Chapter 1 Should Patients with Acute Spinal Cord Injuries Receive Steroids?
The annual incidence of spinal cord injuries (SCIs) is estimated between 11.5 and 53.4 per 1 million people,1–8 and prevalence is estimated at around 700 SCI cases per 1 million people in the United States.9 These injuries are characterized by high mortality and morbidity rates. In those individuals who survive to arrive at an acute care institution, mortality rates range between 4.4% and 16.7%.3,5, 8 These survivors typically experience prolonged hospitalization in acute care hospitals and rehabilitation centers.8,10 Patients are typically young (mean and median ages ranging in the late 20s and early 30s) and male (80–85% of patients).8 Approximately 45% of patients experience a complete neurologic injury with no detectable neurologic function below the level of the lesion.11 Fifty-five percent of patients are injured between C1 and C7-T1.8 Hospital admissions of a week or longer are necessary for approximately 10% of patients with SCI every year because of complications including pressure sores, autonomic dysreflexia, pneumonia, atelectasis, deep venous thrombosis, and renal calculi.12–14 Spasticity and pain also add significantly to neurologic disability in 25% of patients.12 Long-term reduced life expectancy is largely accounted for by pneumonia, pulmonary emboli, and septicemia. Furthermore, the financial burden of managing these injuries both to the individual and to society is enormous. The estimated cost to the United States for care of all patients with SCI in 1990 was $4 billion.8
Accordingly, therapies that limit the extent of neurologic dysfunction after SCI (neuroprotection) or that improve recovery of function (neuroregeneration and neuroaugmentation) would have a huge impact on this patient population. Significant research interest in these strategies has identified many potential therapeutic targets in animal models. Of these, several have been applied to high-quality human investigations. Unfortunately, none has been proved effective in humans. The American Association of Neurological Surgeons and Congress of Neurological Surgeons Joint Section on Disorders of the Spine and Peripheral Nerves’ 2002 Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury15 specifically recognizes methylprednisolone and GM-1 ganglioside as options for treatment in patients with acute SCI. However, these options were qualified “without demonstrated clinical benefit” in the case of GM-1 ganglioside and with “evidence suggesting harmful side effects” that is more consistent than any suggestion of clinical benefit in the case of methylprednisolone. Tirilazad and naloxone have also been studied in humans but without any evidence of efficacy to warrant inclusion in the guidelines.
METHYLPREDNISOLONE AND OTHER CORTICOSTEROIDS IN SPINAL CORD INJURY
Steroids in various forms have been used in the treatment of SCI for many years. Historically, the rationale for the use of corticosteroids in the management of neural trauma extended from their use in decreasing edema in the management of brain tumors. In addition, their anti-inflammatory effect was thought to be beneficial to the secondary injury pathophysiology of SCI. Studies in dogs supported these hypotheses and demonstrated a modest improvement in neurologic outcome with steroid treatment and a modified anti-inflammatory response.16 Subsequently, other animal studies have provided support for improved neurologic recovery after SCI in animal models when methylprednisolone is administered and have provided evidence for inhibition of lipid peroxidation, protection of energy metabolism, reduced post-traumatic ischemia, maintenance of neurofilament structure, and decreased post-traumatic ionic shifts.17
The role of steroids in human SCI management became more rigorously considered after publication of the Second National Acute Spinal Cord Injury Study (NASCIS II). Unfortunately, the initial enthusiasm for an apparent positive effect of methylprednisolone in SCI demonstrated by NASCIS II has not stood up to the extensive scrutiny that ensued.18–21 However, despite significant criticism, this medication continues to be prescribed by many physicians, and a 2002 study suggests that most practitioners prescribe it because of peer pressure or fear of litigation, rather than a firm belief that it is indeed efficacious.22
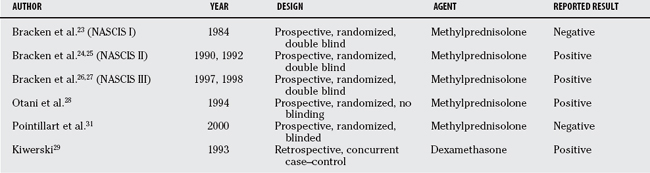
The first NASCIS study compared low- (100 mg/day × 10 days) and high-dose (1000 mg/day × 10 days) methylprednisolone, and did not include a placebo group.23 It failed to demonstrate a difference between the doses tested. The high-dose group exhibited an increased risk for complications. That study was followed by a randomized, controlled trial comparing a 24-hour protocol (30 mg/kg methylprednisolone bolus followed by 5.4 mg/hg/hr until 24 hours) with placebo in NASCIS II.24,25 The dose selected in NASCIS II was greater than that of the original study because of further animal work that suggested a therapeutic threshold of 30 mg/kg.25 NASCIS II concluded that improved neurologic recovery was seen when the methylprednisolone treatment protocol was initiated within 8 hours of injury. That study was then followed by NASCIS III, which compared patients randomized to the 24-hour NASCIS II protocol with those randomized to a 48-hour protocol (5.4 mg/kg/hr methylprednisolone after the 30-mg/kg bolus).26,27 That study concluded that patients for whom therapy was initiated within 3 hours did not gain any benefit from extending treatment to 48 hours, whereas those for whom therapy was initiated between 3 and 8 hours did benefit further. No benefit has been shown if therapy is initiated beyond 8 hours in NASCIS II.
Several concerns have arisen regarding the post hoc analyses of NASCIS II and III. The left-sided motor scores were not published but reported “similar” to right-sided scores. Thus, half the available data were excluded. The statistical analyses failed to correct for multiple statistical comparisons, and it is unclear whether the repeated-measures design was considered. More than 65 methylprednisolone-related t tests were performed in NASCIS II, and more than 100 t tests in NASCIS III. There was, therefore, a high likelihood of type I error (erroneously detecting a statistical difference that does not exist) through random chance. The rationale for an 8-hour subanalysis (NASCIC II) is unclear. It has been claimed that this subgroup was selected based on median time to treatment. However, by definition, 50% of patients should have initiated treatment before the median time of treatment initiation. In fact, only 38% of patients (183/487) were included in this post hoc analysis. The justification for the 3- and 8-hour windows in NASCIS III is similarly obscure. Other observations raise concern about imposing these artificial timerelated stratifications. For example, in NASCIS II, the incompletely injured placebo group when separated into <8- and >8-hour gr-oups differs in recovery, with the latter group showing improved recovery comparable with the <8-hour incompletely injured methylprednisolone group.17 This implies that these patients could be treated with placebo beyond 8 hours and a similar result to treatment with methylprednisolone initiated within 8 hours can be expected. Finally, another common criticism of the NASCIS studies has been the lack of outcomes assessing functional recovery meaningful to the patient’s expected activities.
In addition to the NASCIS studies, Otani and colleagues28 published a prospective, randomized trial investigating the NASCIS II methylprednisolone dosing protocol. The investigators were not blinded to treatment, and the control group was allowed to receive alternate steroids at the physicians’ discretion. Of 158 patients entered, 117 were analyzed. The primary outcome measures (American Spinal Injury Association [ASIA] motor and sensory scores) were not different between treatment groups. Post hoc analyses suggested that more patients improved on the NASCIS II steroid regimen compared with control patients. However, for a greater number of steroid-treated patients to improve, the fewer control patients who also improved must have demonstrated a larger magnitude of recovery (because overall ASIA motor and sensory scores were no different between groups). Thus, such post hoc analyses become difficult to interpret in the face of a negative overall effect.
A retrospective study with concurrent case controls also suggested a benefit with corticosteroid administration.29 This study investigated the use of dexamethasone initiated within 24 hours of injury with the specific dose left to the discretion of the attending physicians. Length of follow-up was not specified, and a new but unvalidated neurologic grading system was used for outcome assessment. This study reports that the percentage of patients who improved was significantly greater in the steroid-treated group. However, there was a much greater mortality rate within the control group, suggesting a selection bias to more severely injured patients in the control arm. The magnitude of the mortality rate is also a concern and suggests that the study population may not be representative and that the results are not generalizable.
A randomized, controlled trial designed to examine the potential therapeutic benefit of nimodipine (a calcium channel antagonist) included an NASCIS II methylprednisolone regimen and a placebo group.30,31 This study, which included approximately 25 patients in each group, failed to show any difference between any of four groups (placebo, nimodipine, methylprednisolone, methylprednisolone and nimodipine) using ASIA scores and ASIA grade outcomes. However, this study was remarkable for an increase in infectious complications in the methylprednisolone group.
Most recently, the data from the five randomized trials of methylprednisolone (NASCIS, NASCIS II, NASCIS III, Otani and colleagues,28 and Pointillart and coworkers31) were subject to a meta-analysis and concluded that high-dose methylprednisolone given within 8 hours of acute SCI is safe and modestly effective. This article estimated a treatment effect of 4.1 motor score points (from ASIA score) over placebo treatment (using the NASCIS II 24-hour protocol). The findings and limitations of the individual articles included in this review are discussed earlier in this chapter (Table 1-1).
TABLE 1-1 Human Clinical Trials in Spinal Cord Injury Investigating Methylprednisolone and Other Corticosteroids
It must also be recognized that corticosteroid administration comes with increased risk for several potential adverse events including pneumonia, sepsis, and steroid-induced myopathy, all of which may negatively impact outcome in patients with SCI, potentially overshadowing any unproven beneficial effect.32 Galandiuk and colleagues33 demonstrate that patients with SCI treated with corticosteroids exhibited a greater rate of pneumonia (79% vs. 50% with placebo treatment) and required a longer hospital stay (44.4 vs. 27.7 days with placebo treatment). Matsumoto and coauthors34 specifically investigated the complication rate in patients treated with the NASCIS II protocol compared with placebo in a small randomized trial. They found a nonstatistically significant trend to greater complication rates with steroid treatment overall, but a significant difference in respiratory and gastrointestinal complications. The majority of these complications were pneumonia and gastric ulcer, respectively. NASCIS II did not find an increased complication rate with steroid treatment, but NASCIS III reported a greater rate of sepsis and pulmonary complications particularly with the 48-hour infusion. The Corticosteroid Randomization after Significant Head Injury (CRASH) trial investigated the use of a corticosteroid regimen similar to that used in NASCIS II in the setting of closed head injury.35 It demonstrated increased mortality with steroid use in that population. One must certainly recognize the possibly of an increased mortality risk in patients with SCI as well. However, it must also be noted that Sauerland and colleagues36 performed a large meta-analysis review of approximately 2500 patients treated with preoperative high-dose steroids (>15 mg/kg methylprednisolone) and found no evidence of increased risk for gastrointestinal bleeding, wound complications, pulmonary complications, or death. They suggest that some reports that claim increased complication rates with high-dose steroid use were subject to selection bias.
CONCLUSIONS
In summary, although well-designed and executed studies have been performed, they have failed to demonstrate convincingly a beneficial effect of methylprednisolone or other corticosteroids in the management of SCI. Post hoc analyses have been used to argue a small effect on motor function in three randomized trials. However, all of these analyses contain significant flaws rendering conclusions of efficacy dubious. These observations have led two national organizations to publish guidelines recommending methylprednisolone administration as a treatment option rather than as a standard of care or recommended treatment (Table 1-2).15,37
| STATEMENT | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION | REFERENCES |
|---|---|---|
*Although data are available from level 1 publications, this item was not the main focus of these articles and the studies were not necessarily powered to address this issue.
1 Botterell EH, Jousse AT, Kraus AS, et al. A model for the future care of acute spinal cord injuries. Can J Neurol Sci. 1975;2:361-380.
2 Gjone R, Nordlie L. Incidence of traumatic paraplegia and tetraplegia in Norway: A statistical survey of the years 1974 and 1975. Paraplegia. 1978;16:88-93.
3 Kraus JF, Franti CE, Riggins RS, et al. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471-492.
4 Kraus JF. A comparison of recent studies on the extent of the head and spinal cord injury problem in the United States. J Neurosurg.; Suppl.; 1980; S35-43.
5 Kraus JF. Injury to the head and spinal cord. The epidemiological relevance of the medical literature published from 1960 to 1978. J Neurosurg.; Suppl.; 1980; S3-10.
6 Kurtzke JF. Epidemiology of spinal cord injury. Exp Neurol. 1975;48:163-236.
7 Minaire P, Castanier M, Girard R, et al. Epidemiology of spinal cord injury in the Rhone-Alpes Region, France, 1970-75. Paraplegia. 1978;16:76-87.
8 Tator CH. Epidemiology and general characteristics of the spinal cord-injured patient. In: Tator CH, Benzel EC, editors. Contemporary management of spinal cord injury: From impact to rehabilitation. Park Ridge, IL: The American Association of Neurological Surgeons Publications Committee; 2000:15-19.
9 Harvey C, Rothschild BB, Asmann AJ, Stripling T. New estimates of traumatic SCI prevalence: A survey-based approach. Paraplegia. 1990;28:537-544.
10 Tator CH, Duncan EG, Edmonds VE, et al. Complications and costs of management of acute spinal cord injury. Paraplegia. 1993;31:700-714.
11 Tator CH, Duncan EG, Edmonds VE, et al. Changes in epidemiology of acute spinal cord injury from 1947 to 1981. Surg Neurol. 1993;40:207-215.
12 Johnson RL, Gerhart KA, McCray J, et al. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998;36:45-50.
13 Krause JS. Aging after spinal cord injury: An exploratory study. Spinal Cord. 2000;38:77-83.
14 McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
15 Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50:S63-72.
16 Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. J Neurosurg. 1969;30:693-697.
17 Zeidman SM, Ling GS, Ducker TB, Ellenbogen RG. Clinical applications of pharmacologic therapies for spinal cord injury. J Spinal Disord. 1996;9:367-380.
18 Nesathurai S. Steroids and spinal cord injury: Revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088-1093.
19 Coleman WP, Benzel D, Cahill DW, et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185-199.
20 Hurlbert RJ. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J Neurosurg. 2000;93:1-7.
21 Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury—a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
22 Hurlbert RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci. 2002;29:236-239.
23 Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. Jama. 1984;251:45-52.
24 Bracken MB, Shepard MJ, Collins WFJr, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23-31.
25 Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405-1411.
26 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. Jama. 1997;277:1597-1604.
27 Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
28 Otani K, Abe H, Kadoya S. Beneficial effect of methylprednisolone sodium succinate in the treatment of acute spinal cord injury. Sekitsui Sekizui J. 1994;7:633-647.
29 Kiwerski JE. Application of dexamethasone in the treatment of acute spinal cord injury. Injury. 1993;24:457-460.
30 Petitjean ME, Pointillart V, Dixmerias F, et al. Medical treatment of spinal cord injury in the acute stage. Ann Fr Anesth Reanim. 1998;17:114-122.
31 Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71-76.
32 Tator CH. Review of treatment trials in human spinal cord injury: Issues, difficulties, and recommendations. Neurosurgery. 2006;59:957-987.
33 Galandiuk S, Raque G, Appel S, Polk HCJr. The two-edged sword of large-dose steroids for spinal cord trauma. Ann Surg. 1993;218:419-427.
34 Matsumoto T, Tamaki T, Kawakami M, et al. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001;26:426-430.
35 Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 1000 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet. 2004;364:1321-1328.
36 Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: A systematic review. Drug Saf. 2000;23:449-461.
37 Hugenholtz H, Cass DE, Dvorak MF, et al. High-dose methylprednisolone for acute closed spinal cord injury—only a treatment option. Can J Neurol Sci. 2002;29:227-235.