Chapter 51 Silicosis and Coal Worker’s Pneumoconiosis
Sources of Exposure
The most common form of crystalline silica is quartz. Quartz is almost pure silicone dioxide but often contains traces of other elements. Other crystalline forms of silica are cristobalite and tridymite. The importance of silica as a health hazard is due to its ubiquity (Table 51-1). Diatomite is a siliceous sedimentary rock used for filtration; for heat and sound insulation; as an adsorbent and filtering agent; as a filler material in plastics, paper, and insecticides; and in the manufacture of floor coverings.
| Occupation | Exposure |
|---|---|
| Sand blaster | Ship building, oil rig maintenance, preparing steel for painting |
| Miner | Surface coal mining, roof bolting, shot firing, drilling, tunneling |
| Miller | Silica flour |
| Glass maker | Polishing with sand and enamel work |
| Potter cleaner | Crushing flint and fettling, foundry work, mold making and vitreous enameling, manufacture of cultured quartz crystal |
| Quarry and stone worker | Cutting of slate, sandstone, and granite |
| Abrasive worker | Inhalation of fine particles during grinding |
Pathophysiology
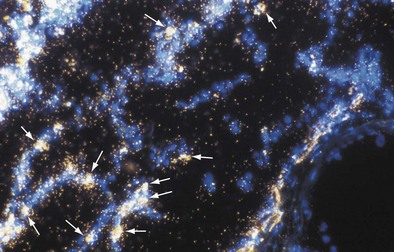
The inflammatory phase is followed by a reparative phase, in which growth factors stimulate the recruitment and proliferation of mesenchymal cells and regulate neovascularization and reepithelialization of injured tissues. During this phase, abnormal or possibly uncontrolled reparative mechanisms may result in the development of fibrosis. Fibrogenic particles activate proinflammatory cytokine production within the respiratory tract. Tumor necrosis factor (TNF)-α seems to play a key role in the recruitment of inflammatory cells induced by toxic dusts (Figure 51-1). In addition, neutrophils recruited in the area of inflammation may contribute to the alveolitis, and respiratory and endothelial cells may play a further role by releasing various chemokines such as interleukin (IL)-8. Finally, growth factors such as platelet-derived growth factor, insulin-like growth factor, fibroblast growth factor, and transforming growth factor-β are involved in the pathogenesis of lung fibrosis and in the proliferative response of type II epithelial cells, which occurs in progressive massive fibrosis (PMF).
Histopathologic Changes
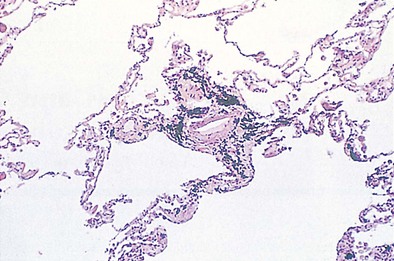
The pleural surfaces of a coal worker’s lung show an irregular pattern of bluish-black pigmentation that corresponds to the junction sites of septal-lymphatic vessels and the pleura. Peribronchial, hilar, and paratracheal lymph nodes are enlarged, black, and firm. The initial lesions in the lung are the coal dust macules, which correspond macroscopically to focal areas of black pigmentation. On microscopic examination, the macule is seen to be composed of coal dust–laden macrophages within the walls of the respiratory bronchioles and adjacent alveoli (Figure 51-2). Focal emphysema around the coal dust macule is common and is considered an integral part of the lesion of simple CWP.
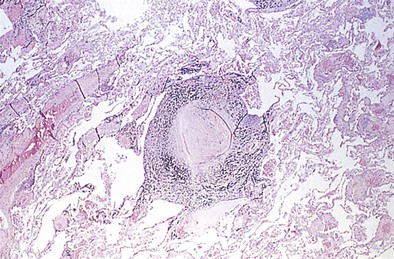
The histopathologic hallmark of simple CWP is the nodule. The nodules are rounded lesions with collagenous centers. On microscopic examination, the nodule can be divided into three zones: a central zone composed of whorls of dense, hyalinized fibrous tissue; a middle zone made up of concentrically arranged collagen fibers (onion-skinning); and a peripheral zone of more randomly oriented collagen fibers mixed with dust-laden macrophages and lymphoid cells (Figure 51-3). “Old” inactive nodules often are relatively acellular. Particles of silica may be demonstrated in the nodules as birefringent particles under polarized light. Nodules represent a form of mixed-dust fibrosis (i.e., coal dust plus silica exposure), usually are found in association with macules, and in some instances may develop from preexisting macules. They are not confined to the respiratory bronchioles but also are seen in the subpleural and peribronchial connective tissues. Nodules tend to cluster and eventually coalesce to produce PMF. Degenerative changes commonly are observed in the nodular lesions, including calcification, cholesterol clefts, and cavitation. In severe silicosis, structural alterations of the pulmonary vasculature may result from the accumulation of dust in the adventitia of large vessels, and involvement of the smaller blood vessels by silicotic nodules also may be seen.
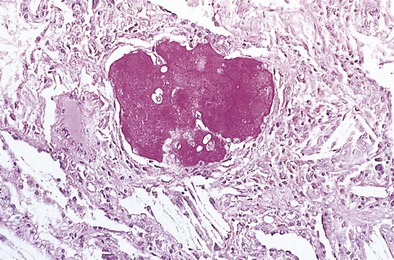
The histopathologic pattern in acute silicosis is quite different from that in the chronic form. Infiltration of the alveolar walls with plasma cells, lymphocytes, and fibroblasts, with some collagenation, is typical. The alveoli are filled with an eosinophilic coagulum (Figure 51-4). Electron microscopy shows widening of alveolar walls, with some collagen and clusters of type II cells; the alveolar spaces contain degenerating cells that probably are type II alveolar cells and macrophages. Silica particles may be demonstrated in the lungs and lymph nodes; silicotic nodules are few or absent.
Clinical Features and Diagnosis
CWP and silicosis generally are first recognized from characteristic changes on the plain chest radiograph, which also is critical in evaluating disease progression. Requirements for the diagnosis include a history of significant exposure, radiographic features consistent with these illnesses, and the absence of any other disease that may mimic pneumoconioses (primarily infections with a predominantly miliary radiographic pattern, such as tuberculosis, fungal infections, or sarcoidosis). The radiographic appearances are most usefully described using the coding system devised for classification of findings on standard chest films in pneumoconiosis under the auspices of the International Labour Office (ILO) (Table 51-2). In clinical practice, simple CWP is characterized by small rounded opacities (nodules) rather than small irregular opacities, although the latter may be seen in much lesser profusion (as described in Table 51-2, profusion categories 0 to 3 refer to the relative abundance of small opacities apparent on the chest radiograph). For designation of disease characterized by large opacities, either PMF or complicated CWP is in common use.
Table 51-2 International Labour Organization (ILO) Radiographic Classification of Pneumoconioses
| Small Opacities* | Regular | Irregular |
|---|---|---|
| <1.5 mm in diameter | p | s |
| >1.5 mm but <3 mm in diameter | q | t |
| >3 mm but <10 mm in diameter | r | u |
| Categories | ||
| Small Opacities | ||
| The four profusion categories, 0 to 3, refer to the concentration (density) of small opacities apparent on the radiograph: category 0, small opacities are absent or less profuse than in category 1; 1, opacities are few in number; 2, opacities are numerous; 3, opacities are very numerous and obscure the normal radiographic markings. | ||
| Large Opacities | ||
| Complicated pneumoconiosis or progressive massive fibrosis (PMF) is divided into categories A to C based on the size of the large opacities. To be classified as PMF, at least one nodule should be 1 cm or greater in diameter. | ||
* Small opacities are defined by their average size and profusion.
Clinical Features
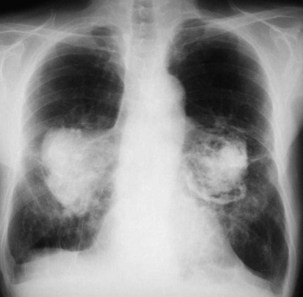
Other than PMF, CWP may be associated with a number of other disorders—most notably the autoimmune disorders of rheumatoid disease and progressive systemic sclerosis. The combination of rheumatoid disease and CWP is known as Caplan syndrome (Figure 51-5). This diagnosis is suggested by the association of coal dust exposure, rheumatoid arthritis, and multiple well-defined large, rounded opacities (nodules greater than 10 mm in diameter) on the chest radiograph. Spontaneous disappearance is common, with or without initial cavitation, and new nodules frequently emerge in different locations. The Caplan nodule also is more likely to cavitate, thereby producing a concentric ring pattern, so this lesion also is known as a necrobiotic nodule. Central necrosis is rare in nodules of CWP, although it may occur in conglomerate lesions.
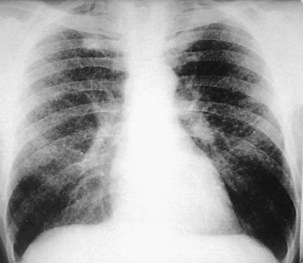
Acute silicosis is rare. Presenting signs and symptoms include cough, weight loss, and fatigue, with rapid progression to fulminant respiratory failure over several months in some cases. Chest auscultation reveals diffuse crackles, and in patients with this clinical picture, the rapid development of cor pulmonale eventuating in respiratory death is characteristic. Survival after the onset of symptoms often is less than 2 years. Diffuse alveolar filling, most apparent at the bases, is the most prominent finding on the chest radiograph (Figure 51-6). Although serial chest radiographs from workers with this illness have been infrequently reported, it seems that the bibasilar filling pattern progresses into large opacities located in the middle zones rather than the upper zones.
Chest Radiology
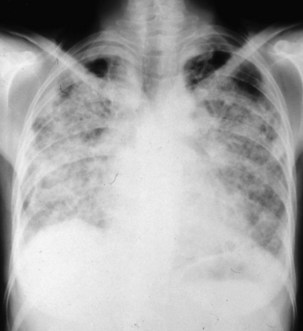
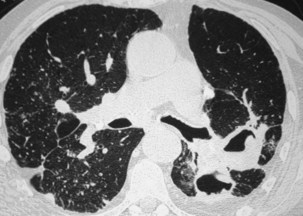
The radiographic pattern in simple CWP typically is one of small rounded opacities that appear first in the upper zones (Figure 51-7). The middle and lower zones become involved as the number of opacities increases. The nodules increase in profusion with increasing dust exposure; a change in profusion after dust exposure has ceased is very unusual. Calcification of the nodules may occur (in 10% to 20% of cases).
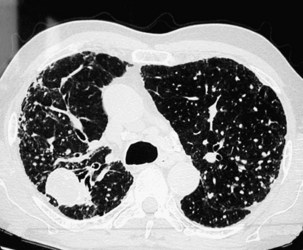
Complicated pneumoconiosis is defined as presence of a lesion of 1 cm or greater in longest diameter. The large opacities usually are predominant in the upper lobes, may be unilateral or bilateral, and are symmetrically or asymmetrically distributed (Figure 51-8). The pattern of change in size is variable and unpredictable. In most but not all cases, PMF occurs on a background of simple pneumoconiosis, and it may appear after dust exposure has ceased. Cavitation can develop within a PMF lesion (Figure 51-9), and occasionally a dense peripheral arc or rim may be seen at its lower pole that represents calcification. Dense calcification with the lesion also is sometimes seen. PMF often is associated with bullous emphysema and fibrotic scarring, leading to distortion of the lung and shift of the trachea and mediastinum to the affected side. Irregular, mainly basal opacities also may be seen on standard radiographs. Eggshell calcification is uncommon in CWP but may occur in intrapulmonary, hilar, or mediastinal lymph nodes, possibly because of concomitant exposure to silica. Pleural effusion is uncommon in CWP. Its presence may be related to an associated infection or an interaction with a systemic collagen vascular disease.
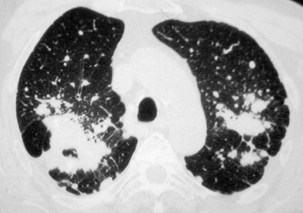
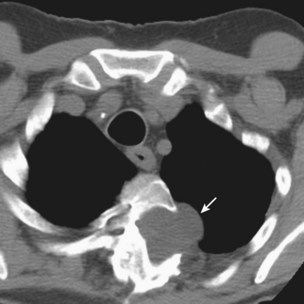
Nodules usually are observed against a background of parenchymal micronodules and generally are associated with subpleural micronodules. Two categories of lesions can be observed in PMF: lesions with irregular borders that are associated with disruption of the pulmonary parenchyma and lead to typical scar emphysema (Figure 51-10) and lesions with regular borders that are unassociated with scar emphysema. When the lesions are greater than 4 cm in diameter, irregular areas of aseptic necrosis can be observed with or without cavitation (Figure 51-11).
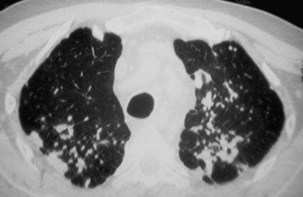
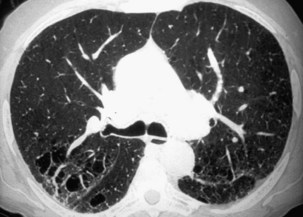
Two major forms of emphysema occurring in coal workers can be detected on the CT scan: bullous changes around PMF lesions, representing paracicatricial or scar emphysema, and nonbullous lesions, defined as irregular emphysema (Figure 51-12). Lesions of diffuse pulmonary fibrosis can be detected on high-resolution CT as honeycombing or areas of ground glass attenuation. Two specific etiopathogenic mechanisms for fibrosis in coal miners should be considered: (1) a direct effect of deposited coal or silica particles and (2) an indirect effect resulting from an association with scleroderma. In addition, determination of the extent of air trapping on expiratory thin-section CT scans may assess obstructive abnormalities.
Akgun M, Araz O. An epidemic of silicosis among former denim sandblasters. Eur Respir J. 2008;32:1295–1303.
Attfield MD, Seixas NS. Prevalence of pneumoconiosis and its relationship to dust exposure in a cohort of U.S. bituminous coal miners and examiners. Am J Ind Med. 1995;27:137–151.
Begin R, Cantin A, Massé S. Recent advances in the pathogenesis and clinical assessment of mineral dust pneumoconioses: asbestosis, silicosis and coal pneumoconiosis. Eur Respir J. 1989;2:988–1001.
Goldsmith DF. Research and policy implications of IARC’s classification of silica as a group 1 carcinogen. Indoor Built Environ. 1999;8:136–142.
Piguet PF, Collart MA, Grau GE, et al. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–247.
Remy-Jardin M, Remy J, Farre I, Marquette CH. Computed tomography evaluation of silicosis and coal worker’s pneumoconiosis. Radiol Clin North Am. 1992;30:1155–1176.
Rom WN, Bitterman PB, Rennard SI, et al. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dust. Am Rev Respir Dis. 1987;136:1429–1434.
Vanhee D, Gosset P, Boitelle A, et al. Cytokines and cytokine network in silicosis and coal workers’ pneumoconiosis. Eur Respir J. 1995;8:1–9.
Wouters EF, Jorna TH, Westenend M. Respiratory effects of coal dust exposure: clinical effects and diagnosis. Exp Lung Res. 1994;20:385–394.
Yu IT, Tse LA. Exploring the joint effects of silicosis and smoking on lung cancer risks. Int J Cancer. 2007;120:133–139.
Yucesoy B, Luster MI. Genetic susceptibility in pneumoconiosis. Toxicol Lett. 2007;168:249–254.
Zhai R, Jetten M, Schins RP, et al. Polymorphisms in the promoter of the tumor necrosis factor alpha in coal miners. Am J Ind Med. 1998;34:318–324.