Chapter 80 Should Thromboprophylaxis Be Used for Lower Limb Joint Replacement Surgery?
THROMBOEMBOLIC DISEASE IN JOINT REPLACEMENT SURGERY
Venous thromboembolic events (VTEs) are among the most feared complication of total hip replacement (THR) and total knee replacement (TKR) surgeries, and these patients are in the greatest surgical risk group for this complication. The development of deep venous thrombosis (DVT) with the potential to propagate a potentially lethal pulmonary embolus (PE) is the complication that presents the greatest risk for perioperative mortality after lower limb joint replacement. This has led to the adoption of routine thromboprophylaxis as a standard of care in joint replacement surgery since the late 1980s.1,2
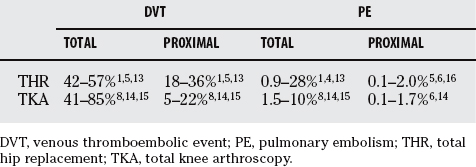
The natural history of VTE disease, after joint replacement surgery, has been well characterized. Based on data before 1980 and from trials with placebo patients, the incidence of lower extremity DVT without thromboprophylaxis in THR and total knee arthroplasty (TKA) has been reported as 40% to 60% and 41% to 85%, respectively (Table 80-1).3–9 Many of these statistics are based on venography end points. At least 50% of these patients had either asymptomatic or distal DVTs that resolved spontaneously without clinical sequelae.10,11 In the absence of thromboprophylaxis, clinically more important proximal DVTs occurred in THR and TKA at 18% to 36% and 5% to 22%, respectively, but again many were asymptomatic. With prophylaxis, symptomatic VTE was seen in 2.4% and 1.7%, respectively, of patients within 3 months of THR or TKA in studies done between 1992 and 1996.12 Many of these thrombotic events occur after hospital discharge, and the VTE risk remains increased for at least 8 weeks after surgery.
The incidence of PEs is less certain. The incidence of asymptomatic PE ranges from 3% to 20% and symptomatic PE from 0.5% to 3%,13,14 and with the current routine use of thromboprophylaxis, fatal PE is uncommon.15 Without thromboprophylaxis, perioperative mortality in THR and TKA from PE was reported from 0.1% to 2%.5,16, 17 With thromboprophylaxis for 7 to 10 days, there is a 0.1% rate of postdischarge fatal PE at 90 days after surgery in THR.18
The ideal VTE prophylaxis includes clear effectiveness compared with placebo or other active interventions; good risk/benefit profile; good compliance by patients, nurses, and physicians; easy administration or intervention; and requiring no need for monitoring whereas being cost effective.19 The best means to attain this goal remains an evolving science.
VENOUS THROMBOEMOBLIC EVENT RISKS
The interaction of age, genetic factors, and secondary, acquired persistent or transient environment or local factors is the accepted model for the pathogenesis of venous thrombotic disease. VTE risk has a direct and major relation to age.19 At ages 20, 50, and 80 years, the prevalence of VTE is 1 in 10,000, 1 in 1000, and 1 in 100 individuals per year, respectively, with a lifetime risk of 10%.20 Ethnicity is a risk factor.21,22 VTE is more common among blacks compared with whites and Asians by 1.4- and 7-fold, respectively.
Up to 15% to 20% of the general population has demonstrable endogenous biochemical hypercoagulable states.23,24 These defects may be on a genetic or acquired basis and include defects or deficiencies in anticoagulant proteins (protein S or C, antithrombin [AT]), altered procoagulant proteins (factor V and prothrombin), or fibrinolytic processes.25 Genetic hypercoagulable states include factor V Leiden (2–4% of the white population) and prothrombin 20210 defect (2–4% of the white population), AT deficiency (1/1000), protein C deficiency (1/2000), and protein S deficiency (1/2000).26 Acquired defects include anti-phospholipid antibodies (2–5%) that can be found in up to 25% of population by age 75.23 Increased factor VIII, found in up to 10% of the well population, has a dose-related relation to VTE and is one of the more recently identified common risk factors.27 Factor V Leiden is the most common cause of inherited thrombophilia, found in up to 20% of VTE cases. In addition, local factors play a key role in thrombosis related to lower limb joint surgery and include vascular injury and thrombotic repair response, as well as venous stasis caused by prolonged immobility.
Independent VTE risk factors identified in studies of surgical populations for VTE included age older than 50 years, varicose veins, prior myocardial infarction, cancer, atrial fibrillation, ischemic stroke, diabetes mellitus, current use of estrogen-containing compounds, and chronic and acute inflammatory states including infections.28 Additional risk factors that increase the risk for surgery-related VTE include previous VTE, obesity, heart failure, and paralysis.29
VTE risk is increased, in a stepwise and fairly linear manner, with multiple risk factors. One surgical study noted a 10% to 15% increased risk for VTE with each additional risk factor identified.30 Multiple risk factors in orthopedic patients should be expected given the age, increasing numbers of patients undergoing joint replacement, and frequency of underlying VTE predispositions.
Medical and surgical patients have been risk stratified into low, intermediate, high, and highest VTE risk groups, to assist in creating robust clinical recommendation for best thromboprophylaxis practice.2,31 Patients undergoing elective joint replacement surgery fall into the highest risk category of this well-accepted clinical model. Patients at highest risk for VTE include surgical patients older than 40 years with multiple risk factors, those with THR or TKA, patients undergoing hip fracture surgery, or patients with major trauma and spinal cord injury. Risk for VTE in this aggregate group include calf DVT (40–80%), proximal DVT (10–20%), clinical PE (4–10%), and fatal PE (0.2–5%).
Prevention of Venous Thromboembolic Events: Introduction
The prophylactic measures most commonly used in surgery include low-dose unfractionated heparin (LDUFH), low-molecular-weight heparin (LMWH), the substituted pentasaccharide, fondaparinux, oral adjusted-dose vitamin K antagonists (VKAs) (international normalized ratio [INR], 2.0–3.0), and lower limb mechanical compression approaches. For joint replacement surgery, LDUFH is not an acceptable option in normal circumstances.2
A large and strong body of evidence-based studies, from randomized trials and meta-analysis, in thromboprophylaxis makes current prophylaxis recommendations robust for most common procedures including joint replacement surgery. A primary reference that is increasingly seen as the authoritative reference body that sets the North American standard of care in thromboprophylaxis is the periodic report on the “Prevention of Venous Thrombosis” for the American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy.2 It is strongly recommended that individual hospitals, as well as subspecialty and physician groups, should develop, adopt, and evolve formal strategies for the prevention of VTE disease for all risk categories for surgical and medical patients.
Drugs Used in Venous Thromboembolic Event Prevention
Low-Dose Unfractionated Heparin.
UFH is a heterogeneous glycosaminoglycan (5000–30,000 molecular weight [MW]) that binds to AT, altering AT so that AT then binds to and inactivates circulating factor Xa and factor IIa (thrombin). UFH then dissociates from these inactive, AT-serpin complexes that are then cleared. This anti–factor Xa and anti–factor IIa activity accounts for UFH anticoagulant activity. UFH inactivates free but not clot-bound factors IIa and Xa in a 1:1 ratio and, to a lesser extent, the other serine proteases.32
Clearance of UFH is dose dependent, with an approximate half-life of 30 to 60 minutes. This short half-life explains the need for two or three times daily dosing when prophylaxis is used. One major biophysical drawback with UFH, but not with LMWH or fondaparinux, is that UFH binds to and is made unavailable by a large number of endogenous, intravascular heparin-binding proteins (von Willebrand factor, fibronectin, platelet factor 4) that reduce its anticoagulant activity, because only free UFH is active.33 This may in part explain LMWH superiority to UFH in high-risk prothrombotic situations such as joint surgery. LDUFH has the advantage that it is relatively inexpensive compared with LMWH and fondaparinux, and is easily administered. Anticoagulant monitoring is not required for prophylaxis.
Limitations of UFH include the pharmacokinetic, biophysical properties and risk for heparin-induced thrombocytopenia (HIT).34 HIT is an immunologic disorder in which antibodies form against heparin-PF4 complex bound to the platelet and endothelial cell surface, and result in vascular cell (platelet and endothelial cell) activation, thrombocytopenia, and arterial and venous thrombosis. These pathologic antibodies form in 1% to 3% of patients receiving UFH, and although more common with full-dose UFH, the syndrome is well described with LDUFH. HIT is associated with significant morbidity (stroke, coronary events, peripheral arterial occlusions) in 10% to 15% of HIT cases. All patients receiving UFH must be followed with regular platelet counts while remaining on UFH, and any unexplained thrombocytopenia (decline in platelets of >50% from baseline or platelet count < 100,000/μL) during UFH use requires investigation and elucidation of its cause. If HIT is suspected, UFH must be stopped and replaced by an alternative anticoagulant not associated with HIT (hirudin, argatroban, or danaparoid) until the diagnosis is established.35 HIT antibodies cross-react with LMWH. Patients diagnosed with HIT should never be exposed to either UFH or LMWH.
Low-Molecular-Weight Heparins.
LMWH is a thromboprophylactic drug of choice for joint replacement surgery. LMWHs are fractionated, low-molecular-weight moieties (3000–5000 MW) of UFH, produced by chemical and physical methods, that are approximately one-third as long as native UFH.32 LMWHs when compared with UFH have a number of desirable pharmacologic and clinical properties that include: (1) superior and predictable pharmacokinetics that allow once or twice daily subcutaneous (SC) dosing based on body weight, (2) no laboratory monitoring, (3) more favorable benefit/risk ratio, and (4) less anti–factor IIa and more anti–factor Xa activity. Because of these properties and its demonstrated improved clinical efficacy in preventing VTE disease, LMWH has replaced UFH for many clinical indications including joint replacement surgery thromboprophylaxis.36
Like UFH, LMWH induces its anticoagulant effect through complex formation with AT and factor Xa, and to a lesser extent, thrombin. The anti–factor IIa/anti–factor Xa ratio is 1:1 for UFH, but 1:2 to 1:5 for different LMWHs. A number of pharmaceutical formulations are available in North America, including nadroparin, enoxaparin, dalteparin, and tinzaparin. They are prepared by different methods resulting in slightly different pharmacokinetic and anticoagulant properties, although clinical efficacy is similar for all indications other than acute coronary syndromes.37 Bleeding risk is similar or slightly lower with LMWH compared with UFH, and there is no true antidote for its anticoagulant action, unlike UFH.
Biophysical properties of LMWH are its major advantage. Because LMWH does not bind to intravascular proteins or cells, they have predictable pharmacokinetics including dose–response relations and an increased plasma half-life. Peak anticoagulant effect is seen at 4 to 8 hours after SC injection. Half-life of this renally cleared agent is much longer than UFH at 3 to 6 hours, and any measurable anticoagulant effect of LMWH is gone in 24 hours in the absence of kidney dysfunction. Depending on the formulation and supporting clinical evidence, LMWHs are administered either once or twice a day for thromboprophylaxis.37
HIT is much less common with LMWH use than UFH, although the precise incidence is not clear. As an example, one randomized, double-blind study of patients after hip surgery found that thrombocytopenia occurred in 9 of 332 patients (2%) receiving UFH compared with none receiving LMWH.38 Because of LMWH cross-reactivity with HIT antibodies, patients with a history of HIT should never be exposed to either LMWH.
Pentasaccharide.
Fondaparinux (Arixtra), a synthetic heparin analog, is a highly sulfated, pentasaccharide sequence, derived from the AT binding region of UFH. It is the newest, currently available anticoagulant for joint surgery thromboprophylaxis and has demonstrated improved efficacy compared with LMWH.39,40 The anticoagulant action of fondaparinux is via a mechanism similar to LMWH in which the drug binds to AT and causes AT to bind to and inactivate factor Xa, but not thrombin. Its action is directed against factor Xa only. Fondaparinux, like LMWH, does not bind to or interact with other plasma proteins or cells resulting in predictable and stable pharmacokinetics. Its half-life at 17 hours is much longer than UFH (0.5–1 hour) or LMWH (4–6 hours), and prophylactic doses (2.5 mg SC) are given daily. Although clinical HIT has not been described with fondaparinux, HIT antibodies do develop with it use.41 Bleeding with prophylactic fondaparinux is slightly more common than with LMWH or VKA, and like LMWH, this agent is not reversible.15,42
Oral Anticoagulants.
VKA has been available in clinical practice since the 1950s and is commonly used for VTE prophylaxis in lower limb joint replacement surgery. Certain anticoagulant properties including long half-life, delayed onset of action, and reversibility have made it a favored drug for joint replacement thromboprophylaxis in North America.43 The VKA warfarin induces an anticoagulant state by interfering with the vitamin K–dependent, post-translational modification of hepatically synthesized blood clotting factors II, VII, IX, and X. This results in nearnormal circulating levels of hypofunctional clotting factors II, VII, IX, and X.
These factors have markedly different half-lives, from 6 to 60 hours. It is the half-lives of these factors that dictate the anticoagulant effect of VKA. The anticoagulant efficacy of VKA most closely correlates with the functional level of factor II (prothrombin) that has the longest half-life (60 hours).44
VKA is rapidly absorbed from the gut and is 90% albumin bound in circulation. Only free, unbound VKA is biologically active. The half-life of VKA is 36 to 48 hours, and it is the hepatic metabolism by microsomal enzymes, including cytochrome P450 variants, that explains, in part, the marked interindividual variation in dosage required for drug effect. Numerous factors affect the biologic activity of VKA, including patient age, activity level, diet, drug absorption, albumin level, vitamin K intake, general state of health, and interfering drugs. The list of drugs known to interact by either potentiating or attenuating the VKA effect is lengthy, and knowledge of their identity and expected interactions is important given its narrow therapeutic index.45 Diet can also impact warfarin effect because ingestion of foodstuffs high in vitamin K will counteract the effects of warfarin within 6 to 12 hours.46
The average oral dosage of VKA is 5 mg/day, and for patients older than 70 years is 4 mg/day. The use of VKA dosing nomograms should be adopted and have been convincingly demonstrated to accelerate the attainment of a target INR whereas simplifying management for healthcare providers.47 The optimal INR target for treatment and prophylaxis of VTE indications is 2.5 (2.0–3.0). For any given indication, there is a trend to lower bleeding risk with no loss in clinical efficacy the lower the INR is kept within the therapeutic range. Target INR should be 2.0 to 3.0 as per published trials as opposed to lower targets. There is no rationale to use a loading dose for the initiation of VKA therapy.43 Given the long half-lives of both VKA (36 hours) and factor II (60 hours), this means that to attain full anticoagulant effect, the INR must be within the therapeutic range for at least 4 to 6 days for all of the vitamin K–dependent proteins to decrease to 20% to 30% of normal.
VKA is generally well tolerated, with few side effects other than the bleeding risk. Gastrointestinal (GI) upset and hair thinning may be seen in 10% to 15% of cases. An outpatient bleeding risk index has been developed that includes independent risk factors: age older than 65, history of GI bleeding, history of stroke.48 Low-, intermediate-, and high-risk patients had, respectively, 3%, 12%, and 53% probability of bleeding. The bleeding risk is directly correlated with INR; it begins to increase quite markedly when the INR increases to more than 3.5, and an INR greater than 5 requires active intervention (hold drug, oral or SC vitamin K1, fresh-frozen plasma) to reverse the drug effect.
Aspirin.
Although aspirin decreases the frequency of VTE after orthopedic surgery when analyzed in meta-analysis, this efficacy is significantly less than that obtained using other anticoagulant agents.49 A large randomized trial involving 4088 patients undergoing THR and TKR received placebo or 160 mg/day aspirin for 35 days, in addition to other thromboprophylactic measures prescribed at the discretion of the treating physician.50 Fatal PE and DVT were both significantly decreased by aspirin: relative risk reduction (RRR) of 40% and absolute risk reduction of 4 fatal PEs per 1000 patients. Wound-related bleeding, GI bleeding, and the need for transfusion were significantly more common in the aspirin-treated group. Thus, although aspirin may have some activity in preventing VTE, its efficacy and safety profile are markedly inferior to other available measures, precluding its use as monotherapy.51 Aspirin cannot currently be recommended for the prophylaxis of VTE.2
New Anticoagulant Agents.
Further improvements in thromboprophylaxis are going to come from the evolution of better pharmacologic agents with not only an increased efficacy in VTE prevention but a lower bleeding risk and improved biologic properties including reversibility and longer half-lives. The quest for this holy grail in anticoagulant therapy among pharmaceutical companies is ongoing. Examples of agents that have demonstrable efficacy with similar benefit/risk profiles when compared with LMWH and have been studied in THR and TKA include the direct thrombin inhibitors parenteral recombinant hirudin, parenteral melagatran, and oral ximelagatran and the new oral direct anti Xa inhibitor rivaroxaban.2,52, 53, 145, 146 Emerging drugs include idraparinux, a long-acting pentasaccharide congener to fondaparinux, currently in trials, that can be given once a week and the biotinylation of pentasaccharides so that their anticoagulant action can be reversed with an infusion of avidin.39 None of these agents has been approved for thromboprophylaxis use in North America.
Mechanical Approaches to Venous Thromboembolic Event Prevention
Compared with drug interventions for thromboprophylaxis, mechanical devices have been studied in fewer patients. Thus, the confidence around the recommendation for their use is weaker. A major practical issue related to using this approach is patient and health provider compliance.54 Intermittent pneumatic leg compression (IPC), graduated compression stockings (GCS), and venous foot pump (VFP) mechanically prevent venous thrombosis by augmenting blood flow and theoretically decreasing venous stasis in the deep veins of the legs. Pneumatic compression has an added biochemical anticoagulant effect by reducing plasminogen activator inhibitor-1 levels, resulting in increased endogenous fibrinolytic activity.55 Thus, mechanical leg compression approaches have both local and systemic effects.
Although IPC56–59 has been demonstrated to reduce the incidence of DVT in moderate-risk surgical patients,60 it is less effective in patients undergoing hip surgery or knee replacement, and although it reduces RRR of total DVTs and prevents calf vein thrombosis, it is not clinically effective for more important proximal DVTs.56,59 IPC use has decreased with decreasing length of hospital stay and rapid ambulation in patients undergoing orthopedic surgery.61 In a review, the duration, degree of compression, and overall compliance were significantly less than expected, even with a concerted education program, in patients undergoing THR when IPC was the only form of prophylaxis.62
GCS16,63–65 reduce venous stasis in the limb by applying decreasing degrees of compression moving proximally from the ankle to calf, but reduce the incidence of postoperative VTE only in low-risk surgical patients.66 In a meta-analysis, GCS in combination with other forms of prophylaxis does not result in any further risk reduction in VTE.67
Evidence of efficacy with VFP65,68 is less developed than the other mechanical methods, and although it does appear to reduce overall rates of DVT, it is inferior to current anticoagulant approaches.9,69 Mechanical devices are not recommended as a sole therapy for thromboprophylaxis in joint replacement surgery, and good comparative studies with these devices as part of multimodal approaches are lacking, making it difficult to make any firm recommendations despite their attractiveness because of their biomechanical action.
TOTAL HIP REPLACEMENT
Thromboprophylaxis has been recommended in all patients undergoing THR for at least 20 years. This recommendation is supported by many randomized, clinical trials between active interventions with and without placebo groups, and guidelines have been well refined. Three pharmacologic approaches have been demonstrated to be effective and safe. LMWH (SC once or twice daily), fondaparinux (SC once daily), or adjusted-dose VKA (target INR, 2.5).2,15,70–76
Mechanical prophylaxes GCS,16,63–65 IPC,56–59 and VFP65,68 have all been studied in THR. Each of these methods provides some level of protection compared with placebo with a RRR of DVT of 20% to 70%. However, this RRR is inferior compared with current pharmacologic interventions in preventing VTE, especially proximal DVTs.2,59, 63 Mechanical, lower limb methods are not recommended as a primary prophylactic modality.2
In THR, both aspirin51 and fixed-dose LDUH77 are superior to placebo in meta-analyses for the prevention of VTE. However, neither of these modalities is recommended as monotherapy because of inferior efficacy when compared with LMWH or VKA.3,53,78–80
In North America, as opposed to the European Union, thromboprophylaxis utilizing adjusted-dose VKA (target INR, 2.5) continues to the most common pharmacologic intervention used in THR.81–86 In the European Union, LMWH has largely replaced VKA because of the lower comparative efficacy of VKA. Advantages of VKA include its later onset of action, physicians’ comfort and familiarity with the drug and its actions, the ability to reverse anticoagulant effect, lower cost compared with fondaparinux and LMWH, and more acceptable bleeding risk. Disadvantages of VKA include the need for frequent blood testing, necessity of complex ongoing infrastructure for monitoring and dose adjustment, wide patient intervariability in drug dosage, variable patient response, narrow therapeutic index, and significant drug and food interactions. There is a delay in full anticoagulant action with VKA,87 for at least 3 to 5 days, that may explain VKA bleeding risk advantage compared with fondaparinux and LMWH.2
Different LMWHs have been studied extensively and demonstrated to be safe and highly efficacious in the thromboprophylaxis of THR.70–73,75,76,88–92 A meta-analysis of 13 studies comparing LMWH with placebo demonstrated that LMWH significantly reduced the risk for asymptomatic DVT (RR, 0.51) without significantly increasing the risk for major bleeding, and indicated that the different LMWH regimens studied and currently recommended are similarly effective and safe.93 Pooled results of a number of large clinical trials75,76, 91, 94 of adjusted-dose VKA versus LMWH in THR reveal that LMWH is more effective, but at the cost of increased bleeding risk. Overall pooled DVT rates were 20.7% versus 13.7% (P = 0.0002), and proximal DVT was 4.8% versus 3.4% (P = 0.08) in the VKA and LMWH groups, respectively. In a trial of greater than 3000 patients with THR treated with LMWH twice daily versus VKA, there was a threefold reduction in in-hospital, symptomatic VTE (0.3% vs. 1.1%), and major bleeding was 1.2% versus 0.5%, respectively, whereas the 3-month overall VTE rates, between treatments, were no different.95 In another major randomized clinical trial, LMWH, started either 2 hours before or 4 hours after surgery, versus adjusted-dose VKA demonstrated a significant reduction in total and proximal DVT, and significant reduction in symptomatic DVT (2.2% vs. 4.4%).76 A meta-analyses of up to 10,000 patients undergoing lower limb orthopedic surgery compared the benefit/risk ratio of VKA versus LMWHs and concluded that VKAs were less effective than LMWH in preventing total and proximal DVTs, and that differences between VKA and LMWH in major hemorrhage and wound hematoma were not significant.96 Thus, LMWHs are more efficacious than VKA in prevention of VTE, but this efficacy comes at a cost of a slightly increased bleeding risk.
The pentasaccharide fondaparinux is the most recently available anticoagulant with indications for thromboprophylaxis with apparent improved efficacy in THR. In the initial European Union study comparing fondaparinux and enoxaparin, in which fondaparinux (2.5 mg SC daily) was started 4 to 8 hours after surgery and LMWH 12 hours before surgery, demonstrated a 50% decrease in overall VTE rates (4% vs. 9%) in favor of fondaparinux.42 In the North American study with LMWH starting 12 to 24 hours after surgery, there was no statistical difference between overall and proximal VTE rates (6% vs. 8% and 2% vs. 1%, respectively).92 There was a nonsignificant trend to more bleeding with fondaparinux versus all LMWH.42,92, 97, 98 Studies directly comparing fondaparinux and VKA have not been performed. A meta-analysis of four multicenter, randomized, double-blind trials for the prevention of VTE after major orthopedic surgery, in which fondaparinux (2.5 mg/day) begun 4 to 8 hours after surgery was compared with enoxaparin, demonstrated a significantly reduced risk for all VTEs by day 11 (6.8% vs. 13.7%; P < 0.001) but at an increased bleeding risk.15
Comparison of bleeding risks across trials of thromboprophylaxis drugs in THR is difficult, because the agents, dosing schedules, dosage, and definition of major and minor bleeding often vary from one trial to another. General conclusion can be inferred that the bleeding risk increases from VKA to LMWH and fondaparinux. First, surgery-related bleeding risk must be put in context by the observation that in the placebo groups of pooled, randomized, clinical trials of THR, the aggregate major bleeding rate was 2% to 4%.99,100 One meta-analysis concluded that there was no significant difference in bleeding risk between VKA and LMWH.96 In four major trials pooled, significant bleeding in VKA and LMWH groups was 3.3% versus 5.3%, respectively,75,76, 91, 94 whereas the rates of major bleeding ranged from 1.8% to 4.1% for fondaparinux and 1.0% to 3.5% for LMWH. A nonsignificant trend to more bleeding with fondaparinux versus all LMWHs was reported.42,92, 97, 98
TOTAL KNEE REPLACEMENT
TKA differs from THR in a number of important ways. The total VTE rate is greater in TKA, whereas more important proximal and symptomatic DVTs are less common. The efficacy of LMWH versus VKA is more pronounced in TKA, and major bleeding is less common but clinically more worrisome. After elective TKR, LMWH, fondaparinux, or adjusted VKA (target INR, 2.5) can be used.2,75, 89, 91, 97, 101 Finally, although the number of patients undergoing TKR now equals the number undergoing THR in North America, there have been fewer trials in patients undergoing knee replacement.
Like THR studies of IPC102,103 and VFP,104 but not GCS,66 mechanical devices show some efficacy. However, issues of compliance and inability to maintain usage limit this efficacy. The relatively smaller number of patients enrolled in mechanical device, randomized, clinical trials compared with pharmacologic studies results in weak confidence around recommendations related to their use. Although IPC may have some adjunctive role in in-hospital prophylaxis, its combined use has not been studied in randomized, controlled studies. Both LDUH99,105 and aspirin7,78, 103 have been studied in TKA and have been shown to be much less efficacious than LMWH, fondaparinux, or adjusted-dose VKA; they are not recommended.
Numerous randomized trials of VKA in TKA have been reported, and confidence around VKA recommendations is high.7,75,91,101,102,106–109 Despite adjusted-dose VKA, thromboprophylaxis total, venographic, DVT rates remain high (25–50%), with treatment out to 2 weeks. However, unlike THR, symptomatic VTE rates are low (1–2%) when assessed at up to 3 months after surgery.86,110 A 2004 meta-analysis of VKA trials in orthopedic surgery demonstrated that VKAs were less effective than LMWH in preventing total and proximal DVTs (9822 patients; RR, 1.51; P < 0.001; and 6131 patients; RR, 1.51; P = 0.028, respectively), whereas the differences between VKA and LMWH in major hemorrhage and wound hematoma were not significant. The authors conclude that VKAs are less effective than LMWH, without any significant difference in the bleeding risk in lower limb orthopedic procedures including TKR and THR.96 When compared with LMWH in patients with TKA, VKA is less efficacious with a similar or slightly lower bleeding risk.
LMWH in TKA is well-studied, and is a safe and effective prophylactic measure with clear superior efficacy compared with VKA. Pooled clinical trials directly comparing VKA and LMWH demonstrate overall DVT rates of 48% versus 33% and proximal DVT rates of 10.4% versus 7.1%, respectively.75,91, 101, 107, 111, 112 A 2001 meta-analysis of thromboprophylaxis studies in TKA evaluated 14 studies (3482 patients) and reported that for proximal DVT rates, LMWH was significantly better than warfarin (P = 0.0002), whereas there was no significant difference for symptomatic PE, fatal PE, major hemorrhage, or total mortality.113 Another meta-analysis confirmed the superior efficacy of LMWH over VKA without increased bleeding.114
Large-scale clinical trials have shown that fondaparinux further reduces the likelihood of VTE complications after major orthopedic surgery.115 Fondaparinux was shown to be superior to LMWH in reduction of total VTE events after TKA in a large, randomized trial of more than 1000 patients.97,115 Fondaparinux (2.5 mg SC daily) started 6 hours after surgery versus LMWH started 12 to 24 hours after surgery resulted in a halving of total VTE (12.5% vs. 27.8%; P < 0.001) and proximal DVT rates (2.4% vs. 5.4%; P = 0.06) respectively. This improved efficacy came at a cost of increased major bleeding (2.1% vs. 0.2%; P = 0.006). In a systematic review of the four thromboprophylaxis studies in major orthopedic surgery, fondaparinux was 50% more effective in reducing VTE than LMWH (enoxaparin), with an overall 1% increased rate of major bleeding, whereas the incidence of fatal bleeding, critical organ bleeding, or bleeding leading to reoperation did not differ between the two treatment groups.39,115
VTE after TKR results in a significant cost burden because of rehospitalization, prolonged stay, morbidity, mortality, and long-term postphlebitic syndrome. Both LMWH and fondaparinux are significantly more costly on a daily basis than VKA for thromboprophylaxis. When cost-effectiveness, comparing VKA and LMWH, has been studied, conflicting conclusions using slightly different methodologies have been drawn, suggesting that there may be little difference in costeffectiveness of VKA versus LMWH.116–118 A review of the pharmacoeconomic evaluations of fondaparinux leads to the conclusion that fondaparinux is a costeffective alternative to LMWHs in VTE prophylaxis.119
OTHER ISSUES
Timing of Prophylaxis
VKA prophylaxis can be commenced at any convenient time perioperatively, usually the night before or 12 to 24 hours after surgery. The onset of anticoagulant effect is delayed until the third or fourth postoperative day.87
There is likely little difference in efficacy in preoperative or postoperative commencement of LMWH.76,88 LMWH at a first injection, 50% dose reduction, started immediately before or early after (<7 hours) surgery in patients undergoing THR, resulted in similar VTE rates of both total and proximal DVT but significantly lower VTE rates when compared with warfarin.120 No difference was found between the preoperative and postoperative LMWH arms with respect to efficacy, but there was more major bleeding in the preoperative LMWH group when compared with warfarin and the postoperative arm. In a meta-analysis comparing preoperative with postoperative initiation of LMWH for prophylaxis of DVT after THR, it was shown that total DVT rates (but not proximal DVT rates) and major bleeding occurred significantly less frequently in the preoperative group compared with those who received postoperative prophylaxis.121 The timing of the first dose of LMWH after THR was further assessed in a meta-analysis of four trials of LMWH given at half the regular dose within 6 to 8 hours compared with full dose at 12 to 24 hours, VKA was associated with a large risk reduction, and major bleeding occurred significantly less frequently in the preoperative group compared with those who received postoperative prophylaxis.121 This study concluded that the timing of initiating LMWH significantly impacts efficacy, and delaying initiation of LMWH prophylaxis results in loss of efficacy without an enhanced safety advantage. The closer to surgery prophylaxis is initiated the better the efficacy, but this is offset by an increased risk for major bleeding.122,123
Findings are similar with fondaparinux with respect to perioperative timing of prophylactic dose.15,39, 122, 124 In a benefit/risk analysis, the incidence of major bleeding was significantly less (2.1% vs. 3.2%) in those patients receiving fondaparinux 6 versus less than 6 hours after surgery, whereas there was no significant difference in efficacy (7.0% vs. 6%, respectively).124 This finding resulted in a regulatory agency recommending fondaparinux initiation at 6 to 8 hours after surgery rather than the timing used in the trials (4–8 hours).
Duration of Prophylaxis
For a patient undergoing THR or TKR, the minimum duration of pharmaco-thromboprophylaxis for all patients should be 7 to 10 days.2 Extending prophylaxis with VKA, LMWH, or fondaparinux beyond this time appears to have greatest efficacy in THR as opposed to TKR. VKA use extended for 4 weeks in THR compared with in-hospital use was associated with a 5- to 10-fold decrease in VTE rates as assessed by DUS at little increased bleeding risk.125 Multiple reports and two meta-analyses clearly support continued prophylaxis with LMWH for 28 to 42 days after THR.70–72,74,126,127 The meta-analyses, when compared with placebo, showed a significant decrease in total DVT and PE without a significant increased risk for major bleeding but increased minor bleeding.121,126, 127 There is less of a need for extended prophylaxis beyond 10 days for patients undergoing TKR because prolonging therapy for an additional 3 to 6 weeks in TKR does not appear to provide significant further benefit.74,75, 126
The benefit of thromboprophylaxis in THR with fondaparinux (2.5 mg/day) for 1 month, rather than 6 to 8 days, was studied in a double-blind, multicenter trial and summarized in two other reviews of all fondaparinux-related joint surgery randomized studies.39,115, 128 Compared with 1 week of fondaparinux followed by 3 weeks of placebo in the PENTHIFRA-PLUS trial, extended fondaparinux resulted in a significant reduction in total VTE from 35.0% to 1.4%, as well as an 87% reduction in symptomatic VTE. However, a nonsignificant trend toward more major bleeding in the 1-month group was found.
SCREENING
It has been clearly demonstrated with numerous studies that postoperative screening for VTE using noninvasive methods is an unacceptable and costly alternative to primary prophylaxis.129 Noninvasive screening methods, such as the DUS, have been demonstrated as having low sensitivity and specificity, after THR, for both distal and proximal DVT.130–132 This has been shown in studies of both TKR and THR.110,133, 134 A meta-analysis demonstrated that venous ultrasound imaging has moderate sensitivity and moderate positive predictive value when used to screen for DVT after orthopedic surgery; thus, ultrasound imaging is not a reliable approach for DVT screening.135 Consequently, DVT screening as secondary prophylaxis is not recommended for patients with lower limb joint replacement.2
NEURAXIAL ANESTHESIA
Lower limb arthroplasty surgery that involves spinal or epidural invasive procedures for regional anesthesia or continued analgesia in conjunction with prophylactic anticoagulants must be used with extreme caution, and current and evolving recommendations of the American Association for Regional Anesthesia should be consulted for evolving recommendations. Spinal hematoma, although a serious concern, is rare.136 Bleeding risk concerns should not outweigh the anticipated benefits of regional anesthesia in joint arthroplasty or its efficacy in thromboprophylaxis, which are much more common complications of lower limb arthroplasty.2,137, 138 A recent meta-analysis of 10 trials showed that compared with general anesthesia (GA), neuraxial block reduced many serious complications in all patients. Pooled results from five trials showed that neuraxial block significantly decreased the incidence of all radiologic DVT or PE in THR. The odds ratio for DVT and PE was 0.27 (confidence interval [CI], 0.17–0.42) and 0.26 (CI, 0.12–0.56), respectively, and the authors concluded that patients undergoing THR with neuraxial anesthesia have better outcomes than those under GA and less frequent VTE events.139 Second, as the 2003 ASRA Consensus Conference statement makes clear, regional anesthesia may be used safely with LMWH prophylaxis136 as long as key factors including timing issues in relation to the regional anesthetic procedure are considered.
COST-EFFECTIVENESS
VTE represents a huge health economic burden of nearly $500 million per year in the United States, and patients undergoing major lower limb surgery are at high risk for development of VTE. Prophylaxis with warfarin substantially reduces the risk for VTE after lower limb arthroplasty and is more cost-effective than no prophylaxis.119 Although LMWHs are more expensive than VKA, they are superior to warfarin in VTE prevention and are cost-effective.140,141 A review of the pharmacoeconomic evaluations of fondaparinux leads to the conclusion that fondaparinux is a cost-effective alternative to LMWHs in lower limb joint surgery.119 Fondaparinux, LMWH, and VKA in extended prophylaxis for 28 days all appear to be cost-effective.142–144
In making overall recommendations for thromboprophylaxis, an increased weight/value is placed on the risks (bleeding) of thromboprophylaxis given the relative low incidence of symptomatic VTE, proximal DVT, and PE with current pharmacologic interventions. VKA, fondaparinux, and LMWH are all efficacious in the prevention of VTE in lower limb joint replacement surgery. This improved efficacy, moving progressively from VKA to LMWH to fondaparinux, is offset by a similar trend toward increasing bleeding rates. This risk/benefit ratio for each intervention needs to be weighed on an individual patient basis. Patients who, on clinical analysis, are at greater risk for bleeding or clotting should be considered for prophylaxis with an approach that carries a lower risk for these respective deleterious complications.2
Specific decisions of thromboprophylaxis approach for THR and TKA should be made at a hospital level, ideally both through a professional consensus approach and at an individual patient level. Decision should be based on issues of local cost, infrastructure support for monitoring of VKA if being considered, patient compliance, and duration of prophylaxis. Table 80-2 provides a summary of recommendations.
| RECOMMENDATIONS | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|
| Elective THR | |
| The routine use of one of the following is recommended for all patients undergoing elective THR: (1) LMWH, at standard high-risk prophylactic dosage, started either 12 hours before or 12 to 24 hours after surgery, or a 50% dose reduction at 4 to 6 hours after surgery escalating to full-dose prophylaxis at 24 hours and daily thereafter; (2) VKA, dose adjusted with INR-warfarin nomogram to a target INR of 2.5, started before surgery or the evening after surgery; or (3) fondaparinux (2.5 mg) started 6 to 8 hours after surgery and daily thereafter. | A |
| The use of aspirin, LDUFH, GCS, IPC, or VFP as monotherapy for thromboprophylaxis is not recommended. | A |
| Elective TKR | |
| The routine use of one of the following is recommended for all patients undergoing elective TKR: (1) LMWH, at standard high-risk prophylactic dosage, started either 12 hours before or 12 to 24 hours after surgery, or a 50% dose reduction at 4 to 6 hours after surgery escalating to full-dose prophylaxis at 24 hours and daily thereafter; (2) VKA, dose adjusted with INR-warfarin nomogram to a target INR of 2.5, started before surgery or the evening after surgery; or (3) fondaparinux (2.5 mg) started 6 to 8 hours after surgery and daily thereafter. | A |
| The use of aspirin, LDUFH, GCS, IPC, or VFP as monotherapy for thromboprophylaxis is not recommended. | A |
| Timing | |
| The efficacy-to-bleeding risk needs to be individualized for all patients at increased risk to bleed or clot based on clinical assessment and on the bases of each agent. Preoperative or postoperative initiation off all three agents (fondaparinux, LMWH, or VKA) is acceptable. | A |
| Screening | |
| The routine use of postoperative VTE screening in asymptomatic patients is not recommended. | A |
| Length of Prophylaxis | |
| In elective THR and TKA, thromboprophylaxis with LMWH, VKA, or fondaparinux should be continued for a minimum of 7 to 10 days for patients. Patients undergoing THR and patients at high risk for thrombosis undergoing TKA should be given extended prophylaxis for up to 28 to 42 days after surgery. | A |
| Neuraxial Anesthesia/Analgesia | |
| Special caution should be used with concurrent use of anticoagulant prophylaxis. | B |
DVT, venous thromboembolic event; GCS, graduated compression stockings; INR, international normalized ratio; IPC, intermittent pneumatic leg compression; LDUFH, low-dose unfractionated heparin; LMWH, low-molecular-weight heparin; PE, pulmonary embolism; THR, total hip replacement; TKA, total knee arthros-copy; TKR, total knee replacement; VFP, venous foot pump; VKA, vitamin K antagonist.
1 NIH Consensus Conference. Prevention of venous thrombosis and pulmonary embolism. JAMA. 1986;256:744-749.
2 Geerts W, Pineo GF, Heit JA, et al. Prevention of venous thrombosis: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S-400S.
3 Freedman KB, Brookenthal KR, Fitzgerald RHJr, Williams S, Lonner JH. A meta-analysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am. 2000;82-A:929-938.
4 Beisaw NE, Groth HE, Merli GJ, Weitz HH, et al. Dihydroergotamine/heparin in the prevention of deep-vein thrombosis after total hip replacement. A controlled, prospective, randomized multicenter trial. J Bone Joint Surg Am. 1988;70:2-10.
5 Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res.; 242; 1989; 212-231.
6 Mohr DN, Silverstein MD, Ilstrup DM, Heit JA, Morrey BF. Venous thromboembolism associated with hip and knee arthroplasty: Current prophylactic practices and outcomes. Mayo Clin Proc. 1992;67:861-870.
7 Lotke PA, Palevsky H, Keenan AM, et al. Aspirin and warfarin for thromboembolic disease after total joint arthroplasty. Clin Orthop Relat Res.; 324; 1996; 251-258.
8 Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66:194-201.
9 Warwick D, Harrison J, Whitehouse S, Mitchelmore A, Thornton M. A randomised comparison of a foot pump and low-molecular-weight heparin in the prevention of deep-vein thrombosis after total knee replacement. J Bone Joint Surg Br. 2002;84:344-350.
10 Ginsberg JS, Turkstra F, Buller HR, MacKinnon B, Magier D, et al. Postthrombotic syndrome after hip or knee arthroplasty: A cross-sectional study. Arch Intern Med. 2000;160:669-672.
11 Kim YH, Oh SH, Kim JS. Incidence and natural history of deep-vein thrombosis after total hip arthroplasty. A prospective and randomised clinical study. J Bone Joint Surg Br. 2003;85:661-665.
12 White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158:1525-1531.
13 Eriksson BI, Kälebo P, Anthymyr BA, Wadenvik H, Tengborn L. Prevention of deep-vein thrombosis and pulmonary embolism after total hip replacement: Comparison of low-molecular-weight heparin and unfractionated heparin. J Bone Joint Surg Am. 1991;73:484-493.
14 Khaw FM, Moran CG, Pinder IM, Smith SR. The incidence of fatal pulmonary embolism after knee replacement with no prophylactic anticoagulation. J Bone Joint Surg Br. 1993;75:940-941.
15 Turpie AGG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: A meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833-1840.
16 Warwick D, Bannister GC, Glew D, Mitchelmore A, Thornton M, et al. Perioperative low-molecular-weight heparin. Is it effective and safe. J Bone Joint Surg Br. 1995;77:715-719.
17 Fender D, Harper WM, Thompson JR, Gregg PJ. Mortality and fatal pulmonary embolism after primary total hip replacement. Results from a regional hip register. J Bone Joint Surg Br. 1997;79:896-899.
18 Paiement G. Prevention and treatment of venous thromboembolic disease complications in primary hip arthroplasty patients. Instr Course Lect. 1998;47:331-335.
19 Montgomery KD, Geerts WH, Potter HG, Helfet DL. Practical management of venous thromboembolism following pelvic fractures. Orthop Clin North Am. 1997;28:397.
20 Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: A systematic review. Thromb Haemost. 2003;90:17-26.
21 Moores L, Bilello KL, Murin S. Sex and gender issues and venous thromboembolism. Clin Chest Med. 2004;25:281-297.
22 White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93:298-305.
23 Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost. 1997;77:444-451.
24 Crowther MA, Kelton JG. Congenital thrombophilic states associated with venous thrombosis: A qualitative overview and proposed classification system. Ann Intern Med. 2003;138:128-134.
25 Kearon C, Crowther M, Hirsh J. Management of patients with hereditary hypercoagulable disorders. Annu Rev Med. 2000;51:169-185.
26 Emmerich J, Rosendaal FR, Cattaneo M, Margaglione M, De Stefano V, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism—pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86:809-816.
27 Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000;343:457-462.
28 Zaw HM, Osborne IC, Pettit PN, Cohen AT. Risk factors for venous thromboembolism in orthopedic surgery. Isr Med Assoc J. 2002;4:1040-1042.
29 Vaughan P, Gardner J, Peters F, Wilmott R. Risk factors for venous thromboembolism in general surgical patients. Isr Med Assoc J. 2002;4:1037-1039.
30 Spencer FA, Emery C, Lessard D, Anderson F, Emani S, et al. The Worcester Venous Thromboembolism study: A population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722-727.
31 Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38(2 suppl 5):12-19.
32 Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):188S-203S.
33 Turpie AG. Pharmacology of the low-molecular-weight heparins. Am Heart J. 1998;135(6 pt 3 suppl):S329-S335.
34 Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):311S-337S.
35 Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: Review and update. Thromb Res. 2006;118:165-176.
36 Prandoni P. Heparins and venous thromboembolism: Current practice and future directions. Thromb Haemost. 2001;86:488-498.
37 White RH, Ginsberg JS. Low-molecular-weight heparins: Are they all the same? Br J Haematol. 2003;121:12-20.
38 Warkentin TE, Roberts RS, Hirsh J, Kelton JG. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Arch Intern Med. 2003;163:2518-2524.
39 Nijkeuter M, Huisman MV. Pentasaccharides in the prophylaxis and treatment of venous thromboembolism: A systematic review. Curr Opin Pulm Med. 2004;10:338-344.
40 Turpie AG, Gallus AS, Hoek JA, Pentasaccharide Investigators. A synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med. 2001;344:619-625.
41 Warkentin TE, Cook RJ, Marder VJ, Sheppard JA, Moore JC, et al. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106:3791-3796.
42 Lassen MR, Bauer KA, Eriksson BI, Turpie AG, European Pentasaccharide Elective Surgery Study (EPHESUS) Steering Committee. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: A randomised double-blind comparison. Lancet. 2002;359:1715-1720.
43 Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):204S-233S.
44 Freedman MD. Oral anticoagulants: Pharmacodynamics, clinical indications and adverse effects. J Clin Pharmacol. 1992;32:196-209.
45 Juurlink D. Drug interactions with warfarin: What clinicians need to know. CMAJ. 2007;177:369-371.
46 Schurgers LJ, Shearer MJ, Hamulyák K, Stöcklin E, Vermeer C. Effect of vitamin K intake on the stability of oral anticoagulant treatment: Dose-response relationships in healthy subjects. Blood. 2004;104:2682-2689.
47 Ebell MH. Evidence-based initiation of warfarin (Coumadin). Am Fam Physician. 2005;71:763-765.
48 Aspinall SL, DeSanzo BE, Trilli LE, Good CB. Bleeding Risk Index in an anticoagulation clinic. Assessment by indication and implications for care. J Gen Intern Med. 2005;20:1008-1013.
49 Collaborative overview of randomised trials of antiplatelet therapy—I. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81-106.
50 Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin. Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295-1302.
51 Hovens MM, Snoep JD, Tamsma JT, Huisman MV. Aspirin in the prevention and treatment of venous thromboembolism. J Thromb Haemost. 2006;4:1470-1475.
52 Eriksson BI, Kälebo P, Ekman S, Lindbratt S, Kerry R, et al. Direct thrombin inhibitor melagatran followed by oral ximelagatran in comparison with enoxaparin for prevention of venous thromboembolism after total hip or knee replacement. Thromb Haemost. 2003;89:288-296.
53 Eriksson BI, Ekman S, Kalebo P, Zachrisson B, Bach D, et al. Prevention of deep-vein thrombosis after total hip replacement: Direct thrombin inhibition with recombinant hirudin, CGP 39393. Lancet. 1996;347:635-639.
54 Westrich GH, Specht LM, Sharrock NE, Sculco TP, Salvati EA, et al. Pneumatic compression hemodynamics in total hip arthroplasty. Clin Orthop Relat Res.; 372; 2000; 180-191.
55 Comerota AJ, Chouhan V, Harada RN, Sun L, Hosking J, Veermansunemi R, et al. The fibrinolytic effects of intermittent pneumatic compression: Mechanism of enhanced fibrinolysis. Ann Surg. 1997;226:306-314.
56 Hull RD, Raskob GE, Gent M, McLoughlin D, Julian D, et al. Effectiveness of intermittent pneumatic leg compression for preventing deep vein thrombosis after total hip replacement. Jama. 1990;263:2313-2317.
57 Paiement G, Wessinger SJ, Waltman AC, Harris WH. Low-dose warfarin versus external pneumatic compression for prophylaxis against venous thromboembolism following total hip replacement. J Arthroplasty. 1987;2:23-26.
58 Norgren L, et al. Low incidence of deep vein thrombosis after total hip replacement: An interim analysis of patients on low molecular weight heparin vs sequential gradient compression prophylaxis. Int Angiol. 1996;15(3 suppl 1):11-14.
59 Francis CW, Pellegrini VDJr, Marder VJ, Totterman S, Harris CM, et al. Comparison of warfarin and external pneumatic compression in prevention of venous thrombosis after total hip replacement. Jama. 1992;267:2911-2915.
60 Ramos R, Salem BI, De Pawlikowski MP, Coordes C, Eisenberg S, et al. The efficacy of pneumatic compression stockings in the prevention of pulmonary embolism after cardiac surgery. Chest. 1996;109:82-85.
61 White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343:1758-1764.
62 Haddad FS, Kerry RM, McEwen JA, Appleton L, Garbuz DS, et al. Unanticipated variations between expected and delivered pneumatic compression therapy after elective hip surgery: A possible source of variation in reported patient outcomes. J Arthroplasty. 2001;16:37-46.
63 Samama CM, Clergue F, Barre J, Montefiore A, Ill P, Samii K. Low molecular weight heparin associated with spinal anaesthesia and gradual compression stockings in total hip replacement surgery. Br J Anaesth. 1997;78:660-665.
64 Barnes RW, Brand RA, Clarke W, Hartley N, Hoak JC. Efficacy of graded-compression antiembolism stockings in patients undergoing total hip arthroplasty. Clin Orthop Relat Res.; 132; 1978; 61-67.
65 Fordyce MJ, Ling RS. A venous foot pump reduces thrombosis after total hip replacement. J Bone Joint Surg Br. 1992;74:45-49.
66 Hui ACW, Heras-Palou C, Dunn I, Triffitt PD, Crozier A, Imeson J, et al. Graded compression stockings for prevention of deep-vein thrombosis after hip and knee replacement. J Bone Joint Surg Br. 1996;78:550-554.
67 Wells PS, Lensing AW, Hirsh J. Graduated compression stockings in the prevention of postoperative venous thromboembolism. A meta-analysis. Arch Intern Med. 1994;154:67-72.
68 Warwick D, Harrison J, Glew D, Mitchelmore A, Peters TJ, Donovan J. Comparison of the use of a foot pump with the use of low-molecular-weight heparin for the prevention of deep-vein thrombosis after total hip replacement. A prospective, randomized trial. J Bone Joint Surg Am. 1998;80:1158-1166.
69 Pitto RP, Hamer H, Heiss-Dunlop W, Kuehle J. Mechanical prophylaxis of deep-vein thrombosis after total hip replacement a randomised clinical trial. J Bone Joint Surg Br. 2004;86:639-642.
70 Lassen MR, Borris LC, Anderson BS, Jensen HP, Skejø Bro HP, et al. Efficacy and safety of prolonged thromboprophylaxis with a low molecular weight heparin (dalteparin) after total hip arthroplasty—the Danish Prolonged Prophylaxis (DaPP) Study. Thromb Res. 1998;89:281-287.
71 Dahl OE, Andreassen G, Aspelin T, Müller C, Mathiesen P, et al. Prolonged thromboprophylaxis following hip replacement surgery: Results of a double-blind, prospective, randomised, placebo-controlled study with dalteparin (Fragmin(TM)). Thromb Haemost. 1997;77:26-31.
72 Planes A, Vochelle N, Darmon JY, Fagola M, Bellaud M, et al. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: Double-blind randomised comparison of enoxaparin versus placebo. Lancet. 1996;348:224-228.
73 Bergqvist D, Benoni G, Björgell O, Fredin H, Hedlundh U, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335:696-700.
74 Comp PC, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. J Bone Joint Surg Am. 2001;83-A:336-345.
75 Hull R, et al. A comparison of subcutaneous low-molecular-weight heparin with warfarin sodium for prophylaxis against deep-vein thrombosis after hip or knee implantation. N Engl J Med. 1993;329:1370-1376.
76 Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: A double-blind, randomized comparison. The North American Fragmin Trial Investigators. Arch Intern Med. 2000;160:2199-2207.
77 Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
78 Anonymous. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295-1302.
79 Anderson DR, O’Brien BJ, Levine MN, Roberts R, Wells PS, et al. Efficacy and cost of low-molecular-weight heparin compared with standard heparin for the prevention of deep vein thrombosis after total hip arthroplasty. Ann Intern Med. 1993;119:1105-1112.
80 Kakkar VV, Howes J, Sharma V, Kadziola Z. A comparative double-blind, randomised trial of a new second generation LMWH (bemiparin) and UFH in the prevention of post-operative venous thromboembolism. The Bemiparin Assessment group. Thromb Haemost. 2000;83:523-529.
81 Paiement GD, Wessinger SJ, Hughes R, Harris WH. Routine use of adjusted low-dose warfarin to prevent venous thromboembolism after total hip replacement. J Bone Joint Surg Am. 1993;75:893-898.
82 Mesko JW, Brand RA, Iorio R, Gradisar I, Heekin R, et al. Venous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: A survey of the AAHKS membership. J Arthroplasty. 2001;16:679-688.
83 Gross M, Anderson DR, Nagpal S, O’Brien B. Venous thromboembolism prophylaxis after total hip or knee arthroplasty: A survey of Canadian orthopedic surgeons. Can J Surg. 1999;42:457-461.
84 Amstutz HC, Friscia DA, Dorey F, Carney BT. Warfarin prophylaxis to prevent mortality from pulmonary embolism after total hip replacement. J Bone Joint Surg Am. 1989;71:321-326.
85 Janku GV, Paiement GD, Green HD. Prevention of venous thromboembolism in orthopaedics in the United States. Clin Orthop Relat Res.; 325; 1996; 313-321.
86 Lieberman JR, Wollaeger J, Dorey F, Thomas BJ, Kilgus DJ, et al. The efficacy of prophylaxis with low-dose warfarin for prevention of pulmonary embolism following total hip arthroplasty. J Bone Joint Surg Am. 1997;79:319-325.
87 Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost. 2004;92:1012-1017.
88 Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, et al. Low-molecular-weight heparin prophylaxis using dalteparin extended out-of-hospital vs in-hospital warfarin/out-of-hospital placebo in hip arthroplasty patients: A double-blind, randomized comparison. North American Fragmin Trial Investigators. Arch Intern Med. 2000;160:2208-2215.
89 Comp PC, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. J Bone Joint Surg Am. 2001;83-A:336-345.
90 Samama CM, Vray M, Barré J, Fiessinger JN, Rosencher N, et al. Extended venous thromboembolism prophylaxis after total hip replacement: A comparison of low-molecular-weight heparin with oral anticoagulant. Arch Intern Med. 2002;162:2191-2196.
91 Hamulyak K, Lensing AW, van der Meer J, Smid WM, van Ooy A, et al. Subcutaneous low-molecular weight heparin or oral anticoagulants for the prevention of deep-vein thrombosis in elective hip and knee replacement? Thromb Haemost. 1995;74:1428-1431.
92 Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, PENTATHALON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: A randomised double-blind trial [erratum appears in Lancet 2002 Oct 5;360(9339):1102]. Lancet. 2002;359:1721-1726.
93 Zufferey P, Laporte S, Quenet S, Molliex S, Auboyer C, Decousus H, et al. Optimal low-molecular-weight heparin regimen in major orthopaedic surgery: A meta-analysis of randomised trials. Thromb Haemost. 2003;90:654-661.
94 Francis CW, Pellegrini VDJr, Totterman S, Boyd ADJr, Marder VJ, et al. Prevention of deep-vein thrombosis after total hip arthroplasty: Comparison of warfarin and dalteparin. J Bone Joint Surg Am. 1997;79:1365-1372.
95 Colwell CWJr, Collis DK, Paulson R, McCutchen JW, Bigler GT, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization three months after discharge. J Bone Joint Surg Am. 1999;81:932-940.
96 Mismetti P, Laporte S, Zufferey P, Epinat M, Decousus H, Cucherat M. Prevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: A meta-analysis. J Thromb Haemost. 2004;2:1058-1070.
97 Bauer KA, Eriksson BI, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305-1310.
98 Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345:1298-1304.
99 Colwell CWJr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res. 1995;321:19-27.
100 Turpie AG, Levine MN, Hirsh J, Carter CJ, Jay RM, Powers PJ, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315:925-929.
101 Fitzgerald RHJr, Spiro TE, Trowbridge AA, Gardiner GAJr, Whitsett TL, et al. J Bone Joint Surg Am. 2001;83-A:900-906.
102 Kaempffe FA, Lifeso RM, Meinking C. Intermittent pneumatic compression versus coumadin: Prevention of deep vein thrombosis in lower-extremity total joint arthroplasty. Clin Orthop Relat Res.; 269; 1991; 89-97.
103 Haas SB, Insall JN, Scuderi GR, Windsor RE, Ghelman B. Pneumatic sequential-compression boots compared with aspirin prophylaxis of deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Am. 1990;72:27-31.
104 Westrich GH, Specht LM, Sharrock NE, Windsor RE, Sculco TP, et al. Venous haemodynamics after total knee arthroplasty: Evaluation of active dorsal to plantar flexion and several mechanical compression devices. J Bone Joint Surg Br. 1998;80:1057-1066.
105 Fauno P, Suomalainen O, Rehnberg V, Hansen TB, Krøner K, et al. Prophylaxis for the prevention of venous thromboembolism after total knee arthroplasty. A comparison between unfractionated and low-molecular-weight heparin. J Bone Joint Surg Am. 1994;76:1814-1818.
106 Francis CW, Pellegrini VDJr, Leibert KM, Totterman S, Azodo MV, et al. Comparison of two warfarin regimens in the prevention of venous thrombosis following total knee replacement. Thromb Haemost. 1996;75:706-711.
107 Leclerc JR, Geerts WH, Desjardins L, Laflamme GH, L’Espérance B, et al. Prevention of venous thromboembolism after knee arthroplasty. A randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med. 1996;124:619-626.
108 Francis CW, Davidson BL, Berkowitz SD, Lotke PA, Ginsberg JS, et al. Ximelagatran versus warfarin for the prevention of venous thromboembolism after total knee arthroplasty. A randomized, double-blind trial. Ann Intern Med. 2002;137:648-655.
109 Francis CW, Berkowitz SD, Comp PC, Lieberman JR, Ginsberg JS, et al. Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med. 2003;349:1703-1712.
110 Robinson KS, Anderson DR, Gross M, Petrie D, Leighton R, et al. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty: The post-arthroplasty screening study. A randomized, controlled trial. Ann Intern Med. 1997;127:439-445.
111 RD Heparin Arthroplasty Group. RD heparin compared with warfarin for prevention of venous thromboembolic disease following total hip or knee arthroplasty. J Bone Joint Surg Am. 1994;76:1174-1185.
112 Heit JA, Berkowitz SD, Bona R, Cabanas V, Corson JD, et al. Efficacy and safety of low molecular weight heparin (ardeparin sodium) compared to warfarin for the prevention of venous thromboembolism after total knee replacement surgery: A double-blind, dose-ranging study. Thromb Haemost. 1997;77:32-38.
113 Brookenthal KR, et al. A meta-analysis of thromboembolic prophylaxis in total knee arthroplasty. J Arthroplasty. 2001;16:293-300.
114 Howard AW, Aaron SD. Low molecular weight heparin decreases proximal and distal deep venous thrombosis following total knee arthroplasty. A meta-analysis of randomized trials. Thromb Haemost. 1998;79:902-906.
115 Turpie AGG. Venous thromboembolism prophylaxis: Role of factor Xa inhibition by fondaparinux. Surg Technol Int. 2004;13:261-267.
116 Menzin J, Colditz GA, Regan MM, Richner RE, Oster G, et al. Cost-effectiveness of enoxaparin vs low-dose warfarin in the prevention of deep-vein thrombosis after total hip replacement surgery. Arch Intern Med. 1995;155:757-764.
117 Hull RD, Raskob GE, Pineo GF, Feldstein W, Rosenbloom D, et al. Subcutaneous low-molecular-weight heparin vs warfarin for prophylaxis of deep vein thrombosis after hip or knee implantation. An economic perspective. Arch Intern Med. 1997;157:298-303.
118 Friedman RJ, Dunsworth GA. Cost analyses of extended prophylaxis with enoxaparin after hip arthroplasty. Clin Orthop Relat Res.; 370; 2000; 171-182.
119 Hawkins D. Pharmacoeconomics of thrombosis management. Pharmacotherapy. 2004;24(7 Pt 2):95S-99S.
120 Hull RD, Brant RF, Pineo GF, Stein PD, Raskob GE, Valentine KA. Preoperative vs postoperative initiation of low-molecular-weight heparin prophylaxis against venous thromboembolism in patients undergoing elective hip replacement. Arch Intern Med. 1999;159:137-141.
121 Hull RD, Pineo GF, Stein PD, Mah AF, MacIsaac SM, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: A systematic review. Arch Intern Med. 2001;161:1952-1960.
122 Raskob GE, Hirsh J. Controversies in timing of the first dose of anticoagulant prophylaxis against venous thromboembolism after major orthopedic surgery. Chest. 2003;124(6 suppl):379S-385S.
123 Strebel N, Prins M, Agnelli G, Büller HR. Preoperative or postoperative start of prophylaxis for venous thromboembolism with low-molecular-weight heparin in elective hip surgery? Arch Intern Med. 2002;162:1451-1456.
124 Turpie A, Bauer K, Eriksson B, Lassen M, Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Efficacy and safety of fondaparinux in major orthopedic surgery according to the timing of its first administration. Thromb Haemost. 2003;90:364-366.
125 Prandoni P, Bruchi O, Sabbion P, Tanduo C, Scudeller A, et al. Prolonged thromboprophylaxis with oral anticoagulants after total hip arthroplasty: A prospective controlled randomized study. Arch Intern Med. 2002;162:1966-1971.
126 Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: A meta-analysis of the randomised trials. Lancet. 2001;358:9-15.
127 Hull RD, Pineo GF, Stein PD, Mah AF, MacIsaac SM, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: A systematic review. Intern Med. 2001;135:858-869.
128 Turpie AGG, Eriksson BI, Bauer KA, Lassen MR. New pentasaccharides for the prophylaxis of venous thromboembolism: Clinical studies. Chest. 2003;124(6 suppl):371S-378S.
129 Kearon C. Noninvasive diagnosis of deep vein thrombosis in postoperative patients. Semin Thromb Hemost. 2001;27:3-8.
130 Agnelli G, Cosmi B, Ranucci V, Renga C, Mosca S, et al. Impedance plethysmography in the diagnosis of asymptomatic deep vein thrombosis in hip surgery. A venography-controlled study. Arch Intern Med. 1991;151:2167-2171.
131 Ciccone WJ2nd, Fox PS, Neumyer M, Rubens D, Parrish WM, Pellegrini VDJr. Ultrasound surveillance for asymptomatic deep venous thrombosis after total joint replacement. J Bone Joint Surg Am. 1998;80:1167-1174.
132 Magnusson M, Eriksson BI, Kälebo P, Sivertsson R. Is colour Doppler ultrasound a sensitive screening method in diagnosing deep vein thrombosis after hip surgery? Thromb Haemost. 1996;75:242-245.
133 Schmidt B, et al. Ultrasound screening for distal vein thrombosis is not beneficial after major orthopedic surgery. A randomized controlled trial. Thromb Haemost. 2003;90:949-954.
134 Leclerc JR, et al. The incidence of symptomatic venous thromboembolism during and after prophylaxis with enoxaparin: A multi-institutional cohort study of patients who underwent hip or knee arthroplasty. Canadian Collaborative Group. Arch Intern Med. 1998;158:873-878.
135 Wells PS, Lensing AW, Davidson BL, Prins MH, Hirsh J. Accuracy of ultrasound for the diagnosis of deep venous thrombosis in asymptomatic patients after orthopedic surgery. A meta-analysis. Ann Intern Med. 1995;122:47-53.
136 Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, et al. Regional anesthesia in the anticoagulated patient: Defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28:172-197.
137 Modig J. The role of lumbar epidural anaesthesia as antithrombotic prophylaxis in total hip replacement. Acta Chir Scand. 1985;151:589-594.
138 Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8-15.
139 Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: A meta-analysis. Anesth Analg. 2006;103:1018-1025.
140 Botteman MF, Caprini J, Stephens JM, Nadipelli V, Bell CF, et al. Results of an economic model to assess the cost-effectiveness of enoxaparin, a low-molecular-weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long-term complications in total hip replacement surgery in the United States. Clin Ther. 2002;24:1960-1986. discussion 1938,
141 Matzsch T. Thromboprophylaxis with low-molecular-weight heparin: Economic considerations. Haemostasis. 2000;30(suppl 2):141-145. discussion 128–129,
142 Tran AH, Lee G. Fondaparinux for prevention of venous thromboembolism in major orthopedic surgery. Ann Pharmacother. 2003;37:1632-1643.
143 Skedgel C, Goeree R, Pleasance S, Thompson K, O’Brien B, Anderson D. The cost-effectiveness of extended-duration antithrombotic prophylaxis after total hip arthroplasty. J Bone Joint Surg Am. 2007;89:819-828.
144 Dahl OE, Pleil AM. Investment in prolonged thromboprophylaxis with dalteparin improves clinical outcomes after hip replacement. J Thromb Haemost. 2003;1:896-906.
145 Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;26;358(26):2765-2775.
146 Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban verus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776-2786. Jun 26