum removal |
| Good cleansing; commonly combined with triclosan topical antibacterial; good choice for high-risk wound infections or contaminated body areas |
The need for good hygiene in a compromised barrier situation has led to the development of synthetic detergents, known as syndets (Fig. 2.5). The most popular syndet cleansers contain sodium cocoyl isethionate with a neutral pH of 5.5–7. This more neutral pH removes fewer intercellular lipids, preventing further barrier damage during cleansing. These products, particularly in the form of a foaming face wash, are the best post-procedure cleansers.
Moisturizers
Moisturizers (Fig. 2.6)4–7 are applied to the skin following cleansing in the post-surgical patient to minimize transepidermal water loss, so creating an environment that is optimal for skin healing.
Sunscreens
Sunscreens8–19 are an important part of post-surgical skin care. Inflammation resulting from a face peel or other skin wounding procedure can cause hyperpigmentation, especially in the presence of UVA radiation, which stimulates melanin production by melanocytes.
Photoprotective mechanisms may be endogenous (Table 2.3) or externally applied (Table 2.4).
| Cutaneous structure | Sun protective mechanism |
|---|---|
| Compact horny layer | Absorbs and scatters UV |
| Keratinocyte melanin |
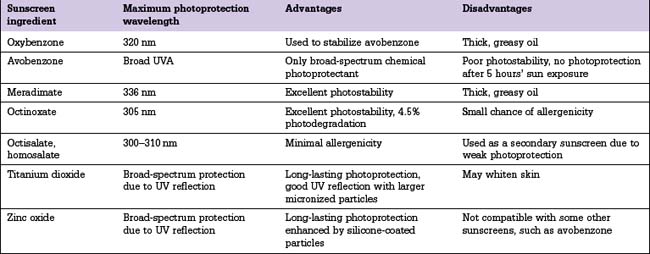
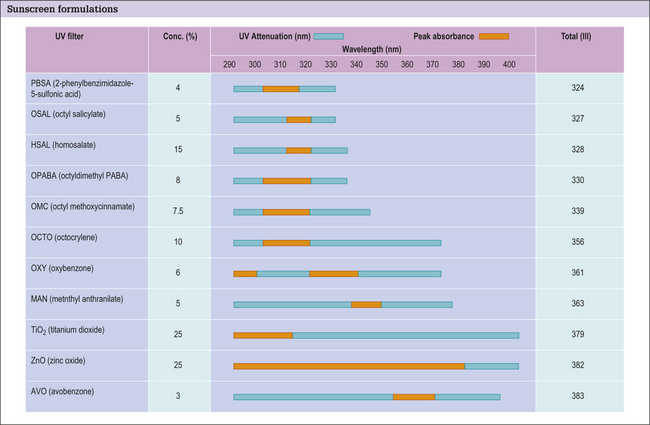
Sunscreen ingredients can be classified into two major categories.
Sunscreen ingredients can be divided into the following three groups:
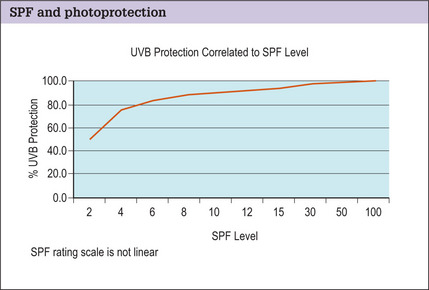
Most modern sunscreen formulations are a blend of two to three different substances carefully selected to compliment one another and enhance product performance (Fig. 2.7). However, raising the SPF above 30 only confers an incremental increase in photoprotection (Fig. 2.8).
UVA sunscreen ingredients
Oxybenzone may also be combined with menthyl anthranilate (also known as meradimate) to extend UVA photoprotection with a peak absorption at 336 nm. Meradimate is commonly used as a secondary UVA photoprotectant because it is a sticky oil and can decrease sunscreen aesthetics.
Physical UVA/UVB absorbers
The physical UVA/UVB absorbers are titanium dioxide and zinc oxide.
1. Willcox M.J., Crichton W.P. The soap market. Cosmet Toilet. 1989;104:61-63.
2. Wortzman M.S. Evaluation of mild skin cleansers. Dermatol Clin. 1991;9:35-44.
3. Wortzman M.S., Scott R.A., Wong P.S., et al. Soap and detergent bar rinsability. J Soc Cosmet Chem. 1986;37:89-97.
4. Draelos Z.D. Therapeutic moisturizers. Dermatol Clin. 2000;18:597-607.
5. Flynn T.C., Petros J., Clark R.E., et al. Dry skin and moisturizers. Clin Dermatol. 2001;19:387-392.
6. Rawlings A.V., Harding C.R., Watkinson A., et al. Dry and xerotic skin conditions. In: Leyden J.J., Rawlings A.V., editors. Skin moisturization. New York: Marcel Dekker; 2002:119-144.
7. Draelos Z.D. Moisturizers. In: Draelos Z.D., editor. Cosmetics in dermatology. 2nd edn. New York: Churchill-Livingstone; 1995:83-95.
8. Ichihashi M., Ueda M., Budiyanto A., et al. UV-induced skin damage. Toxicology. 2003;189:21-39.
9. Young A.R. Cumulative effects of ultraviolet radiation on the skin: cancer and photoaging. Semin Dermatol. 1990;9:25-31.
10. Gilchrest B.A. A review of skin ageing and its medical therapy. Br J Dermatol. 1996;135:867-875.
11. Matsumura Y., Ananthaswamy H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298-308.
12. Wulf H.C., Sandby-Møller J., Kobayasi T., et al. Skin aging and natural photoprotection. Micron. 2004;35:185-191.
13. Kawada A., Noda T., Hiruma M., et al. The relationship of sun protection factor to minimal erythema dose, Japanese skin type, and skin color. J Dermatol. 1993;20:514-516.
14. Roelandts R., Sohrabvand N., Garmyn M. Evaluating the UVA protection of sunscreens. J Am Acad Dermatol. 1989;21:56-62.
15. Diffey B.L. A method for broad spectrum classification of sunscreens. Int J Cosmet Sci. 1994;16:47-52.
16. Nash J.F. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
17. Gasparro F.P., Mitchnick M., Nash J.F. A review of sunscreen safety and efficacy. Photochem Photobiol. 1998;68:243-256.
18. Moloney F.J., Collins S., Murphy G.M. Sunscreens: safety, efficacy and appropriate use. Am J Clin Dermatol. 2002;3:185-191.
19. Tanner P.R. Sunscreen product formulation. Dermatol Clin. 2006;24:53-62.