CHAPTER 7 Skin and its appendages
In this chapter, the types and functions of skin in different parts of the body are described first, followed by the microstructure of the epidermis and dermis, and the appendages of skin including the pilosebaceous units and the sweat glands and nails. The development of skin, natural skin lines and age-related changes, and clinical aspects of skin, e.g. grafts, surgical skin flaps and wound healing, are also described. The integumental system includes the skin and its derivatives, hairs, nails, sweat and sebaceous glands; subcutaneous fat and deep fascia; the mucocutaneous junctions around the openings of the body orifices; and the breasts. Mucocutaneous junctions and breast tissues are covered in the appropriate regional sections.
TYPES AND FUNCTIONS OF SKIN
CLASSIFICATION OF SKIN
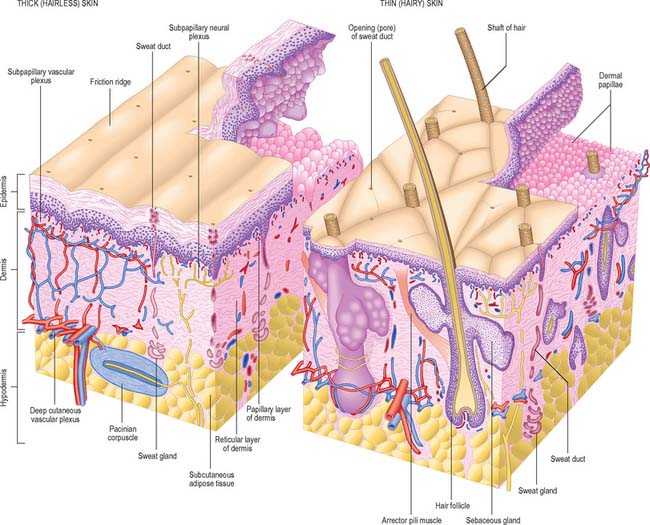
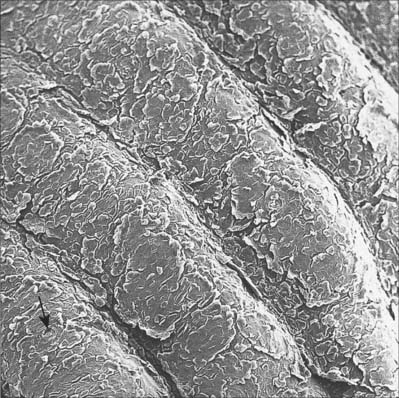
Although skin in different parts of the body is fundamentally of similar structure, there are many local variations in parameters such as thickness, mechanical strength, softness, flexibility, degree of keratinization (cornification), sizes and numbers of hairs, frequency and types of glands, pigmentation, vascularity, innervation. Two major classes of skin are distinguished: they cover large areas of the body and show important differences of detailed structure and functional properties. These are thin, hairy (hirsute) skin, which covers the greater part of the body, and thick, hairless (glabrous) skin, which forms the surfaces of the palms of the hands, soles of the feet, and flexor surfaces of the digits (Fig. 7.1, Fig. 7.2, see Fig. 7.4).
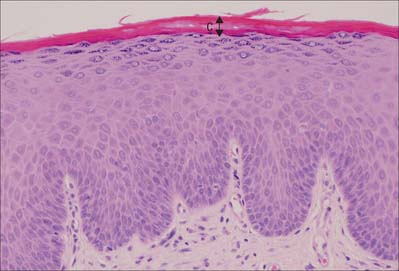
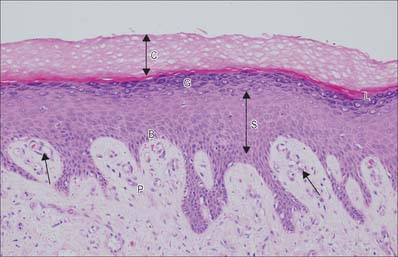
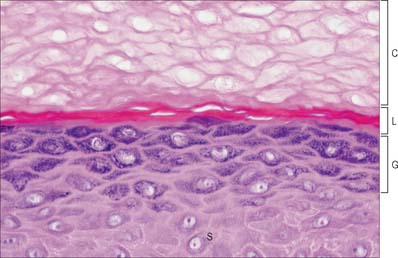
Fig. 7.2 The interfollicular epidermis of human thin skin. Note the thin cornified layer (C) in comparison with Fig. 7.4 (which is also at a lower magnification).
MICROSTRUCTURE OF SKIN AND SKIN APPENDAGES
EPIDERMIS
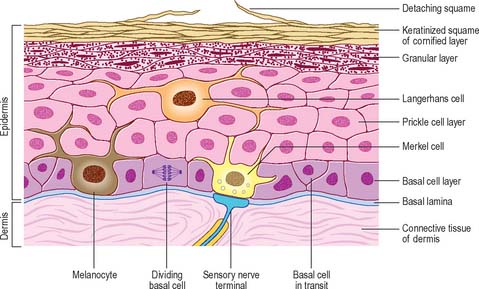
The epidermis (Fig. 7.2, Fig. 7.3) is a compound tissue consisting mainly of a continuously self-renewing, keratinized, stratified squamous epithelium: the principal cells are called keratinocytes. Nonkeratinocytes within the mature epidermis include melanocytes (pigment-forming cells from the embryonic neural crest), Langerhans cells (immature antigen-presenting dendritic cells derived from bone marrow), and lymphocytes. Merkel cells, which may function as sensory mechanoreceptors or possibly as part of the dispersed neuroendocrine system, are associated with nerve endings. Free sensory nerve endings are sparsely present within the epidermis. In routine histological preparations, the non-keratinocytes and Merkel cells are almost indistinguishable, and appear as clear cells surrounded by a clear space produced by shrinkage during processing. Their cytoplasm lacks prominent filament bundles.
The epidermis can be divided into a number of layers from deep to superficial as follows: basal layer (stratum basale), spinous or prickle cell layer (stratum spinosum), granular layer (stratum granulosum), clear layer (stratum lucidum) and cornified layer (stratum corneum) (Fig. 7.4). The first three of these layers are metabolically active compartments through which cells pass and change their form as they progressively differentiate. The more superficial layers of cells undergo terminal keratinization, or cornification, which involves not only structural changes in keratinocytes, but also alterations in their relationships with each other and with non-keratinocytes, and molecular changes within the intercellular space.
Keratinocytes
Basal layer
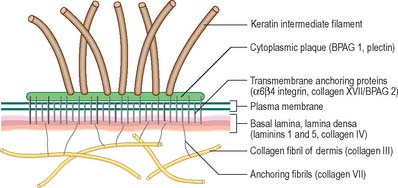
The basal or deepest layer of cells, adjacent to the dermis, is the layer where cell proliferation in the epidermis takes place. This layer contacts a basal lamina (Fig. 7.5, see Fig. 2.7), which is a thin layer of specialized extracellular matrix, not usually visible by light microscopy. By routine electron microscopy the basal lamina appears as a clear lamina lucida (adjacent to the basal cell plasma membrane) and a darker lamina densa. The basal plasma membrane of the basal keratinocytes, together with the extracellular basal lamina (lamina lucida and lamina densa) and anchoring fibrils within the subjacent dermal matrix (the lamina fibroreticularis), which insert into the lamina densa and loop around bundles of collagen, collectively form the basement membrane zone (BMZ) which constitutes the dermo-epidermal junction. This is a highly convoluted interface, particularly in thick, hairless skin, where dermal papillae (rete ridges) project superficially into the epidermal region, interlocking with adjacent downward projections of the epidermis (rete pegs) (Fig. 7.4).
The majority of basal layer cells (Fig. 7.3) are columnar to cuboidal in shape, with large (relative to their cytoplasmic volume) mainly euchromatic nuclei and prominent nucleoli. The cytoplasm contains variable numbers of melanosomes and, characteristically, keratin filament bundles corresponding to the tonofilaments of classic electron microscopy. In the basal keratinocytes these keratins are mostly K5 and K14 proteins. The plasma membranes of apposed cells are connected by desmosomes, and the basal plasma membrane is linked to the basal lamina at intervals by hemidesmosomes (Fig. 7.5, see Fig. 1.5). Melanocytes (see Fig. 7.9), occasional Langerhans cells (see Fig. 7.3) and Merkel cells (see Fig. 3.30) are interspersed among the basal keratinocytes. Merkel cells are connected to keratinocytes by desmosomes, but melanocytes and Langerhans cells lack these specialized contacts. Intraepithelial lymphocytes are present in small numbers.
At any one time the basal layer of the epidermis contains keratinocytes with different fates. These include multipotent stem cells. On division these may self-renew or produce a daughter cell which is committed to differentiate after undergoing further transit amplifying cell divisions. The activity of stem cells and transit amplifying cells in the basal layer provides a continuous supply of differentiating cells which enter the prickle cell layer. The great majority of these cells are postmitotic, although some cell division may occur in the more basal regions of the prickle cell layer. Stem cells are thought to reside mainly in the troughs of rete pegs, and in the outer root sheath bulge of the hair follicle, but they cannot easily be distinguished morphologically. The distribution of stem cells and the size of their proliferative units (see below) may be quite variable in human skin (Ghazizadeh & Taichman 2005).
The organization of the basal layer and overlying progeny cells is thought to form a series of columns. Several layers of prickle and granular cells overlie a cluster of six to eight basal cells, forming a columnar proliferative unit. Each group of basal cells consists of a central stem cell with an encircling ring of transit amplifying proliferative cells and postmitotic maturing cells. From the periphery of this unit, postmitotic cells transfer into the prickle cell layer. The normal total epidermal turnover time is between 52 and 75 days. In some pathologies of skin, turnover rates and transit times can be exceedingly rapid, e.g. in psoriasis, total epidermal turnover time may be as little as 8 days. The control of keratinocyte proliferation and differentiation is beyond the scope of this publication but is reviewed in Niemann & Watt (2002) and Byrne et al (2003).
Prickle cell layer
The prickle cell layer (Fig. 7.3, Fig. 7.6) consists of several layers of closely packed keratinocytes that interdigitate with each other by means of numerous cell surface projections. The cells are anchored to each other by desmosomes that provide tensile strength and cohesion to the layer. These suprabasal cells are committed to terminal differentiation and gradually move upwards towards the cornified layer as more cells are produced in the basal layer. When skin is processed for routine light microscopy, the cells tend to shrink away from each other except where they are joined by desmosomes, which gives them their characteristic spiny appearance. Prickle cell cytoplasm contains prominent bundles of keratin filaments, (mostly K1 and K10 keratin proteins) arranged concentrically around a euchromatic nucleus, and attached to the dense plaques of desmosomes. The cytoplasm also contains melanosomes, either singly or aggregated within membrane-bound organelles (compound melanosomes). Langerhans cells (see Fig. 7.11) and the occasional associated lymphocyte are the only non-keratinocytes present in the prickle cell layer.
Granular layer
Extensive changes in keratinocyte structure occur in the three to four layers of flattened cells in the granular layer. The nuclei become pyknotic and begin to disintegrate; organelles such as ribosomes and membrane-bound mitochondria and Golgi bodies degenerate; and keratin filament bundles become more compact and associated with irregular, densely staining keratohyalin granules (Fig. 7.6). Small round granules (100 × 300 nm) with a lamellar internal structure (lamellar granules, Odland bodies, keratinosomes) also appear in the cytoplasm. Keratohyalin granules contain a histidine-rich, sulphur-poor protein (profilaggrin) which, when the cell reaches the cornified layer, becomes modified to filaggrin. The lamellar granules are concentrated deep to the plasma membrane, with which they fuse, releasing their hydrophobic glycophospholipid contents into the intercellular space within the layer and also between it and the cornified layer. They form an important component of the permeability barrier of the epidermis, rendering it relatively waterproof.
Clear layer
The clear layer is only found in thick palmar or plantar skin. It represents a poorly understood stage in keratinocyte differentiation. It stains more strongly than the cornified layer with acidic dyes (Fig. 7.6), is more refractile optically, and often contains nuclear debris. Ultrastructurally, its cells contain compacted keratin filaments and resemble the incompletely keratinized cells which are occasionally seen in the innermost part of the cornified layer of thin skin.
Cornified layer
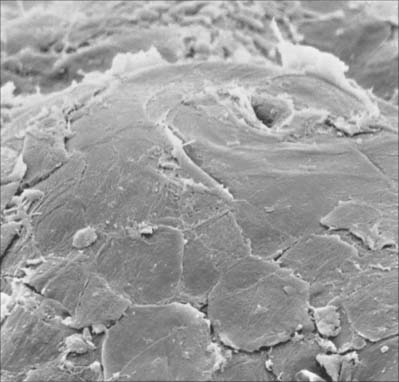
The cornified layer (Fig. 7.3, Fig. 7.6) is the final product of epidermal differentiation, or cornification. It consists of closely packed layers of flattened polyhedral squames (Fig. 7.7), ranging in surface area from 800 to 1100 μm2. These cells overlap at their lateral margins and interlock with cells of apposed layers by ridges, grooves and microvilli. In thin skin this layer may be only a few cells deep, but in thick skin it may be more than 50 cells deep. The plasma membrane of the squame appears thicker than that of other keratinocytes, partly due to the cross-linking of a soluble precursor, involucrin, at the cytoplasmic face of the plasma membrane, in the complex insoluble cornified envelope. The outer surface is also covered by a monolayer of bound lipid. The intercellular region contains extensive lamellar sheets of glycolipid derived from the lamellar granules of the granular layer. The cells lack a nucleus and membranous organelles, and consist solely of a dense array of keratin filaments embedded in a cytoplasmic matrix which is partly composed of filaggrin derived from keratohyalin granules.
Keratins
Keratins are the intermediate filament proteins found in all epithelial cells. There are two types, type I (acidic) and type II (neutral/basic); they form heteropolymers, are coexpressed in specific pairs and are assembled into 10 nm intermediate filaments. Fifty-four different keratin genes have been recognized and their protein products are numbered. The nomenclature for human keratins and keratin genes has recently been revised and is given in Schweizer et al (2006). Different keratin pairs are expressed according to epithelial cell differentiation; antibodies to individual keratins are useful analytical tools (Fig. 7.8). Keratins K5 and K14 are expressed by basal keratinocytes. New keratins, K1 and K10, are synthesized suprabasally. In the granular layer the filaments become associated with keratohyalin granules containing profilaggrin, a histidine-rich phosphorylated protein. As the cells pass into the cornified layer, profilaggrin is cleaved by phosphatases into filaggrin which causes aggregation of the filaments and forms the matrix in which they are embedded. Other types of keratin expression occur elsewhere, particularly in hair and nails, where highly specialized hard, or trichocyte, keratin is expressed. This becomes chemically modified and is much tougher than in the general epidermis. For a recent review of keratin function see Gu & Coulombe 2007.
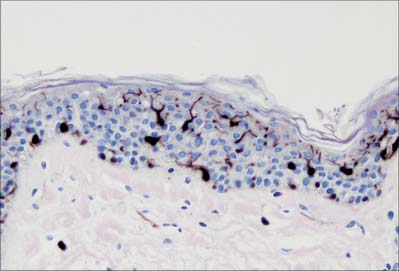
Melanocytes
Melanocytes are melanin pigment-forming cells derived from the neural crest (Fig. 7.9, Fig. 7.10). They are present in the epidermis and its appendages, in oral epithelium, some mucous membranes, the uveal tract (choroid coat) of the eyeball, parts of the middle and internal ear and in the pial and arachnoid meninges at the base of the brain. The cells of the retinal pigment epithelium, developed from the outer wall of the optic cup, also produce melanin, and neurones in different locations within the brainstem (e.g. the locus coeruleus and substantia nigra) synthesize a variety of melanin called neuromelanin. True melanins are high molecular weight heteropolymers attached to structural protein. In humans there are two classes, the brown-black eumelanin, and the red-yellow phaeomelanin, both derived from the substrate tyrosine. Most natural melanins are mixtures of eumelanin and phaeomelanin, and phaeomelanic pigments, trichochromes, occur in red hair.
In albinism, the tyrosinase required for melanin synthesis is either absent or inactive, and melanocytes, though present, are relatively quiescent cells in an otherwise normal epidermis. Melanocytes decrease significantly in numbers in old age, and are absent from grey-white hair. For further reading on melanocyte function in health and disease, see Goding (2007).
Langerhans cells
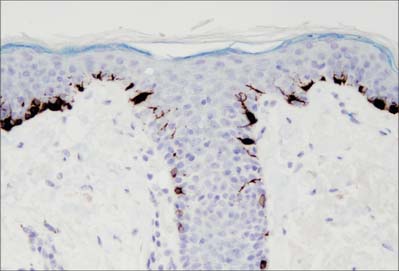
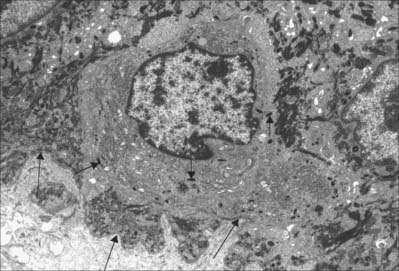
Langerhans cells (Fig. 7.11) are immature dendritic antigen-presenting cells (see p. 79) regularly distributed throughout the basal and prickle cell layers of the epidermis and its appendages, apart from the sweat gland. They are also present in other stratified squamous epithelia, including the buccal, tonsillar and oesophageal epithelia, as well as the cervical and vaginal mucosae and the transitional epithelium of the bladder. They are found in the conjunctiva, but not in the cornea. In routine preparations they appear as clear cells, relatively high in the stratified layer. They enter the epidermis from the bone marrow during development to establish the postnatal population (460–1000/mm2, 2–3% of all epidermal cells, with regional variations), and this is maintained by continual replacement from the marrow.
The nucleus is euchromatic and markedly indented and the cytoplasm contains a well-developed Golgi complex, lysosomes (which often contain ingested melanosomes), and a characteristic organelle, the Birbeck granule. The latter are discoid, cup-shaped, or have a distended vesicle resembling the head of a tennis racket; in section they often appear as a cross-striated rod 0.5 μm long and 30 nm wide. When stimulated by antigen, Langerhans cells migrate out of the epidermis to lymphoid tissues (see Fig. 4.14). Their numbers are increased in chronic skin inflammatory disorders, particularly of an immune aetiology, such as some forms of dermatitis.
DERMIS
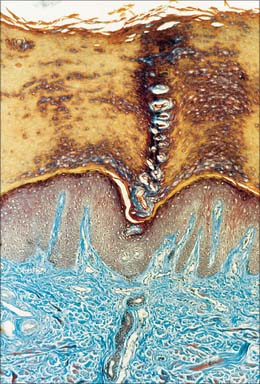
The dermis (Fig. 7.1, Fig. 7.4, see Fig. 7.17) is an irregular, moderately dense connective tissue. It has a matrix composed of an interwoven collagenous and elastic network in an amorphous ground substance of glycosaminoglycans, glycoproteins and bound water, which accommodates nerves, blood vessels, lymphatics, epidermal appendages and a changing population of cells. Mechanically, the dermis provides considerable strength to the skin by virtue of the number and arrangement of its collagen fibres (which give it tensile strength), and its elastic fibres (which give it elastic recoil). The density of its fibre meshwork, and therefore its physical properties, varies within an area, in different parts of the body, and with age and sex. The dermis is vital for the survival of the epidermis, and important morphogenetic signals are exchanged at the interface between the two both during development and postnatally. The dermis can be divided into two zones, a narrow superficial papillary layer and a deeper reticular layer: the boundary between them is indistinct.
Two major categories of cell are present in postnatal dermis, permanent and migrant, as is typical of all general connective tissues (see Ch. 2). The permanent resident cells include cells of organized structures such as nerves, vessels and cells of the arrector pili muscles, and the fibroblasts, which synthesize all components of the dermal extracellular matrix. The migrant cells originate in the bone marrow (Fig. 4.12) and include macrophages, mast cells, eosinophils, neutrophils, T and B cells (including antibody-secreting plasma cells), and dermal interstitial dendritic cells which are capable of immune surveillance and antigen presentation.
Layers of the dermis
Papillary layer
The papillary layer is immediately deep to the epidermis (Fig. 7.4), and is specialized to provide mechanical anchorage, metabolic support, and trophic maintenance to the overlying epidermis, as well as supplying sensory nerve endings and blood vessels. The cytoskeleton of basal epidermal keratinocytes is linked to the fibrous matrix of the papillary dermis through the attachment of keratin filament bundles to hemidesmosomes, then via anchoring filaments of the basal lamina, to the anchoring fibrils of type VII collagen which extend deep into the papillary dermis (Fig. 7.5). This arrangement provides a mechanically stable substratum for the epidermis.
The superficial surface of the dermis is shaped into numerous papillae or rete ridges, which interdigitate with rete pegs in the base of the epidermis and form the dermo-epidermal junction at their interface. The papillae have round or blunt apices which may be divided into several cusps. In thin skin, especially in regions with little mechanical stress and minimal sensitivity, papillae are few and very small, while in the thick skin of the palm and sole of the foot they are much larger, closely aggregated, and arranged in curved parallel lines following the pattern of ridges and grooves on these surfaces (Fig. 7.1). Lying under each epidermal surface ridge are two longitudinal rows of papillae, one on either side of the epidermal rete pegs through which the sweat ducts pass on the way to the surface. Each papilla contains densely interwoven, fine bundles of types I and III collagen fibres and some elastic fibrils. Also present is a capillary loop (Fig. 7.4), and in some sites, especially in thick hairless skin, Meissner’s corpuscle nerve endings.
Hypodermis
The quantity and distribution of subcutaneous fat differs in the sexes. It is generally more abundant and widely distributed in females. In males it diminishes from the trunk to the extremities, and this distribution is more obvious in middle age, when the total amount increases in both sexes. The amount of adipose tissue in the hypodermis, as elsewhere, reflects the quantity of lipid stored in its adipocytes rather than a change in the number of cells. There is an association with climate (rather than race), and superficial fat is more abundant in colder geographical regions. The hypodermis is most distinct on the lower anterior abdominal wall, where it contains much elastic tissue and appears many-layered as it passes through the inguinal regions into the thighs. It is similar in the limbs and the perineum, but is thin where it passes over the dorsal aspects of the hands and feet, the sides of the neck and face, around the anus, and over the penis and scrotum. It is almost absent from the external ears but is particularly dense in the scalp, palms and soles, where it is crossed by numerous strong connective tissue bands binding the hypodermis and skin to underlying structures: these are part of the deep fascia, but are known regionally as aponeuroses of the scalp, palm and sole.
PILOSEBACEOUS UNIT
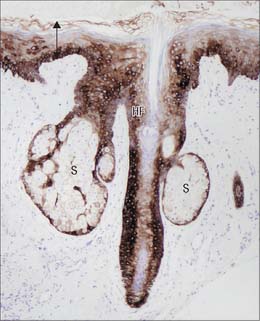
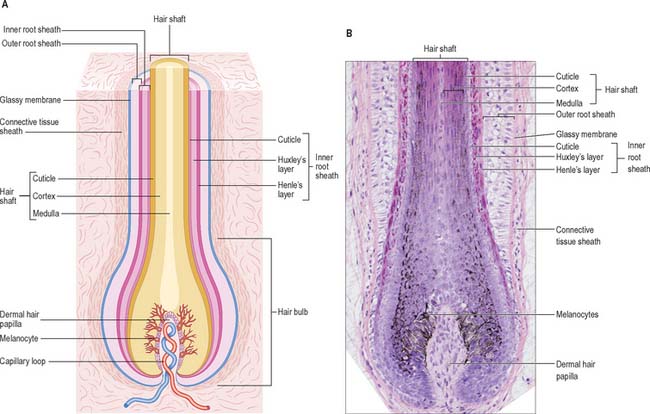
The pilosebaceous unit consists of the hair and its follicle with an associated arrector pili muscle, sebaceous gland, and sometimes an apocrine gland (Fig. 7.1, Fig. 7.12). Not all elements of the unit occur together in all body regions.
Hairs
Hairs are filamentous cornified structures present over almost the entire body surface. They grow out of the skin at a slant (Fig. 42.1) as is evident in the sloping of the hairs on the dorsum of forearm, hand and fingers towards the ulnar side. Hairs are absent from several areas of the body, including the thick skin of the palms, soles, the flexor surfaces of the digits, the thin skin of the umbilicus, nipples, glans penis and clitoris, the labia minora and the inner aspects of the labia majora and prepuce. The presence, distribution and relative abundance of hair in certain regions such as the face (in males), pubis and axillae, are secondary sexual characteristics which play subtle roles in sociosexual communication. There are racial variations in density, form, distribution and pigmentation, as well as individual variations. Hairs assist minimally in thermoregulation: on the scalp they provide some protection against injury and the harmful effects of solar radiation. They have a sensory function.
Hair follicle
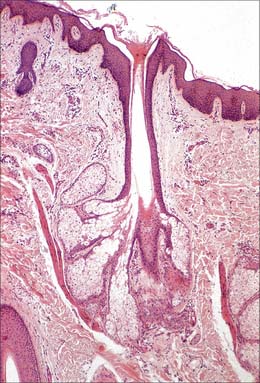
The hair follicle (Fig. 7.1, Fig. 7.12, Fig. 7.13A) is a downgrowth of the epidermis containing a hair, which may extend deeply (3 mm) into the hypodermis, or may be more superficial (1 mm) within the dermis. Typically, the long axis of the follicle is oblique to the skin surface; with curly hairs it is also curved. There are cycles of hair growth and loss, during which the follicle presents different appearances. In the anagen phase the hair is actively growing and the follicle is at its maximum extent of development. In the involuting or catagen phase, hair growth ceases and the follicle shrinks. During the resting or telogen phase the inferior segment of the follicle is absent. This is succeeded by the next anagen phase. Further details of the hair growth cycle are given below, after the description of the anagen follicle and hair.
Hair bulb
The hair bulb forms the lowermost portion of the follicular epithelium and encloses the dermal hair papilla of connective tissue cells (Fig. 7.13B). The dermal hair papilla is an important cluster of inductive mesenchymal cells which is required for hair follicle growth in each cycle throughout adult life: it is a continuation of the layer of adventitious mesenchyme that follows the contours of the hair follicle. The hair bulb generates the hair and its inner root sheath. A hypothetical line drawn across the widest part of the hair bulb divides it into a lower germinal matrix and an upper bulb. The germinal matrix is formed of closely packed, mitotically active pluripotential keratinocytes, among which are interspersed melanocytes, and some Langerhans cells. The upper bulb consists of cells arising from the matrix. These migrate apically and differentiate along several lines. Those arising centrally form the hair medulla. Radially, successive concentric rings of cells give rise to the cortex and cuticle of the hair and outside this, to the three layers of the inner root sheath. The latter are, from within out, the cuticle of the inner root sheath, Huxley’s layer and Henle’s layer. Henle’s layer is surrounded by the outer root sheath, which forms the cellular wall of the follicle (Fig. 7.13). Differentiation of cells in the various layers of the hair and its inner root sheath begins at the level of the upper bulb and is asynchronous, beginning earliest in Henle’s layer and Huxley’s layer.
Structure of hair and its sheaths
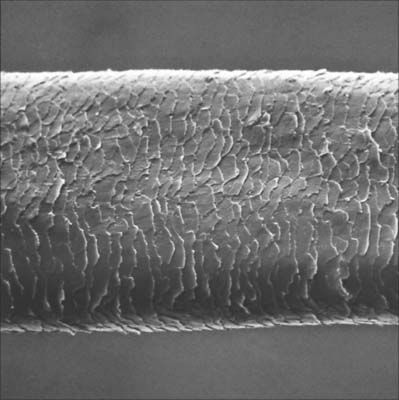
A fully developed hair shaft consists of three concentric zones which are, from outwards in, the cuticle, cortex and medulla. Each has different types of keratin filament proteins and different patterns of cornification. In finer hairs the medulla is usually absent. The cuticle forms the hair surface and consists of several layers of overlapping cornified squames directed apically and slightly outwards (Fig. 7.14). Immature cuticle cells have dense amorphous granules aligned predominantly along the outer plasma membrane with a few filaments. The cortex forms the greater part of the hair shaft and consists of numerous closely packed, elongated squames which may contain nuclear remnants and melanosomes. Immature cortical cells contain bundles of closely packed filaments but no dense granules, and when fully cornified, they have a characteristic thumb-print appearance with filaments arranged in whorls. The medulla, when present, is composed of loosely aggregated and often discontinuous columns of partially disintegrated cells containing vacuoles, scattered filaments, granular material and melanosomes. Air cavities lie between the cells or even within them.
Henle’s layer and Huxley’s layer of the inner root sheath contain irregular dense keratohyalin granules and associated filaments in the precornified state. At the level of the upper bulb, Henle’s layer begins to cornify, as does Huxley’s layer at the middle of the inferior follicle. When fully differentiated, cells of both layers have a thickened cornified envelope enclosing keratin filaments embedded in a matrix. The cells of the inner root sheath cuticle undergo terminal differentiation at a level closer to the hair bulb than that of Huxley’s layer, but lack a clear-cut filament pattern such as is seen in the cortical cells of the hair shaft. As they cornify, the cuticle cells of the inner root sheath and hair become interlocked. At about the level of entry of the sebaceous duct, above the isthmus, the inner root sheath undergoes fragmentation, and the hair then lies free in the pilary canal.
Hair cycle and growth of hair
Postnatally, hairs exhibit regional asynchrony of cycle duration and phase leading to an irregular pattern of growth and replacement. In some regions, such as the scalp, the cycle is measured in years; in others, such as general body hair, the cycle is much shorter and hairs are therefore limited in length. At puberty, hair growth and generation of much thicker hairs occurs on the pubes and axillae in both sexes, and on the face and trunk in males. The actions of hormones on hair growth are complex, and involve not only sex hormones, but also those of the thyroid, suprarenal cortex and pituitary glands. Androgens stimulate facial and general body hair formation. After about the first 30 years, they tend to cause the thick terminal hairs of the scalp to change to small vellus hairs, which produces recession from the forehead and sometimes almost complete male pattern baldness. In females, oestrogens tend to maintain vellus hairs: postmenopausal reduction of oestrogens may permit stronger facial and bodily hair growth. In mid-pregnancy, hair growth may be particularly active but later, often post-partum, an unusually large number of hairs enter the telogen phase and are shed before the growth cycle recommences. In older men, growth of hairs on the eyebrows and within the nostrils and external ear canals increases, whereas elsewhere on the body, growth slows and the hairs become much finer.
Sebaceous glands
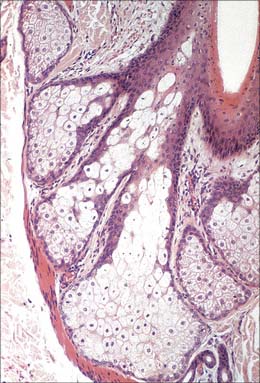
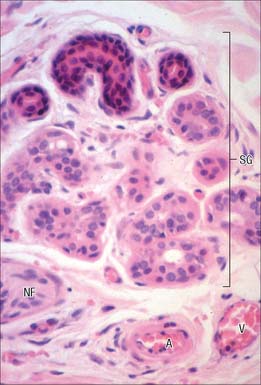
Sebaceous glands are small saccular structures (Fig. 7.1, Fig. 7.12, Fig. 7.15) lying in the dermis; together with the hair follicle and arrector pili muscle, they constitute the pilosebaceous unit. They are present over the whole body except the thick hairless skin of the palm, soles and flexor surfaces of digits. Typically, they consist of a cluster of secretory acini which open by a short common duct into the dermal pilary canal of the hair follicle. They release their lipid secretory product, sebum, into the canal by a holocrine mechanism (see Ch. 2). In some areas of thin skin which lack hair follicles, their ducts open instead directly on to the skin surface, e.g. on the lips and corners of the mouth, the buccal mucosa, nipples, female breast areolae, penis, inner surface of the prepuce, clitoris and labia minora. At the margins of the eyelids, the large complex palpebral tarsal glands (Meibomian glands) are of this type. They are also present in the external auditory meatus.
Microscopically, the glandular acini are enclosed in a basal lamina supported by a thin dermal capsule and a rich capillary network. Each acinus is lined by a single layer of small, flat, polygonal epithelial cells (sebocytes) which ultrastructurally resemble undifferentiated basal keratinocytes of interfollicular epidermis. They possess euchromatic nuclei and large nucleoli, scattered keratin filaments, free ribosomes, smooth endoplasmic reticulum and rounded mitochondria, and are attached to each other by desmosomes. Functionally, they are mitotically active stem cells whose progeny move gradually towards the centre of the acinus, increasing in volume and accumulating increasingly large lipid vacuoles. The nuclei become pyknotic as the cells mature. The huge distended cells ultimately disintegrate, filling the central cavity and its duct with a mass of fatty cellular debris (Fig. 7.15). The process takes 2–3 weeks. The secretory products pass through a wide duct lined with keratinized stratified squamous epithelium into the infundibulum of the hair follicle and then to the surface of the hair and the general epidermis.
Apocrine glands
An apocrine gland consists of a basal secretory coil and a straight duct which opens into either the pilary canal above the duct of the sebaceous gland, or directly onto the skin surface if there is no associated hair. The secretory region may be as much as 2 mm wide and its coils often anastomose to form a labyrinthine network. Each coil is lined by cuboidal secretory cells whose apical cytoplasm projects into the lumen and basally is in contact with a layer of myoepithelial cells within a thick basal lamina. The secretory cells contain vacuoles, vesicles and dense granules of varying size and internal structure: the numbers and character vary with the cycle of synthesis and discharge. The mechanism of secretion is still not clear, but may involve merocrine secretion of granules, apocrine secretion or complete holocrine disintegration of the cells (see Fig. 2.5).
Arrector pili muscles
The arrector pili muscles are small bundles of closely packed smooth muscle cells which form oblique links between the dermal sheaths of hair follicles and the papillary layer of the dermis (Fig. 7.1, Fig. 7.12). They show the typical features of smooth muscle cells and are separated by narrow spaces containing collagen fibres and unmyelinated noradrenergic sympathetic axons.
SWEAT GLANDS
The vast majority of sweat glands (Fig. 7.16) are often classified as eccrine, although their mode of secretion includes typical merocrine mechanisms (see Ch. 2). They are long unbranched tubular structures, each with a highly coiled, secretory portion up to 0.4 mm in diameter, situated deep in the dermis or hypodermis. From there, a narrower, straight or slightly helical ductal portion emerges (Fig. 7.1). The walls of the duct fuse with the base of epidermal rete pegs and the lumen passes between the keratinocytes, often in a tight spiral, particularly in thick hairless skin (Fig. 7.17), and opens via a rounded aperture (pore) onto the skin surface (Fig. 7.7). In thick hairless skin, sweat glands discharge along the centres of friction ridges, incidentally providing fingerprint patterns for forensic analysis. Sweat glands have an important thermoregulatory function, they contribute significantly to excretion and their secretion enhances grip and sensitivity of the palms and soles.
NAIL APPARATUS
Nails (Fig. 7.18) are homologous with the cornified layer of the general epidermis. They consist of compacted, anucleate, keratin-filled squames in two or three horizontal layers. Ultrastructurally, the squames contain closely packed filaments which lie transversely to the direction of proximodistal growth, and are embedded in a dense protein matrix. Unlike the general epidermis, squames are not shed from the nail plate surface. A variety of mineral elements are present in nail, including calcium. Calcium is not responsible for the hardness of nail: this is determined by the arrangement and cohesion of the layers of squames, and their internal fibres. The water content of nail is low, but nail is 10 times more permeable to water than the general epidermis. The softness and elasticity of the nail plate is related to its degree of hydration.
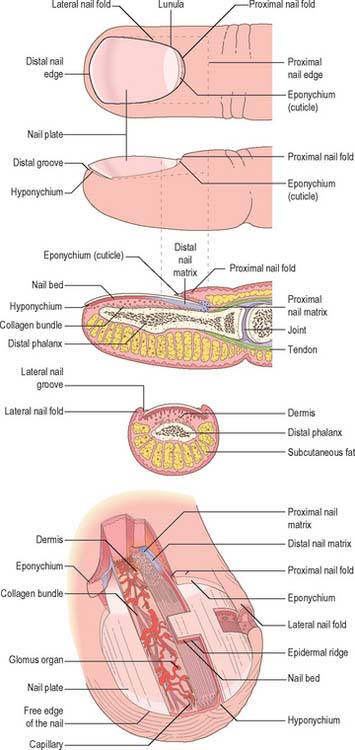
Fig. 7.18 The organization and terminology of the structures associated with a fingernail.
(By permission from Paus R, Peker S 2003 Biology of hair and nails. In: Bolognia JL, Jorizzo JL, Rapini RP (eds) Dermatology. London: Mosby.)
Nail plate
The nail plate is embedded within the proximal and lateral nail folds. It is approximately rectangular in shape and is mostly convex in both longitudinal and transverse axes: there is considerable inter- and intra-individual variation (Fig. 7.18). The thickness of the plate increases proximodistally from about 0.7 mm to 1.6 mm: the terminal thickness varies between individuals. The surface of the nail plate may show fine longitudinal ridges, and its undersurface is grooved by corresponding ridges in the nail bed. Disturbances of growth pattern or disease may lead to transverse ridging or grooves, and minute trapped air bubbles may produce white flecks. These defects move distally with growth of the plate.
Nail folds
The sides of the nail plate are bordered by lateral nail folds which are continuous with the proximal fold (Fig. 7.18). The lateral nail folds enclose the lateral free edges of the nail plate and are bounded by the attachment of the skin to the lateral aspect of the distal phalanx margin and the lateral nail. The proximal nail fold provides the visible proximal border to the nail apparatus. It consists of two epidermal layers, superficial and deep, separated by a core of dermis. The epidermis of the superficial layer lacks hair follicles and epidermal ridges: its cornified distal margin extends over the nail plate for a little distance as the cuticle or eponychium. The deep layer merges with the nail matrix.
Nail bed
The dermis of the nail bed is anchored to the periosteum of the distal phalanx without any intervening subcutaneous layer. It forms a distinct compartment, which means that infections of the nail bed, or other local causes of a rise in pressure (e.g. haematoma) may cause severe pain which is only relieved by excision of part or all of the nail plate. The dermis is richly vascularized. The blood vessels are arranged longitudinally and display numerous glomus bodies, which are encapsulated arteriovenous anastomoses involved in the physiological control of peripheral blood flow in relation to temperature (see Ch. 6 and Ch. 50). The dermis is well-innervated, and contains numerous sensory nerve endings, including Merkel endings and Meissner’s corpuscles.
Nail bed cells differentiate towards the nail plate, and contribute to its thickness ventrally.
Hyponychium
The hyponychium is the area under the free nail between the onychodermal band proximally and the distal groove. It is an epidermal ridge which demarcates the junction between the finger pulp and the subungual structures.
VASCULAR SUPPLY, LYMPHATIC DRAINAGE AND INNERVATION
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
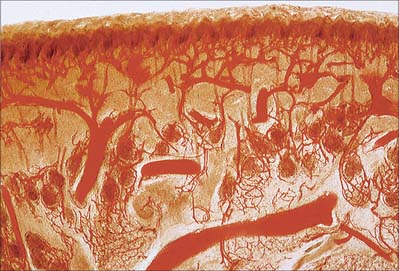
The direct cutaneous vessels, the musculocutaneous perforators and the fasciocutaneous perforators each contribute to six anastomosing horizontal reticular plexi of arterioles (Fig. 7.19) which have vascular connections between them and which ultimately provide the blood supply to the skin. Three plexi are located in the skin itself and supply all elements including the sweat glands and pilosebaceous units. The subpapillary plexus is located at the junction of the papillary and reticular layers of the dermis. It gives off small branches which form capillary loops in the dermal papillae (usually one loop per papilla) which are perpendicular to the skin surface (Fig. 7.1, Fig. 7.4, Fig. 7.20). The reticular dermal plexus is located in the middle portion of the dermis and is primarily venous. The deep dermal plexus is located in the deepest part of the reticular dermis and on the undersurface of the dermis. The close association between arteriolar and venous plexi permits exchange of heat between blood in vessels at different temperatures flowing in opposite directions (counter-current heat exchange).
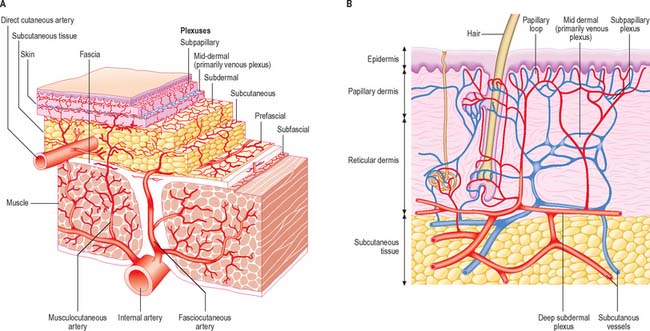
(A, redrawn from McCarthy JG (ed) Chapter 9 in Plastic Surgery, Vol 1. Philadelphia: Saunders. B, redrawn from Cormack GC, Lamberty BGH 1994 The Arterial Anatomy of Skin Flaps, 2nd edition. Edinburgh: Churchill Livingstone.)
The general structure and arrangement of the microvasculature is described in detail in Ch. 6, and so only features particular to skin will be considered here. In the deeper layers of the dermis, arteriovenous anastomoses are common, particularly in the extremities (hands, feet, ears, lips, nose), where, as glomera, they are surrounded by thick muscular coats. Under autonomic vasomotor control, these vascular shunts, when relaxed, divert blood away from the superficial plexus and so reduce heat loss, while at the same time ensuring some deep cutaneous circulation and preventing anoxia of structures such as nerves. Extensive capillary anastomoses are present. Generally, cutaneous blood flow is regulated according to thermoregulatory need, and also, in some areas of the body, according to emotional state. In very cold conditions, the peripheral circulation is greatly reduced by vasoconstriction, but intermittent spontaneous vasodilatation results in periodic increases in temperature which prevent cooling to the level at which frostbite might occur. This is thought to be due to a direct effect of oxygen lack on the arteriolar constrictor muscle, rather than to a neural influence.
The lymphatics of the skin, as elsewhere, are small terminal vessels which collect interstitial fluid and macromolecules for return to the circulation via larger vessels. They also convey lymphocytes, Langerhans cells and macrophages to regional lymph nodes. They begin as blind-ended, endothelial-lined tubes or loops just below the papillary dermis. These drain into a superficial plexus below the subpapillary venous plexus, which drains via collecting vessels into a deeper plexus at the junction of the reticular dermis and subcutis, and this, in turn, drains into the larger subcutaneous channels.
INNERVATION
The primary input is transmitted by neurones whose cell bodies lie in the spinal and cranial ganglia (see p. 55), and whose myelinated or unmyelinated axons are terminally distributed, mainly within the dermis. Efferent autonomic fibres are unmyelinated and noradrenergic or cholinergic. They innervate the arterioles, arrector pili muscles, and the myoepithelial cells of sweat and apocrine glands. In the scrotum, labia minora, perineal skin and nipples they also supply smooth muscle fasciculi of the dermis and adjacent connective tissue. Except in the nipples and genital area, activity of the autonomic efferent nerves is mainly concerned with regulation of heat loss by vasodilation and vasoconstriction, sweat production, and pilo-erection (although this is a minor function in humans).
On reaching the dermis, nerve fasciculi branch extensively to form a deep reticular plexus which serves much of the dermis, including most sweat glands, hair follicles and the larger arterioles. Many small fasciculi pass from this plexus to ramify in another superficial papillary plexus at the junction between the reticular and papillary layers of the dermis. Branches from this pass superficially into the papillary layer, ramifying horizontally and vertically, and terminate either in relation to encapsulated receptors, or as terminals reaching the level of the basal lamina. In some instances, they enter the epidermis as free endings, responsive to light pressure and touch sensation or to nociceptive stimuli. As these latter fasciculi terminate, they lose their epineurial and perineurial sheaths, leaving Schwann cell axonal complexes or naked axons enveloped by basal lamina, in direct contact with the matrix. These naked distal axonal terminals may be vulnerable to pathogens entering via a skin abrasion. The structure and classification of sensory endings are described in detail Chapter 3.
The segmental arrangement of the spinal nerves is reflected in the sensory supply of the skin: a dermatome is the area supplied by all the cutaneous branches of an individual spinal nerve through its dorsal and ventral rami (see Ch. 15 and Fig. 15.12). Typically, dermatomes extend round the body from the posterior to the anterior median line. The upper half of each zone is supplemented by the nerve above, the lower half by the nerve below. Dermatomes of adjacent spinal nerves overlap markedly, particularly in the segments least affected by development of the limbs.
DEVELOPMENT OF SKIN AND SKIN APPENDAGES
EPIDERMIS AND APPENDAGES
General (interfollicular) epidermis
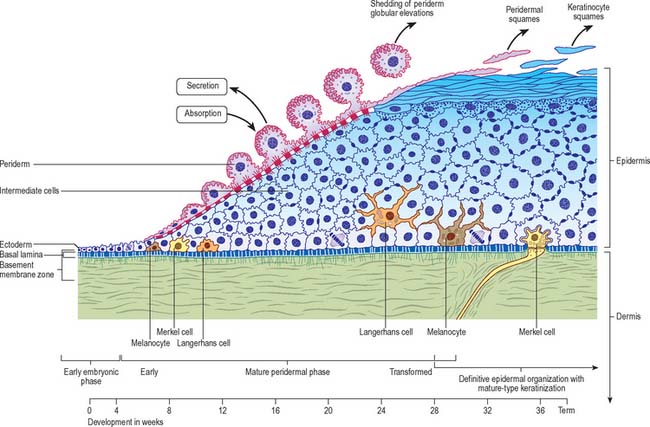
In the first 4–5 weeks, embryonic skin consists of a single layer of ectodermal cells overlying a mesenchyme containing cells of stellate dendritic appearance interconnected by slender processes and sparsely distributed in a loosely arranged microfibrillar matrix (Fig. 7.21). The interface between ectoderm and mesenchyme, known as the basement membrane zone (BMZ), is an important site of mutual interactions upon which the maintenance of the two tissues depends, both in prenatal and postnatal life. Ectodermal cells, which characteristically contain glycogen deposits, contact each other at gap and tight junctions. The layer so formed soon develops into a bilaminar epithelium, and desmosomes also appear. The basal germinative layer gives rise to the definitive postnatal epidermis, and the superficial layer to the periderm, a transient layer confined to fetal life. The periderm maintains itself, expresses different keratin polypeptides, and grows by the mitotic activity of its own cells, independent of those of the germinative layer. Originally flattened, the periderm cells increase in depth: the central area containing the nucleus becomes elevated and projects as a globular elevation towards the amniotic cavity. The plasma membrane develops numerous surface microvilli with an extraneous coat of glycosaminoglycans, and cytoplasmic vesicles become prominent deep to it. These developments reach a peak over the period 12–18 weeks, at which time the periderm is a major source of the amniotic fluid to which it may contribute glucose; it also has an absorptive function. From about 20 weeks onwards, the globular protrusions become undermined and pinched off to float free in the amniotic fluid. The now flattened periderm cells undergo a type of terminal differentiation to form what is regarded as a temporary protective layer for the underlying developing epidermis proper, against an amniotic fluid of changing composition as a result of the accumulation of products of fetal renal excretion. Up to parturition, periderm squames continue to be cast off into the amniotic fluid, and they contribute to the vernix caseosa, a layer of cellular debris which covers the fetal skin at birth.
Proliferation in the germinative layer leads to a stratified appearance with successive layers of intermediate cells between it and the periderm. From an early stage, cells of all layers are packed with glycogen granules, presumably a source of energy during this early replicative stage of differentiation. Differentiation of these layers is not synchronous throughout all regions of the developing skin, being more advanced cranially than caudally, and progressing on the body from the midaxillary line ventrally. Reduction in glycogen content of the cells is associated with a shift towards biosynthetic activity connected with terminal (cornifying) differentiation, manifested by the presence of different enzymes and expression of keratins. Simple epithelial keratins present from before implantation (K8 and K18) are replaced by typical keratinocyte basal cell keratins (K5 and K14), followed in the first suprabasal cell layer by those of higher molecular weight associated with differentiation (K1 and K10) at 10–12 weeks. This is soon followed by expression of profilaggrin and filaggrin, and the appearance of keratohyalin granules among filamentous bundles of the uppermost intermediate layer cells at approximately 20 weeks. The first fully differentiated keratinocytes appear shortly afterwards. By 24–26 weeks a definite cornified layer exists in some areas, and by 30 weeks or so, apart from some lingering glycogen in intermediate cells, the interfollicular epidermis is essentially similar to its postnatal counterpart (see Holbrook & Odland 1980, for further details).
Merkel cells originate from migratory neural crest cells (Szeder et al 2003) and begin to appear in the epidermis of the palm and sole of the foot between 8 and 12 weeks, and later in association with some hairs and with dermal axonal–Schwann cell complexes.
Pilosebaceous unit
Eccrine sweat glands
Eccrine (merocrine) sweat glands are one type of sudoriferous gland. Sweat gland rudiments appear in the second and third months as cell buds associated with the primary epidermal ridges of the finger and toe pads of terminal digits. They elongate into the dermis and by 16 weeks the lower end begins to form the secretory coil, within which, by 22 weeks, secretory and myoepithelial cells are evident. The solid cord of cells connecting the coil to the epidermis becomes the intradermal duct, and the lumina of both are formed by dissolution of desmosomal contacts between the cells. The intraepidermal duct is foreshadowed by a coiled column of concentrically arranged inner and outer cells, within which, by fusion of lysosomal vacuoles, a lumen is formed which opens on the surface at 22 weeks. As with hair follicles, no new eccrine sweat glands develop postnatally. Emotional sweating, detected by skin conductance changes, occurs in preterm infants from 29 weeks’ gestational age.
Abnormalities of the epidermis
Anomalous development of the epidermis and its derivatives is relatively common. Excessive or diminished growth, or even complete absence, may affect sebaceous or sudoriferous glands and hair, either locally or generally. Similarly, the epidermis may be excessively pigmented (melanism). Excessive production of the cornified layer leads to ichthyosis. A naevus or mole is a benign proliferation of melanocytes in the basal layer of the epidermis. Ectodermal dysplasia is a rare condition characterized by fine blond and scanty hair, reduced or absent eyelashes and eyebrows. The skin has deficient sweat and sebaceous glands. Teeth are usually peg- or cone-shaped, and absence of major salivary glands may occur. For information on keratin disorders, see Irvine & McLean 1999.
NEONATAL GROWTH
The surface area of the skin increases with growth. It has been estimated that the surface area of a premature neonate weighing 1505 g is approximately 1266 cm2, whereas a neonate of 2980 g has a surface area of 2129 cm2. The skin of the neonate is thinner than that of older infants and children. It cornifies over a period of 2–3 weeks which provides protection; however, in the premature infant the thin epidermal layer allows absorption of a variety of substances, e.g. chlorhexidine and boric acid and also permits a significantly higher transepidermal water loss than occurs in full term neonates. At birth the skin is richly vascularized by a dense subepidermal plexus. The mature pattern of capillary loops and of the subpapillary venous plexus is not present at birth but develops as a result of capillary budding with migration of endothelia at some sites and the absorption of vessels from other sites. Some regions mature faster than others. With the exceptions of the palms, soles and nail beds, the skin of the neonate has almost no papillary loops. It has a disordered capillary network which becomes more orderly from the second week when papillary loops appear; defined loops are not present until the fourth or fifth week, and all areas possess loops by 14–17 weeks postnatally.
NATURAL SKIN CREASES AND WRINKLES
SKIN LINES
Surface pattern lines, tension lines and skin creases
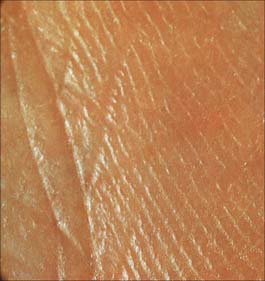
Externally visible skin lines are related to various patterns of epidermal creasing, ridge formation, scarring and pigmentation. A simple lattice pattern of lines occurs on all major areas of the body other than the thick skin of volar and plantar surfaces. The lattice pattern typically consists of polygons formed by relatively deep primary creases visible to the naked eye, which are irregularly divided by finer secondary creases into triangular areas (Fig. 7.22, Fig. 7.23). These, in turn, are further subdivided by tertiary creases limited to the cornified layer of the epidermis, and, finally, at the microscopic level, by quaternary lines which are simply the outlines of individual squames (Fig. 7.7). Apart from the quaternary lines, all the others increase the surface area of the skin, permit considerable stretching and recoil and distribute stresses more evenly. Details of the pattern vary according to the region of the body; e.g. on the cheek the primary creases radiate from the hair follicles, on the scalp they form hexagons, while on the calf and thigh they form parallelograms. There is a relationship between type of pattern and local skin extensibility.
Flexure (joint) lines
Flexure (joint) lines are major markings found in the vicinity of synovial joints, where the skin is attached strongly to the underlying deep fascia (Fig. 7.22). They are conspicuous on the flexor surfaces of the palms, soles, and digits, and in combination with associated skin folds, they facilitate movement. The skin lines do not necessarily coincide with the associated underlying joint line. For example, the flexure lines demarcating the extended fingers from the palm lie approximately half an inch distal to the metacarpophalangeal joints, the positions of which are more closely related to the distal palmar crease (heart-line of palmistry). The patterns of flexure lines on the palms and soles may vary and are to some extent genetically determined. In Down syndrome, the distal and middle palmar creases tend to be united into a prominent single transverse crease, a sign which is of diagnostic importance.
Papillary ridges
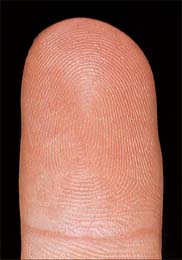
Papillary ridges are confined to the palms and soles and the flexor surfaces of the digits, where they form narrow parallel or curved arrays separated by narrow furrows (Fig. 7.24, Fig. 7.25). The apertures of sweat ducts open at regular intervals along the summit of each ridge. The epidermal ridges correspond to an underlying interlocking pattern of dermal papillae, an arrangement which helps to anchor the two layers firmly together. The pattern of dermal papillae determines the early development of the epidermal ridges. This arrangement is stable throughout life, unique to the individual, and therefore significant as a means of identification. The ridge pattern can be affected by certain abnormalities of early development, including genetic disorders such as Down syndrome, and skeletal malformations such as polydactyly. Absence of epidermal ridges is extremely rare. Functionally, epidermal ridges increase the gripping ability of hands and feet, preventing slipping. The great density of tactile nerve endings beneath them means that they are also important sensory structures.
The analysis of ridge patterns by studying prints of them (fingerprints) is known as dermatoglyphics and is of considerable forensic importance. Measurable parameters include the frequency of ridges in particular patterns and the disposition of tri-radii, which are junctional areas where three sets of parallel ridges meet. Fingerprint ridge patterns can be separated into three major types (Fig. 7.24), arches (5%), loops (70%), and whorls (25%). Arches have no tri-radii, loops have one tri-radius, and whorls have two or more. Whorl finger patterns are more common on the right hand, and males generally have more whorls and fewer arches than females, in whom the ridges are relatively narrower. The frequency of individual patterns varies with particular fingers. Similar patterns are seen on the toes.
Lines detectable after manipulation or incision
In certain regions of the body, surgical wounds heal with a better and less conspicuous scar if they are lying in a particular direction. This finding is related to a number of factors including skin tension and naturally formed wrinkle lines. Skin is normally under tension and the direction in which this is greatest varies regionally. Tension is dependent on the protrusion of underlying structures, the direction of underlying muscles, and on joint movements. Many anatomists and surgeons have therefore attempted to produce a body map to indicate the best direction in which to make an elective incision to obtain the most aesthetic scar. These maps frequently differ, especially in the region of the face. Out of the multitude of described cleavage lines, the most commonly referred to are relaxed skin tension lines (RSTLs), Langer’s lines, and Kraissl’s lines (Borges 1984). Of these, the RSTLs and Kraissl’s lines are probably more appropriate lines for surgical incision.
Lines of Langer and Kraissl
Kraissl’s lines are essentially exaggerated wrinkle lines obtained by studying the loose skin of elderly faces whilst contracting the muscles of facial expression. These lines for the most part correspond to RSTLs, but slight variation exists on the face, especially on the lateral side of the nose, the lateral aspect of the orbit, and the chin. For further information on lines of Langer and Kraissl, see Chapter 29.
AGE-RELATED SKIN CHANGES
Intrinsic ageing
From about the third decade onwards there are gradual changes in the appearance and mechanical properties of the skin which reflect natural ageing processes. These become very marked in old age. Normal ageing is accompanied by epidermal and dermal atrophy, which result in some changes in the appearance, microstructure and function of the skin. Alterations include wrinkling, dryness, loss of elasticity, thinning and a tendency towards purpura on minor injury. Epidermal atrophy is expressed by general thinning and loss of the basal rete pegs with flattening of the dermo-epidermal junction, and this results in a reduction in contact area between the two which may affect epidermal nutrition. Flattening of the junction decreases resistance to shear, leading to poor adhesion of epidermis and its separation following minor injury. The thickness of the cornified layer is not reduced in old age, and its permeability characteristics seem little affected. Epidermal proliferative activity and rate of cell replacement decline with age, being reduced by up to 50% in elderly skin. Synthesis of vitamin D is also reduced. After middle age there is a 10–20% decline in the number of melanocytes, and Langerhans cells become sparser, which is associated with a reduction in immune responsiveness. Depigmentation and loss of hair, and some local increases (eyebrows, nose and ears in males, and face and upper lip in females) are commonly observed. Alterations in non-keratinocytes may be aggravated by chronic exposure to UV irradiation.
CUTANEOUS WOUND HEALING AND SCARRING
The molecular biology of cutaneous repair involves the coordination of numerous cell types, signalling molecules and matrix proteins. Many such factors are pleiotropic in their effects and it is the complex balance of such mediators, rather than their individual action, that determines events in wound repair. Wound healing is often considered in four overlapping temporal phases, namely haemostasis, inflammation, proliferation and remodelling (Fig. 7.26). These events will be discussed separately for clarity, although this is an artificial division of the complex, inter-related processes that constitute the wound healing response. For further reading, see Miller & Nanchahal (2005).
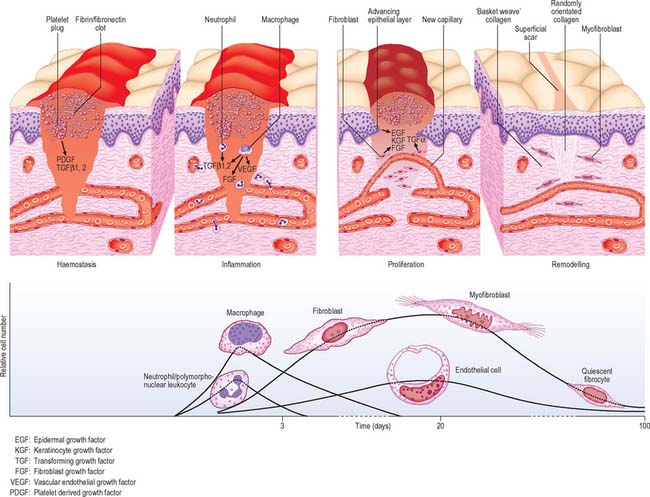
Fig. 7.26 Schematic illustration of the processes involved in the healing of a normal cutaneous wound.
HAEMOSTASIS
Wounding causing vascular injury initiates fibrin-fibronectin clot formation. The clot consists of platelets (promoted to aggregate by fibrillar collagens type I and III) embedded in a mesh of cross-linked fibrin and fibronectin fibres. It serves as a haemostatic plug, protects the denuded wound with a temporary physical shield, and forms a provisional matrix through and over which cells migrate during the repair process. The clot also acts as a reservoir of growth factors and cytokines, which are released as platelets de-granulate, and contribute to inflammatory cell recruitment.
SKIN GRAFTS AND FLAPS
A graft is a piece of tissue which has been detached from its blood supply and therefore needs to regain a blood supply from the bed in which it is placed in order to survive. In contrast, a flap is a piece of tissue which is surgically raised and transferred from one location in the body to another whilst maintaining its blood supply, which enters the base (pedicle) of the flap when it is transplanted.
FLAPS
A free flap (free tissue transfer) is a specific type of flap in which the tissue, whether skin, fascia, muscle, bone or a combination of these, is completely removed from its original location in the body along with a single identifiable artery and vein and transferred to a remote site. The blood vessels in the flap are anastomosed to vessels located in the new site using microsurgical techniques. This often allows for greater flexibility in performing reconstructive surgery. (The arterial perforators in the lower limb are described in Ch. 79.)
Borges AF. Relaxed skin tension lines (RSTL) versus other skin lines. Plast Reconstr Surg. 1984;73:144-150.
Byrne C, Hardman M, Nield K. Covering the limb – formation of the integument. J Anat. 2002;1:113-124. In: Lane EB, Tickle C (eds) Symposium issue: how to make a hand
Current views on the differentiation of skin and its appendages during embryogenesis..
Ghazizadeh S, Taichman LB. Organization of stem cells and their progeny in human epidermis. J Invest Dermatol. 2005;124:367-372.
Goding CR. Melanocytes: the new black. Int J Biochem Cell Biol. 2007;39:275-279.
Gu L-H, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Op Cell Biol. 2007;19:13-23.
Holbrook KA, Odland GF. Regional development of the human epidermis in the first trimester embryo and the second trimester fetus (ages related to the timing of amniocentesis and fetal biopsy). J Dermatol. 1980;4(3):161-168.
Irvine AD, McLean WHI. Human keratin diseases: increasing spectrum of disease and subtlety of phenotype-genotype correlation. Br J Dermatol. 1999;140:815-828.
Miller M-C, Nanchahal J. Advances in the modulation of cutaneous wound healing and scarring. Biodrugs. 2005;19:363-381.
Montagna W, Kligman AM, Carlisle KS. Atlas of Normal Human Skin. New York: Springer-Verlag, 1992.
Niemann C, Watt FM. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 2002;12(4):185-192.
Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DAD, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169-174.
Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Dev Biol. 2003;253:258-263.