Chapter 11 Silicone Stent Insertion for Focal Crescent–Type Tracheomalacia in a Patient with Sarcoidosis
Case Description
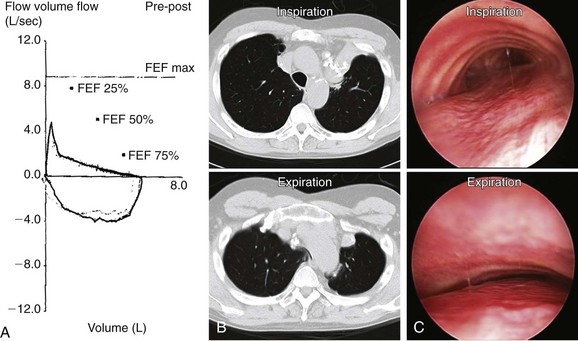
A 62-year-old man with a history of pulmonary sarcoidosis diagnosed 10 years earlier presented with chronic cough and inability to clear secretions. He described his cough as “seal barking.” He had also noted increasing dyspnea with normal physical activities. For the past month, his primary care physician had treated him with short- and long-acting bronchodilators, as well as inhaled and systemic steroids (prednisone 20 mg/day) for suspected adult-onset asthma. He was a nonsmoker, worked as an electrician, and had a history of hypertension and supraventricular tachycardia controlled by diltiazem. Electrocardiogram (ECG) and two-dimensional (2D) echocardiogram were unremarkable. Pulmonary function testing (PFT) revealed moderate obstructive ventilatory impairment without improvement after bronchodilators. Flow volume loop (FVL) showed an airway collapse pattern on the expiratory curve (Figure 11-1). Paired inspiratory/expiratory dynamic computed tomography showed bilateral upper lobe fibrosis, tracheomegaly, and localized narrowing of the lower trachea during dynamic expiration (see Figure 11-1). Flexible bronchoscopy revealed fibrotic closure of the right and left upper lobar bronchi and complete expiratory collapse of the anterior cartilaginous structures in the lower trachea, giving the airway lumen the shape of a crescent (see Figure 11-1).
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient presented with dyspnea, cough, and mucus retention. These nonspecific symptoms of pulmonary disorders are seen primarily in patients suffering from asthma, chronic obstructive pulmonary disorder (COPD), or bronchiectasis. His cough, however, was described as “seal barking.” This pattern is suggestive of expiratory central airway collapse (ECAC) and presumably is caused by vibration of the floppy membranous posterior wall against the anterior airway wall during expiration.1 As in our patient, symptoms are usually refractory to corticosteroids and bronchodilators.2 In addition, patients with ECAC may have recurrent bronchitis, pneumonia, and even respiratory failure.3 Some patients experience cough-syncope, presumably as a result of a significant rise in intrathoracic pressure and a sudden drop in venous return and cardiac output4 during violent coughing episodes.
Spirometry showed obstructive ventilatory impairment. In ECAC, spirometry usually demonstrates obstruction that is proportionate to the severity of the disease.5 The expiratory FVL seen in this patient suggested compression of the central airways (see Figure 11-1). This “airway collapse” pattern occurs when maximal flow is quickly reached after expiration of a small volume of air. Following maximal flow, a large fall in flow occurs, although only a small volume is exhaled. Subsequently, the flow rate falls very little during the remainder of expiration. This phase is responsible for the long plateau seen on the FVL (see Figure 11-1).6 However, the FVL is neither sensitive nor specific; it can be seen in almost 40% of patients with severe COPD.7 Flow oscillations on the FVL have also been described. These take on a sawtooth appearance, defined as a reproducible sequence of alternating decelerations and accelerations of flow. This pattern is nonspecific because it can be seen in patients with obstructive sleep apnea, structural or functional disorders of the upper airway, and neuromuscular diseases.8 Lack of clinical and spirometric improvement after bronchodilator administration might be explained by the effects of bronchodilators on central airways. Bronchodilators cause smooth muscle relaxation, further decreasing tracheobronchial wall stiffness and worsening airway obstruction. Bronchodilators increase airway wall compliance. The resulting increased airway compressibility will cause the choke point to be localized to a point closer to the thoracic outlet. The increased length of the upstream segment (from the alveoli to the choke point) and the decreased cross-sectional area at the choke point offset any advantages gained by bronchodilator-induced caliber increase in upstream airways with respect to maximal expiratory flow.9 In fact, although this effect has not been studied in adults, a dramatic fall in peak flow in response to bronchodilators has been described in pediatric cases of tracheobronchomalacia (TBM).10*
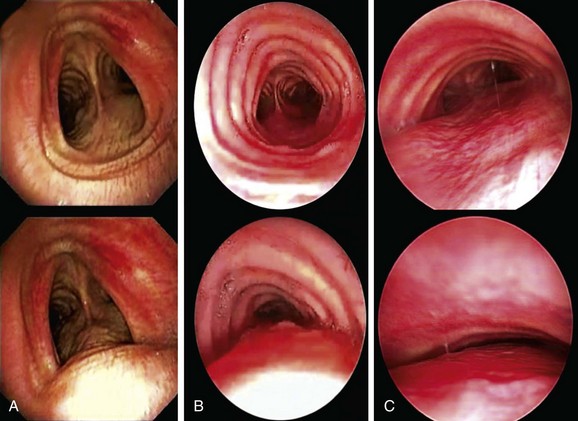
In our patient, a diagnosis of expiratory central airway collapse was made bronchoscopically and on paired dynamic inspiratory/expiratory computed tomography (CT) scanning. Because of the obvious weakness of the lower anterior tracheal cartilaginous wall, this patient had focal crescent tracheomalacia.11 The disorder was characterized by a severe (100%) degree of airway narrowing. During dynamic (functional) bronchoscopy, the airways were visualized by moving the patient into supine, upright, and lateral decubitus positions during spontaneous breathing, as well as during cough, forced expiration, and deep inspiration. During these bronchoscopic assessments, changes in airway lumen can be measured and the extent of collapse noted, and narrowing can be classified as being of the crescent, saber-sheath, or circumferential type. Regions of cartilaginous weakness (tracheomalacia) can be differentiated from areas of excessive dynamic airway collapse (EDAC) or normal physiologic dynamic airway collapse (DAC) (Figure 11-2).
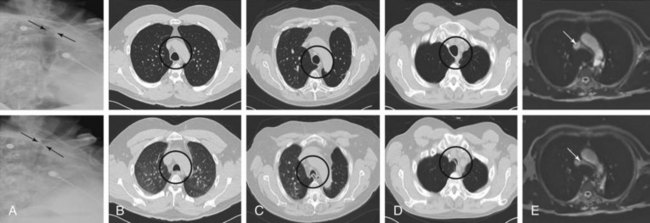
Among radiologic studies, fluoroscopy was used to diagnose tracheomalacia in the past. During fluoroscopy, one can visualize the airway collapse, but it is difficult to appreciate whether it is due to softening of the cartilage or to excessive bulging of the posterior membrane (Figure 11-3).12 Today, with the use of dynamic CT, one more easily distinguishes cartilaginous from posterior wall collapse (see Figure 11-3). Results from studies show that dynamic CT correlates well with bronchoscopy findings,13 offers excellent display of anatomic details of the airway and adjacent structures,14 and allows objective interpretation and quantitative measurements of the degree of airway wall collapse before and after airway splinting interventions.14,15 Dynamic magnetic resonance imaging (MRI) has been insufficiently studied, but small case series support its use for quantifying the degree of airway collapse16 (see Figure 11-3). Its main advantage over CT is that radiation is avoided and contrast materials used are not nephrotoxic.
We quantified this patient’s functional impairment on the basis of World Health Organization (WHO) functional class criteria.17 He had WHO class II functional impairment defined as mild limitation of physical activity without discomfort at rest, but with normal physical activity causing increased symptoms.18 The use of other dyspnea or quality of life (QOL) instruments (St. George’s Respiratory Questionnaire [SGRQ], American Thoracic Society [ATS] Dyspnea Scale, Baseline Dyspnea Index [BDI]/Transitional Dyspnea Index [TDI], Karnofsky Performance Scale [KPS], and a 6-minute walk test [6MWT]) in these patients to objectively determine response to interventions has been studied.19
Comorbidities
The patient had controlled cardiac arrhythmia and no evidence of systolic or diastolic dysfunction on 2D echocardiogram. Although moderate obstructive ventilatory impairment was noted, clinical evaluation and the 2D echocardiogram showed no evidence of pulmonary hypertension, which might prompt hemodynamic instability in case of general anesthesia.20
The patient had been on prednisone 20 mg/day for 4 weeks. Traditionally, any patient who has received the equivalent of 15 mg/day of prednisone for longer than 3 weeks should be suspected of having hypothalamic-adrenal axis suppression.21 As a general rule, any patient who has received glucocorticoids in doses equivalent to at least 20 mg/day of prednisone for longer than 5 days is at risk for hypothalamic-pituitary-adrenal (HPA) axis suppression.22 Furthermore, this patient had been on high-dose inhaled fluticasone (440 mcg twice daily) for several years. The development of adrenal suppression from inhaled steroids is known to be related to dose, duration of therapy, and use of a potent agent (such as fluticasone).23
Support System
The patient is married, but his wife suffers from severe COPD and is on continuous home oxygen supplementation. He is her caregiver. Investigators have found that informal caregivers (defined as untrained and unpaid persons who provide care to an ill person) have needs that go largely unmet, and that they usually receive insufficient help from services with regard to daily physical caring and emotional issues, as well as regarding information about available health and social services. Caring for a patient with dyspnea is particularly challenging and is most difficult to cope with.24
Patient Preferences and Expectations
Similar to other people caring for patients with dyspnea, this patient expressed uncertainty about the future and feared there would come a time when he could no longer help his spouse, or that helping her would be done at the expense of his own health. Active participation and availability of health care professionals are needed to assist informal caregivers with coping strategies they are already using and to address future challenges. Greater involvement of health care professionals leads to sharing of information and advice, thereby enhancing the informal caregiver’s competence, especially in managing difficult symptoms such as dyspnea.24 This patient was particularly motivated to improve his own symptoms so that he could maintain and improve his caregiving role for his wife. He had clearly expressed his willingness for treatment and was ready to consider all available treatment options, including noninvasive positive-pressure ventilation, airway stent insertion, and open surgical resection if necessary.
Procedural Strategies
Indications
Focal Tracheomalacia Resulting in Functional Impairment
The treatment of severe malacia often involves the use of minimally invasive or open surgical procedures.25,26 The exact degree of expiratory airway narrowing responsible for symptoms and requiring intervention remains unknown. Many investigators use 50% or greater reduction in airway caliber between inspiration and expiration to identify abnormal central airway collapse on dynamic CT or bronchoscopy.27–29 This criterion, however, is applied during coughing or forced exhalation, and its use may lead to false-positive results: 78% of normal individuals exceed the current diagnostic criterion for tracheomalacia in the upper and/or lower trachea.30 Furthermore, patients with other airway disorders such as bronchiolitis, COPD, or cystic fibrosis have peripheral airway obstruction, and the expiratory airway collapse seen in the central airways represents normal physiology.31–33 Indeed, during flow-limited breathing, the central airways become severely compressed, particularly during forced expiration and cough—maneuvers often used in diagnosing this entity on dynamic imaging studies. The Starling resistor model* shows that the pressure drop occurs across a very short length of airway, and that proximal airway (downstream from the choke point, mouth ward) resistance should not affect airflow. Pressure catheter measurements demonstrate this flow-limiting choke point and lack of further pressure drop in airways between the mouth and the flow-limiting segment.34 Because the choke point in adult humans is often located at the level of the lobar bronchi and is even more peripheral in patients with COPD,35 central airway collapsibility should not impede airflow.32
Our patient had severe (100%) collapse, resulting not just in exertional dyspnea but also in refractory cough and inability to raise secretions. For these reasons, after taking into account the patient’s goals of care, we elected to proceed with rigid bronchoscopy under general anesthesia to place an indwelling silicone stent that would splint open the lower trachea. Our goal was to improve airway lumen patency to less than 50% collapse during exhalation, which, by convention, is currently considered by most investigators to be within normal limits.17
Expected Results
In the short term (up to 10 to 14 days), airway stabilization with silicone stents in patients with various forms of expiratory central airway collapse was shown to improve respiratory symptoms, quality of life, and functional status.18,19 In one large study evaluating stent insertion for this disease, 45 of 58 patients (77%) reported symptomatic improvement; quality of life scores improved in 19 of 27 patients (70%) (P = .002), dyspnea scores improved in 22 of 24 patients (91%) (P = .001), and functional status scores improved in 18 of 26 patients (70%) (P = .002).19
Team Experience
Silicone stent insertion requires rigid bronchoscopy—a skill possessed by a minority of pulmonologists.36 Even for those who have undergone training, the technique of gentle, atraumatic rigid intubation and stent insertion is one that is gradually perfected over time.
Therapeutic Alternatives for Restoring Airway Patency
These include conservative, minimally invasive, and open surgical therapies.
• Continuous positive airway pressure (CPAP): Excessive airway narrowing and the resulting turbulent flow result in increased airway resistance, which requires greater transpulmonary pressures to maintain expiratory airflow. This will increase the work of breathing and will result in dyspnea. Adjunctive noninvasive positive-pressure ventilation decreases pulmonary resistance and can be used to maintain airway patency, facilitate secretion drainage, and improve expiratory flow. Small studies have showed that the addition of nasal CPAP improves spirometry values, sputum production, atelectasis, and exercise tolerance, but its long-term efficiency has not been clearly demonstrated.37,38
• Metal stent insertion: This approach has been used in the past with variable success.11 Advantages include placement by flexible bronchoscopy, dynamic expansion, and preservation of airway mucociliary function with uncovered stents. In some studies, however, metal stents had to be removed because of stent failure, or because of stent-related complications. Stent fracture and fatal hemorrhage from perforation, for example, have been reported.39 The Food and Drug Administration (FDA) recommends against the use of metallic stents for histologically benign forms of airway obstruction, as in the case described herein.40
• Open surgical interventions: Airway splinting has been used to consolidate and reshape the airway wall. Before an open surgical intervention is proposed, a stent trial has been recommended to identify those patients who are likely to benefit from surgery over the long term. Among surgical interventions, membranous tracheoplasty seems to provide a favorable outcome in uncontrolled studies. This procedure reinforces the membranous portion of the trachea in severe diffuse expiratory central airway collapse.41,42 However, our patient had mild functional impairment and focal disease limited to the lower trachea, probably warranting a more conservative approach.
• Tracheostomy: This technique may be used to splint the malacic airway and provide invasive ventilatory support if necessary; because it can be complicated by secondary tracheomalacia and stenosis at the stoma site, it should not be considered a first-line treatment in elective cases. In our case, the malacic segment would not have been bypassed by tracheostomy because it was localized in the lower trachea.
• Tracheal resection has been proposed for focal tracheomalacia with good outcome and low mortality in experienced centers.43 This procedure, however, has been performed for post-tracheostomy–related malacia, not for lesions localized in the lower trachea.
Cost-Effectiveness
Therapeutic alternatives offered to patients depend on the extent, type, and severity of airway collapse; the degree of functional impairment; and access to and the level of medical or surgical expertise available.11 Although no formal cost-effectiveness evaluations of these various modalities have yet been published, future studies may examine this topic if a consensus is reached regarding classification of this syndrome. Such an assessment would provide clinicians and researchers with an objective tool that can be used in designing outcome studies.17
Informed Consent
With the shift toward a more “consumer-centric” health care system as part of an overall effort to improve the quality of health care and to reduce costs, people need to take an even more active role in health care–related decisions. To accomplish this in an age of shared responsibility between physician and patient, patients must display strong decision-making skills.44 Although the impact of health literacy levels and outcomes has not been addressed for this patient population, it is known that poor health literacy is “a stronger predictor of a person’s health than age, income, employment status, education level, and race.”45
Techniques and Results
Anesthesia and Perioperative Care
Upper airway instrumentation such as intubation and inhalation of irritants (e.g., desflurane) may trigger vagally mediated bronchospasm, thereby promoting expiratory collapse of the peripheral airways with incomplete lung alveolar emptying (air trapping).46 This can potentially lead to hypercarbia, hypoxemia, and hemodynamic instability from high intrathoracic pressures and reduced cardiac output.
Also of concern is the uncommon event of perioperative hypotension and death in patients treated on a long-term basis with glucocorticoids. Although it is well known that patients with adrenal suppression require perioperative glucocorticoid supplementation therapy, serious omissions can occur as a result of inadequate or unclear instructions from the treating team. Therefore, it is important to clearly document and institute an appropriate perioperative glucocorticoid management plan.21 Current recommendations address the need for increased glucocorticoid supplementation in patients with adrenal suppression during medical and surgical stress without exposing patients to excessive or prolonged steroid dosing. Although the level of physiologic stress has not been studied for rigid bronchoscopy, by extrapolating from available guidelines, this can be considered a minor to moderate surgical stress. The recommended glucocorticoid dosage for such situations is intravenous hydrocortisone 75 mg/day on the day of the procedure (e.g., 25 mg every 8 hours) with taper over the next 1 to 2 days to the usual replacement dose in uncomplicated cases (for minor stress), and intravenous hydrocortisone 150 mg/day (e.g., 50 mg every 8 hours) with taper over the next 2 to 3 days to the usual replacement dose in uncomplicated cases (for moderate stress). Short courses (<48 hours) of increased glucocorticoid therapy rarely cause significant complications. Our patient was treated with a moderate stress dose of hydrocortisone (150 mg divided in three doses).
The anesthesiologist should be made aware of the patient’s adrenal suppression, so that safe drugs are used for induction. Etomidate, for instance, is a parenteral hypnotic agent that decreases cortisol synthesis by inhibiting 11-beta hydroxylase, a mitochondrial enzyme in the final step of cortisol synthesis. Single-dose etomidate is common for induction of anesthesia, especially for hemodynamically unstable patients and patients who may not tolerate wide variance in heart rate or blood pressure. A single bolus dose of etomidate has been shown to cause adrenal insufficiency in elective cardiopulmonary bypass patients and may induce postoperative vasopressor dependency.47,48 Use of etomidate is a modifiable risk factor for the development of adrenal insufficiency that should be avoided because safer alternatives are available.
The anesthesiologist should also be aware that worsening airway obstruction may occur during induction. This phenomenon has been reported with the use of both intravenous and inhalational induction.49 The use of neuromuscular blocking agents should be avoided because these drugs may eliminate the only muscle tone that keeps the airway open. Spontaneous assisted ventilation is in fact preferred because of its safety profile.50 Patients with ECAC may not tolerate the supine position after induction; therefore the bronchoscopist should be stationed at the head of the bed throughout induction of anesthesia in case emergent bronchoscopic intervention is required to secure and control the airway.
Instrumentation
We used a 13 mm Efer-Dumon rigid nonventilating bronchoscope (Bryan Corp., Woburn, Mass). This large-diameter scope allows insertion of a silicone stent larger than 12 mm. Based on bronchoscopy and CT scanning, the patient had secondary tracheomegaly caused by bilateral upper lobe fibrosis (see Figure 11-1). Because of this, we intended to use a 20 mm diameter stent. Based on measurements performed during flexible bronchoscopy, the extent of the collapse was 4 cm in the lower trachea. We therefore chose a 20 × 60 mm straight studded silicone stent to properly cover the abnormal collapsing airway (1 cm above and 1 cm below).
Results and Procedure-Related Complications
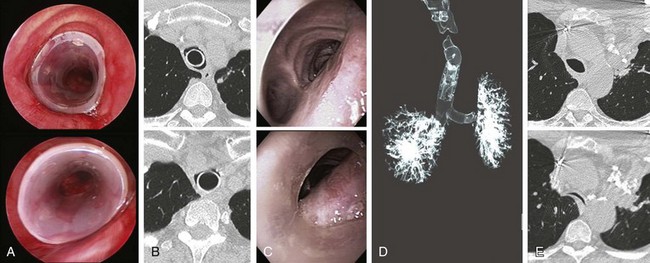
The patient was atraumatically intubated, and airway collapse could be accurately assessed in terms of exact location, type, extent, and severity. It was indeed noted that the patient had severe airway collapse in the lower trachea, characterized by flattening of the anterior cartilaginous wall, giving the trachea the shape of a crescent. The collapsing airway segment ended 2 cm above the main carina. The rigid bronchoscope was gently advanced distal to the obstruction, and the 20 × 60 mm stent was deployed with the distal extremity placed 1 cm above the main carina (see video on ExpertConsult.com) (Video III.11.1![]() ). Airway patency was restored and the collapsing index was estimated at less than 50%; this was later confirmed by morphometric bronchoscopic analysis (Figure 11-4). The case lasted 20 minutes. The patient was extubated without difficulty and was transferred to the postanesthesia care unit (PACU) for 24 hours, during which no postoperative stridor or hypotension was noticed. He was kept NPO until the following day in case immediate stent migration might prompt urgent rigid bronchoscopy for stent revision. This did not occur, so the next day the patient was discharged home after stent instructions were provided to the patient and his daughter.
). Airway patency was restored and the collapsing index was estimated at less than 50%; this was later confirmed by morphometric bronchoscopic analysis (Figure 11-4). The case lasted 20 minutes. The patient was extubated without difficulty and was transferred to the postanesthesia care unit (PACU) for 24 hours, during which no postoperative stridor or hypotension was noticed. He was kept NPO until the following day in case immediate stent migration might prompt urgent rigid bronchoscopy for stent revision. This did not occur, so the next day the patient was discharged home after stent instructions were provided to the patient and his daughter.
No immediate anesthesia- or stent-related complications were reported. Because the dynamic features of expiratory central airway collapse continuously alter the shape of the central airways, as well as the contact between a stent and the airway walls, stent-related complications may occur more frequently in this setting than in other benign disorders or malignancy. Indeed, 26 stent-related complications (12 mucus plugs, 8 migrations, and 6 granulation tissues) were seen in 10 of the 12 patients (83%) who underwent silicone stent insertion for ECAC.18 In another study with 57 patients, authors had 21 partial stent obstructions, 14 infections, and 10 stent migrations.19 These complications usually are not life threatening, but they do require repeated bronchoscopic interventions.
Long-Term Management
Outcome Assessment
Relief of airway obstruction was successfully achieved, as assessed bronchoscopically. The patient had noticed immediate improvement in his ability to raise secretions, and his seal barking cough had subsided. A less than 50% airway collapse should have also improved the patient’s exercise capacity. His exercise capacity and QOL scores can be objectively documented at follow-up clinic visits by using one of the previously applied scales for tracheobronchomalacia, such as the ATS Dyspnea Scale, BDI/TDI, SGRQ, KPS, or WHO functional class impairment.17–19
Follow-up Tests and Procedures
Follow up bronchoscopy is warranted for at least two reasons:
1. It may detect the evolution of the disease process. The prognosis of pure malacia is not completely understood, in part because previous studies did not differentiate dynamic abnormalities of the posterior membrane from abnormalities of the cartilaginous airway wall. Results of older studies demonstrate that expiratory central airway collapse is progressive in most patients.2,29 In one study of 17 patients, 76% of patients had worse airway narrowing detected on repeat bronchoscopy.2 A larger study followed 94 patients with tracheomalacia (TM), TBM, or bronchomalacia for an average of 5.2 years. Among those who underwent repeat bronchoscopy, most had worsening disease, some had stable disease, and none improved spontaneously. Six of the nine patients (66%) with what was called tracheomalacia progressed to diffuse tracheobronchomalacia.5
2. It may detect possible stent-related complications or migrated choke points requiring further intervention.11 In one study, stent-related adverse events were common and usually occurred within the first few weeks after stent insertion (median of 29 days).18 Another study showed that complications occurred within the first 3 months after stent insertion (median time, 26 days; range, 3 to 865 days).19 These results certainly seem to justify follow-up bronchoscopy 4 weeks after stent insertion.
Our patient had a surveillance bronchoscopy 4 weeks after stent insertion. This showed no evidence of stent-related complications. The airway lumen had remained patent, and less than 50% collapse occurred during expiration. These findings were also seen on follow-up dynamic CT scanning 8 weeks later (see Figure 11-4), confirming preliminary results suggesting a potentially important role for CT in preintervention and postintervention assessments of patients with tracheobronchomalacia.15
Two years after initial presentation to our department, this patient developed newly recurrent dyspnea and an inability to raise secretions. Bronchoscopy revealed that the stent had migrated 1 centimeter proximally and that a small amount of associated granulation tissue was evident (see video on ExpertConsult.com) (Video III.11.2![]() ). Rigid bronchoscopy with stent revision was performed, and the stent was repositioned in its original location. Surveillance bronchoscopy and dynamic CT 1 year later showed that the patient had no stent-related complications, but the choke point migrated distal to the stent with evidence of a moderate degree of airway narrowing (see Figure 11-4), which was associated with exertional dyspnea. After readdressing the treatment alternatives, we decided to place the patient on CPAP 10 cm H2O as an adjuvant treatment to splint open residual collapsing airway in the distal trachea.
). Rigid bronchoscopy with stent revision was performed, and the stent was repositioned in its original location. Surveillance bronchoscopy and dynamic CT 1 year later showed that the patient had no stent-related complications, but the choke point migrated distal to the stent with evidence of a moderate degree of airway narrowing (see Figure 11-4), which was associated with exertional dyspnea. After readdressing the treatment alternatives, we decided to place the patient on CPAP 10 cm H2O as an adjuvant treatment to splint open residual collapsing airway in the distal trachea.
Quality Improvement
Airway patency was restored and symptoms improved, but during the 3 year follow-up, our patient developed both stent-related complications and choke point migration requiring further therapy.11 We discussed whether follow-up flexible bronchoscopy would be performed on CPAP to determine an optimal pressure level that would maintain airway patency.51 We concluded that this will be performed if the patient does not tolerate the current CPAP pressure, and/or if no symptomatic improvement is noted.
Discussion Points
1. Describe five criteria used in classifying expiratory central airway collapse.
No classification system for this disease has been universally accepted. After synthesizing various criteria proposed in the literature in a multidimensional classification system,17 we use the following parameters:
2. List three imaging modalities used to diagnose expiratory central airway collapse.
3. List three indications for treatment in this patient.
Because the criteria used to define the exact degree of airway narrowing that leads to symptoms remain vague at this time, physicians should treat only patients who are functionally impaired. Patients with incidental abnormal airway collapse on bronchoscopy or CT scanning performed for other reasons probably should not undergo treatment.25,26 Functional impairment may result from at least three causes:
Dyspnea and cough are the main presenting complaints in TBM in at least three older studies.2,5,52 More recent evidence shows that almost all patients have dyspnea as a main symptom or in combination with cough or mucus retention.17,19 Therefore as for other pulmonary disorders, QOL and functional impairment scales may be appropriate to measure the impact of respiratory symptoms on overall health, daily life, and perceived well-being in patients suffering from malacia.
1. Wright CD. Tracheomalacia. Chest Surg Clin North Am. 2003;13:349-357.
2. Jokinen K, Palva T, Sutinen S, et al. Acquired tracheobronchomalacia. Ann Clin Res. 1977;9:52-57.
3. Collard P, Freitag L, Reynaert MS, et al. Respiratory failure due to tracheobronchomalacia. Thorax. 1996;51:224-226.
4. Koziej M, Górecka D. Cough-syncope syndrome in tracheobronchomalacia. Pneumonol Alergol Pol. 1992;60:89-91.
5. Nuutinen J. Acquired tracheobronchomalacia: a clinical study with bronchological correlations. Ann Clin Res. 1977;9:350-355.
6. Campbell AH, Faulks LW. Expiratory air-flow pattern in tracheobronchial collapse. Am Rev Respir Dis. 1965;92:781-791.
7. Healy F, Wilson AF, Fairshter RD. Physiologic correlates of airway collapse in chronic airflow obstruction. Chest. 1984;85:476-481.
8. Vincken WG, Cosio MG. Flow oscillations on the flow-volume loop: clinical and physiological implications. Eur Respir J. 1989;2:543-549.
9. Bouhuys A, van de Woestijne KP. Mechanical consequences of airway smooth muscle relaxation. J Appl Physiol. 1971;30:670-676.
10. Finder JD. Primary bronchomalacia in infants and children. J Pediatr. 1997;130:59-66.
11. Murgu SD, Colt HG. Treatment of adult tracheobronchomalacia and excessive dynamic airway collapse: an update. Treat Respir Med. 2006;5:103-115.
12. Murgu SD, Colt HG. A 68-year-old man with intractable dyspnea and wheezing 45 years after a pneumonectomy. Chest. 2006;129:1107-1111.
13. Lee KS, Sun MR, Ernst A, et al. Comparison of dynamic expiratory CT with bronchoscopy for diagnosing airway malacia: a pilot evaluation. Chest. 2007;131:758-764.
14. Boiselle PM, Feller-Kopman D, Ashiku S, et al. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am. 2003;41:627-636.
15. Baroni RH, Ashiku S, Boiselle PM. Dynamic CT evaluation of the central airways in patients undergoing tracheoplasty for tracheobronchomalacia. AJR Am J Roentgenol. 2005;184:1444-1449.
16. Suto Y, Tanabe Y. Evaluation of tracheal collapsibility in patients with tracheomalacia using dynamic MR imaging during coughing. AJR Am J Roentgenol. 1998;171:393-394.
17. Murgu SD, Colt HG. Description of a multidimensional classification system for patients with expiratory central airway collapse. Respirology. 2007;12:543-550.
18. Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg. 2007;84:1870-1877.
19. Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest. 2007;132:609-616.
20. Mercer M. Anesthesia for the patient with respiratory disease. Update in Anaesthesia. 2000;12:1-3.
21. Jung C, Inder JW. Management of adrenal insufficiency during the stress of medical illness and surgery. Med J Aust. 2008;188:409-413.
22. Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin N Am. 2003;32:367-383.
23. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159:941-955.
24. Gysels MH, Higginson IJ. Caring for a person in advanced illness and suffering from breathlessness at home: threats and resources. Palliat Support Care. 2009;7:153-162.
25. Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology. 2006;11:388-406.
26. Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984-1005.
27. Zhang J, Hasegawa I, Feller-Kopman D, Boiselle PM. 2003 AUR Memorial Award. Dynamic expiratory volumetric CT imaging of the central airways: comparison of standard-dose and low-dose techniques. Acad Radiol. 2003;10:719-724.
28. Gilkeson RC, Ciancibello LM, Hejal RB, et al. Tracheobronchomalacia: dynamic airway evaluation with multidetector CT. AJR Am J Roentgenol. 2001;176:205-210.
29. Nuutinen J. Acquired tracheobronchomalacia. Eur J Respir Dis. 1982;63:380-387.
30. Boiselle PM, O’Donnell CR, Bankier AA, et al. Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology. 2009;252:255-262.
31. Park JG, Edell ES. Dynamic airway collapse: different from tracheomalacia. Rev Port Pneumol. 2005;11:600-602.
32. Baram D, Smaldone G. Tracheal collapse versus tracheobronchomalacia: normal function versus disease. Am J Respir Crit Care Med. 2006;174:724. author reply 724-725
33. Park J, Edell E. It’s in the definition. Chest. 2006;129:497. author reply 497-498
34. Smaldone GC, Smith PL. Location of flow-limiting segments via airway catheters near residual volume in humans. J Appl Physiol. 1985;59:502-508.
35. Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355-1360.
36. Colt H, Prakash U, Offord K. Bronchoscopy in North America: survey by the American Association for Bronchology. J Bronchol. 1999;2000:8-25.
37. Ferguson GT, Benoist J. Nasal continuous positive airway pressure in the treatment of tracheobronchomalacia. Am Rev Respir Dis. 1993;147:457-461.
38. Adliff M, Ngato D, Keshavjee S, et al. Treatment of diffuse tracheomalacia secondary to relapsing polychondritis with continuous positive airway pressure. Chest. 1997;112:1701-1704.
39. Bolot G, Poupart M, Pignat JC, et al. Self-expanding metal stents for the management of bronchial stenosis and bronchomalacia after lung transplantation. Laryngoscope. 1998;108:1230-1233.
40. FDA Public Health Notification. Complications from metallic tracheal stents in patients with benign airway disorders. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062115.htm, 2005. Accessed February 21
41. Wright CD, Grillo HC, Hammoud ZT, et al. Tracheoplasty for expiratory collapse of central airways. Ann Thorac Surg. 2005;80:259-267.
42. Majid A, Guerrero J, Gangadharan S, et al. Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest. 2008;134:801-807.
43. Grillo HC. Surgical treatment of postintubation tracheal injuries. J Thorac Cardiovasc Surg. 1979;78:860-875.
44. National Network of Libraries of Medicine. Health literacy. Available at http://nnlm.gov/outreach/consumer/hlthlit.html#A1, 2011. Accessed February 21
45. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health literacy: report of the Council on Scientific Affairs. JAMA. 1999;281:552-557.
46. Licker M, Schweizer A, Ellenberger C, et al. Perioperative medical management of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:493-515.
47. Iribarren JL, Jiménez JJ, Hernández D, et al. Relative adrenal insufficiency and hemodynamic status in cardiopulmonary bypass surgery patients: a prospective cohort study. J Cardiothorac Surg. 2010;5:26.
48. Seravalli L, Pralong F, Revelly JP, et al. Adrenal function after induction of cardiac surgery patients with an etomidate bolus: a retrospective study. Ann Fr Anesth Reanim. 2009;28:743-747.
49. McMahon CC, Rainey L, Fulton B, Conacher ID. Central airway compression: anaesthetic and intensive care consequences. Anaesthesia. 1997;52:158-162.
50. Perrin G, Colt HG, Martin C, et al. Safety of interventional rigid bronchoscopy using intravenous anesthesia and spontaneous assisted ventilation: a prospective study. Chest. 1992;102:1526-1530.
51. Murgu SD, Pecson J, Colt HG. Bronchoscopy on noninvasive positive pressure ventilation: indications and technique. Respir Care. 2010;55:595-600.
52. Johnson TH, Mikita JJ, Wilson RJ, Feist JH. Acquired tracheomalacia. Radiology. 1973;109:576-580.
53. Boiselle PM, Reynolds KF, Ernst A. Multiplanar and three-dimensional imaging of the central airways with multidetector CT. AJR Am J Roentgenol. 2002;179:301-308.
* In children, the primary or congenital form of malacia is believed to be a consequence of the inadequate maturity of tracheobronchial cartilage, resulting from premature delivery or from an innate immaturity despite normal gestation. It also could be caused by disease processes that result in the formation of an abnormal cartilaginous matrix of the trachea, as is seen with several genetic syndromes. Most infants outgrow the condition by the age of 2 years because the cartilage strengthens and stiffens, but the disease often persists in children with connective tissue disorders and congenital syndromes. Current management following diagnosis includes medical approaches aimed at reducing associated symptoms of tracheomalacia, ventilation modalities of continuous positive airway pressure (CPAP) and bilateral positive airway pressure (BiPAP), and, in the most severe forms of malacia, surgical approaches aimed at improving the caliber of the airway, such as airway stenting, aortopexy, or tracheopexy. For comprehensive reviews of pediatric malacia, interested readers may wish to refer to articles by I.B. Masters (Cochrane Database Syst Rev. 2005;[4]:CD005304), K. Carden (Chest. 2005;127:984-1005), R.D. Jaquiss (Semin Thorac Cardiovasc Surg. 2004;16:220-224), and J. Austin (Paediatr Anaesth. 2003;13:3-11).
* The Starling resistor is a simple model of the lung that comprises an elastic tube mounted between two rigid tubes inside a chamber filled with air; airflow is driven through the system. This model has been used to explain expiratory flow limitations.