chapter 61 Sexual health
COMMUNICATING WITH PATIENTS ABOUT SEX
Despite the constant stream of sexualised messages, talking meaningfully and frankly about an individual’s personal sexual matters remains difficult for many patients and healthcare professionals. Open, confident and personal communication about sexual matters is not well entrenched in most societies, and may be more difficult with individuals from some cultural and religious backgrounds. Despite this, facilitating effective communication about sex is professionally rewarding to the provider, can be a therapeutic intervention in its own right and is appreciated by anxious patients.
The exhaustive list of questions to ask during a full sexual health history is shown in Box 61.1. Such a history is really only suitable for sexual health clinics and special circumstances such as symptomatic or otherwise high-risk patients. There is growing appreciation that a full sexual history for all patients in all scenarios is unnecessary and intrusive, and yields false responses from a significant proportion of patients—an observation that would probably not surprise most experienced GPs.
BOX 61.1 Sexual health history*
A recent Australian study illustrates this point well.1 The authors examined the attitudes of women to chlamydia testing in general practice. Acceptance of age-based chlamydia testing was high, but women did not want to be asked to provide a sexual history as part of being asked to have a chlamydia test. Some reported that they would lie if asked how many partners they had had. The authors conclude that chlamydia screening in general practice needs to be normalised and destigmatised.
It falls to the person taking a sexual history to employ non-intrusive strategies that distinguish low-risk from high-risk settings, and then alter the direction of the history accordingly. This is a complex task, and it is reasonable to initially find the process daunting. The task, however, gets easier with experience and practice. In turn most patients will read the comfort level of the interviewer and respond in kind: the interviewer who can respectfully and confidently collect an intimate history provides an obvious cue for the patient to respond with similar openness and confidence (see Box 61.2, for example).
SAFER SEX AND STI PREVENTION
The World Health Organization ranks unsafe sex as the world’s fifth most prevalent cause of mortality.2,3 This includes mortality from HIV infection, consequences of pregnancy (predominantly lack of access to safe abortion and contraception), cervical and anal cancers and bacterial STI. Disease acquired through sexual activity accounts for 0.5% of the burden of disease in Australia.4 Lower rates in developed countries are attributable to education, access to contraception (particularly condoms) and safe abortion, cervical screening and better healthcare for those who acquire STIs.
Between 16% and 20% of Australian couples reported using condoms with their regular partner in the previous 3 months, and condom use was higher with casual partners than with regular partners. Gay men reported higher rates of condom use than heterosexuals. Condom use has increased significantly among people having sex for the first time.5
Abstinence, like condoms, is effective only if used consistently. While celibacy is a valid personal choice for some people, there is incontrovertible evidence that it is ineffective as a public health strategy to reduce STIs or unintended pregnancies.6
SEXUALLY TRANSMISSIBLE INFECTIONS
Sexually transmissible infections are caused by a diverse group of organisms. They range in size from viruses, usually measured in micrometres, to ectoparasites such as pubic lice, which are visible to the human eye. Their life cycles differ enormously, and may include stages of latency, asymptomatic carriage, systemic spread, immune evasion or neural invasion. Some are extraordinarily well adapted to coexist with humans and cause their host such little damage that they are spread widely from person to person. Some cause disease so severe that they damage their own chances of onward transmission by causing disabling symptoms, or even remove their host from the population altogether.
ASYMPTOMATIC SEXUAL HEALTH CHECKS
‘Window periods’ exist for serological tests, and tests may need to be repeated once the window period has elapsed, to completely exclude infection after a particular exposure:
GENITAL SYNDROMES
URETHRITIS
Non-infective causes of urethritis include trauma from, for example, vigorous sexual activity, urethral stricture (fortunately rare these days), foreign body and Reiter syndrome. The anxious patient who ‘milks’ his urethra in search of discharge will, if he is diligent enough, cause a traumatic urethritis.
VAGINAL DISCHARGE
Recurrent candidiasis (four or more microbiologically confirmed episodes per year) is more difficult to treat. Weekly oral fluconazole for up to 6 months may help to reduce the bowel carriage of Candida from which recurrences are thought to arise. Alternatively, doubling the duration of standard doses may help. Attention to predisposing factors is recommended. Care should be taken to ensure that the Candida species recovered by the laboratory are albicans, as non-albicans species such as C. glabrata and C. krusei are often resistant to azoles. Liaison with a sexual health physician, gynaecologist or microbiologist is recommended. The available evidence for use of probiotics for prevention of recurrent vulvovaginal candidiasis is limited. There are some small clinical trials supporting the effectiveness of oral or intravaginal administration of lactobacilli, particularly the strains acidophilus, rhamnosus GR-1 and fermentum RC-14. In vitro studies have shown that lactobacilli inhibit the growth of C. albicans and its adherence to the vaginal epithelium. A recent review of probiotics for recurrent vulvovaginal candidiasis concluded that there is limited beneficial evidence, but as adverse effects are rare, it may be recommended.7
Garlic has antifungal properties, and wrapping a single clove in unbleached gauze may be effective in treating candidiasis. Side effects, however, include local irritant and allergic reactions, as well as odour.8 Tea tree oil is ineffective for vulvovaginal candidiasis, and can lead to severe contact reactions.8 Gentian violet has antifungal properties, but there are no randomised controlled trials of its efficacy, and side effects include vulval irritation and staining of clothes.8
Douching is not a recommended treatment of candidiasis. Douching can in fact be quite harmful and has no place in women’s hygiene practices. It may remove the normal vaginal bacteria, leading to an overgrowth of pathogenic species and a predisposition to STIs, such as Neisseria gonorrhoeae or Chlamydia trachomatis. The pressurised fluid can force pathogens upwards from the lower genital tract into the cervix, uterus, uterine tubes and abdominal cavity, potentially causing pelvic inflammatory disease. Douching is also linked to vulval dermatitis and chemical burns, ectopic pregnancy and bacterial vaginosis.9
Cervicitis is most frequently caused by chlamydia, but gonorrhoea and trichomoniasis are less common causes. Cervicitis may present with vaginal discharge, dyspareunia, abnormal menstrual bleeding, postcoital bleeding or intermenstrual bleeding. Chlamydia cervicitis is treated with azithromycin 1 g orally statim. Alternatively, doxycycline (100 mg b.i.d. for 7 days) can be used in women with macrolide allergy.
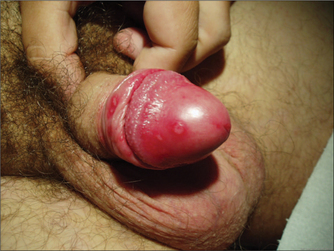
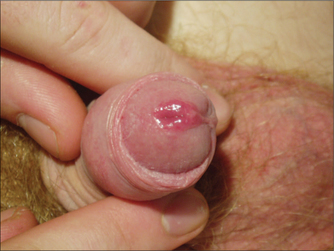
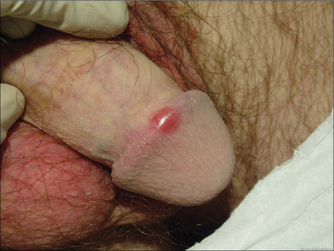
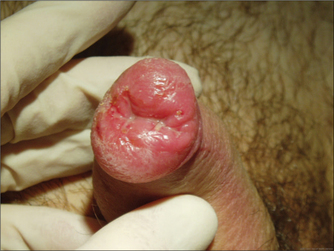
GENITAL LUMPS
Medical treatments for warts include imiquimod and podophyllotoxin, both of which can be applied by the patient at home. Practitioner-applied medical therapy includes podophyllin resin and trichloroacetic acid, but training is required for the use of these. Commercially available over-the-counter preparations for warts are not suitable for genital warts as the concentrations of salicylic acid, podophyllin derivatives and other ingredients are too high. Care should be taken to avoid using imiquimod or podophyllin/toxin in pregnant women.
Zinc supplementation has been examined in one double-blind randomised controlled trial10 and showed a significantly greater response to zinc than to placebo. The dose of zinc studies was reasonable high, and side effects included gastrointestinal symptoms such as nausea, vomiting and abdominal pain.
Lifestyle modification may be useful in managing warts, particularly those that are difficult to treat. Smoking and stress are likely contributors to ongoing genital warts. Hypnosis was found to be an effective treatment for warts in one trial.11 In this study, hypnosis resulted in greater wart regression than placebo or salicylic acid.
MOLLUSCUM CONTAGIOSUM
Other causes of genital bumps include skin tags (acrochordon), seborrhoeic keratoses and tumours.
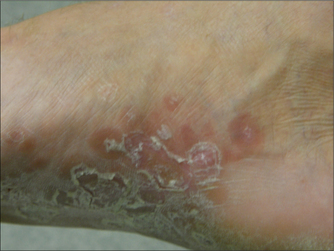
GENITAL ULCERS
Ulcers on genital skin and mucosal membranes are a common presentation in primary care. The causes of genital herpes are listed in Box 61.4. Almost all genital ulcers in developed communities are caused by the herpes simplex viruses. Other causes of genital ulcers are important because of their public health implications, and the seriousness of their complications if left untreated.
Herpes is the most common cause of genital ulceration worldwide.
Virtually all patients who have herpes infections will experience virological recurrence, whereby there is viral replication and shedding of virus from the skin. The ‘classic’ clinical appearance of a recurrence is of grouped vesicles on an erythematous base that burst to form painful ulcers. These recurrences are very easy to recognise clinically, but only about one-fifth of people with genital herpes will experience these classic recurrences. Another fifth will periodically shed virus, but remain completely asymptomatic. The other 60% will have ‘atypical’ recurrences and have a wide variety of clinical manifestations. Atypical lesions include fissures, painless ulcers, erythema or altered sensation without any visible changes. Unsurprisingly, most people with herpes simplex infections on their genitals remain unaware of the infection.
SYPHILIS
Unfortunately, clinicians’ unfamiliarity with syphilis has led to delays in treatment and the risk of ongoing transmission in many cases. While a detailed description of the clinical spectrum of syphilis is beyond the scope of this book, the key clinical features of early syphilis are listed in Box 61.5.
SPECIAL POPULATIONS
YOUTH
Young people have a number of factors that predispose them to acquiring STIs. The comparatively high rate of partner change experienced by young people still learning about relationships, sex and themselves provides an epidemiological opportunity for disease acquisition. Biological factors such as cervical ectropion may predispose younger women, and those using oestrogen-containing contraceptives may be at increased risk of chlamydia acquisition. Young people have a greater chance of encountering new pathogens to which they have not mounted immunological defences, further predisposing them to new infections.
Opportunistic screening is recommended for people aged between 15 and 29 years.
WOMEN WHO HAVE SEX WITH WOMEN
STIs can be transmitted between women through the sharing of cervical, vaginal and anal secretions. Reported rates of most STIs among women who have sex with women (WSW) are probably no less than rates among heterosexual women. Rates of HIV, hepatitis B, hepatitis C and syphilis, however, are reported at very low rates. Bacterial vaginosis is more commonly found among WSW and, unlike heterosexual contact, it appears that bacterial vaginosis can be sexually transmitted. Cervical abnormalities are reported as frequently as in women in general, but uptake of screening among younger women is suboptimal. Healthcare practitioners play an important role in encouraging WSW to participate in cervical screening. Lesbians need Pap smears even if they have never had sex with a man.12
COMMERCIAL SEX WORKERS
In the early days of the HIV epidemic, commercial sex workers (CSW) in Australia were pivotal in preventing the spread of HIV into the general community. Sex worker organisations vigorously promoted safer sex practices, and CSW incorporated condoms for all penetrative services with an outstanding level of uniformity and consistency. Consequently, STIs are infrequently seen in CSW, and when they do occur they have often been acquired from private, rather than professional, life.
MANAGING PARTNERS
A common pitfall in the management of contacts is not treating them on presentation. Where there is an established risk of contact with a treatable STI, treatment should be offered immediately, rather than waiting for test results. Testing should still occur, but should not delay treatment. Box 61.6 summarises the management of partners.
BOX 61.6 Management of contacts of patients with STI
Adapted from the Australasian Contact Tracing Manual14
The Australasian Contact Tracing Manual14 contains more information about contact tracing and such useful additions as patient handouts, case studies, privacy legislation considerations and sample contact tracing letters.
GENITAL DERMATOLOGY
Soaps, detergents and over-washing are the most frequent irritants. Increasing one’s washing habits is, unfortunately, a common reaction to irritation or rash on genital skin. Over-the-counter preparations, such as antifungals, tea tree oil, paw-paw ointment and antiseptics can all be potent irritants, especially when applied to non-intact skin. Often, by the time patients present, the clinical picture has become complicated.
Treatment of dermatological conditions is described in Chapter 42, Skin. Similar treatment strategies can be employed in the genitals, but care should be taken to account for the occluded conditions and thinness of genital skin. Tar solutions and salicylic acid concentrations should not exceed 3%, and care should be taken with the use of calcipotriol. Ointments are better tolerated than creams on genital surfaces, and provoke less irritation.
SEXUAL DIFFICULTIES
It is not possible to give a detailed account of sexual difficulties here, but some of the common themes and problems are introduced. Erectile dysfunction is covered separately in Chapter 49, reflecting the recent increase in research and availability of newer treatments in recent times.
Dyspareunia
Pain during sex is known as dyspareunia. It is much more common in women, and seldom seen in men. It can arise from multiple causes including vaginismus (see below), pelvic pathology, postmenopausal vaginal dryness, dermatological problems and penetration when the woman is not aroused. See also Ch 52.
Premature ejaculation
It does not take much time in general practice to gain an awareness of how common sexual difficulties are. The prevalence of sexual difficulties was illustrated in the landmark Study of Health and Relationships5 and confirms that sexual difficulties are common, perhaps surprisingly so. Table 61.1 describes the frequency of various sexual difficulties.
TABLE 61.1 Sexual difficulties for at least one month in the previous year
| Sexual difficulties | Men (%) | Women (%) |
|---|---|---|
| Lacked interest in having sex | 25 | 55 |
| Came to orgasm too quickly | 24 | 12 |
| Worried during sex that body looked unattractive | 14 | 36 |
| Unable to orgasm | 6 | 29 |
| Felt anxious about ability to perform | 16 | 17 |
| Did not find sex pleasurable | 6 | 27 |
| Physical pain during intercourse | 2 | 20 |
| Vaginal dryness | – | 24 |
| Trouble keeping erection | 10 | – |
| Used treatment to aid erections | 2 | – |
Adapted from Richters & Rissel 2005,5 p 85
Al-Gurairi FT, Al-Waiz M, Sharquie KE. Oral zinc sulphate in the treatment of recalcitrant viral warts: randomized placebo-controlled clinical trial. Br J Dermatol. 2002;146:423-431.
Australian Herpes Management Forum. http://www.ahmf.com.au.
Bradford D, Russell D. Talking with clients about sex: a health professional’s guide. Melbourne: IP Communications, 2006.
Cunningham AL, Taylor J. Prevalence of infection with herpes simplex virus types 1 and 2 in Australia: a nationwide population-based survey. Sex Trans Infect. 2006;82:65-68.
dialog, a comprehensive Australian website addressing healthcare for lesbian, bisexual and same-sex attracted women, with many links to other sites, research and specific resources. http://www.dialog.unimelb.edu.au.
girl2girl, a website for patient-orientated information regarding sexual activity. http://www.girl2girl.info.
Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:2.
Russell D, Bradford D, Fairley C. Sexual health medicine. Melbourne: IP Communications, 2005.
Scarlet Alliance: Australian Sex Workers’ Association. http://www.scarletalliance.org.au.
Spanos NP, Williams V, Gwynn MI. Effects of hypnotic, placebo, and salicylic acid treatments on wart progression. Psychosom Med. 1990;52:109-114.
STI in Gay Men Action Group. http://www.whytest.org.
STIGMA Guidelines. http://www.ashm.org.au/uploads/STIGMA_STI_Testing_Guidelines_for_MSM.pdf.
Temple-Smith M, Gifford S. Sexual health: an Australian perspective. Melbourne: IP Communications, 2005.
Tomblin FA, Lucas KH. Lysine for the management of herpes labialis. Am J Health-Syst Pharm. 2001;58(4):298-304.
Venereology Society of Victoria and Australasian Chapter of Sexual Health Medicine, Royal Australasian College of Physicians. National management guidelines for sexually transmissible infections. Melbourne: Venereology Society of Victoria, 2008.
1 Pavlin NL, Parker R, Fairley CK, et al. Take the sex out of STI screening! Views of young women on implementing chlamydia screening in general practice. BMC Infectious Disease. 2008;8:62.
2 World Health Organization. Gender and reproductive health. Sexuality. Online. Available: www.who.int/reproductive-health/gender/sexual_health.html#2.
3 World Health Organization. Reducing risks, promoting healthy life; 2002.
4 Australian Institute of Health and Welfare. Australia’s Health 2006. AIHW Cat. no. AUS 73. Canberra: AIHW, 2006;146.
5 Richters J, Rissel C. Doing it down under: the sexual lives of Australians. Crows Nest, New South Wales: Allen & Unwin, 2005.
6 Mindel A, Sawleshwarkar S. Condoms for sexually transmissible infection prevention: politics versus science. Sexual Health. 2007;5(1):1-8.
7 Falagas ME, Gregoria IB, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrobial Chemotherapy. 2006;58(2):266-272.
8 Watson C, Calabretto H. Comprehensive review of conventional and non-conventional methods of management of recurrent vulvovaginal candidiasis. Aus NZ J Obstet Gynaecol. 2007;47:262-272.
9 Martino JL, Vermund SH. Vaginal douching: evidence for risks or benefits to women’s health. Epidemiol Rev. 2002;24(2):110-116.
10 Al-Gurairi FT, Al-Waiz M, Sharquie KE. Oral zinc sulphate in the treatment of recalcitrant viral warts: randomized placebo-controlled clinical trial. Br J Dermatol. 2002;146:423-431.
11 Spanos NP, Williams V, Gwynn MI. Effects of hypnotic, placebo, and salicylic acid treatments on wart regression. Psychosom Med. 1990;52(1):109-114.
12 Marrazzo JM, Koutsky LA, Kiviat NB, et al. Papanicolaou test screening and prevalence of genital human papilloma virus among women who have sex with women. Am J Pub Health. 2001;91:947-952.
13 Martin S. Lesbian sexual health needs. Medical Observer 2009; 9 October. Online. Available: http://www.medicalobserver.com.au/news/lesbian-sexual-health-needs.
14 Australian Government, Department of Health and Ageing. Australasian Contact Tracing Manual, 3rd edn; 2006. Online. Available: http://www.ashm.org.au/images/publications/aust-contact-tracing.pdf.