82 Severe Heart Failure
Heart failure is a very common condition with high mortality and morbidity rates. Data from the Framingham heart study suggest that at 40 years of age, the lifetime risk for congestive heart failure is 21.0% (95% confidence interval [CI], 18.7%-23.2%) for men and 20.3% (95% CI, 18.2%-22.5%) for women.1 The prevalence of heart failure is between 2% and 3% and reaches 10% and 20% in those older than 70 years.2 In the United States, approximately 5 million patients have heart failure, with over 550,000 new cases diagnosed each year.3 Despite improvements in treatment, the overall prevalence of heart failure is increasing because of the aging of the population and better survival following myocardial infarction (MI).3,4 Total heart failure–related costs were about $28 billion in 2005 and consume approximately 2% of national expenditure on health in Europe.5 Patients with severe heart failure often present in extremis, and their condition may deteriorate rapidly, so a sound knowledge of immediate treatment is vital for critical care and emergency physicians. Such patients often respond rapidly to appropriate treatment, making this a very satisfying condition to treat. However, it is important to note that outlook remains poor despite initial clinical improvement.
 Etiology
Etiology
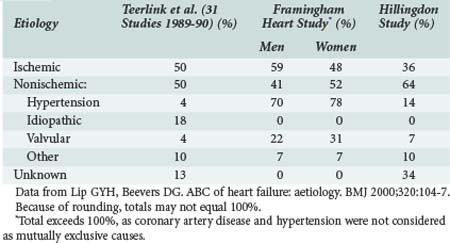
Ischemic heart disease is the most common cause of heart failure, commonly related to previous MI. Although epidemiologic surveys such as the Framingham study suggest a high prevalence of hypertension as the “cause” of heart failure, it is likely that associated ischemic heart disease or arrhythmias also contribute. Other studies have demonstrated similar findings (Table-82-1).
Ischemic Heart Disease
Ischemic heart disease is the most common cause of heart failure in the Western world. Many patients presenting with severe heart failure will give a history of previous MI. However, an episode of severe heart failure may also be the first manifestation of ischemic heart disease, either due to massive MI causing cardiogenic shock6 or as a result of previous silent (or unreported) episodes of ischemia/infarction. It is therefore important to exclude MI in all patients presenting with severe heart failure. Additionally, once the patient is stabilized, adequate secondary preventive strategies are vital to prevent further ischemia or infarction. Some patients with ischemic cardiomyopathy may show evidence of “hibernation” of segments of myocardium,7 and cardiac function in these patients may improve with revascularization (see later discussion).
Dilated Cardiomyopathy
Dilated cardiomyopathy is defined as left ventricular dysfunction of unknown cause. It is therefore a diagnosis of exclusion, and a firm diagnosis of dilated cardiomyopathy can only be made in the presence of a normal coronary angiogram. Intensive investigation of patients with a label of dilated cardiomyopathy may yield a definite cause in at least 50% of cases (Table 82-2). As many as 30% of patients with dilated cardiomyopathy may have a genetic cause of the disease.8
TABLE 82-2 Final Diagnoses in 1230 Patients with Initially Unexplained Cardiomyopathy
| Diagnosis | Number | Percentage |
|---|---|---|
| Idiopathic dilated cardiomyopathy | 616 | 50 |
| Myocarditis | 111 | 9 |
| Ischemic heart disease | 91 | 7 |
| Infiltrative cardiomyopathy | 59 | 5 |
| Peripartum cardiomyopathy | 51 | 4 |
| Hypertension | 49 | 4 |
| Human immunodeficiency virus infection | 45 | 4 |
| Connective tissue disease | 39 | 3 |
| Substance abuse | 37 | 3 |
| Familial | 25 | 2 |
| Valvular disease | 19 | 1.5 |
| Doxorubicin therapy | 15 | 1 |
| Endocrine disorder | 11 | 1 |
| Others | 62 | 5.5 |
Data from Felker GM et al. Underlying causes and long term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077-84.
Diabetes
In addition to the role of diabetes as a risk factor for the development of ischemic heart disease and resultant heart failure, there is evidence for a distinct diabetic cardiomyopathy.8 Current guidelines recognize diabetes as a risk factor for heart failure.8 Hemoglobin A1c levels have been shown to be an independent progressive risk factor for cardiovascular and total mortality and rehospitalization rate in heart failure patients.9,10 All patients presenting with heart failure should be screened for diabetes, both for this reason and so that appropriate secondary prevention can be instituted.
Other Possible Causes or Exacerbating Factors
Patients with a long history of stable heart failure may decompensate as the result of a number of different factors. Intercurrent infection is a common cause of decompensation, and prompt recognition and treatment are important. Arrhythmias are also a common cause for decompensation of a previously stable heart failure patient, and a recent meta-analyses of 16 randomized clinical trials (53,969 patients) revealed that the presence of AF is associated with an adverse effect on total mortality, with an odds ratio of 1.40 (95% CI, 1.32-1.48).11 Another common cause of decompensation is anemia, which is often poorly tolerated in patients with heart failure. A meta-analysis of 34 studies comprising 153,180 patients, of whom 37.2% were anemic, showed that adjusted mortality risk of anemia had a hazard ratio of 1.46 (95% CI, 1.26-1.69).12 Poor prognosis associated with presence of atrial fibrillation or anemia was irrespective of left ventricular systolic function, either preserved or impaired.11,12 It is vital to consider and treat such exacerbating conditions where appropriate.
 Presentations of Severe Heart Failure
Presentations of Severe Heart Failure
Acute Presentation: Pulmonary Edema
Cardiovascular Examination
Examination of the pulse may reveal atrial fibrillation. Patients in sinus rhythm are usually tachycardic, although patients with a history of ischemic heart disease may well be taking beta-blockers, which mask tachycardia. Heart rate appears to be an independent and powerful factor of prognosis in heart failure. In the BEAUTIFUL study, heart failure patients with heart rates of 70 bpm or greater had 34% higher risk for cardiovascular death and 53% higher risk admission to hospital than those with heart rate blow 70 bpm. The study has shown 8% and 15% increments of cardiovascular death and hospital admission for every increase of 5 bpm.13
Subacute Presentation: Shortness of Breath/Peripheral Edema
Many patients with severe heart failure present less acutely with varying combinations of breathlessness and edema. This is often the case in patients with a previous diagnosis of heart failure and can be precipitated by intercurrent infection or withdrawal of diuretic or other medication (by the patient or a physician). In the early stages, edema may be more prominent unilaterally, and this may result in diagnostic difficulty. Such patients may be referred for exclusion of deep venous thrombosis (and it is important to be aware that the two conditions can coexist). These patients often report gradually increasing breathlessness with symptoms of orthopnea (shortness of breath occurring when lying supine) and paroxysmal nocturnal dyspnea (sudden shortness of breath waking the patient from sleep). Patients may resort to sleeping in a chair, leading to additional gravitational edema. Edema of the bowel can lead to reduced appetite, so called “cardiac cachexia,” and further edema from hypoproteinemia. Peripheral edema is therefore often multifactorial in patients with heart failure. Differential diagnoses of peripheral edema are listed in Table 82-3.
Collapse/Cardiac Arrest
Patients with severe heart failure of any cause are at high risk for malignant arrhythmias and thromboembolic disease such as pulmonary embolism. It is therefore not unusual for patients with severe heart failure to present with collapse or cardiac arrest. In such patients, the outlook is extremely poor. Even for patients presenting with ventricular tachycardia or ventricular fibrillation who are successfully cardioverted, the chance of surviving to discharge from hospital is low. Such patients can be considered for implantable cardioverter-defibrillators (see later and Chapter 81). Pulmonary embolism and ventricular arrhythmias are covered in Chapters 62 and 79, respectively, so will not be discussed in detail here.
 Investigations
Investigations
Electrocardiography
All patients presenting with severe heart failure require at least one electrocardiogram (ECG). In cases of diagnostic difficulty, an entirely normal ECG virtually excludes systolic heart failure as the cause of symptoms.14 In heart failure, an ECG is essential to diagnose arrhythmias such as atrial fibrillation, which may complicate management, as well as to look for evidence of myocardial ischemia or infarction and conduction abnormalities such as left bundle branch block or bradycardia due to high-degree atrioventricular block, which may respond to pacing. In patients in whom ischemia is suspected, serial ECGs are recommended, as changes may evolve during the course of the patient’s treatment. Patients with acute severe heart failure should have continuous ECG monitoring during the acute phase, as they are at high risk for malignant ventricular arrhythmias. Patients with biventricular pacemakers (discussed later) in situ may have paced QRS complexes that are narrower than in those with single-chamber right ventricular leads.
Echocardiography
Echocardiography is useful both to determine the extent of left ventricular dysfunction and to identify the cause. In cases of ischemic cardiomyopathy, regional wall motion abnormalities are commonly seen (although these can occasionally occur in cases of cardiomyopathy of other causes). Valve disease is readily identified by echocardiography. Echocardiography can be used to calculate the left ventricular ejection fraction, but in experienced hands, a qualitative assessment of left ventricular function can be equally useful. Some patients presenting with severe heart failure have preserved systolic function, and echocardiography can also be used to assess diastolic function. In the patient presenting with shortness of breath, in whom the cause is unclear, echocardiography can readily determine the presence or absence of systolic heart failure. Although a number of diagnostic criteria have been developed for assessment of the diastolic left ventricular function, their reliability in predicting intracardiac filling pressures is not always satisfactory and needs further development.15 A clear distinction can sometimes be made only by measurements of gas exchange or blood oxygen saturation or by invasive hemodynamic measurements during graded levels of exercise following the clinical stabilization.
Brain Natriuretic Peptide
Natriuretic peptides are currently emerging as a novel test in cases of heart failure. The group includes three structurally related peptides, with variable activity at three distinct natriuretic peptide receptor subtypes, of which two are of potential use in patients with heart failure. Atrial natriuretic peptide is released from the atria in response to wall stretch. Brain natriuretic peptide (BNP), so called because it was first identified in brain tissue, is mainly released by the cardiac ventricles in response to wall stretch.16 All the natriuretic peptides are elevated in acute coronary syndromes and MI, owing to release from myocytes. In addition, decompensated heart failure is associated with elevations of natriuretic peptide levels. Many possible applications for assays of these peptides have been proposed, but at present the most widely accepted indications for use of BNP (which appears to have the best sensitivity/specificity of all the natriuretic peptides) are as follows:
The use of BNP for monitoring progress in heart failure is controversial; some studies have suggested that BNP may be useful to guide treatment. Indeed, many studies have found that BNP levels may have prognostic implications. It is also important to note that BNP levels must be used in conjunction with clinical assessment of the patient, as unexpected values may occur in some patients, such as a high BNP level in a stable patient. Recent findings do not support routine use of the peptides in all dyspneic patients admitted to the emergency department.17 Of particular note is the fact that patients with severe heart failure due to cardiogenic shock may exhibit a paradoxically normal or even low BNP level. It has been suggested that myocytes in such a situation are unable to produce BNP. This theory is supported by studies of serial BNP levels in patients recovering from an episode of cardiogenic shock. An initially low BNP level is followed by a high level as recovery of myocardial function begins, and as recovery continues, the level returns to normal.
However, the measurement of BNP during admission with acute decompensated heart failure may help to assess prognosis and guide therapy following stabilization.18,19 A soluble form of ST2, an interleukin 1 (IL-1) receptor family member, has also been found to be potentially useful for identifying heart failure patients at risk of sudden cardiac death and may provide additional information to BNP.20
 Treatment
Treatment
Acute Treatment
Thromboprophylaxis
Patients with severe heart failure are often poorly mobile due to breathlessness, peripheral edema, and the presence of monitoring and treatment equipment. They are at high risk for the development of deep venous thrombosis and pulmonary embolism. The MEDENOX (prophylaxis in MEDical patients with ENOXaparin) trial, which included 1102 hospitalized patients, including 376 with NYHA class III/IV heart failure, found that 14.9% of placebo-treated patients suffered venous thromboembolism. Importantly, in the group treated with enoxaparin, only 5.5% suffered venous thromboembolism.21 This trial also included patients with other serious medical illnesses including cancer, so this may be an overestimate of the risk of thromboembolism in heart failure. In cases of moderate to severe heart failure, particularly in hospitalized patients, some of this increased risk may be related to immobility, which is a well-known risk factor for deep venous thrombosis. Indeed, in previous years when bed rest was standard treatment for patients with heart failure, the rate of pulmonary embolism was very high. All patients with severe heart failure who are not anticoagulated and in whom there are no contraindications (such as active bleeding) should receive thromboprophylaxis with unfractionated or low-molecular-weight heparin, with the dose adjusted according to the patient’s body weight.
Nesiritide
The natriuretic peptides have a variety of beneficial effects on the heart and circulation, causing diuresis, increasing sodium excretion, and reducing pre- and afterload by causing venous and arterial dilatation. They may also reduce left ventricular remodeling and fibrosis. These attributes have recently led to the therapeutic use of natriuretic peptides in heart failure, and short-term studies have shown that nesiritide infusion is at least as efficacious as standard therapy (dobutamine, milrinone, or glyceryl trinitrate) and is associated with reduced diuretic use in patients with acutely decompensated heart failure.22 Nesiritide (recombinant human BNP) has been approved by the U.S. Food and Drug Administration (FDA) for use in patients with acutely decompensated heart failure in whom systolic blood pressure is above 90 mm Hg. It is given by IV bolus (2 µg/kg) followed by continuous infusion (0.01 µg/kg/min) as an alternative to glyceryl trinitrate. Treatment is usually continued for 24 to 48 hours.
Inotropes
Approximately 80% of patients presenting with acute decompensated congestive heart failure have preserved blood pressure and can therefore receive cardiac load–reducing therapy such as glyceryl trinitrate or nesiritide. However, these treatments are contraindicated in hypotensive patients with heart failure. If such patients do not respond to initial diuretic therapy favorably or show evidence of deterioration, inotropic therapy may be considered. Long-term use of inotropic therapy is likely to be harmful in patients with heart failure,23 but potentially appropriate uses of inotropes include use as temporary treatment of diuretic-refractory acute heart failure decompensations or as a bridge to definitive treatment such as revascularization or cardiac transplantation.
Experimental Agents
A number of novel and promising treatments for acute decompensated heart failure are currently under clinical development. Infusion of cinaciguat (BAY 58-2667), the first of a new class of soluble guanylate cyclase activators, has potent preload- and afterload-reducing effects, thereby increasing cardiac output.24 Administration of relaxin, a natural human peptide that affects multiple vascular control pathways, improved symptoms and reduced cardiovascular death or readmission due to heart or renal failure at day 60 compared with placebo (2.6% [95% CI, 0.4-16.8] versus 17.2% [9.6-29.6]; P = 0.053). Both agents were safe and well tolerated, and further clinical development is warranted.25
Intraaortic Balloon Counterpulsation
Intraaortic balloon counterpulsation is an invasive strategy to preserve coronary flow in the presence of very poor cardiac output. A percutaneous approach is used to position a balloon in the descending aorta. The balloon is inflated during diastole, diverting blood into the coronary arteries. This technique may be used to maintain circulation to the heart and brain at the expense of other tissues as a bridge to transplantation or other surgical intervention. Use of intraaortic balloon counterpulsation is associated with a significant adverse event rate—up to 60% in one study of patients with cardiogenic shock.26 There is no definite evidence that use of intraaortic balloon counterpulsation improves the mortality rate among patients in heart failure; however, a comparison of patients from the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) study showed a significantly lower rate of mortality in those undergoing intraaortic balloon counterpulsation up to 1 day after admission as compared with all other patients (57% versus 67%).27 It has been recently shown that continuous aortic flow augmentation improved cardiac performance and pulmonary capillary wedge pressure, but not clinical outcomes.28
Assisted Ventilation
Noninvasive Ventilation
Continuous positive airway pressure (CPAP) has an accepted role in the treatment of sleep apnea syndromes. It is now recognized that sleep apnea is prevalent in patients with heart failure and may play a role in the development and progression of heart failure. In addition, noninvasive ventilation has favorable effects on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Noninvasive ventilation has been used to treat acute heart failure. Several randomized trials have suggested that use of CPAP results in more rapid increase in PaO2, decrease in PCO2, and lower rates of intubation compared with standard treatment.29 Noninvasive ventilation may be considered in patients with rising PCO2 levels despite adequate medical therapy. Its use results in decreased blood pressure, so it may have a deleterious effect in patients who are already hypotensive. To be used successfully, noninvasive ventilation requires careful attention to mask fitting and close patient observation. It should be used only in a high-dependency setting with appropriately trained staff.
Surgery
Left Ventricular Assist Device
Left ventricular assist devices (LVADs) are surgically implanted devices developed to allow short- or long-term support to the failing left ventricle. Commonly, an inflow cannula receives blood from the left ventricle, which is then pumped out through a cannula in the ascending aorta. Although initially used as a bridge to transplantation, some studies have demonstrated recovery of function, allowing explantation of the device after a period of left ventricular support in certain subgroups of patients together with appropriate pharmacologic therapy.30 LVAD therapy for patients with terminal heart failure but who are not eligible for heart transplantation has been shown in the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial31 to be superior to medical therapy in ameliorating symptoms and to produce a 48% mortality reduction at 2 years’ follow-up. However, the frequency of serious adverse events in the LVAD group was more than twice that in the medical therapy group, mainly due to infection, bleeding, and malfunction of the device. A number of devices are available. Choice depends on availability and local expertise.
New continuous-flow devices are smaller and may be more durable than the pulsatile-flow devices. In one randomized trial,the continuous-flow device improved 2-year survival of patients with severe heart failure by 24% to 58%.32
Revascularization
In recent years, the phenomena of “stunned” and “hibernating” myocardium have been recognized and widely investigated. Hibernating myocardium is defined as poorly functioning myocardium caused by reduced perfusion, which may recover function if perfusion is restored. Stunned myocardium results from an episode of ischemia. The segment of myocardium regains normal blood flow after the episode, but recovery of function is delayed (although recovery occurs spontaneously). In patients with chronic ischemic cardiomyopathy, revascularization may therefore result in improvement in left ventricular function. Patients with cardiogenic shock due to acute MI have a very poor prognosis (see later discussion), and in recent years several studies have addressed the possible benefits of acute revascularization in such patients. Retrospective analysis of the patients from the GUSTO-I study with cardiogenic shock (7.2%) showed that revascularization was associated with decreased mortality rate (overall 30-day mortality, 55%; patients undergoing coronary artery bypass grafting, 29%; patients undergoing percutaneous transluminal coronary angioplasty, 22%).33
The treatments were not allocated randomly, however. The two randomized controlled trials of medical therapy versus revascularization (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock [SHOCK]34 and Swiss Multicenter Angioplasty for SHOCK [SMASH]35) had difficulties in recruitment, and both reported no significant difference in early mortality, although the SHOCK trial did show decreased mortality rate at 6 months in the intervention group. It is important to note that results from the SHOCK trial registry, which showed that patients selected to undergo angiography had better outcomes whether or not they went on to be revascularized, suggest that bias may be involved in the results of these studies. Furthermore, adding surgical ventricular reconstruction to coronary revascularization reduced the left ventricular volume, as compared with revascularization alone, but not the rate of death or hospitalization for cardiac causes.36 Current evidence certainly does not support aggressive revascularization of all patients with cardiogenic shock, but revascularization may be appropriate in selected patients.
Stabilization and Chronic Treatment
Angiotensin-Converting Enzyme Inhibitors/Angiotensin II Receptor Blockers
Multiple large randomized trials have shown that ACE inhibitors (e.g., ramipril, perindopril, lisinopril) are of unequivocal benefit in patients with heart failure and asymptomatic left ventricular dysfunction.37 All patients should be started on an ACE inhibitor as soon as possible after a diagnosis of heart failure has been made—this is almost always during the index admission. Most patients will require gradual introduction of the drug, with monitoring of blood pressure and renal function. Effort should be made to achieve the highest tolerated dose of the chosen ACE inhibitor.
Some patients are unable to tolerate ACE inhibitors as a consequence of cough due to elevated levels of bradykinin, which is usually degraded by ACE. An alternative in such patients are angiotensin II receptor blockers, which directly block the angiotensin II receptor and do not cause bradykinin buildup. There is not yet sufficient evidence on angiotensin II receptor blockers to recommend them over ACE inhibitors as first-line therapy in heart failure patients. However, the recent Candesartan in Heart Failure—Assessment of Reduction in Mortality (CHARM) study demonstrated that in patients unable to tolerate ACE inhibitors, the angiotensin II receptor blocker, candesartan, provided similar mortality benefit.38 Another arm of the CHARM study (CHARM-ADDED) showed additional benefit (reduction in the primary endpoint of cardiovascular death or hospital admission for congestive heart failure) when candesartan was added to ACE inhibitors.
Recently the Heart Failure End Point Evaluation of Angiotensin II Antagonist Losartan (HEAAL) trial showed that triple increase of losartan dose (150 mg daily compared with traditional 50 mg) is required to achieve maximal reduction of rate of death or admission in patients with heart failure.39
Beta-Blockers
For many years, beta-blockers were thought to be harmful in patients with heart failure because of their negative inotropic effect. More recently, however, several large randomized trials have demonstrated consistent benefit of beta-blockers such as carvedilol,40 bisoprolol,41 and metoprolol.42 Beta-blockers are indicated in patients with stabilized heart failure and are rarely started during the index admission. Their use involves careful dose titration, best supervised in a specialist heart failure clinic. The degree of heart rate reduction is more important than the maximal dose of beta-blocker achieved, and for every heart rate reduction of 5 bpm, there is an 18% reduction in the risk for death (but no significant relationship between all-cause mortality and beta-blocker dosing).39
Aldosterone Inhibitors
The landmark Randomised Aldactone Evaluation Study (RALES) showed that in patients with severe heart failure, spironolactone reduced mortality by 30%.43 More recently, a more selective aldosterone inhibitor, eplerenone, has been developed, which (because of its lack of action at sex hormone and glucocorticoid receptor sites) lacks the unpleasant side effects of spironolactone, such as painful gynecomastia. The recent EPHESUS study, which recruited 6642 post-MI patients with left ventricular ejection fraction less than 40% and clinical heart failure and randomized them to receive eplerenone or placebo (in addition to otherwise optimized medical therapy), demonstrated a 15% reduction in all-cause mortality among the eplerenone group after a mean follow-up period of 16 months.44 It is likely that aldosterone antagonists will be used increasingly in the management of patients with chronic heart failure.
Aldosterone blockers may cause hyperkalemia, particularly in combination with ACE inhibitors; patients on this combination should have regular renal function testing. In the EPHESUS study, the eplerenone added to standard therapy significantly improved outcomes, without an excess risk of hyperkalemia when periodic monitoring of serum potassium was performed.45
Antithrombotic Therapy
Patients with heart failure and atrial fibrillation have clear indications for anticoagulation with adjusted-dose warfarin. There is no clear evidence for the use of antithrombotic therapy in patients with heart failure in sinus rhythm, although such patients fulfill Virchow’s triad (abnormal flow, abnormal vessel wall, abnormal blood constituents) for a prothrombotic state. Recently completed trials also failed to demonstrate benefits of warfarin over antiplatelet agents in heart failure patients.46
Digoxin
Digoxin therapy in patients with heart failure in sinus rhythm (i.e., for inotropic effect) is common practice in North America but is less frequently used in Europe. Evidence of benefit is somewhat limited. Withdrawal of digoxin from patients with symptomatic heart failure resulted in increased risk of heart failure decompensation.47 The Digitalis Investigation Group (DIG) trial48 demonstrated no difference in survival associated with the use of digoxin. A reduction in the risk of death from progressive heart failure in the DIG trial was balanced by an increase in the risk of sudden cardiac death. Digoxin may therefore be considered as additional therapy for patients on ACE inhibitors and beta-blockers but is not an alternative to these drugs.
Cardiac Resynchronization Therapy
Patients with heart failure may exhibit dyssynchronous contraction of the left ventricle resulting from abnormal electrical conduction pathways. Typically this results in septal contraction occurring some time before contraction of the free wall of the left ventricle. Such dyssynchronous contraction results in significant circulation of blood in the left ventricular cavity, rather than forward flow of blood. The use of biventricular pacing to restore synchronous contraction of the left ventricle (cardiac resynchronization therapy) has increased in popularity in recent years. However, the optimal method for selecting patients for cardiac resynchronization therapy is unclear. Current guidelines use duration of the ECG QRS complex, but recent studies have shown that some patients with narrow QRS complexes or even less advanced heart failure (i.e., with NYHA I-II functional class) may benefit from cardiac resynchronization therapy, and equally, not all patients with wide QRS complexes benefit.49 Echocardiographic evidence of dyssynchronous contraction in combination with the ECG might prove to be a better method of selecting candidates for cardiac resynchronization therapy. More recent findings indicate that the benefit of cardiac resynchronization therapy can be maximized by simultaneous implantation of a defibrillator.50,51
Arrhythmia Therapy
Ventricular Arrhythmias
Patients with heart failure frequently suffer from sudden death. Although it is now recognized that some episodes of sudden death are caused by thrombosis such as pulmonary embolism, it is clear that malignant arrhythmias are a common cause of death in heart failure. Surprisingly, therefore, multiple trials of a variety of antiarrhythmic drugs in patients with heart failure have failed to show a mortality benefit (amiodarone)52 or have even shown a worsening of mortality (e.g., flecainide).53 Routine use of antiarrhythmic drugs in patients with heart failure is therefore not recommended. In contrast, recent studies involving the use of ICD devices in patients with reduced ejection fraction following MI have shown reduced mortality. The recent COMPANION study,54 which compared optimal medical treatment alone to optimal medical treatment plus cardiac resynchronization therapy plus or minus ICD therapy, found that combined cardiac resynchronization therapy/ICD reduced mortality but not hospitalization as compared with cardiac resynchronization therapy alone. At present, routine use of implantable cardioverter defibrillators (which in any case would be prohibitively expensive in most countries) in all heart failure patients cannot be recommended. Current indications for ICD therapy in heart failure are listed in Table 82-4.
TABLE 82-4 Indications for Implantable Cardioverter-Defibrillator Therapy in Patients with Heart Failure
|
Syncope of unknown origin with inducible ventricular tachycardia or ventricular fibrillation at electrophysiologic study
|
Other Therapies
Anemia is common in heart failure and associated with a worse prognosis, but interventions directed at increasing hemoglobin levels (e.g., using erythropoietin) have shown inconsistent results. However a meta-analysis of randomized clinical trials suggests that administration of erythropoiesis-stimulating proteins is associated with a significantly lower risk of heart failure–linked hospitalizations.55 Similar benefits were seen with IV ferric carboxymaltose.56
Although uniform conclusions cannot be drawn at present, statin use in patients with heart failure may be indicated. Some data are suggestive of potential benefits of administration of atorvastatin and possible simvastatin in terms of the prognosis, while rosuvastatin only appears to be of use in patients with heart failure due to ischemic heart disease who have relatively low levels of BNP.57 However, treatment with statins is probably useful in those with hypercholesterolemia.
Of interest, long-acting testosterone administration has been shown to improve exercise capacity, muscle strength, and glucose metabolism in men with moderately severe heart failure, providing the basis for further development in this direction.58
 Further Management: Specialist Heart Failure Clinic
Further Management: Specialist Heart Failure Clinic
Patients with heart failure are at high risk of further admissions and sudden death. Careful follow-up and adequate secondary prevention using the drugs and devices detailed here is essential to reduce the risk of readmission and other complications of heart failure. Ideally, such patients should be followed in a specialist heart failure clinic, with access to a cardiologist specializing in heart failure, specialist heart failure nursing, and access to investigations such as echocardiography, cardiac catheterization, and BNP. Nurse-led clinics are ideal for dose titration of beta-blockers and ACE inhibitors and also provide opportunities for monitoring of fluid status and symptoms. A recent meta-analysis has shown that remote patient monitoring may facilitate reduction of deaths and hospitalizations.59 This can take place via different methods, including regular structured telephone contact between patients and healthcare providers and electronic transfer of physiologic data using remote-access technology via remote external, wearable, or implantable electronic devices. Intriguingly, patient self-monitoring of atrial pressures with implanted investigational left atrial pressure monitoring, followed by appropriate individualized adjustments of the therapy guided by these pressures, was associated with highly significant improvement of event-free survival over a median follow-up of 25 months (hazard ratio, 0.16 [95% CI, 0.04-0.68], P = 0.012).60
 Prognosis
Prognosis
Heart failure has a poor prognosis—diagnosis of chronic heart failure is associated with a mortality rate worse than that of many cancers.61 As noted earlier, patients with severe heart failure often present in extremis but may respond rapidly to prompt effective management. However, their inpatient course is associated with a high risk of complications such as thromboembolism (particularly in the presence of atrial fibrillation) and sudden death, even in patients who show signs of recovery from their initial event. Close follow-up and secondary preventive measures are essential to improve prognosis in this high-risk group.
Key Points
ESC Committee for Practice Guidelines (CPG)Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson Pa, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Eur Heart J. 2008;29:2388-2442.
Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-90.
Halperin JL, the Executive Steering Committee, SPORTIF III and V Study Investigators. Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: rationale, objectives, and design of a pair of clinical studies and baseline patient characteristics (SPORTIF III and V). Am Heart J. 2003;146:431-438.
MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
1 Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072.
2 Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of CardiologyDickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Eur Heart J. 2008;29:2388-2442.
3 American Heart Association. Heart Disease and Stroke Statistics–2005 Update. Dallas, TX: American Heart Association; 2005.
4 Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29-34.
5 Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4:361-371.
6 Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction: Etiologies, management and outcome. A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36(3 Suppl A):1063-1070.
7 Dutka DP, Camici PG. Hibernation and congestive heart failure. Heart Fail Rev. 2003;8:167-173.
8 Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-90.
9 Sack MN, Yellon DM. Insulin therapy as an adjunct to reperfusion after acute coronary ischemia: A proposed direct myocardial cell survival effect independent of metabolic modulation. J Am Coll Cardiol. 2003;41:1404-1407.
10 Gerstein HC, Swedberg K, Carlsson J, et al. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699-1704.
11 Mamas MA, Caldwell JC, Chacko S, et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676-683.
12 Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818-827.
13 Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817-821.
14 Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.
15 Mullens W, Borowski AG, Curtin RJ, et al. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62-70.
16 Cowie MR, Jourdain P, Maisel A, et al. Clinical applications of B-type natriuretic peptide (BNP) testing. Eur Heart J. 2003;24:1710-1718.
17 Schneider HG, Lam L, Lokuge A, et al. B-type natriuretic peptide testing, clinical outcomes, and health services use in emergency department patients with dyspnea: a randomized trial. Ann Intern Med. 2009;150:365-371.
18 Pfisterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383-392.
19 Berger R, Moertl D, Peter S, et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55:645-653.
20 Pascual-Figal DA, Ordoñez-Llanos J, Tornel PL, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174-2179.
21 Turpie AG. Thrombosis prophylaxis in the acutely ill medical patient: Insights from the prophylaxis in MEDical patients with ENOXaparin (MEDENOX) trial. Am J Cardiol. 2000;86:48M-52M.
22 Colucci WS, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of congestive cardiac failure. N Engl J Med. 2000;343:246-253.
23 Dies F. Intermittent dobutamine in ambulatory patients with chronic cardiac failure. Br J Clin Pract. 1986;40(suppl):37-39.
24 Lapp H, Mitrovic V, Franz N, et al. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation. 2009;119:2781-2788.
25 Teerlink JR, Metra M, Felker GM, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429-1439.
26 Allen BS, Rosenkranz E, Buckberg GD, et al. Studies on prolonged acute regional ischaemia VI. Myocardial infarction with left ventricular power failure. J Thorac Cardiovasc Surg. 1989;98:691-703.
27 Anderson DR, Ohman M, Holmes DR, et al. Use of intraaortic balloon counterpulsation in patients with cardiogenic shock: Observations from the GUSTO-I study. J Am Coll Cardiol. 1997;30:708-715.
28 Greenberg B, Czerska B, Delgado RM, et al. Effects of continuous aortic flow augmentation in patients with exacerbation of heart failure inadequately responsive to medical therapy: results of the Multicenter Trial of the Orqis Medical Cancion System for the Enhanced Treatment of Heart Failure Unresponsive to Medical Therapy (MOMENTUM). Circulation. 2008;118:1241-1249.
29 Yan AT, Bradley TD, Liu PP. The role of continuous positive airways pressure in the treatment of congestive heart failure. Chest. 2001;120:1675-1685.
30 Hon JK, Yacoub MH. Bridge to recovery with the use of left ventricular assist device and clenbuterol. Ann Thorac Surg. 2003;75(6 Suppl):S36-S41.
31 Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group: Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435-1443.
32 Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241-2251.
33 Holmes DR, Bates ER, Kleiman NS, et al. Contemporary reperfusion therapy for cardiogenic shock: The GUSTO-1 trial experience. for the GUSTO-1 Investigators. J Am Coll Cardiol. 1995;26:668-674.
34 Hochman JS, Boland J, Sleeper LA, et al. Current spectrum of cardiogenic shock and effect of early revascularisation on mortality: Results of an international registry. and the SHOCK Registry Investigators. Circulation. 1995;91:873-881.
35 Urban P, Stauffer JC, Bleed D, et al. A randomized evaluation of early revascularization to treat shock complicating acute myocardial infarction. The (Swiss) Multicenter Trial of Angioplasty for Shock (SMASH). Eur Heart J. 1999;20:1030-1038.
36 Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009 Apr 23;360(17):1705-1717.
37 Garg R, Yusuf S, for the Collaborative Group on ACE Inhibitor Trials. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995;273:1450-1456.
38 Granger CB, McMurray JJ, Yusuf S, et al. CHARM Investigators and Committees: Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet. 2003;362:772-776.
39 Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840-1848.
40 Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study. N Engl J Med. 1996;334:1349-1355.
41 CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II. Lancet. 1999;353:9-13.
42 MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
43 Pitt B, Zannad F, Remm WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. for the Randomised Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717.
44 Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. N Engl J Med. 2003;348:1309-1321.
45 Pitt B, Bakris G, Ruilope LM, et al. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation. 2008;118:1643-1650.
46 Massie BM, Collins JF, Ammon SE, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616-1624.
47 Packer M, Cheorhiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE study. N Engl J Med. 1993;329:1-7.
48 The Digitalis Investigation Group (DIG). The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525-533.
49 St John Sutton M, Ghio S, Plappert T, et al. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation. 2009;120:1858-1865.
50 Anand IS, Carson P, Galle E, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119:969-977.
51 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
52 Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: Meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350:1417-1424.
53 Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781-788.
54 Bristow MR, Feldman AM, Saxon LA, et al. Heart failure management using implantable devices for ventricular resynchronisation: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial. J Card Fail. 2000;6:276-285.
55 van der Meer P, Groenveld HF, Januzzi JLJr, et al. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95:1309-1314.
56 Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436-2448.
57 Cleland JG, McMurray JJ, Kjekshus J, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). J Am Coll Cardiol. 2009;54:1850-1859.
58 Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919-927.
59 Klersy C, De Silvestri A, Gabutti G, et al. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54:1683-1694.
60 Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086-1095.
61 Gillum RF. Heart and stroke facts. Am Heart J. 1993;126:1042-1047.