CHAPTER 20 Side Effects and Complications of Injection Procedures: Anticipation and Management
GENERAL COMPLICATIONS
Anaphylactic and allergic reactions
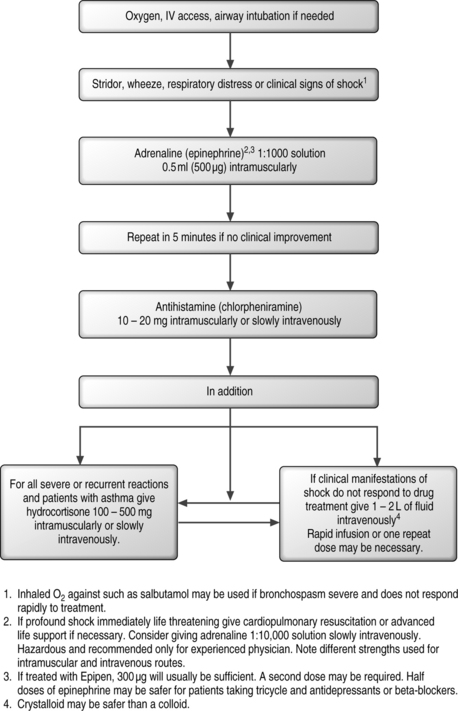
Anaphylactic and allergic reactions are exceedingly rare in interventional spinal procedures. These systemic reactions can occur with anesthetics, corticosteroids, or contrast media. Anaphylactic reactions occur most often within 2 hours after an epidural is performed.1 This can result in sudden cardiovascular and respiratory collapse, which can possibly be followed by death. Abnormal rates of absorption and bi-transformation of the local anesthetic can also lead to such a reaction.
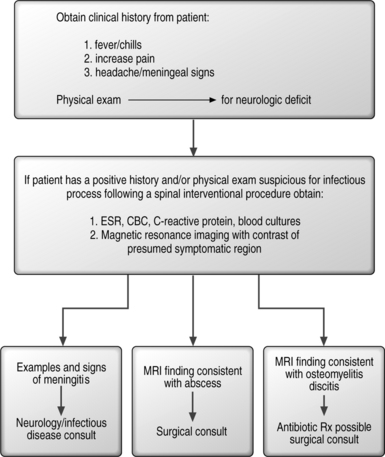
Clinical management of anaphylactic reaction is seen in algorithmic form in Figure 20.1.
Anesthetics
Allergic reactions to local anesthetic, although rare, may occur either from the local anesthetic agent, or if present, the methylparaben used as a preservative. There is a higher likelihood of reaction to procaine than amide-based local anesthetics. In a prospective review of 10 440 patients who received lidocaine for spinal anesthesia, the incidence of adverse reactions was reported to be about 3% for positional headaches, hypotension, and backache, 2% from shivering, and less than 1% each for peripheral nerve symptoms, nausea, respiratory inadequacy, and diploplia. Many of these observations may be related to injection techniques, with or without a contribution from the local anesthetic used.2
Inadvertent puncture of the thecal sac while injecting local anesthetic for an epidural can result in prolonged paresthesia, pains in the legs, and even transient paralysis.3 Bupivacaine can cause degeneration of muscle fibers after a single injection into a skeletal muscle.4 There have been reports of anesthetic injections outside of the neurofascicle which can lead to alterations in the blood–nerve barrier and consequent edema and swelling of the nerve.5 This can lead to a progressive decrease in nerve blood flow as the concentration increases.6 If a local anesthetic is injected intrafascicularly there can be devastating consequences from cytotoxicity and edema.7,8 These possible complications can be avoided using appropriate techniques, and utilizing contrast media prior to introduction of anesthetic near a nerve.
Attention must be given to the amount of anesthetic administered in order to avoid the possibility of central nervous system toxicity. The total dosage of lidocaine should remain below 400–500 mg or 40 mL of 1% lidocaine. Bupivacaine is about 4 times more toxic than 1% lidocaine with a toxic bolus of 175 mg (or 17.5 cc) in a 70 kg patient.9 The signs of central nervous toxicity are disorientation, light-headedness, nystagmus, tinnitus, and muscle twitching in the face or distal extremities. Peak plasma concentrations occur 10–20 minutes after injection. Therefore, ongoing monitoring of the patient for the postoperative period for over 30 minutes is suggested strongly to avoid any possible potential nervous system toxicity from anesthetics.
Other complications which can arise from local anesthetics for epidural analgesia include motor block, hypotension, urinary retention, and pressure necrosis.10 Higher doses of local anesthetics appear to increase the risk of motor block and hypotension.11,12
Cardiovascular complications
If high systemic levels of local anesthetics are injected intravascularly and/or intrathecally during a spinal interventional procedure cardiovascular complications can arise. Usually, such reactions are preceded by central nervous system toxicity prior to cardiovascular complications arising. The cardiovascular toxicity is a direct result of anesthetics acting upon the myocardium and peripheral vasculature. In vitro studies using isolated myocardial tissue have shown anesthetics to have a dose-dependent negative inotropic action.13,14 The effects of local anesthetics on the cardiovascular system when at low levels can slightly increase blood pressure due to an increase in cardiac output and heart rate, which can enhance sympathetic activity and cause direct vasoconstrictor action. At higher concentrations, which produce CNS toxicity, they can cause a marked increase in heart rate, cardiac output, peripheral resistance, and blood pressure. With further increase in dosage there can be severe hypotension and cardiovascular collapse due to decreased cardiac output and peripheral vascular dilation. Prevention of such complications certainly depends on avoiding intravascular injection. Thus, appropriate needle localization with fluoroscopy and contrast media is essential.
Once CNS toxicity symptoms are present, the interventionist needs to prepare for possible cardiovascular complications. Institution of appropriate cardiopulmonary resuscitation is imperative for the management of such complications. Management of the patient using advanced cardiac life support (ACLS) would be implemented any time there is such a need. Appropriate management through emergency cardiac care algorithms is recommended and the reader is referred to more detailed sources for further information with regard to these algorithms and management.15–17
Corticosteroids
Several injectable corticosteroid preparations have been utilized in interventional spinal procedures. These include, most commonly, triamcinolone, methylprednisolone, and betamethasone. Each of these corticosteroids has a different chemical composition. There have been several reports in the literature that have stated arachnoiditis may be related to the use of intrathecal steroids.18–22 A study by Latham23 has been done to assess intrathecally injected betamethasone in sheep, which did not reveal any evidence of arachnoiditis until high doses were utilized, and thus the study concluded intrathecal injection of betamethasone in low doses, such as those given in humans, is unlikely to cause arachnoiditis.23 Several studies have examined the effects of epidural and subarachnoid-deposited injections into the meninges and spinal cord.
Cicala et al.24 looked for evidence of acute neurotoxicity (within 30 days) after injection of epidural corticosteroids. They examined the effects of a mixture of 1% lidocaine and methylprednisolone on the meningeal membranes and nerve roots 4 or 10 days after epidural injection. Microscopic examination revealed a complete lack of inflammatory changes in neural tissue and meningeal thickening.
Abram et al.25 examined histological sections of spinal cords of rats that received 4 subarachnoid injections of triamcinolone at 5-day intervals and compared them with sections from animals that received subarachnoid saline solution during the same time periods. They found a few large, common dark-stained neurons in several preparations, but these were invariably in areas adjacent to spinal catheters, where mechanical compression of the cord was evident. There was no difference in the incidence of such occurrences between steroid-treated and saline-treated animals.
A study by Delaney and colleagues26 evaluated possible long-term histological effects of a mixture of 2% lidocaine and triamcinolone following epidural injection and catheterizations. They found that the nerve root and the root entry zone in the meninges when examined by light microscopy 30 and 100 days after injection showed only a minor inflammatory response at the 30 day period. The reaction was completely resolved at 100 days.
It has been proposed that polyethylene glycol (PEG), a preservative agent in methylprednisolone, has been implicated in cases of sterile meningitis and arachnoiditis when injected intrathecally.20 A study performed on the toxicity of PEG was performed by Benzon et al.27 They showed a concentration of 20% PEG or greater resulted in a swelling and a reduction in compound muscle potential in isolated nerve preparations. However, this concentration is clearly higher than that found in commercial corticosteroids preparations, which is 3–9%. These preparations are subsequently diluted with saline and local anesthetics, which would indicate an even lower predisposition for any neurotoxicity.
Epidural administration of corticosteroids has several known side effects. These include fluid retention, which can lead to congestive heart failure.28–30 Case reports of steroid myopathy have been reported.13 There have also been reports of irregular menses.31 Epidural lipomatosis has been associated with prolonged corticosteroid therapy.32–34 Minor digestive disturbances and occasional minor changes in serum glucose have been documented.35 Less-common side effects are elevated temperature, euphoria, depression, mood swings, local fat atrophy, deep pigmentation of the skin, and pain flare. Other rare but reported side effects include weight gain,28 deep vein thrombosis,36 and hypertension,37 and allergic reactions to triamcinolone.1
An uncommon but serious complication is development of Cushing’s syndrome from excess glucocorticoid administration.38 This syndrome usually occurs when exceptionally high dosages of corticosteroid are given over a short period of time. One such case occurred when a patient received two 80 mg injections of methylprednisolone 1 week apart.39 Serum cortisol levels have been shown to be depressed for 1–2 weeks after epidural injections but may return to normal after 3 weeks.40,41 The recommended dose to avoid systemic side effects from epidural steroids has been stated by Knight and Burrell42 to be no more than 3 mL/kg of methylprednisolone. Despite adhering to this, there have been reports of cumulative effects from long durations of steroid preparations. Kay43 and coworkers have shown both acute and chronic suppression of the hypothalamic–pituitary–adrenal axis in a clinically relevant study of 14 patients who received a total of three epidural steroid injections once weekly with 80 mg of triamcinolone in 7 mL of 1% lidocaine. Within 15 minutes of injection, there was evidence of the suppression of endogenous corticosteroids, manifested by a significant decrease in both ACTH and cortisol. The median duration of such effect was 1 month, but 5 of 14 patients showed suppression for up to 3 months. Adrenal function returned to a normal status after 3 months.
In a study by Manchikanti44 it was concluded that low-dose administration of neuroaxial steroids are not deleterious and did not result in any significant deterioration in bone mineral density, causing neither osteopenia or osteoporosis.
Contrast media
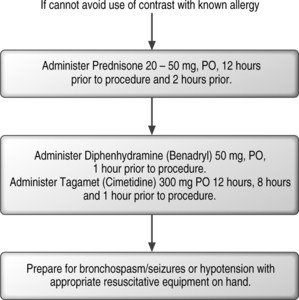
Because fluoroscopic procedures are combined with introduction of radiographic contrast media, it is important to know the potential adverse effects of contrast media. The largest study to compare the effects of intrathecal nonionic (iopmanidol) to ionic (metrizamide) contrast found substantially fewer adverse reactions in the nonionic group.45
In 337 647 cases in which iso-osmolar contrast was used, only 1 death occurred.46 Patients with a known sensitivity to iodine, prior reaction to contrast media, food allergies, asthma, and hay fever have a higher incidence of anaphylactic or cardiovascular reactions. Premedication with histamine H1 and histamine H2 blockers and corticosteroids may reduce the incidence and severity of life-threatening reactions in patients with a known allergy. Controlled studies on nonionic contrast agents used intrathecally in myelography have shown adverse reactions including headache (18%), nausea (7.3%), muscular pain (3.7%), and hypotension (1.1%).47 These studies were performed using 10–15 mL of contrast injectate, which is a larger dose than the 1–3 mL used during fluoroscopic injections.
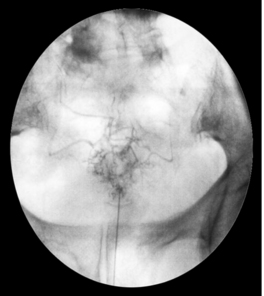
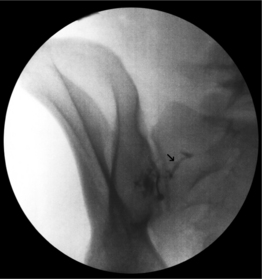
The fluoroscopic technique in combination with extradural myelography can avoid intrathecal injections. Intravascular injection can occur in 9.2% of cases despite a negative aspiration for blood in lumbar epidural injections, without fluoroscopic guidance.48 When utilizing fluoroscopy and contrast media, Sullivan et al.49 found a similar overall incidence of 8.5% intravascular uptake in lumbar spinal injections. The caudal and transforaminal routes were intravascular 10.9% (Fig. 20.2) and 10.8% of the time, respectively, sacroiliac joint injections 6.3% (Fig. 20.3), zygapophyseal joint injections 6.1%, and interlaminar injections 1.9%. The intravascular pattern in lumbar transforaminal epidural injections was also described by Furman to be 11.2%50 and in cervical transforaminal injections, and intravascular uptake is 19.4%.51 These studies unequivocally demonstrate the importance of introducing contrast media prior to the infusion of anesthetics or corticosteroids in spinal interventional injections. Prophylaxis may be utilized in patients with known allergy to contrast media (Fig. 20.4). An alternative in patients allergic to iodinated contrast is to utilize gadolinium contrast.52
Infectious complications
Infectious complications can arise from spinal injections. These complications can include epidural abscess,53–55 meningitis56–58 and osteomyelits/discitis.59
Epidural abscess
These have been reported after epidural injections in the cervical, thoracic, and lumbar spine. There have been cases of epidural abscess following thoracic epidural injections of steroids and bupivacaine to treat postherpetic neuralgia.57,60 A thoracic epidural abscess has also been reported after four epidural injections in a patient with metastatic disease.53 Five other cases of abscesses were reported, three after lumbar, one after cervical, and one after caudal epidural injections.54,55,61–63 The bacteria identified in these cases was Staphylococcus aureus. Of these patients, three patients had diabetes mellitus, one had a surgical infection with S. aureus 2 weeks prior to the epidural injection, and one had carcinoma with spinal metastasis. All of these patients presented with fever, spinal pain, radicular pain, and/or progressive neurologic deficit 3 days to 3 weeks following their injections. It is presumed that the most common bacterial etiology for spinal infection from a spinal injection is S. aureus. The likely mechanism of infection is introduction by the needle, carrying an inoculum from the skin to deeper structures.
Laboratory evaluation with erythrocyte sedimentation rate (ESR), C-reactive protein, and complete blood count (CBC) needs to be obtained. Magnetic resonance imaging (MRI) with contrast is the radiological procedure of choice for the diagnosis of epidural abscess.55,64 Once diagnosed, appropriate antibiotic therapy can be administered and surgical debridement performed if necessary.
Meningitis
This is a rare phenomenon, which can occur following spinal injections. It can occur when the dura is accidentally punctured during epidural procedures or other spinal interventional procedures. There are two types of meningitis that have been reported following spinal injections: bacterial and aseptic. Several cases of bacterial meningitis have been reported following lumbar epidural injections.57 Of the two cases reported by Dougherty, one patient had a documented dural puncture and the other patient had an inadvertent unrecognized dural puncture.
Cases of aseptic meningitis have been reported by Nelson et al.19 following subarachnoid injection with methylprednisolone. The manifestations included headache, fever, nausea, leg pain, and convulsions. Cerebrospinal fluid (CSF) analysis revealed elevated protein, leukocytes, and red blood cells, while CSF culture was negative. Spontaneous recovery ensued over a 3-week period. Several other cases of aseptic meningitis have been reported after a subarachnoid injection.25,64,65 In each instance the symptoms resolved within several days. A case report by Morris66 of aseptic meningitis began 48 hours after an epidural injection of methylprednisolone without any anesthetic. The symptoms included fever, headaches, lethargy, and meningismus. CSF evaluation in this case revealed pleocytosis, elevated protein values, and diminished glucose levels. CSF culture was negative.
Osteomylitis/discitis
Cases of osteomyelitis and discitis have been reported following cervical, thoracic, and lumbar discography. This is a potential life-threatening complication if it develops in the cervical or thoracic spine. As previously mentioned, these infectious complications can be minimized by adhering to sterile technique along with the use of intravenous and intradiscal antibiotics. The use of antibiotics are further discussed in the section on discography. Patients who have undergone a discographic procedure should be observed clinically for symptoms of osteomyelitis or discitis. The typical signs of infection include fever, malaise, lethargy, pain, and/or neurological symptoms. These typically present 1–4 weeks after injection although neurological symptoms are uncommon. Laboratory studies can reveal leukocytosis, elevated sedimentation rate, and/or an increase in C-reactive protein. MRI with contrast has been shown to be superior to bone scan for the early diagnosis of discitis and osteomyelitis.67
In summary, early detection of infectious complications from injection procedures is necessary in order to avoid morbidity and mortality. In patients who have a history of prior infection, consideration should be given to prophylactic antibiotic treatment in these patients, prior to their procedure. An algorithmic approach to managing these infectious complications is seen in Figure 20.5.
Hematologic complications
Many factors are involved in evaluating the bleeding risk in patients who are undergoing spinal interventional procedures. Patients who suffer from coagulation disorders are at increased risk of bleeding.68 These include patients with hemophilia and von Willebrand’s disease. Spinal blocks have been performed in hemophiliacs utilizing recombinant factor VIIa.69 Von Willebrand’s disease can lead to spontaneous bleeding following epidural procedures.70 However, there are cases of epidural catheterization which have been performed in patients with von Willebrand’s disease without complications.71–73 Patients with thrombocytopenia and idiopathic thrombocytopenic purpura can be at risk for complications from spinal injections. Epidural catheterization is believed to be safe in patients with platelet counts of greater then 100 000.74 There are, however, case reports of patients safely receiving epidural injections with platelet counts below 100, 000.75,76 Obtaining a baseline CBC prior to a spinal injection can identify a patient with thrombocytopenia.
Patients who have liver and renal disease may have altered coagulation, which could lead to a complication with hepatic dysfunction accompanied with portal hypertension, resulting in increased epidural venous congestion, which can lead to increased likelihood of bleeding.77 Epidural hematomas following lumbar puncture78 and epidural catheter placement79,80 have been reported in patients with known liver disease. They have also been reported in patients with renal disease.81,82
Epidural hematoma
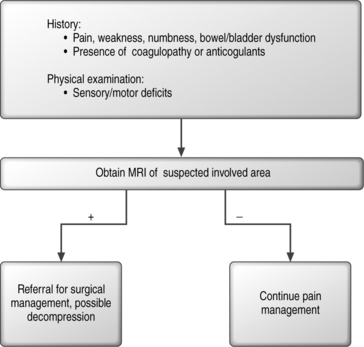
Epidural hematomas can develop spontaneously and in patients with no evidence of any bleeding tendency, anticoagulation, or traumatic needle insertion.83,84 In a review of epidural steroid injections in 65 published series with a total of 69 047 patients receiving one or more epidural injections, there was only one case of an epidural hematoma.85 Hematomas can be localized in the cervical, thoracic, or lumbosacral spine. Symptoms will vary depending on the location and size f the hematoma. The presentation can be immediate or delayed up to several days.86,87 In order to diagnose patients with such a complication, prompt spinal imaging is recommended. MRI is the most sensitive modality in order to diagnose a hematoma, define the extent of its spread, and distinguish it from other space-occupying lesions.88–90 Once identified, treatment of the hematoma can involve high-dose corticosteroid therapy and/or emergency decompressive surgery in order to prevent further compromise of neurologic function. If an epidural catheter is present with a hematoma, infusion should be stopped and the catheter removed in order to avoid increasing compression.84 In order to minimize the risks to the patient when performing spinal interventional procedures, the interventionist can do several things that can minimize the potential possibility of causing an epidural hematoma. These include minimizing the passes of the needle (needle trauma) utilizing fluoroscopic guidance, use of smaller-caliber needles,91 and aspiration can be performed in order to determine if the needle is intravascular. See Figure 20.6 for an algorithmic approach to the evaluation of a patient with a presumptive epidural hematoma.
Management of anticoagulant medications in interventional spinal procedures
Clinically apparent epidural hematomas are rare, with an incidence between 1:40 000 and 1:150 000.84,85,92,93 Aspirin and nonsteroidal antiinflammatory drugs may effect platelet function and subsequently increase the risk of an epidural hematoma. These medications have been reported to increase the occurrence of epidural hematoma from both spinal anesthesia and epidural injection.94–97 It should be noted, however, that there are other studies, which report no increased incidence of epidural hematomas in patients who are receiving aspirin and nonsteroidal antiinflammatory medications.98–101
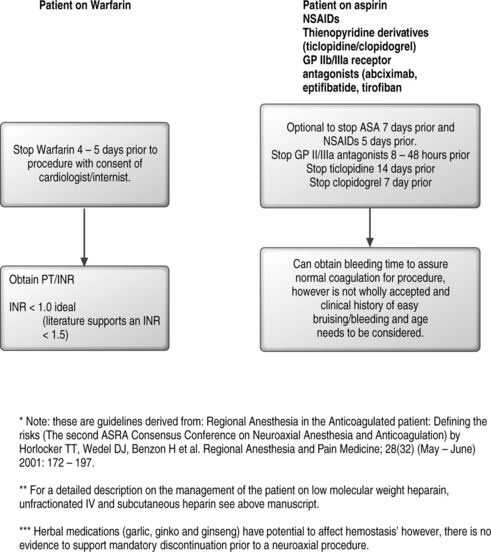
Patients using thienopyridine derivatives and glycoprotein receptor antagonists have developed hematomas from interventional spinal injections.86,102 Since the risk associated with these medications is unknown, the American Society of Regional Anesthesia recommends withholding ticlopidine 14 days, clopidogrel 7 days, and Gp antagonists for 4 weeks prior to procedure.87 Oral anticoagulation with warfarin is a contraindication to spinal anesthesia.87 The American Association of Regional Anesthesia suggests that warfarin should ideally be stopped 4–5 days prior to spinal injection. The prothrombin time (PT) and international normalized ratio (INR) should be checked prior to a spinal injection. There is no consensus regarding the delineation of a safe PT/INR prior to performing a spinal injection. There is some literature that supports an INR of 1.5 as safe for major surgical procedures including spinal injections while an INR greater than 1.5 is unsafe.103,104 It should be noted that identification of risk factors for bleeding disorders does not eliminate the risk of a spinal hematoma. This has been shown in the study by Vandermeulen94 in which 87% of patients who had spinal hematomas had a hemostatic abnormality but, 13% had no identifiable risks. See Figure 20.7 for an algorithmic approach to the management of patients on warfarin, aspirin, or NSAIDs.
Neurological complications
Several studies have shown prospectively that the incidence of neurological injuries is approximately 0.002–0.7% and that they are usually self-limiting.105–107 Placement of thoracic epidural catheters has been shown to have an incidence of neurological injury of 0.7%.107 There have been case reports of upper limb weakness and nerve root injury and even intrinsic spinal cord damage from spinal injections.54,108–110 Neurological compromise can also occur from spinal infection such as epidural abscess or epidural hematoma.
There have been case reports of transiently increased sciatic pain and paresthesia following spinal injections.111–119
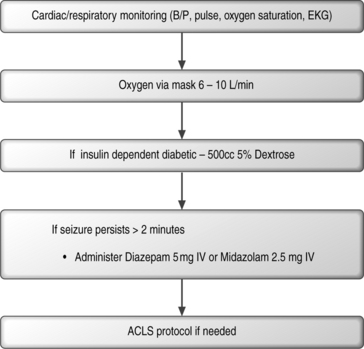
Seizures can also result from spinal injections. These seizures may be a result of anesthetic or cardiac-induced hypotension secondary to neural blockade. Prolonged seizures can result in brain injury if they last longer than 30 minutes.120–122 In order to possibly prevent such consequences the interventional physician should carry out a comprehensive neurologic and physical examination and immediately treat the seizure activity. See Figure 20.8 for an algorithmic approach in the management of a seizure.
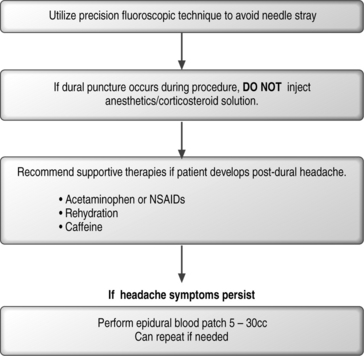
Dural puncture and post-dural puncture headache
The potential complication of entering the subarachnoid space due to penetration of the dura exists with any spinal injection. This can occur in any region of the spine (cervical, thoracic, or lumbosacral). The incidence of an inadvertent dural puncture in lumbar epidural injections has been reported to be as low as 0.5% and as high as 5%.109,123 Incidence of dural puncture in the cervical spine can be as high as 20%.108 The incidence of headache following dural puncture in the lumbar spine has been reported to be 7.5–75% depending on technique, experience, and size of needle used.124,125 These headaches may emanate from an unrecognized dural puncture causing a leakage of cerebrospinal fluid (CSF) or they may result from inadvertent injection of air into the subarachnoid space.126,127
When small-gauge needles are used in spinal anesthesia there is a lower incidence of postdural puncture headache. The incidence is approximately 40% with a 22-gauge (ga) needle, 25% with a 25-ga needle,128,129 2–12% with a 26-ga Quincke needle,130 and less than 2% with a 29-ga needle.131
If a postdural puncture headache occurs, the headache will usually occur within 3 days of the procedure. Up to 66% start within the first 48 hours.132,133 The headache can also present immediately following a dural puncture.134
The pain associated with the headache is described as being a shearing and severe spreading pain. Distribution of the pain appears to be in the frontal and occipital areas, which can radiate into the neck and shoulders. There tends to be stiffness in the neck and the pain is exacerbated with head movements and relieved by lying down.134,135 Symptoms that can also appear in dural puncture headaches include nausea, vomiting, hearing loss, tinnitus, vertigo, dizziness, paresthesias of the scalp, and upper/lower limb pains. There have also been reports of visual disturbances such as diplopia or cortical blindness.3,136,137 There are also reports of intracranial subdural hematomas, cerebral herniation, and death.138
A review of the literature has revealed that the spontaneous rate of recovery from postdural puncture headache is typical with 85% recovering within 6 weeks.3,139,140 Even though this side effect can spontaneously resolve, there are frequent circumstances during which the severity of the headaches necessitates treatment. A variety of strategies can be undertaken including rehydration, acetaminophen, nonsteroidal antiinflammatory drugs, opioids, and antiemetics.141 Caffeine has been utilized in the treatment of postdural headache at a dose of 300–500 mg of oral or i.v. caffeine once or twice daily.142,143
The most definitive method of treatment, however, is an epidural blood patch.144–146 The first report of epidural blood patch for treatment of postdural puncture headache was described by Gormley in 1960.147 This technique has a success rate of 70–98%.148 The exact mechanism by which the epidural blood patch eliminates the headache is unknown. However, it has been postulated that the headache is believed to be due to a reduction of CSF volume or pressure, which leads to traction and tension across the pain-sensitive dural sinuses that occurs in the upright position.149 It should be noted that lumbar extradural blood patch has been shown to be effective even in the treatment of a cervical postdural puncture headache, supporting this theory.149
Slipman et al.150 have reported the successful use of a cervical transforaminal epidural blood patch in a patient with a postdural puncture headache from an interlaminar cervical epidural injection in which cervical interlaminar epidural blood patch failed to relieve symptoms.
Another theory for how the blood patch works is that the blood clot adheres to the dura matter which may perhaps seal the hole in the dura and prevent CSF leak.151 Another possible mechanism of action is that the mixing of blood with the CSF lends to rapid coagulation response, sealing the dural tear even if it is far from the blood patch site.151
Complications can arise as well from the epidural blood patch. These can include exacerbation of symptoms and radicular pain.152 Pneumocephalus has also been described following epidural blood patch.153 An inadvertent subdural blood patch has been described, resulting in a nonpositional persistent headache with resulting lower extremity discomfort.132
An algorithmic approach to avoiding dural puncture, and the treatment of dural puncture can be seen in Figure 20.9.
Respiratory complications
Pneumothorax can occur in a lower cervical or any thoracic spinal interventional procedure. A pneumothorax tends to be self-limiting and the treatment of this potential complication would require supportive care, observation, and follow-up serial chest radiographs. If a pneumothorax becomes significant, with greater than 25% collapse of the lung, then a chest tube may need to be placed to maintain respiration.154 Cervical spinal injections can injure the recurrent laryngeal nerve. A patient with such an injury may present with a reduction in the ability to protect the airway and may also have hoarseness. This condition is usually self-limiting and resolves. Vocal cord dysfunction has been reported transiently with a 12% incidence in patients receiving steroid injections in the axial skeleton.155
Respiratory depression can arise from all neuraxial opioids. The incidence of clinical respiratory depression with a bolus of morphine is 0.2%.156,157
Urological complications
The incidence of urological complications are very few, if any, from interventional spinal procedures. The most common problem is urinary retention, which may follow injections of spinal local anesthetic.158 The other possible cause of urological problems can be an infection such as an epidural abscess or epidural hematoma, which can compromise the spinal cord and/or cauda equina.
SPECIFIC COMPLICATIONS OF CERVICAL SPINAL INJECTIONS
Cervical spinal injections
Cervical epidural steroid injections
The complications of cervical epidural steroid injections include dural puncture headache, pneumothorax, pneumocephalus,159 bloating, nausea,108,160 vomiting, fever, spinal nerve injury, epidural hematoma,93 subdural hematoma,161 hypotension, transient paresis, and respiratory insuffiency.162 Facial flushing has a 9.3% incidence and a stiff neck has a 13.2% incidence.108,163 Cervical epidural abscess61,164,165 and complex regional pain syndrome166 have also been reported. Subdural or subarachnoid injections of large doses of anesthetic may cause loss of consciousness,163 hypotension, spinal anesthesia, cardiovascular arrest, apnea, or death.
An infrequent although serious complication of cervical interlaminar epidural steroid injection is spinal cord injury from direct needle trauma.53,54,110,164 Spinal cord injuries from direct trauma have occurred when patients were sedated to such a degree that they did not report pain.110 Therefore, heavy sedation is contraindicated for cervical interlaminar epidurals. Botwin et al.167 found an incidence of minor complications in fluoroscopically guided interlaminar cervical epidural injections of 16.8%. The most common complications were neck pain 6.7%, nonpositional headache 4.6%, insomnia 1.7%, vasovagal reactions 1.7%, facial flushing 1.5%, and dural puncture 0.3%.
Spinal cord infarction has been reported in cervical transforaminal epidurals, possibly through intravascular injection into a radicular artery.166,168,169 Brouwers et al. described a cervical anterior spinal artery syndrome after a diagnostic block of the right C6 nerve.170 Similarly, Ludwig169 described spinal cord infarction following a left C6 transforaminal epidural steroid injection. A case of quadriparesis and brainstem herniation after a selective cervical transforaminal injection on the right at C5–6 has been reported by Tiso et al.171 Brady et al.163 found two complications following 357 transforaminal epidural steroid injections. Both complications were transient loss of consciousness followed by nausea and vomiting. A study by Slipman et al.172 reported no complications in 20 patients with cervical spondylitic radicular pain who underwent cervical transforaminal injection. Slipman et al.,173 in a prospective study of 89 cervical selective nerve root injections found immediately following the procedure, found that 22.7% of injections resulted in increased pain at the injection site, 18.2% had increased radicular pain, 13% had light-headedness, 9.1% had increased spine pain, 4.5% had non-specific headache, and 3.4% had nausea. Ninety-one percent experienced no complications or side effects during the procedure.
The incidence of dural puncture using the cervical interlaminar technique is 0.25–2.0%.108,164 Slipman173 reported an incidence of dural puncture in cervical selective nerve root injections of 0.33%. Dural headaches may be from unrecognized dural punctures causing leakage of cerebral spinal fluid or they may result from inadvertent injection of air into the subarachnoid space.126,127 A study injecting 10 mL of intrathecal normal saline prophylactically showed a favorable response in reducing the severity of postdural puncture headaches.173 Many headaches resolve with conservative management including bed rest and fluids. Persistent postural headache may require an epidural blood patch using 5–30 mL of autologous blood under aseptic conditions and using fluoroscopy.
The cervical epidural space is densely vascularized.174 The incidence of intravascular penetration in cervical transforaminal injections is 19.4%.50 The incidence of intravascular injection using interlaminar technique is considered to be much lower but has not been studied. Unrecognized inadvertent intravascular needle placement delivers the medication systemically instead of locally and the treatment effect may not be obtained. Misplacement of the anesthetic intravascularly may result in local anesthetic toxicity, requiring immediate supportive measures.174
Neuropathic pain has been reported after cervical epidurals and may result from spinal nerve irritation caused by the injectate or by compression of neural elements.175–177
Cervical median branch block/radiofrequency ablation
According to the literature, complications from third occipital nerve (TON) lesioning include ataxia, numbness, dysesthesias and hypersensitivity in the area of the treated nerves.178,179 Numbness is a predictable side effect which is usually well tolerated by patients.178–180 In fact, absence of numbness indicates a technical failure.178 The dysesthesia is temporary and not usually distressing to the patient.178 Tricyclic antidepressants and anticonvulsants can be used to manage these symptoms. Ataxia can be expected due to the interruption of the tonic neck reflexes and proprioception when the semispinalis is denervated in TON neurotomy.181 The ataxia causes unsteadiness when looking down and, according to Govind, was not disabling to the patient.178
Cervical discography
Discitis is the most widely recognized complication associated with discography and can be difficult to eradicate due to the limited blood supply of the disc. The most common infectious etiologic agent is S. aureus followed by other staphylococcus species and anaerobic organisms.170,177 Inadvertently piercing the esophagus during the procedure may seed the disc with Gram-negative and anaerobic organisms. The patient usually presents 1–4 weeks after the procedure with increased neck pain. Initially, there is usually no change at neurologic examination.
Epidural abscess may follow cervical discography and generally presents within 24–48 hours. The signs and symptoms are high fever, spine pain, and progressive neurologic deficit. White blood cell count (WBC), ESR, and C-reactive protein should be obtained if either discitis or epidural abscess is suspected. WBC is an unreliable marker. ESR and the C-reactive protein are the most sensitive laboratory markers.181 MRI with contrast is the radiographic imaging modality of choice in diagnosing postprocedural discitis and epidural abscess. MRI has a reported sensitivity of 93% and specificity of 97% in suspected cases of discitis.182 Blood cultures have a 55.6% positive rate in the setting of a culture-positive discitis.183 When the organism is determined, antibiotics should be instituted. Surgical intervention is required with large lesions or lack of improvement with antibiotics and immobilization.
Pneumothorax is another rare complication of cervical discography. It usually is self-limited and causes only minor lung collapses (10%).184 Treatment includes supportive care in a hospital setting with serial chest X-rays and possibly placement of a chest tube.
Thoracic spinal injections
Potential complications of thoracic spinal injections include dural puncture, infection, vascular and neurological injury.107,184 Thoracic spinal procedures have greater propensity for occurrence of complications secondary to unique inherent anatomical properties of the thoracic region. These include a narrow epidural space, which leads to a closer proximity to the spinal cord. The proximity of the lung poses a significant risk of developing a pneumothorax. Injection of local anesthetic in the mid-thoracic epidural space may cause inhibition of the cardiac accelerator leading to bradycardia and/or hypotension.185 Thoracic motor blockade can impair ventilation with up to 50% reduction in tidal volume.185 Slipman et al.186 have reported persistent hiccups associated with thoracic epidural injection. Arterial supply by means of the artery of Adamkiewicz and its anatomical variations make it vulnerable to injury during lower thoracic or upper lumbar transforaminal epidural injections.187–189
Lumbar spinal injections
Lumbar epidural steroid injections
Interlaminar lumbar epidural injections
Complications following interlaminar lumbar epidural injections can be broadly divided into septic, vascular, neurological, and idiopathic. Vascular complications include vascular damage and development of epidural hematoma. Vascular infiltration can lead to subsequent cardiorespiratory and/or neurologic complications (i.e. seizures), which have been discussed in depth in the beginning of this chapter. Since the lumbar epidural space is highly vascularized, inadvertent intravenous placement of the epidural needle can occur in 0.5–1% of patients undergoing lumbar epidural injection.123 When fluoroscopy is used, the injection of contrast can rule out any significant intravascular injection. Development of an epidural hematoma is a potentially dangerous complication of vascular injury. Any rapidly progressive neurological deficit or cauda equina syndrome should elevate the suspicion of the development of an epidural hematoma and CT/MRI scan should be done to rule this out.190,191 The presence of an epidural hematoma should be dealt with by prompt surgical decompression.
Neurologic complications include dural puncture and injury to the cauda equina or nerve roots. The former has been discussed in detail earlier in this chapter. Direct nerve injury usually elicits painful symptoms. Therefore, increased pain during insertion of the spinal needle should alarm the interventionist to reconfirm needle placement to avoid possible nerve injury.123 McLain et al.192 reported transient, profound paralysis after an epidural steroid injection, which was carried out without fluoroscopic control and was complicated by a puncture of the thecal sac. Radiographic studies suggested three possible explanations for the paralysis: (1) inadvertent thecal penetration during injection may have produced an atypical anesthetic block; (2) location of the injected fluid may have caused a transient compressive lesion; or (3) intrathecal injection may have produced an iatrogenic arachnoid cyst. Conscious sedation or general anesthesia prior to epidural injection can reduce the patient’s ability to provide optimal feedback to painful stimuli; thus, greater caution should be exercised while using them.193,194
Idiopathic complications of lumbar epidural steroid injections include acute retinal necrosis and retinal hemorrhage. The retinal adverse effects are related to rapid increase in intracranial pressure. Browning195 reported acute retinal necrosis following epidural steroid injections. Young196 published a case report and literature review of transient blindness after lumbar epidural steroid injection and suggested strategies for prevention of this complication. This included avoidance of rapid injection into the epidural space and limiting of the amount of fluid injected during the procedure. Although these complications were noted with volumes of injectate as high as 20 mL, the literature fails to advocate ideal volume of injectate to avoid such complications.
Caudal epidural injections
Specific complications of caudal epidural steroid injections include dural puncture, infection, and effects of local anesthesia. Failure of correct needle placement has been reported to be as high as 25–40%197–200 using a blind technique. This can lead to the injection of air or fluid into subcutaneous tissues, periosteum, sacrococcygeal ligament, sacral marrow cavity, and pelvic cavity with the likelihood of penetrating the rectum or vagina. Renfrew et al.48 reported 9.2% of vascular infiltration with caudal epidural injections. Figure 20.2 shows a case of vascular infiltration during a caudal epidural injection. Fluoroscopy can help recognize these potential pitfalls. El Khoury et al.201 supported this, as they found the incidence of incorrect placement of needle during caudal injections was reduced to 2.5% when fluoroscopy was utilized. Dural puncture following caudal epidural injection is very rare. Although the thecal sac usually ends at the S2 bony level, in rare variants it may end as low as S4. Hence, the needle should not be placed superior than absolutely necessary to assure epidural injection.188
The use of anesthesia in caudal injections can anesthetize the sacral nerve roots. This can lead to an increased incidence of urinary retention. The use of smaller doses of local anesthetic can minimize this complication without adversely affecting the efficacy of caudal epidural steroid injections when treating painful conditions.188
Earlier literature mentions use of higher volumes of injectate for caudal epidural injections.202,203 Surprisingly, the adverse effects noted with higher volumes of injectate during the interlaminar epidural approach do not necessarily translate to the caudal epidural approach. Cyriax203 reported on the use of 50 mL of procaine following 50 000 caudal injections and had only five adverse effects; one case of hypersensitivity, two cases of transient paraplegia, and two cases of chemical meningitis. All these recovered without any residual damage. Bogduk204 suggested volumes of 10 mL and 15 mL of epidural injectate are adequate to reach L5 and L4 levels, respectively, during a caudal approach.
Bryan et al.205 found that contrast reaches the L4–5 intervertebral level in 85% of patients with a volume of <8 mL during caudal approach.
Botwin et al.206 reported a 15.6% incidence of minor complications in fluoroscopically guided caudal epidural injections. The most common minor complications observed was 4.7% insomnia the night of injection, 3.5% nonpositional headache, 3.1% increased back pain at injection site, and 0.4% increased leg pain.
Lumbar transforaminal epidural steroid injections
Complications related to the lumbar transforaminal technique are relatively fewer as compared to the interlaminar technique. Lutz and Wisneski207 as well as Kraemer et al.208 reported no dural punctures or any other major complications related to this technique. The placement of the needle in the safe triangle described by Derby et al.209 reduces the chances of dural puncture or nerve injury. Botwin et al.,210 in their study of 322 lumbar transforaminal injections, reported a 9.6% incidence of minor complications, which were entirely transient and resolved without morbidity. Complications included postprocedural back pain at the injection site, increased leg pain, and transient leg weakness.
Slipman et al.,173 in a prospective study of 217 lumbosacral nerve root injections, found no major complications but did report an immediate postprocedure incidence of 17.1% increased pain at injection site, 8.8% increased radicular pain, 6.5% light-headedness, 5.1% increased spine pain, 3.7% nausea, 1.4% non-specific headache, and 0.5% vomiting per injection.
The potential complications of lumbar transforaminal epidural injections include infection, dural puncture, nerve injury, and vascular infiltration. Furman et al.50 reported an 11.2% incidence of intravascular injections in 761 transforaminal epidural injections with a significantly higher rate of intravascular injections (21.3%) at S1 level. They also concluded that contrast enhancement is mandatory to rule out vascular infiltration, as negative flash or aspiration was found to be unreliable. There have been reports of acute paraplegia following left-sided high lumbar transforaminal injections, possibly secondary to disruption of the artery of Adamkiewicz leading to anterior spinal artery ischemia and cord infarction.189,211 The artery of Adamkiewicz is the principal blood supply to the lower spinal cord and like other radicular arteries typically enters the spinal canal along the anterior, superior aspect of the spinal nerve.212 The usual technique of placing a needle tip for a left transforaminal epidural injection at the mid-thoracic to upper lumbar region can place the artery of Adamkiewicz at risk. A modified technique has been suggested to keep the needle tip in the posterior, inferior location within the foramen in order to reduce disruption of the radicular artery.185 More research is needed to reduce the risk of entering the artery of Adamkiewicz. Extreme caution should also be exercised in rare conditions such as far lateral/foraminal disc herniations where there can be likelihood of injecting the disc itself if the needle tip is too far medial in the intervertebral foramen.
Lumbar zygapophyseal joint injections
Potential complications include infections and inadvertent entry into intervertebral foramen or spinal canal causing dural puncture and/or nerve injury. Infectious complications range from delayed septic arthritis of facet joints to development of an epidural abscess.213–215 The most often noted complication (2%) is transient exacerbation of pain.216 Spinal anesthesia can occur if there is an abnormal communication between facet joint capsule and the thecal sac. Authors have reported chemical meningitis following facet joint blocks.217,218
Lumbar medial branch block/radiofrequency ablation
Potential complications of radiofrequency zygapophyseal denervation or medial branch block include infection, nerve root injury, and dural puncture. The reported complication rate for radiofrequency zygapophyseal denervation is quite low.219–223 Superficial infections and reactions to the local anesthetic are the most common complications incurred.224
In a recent study of 92 patients who received 616 radiofrequency lesions, six complications arose for a 1% complication rate per lesion site. Among these complications were three cases of localized pain at lesion site lasting more than 2 weeks and three cases of neuropathic pain lasting less than 2 weeks.225
Lumbar discography
Potential complications of discography include nerve root injury, dural puncture, intradural injection, meningitis, arachnoiditis, intrathecal hemorrhage, and discitis. Discitis is the single most significant complication of discography with the incidence ranging from 0% to 4.9%.2,224,226–229 Osti et al.227 performed a prospective clinical study of 127 patients treated with intradiscal cefazolin 1 mg/mL mixed with diatrozoate contrast. None of these patients developed discitis. Klessig et al.230 indicated that intradiscal antibiotics mixed with iohexol provided adequate antibacterial prophylaxis against discitis. Use of intradiscal antibiotics has been recommended as an integral part of practice guidelines issued by the International Spinal Injection Society.231 Other rare complications of discography include vascular injury, retroperitoneal hemorrhage,232 disc herniation,233 and pulmonary embolism.234 DeSeze235 and Bernard228 have raised the possibility of discography causing degenerative changes; however, various other studies have disputed these claims.235–238 There has been a case of seizure and death from an inadvertent injection of 12.5 mg/mL of cefazolin intrathecally during a discogram at L5/S1.239
Lumbar intradiscal electrothermal annuloplasty/nucleoplasty
Potential complications of intradiscal annuloplasty would include those incurred with discography. Reported complications of intradiscal electrothermal annulopasty (IDEA) include cauda equina syndrome240 and disc herniation.241 There have been no reports of infection, bleeding, or other equipment- or technique-related complications so far mentioned in the literature.242,243 Cohen et al.,244 in their study of 78 patients treated with IDEA, reported a 10% incidence of transient complications ranging from paresthesia radicular pain to weakness.
No complications have been reported in two prospective outcome studies evaluating percutaneous disc decompression using nucleoplasty.245,246
Lumbar sympathetic blocks
Potential complications of lumbar sympathetic blocks consist of accidental spread of anesthetic to the neuraxis causing a blockade of somatic nerves (lumbar plexus). Vascular injury/infiltration, lymphatic infiltration, or nerve root injury can occur as well.247–252 If the transverse process is mistakenly identified as the vertebral body, and a large volume of drug is injected into the intervertebral foramen, an ipsilateral lumbar plexus block and/or epidural spread of solution may occur. Therefore, authors advocate diluting the concentration of anesthetic so as not to produce motor blockade.248 Anterior advancement of needle has the potential danger of penetrating either the aorta or the inferior vena cava where injection of local anesthetic could produce systemic toxicity.252 Fluoroscopic guidance can help minimize these potential complications.
1 Simon DL, Kunz RD, German JD. Allergic or pseudo-allergic reaction following epidural steroid deposition and skin testing. Reg Anesth. 1989;14:253-255.
2 Astra Pharmaceuticals. Package insertion: Xylocaine. Westborough, MA: Astra Pharmaceuticals, 1997.
3 Vandam LD, Dripps RD. Long-term follow up of patients who received 10 098 spinal anesthetics. JAMA. 1960;1972:1483-1487.
4 Benoit PW, Belt WD. Destruction and regeneration of skeletal muscle after treatment with a local anesthetic, bupivacaine (Marcaine). J Anat. 1970;107:547-556.
5 Myers RR, Kalichman MW, Reisner LS. Neurotoxicity of local anesthetics: Altered perineural permeability, edema and nerve fiber injury. Anesthesiology. 1986;64:29-35.
6 Myers RR, Heckmann HM. Effects of local anesthesia on nerve blood flow: Studies using lidocaine with and without epinephrine. Anesthesiology. 1989;71:757-762.
7 Selander D, Branttsand R, Lundborg G. Local anesthetics: Importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. Acta Anesth Scand. 1979;23:127-136.
8 Gentill F, Hudson AR, Hunter D. Nerve injection injury with local anesthetic agents: A light and electron microscopic, fluorescent microscopic and horseradish peroxidase study. Neurosurgery. 1980;6:263-272.
9 Covino BG. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, Bridenbaugh MJ, Phillip O, editors. Neural blockade in clinical anesthesia and management of pain. Philadelphia: Lippincott; 1988:111-144.
10 Liu SS, Carpenter RL, Neal JM. Epidural anesthesia and analgesia: Their role in postoperative outcome. Anesthesiology. 1995;82:1474. 1150
11 Scott DA, Chanley DM, Mooney PH. Epidural ropivacaine infusion for postoperative analgesia after major lower abdominal surgery: A dose finding study. Anesth Analg. 1995;81:982-986.
12 Liu S, Angel JM, Owens BD. Effects of epidural bupivacaine after thoracotomy. Reg Anesth. 1995;20:303-310.
13 Block A, Covino BG. Effect of local anesthetic agents on cardiac conduction and contractility. Reg Anesth. 1982;6:55-59.
14 Feldman HS, Covino BM, Sage DJ. Direct chronotropic and inotropic effects of local anesthetic agents in isolated guinea pig atria. Reg Anesth. 1982;7:149.
15 American Heart Association. Textbook of advanced cardiac life support, 2nd ed. American Heart Association, 1993.
16 American Heart Association. Guidelines for cardiopulmonary resuscitation and emergency cardiac care. JAMA. 1992;268:2171-2302.
17 Householder-Hughes S. Advanced cardiac life support for the new millennium. J Cardiovasc Nurs. 2002;16:9-23.
18 Boonen S, Van Distel G, Westhovens R. Steroid myopathy induced by epidural triamcinolone injection. Br J Rheum. 1995;24:385-386.
19 Nelson DA, Vates TSJr, Rhomas RBJr. Complications from intrathecal steroid therapy in patients with multiple sclerosis. Acta Neurol Scand. 1973;49:176-188.
20 Nelson DA. Dangers from methylprednisolone acetate therapy by intraspinal injection. Arch Neurol. 1988;45:804-806.
21 Bernat IL, Sadowsky CH, Vincent FM. Sclerosing pachymeningitis. A complication of intrathecal administration of Depo-Medrol for multiple sclerosis. J. Neurol Neurosurg Psychiatry. 1976;39:1124-1128.
22 Carta F, Canu C, Datti R. Calcification and ossification of spinal arachnoid after intrathecal administration of DepoMedrol. Zentralbl Neurochir. 1987;48:256-261.
23 Latham IM, Fraser RD, Moore RI. The pathologic effects of intrathecal betamethasone. Spine. 1997;22:1558-1562.
24 Cicala RS, Turner R, Moran E. Methylprednisolone acetate does not cause inflammatory changes in the epidural space. Anesthesiology. 1990;72:556-558.
25 Abram SE, Marsala M, Yaksh TL. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994;81:1198-1205.
26 Delaney TJ, Rowlingson JC, Carron H. Epidural steroid effects on nerves and meninges. Anesth Analg. 1980;59:610-614.
27 Benzon HT. Epidural steroid injection for low back pain and lumbosacral radiculopathy. Pain. 1986;24:277-295.
28 Goebert HW, Jallo SJ, Gardner WJ. Painful radiculopathy treated with epidural injections of procaine and hydrocortisone acetate. Results in 113 patients. Anesth Analg. 1961;40:130-134.
29 Wallace G, Solove GJ. Epidural steroid therapy for low back pain. Postgrad Med. 1985;78:213-215. 218
30 Abram SE. Subarachnoid corticosteroid injection following inadequate response to epidural steroids for sciatica. Anesth Analg. 1978;57:313-315.
31 Bush K, Hillier S. A controlled study of caudal epidural injection of triamcinolone plus procaine for the management of intractable sciatica. Spine. 1991;16:572-575.
32 Humblot S, Weber JC, Korganow AS. Lipomatosis induced by corticosteroid therapy. Rev Med Interne. 1997;18:396-401.
33 Roy-Camille R, Mazel C, Husson I. Symptomatic effects of epidural dexamethasone injections. Rev Rhum Engl Ed. 1995;62:428-432.
34 McCullen GN, Spurling FR, Webster JS. Epidural lipomatosis complicating lumbar steroid injections. J Spinal Disord. 1999;12:526-529.
35 Jurmand SH. Corticotherapie peridural des lombalgies et des sciatiques d’origine disccale. Concours Med. 1972;94:5061-5070.
36 Harley C. Extradural corticosteroid infiltration. Ann Phys Med. 1967;9:22-28.
37 Forrest JB. The response to epidural steroid injections in chronic dorsal root pain. Can J Anaesth. 1980;27:40-46.
38 Tuel SM, Meythaler JM, Cross LL. Cushing’s syndrome from epidural methylprednisolone. Pain. 1990;40:81-84.
39 Stambough JL, Booth RE, Rothman RH. Transient hypercorticism after epidural steroid injection. J Bone Joint Surg Am. 1984;66:115-116.
40 Burn JM, Langdon L. Duration of action of epidural methylprednisolone, a study in patients with the lumbosciatic syndrome. Am J Phys Med. 1974;53:29-34.
41 Jacobs S, Pullan PT, Potter JM. Adrenal suppression following extradural steroids. Anaesthesia. 1983;38:953-956.
42 Knight CL, Burnell JC. Systemic side effects of extradural steroids. Anasthesia. 1980;35:593-594.
43 Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary–adrenal axis in human subjects. Anesth Analg. 1994;78:501-505.
44 Manchikanti L. The effect of neuroaxial steroids of weight and bone mass density; a prospective evaluation. Pain Physician. 2000;3:357-366.
45 Squibb Diagnostic Products Monograph for Isovue 1984. Data on file. Princeton, NJ: Squibb Institute for Medical Research, ER Squibb & Sons, 1984.
46 Yamaguchi K, Kozuka T, Takashima T, et al. Adverse reactions to ionic and non-ionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media [Comment]. Radiology. 1990;175:616-618.
47 Drayer BP. A double blind clinical trial of iopamidol versus metrizomide for lumbosacral myelography. J. Neurosurg. 1983;32:320-321.
48 Renfrew DL, Moore TE, Kathol MH, et al. Correct placement of epidural steroid injections: fluoroscopic guidance and contrast administration. Am J Neuroradiol. 1991;12:1003-1007.
49 Sullivan WJ, Willick SE, Chira-Adisai W, et al. Incidence of intravascular uptake in lumbar spinal injection procedures. Spine. 2000;25:481-486.
50 Furman MB, O’Brien EM, Zgleszewski TM. Incidence of intravascular penetration in transforaminal lumbosacral epidural steroid injections. Spine. 2000;25:2628-2632.
51 Furman MB, Giovanniello MT, O’Brien EM. Incidence of intravascular penetration in transforaminal cervical epidural steroid injections. Spine. 2003;28:21-25.
52 Falco FJ, Rubbani M. Visualization of spinal injection procedures using gadolinium contrast. Spine. 2003;28:E496-E498.
53 Shealy CN. Dangers of spinal injections without proper diagnosis. JAMA. 1966;197:1104-1106.
54 Chan ST, Leung S. Spinal epidural abscess following steroid injection for sciatica. Case report. Spine. 1989;14:106-108.
55 Goucke CR, Craziotti P. Extradural abscess following local anesthetic and steroid injection for chronic low back pain. Br J Anaesth. 1990;65:427-429.
56 Cooper AB, Sharpe MD. Bacterial meningitis and cauda equina syndrome after epidural steroid injections. Can J Anaesth. 1996;43:471-474.
57 Dougherty JH, Fraser RA. Complications following intraspinal injections of steroids. Reports of two cases. J Neurosurg. 1978;48:1023-1025.
58 Roberts M, Sheppard GL, McCormick RC. Tuberculous meningitis after intrathecally administered methylprednisolone acetate. JAMA. 1967;200:894-896.
59 Tham E, Stoodley M, Macintyre P. Back pain following postoperative epidural analgesia; An indicator of possible infection. Anesth Intensive Care. 1997;25:297-301.
60 Bromage PR. Spinal extradural abscess: pursuit of vigilance. Br J Anaesth. 1993;70:471-474.
61 Waldman SD. Cervical epidural abscess after cervical epidural nerve block with steroids (Letter). Anesth Analg. 1991;72:717.
62 Mamourian AC, Dickman CA, Drayer BP. Spinal epidural abscess: Three cases following spinal epidural injection demonstrated with magnetic resonance imaging. Anesthesiology. 1993;78:204-206.
63 Knight JW, Cordingley JJ, Palazzo MGA. Epidural abscess following epidural steroid and local anesthetic injection. Anesthesia. 1997;52:576-579.
64 Gutknecht DR. Chemical meningitis following epidural injections of corticosteroids. Am J Med. 1987;82:507-509.
65 Plumb VJ, Dismukes WE. Chemical meningitis related to intrathecal corticosteroid therapy. South Med J. 1977;70:1241-1247.
66 Morris JT, Konkol KA, Longfield RN. Chemical meningitis following epidural methylprednisolone injection. Infect Med. 1994;11:439-440.
67 Meyers SP, Weiner SN. Diagnosis of hematogenous pyogenic vertebral osteomyelitis by magnetic resonance imaging. Arch Intern Med. 1991;151:683-689.
68 Gold MS. The effect of the epidural/general and cervical plexus block on activated clotting time in patients undergoing vascular surgery. Anesth Analg. 1993;74:701-704.
69 Aldouri M. The use of recombinant factor VIIa in controlling surgical bleeding in non-haemophiliac patients. Haemost Thromb. 2002;32:41-46.
70 Kakazu K, Ohira N, Ojima T, et al. Extensive spinal epidural hemorrhage associated with von Willebrand’s disease – a case report. Nippon Seikeigeka Gakkai Zasshi. 1980;54:501-505.
71 Stedeford JC, Pittman JA. von Willebrand’s disease and neuroaxial anaesthesia. Anaesthesia. 2000;55:1228-1229.
72 Cohen S, Daitch JS, Amar D. Epidural analgesia for labor and delivery in a patient with von Willebrand’s disease. Reg Anesth. 1989;14:95-97.
73 Milaskeiwicz RM, Holdcroft A, Letsky E. Epidural anesthesia and von Willebrand’s disease. Anasthesia. 1990;45:462-464.
74 Rolbin SH, Abbott D, Musclow E. Epidural anesthesia in pregnant patients with low platelet counts. Obstet Gynecol. 1988;71:918-920.
75 Waldman SD, Feldstein G. Caudal administration of morphine sulfate in anticoagulated and thrombocytopenic patients. Anesth Analg. 1987;66:267-268.
76 Rasmus KT, Rottman RL, Kotelko DM. Unrecognized thrombocytopenia and regional anesthesia in parturients: a retrospective review. Obstet Gynecol. 1989;73:943-946.
77 Harvey SC, Roland PJ, Cure JK. Spinal epidural hematoma detected by lumbar epidural puncture. Anesth Analg. 1997;84:1136-1139.
78 Laglia AG, Eisenberg RL, Weinstein PR. Spinal epidural hematoma after lumbar puncture in liver disease. Ann Intern Med. 1978;88:515-516.
79 Yarnell RW, D’Alton ME. Epidural hematoma complicating cholestasis of pregnancy. Curr Opin Obstet Gynecol. 1996;8:239-242.
80 Morisaki H, Doi J, Ochial R. Epidural hematoma after epidural anesthesia in a patient with hepatic cirrhosis. Anesth Analg. 1995;80:1033-1035.
81 Takahaski K, Loiwa F, Tayama H. A case of acute spontaneous epidural haematoma in a chronic renal failure patient undergoing haemodialysis: successful outcome with surgical management. Nephrol Dial Transplant. 1999;14:2499-2501.
82 Ziyal IM, Aydin S, Inci S. Multilevel acute spinal epidural hematoma in a patient with chronic renal failure; case report. Neurol Med Chir (Toyko). 2003;43:409-412.
83 Groen RJM, Van Alphen HAM. Operative treatment of spontaneous spinal epidural hematomas: A study of the factors determining postoperative outcome. Neurosurgery. 1996;39:484-509.
84 Inoue K, Yokoyama M, Nakatsuka H. Spontaneous resolution of epidural hematoma after continuous epidural analgesia in a patient without bleeding tendency. Anesthesiology. 2002;97:735-737.
85 Abram SE, O’Connor TC. Complications associated with epidural steroid injections. Reg Anesth. 1996;21:149-162.
86 Kawaguchi S, Tokutomi S. A case of epidural hematoma associated with epidural catheterization which occurred on 12th day after the last medication of ticlopidine hydrochloride. Masui. 2002;51:526-528.
87 Horlocker TT, Wedel DJ, Schlicting JL. Postoperative epidural analgesia and oral anticoagulant therapy. Anesth Analg. 1994;79:89-93.
88 Ng WH, Lim CC, Ng PY. Spinal epidural hematoma: MRI aided diagnosis. J Clin Neurosci. 2002;9:92-94.
89 Grejda S, Ellis K, Arino P. Paraplegia following spinal anesthesia in a patient with chronic renal failure. Regional Anesthesia. 1989;14:155-157.
90 Alexiadou-Rudolf C, Ernestus RI, Nanassis K. Acute nontraumatic spinal epidural hematomas: An important differential diagnosis in spinal emergencies. Spine. 1998;23:1810-1813.
91 Selander D, Dhuner KG, Lundoborg G. Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand. 1977;21:182-188.
92 Odom J, Sih I. Epidural analgesia and anticoagulant therapy: Experience with one thousand cases of continuous epidurals. Anesthesia. 1983;38:550-551.
93 Rao T, El-Etr A. Anticoagulation following placement of epidural and subarachnoid catheters. Anesthesiology. 1981;55:618-620.
94 Vandermeulen EP, VanAken H, Vermylen J. Anticoagulants and spinal epidural anesthesia. Anesth Analg. 1994;79:165-177.
95 Williams KN, Jackowski A, Evans PJ. Epidural hematoma requiring surgical decompression following repeated cervical epidural steroid injections for chronic pain. Pain. 1990;42:197-199.
96 Wagner S, Forsting M, Hache W. Spontaneous resolution of a large spinal epidural hematoma: Case report. Neurosurgery. 1996;38:816-818.
97 Locke GE, Giorgio AJ, Biggers SLJr. Acute spinal epidural hematoma secondary to aspirin induced prolonged bleeding. Surg Neurol. 1976;5:293-296.
98 Urmey WF, Rowlingson JC. Do anti-platelet agents contribute to the development of perioperative spinal hematoma? Reg Anesth Pain Med. 1998;23:146-151.
99 Horlocker TT, Bajawa ZH, Ashraf Z. Risk assessment of hemorrhagic complications associated with non-steroidal anti-inflammatory medications in ambulatory pain clinic patients undergoing epidural steroid injection. Anesth Analg. 2002;95:1691-1697.
100 Horlocker TT, Wedel DJ, Offord KP. Does preoperative antiplatelet therapy increase the risk of hemorrhagic complications associated with regional anesthesia? Anesth Analg. 1990;70:631-634.
101 Horlocker TT, Wedel DJ, Schroeder DR. Preoperative anti-platelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg. 1995;80:303-309.
102 Mayumi T, Dohi S, Takahashi T. Spinal subarachnoid hematoma after lumbar puncture in a patient receiving anti-platelet therapy. Anesth Analg. 1983;62:777-779.
103 Watts SA, Gibbs NM. Outpatient management of the chronically anti-coagulated patient for elective surgery. Anaesth Intensive Care. 2003;31:145-154.
104 Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1506-1511.
105 DeLeon-Casasola OA, Parker B, Lema MJ. Postoperative epidural bupivacaine-morphine therapy: Experience with 4227 surgical cancer patients. Anesthesiology. 1994;81:368-375.
106 Auroy Y, Narchi P, Messiah A. Serious complications related to regional anesthesia. Anesthesiology. 1997;87:479-486.
107 Giebler RM, Scherer R, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization. Anesthesiology. 1997;86:55-63.
108 Cicala RS, Westbrook L, Angel JJ. Side effects and complications of cervical epidural steroid injections. J Pain Symptom Manage. 1989;4:64-66.
109 Purkis IE. Cervical epidural steroids. Pain Clin. 1986;1:3-7.
110 Hodges SD, Castleberg RL, Miller T, et al. Cervical epidural steroid injection with intrinsic spinal cord damage. Spine. 1998;19:2137-2142.
111 Winnie AP, Hartman JT, Meyers HL. Pain clinic: Intradural and extradural corticosteroids for sciatica. Anesth Analg. 1972;5:990-1003.
112 Warr AC, Wilkinson JA, Burn JMB. Chronic lumbosciatic syndrome treated by epidural steroid injection and manipulation. Practitioner. 1972;209:53-59.
113 Berman AT, Garbarinbo JL, Fisher SM. The effects of epidural injection of local anesthetics and corticosteroids on patients with lumbosciatic pain. Clin Orthop. 1984;1898:144-148.
114 Allum RL, Parker BC. Epidural steroids in the treatment of low back pain and sciatica. J Bone Joint Surg Br. 1982;64:249-253.
115 Gardner WJ, Goebert HW, Sehgal AD. Intraspinal corticosteroids in the treatment of sciatica. Trans Am Neurol Assoc. 1961;86:214-215.
116 Ryan MD, Taylor TKF. Management of lumbar nerve root pain by intrathecal and epidural injection of deport methylprednisolone acetate. Med J Aust. 1981;2:532-534.
117 Sehgal AD, Gardner WJ. Corticosteroids administered intradurally for relief of sciatica. Cleve Clin J Med. 1960;27:198-201.
118 Ito R. The treatment of low back pain and sciatica with epidural corticosteroids injection and its pathological basis. J Jpn Orthop Assoc. 1971;45:769-777.
119 Kim SIK, Sadove MS. Caudal epidural corticosteroids in postlaminectomy syndrome: Treatment for low back pain. Compr Ther. 1975;1:57-60.
120 Holtkamp M, Masuhr F, Harms L. The management of refractory generalized convulsive and complex partial status epilepticus in three European countries: A survey among epileptologists and critical care neurologists. J Neurol Neurosurg Psychiatry. 2003;74:1095-1099.
121 Outin H, Liot P, DeJonghe B. Management of adult refractory convulsive status epilepticus in the intensive care unit. Rev Neurol. 2002;158:1059-1068.
122 Lacey D. Status epilepticus in children and adults. J Clin Psychiatry. 1988;49:33-36.
123 Bromage PR. Complications and contraindications. In: Bromage PR, editor. Epidural analgesia. Philadelphia: WB Saunders; 1978:654-711.
124 Nishimura N. The spread of lidocaine and I-131 solution in the epidural space. Anesthesiology. 1959;20:785-789.
125 Boys JE. Accidental subdural analgesia. A case report, possible clinical implications and relevance to ‘massive extradurals’. Br J Anesth. 1975;47:111-113.
126 Abram SE, Cherwenka RW. Transient headache immediately following epidural steroid injections. Anesthesiology. 1979;50:461-462.
127 Katz JA, Lukin R, Bridenbaugh PO, et al. Subdural intracranial air: an unusual cause of headache after epidural steroid injection. Anesthesiology. 1991;74:615-618.
128 Barker P. Headache after dural puncture. Anaesthesia. 1989;44:696-697.
129 Flaatten H, Rodt S, Rosland J, et al. Postoperative headache in young patients after spinal anaesthesia. Anesthesia. 1987;42:202-205.
130 Flaatten H, Rodt SA, Vamnes J, et al. Postdural puncture headache. A comparison between 26 and 29 gauge needles in young patients. Anaesthesia. 1989;44:147-149.
131 Geurts JW, Haanschoten MC, vanWijk RM, et al. Postdural puncture headache in young patients. A comparative study between the use of 0.52 mm (25 gauge) and 0.33 mm (29 gauge) spinal needles. Acta Anaesthesiol Scand. 1990;234:350-353.
132 Reynolds AFJr, Hameroff SR, Blitt CD, et al. Spinal epidural epiarachnoid hematoma; a complication of a novel epidural blood patch technique. Anesth Analg. 1980;59:702-703.
133 Leibold RA, Yealy DM, Copppola M, et al. Post-dural puncture headache: characteristics, management, and prevention. Ann Emerg Med. 1993;22:1863-1870.
134 Weir EC. The sharp end of the dural puncture. Br Med J. 2000;320:127-128.
135 McGrady EM, Freshwater JV. ‘Spinal’ headache with no headache. Anaesthesia. 1991;46:794-797.
136 Lybecker H, Anderson T. Repetitive hearing loss following dural puncture treated with autologous epidural blood patch. Acta Anaesthesiol Scand. 1995;39:987-989.
137 Schabel JE, Wang ED, Glass PS. Arm pain as an unusual presentation of postdural puncture intracranial hypotension. Anesth Analg. 2000;91:910-912.
138 Eerola M, Kaukinen L, Kaukinen S. Fatal brain lesion following spinal anesthesia. Reports of a case. Acta Anaesthesiol Scand. 1981;25:115-116.
139 Costigan SN, Sprigge JS. Dural puncture: the patients’ perspective. A patient survey of cases at a DGH maternity unit 1983–1993. Acta Anaethesiol Scan. 1996;40:710-714.
140 MacArthur C, Lewis M, Knox EG. Accidental dural puncture in obstetric patients and long-term symptoms. Br Med J. 1992;304:1279-1282.
141 Ostheimer GW, Palahniuk RJ, Shnider SM. Epidural blood patch for postlumbar puncture headache. Anesthesiology. 1974;41:307-308.
142 Camann WR, Murray RS, Mushlin PS, et al. Effects of oral caffeine on post-dural puncture headache. A double blind placebo controlled trial. Anesth Analg. 1990;70:181-184.
143 Jarvis AP, Greenawalt JW, Fagraeus L. Intravenous caffeine for post-dural puncture headache. Anesth Analg. 1986;65:316-317.
144 Brownridge P. The management of headache following accidental dural puncture in obstetric patients. Anesth Intensive Care. 1983;11:4-15.
145 Deisenhammer EL. Clinical and experimental studies on headache after myelography. Neuroradiology. 1985;74:615-618.
146 Digiovanni A. Epidural injection of autologous blood for post-lumbar puncture headache. Anesth Analg. 1979;49:268-271.
147 Gormly JB. Treatment of post-spinal headache. Anesthesiology. 1960;39:613-617.
148 Abouleish E, Vega S, Blendinger I, et al. Long-term follow-up of epidural blood patch. Anesth Analg. 1975;54:459-463.
149 Colonna-Romano P, Linton P. Cervical dural puncture and lumbar extradural blood patch. Can J Anesth. 1995;42:1143-1144.
150 Slipman CW, Ed Abd OH, Bhargava A, et al. Transforaminal cervical blood patch for the treatment of postdural puncture headache. Am J Phys Med Rehabil. 2005;84:76-80.
151 Cook M, Watkins-Pitchford J. Epidural blood patch: A rapid coagulation response. Anesth Analg. 1990;70:567-568.
152 Woodward WM, Levy DM, Dixon AM. Exacerbation of postdural puncture headache after epidural blood patch. Can J Anaesth. 1994;41:628-631.
153 Kawamata T, Omote K, Matsumoto M, et al. Pneumocephalus following an epidural blood patch. Acta Anaesthesiologica Scandinavica. 2003;4:907-909.
154 Idrees M, Ingleby A, Wali S. Evaluation and management of pneumothorax. Saudi Med J. 2003;24:447-452.
155 Slipman CW, Bhat AL, Chow DW, et al. Incidence of vocal cord dysfunction following fluoroscopically guided steroid injection in the axial skeleton. Arch Phys Med Rehabil. 2005;86:1330-1332.
156 Chaney MA. Side effects of intrathecal and epidural opioids. Anaesth. 1995;42:891-903.
157 Ready LB, Loper KA, Nessly M. Postoperative epidural is safe on surgical wards. Anesthesiology. 1991;75:452-456.
158 Armitage EN. Lumbar and thoracic epidural. In: Wildsmith JAW, Armitage EN, editors. Principles and practice of regional anesthesia. New York: Churchill Livingstone; 1987:109.
159 Simopoulos T, Peeters-Asdourian C. Pneumocephalus after cervical epidural steroid injection. Anesth Analg. 2001;92:1576-1577.
160 Shulman M. Treatment of neck pain with cervical epidural steroid injections. Reg Anesth. 1986;11:92-94.
161 Reitman C, Watters W. Subdural hematoma after cervical epidural steroid injection. Spine. 2002;3:E174-E176.
162 Catchlove FR, Braha R. The use of cervical epidural nerve blocks in the management of head and neck pain. Can Anaesth Soc J. 1984;31:188-191.
163 Brady RD, Wolff MW. Complications of transforaminal cervical epidural steroid injections. North American Spine Society Proceedings 14th annual meeting, p142. Chicago, Oct 20–23, 1999.
164 Waldman SD. Complication of cervical epidural nerve blocks with steroids: a prospective study of 790 consecutive blocks. Reg Anesth. 1989;14:149-151.
165 Huang RC, Shapiro GS, Lim M, et al. Cervical epidural abscess after epidural steroid injection. Spine. 2004;29:E7-E9.
166 Siegried RN. Development of complex regional pain syndrome after a cervical epidural steroid injection. Anesthesiology. 1997;86:1394-1396.
167 Botwin KP. Complications of fluoroscopically guided interlaminar cervical epidural injections. Arch Phys Med Rehabil. 2003;84:627-633.
168 Oza RM, Oleson CV, Formal CS. Tetraplegia after cervical epidural steroid injection: A case report. Am J Phys Rehabil. 2000;81:552.
169 Ludwig MA, Burns S. Spinal cord infarction after cervical transforaminal epidural injection: a case report. Arch Phy Med Rehabil. 2003;84:A37.
170 Brouwers PJAM, Kottink EJBL, Simmon MAM. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6 nerve root. Pain. 2001;19:397-399.
171 Tiso RI, Cutler T, Catania JA, et al. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine. 2004;4:468-474.
172 Slipman CW, Lipetz JS, Jackson HB, et al. Therapeutic selective nerve root block in the nonsurgical treatment of atraumatic cervical spondylitic radicular pain: A retrospective analysis with independent clinical review. Arch Phys Med Rehabil. 2000;81:741-746.
173 Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86:277-283.
174 Carpenter M. Satterfield T, editor. Core text of neuroanatomy, 4th edn.. Williams & Wilkins, Baltimore, 1991;434-438.
175 Stav A, Ovadia L, Sternberg A, et al. Cervical epidural steroid injection for cervicobrachialgia. Acta Anaesthesiol Scand. 1993;37:562-566.
176 Field J, Rathmell JP, Stephenson JH, et al. Neuropathic pain following cervical epidural steroid injection. Anesthesiology. 2000;93:885-888.
177 Baker R, Dreyfuss P, Mercer S, et al. Cervical transforaminal injection of corticosteroids into a radicular artery: a possible mechanism for spinal cord injury. Pain. 2003;103:211-215.
178 Govind J, King W, Bailey B, et al. Radiofrequency neurotomy for the treatment of third occipital headache. J Neuro Neurosgy Psychiatry. 2003;74:88-93.
179 Lord SM, Barnsley L, Wallis B, et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721-1726.
180 McDonald GJ, Lord SM, Bogduk N. Long-term follow-up of patients treated with cervical radiofrequency neurotomy of chronic neck pain. Neurosurgery. 1999;45:61-67.
181 Lord S, Barnsley L, Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophyseal joint pain: A caution. Neurosurgy. 1995;36:732-739.
182 Guyer RD, Colleir RC, Smith WJ. Discitis after discography. Spine. 1988;13:1352-1354.
183 Silber JS, Anderson DG. Management of postprocedural discitis. Spine J. 2002;3:279-287.
184 Zaugg M, Stoehr S, Weder W. Accidental plural puncture by a thoracic epidural catheter. Anesthesia. 1998;53:69-78.
185 Giebler RN, Scherer RU, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization related to thoracic epidural catheterization. Anesthesiology. 1997;86:55-56.
186 Slipman CW, Shin CH, Patel RK, et al. Persistent hiccup associated with thoracic epidural injection. Am J Phy Med Rehab. 2001;80:618-621.
187 Lo D, Vallee J, Spelle L. Unusual origin of the artery of Adamkiewicz from the fourth lumbar artery. Neurorodiology. 2002;44:153-157.
188 Windsor RE, Storm S, Sugar R. Prevention and management of complications resulting from common spinal injections. Pain Physician. 2003;6:473-483.
189 Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J. 2002;2:70-75.
190 Cousins M. Hematoma following epidural block. Anesthesiology. 1972;37:263.
191 Lerner S, Gutterman P, Jenkins F. Epidural hematoma and paraplegia after numerous lumbar punctures. Anesthesia. 1973;39:550-553.
192 McLain RF, Fry M, Hecht ST. Transient paralysis associated with epidural steroid injection. J Spinal Disord. 1997;10:441-444.
193 Braid D, Scott D. Dosage of lignocaine in epidural block in relation to toxicity. Br J Anaesth. 1966;38:596-602.
194 Cousins M. Epidural neural blockade. In: Cousins MJ, Bridenbaugh MJ, Phillip O, editors. Neural blockade in clinical anesthesia and management of pain. Philadelphia: Lippincott; 1988:340-341.
195 Browning DJ. Acute retinal necrosis following epidural steroid injections. Am J Ophthalmol. 2003;136:192-194.
196 Young WF. Transient blindness after lumbar epidural steroid injection: a case report and literature review. Spine. 2002;27:E476-E477.
197 Singh V, Manchikanti L. Role of caudal epidural injections in the management of chronic low back pain. Pain Physician. 2002;5:133-148.
198 Bogduk N. Epidural steroids for low back pain and sciatica. Pain Digest. 1999;9:226-227.
199 White A. Injection techniques for the diagnosis and treatment of low back pain. Orthop Clin North Am. 1983;14:553-567.
200 Manchikanti L, Staats P, Singh V, et al. Evidence-based practice guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2003;6:3-80.
201 el-Khoury GY, Ehara S, Weinstein JN, et al. Epidural steroid injection: a procedure ideally performed with fluoroscopic control. Radiology. 1988;168:554. 547
202 Evans W. Intrasacral epidural injection in treatment of sciatica. Lancet. 1930;2:1225-1229.
203 Cyriax JH. Epidural anesthesia and bed rest in sciatica. Br Med J. 1961;1:20-24.
204 Bogduk N, Christophifid N, Cherry D. Epidural use of steroids in the management of back pain. Canberra: Commonhealth of Australia: National Health and Medical Research Council, 1994;1-76.
205 Bryan BM, Lutz C, Lutz GE. Fluoroscopic assessment of epidural contrast spread after caudal injection. J Orthoped Med. 2000;22:38-41.
206 Botwin KP, Gruber RD, Bouchlas CG, et al. Complications of fluoroscopically guided caudal epidural injections. Am J Phys Med Rehab. 2001;80:416-424.
207 Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79:1362-1366.
208 Kraemer J, Ludwig J, Bickert U, et al. Lumbar epidural perineural injection: a new technique. Eur Spine J. 1997;6:357-361.
209 Derby R, Bogduk N, Kine G. Precision percutaneous blocking procedures for localizing spinal pain: Part 2. The lumbar neuroaxial compartment. Pain Digest. 1993;3:175-188.
210 Botwin KP, Gruber RD, Bouchlas CG, et al. Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil. 2003;81:1045-1050.
211 Windsor RE, Falco FJ. Paraplegia following selective nerve root blocks. Int Spine Injection Soc; Scientific Newsletter. 2001;4:53-54.
212 Alleyne C, Cawley C, Shengelaia. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurgery. 1998;89:791-795.
213 Cook NJ, Hanrahan P, Song S. Paraspinal abscess following facet joint injection. Clin Rheumatol. 1999;18:52-53.
214 Magee M, Kannangara S, Dennien B. Paraspinal abscess complicating facet joint injection. Clin Nucl Med. 2000;25:71-77.
215 Alcock E, Regaard A, Browne J. Facet joint injection: a rare form of epidural abscess formation. Pain. 2003;103:209-210.
216 Bous R. Facet joint injections. In: Stanton-Hicks M, Bous R, editors. Chronic low back pain. New York: Raven Press; 1982:199-211.
217 Thomson SJ, Lomax DM, Collett BJ. Chemical meningism after lumbar facet joint nerve block with local anesthetic and steroids. Anesthesia. 1991;46:563-564.
218 Berrigan T. Chemical meningism after lumbar facet joint block. Anesthesia. 1992;47:905-906.
219 North RB, Han M, Zahurak M. Radiofrequency lumbar facet denervation: Analysis of prognostic factors. Pain. 1994;57:77-83.
220 Shealy CN. Percutaneous radiofrequency denervation of spinal facets. J Neurosurg. 1979;43:448-451.
221 Lora J, Long D. So-called facet denervation in the management of intractable back pain. Spine. 1976;1:121-126.
222 Burton CV. Percutaneous radiofrequency facet denervation. Appl Neurophysiol. 1976;39:80-86.
223 Dreyfuss P, Halbrook B, Pauza K, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophyseal joint pain. Spine. 2000;25:1270-1277.
224 Guyer RD, Ohnmeiss DD. Lumbar discography. Spine J. 2003;3:11S-27S.
225 Kormick C, Klromarich S, Lamer TJ, et al. Complications of lumbar facet radiofrequency denervation. Spine. 2004;29:1352-1354.
226 Jackson RP, Cain JE, Jacobs RR. The neuroradiographic diagnosis of lumbar herniated nucleus pulposus. A comparison of computed tomography (CT), myelography, CT-myelography, discography and CT-discography. Spine. 1989;14:1356-1361.
227 Osti OL, Fraser RD, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg [Br]. 1990;72-B:271-274.
228 Bernard TN. Lumbar discography and post-discography computerized tomography: refining the diagnosis of low back pain. Spine. 1990;15:690-707.
229 Fraser RD, Osti OL, Veron-Roberts B. Discitis after discography. J Bone Joint Surg [Br]. 1987;69:31-35.
230 Klessig HT, Showsh SA, Sekorski A. The use of intradiscal antibiotics for discography: an in vitro study of gentamicin, cefazolin, and clindamycin. Spine. 2003;28:1735-1738.
231 Endres SM, Bogduk N. International Spinal Injection Society Practice Guidelines and Protocols: Lumbar Disc Stimulation. ISIS 9th Annual Scientific Meeting. Boston, MA: Syllabus, 2001;56-75.
232 McCulloch JA, Waddell G. Lateral lumbar discography. Br J Radiol. 1978;51:498-502.
233 Grubb SA, Lipscomb HJ, Guilford WB. The relative value of lumbar roentgenograms, metrizamide myelography, and discography in the assessment of patients with chronic low-back pain syndrome. Spine. 1987;12:282-286.
234 Schreck RI, Manion WL, Kambin P, et al. Nucleus pulposus pulmonary embolism: a case report. Spine. 1997;22:2191-2193.
235 DeSeze S, Levernieux J. Les accidents de la discographie. Rev Rheum Mal Osteoartic. 1952;19:1027-1033.
236 Johnson RG. Does discography injure normal discs? An analysis of repeat discograms. Spine. 1989;14:424-426.
237 Kahanovitz N, Arnoczky SP, Sissons HA. The effect of discography on canine intervertebral disc. Spine. 1986;11:26-27.
238 Saifuddin A, Renton P, Taylor BA. Effects on the vertebral end plate of uncomplicated lumbar discography: an MRI study. Eur Spine J. 1998;7:36-39.
239 Boswell MV, Wolfe JR. Intrathecal cefazolin induced seizures following attempted discography. Pain Physician. 2004;7:103-106.
240 Hsia AW, Isaac K, Katz JS. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2000;55:320-326.
241 Cohen SP. A giant herniated disc following intradiscal electrothermal therapy. J Spinal Disord. 2002;15:537-541.
242 Biyani A, Anderson GB, Chaudhary H, et al. Intradiscal electrothermal therapy: a treatment option in patients with internal disc disruption. Spine. 2003;28:S8-S14.
243 Heary RF. Intradiscal electrothermal annuloplasty: the IDET procedure. J Spinal Disord. 2001;14:353-360.
244 Cohen SP, Larkin T, Abdi S, et al. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine. 2003;28(11):1142-1147.
245 Sharps LS, Isaac Z. Percutaneous disc decompression using nucleoplasty. Pain Physician. 2002;5:121-126.
246 Singh V, Piryani C, Ligo K, et al. Percutaneous disc decompression using coblation (nucleoplasty) in the treatment of chronic discogenic pain. Pain Physician. 2002;5:250-259.
247 Schmidt S, Gibbons J. Postdural puncture headache after fluoroscopically guided lumbar paravertebral sympathetic block. Anesthesiology. 1993;78:78-98.
248 Murphy TM. Chronic pain. In: Miller RD, editor. Anesthesia. 4th edn. New York: Churchill Livingstone; 1994:2345.
249 Hogan QH. Neural blockade for diagnosis and treatment of painful conditions. In: Ashburn MA, Rice LJ, editors. The management of pain. New York: Churchill Livingstone; 1998:275.
250 Bonica JJ, Buckley FP. Regional analgesia with local anesthetics. In: Bonica JJ, editor. The management of pain. 2nd edn. Philadelphia: Lea & Febiger; 1990:1883.
251 Rowlingson JC. Lumbar ganglion. In: Hahn MB, McQuillan PM, Sheplock GJ, editors. Regional anesthesia: an atlas of anatomy and techniques. St. Louis: CV Mosby; 1996:183.
252 Miller RD. The treatment of chronic pain. Anesthesia, 5th edn., New York: Churchill Livingstone; 2000:2363-2367.