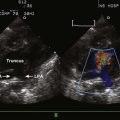
5 Shunting Lesions
Atrial Septal Defects
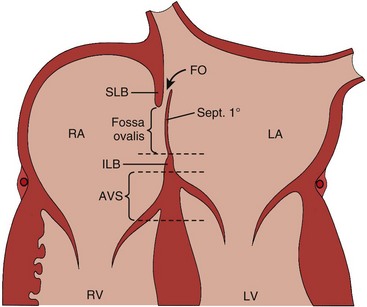
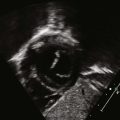
Atrial deptal defects represent defects in septation of the atria. They are common, representing as much as 10% of cases of congenital heart disease. Anatomic types of defects include (Fig. 5-1):
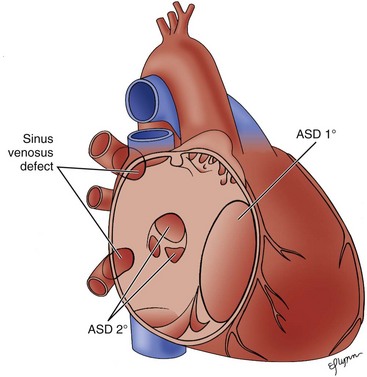
Figure 5-1 Diagram of the atrial septum showing several types of atrial septal defects.
(Adapted from Fyler DC [ed], Nadas’ Pediatric Cardiology. Philadadelphia: Hanley & Belfus, 1992.)
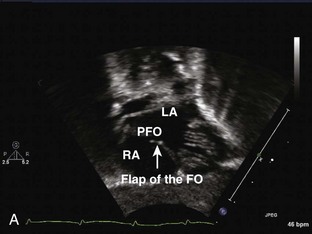
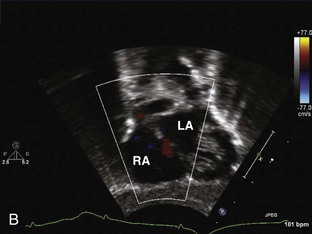
Patent Foramen Ovale
Ostium Primum Atrial Septal Defect
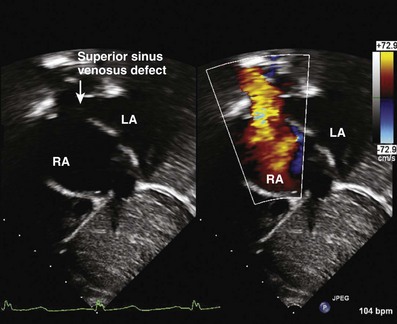
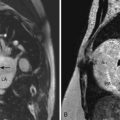
Sinus Venosus Atrial Septal Defects: Two Types
The Echocardiography (Echo) Exam: Step-by-Step Approach
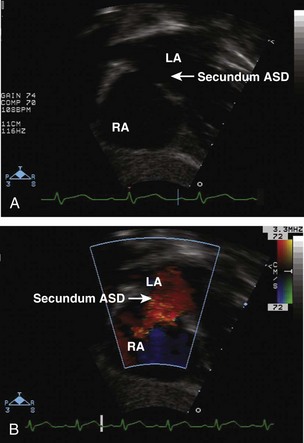
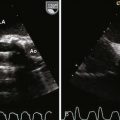
Step 1: Evaluate the Location of the Atrial Septal Defect
Key Points
Step 2: Evaluate the Atrial Septal Defect Dimensions and Position
Step 4: Determine the Size of the Shunt
Post-Device Atrial Septum Defect Closure Evaluation: Special Considerations
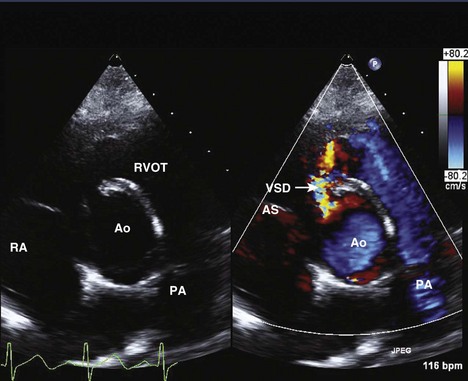
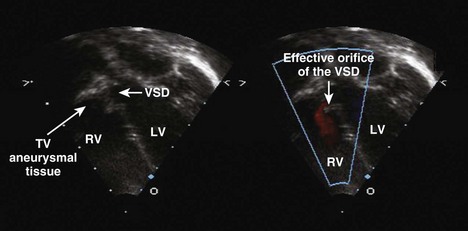
Ventricular Septal Defects
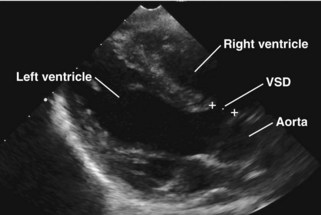
A ventricular septal defect is a communication within the interventricular septum that seperates the left ventricle (LV) and RV, allowing for shunting for blood between the ventricles. VSDs represent 20% of congenital heart disease. VSDs can be classified as follows (Fig. 5-6):
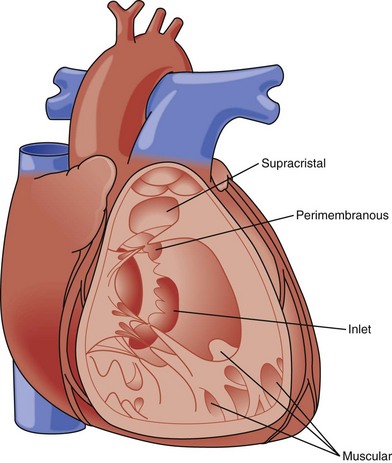
Figure 5-6 Diagram of types of VSDs as viewed from the right ventricle.
(Adapted from Fyler DC [ed], Nadas’ Pediatric Cardiology. Philadadelphia: Hanley & Belfus, 1992.)
Key Points
Membranous
Perimembranous
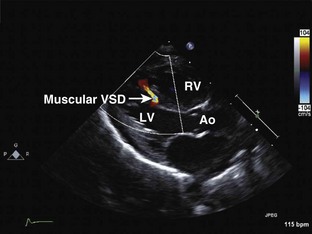
Muscular
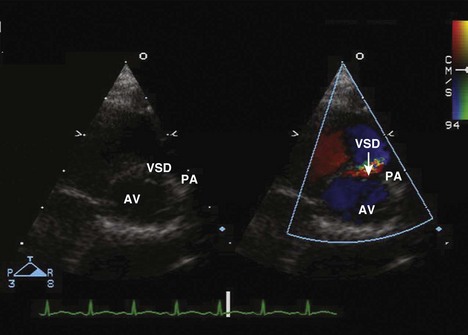
Supracristal (Subpulmonary)
The Echo Exam: Step-by-Step Approach
Step 1: Evaluate the Ventricular Septal Defect Location
Key Points
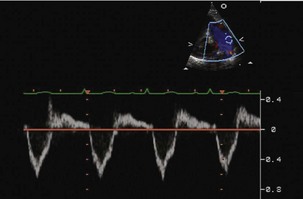
Step 2: Evaluate Ventricular Septal Defect Size
Step 4: Determine the Size of the Shunt
Key Points
Step 5: Evaluate the Effects of Shunting
Key Points
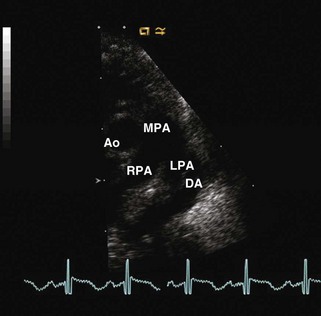
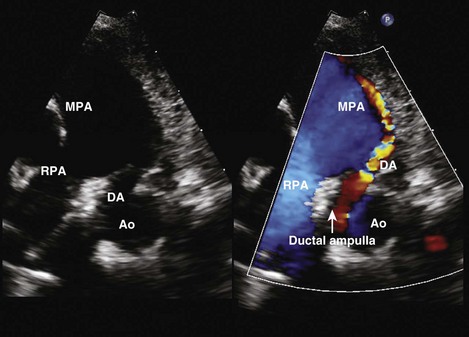
Patent Ductus Arteriosus
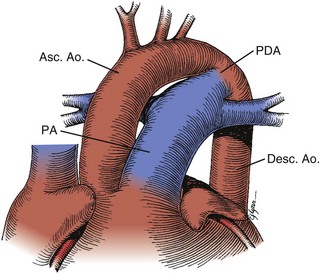
Figure 5-13 Anatomic drawing of a large persistent patent ductus arteriosus.
(Adapted from Fyler DC [ed], Nadas’ Pediatric Cardiology. Philaladelphia: Hanley & Belfus, 1992.)
The Echo Exam: Step-by-Step Approach
Step 1: Determine the Presence of a Patent Ductus Arteriosus
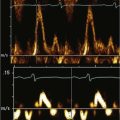
Step 2: Evaluate the Size of the Patent Ductus Arteriosus
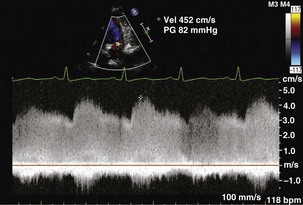
Step 3: Evaluate the Direction of the Shunt
Step 4: Determine Whether the Shunt Is Hemodynamically Significant
Step 5: Evaluate for Other Cardiac Disease
1 Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, editors Moss and Adams’ Heart Disease in Infants, Children and Adolescents: Including the Fetus and Young Adult. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2007: 632-645, 667-701.
2 Keane JF, Fyler DC, Lock JE. Nadas’ Pediatric Cardiology, 2nd ed. Philadelphia: Saunders; 2006. 527-548, 603-626
3 Lai W, Mertens L, Cohen M, Geva T, editors Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult. Hoboken, NJ: Wiley-Blackwell; 2009:158-187, 283-296
Includes a description of the anatomic lesions and additional imaging techniques.
4 Praagh VS, Carrera ME, Sanders SP, et al. Sinus venosus defects: unroofing the right pulmonary veins—anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994;128:365-379.
Key paper describing the anatomic findings of a sinus venosus defect seen by echo.
5 Hubail Z, Lemler M, Ramaciotti C, et al. Diagnosing a patent foramen ovale in children: is transesophageal echocardiography necessary. Stroke. 2011;42:98-101.
6 Butera G, Romagnoli E, Carminati M, et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1,013 consecutive patients. Am Heart J. 2008;156:706-712.
7 Radzik D, Davignon A, van Doesburg N, et al. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol. 1994;23:851-853.
8 Berger F, Ewert P, Biornstad P, et al. Transcatheter closure as standard treatment for most interatrial defects: experience in 200 patients treated with Amplatzer Septal Occluder. Cardiol Young. 1999;9:468-473.
9 Chiu SN, Wang JK, Lin MT, et al. Aortic valve prolapse associated with outlet-type ventricular septal defect. Ann Thorac Surg. 2005;79:1366-1371.
10 Canale JM, Sahn DF, Allen HD, et al. Factors affecting real-time, cross-sectional echocardiographic imaging of perimembranous ventricular septal defects. Circulation. 1981;63:689-697.
11 Mehta AV, Chidambaram B. Ventricular septal defect in the first year of life. Am J Cardiol. 1992;70:364-366.
12 Glen S, Burns J, Bloomfield P. Prevalence and development of additional cardiac abnormalities in 1448 patients with congenital ventricular septal defects. Heart. 2004;90:1321-1325.
13 Ramaciotti C, Vetter JM, Bornemeier RA, et al. Prevalence, relation to spontaneous closure and association of muscular ventricular septal defects with other cardiac defects. Am J Cardiol. 1995;75:61-65.
14 Turner SW, Hunter S, Wyllie JP. The natural history of ventricular septal defects. Arch Dis Child. 1999;81:413-416.
15 Sutherland GR, Godman M, Smallhorn F, et al. Ventricular septal defects: two dimensional echocardiographic and morphological correlations. Br Heart J. 1982;47:316-328.
Validates the use of echo in identifying VSDs.
16 Minette MS, Sahn DF. Ventricular septal defect. Circulation. 2006;114:2190-2197.
17 Su BH, Watanabe T, Mitsumasa S, et al. Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch Dis Child. 1997;77:F36-F40.
18 Sehgal A, McNamara PJ. Does echocardiography facilitate determination of hemodynamic significance attributable to the ductus arteriosus? Eur J Pediatr. 2009;168:907-914.
Methods for ductal assessment including echocardiographic markers of hemodynamically shunting.