CHAPTER 10 SIEA Flap Breast Reconstruction
Key Points
Introduction
Historically, the first microvascular transfer of abdominal tissue based on the SIEA pedicle is credited to Antia and Buch in 1971,1 using it to reconstruct a soft tissue deformity of the face. Since then the SIEA flap has often been described for various reconstructive purposes. Holmström2 first reported the use of a free SIEA flap for breast reconstruction, but it was not until 1991 that Grotting3 popularized the SIEA flap for immediate breast reconstruction. Since then the microvascular transfer of the SIEA flap has been reported by several groups.4–9
Indications
In our institute for breast reconstruction the lower abdominal tissue is the favored donor site. The SIEA flap is our flap of choice when the appropriate criteria for its use are met; if these criteria are not met we will use the DIEP flap. Therefore, we have devised an algorithm for choosing the SIEA flap,7 a detailed overview of the different parameters involved in choosing the appropriate flap is presented later on in this chapter. When patients are not candidates for abdominal tissue reconstruction we will reconstruct the breast with either a superior gluteal artery perforator flap (SGAP), a latissimus dorsi flap combined with an implant, or infrequently with a breast implant only (Boxes 10.1 and 10.2).
Box 10.1 Possible applications for the SIEA flap in breast surgery
Operative Technique (Box 10.3)
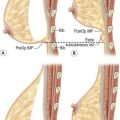
Surgical anatomy (Fig. 10.1)
Box 10.3 Operative summary
Inferior to the inguinal ligament the vessels lie deep to Scarpa’s fascia, as they course superiorly they penetrate Scarpa’s fascia and lie in the superficial subcutaneous tissue. The SIEA and its venae comitantes typically run laterally to the lateral deep inferior epigastric perforator row. The venous drainage of the SIEA flap abdominal territory is mainly via the superficial inferior epigastric vein (SIEV) which empties into the saphenous bulb, and via two venae comitantes running with the SIEA emptying into the femoral vein or occasionally the saphenous bulb. The average length of the main pedicle (artery and two venae comitantes) varies from 5–8 cm, with an arterial diameter range of 1.1–1.9 mm, depending on the site of measurement. The sensory innervation of the SIEA flap is derived from the 10th to 12th intercostal nerves.10–13
In Taylor and Daniel’s original anatomical study of the SIEA in 100 cadavers, they found the SIEA to be totally absent in 35% of their dissections; they also noted that the origin of the SIEA flap in the remaining cadavers was variable with 48% originating as a common trunk with the superficial circumflex from the common femoral artery, while 17% originated directly from the common femoral artery.10 Our published series of 278 clinical dissections during breast reconstruction procedures correlates with this data, showing absence of the SIEA in 42% (n = 118).7 Further assessment of the remaining 58% (n = 160) that did have an obvious artery, revealed that 54% (n = 87) of the arteries had an external diameter greater than or equal to 1.5 mm at the level of the lower abdominal incision. Using our criteria for choosing a SIEA flap, requiring the artery to have an external diameter of 1.5 mm or more at the level of the lower abdominal incision line, the SIEA was adequate for use in approximately 31% of all our cases.
Debate exists in the literature concerning the reliability of tissue perfusion and venous drainage when attempting to harvest unipedicled SIEA flaps across the midline. While a few anatomical and clinical studies have commented on the unpredictability of the arterial perfusion and venous drainage of SIEA flaps across the midline,5,6,14,15 others have demonstrated the possibility of reliably transferring this extended adipocutaneous tissue.8,9,11 In our experience we have observed that the amount of tissue reliably harvested across the midline in an SIEA flap varies with each patient, and we make that determination by intraoperatively evaluating flap perfusion. Therefore, in some patients the zone of demarcation is present at a point roughly corresponding to the medial border of the contralateral rectus muscle, while in others the demarcation is seen at the lateral border of the contralateral rectus muscle.
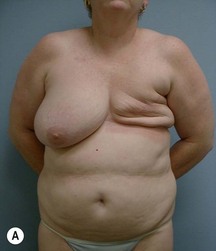
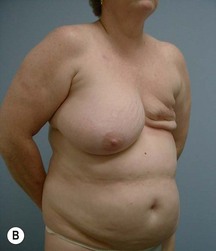
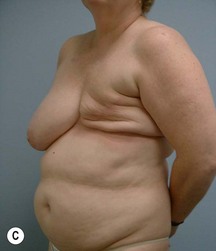
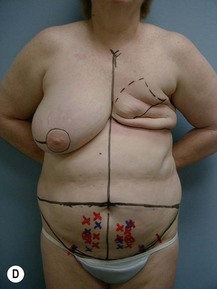
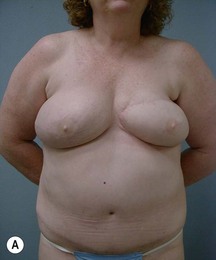
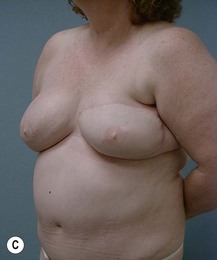
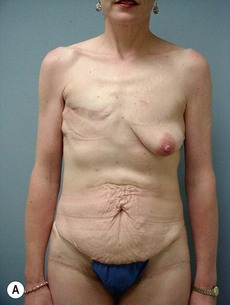
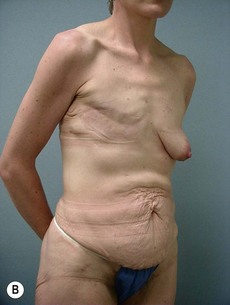
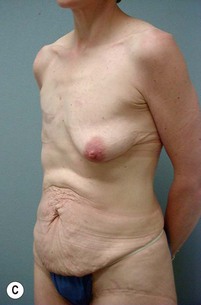
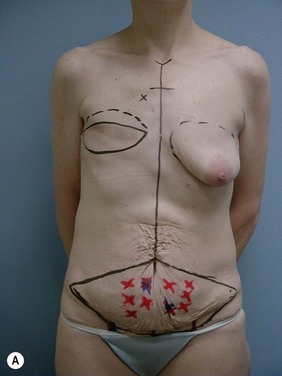
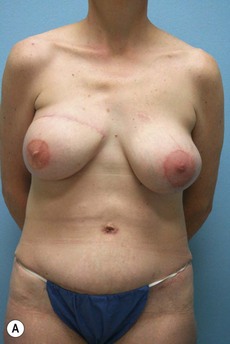
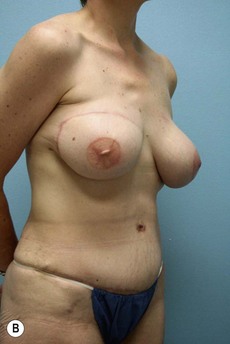
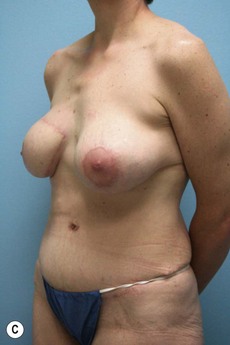
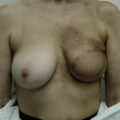
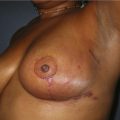
Case Presentations I and II (Figs 10.2–10.7)
Two case studies are now presented, which illustrate the following procedures.
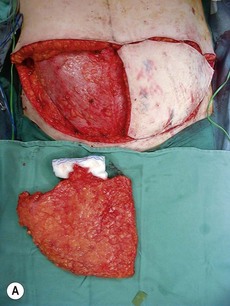
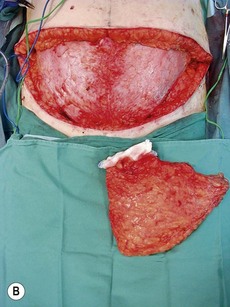
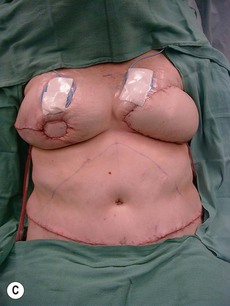
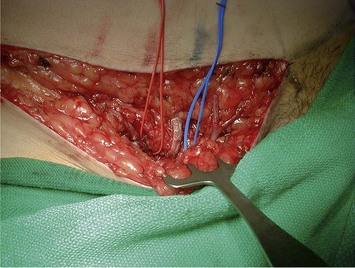
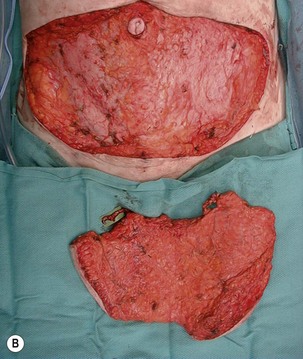
Fig. 10.3 A, B Intraoperative view of dissected flaps, case I.
Operative procedure
Flap harvest (Figs 10.3a–b and 10.6b)
We have previously published our algorithm for choosing the SIEA flap in breast reconstruction.7 In this review of our clinical experience with the SIEA flap, we have noted that whenever the diameter of the SIEA was smaller than 1.5 mm as the pedicle entered the flap (lower line of abdominal incision), there exists an increased chance of vascular compromise. Thus, when opting to use the SIEA flap we precede in the following manner; using a vessel sizer provided with the venous coupler, we measure the SIEA’s external diameter; if its external diameter is larger than 1.5 mm and there is a visible, palpable and audible Doppler pulse, the artery and its venae comitantes are dissected in a retrograde fashion. We do not necessarily dissect the vessels to their origin; rather we continue the dissection as long as we can perceive an increase in the vessel diameter. Once a point is reached at which the diameter is constant there is no need for further dissection since pedicle length is rarely an issue with SIEA flaps (contrary to DIEP flaps). Another key point to keep in mind during pedicle dissection is not to skeletonize the vessels. Preferably a narrow cuff of tissue (e.g., fat) is left just deep to the vessels to protect the vascular pedicle. One should refrain from adding too much surrounding tissue that could unnecessarily lead to prolonged seroma formation due to removal of lymphatic nodes and ducts. Only the distalmost part of the vessels is fully skeletonized, at the area closest to the anastomotic site.
Since we published our original description of the algorithm for choosing the SIEA flap7 we have changed our preferred hemi-abdomen for unilateral reconstructions, and are now preferentially using the ipsilateral side. The rationale for preferring an ipsilateral flap is threefold. First, the SIEA pedicle enters the abdominal flap on its lateral aspect (usually lateral to the lateral row of the DIEA perforators), thus, since the flaps are rotated 180° for their final inset, vessel proximity to the recipient internal mammary vessels is preferable in ipsilateral flaps. Second, the final positioning of the least reliable zone of blood supply (across the midline) will be at the lateral aspect of the reconstructed breast and not medially (should there be any flap loss preferably it should be laterally). Lastly, suturing of the flap at the previous umbilical excision site will cone and shape the breast at the desired position laterally. In unilateral cases both SIEA’s are explored before making the final decision as to the preferred pedicle side which is mainly based on arterial caliber.
Pitfalls – Avoidance and Correction
1 Antia NH, Buch VI. Transfer of an abdominal dermo-fat graft by direct anastomosis of blood vessels. Br J Plast Surg. 1971;24:15-19.
2 Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13:423-427.
3 Grotting JC. The free abdominoplasty flap for immediate breast reconstruction. Ann Plast Surg. 1991;27:351-354.
4 Arnez ZM, Khan U, Pogorelec D, Planinsek F. Breast reconstruction using the free superficial inferior epigastric artery (SIEA) flap. Br J Plast Surg. 1999;52:276-279.
5 Chevray PM. Breast reconstruction with superficial inferior epigastric artery flaps: a prospective comparison with TRAM and DIEP flaps. Plast Reconstr Surg. 2004;114:1077-1083.
6 Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction using perforator flaps. J Surg Oncol. 2006;94:441-454.
7 Spiegel AJ, Khan FN. An intraoperative algorithm for use of the SIEA flap for breast reconstruction. Plast Reconstr Surg. 2007;120:1450-1459.
8 Volpe AG, Rothkopf DM, Walton RL. The versatile superficial inferior epigastric flap for breast reconstruction. Ann Plast Surg. 1994;32:113-117.
9 Ulusal BG, Cheng MH, Wei FC, Ho-Asjoe M, Song D. Breast reconstruction using the entire transverse abdominal adipocutaneous flap based on unilateral superficial or deep inferior epigastric vessels. Plast Reconstr Surg. 2006;117:1395-1403.
10 Taylor GI, Daniel RK. The anatomy of several free flap donor sites. Plast Reconstr Surg. 1975;56:243-253.
11 Hester TRJr, Nahai F, Beegle PE, Bostwick J. Blood supply of the abdomen revisited, with emphasis on the superficial inferior epigastric artery. Plast Reconstr Surg. 1984;4:657-670.
12 Reardon CM, O’Ceallaigh S, O’Sullivan ST. An anatomical study of the superficial inferior epigastric vessels in humans. Br J Plast Surg. 2004;57:515-519.
13 Stern HS, Nahai F. The versatile superficial inferior epigastric artery free flap. Br J Plast Surg. 1992;45:270-274.
14 Holm C, Mayr M, Höfter E, Ninkovic M. The versatility of the SIEA flap: a clinical assessment of the vascular territory of the superficial epigastric inferior artery. J Plast Reconstr Aesthet Surg. 2007;60:946-951.
15 Schaverien M, Saint-Cyr M, Arbique G, Brown SA. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg. 2008;121:1909-1919.