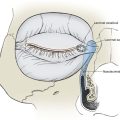
Skin and Composite Grafts
Skin Anatomy and Physiology
The epidermis is attached to the dermis by a basement membrane zone that extends from the epidermis to pilosebaceous units and sweat ducts located in the dermis. Each pilosebaceous unit contains sebaceous glands, a hair shaft and follicle with associated arrector pili muscle, and a sensory end organ. Epithelialization of partial-thickness skin wounds occurs from wound edges and the basement membrane zone around the hair follicles, sebaceous glands, and sweat ducts.1
The dermis is divided into a thin papillary and a thicker reticular dermis. The overall thickness of the dermis is variable, depending on its location. Eyelid skin has the thinnest dermis, measuring less than 1 mm thick. Dermal thickness measures 1.5 mm on the temple, 2.5 mm on the scalp, and more than 4 mm on the back. The dermis is thin at birth, increases in thickness until the fourth or fifth decade, and then decreases with further aging. On average, men have a thicker dermis than women do.1
Cutaneous blood flow is directed toward the more metabolically active epidermis through dermal papillae, hair papillae, and adnexal structures. Two vascular plexuses connected by communicating vessels are present in the reticular dermis. A deep plexus lies at the junction of dermis and fat; the superficial plexus gives rise to a rich capillary loop system in the superficial dermal papillae. This system provides nutrients to the epidermis through diffusion.2
Skin Grafts
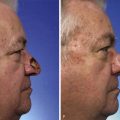
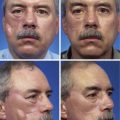
On occasion, superficial facial defects are best addressed by repair with a skin or composite graft. Very young patients with tight facial skin presenting with large cutaneous defects are appropriate candidates for reconstruction with a full-thickness skin graft. Use of a skin graft is usually reserved for those cases that cannot reasonably be reconstructed with a local flap because of the size of the defect. The skin graft obviates the need for additional scars on the face or the use of a distant or microsurgical flap. Unfortunately, full-thickness skin grafts used in such cases frequently have a “patch” appearance, with discrepancies of color and texture between native and grafted skin (Fig. 15-1). Color and texture discrepancies between facial skin and skin grafts may also be a problem in elderly patients (Fig. 15-2). However, because of the tendency for elderly patients to have thin skin, grafts used to repair facial defects are more likely to display a better color and textural match with native facial skin than when similar grafts are used in younger patients (Fig. 15-3). Postoperative dermabrasion assists in minimizing color and texture discrepancies between skin grafts and facial skin.
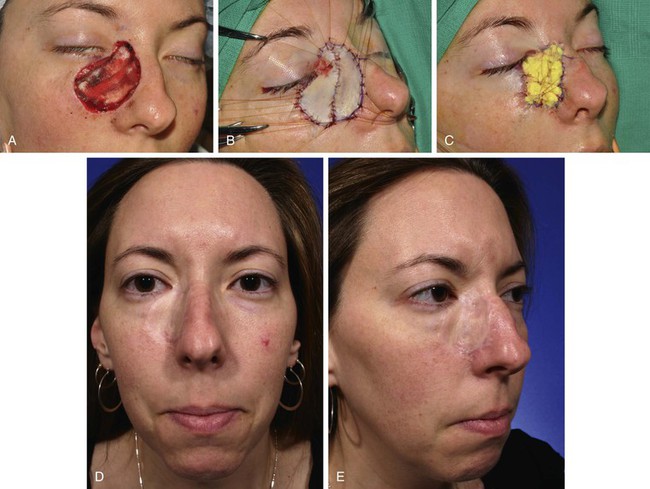
FIGURE 15-1 A, A 4.5 × 4.2-cm skin defect after micrographic excision of basal cell carcinoma in a 34-year-old woman. B, C, Repair with full-thickness skin graft from supraclavicular fossa. D, E, Postoperative views at 11 months. No adjunctive procedures performed. Graft has poor color and textural match with facial skin. (Courtesy Shan R. Baker, MD.)
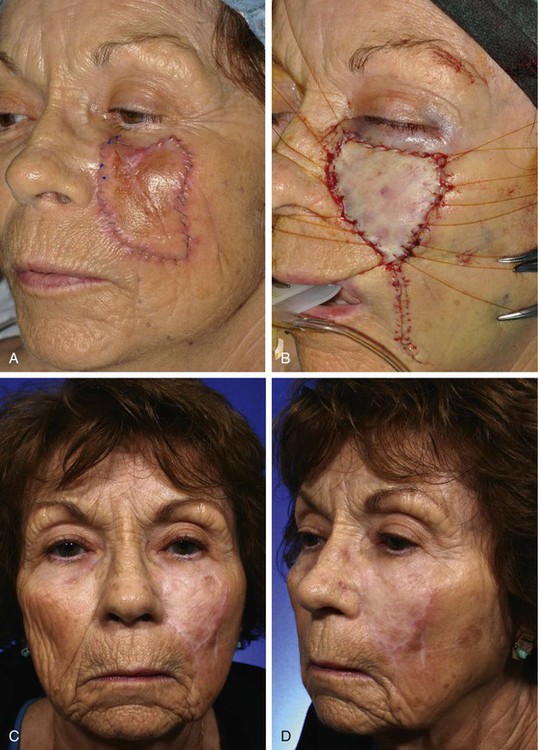
FIGURE 15-2 A, A 5 × 4-cm melanoma in situ. Suture line marks planned excision. B, Melanoma excised. Resulting wound partially closed primarily. Remaining wound covered with 3.5 × 4-cm full-thickness skin graft. C, D, Postoperative views at 1 year. Z-plasties performed at superior border of graft. (Courtesy Shan R. Baker, MD.)
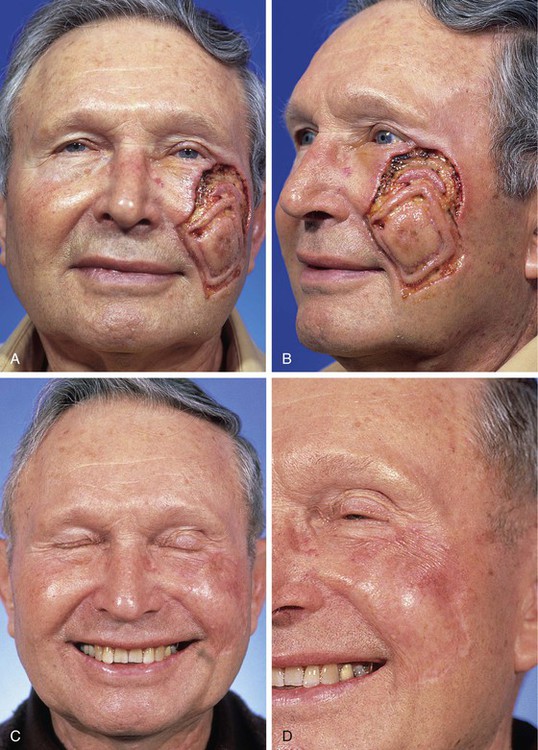
FIGURE 15-3 A, B, Lentigo maligna of cheek outlined by linear incisions from attempts to obtain tumor-free margins before complete resection (square technique; see Chapter 8). Resected area (7 × 6 cm) was reconstructed with full-thickness skin graft harvested from supraclavicular fossa. C, D, Postoperative views at 10 months. Skin graft provided an excellent color and textural match with native facial skin. No adjunctive procedures performed. (Courtesy Shan R. Baker, MD.)
Contact between the skin graft and recipient site is essential. A bolster dressing is helpful to prevent fluid collections beneath the graft postoperatively (Fig. 15-4). Bolsters also prevent shearing forces from disrupting vascular connections between graft and wound bed. Systemic illnesses that may compromise graft survival include collagen vascular diseases, hematologic disorders, diabetes, nutritional deficiencies, and hypoxemia.3 Use of tobacco products is also detrimental to the survival of skin grafts.
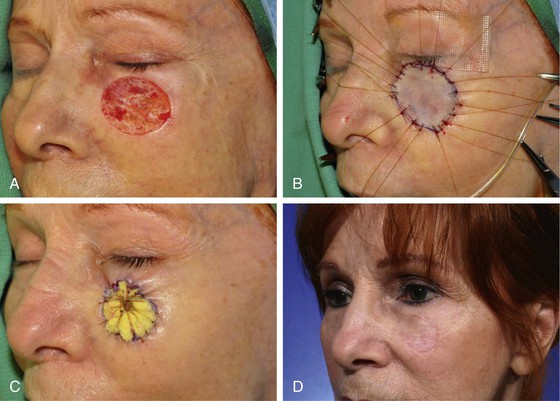
FIGURE 15-4 A, A 3 × 2.7-cm skin defect of cheek after micrographic excision of squamous cell carcinoma. B, Defect repaired with full-thickness skin graft from supraclavicular fossa. C, Gauze bolster dressing secures graft in place for 5 days. D, Postoperative view at 1 year. (Courtesy Shan R. Baker, MD.)
Recipient site conditions that are not favorable to skin graft survival include irradiated tissue; scar; exposed bone, cartilage, or tendon; and bleeding wounds. Grafts placed over avascular defects smaller than 1 cm2 may survive by nutritional support through the wound edges; however, grafting over larger avascular wounds is unlikely to succeed.3 Skin graft survival on bone is enhanced if a thin layer of bone is removed with a diamond fraise bur until punctuate bleeding is achieved. On the skull, holes can be made in the outer bone table to allow communication with the inner diploë. Large areas of exposed bone, cartilage, or tendon may require coverage with a muscle or fascial flap before grafting. Another technique that has proved successful in skin grafting auricular cartilage devoid of perichondrium is to create windows through the cartilage, exposing perichondrium and subcutaneous tissue on the opposing surface of the ear. The windows are made as large as possible through the cartilage in areas that can be resected without jeopardizing the structural integrity of the ear. The windows provide a port for vascular ingrowth to revascularize the graft (Fig. 15-5). Only very thin full-thickness skin grafts should be used for covering auricular cartilage so that the graft will not obscure the complex and delicate topography of the cartilage. Thin skin grafts are more likely to survive compared with thick full-thickness skin grafts, given the limited contact with a vascular source for nourishment.
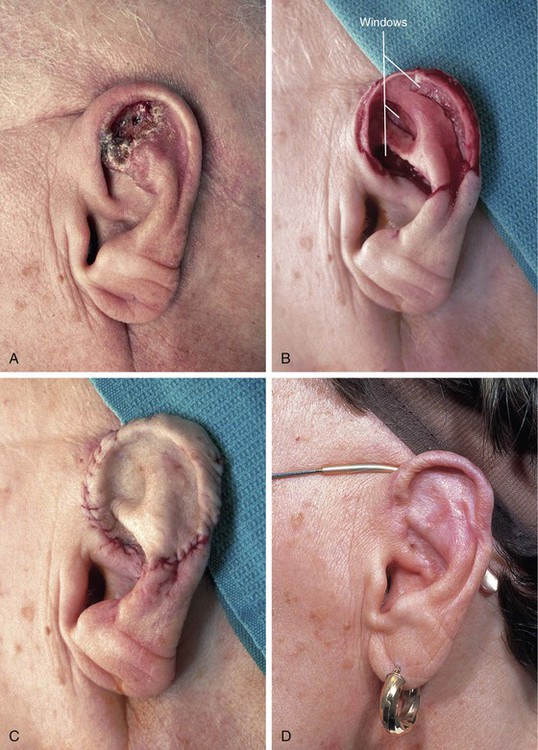
FIGURE 15-5 A, Basal cell carcinoma of auricle. B, After tumor resection, windows made through cartilage for portals of ingrowth of blood vessels to nourish skin graft. C, Thin full-thickness skin graft in place. D, Postoperative view at 6 months. No adjunctive procedures performed. (Courtesy Shan R. Baker, MD.)
For deeper wounds, skin grafting may be delayed until granulation tissue has filled the wound bed (2-3 weeks). Any epithelium on the surface of the granulation tissue is removed before grafting, and the tissue is crosshatched so that myofibrils are released. Granulating wounds normally contain bacteria. Bacterial counts greater than 105 organisms per gram of tissue often lead to graft loss.4 When delayed grafting is planned, the patient is prescribed a 10-day course of an antistaphylococcal antibiotic to begin 3 days before skin grafting. When necessary, wound culture specimens are obtained to direct antibiotic selection.
Defect Preparation
The aesthetic facial regions and their individual aesthetic units are based on variations in skin thickness and texture as well as on variations in contour created by the underlying facial framework. Optimal repair of a defect may require repositioning of skin and soft tissue within an involved aesthetic unit, thereby allowing eventual scars to lie within zones of transition between adjacent units. In addition, small defects may be enlarged to facilitate resurfacing of an entire aesthetic unit by a single repair method.
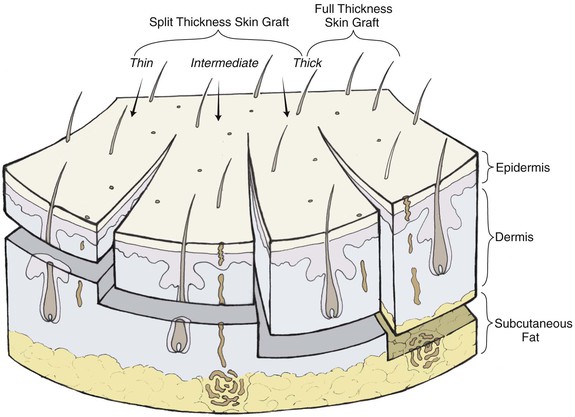
Full-Thickness Skin Grafts
Full-thickness skin grafts consist of epidermis and full-thickness dermis (Fig. 15-6). They resist contraction, have texture and pigmentation similar to normal skin, and require a well-vascularized, uncontaminated recipient site for survival. Full-thickness grafts survive initially by diffusion of nutrition from fluid at the recipient site, a process known as plasma imbibition. Vascular inosculation occurs during the first 24 to 48 hours. After 48 to 72 hours, capillaries in the recipient site begin to grow into the graft to provide new circulation. By 3 to 5 days, a new blood supply has been established. Initially, full-thickness skin grafts appear blanched; however, during 3 to 7 days, a pink color develops, signaling neovascularization. After 4 to 6 weeks, the pink color begins to fade, and the graft will often remain lighter than the surrounding skin, especially in dark-skinned individuals.
Compared with split-thickness skin grafts, full-thickness skin grafts have the advantage of better color and texture match, fewer contour irregularities, no need for special equipment to harvest the graft, and easier donor site wound care. The disadvantages include reduced survival rate for larger grafts and longer healing time.3
Full-thickness skin grafts are well suited for defects located in thin-skinned areas of the face (eyelids, nasal sidewall, columella) and for defects involving concavities (temple, medial canthus) (Figs. 15-7 and 15-8). Full-thickness skin grafts used to repair defects of the face in regions of thicker skin tend to heal with a contour depression and noticeable textural discrepancies between graft and adjacent skin. This is because the skin in these areas contains more sebaceous glands and has a thicker dermis than the graft. However, contour discrepancies between skin grafts and adjacent nasal skin frequently improve with time as scar tissue forms beneath the graft (Figs. 15-9 and 15-10). If a skin graft has resulted in a persistent contour depression, the appearance of the graft may be improved by placement of a dermal fat graft beneath the skin graft after it has healed (Fig. 15-11). Meyers et al5 described the use of dermal grafts at the time of skin graft placement to help prevent contour depression after repair of deeper defects. Their technique involves the placement of dermal tissue in linear strips within the wound bed, leaving adequate exposure of the wound bed to provide nourishment to an overlying skin graft.5 The author’s preference is to perform a delayed dermal fat graft 3 months postoperatively if a significant contour deformity exists.
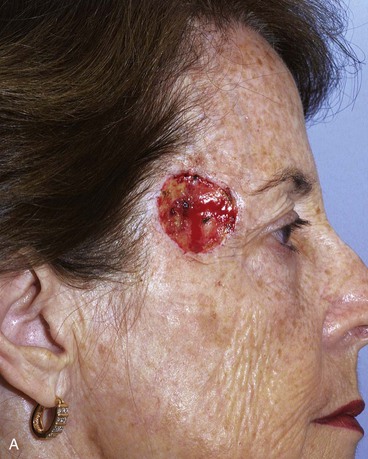
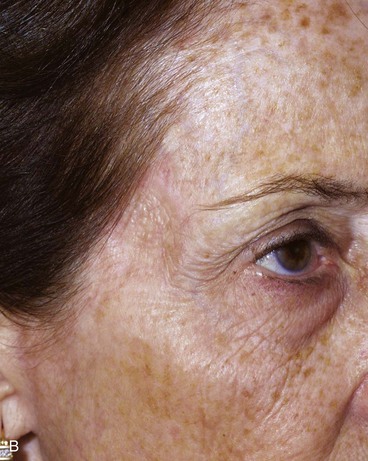
FIGURE 15-7 A, A 2 × 2-cm skin defect of temple. B, Six months after repair with preauricular full-thickness skin graft. No adjunctive procedures performed.
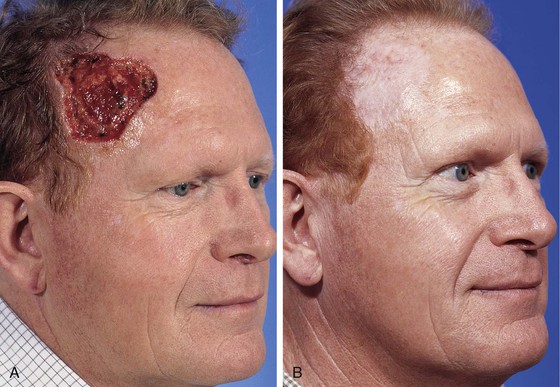
FIGURE 15-8 A, A 6 × 6-cm skin defect of temple after micrographic surgery for basal cell carcinoma. B, Five months after repair with full-thickness skin graft from supraclavicular fossa. No adjunctive procedures performed. (Courtesy Shan R. Baker, MD.)
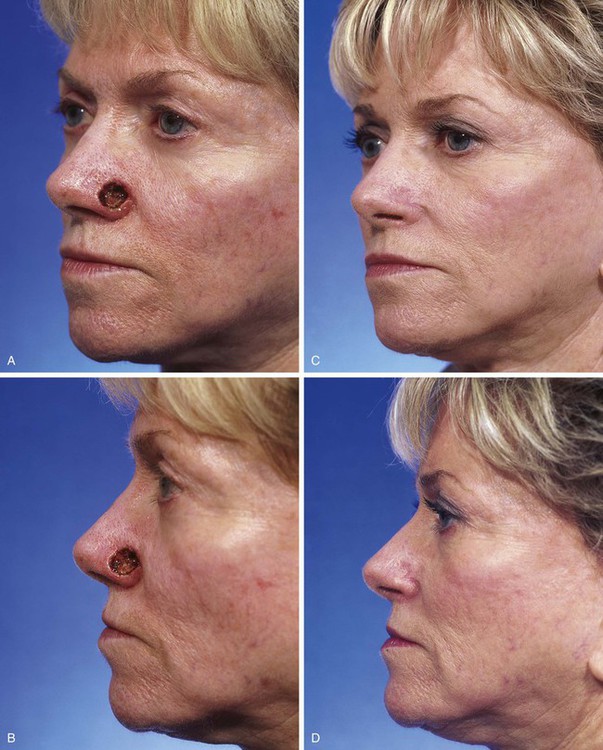
FIGURE 15-9 A, B, A 1.5 × 1-cm-deep defect of ala repaired with full-thickness skin graft from supraclavicular fossa. C, D, Postoperative views at 7 months. Scar tissue beneath graft assists with filling depth of wound, preventing contour discrepancy between graft and adjacent nasal skin. Graft was dermabraded. (Courtesy Shan R. Baker, MD.)
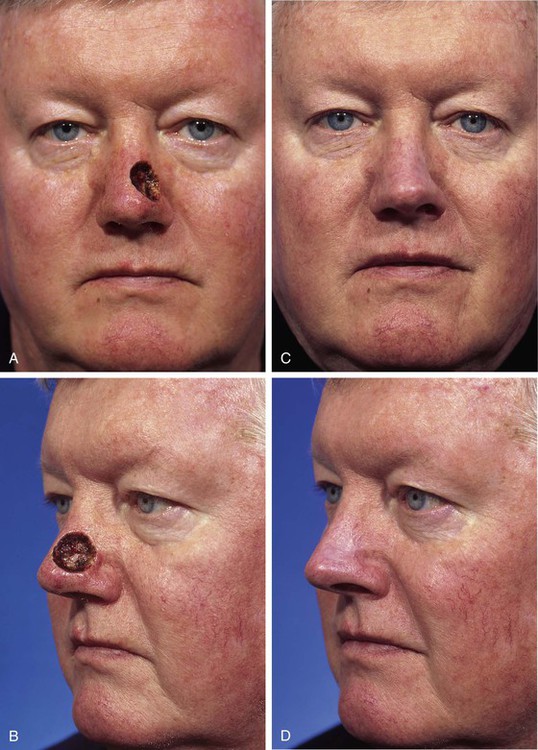
FIGURE 15-10 A, B, A 2 × 2-cm-deep skin and soft tissue defect of nasal tip and sidewall. Repair with paramedian forehead flap recommended but patient declined. Defect repaired with full-thickness skin graft from supraclavicular fossa. C, D, Postoperative result at 5 months. No adjunctive surgery performed. No contour deformity occurred secondary to scar tissue deposition beneath skin graft. (Courtesy Shan R. Baker, MD.)
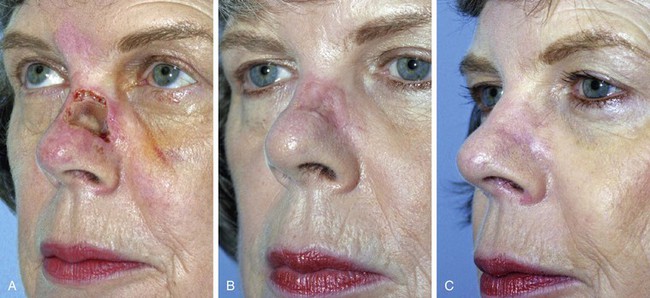
FIGURE 15-11 A, A 2 × 1.5-cm skin defect of nasal sidewall with exposed periosteum. Defect repaired with full-thickness skin graft. B, Depressed contour of graft 3 months later. C, One year after staged dermal fat graft placed beneath skin graft and subsequent dermabrasion of skin graft.
There is a wide variation of skin thickness among individuals, and the overall thickness of the facial skin is an important preoperative consideration. For similar defects, a skin graft may provide a perfect match in terms of thickness for one person and a poor match for another. There are individuals who have thin skin covering the entire face. These individuals are often fair-skinned elderly women, and full-thickness skin grafts may be used in these patients for superficial cutaneous defects anywhere on the face without concern for color or textural discrepancies between graft and facial skin (Fig. 15-12). The only exception to this rule is in the area of the margins of the nostril, eyelid, and vermilion; scar contraction after skin grafting will likely distort the border of these structures.
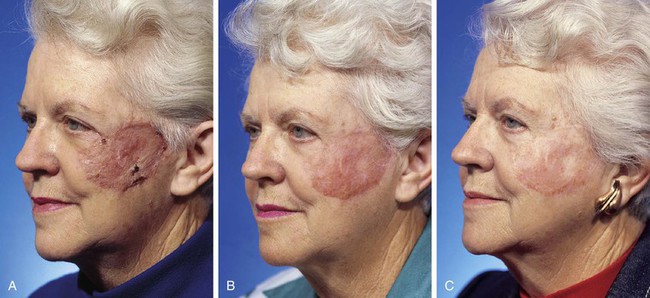
FIGURE 15-12 A, One month after full-thickness skin grafting of cheek. Graft harvested from supraclavicular fossa. B, Three months later. Graft has developed postinflammatory hyperpigmentation. C, Postoperative view at 10 months. Hyperpigmentation has resolved. There are fewer skin color and textural discrepancies between graft and native skin in fair-skinned elderly patients. (Courtesy Shan R. Baker, MD.)
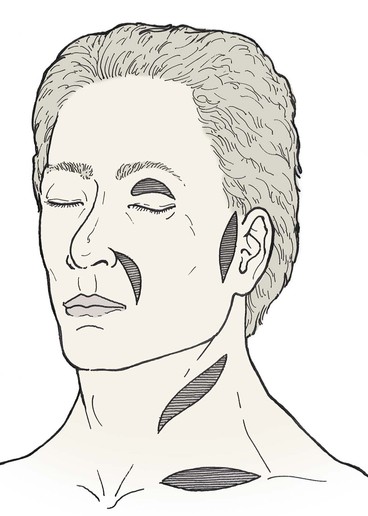
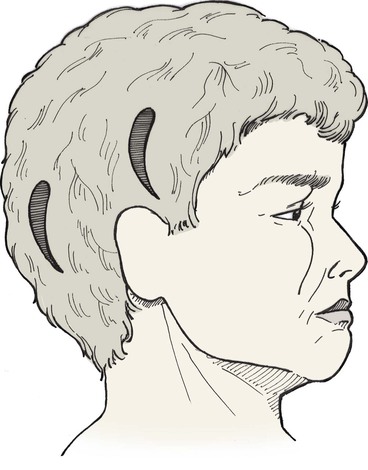
A number of donor sites for skin grafts are available in most individuals, depending on the location and size of the facial defect. Donor sites include upper eyelid, forehead, melolabial fold, preauricular, postauricular, and supraclavicular areas (Fig. 15-13). In selecting a donor site, the thickness of the skin surrounding the recipient site is assessed and donor skin is selected accordingly. Regions of the face with thicker skin include the forehead, medial cheek, caudal nose, and chin. Skin defects in these areas can be repaired with grafts from contralateral facial donor sites and periauricular or supraclavicular areas (Fig. 15-14). Regions of the face with thinner skin, such as the eyelids and cephalic two-thirds of the nose, are best repaired with skin grafts obtained from the eyelid or periauricular area that are thinned appropriately. Skin grafts harvested from the postauricular area are often preferred in men because they are hairless. Men tend to have shorter hair than women do, causing the postauricular skin to more likely manifest solar aging, which provides an improved skin color match with the facial skin (Fig. 15-15). In contrast, preauricular skin is often the preferred source for skin grafts in women. Preauricular skin in women is hairless and has more solar aging compared with postauricular skin. The supraclavicular region is an excellent source for skin grafts, especially if a large graft is required (Fig. 15-16). Supraclavicular skin has moderate thickness, but the dermis of this donor skin can be thinned so that the thickness of the graft will match the depth of the recipient site.6
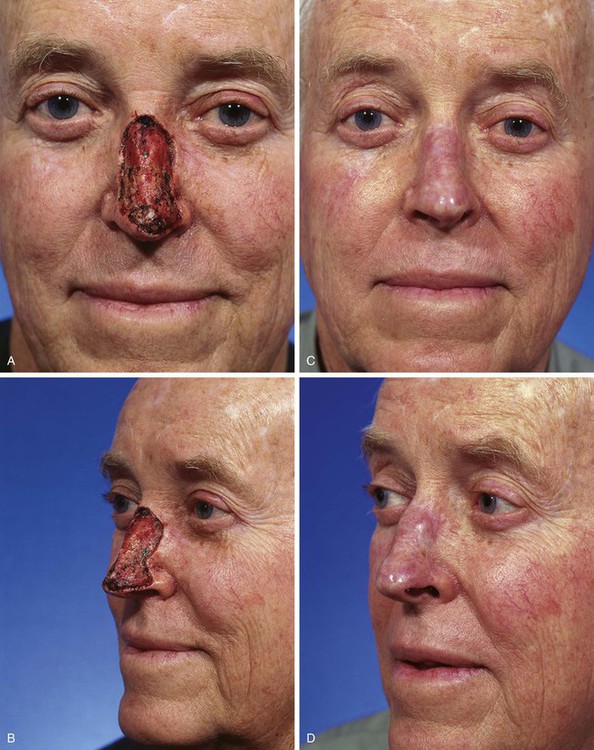
FIGURE 15-14 A, B, A 4 × 3-cm superficial skin defect of nasal dorsum and tip in patient with thin skin. C, D, Eight months after repair with full-thickness skin graft harvested from supraclavicular fossa. Z-plasties performed at border of graft. (Courtesy Shan R. Baker, MD.)
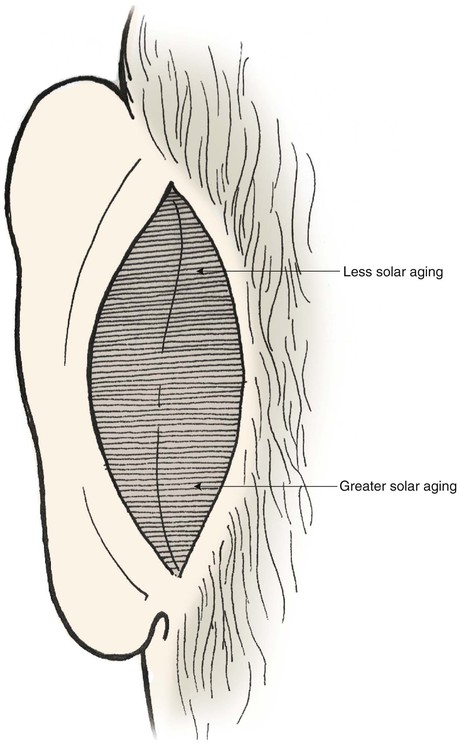
FIGURE 15-15 Skin posterior to auricle is the ideal source of full-thickness grafts to cover cutaneous defects of cephalic nose. Skin from inferior aspect of region has greater solar aging and provides better color match with facial skin.
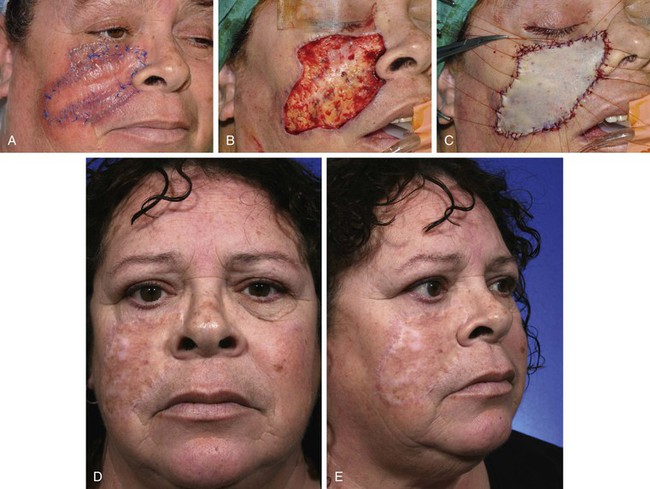
FIGURE 15-16 A, An 8.5 × 4.8-cm melanoma in situ. Peripheral suture line marks planned excision. B, Melanoma excised. C, Defect repaired with full-thickness skin graft from supraclavicular fossa. D, E, Postoperative views at 10 months. (Courtesy Shan R. Baker, MD.)
There are occasions when the author will use skin grafts to repair shallow skin defects of the face even when it is anticipated that the graft will result in a contour depression or noticeable mismatch of skin texture or color with adjacent native skin. These situations often arise in caring for elderly debilitated patients who have life-threatening illnesses. Another indication for use of a skin graft in place of a more desirable local flap is in patients who have skin malignant neoplasms with aggressive histologic growth patterns that predispose the patient to recurrent tumor. In these instances, skin grafts may be used as a temporary covering for a 2- or 3-year interval while the patient is at greatest risk for recurrence of the skin cancer.
Defects involving more than one facial region are often repaired with separate techniques. For example, defects located on the lateral nasal ala immediately adjacent to the nasofacial sulcus may be repaired by a number of options. If the defect extends into the cheek, the cheek component of the defect is usually repaired with a cheek advancement flap. The alar component may be repaired with an interpolated cheek or paramedian forehead flap if the defect is large. For smaller defects, especially in very young patients, full-thickness skin grafts and composite grafts are considered. Full-thickness grafts are best suited for superficial defects not extending to the nostril border. In the case of deep alar defects, skin grafts do not lend any structural support, and subsequent nasal valve collapse may lead to compromise of the nasal airway. One option for patients with small, laterally located deep alar defects is the transfer to the defect of subcutaneous cheek tissue in the form of a hinge flap (Fig. 15-17). The flap partially fills the defect and nourishes a cartilaginous alar framework graft placed deep to the hinge flap to lend structural support to the nostril. A full-thickness skin graft is placed as an external covering over the hinge flap, thereby completing a single-stage reconstruction (Fig. 15-18).7
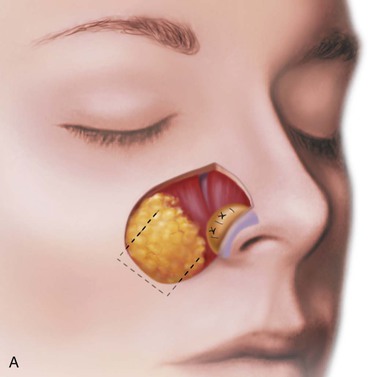
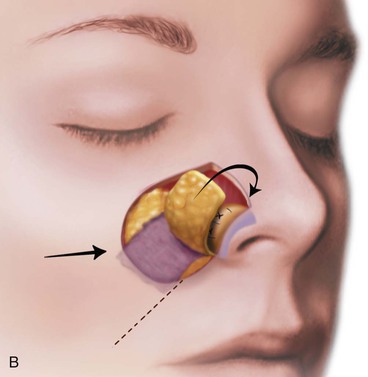
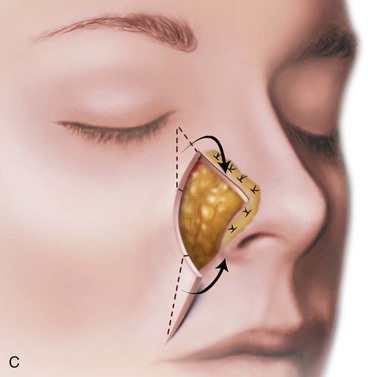
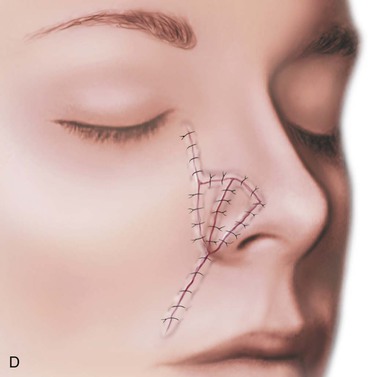
FIGURE 15-17 A, Fat hinge flap outlined by broken line is turned medially to cover cartilage graft placed along alar margins. Flap fills soft tissue void. B, Broken line in melolabial crease represents incision necessary to enable medial advancement of cheek skin to level of nasofacial sulcus. C, Borders of hinge flap tucked beneath nasal skin surrounding alar defect. D, Standing cutaneous deformities from advancement flap excised and used as full-thickness skin grafts to cover hinge flap and to resurface ala. (From Baker SR: Subcutaneous hinge cheek flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011, p 252.)
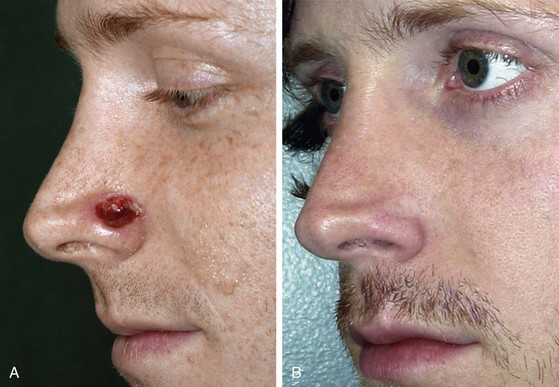
FIGURE 15-18 A, A 1 × 1.5-cm-deep alar defect, extending to but not through vestibular skin. Full-thickness skin graft used for repair because of young age, propensity for future skin cancers, and desire to avoid cheek scar. Wound bed augmented with subcutaneous tissue hinge flap followed by skin grafting. B, Postoperative view at 2 years. No adjunctive surgery performed.
When skin defects of the nasal sidewall that extend to the medial cheek are repaired with a skin graft, the cheek component of the defect is reconstructed with a cutaneous advancement flap developed from the remaining medial cheek skin. The flap is advanced to the level of the nasofacial sulcus and anchored in place with deep sutures that pass from the medial border of the flap to the periosteum of the nasal sidewall. A full-thickness skin graft is then used to resurface the sidewall. The cheek advancement flap enables the placement of scars in the aesthetic boundary between the cheek and nose. If the cheek defect is of considerable size, the standing cutaneous deformities that occur from advancement of the cheek flap may be excised and used as full-thickness skin grafts to cover the sidewall defect (see Fig. 15-17).
Technique
A template is made of the recipient site by outlining the periphery of the wound with a surgical marker and pressing a nonadherent dressing pad over the marking. If the defect is round, the shape may be modified by excising skin to create “corners.” This causes the defect and covering graft to have angulated borders, which lessens the likelihood of development of a trap-door deformity. The template is fashioned and used to design the size and configuration of the graft. Realizing that most full-thickness skin grafts contract 10% to 15% after excision from the donor site,8 the graft is designed slightly larger than the defect to accommodate this contraction. The graft is excised, and all subcutaneous tissue is removed from the deep surface of the graft with use of curved iris scissors. This is best accomplished by placing the graft over the index finger, epidermal side down, and trimming off excess fat until shiny dermis is visible. If reducing the thickness of the graft is desirable, the same scissors are used to remove portions of the exposed deep surface of the dermis.
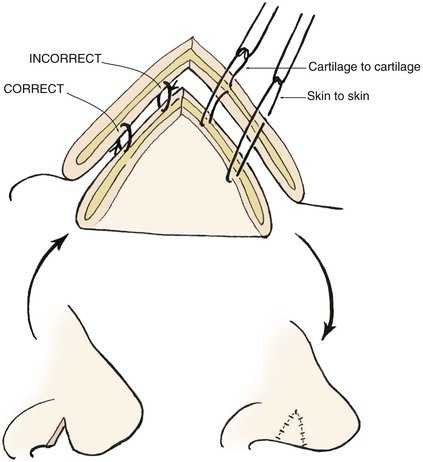
The skin graft is transferred to the recipient site and oriented in a manner to maximize contact between wound and graft. Excess graft is trimmed, leaving a slight redundancy. The graft may be secured with multiple “tacking” sutures that serve to attach the graft to the underlying tissue, thereby maximizing surface adherence and graft survival. Placement of tacking sutures may lead to punctate areas of epidermolysis and eventual textural and color discrepancies; therefore, the author attempts to place these sutures along boundary lines separating aesthetic regions and units whenever possible. Graft edges are secured to the borders of the recipient site with simple interrupted 5-0 polypropylene or chromic sutures. One end of each suture is left sufficiently long to be tied with another suture at the opposite side of the graft (Fig. 15-19). A bolster dressing is made from nonadherent bandage or gauze and secured with opposing sutures. Placement of petroleum-based antibiotic ointment between the dressing layers helps stabilize the bolster during suture fixation. Alternatively, cotton balls covered with petroleum-based antibiotic ointment may be wrapped with petrolatum 3% bismuth tribromophenate (Xeroform) to create a similar bolster material that is particularly helpful in covering larger wounds. Larger bolsters are usually secured with interrupted 3-0 silk sutures placed in pairs into the surrounding skin 1 to 2 mm from the actual wound edge (see Fig. 15-19).
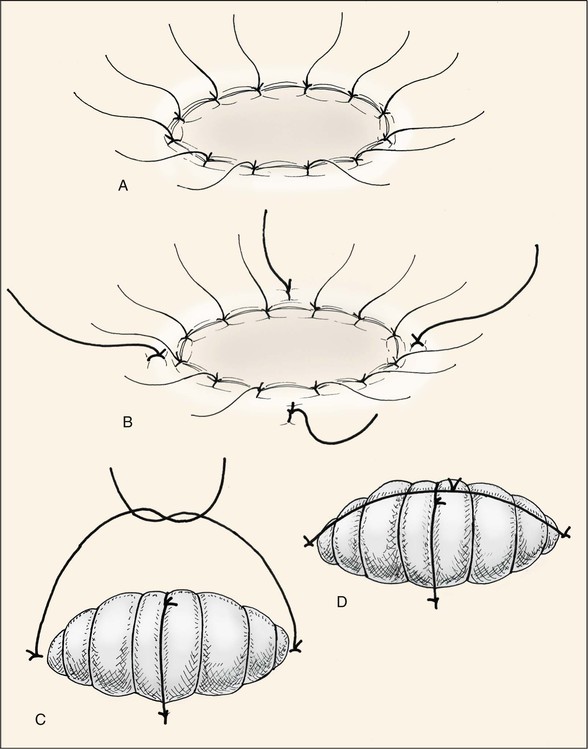
FIGURE 15-19 Variations of tie-down bolster dressing. A, Tie-over sutures placed at wound edge as part of perimeter fixation of graft. B-D, Alternatively, bolster sutures are placed 1 to 2 mm from wound edge.
The graft may appear cyanotic during the first few days after transfer. It then transitions to a hyperemic stage, which fades during weeks to months. On occasion, a cyanotic graft may not survive. If the entire graft dies, it will suppurate and separate from the recipient site within 2 weeks. More commonly, the deeper portion of the graft will survive while the more superficial portion forms an eschar, which remains fixed to the wound bed. When this occurs, the eschar is left in place as a biologic dressing, allowing healing by secondary intention. Re-epithelialization will occur from the wound edges and from the viable deeper dermal component of the graft. Sufficient dermis often survives to prevent the development of a depressed scar after complete healing; however, pigmentary and textural differences between facial skin and the skin graft are usually more apparent than when the graft survives completely.
Adjunctive procedures to optimize the aesthetic appearance of a graft may be performed 6 weeks after the graft has healed. Trap-door deformities usually resolve over time, especially if steroids are injected beneath the graft. Grafts rarely require a surgical contouring procedure, but Z-plasties are occasionally performed at the border of the graft to enhance the appearance of the transition between graft and native skin. Dermabrasion of all full-thickness skin grafts used to resurface nasal cutaneous defects is recommended. In addition to the graft, the skin of the entire aesthetic unit surrounding the graft is dermabraded. Dermabrasion is accomplished in the office with use of local anesthesia and is usually performed 8 weeks after successful grafting. The color and textural match of skin grafts with adjacent facial skin nearly always improves after dermabrasion. Grafts are occasionally dermabraded a second time if the first treatment fails to yield acceptable results.6
Split-Thickness Skin Grafts
Split-thickness skin grafts consist of epidermis and a variable portion of underlying dermis. They have more capillaries exposed on their undersurface compared with full-thickness grafts, permitting greater absorption of nutrients from the wound bed. In addition, split-thickness skin grafts consist of less tissue that requires revascularization. As a result, split-thickness grafts are often used for wounds in which revascularization is a concern. However, because of their likely poor color and texture match with normal skin and their tendency to contract, split-thickness skin grafts are selectively used in facial reconstruction. Some indications for split-thickness skin grafts are to cover defects too large to be repaired with a local flap (Fig. 15-20), to facilitate monitoring for potential cancer recurrence, and to provide temporary coverage of a wound bed before definitive reconstruction. Most split-thickness grafts are between 0.012 and 0.030 inch in thickness; thicker grafts have a relatively better color and texture match with facial skin.3
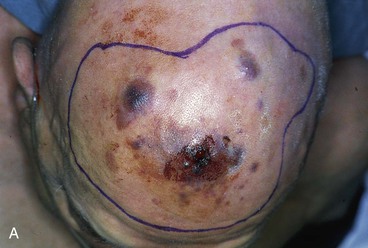
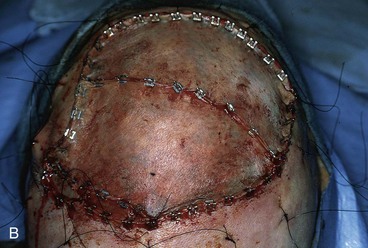
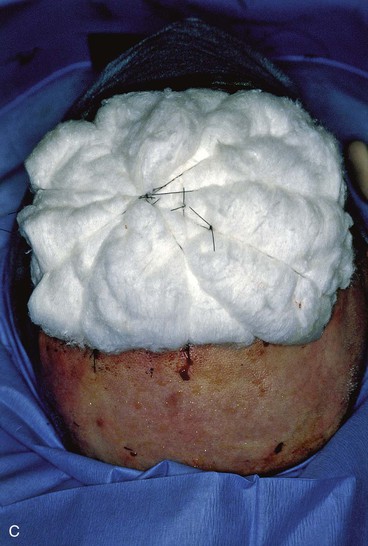
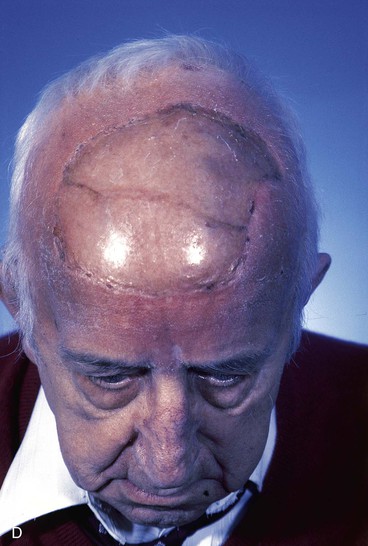
FIGURE 15-20 A, Angiosarcoma of scalp. B, Area resected and covered with split-thickness skin graft. Area too large to be repaired with local flap. C, Cotton bolster dressing. D, Postoperative view at 3 months. (Courtesy Shan R. Baker, MD.)
Technique
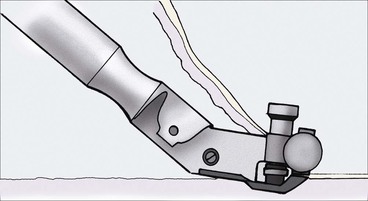
The electric Brown dermatome is a commonly used device to harvest split-thickness skin grafts, usually from the anterolateral thigh (Fig. 15-21). The dermatome is assembled before use, placing the disposable metal blade over the projecting rivets. The screws on the back of the dermatome are tightened, and the device is calibrated. Calibration involves setting the dermatome dials to 0, then moving both dials simultaneously to set the graft thickness dial at 0.015 inch, which corresponds to the passage of the beveled portion of a Bard-Parker No. 15 blade between the blade and casing. The donor site is marked, with the length of the graft equal to the greatest dimension of the recipient site plus an additional 15% to 20% in anticipation of some graft shrinkage.3 Grafts may be harvested under general anesthesia without use of a local anesthetic. If the patient is receiving intravenous sedation or no sedation, a local anesthetic consisting of 1% lidocaine with 1 : 100,000 concentration of epinephrine is injected subcutaneously at the donor site. After skin preparation, mineral oil is placed over the donor site. While the surgeon provides countertraction with his or her nondominant hand, an assistant provides traction with a tongue blade at the superior aspect of the donor area. Light downward pressure is applied to the dermatome on the donor site while the dermatome is advanced. Forceps are used to keep the graft from getting caught in the dermatome; at the end of the donor area, the dermatome is lifted away from the skin while still cutting. This facilitates tapering of the graft in that area. Split-thickness skin grafts are bolstered to the wound bed in a fashion similar to full-thickness skin grafts. Before placement of a compression dressing, small incisions may be made in the graft at evenly spaced intervals to allow fluid egress during the healing phase. Wound care after dressing removal is the same as that for full-thickness grafts.
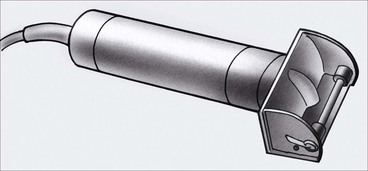
A gas-sterilized dermatome that is commonly used in the operating room is the Padgett dermatome (Fig. 15-22). This dermatome comes with three templates, allowing grafts with widths of 2, 4, or 6 inches to be harvested. No calibration is necessary, and grafts are harvested in a fashion similar to that with the Brown dermatome.
The donor site wound is dressed with gauze soaked in hydrogen peroxide to facilitate hemostasis. The gauze is then removed, the wound is padded dry, and a sterile Tegaderm OpSite dressing is placed. Dry gauze secured by tape is placed over the OpSite as a light compression dressing, with care taken not to wrap the tape circumferentially around the leg. Any fluid collection beneath the dressing may be drained after removal of the gauze at 48 hours. This is accomplished by insertion of a sterile needle through the OpSite to aspirate fluid, then “patching” of the needle hole with a small piece of OpSite. The OpSite is left in place for 5 to 7 days, and on removal, the wound is cared for by daily cleaning with saline and application of bacitracin and a nonadherent gauze dressing. Alternatively, hydrophilic polyurethane dressing (Allevyn), which is highly absorbent and nonadherent, may be used as a donor site dressing.3 This dressing has an external layer similar to OpSite and an inner layer that has an absorbent nature, which helps prevent fluid buildup and leaks. Brodovsky et al9 suggested that Nobecutane spray is an effective temporary dressing for skin graft donor sites. The spray contains a modified acrylic resin in an organic solvent and tetramethylthiuram disulfide, which is a bactericidal-fungicidal agent. When it is sprayed on a wound, the spray forms a transparent plastic film that is shed spontaneously with epidermal regeneration.9
Composite Chondrocutaneous Grafts
Composite grafts contain two or more tissue layers and often are unsuccessful secondary to high metabolic demands.10 They obtain their nourishment through plasma imbibition during the first 24 hours after transfer. This is followed by vascular inosculation. Ingrowth of capillaries from the edges of the graft begins by the third day.11,12
Composite grafts were first described by Konig,13 who used composite auricular grafts to repair alar defects, noting a 53% graft survival. Composite grafts have been used to repair columellar defects14,15 and deficiencies in nasal lining.16 During the first half of the 20th century, Limberg17 advocated the cavum and cymba of the concha as the preferred donor site of composite grafts used to repair the nose, and Gillies18 described the transfer of composite grafts to the undersurface of forehead flaps for nasal reconstruction. Symonds and Crikelair19 also used composite auricular grafts for nasal reconstruction, reporting an 89% graft survival rate.
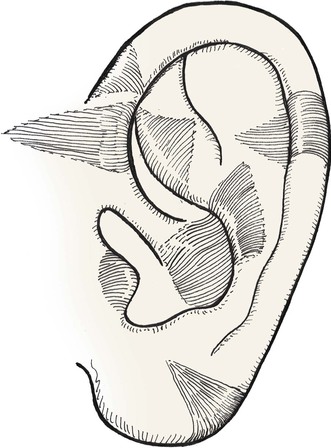
Composite grafts are commonly used to repair small full-thickness nasal defects. The auricle is an excellent donor source for composite grafts used for nasal reconstruction because it provides a contoured graft of skin and cartilage (Fig. 15-23). Certain segments of the auricle can loosely replicate the delicate topography of the columella, facet, and nostril margin, where composite grafts are commonly employed. The skin is tightly attached to the underlying cartilage at the columella and to the fibrofatty tissue of the alae and facets. Likewise, the skin covering the lateral aspect of the auricle is firmly attached to the underlying cartilage. Common auricular donor sites are helical crus, helical rim, antihelix, tragus, antitragus, and fossa triangularis. The helical crus provides a good contour match for small nostril margin defects and provides the option of incorporating in the graft a segment of preauricular skin. Incorporation of preauricular skin with the composite graft is useful for resurfacing of larger nasal defects requiring considerable skin but minimal framework replacement.6
The traditional recommendation is to limit the size of composite grafts to 1 cm or less.20 Considerably larger grafts may be successful if they are placed in a vascular recipient site and the graft is designed so that no portion is more than 1 cm from a wound edge.21–24 Skouge advocated a tongue-and-groove technique when composite grafts are used. This technique involves insetting the border of the graft between two layers of tissue at the recipient site.4 This method of graft attachment has the effect of increasing surface contact between graft and recipient site by 50%.20 If a defect is allowed to heal by secondary intention and then reconstructed with a composite graft, there is a greater success rate. In such instances, a hinged flap of scar and epithelium formed during secondary healing can be dissected to increase the surface area for attachment of a composite graft (Fig. 15-24).25
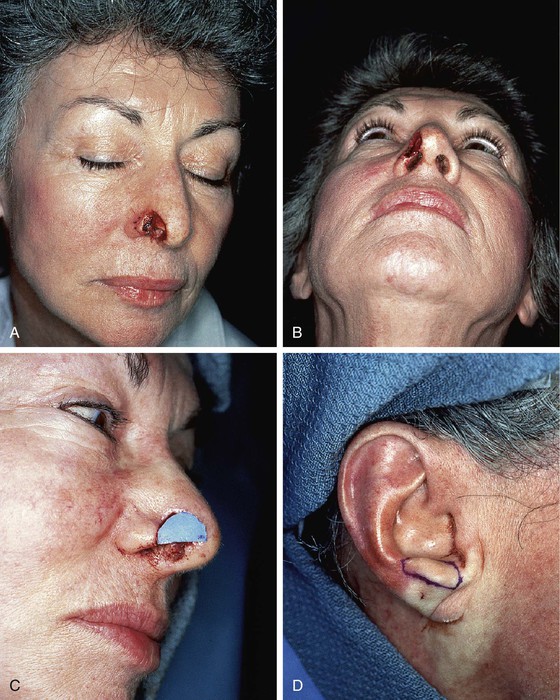
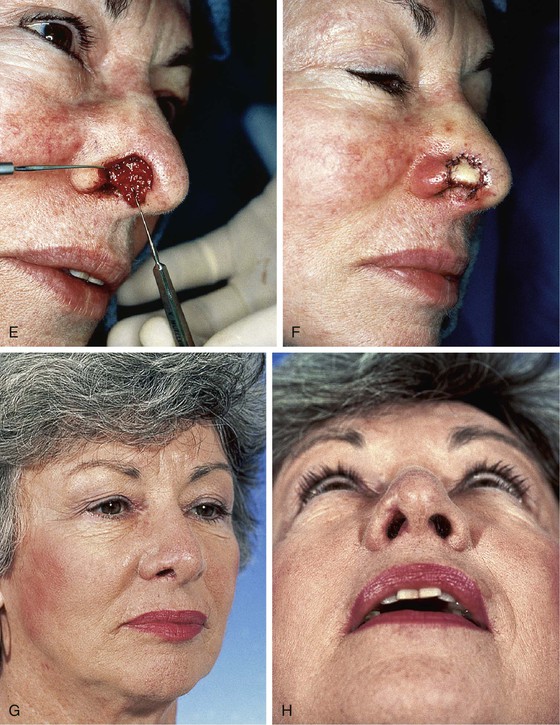
FIGURE 15-24 A, B, Full-thickness defect of nostril margin allowed to heal by secondary intention before repair with composite auricular graft. C, D, Template 0.2 cm larger than defect used to design composite graft of antitragus. E, Flap of soft tissue hinged on border of defect developed to enhance surface contact with graft. F, Composite graft secured to recipient site with limited number of cutaneous sutures. G, H, Six months after grafting.
The ideal nasal defect for a composite auricular graft is a small (1 cm or less) full-thickness defect of the facet, columella, or nostril margin. The nasal skin in these areas is extremely thin, lacking subcutaneous fat, and is tightly attached to the alar cartilage in the case of the columella and fibrous connective tissue in the case of the facets and alae. Skin flaps used to repair the facet or columella convey skin with subcutaneous fat and are thicker than the native nasal skin. In contrast, composite auricular grafts obtained from the helical crus provide a graft with thin skin attached to a delicate segment of cartilage. The cartilage provides structural support, and the skin closely resembles the adjacent nasal skin of the columella and facet (Fig. 15-25).
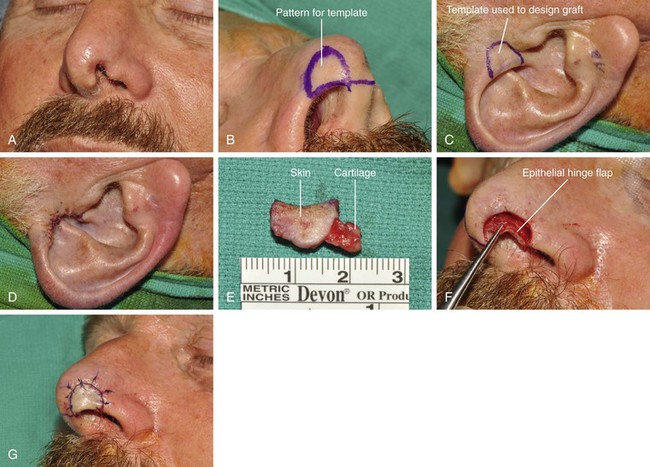

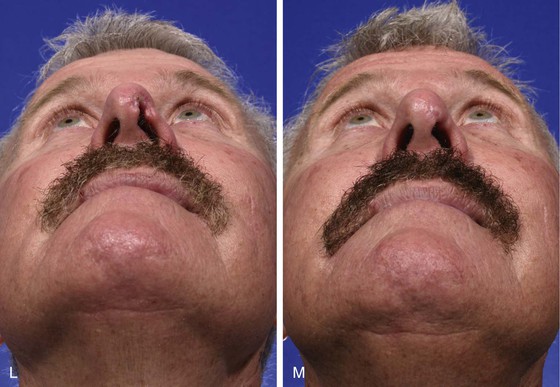
FIGURE 15-25 A, Full-thickness defect of nasal facet. B, Template designed from contralateral facet. C, D, A 1.2 × 1-cm composite graft harvested from helical crus and donor site closed primarily. E, Ear cartilage extended between soft tissue leaves at recipient site margin. F, Epithelial hinge flap created to maximize contact surface between graft and recipient site. G, Graft sutured in place. H-M, Preoperative and 1-year postoperative views. Small Z-plasty at margin of graft and nostril performed 6 months postoperatively. Graft also dermabraded. (Courtesy Shan R. Baker, MD.)
Perhaps more commonly, composite grafts are used to repair small defects of the nostril margin (Fig. 15-26). Deep wounds in this location may lead to notching of the nostril margin. Composite grafts may be used to maintain structural integrity and to provide a smooth continuous border to the nostril.7 Weisberg and Becker26 described the use of auricular composite grafts with stabilizing struts for repair of such defects. The stabilizing struts are extensions of cartilage that are placed beneath the adjacent nasal skin in a tongue-and-groove fashion similar to Skouge’s original description (see Fig. 15-25).
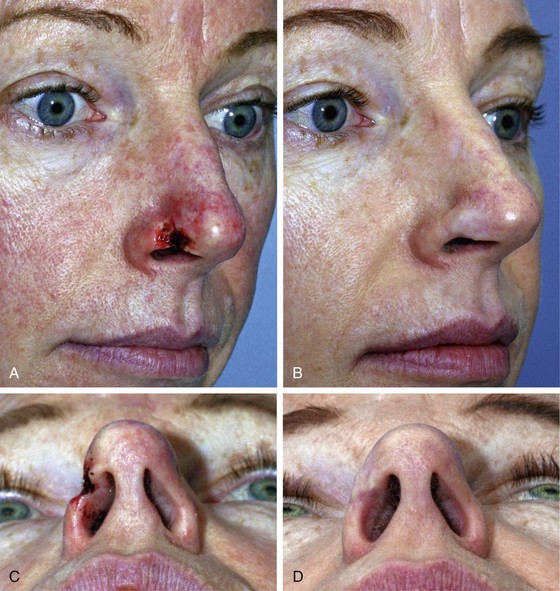
FIGURE 15-26 A, C, Full-thickness defect, extending to nostril rim. Composite graft from ipsilateral antitragus used for reconstruction. B, D, Postoperative views at 18 months. Contouring procedure performed 3 months postoperatively.
The use of perioperative corticosteroids is beneficial in enhancing survival of composite grafts in animals. Rabbits treated with preoperative and postoperative methylprednisolone demonstrated improved graft survival compared with animals receiving no steroids or postoperative doses only. Attempts to salvage compromised grafts with delayed administration of steroids were not successful.27,28 Cooling of composite grafts has also been demonstrated to improve survival. Cooling reduces biologic requirements and improves graft survival in irradiated, atrophic, or scarred recipient sites. Conley demonstrated that constant application of ice and ice compresses for 14 days effected a fall in skin temperature from 38°F to 17°F. Composite grafts ranged in size from 1 × 1-cm to 2 × 2-cm. Of 12 composite grafts transferred to the nose and treated with ice compresses, 10 survived completely. Five of the grafts had been placed in recipient sites with scarring or postirradiation fibrosis.29
Technique
There is an improved survival rate if grafting is delayed until the defect has healed by secondary intention. The defect is then prepared by removal of the epithelium and subepithelial scar tissue, and a template is created that measures 2 mm larger than the defect in all dimensions. Harvesting a graft that is slightly larger than the defect accommodates the inevitable contraction of the graft. Whenever possible, a flap of soft tissue hinged on a border of the defect is developed to enhance surface contact with the graft. A composite graft containing skin and cartilage is harvested from the region of the auricle that most closely matches the contour and thickness of the recipient site (Fig. 15-27). The graft is placed in cold saline, and the donor site is closed primarily with 5-0 polydioxanone sutures to approximate the edges of the auricular cartilage and 5-0 polypropylene vertical mattress sutures for the skin. The graft is transferred to the recipient site and secured in place with 5-0 polypropylene simple cutaneous sutures while limiting the degree of graft manipulation. Subdermal or subcutaneous sutures are not employed. With use of as few sutures as possible, precise approximation of skin edges is accomplished. If the graft is small, no sutures are placed through the cartilage. Larger grafts will require a few sutures approximating the cartilage of the composite graft to the cartilage of the recipient site (Fig. 15-28). Limiting the number of sutures used to fix the graft in place is thought to be beneficial in enabling earlier and more abundant vessel ingrowth.
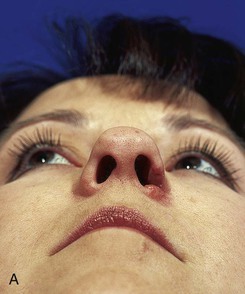
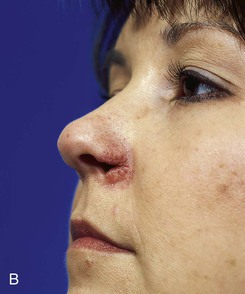
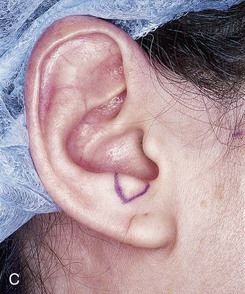
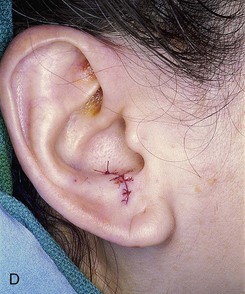
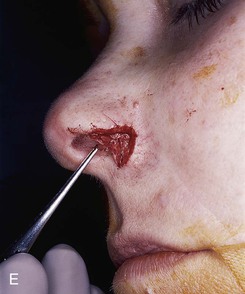
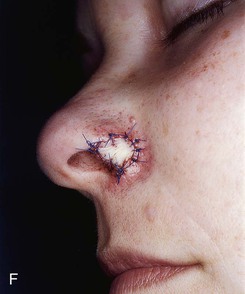
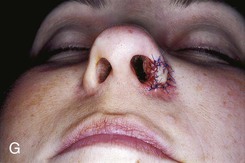
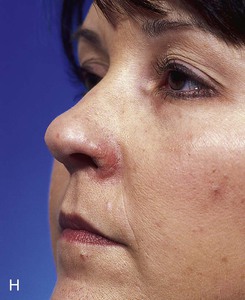
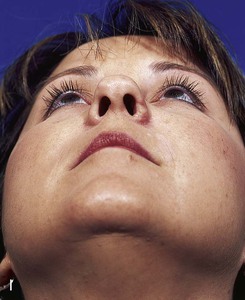
FIGURE 15-27 A, B, Full-thickness defect of ala. C, D, Composite auricular graft harvested from antitragus. E, Hinge flap of epithelium used to line interior of ala and to provide greater surface contact with composite graft. F, G, Composite graft sutured in place with limited cutaneous sutures. H, I, Postoperative views at 3 months. No adjunctive surgery performed. (Courtesy Shan R. Baker, MD.)
Other Composite Grafts
Other types of composite grafts have been described for nasal reconstruction. Composite grafts consisting of skin and subcutaneous fat from the earlobe or full-thickness segments of the contralateral alar base may be used to repair defects of the alar base or nostril margin.13 Stucker and Shaw30 described the use of perichondrocutaneous grafts that consist of epidermis, dermis, scant subcutaneous tissue, and a perichondrial layer. The grafts are harvested from the cavum concha, and an island pedicled skin flap from the postauricular sulcus is used to repair the donor site. These authors reported excellent aesthetic results, with no contraction of the graft.31
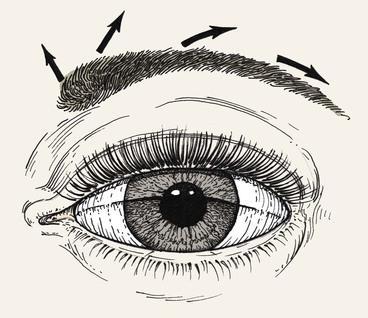
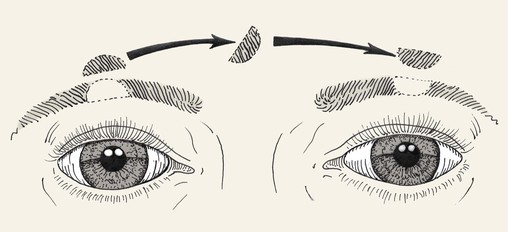
Composite grafts may also be used in eyelid (see discussion in Chapter 17) and eyebrow reconstruction. Tarsoconjunctival grafts are sometimes used for reconstruction of the posterior lamellae of the eyelid. Furthermore, strip grafting, involving the transfer of skin, hair follicles, and fat from the scalp, has been used for eyebrow reconstruction (Fig. 15-29). Survival may be impaired in eyebrow defects that are severely scarred. Although the results are acceptable, strip grafting has been largely replaced by microfollicular hair transfer.3 The multidirectional orientation of eyebrow hair should be considered during eyebrow reconstruction (Figs. 15-30 and 15-31).
References
1. Johnson, TM, Nelson, BR. Anatomy of skin. In: Baker SR, Swanson NA, eds. Local flaps in facial reconstruction. St. Louis: Mosby; 1995:3.
2. Goding, GS, Hom, DB. Skin flap physiology. In: Baker SR, Swanson NA, eds. Local flaps in facial reconstruction. St. Louis: Mosby; 1995:15.
3. Glogau, RG, Haas, AF. Skin grafts. In: Baker SR, Swanson NA, eds. Local flaps in facial reconstruction. St. Louis: Mosby; 1995:247.
4. Skouge, JW. Skin grafting. New York: Churchill Livingstone; 1991.
5. Meyers, S, Rohrer, T, Grande, D. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001; 27:300.
6. Jewett, BS. Skin and composite grafts. In: Baker SR, ed. Principles of nasal reconstruction. St. Louis: Mosby; 2002:58.
7. Jewett, BS. Repair of small nasal defects. Facial Plast Surg Clin North Am. 2005; 13:283.
8. Hill, TG. Reconstruction of nasal defects using full-thickness grafts: a personal reappraisal. J Dermatol Surg Oncol. 1983; 12:995.
9. Brodovsky, S, Dagan, R, Ben-Bassatt, M. Nobecutane spray as temporary dressing of skin graft donor sites. J Dermatol Surg Oncol. 1986; 12:386.
10. Konior, RJ. Free composite grafts. Otolaryngol Clin North Am. 1994; 27:81.
11. Clairmont, AA, Conley, JJ. The uses and limitations of auricular composite grafts. J Otolaryngol. 1978; 7:249.
12. McLaughlin, CR. Composite ear grafts and their blood supply. Br J Plast Surg. 1954; 7:274.
13. Konig, F. Über Nasenplastik. Beitr Klin Chir. 1914; 94:515.
14. Converse, JM. Reconstruction of nasolabial area by composite graft from the concha. Plast Reconstr Surg. 1950; 5:247.
15. Meade, RJ. Composite ear grafts for construction of columella. Plast Reconstr Surg. 1959; 23:134.
16. Dingman, RO, Walter, C. Use of composite grafts in correction of the short nose. Plast Reconstr Surg. 1969; 43:117.
17. Limberg, A. Rhinoplasty using free transplant from concha. Sovet Khir. 1935; 9:70.
18. Gillies, HD. A new free graft applied to the reconstruction of the nostril. Br J Surg. 1943; 30:305.
19. Symonds, FC, Crikelair, GF. Auricular composite grafts in nasal reconstruction. Plast Reconstr Surg. 1956; 37:433.
20. Ballantyne, DL, Converse, JM. Vascularization of composite auricular grafts transplanted to the chorio-allantois of the chick embryo. Transplant Bull. 1958; 5:373.
21. Ruch, M. Utilization of composite free grafts. J Int Coll Surg. 1958; 30:274.
22. Becker, OJ. Extended application of free composite grafts. Trans Am Acad Ophthalmol Otolaryngol. 1960; 64:649.
23. Avelar, JM, Psillakis, JM, Viterbo, F. Use of large composite grafts in the reconstruction of deformities of the nose and ear. Br J Plast Surg. 1984; 37:55.
24. Davenport, G, Bernard, FD. Improving the take of composite grafts. Plast Reconstr Surg. 1959; 24:175.
25. Converse, JM, Reconstructive plastic surgery vol 2. Saunders, Philadelphia, WB, 1964.
26. Weisberg, NK, Becker, DS. Repair of nasal ala defects with conchal bowl composite grafts. Dermatol Surg. 2000; 26:1047.
27. Aden, KK, Biel, MA. The evaluation of pharmacologic agents on composite survival. Arch Otolaryngol Head Neck Surg. 1992; 118:175.
28. Hartman, DF, Good, RL. Pharmacologic enhancement of composite graft survival. Arch Otolaryngol Head Neck Surg. 1987; 113:720.
29. Conley, JJ, Van Fraenkel, P. The principle of cooling applied to the composite graft in the nose. Plast Reconstr Surg. 1956; 17:444.
30. Stucker, FJ, Shaw, GY. The perichondrial cutaneous graft. Arch Otolaryngol Head Neck Surg. 1992; 118:287.
31. Portuese, W, Stucker, F, Grafton, W, et al. Perichondrial cutaneous graft: an alternative in composite skin grafting. Arch Otolaryngol Head Neck Surg. 1989; 115:705.