Chapter 122 Serenoa repens (Saw Palmetto)
Serenoa repens (family: Arecaceae)
Common names: saw palmetto, palmetto scrub, Sabal serrulata
 General Description
General Description
Serenoa repens is a small palm tree native to the West Indies and southeastern United States, particularly Florida. The deep red-brown to black fruits, the medicinal parts, are wrinkled and oblong, measuring 0.5 to 1 in. in length with a diameter of 0.5 in. 1
 Chemical Composition
Chemical Composition
Saw palmetto berries contain about 1.5% of a fruity-smelling oil containing saturated and unsaturated fatty acids and sterols.1 About 63% of this oil is composed of free fatty acids, including capric, caprylic, caproic, lauric, palmitic, and oleic acids. The remaining portion is composed of ethyl esters of these fatty acids and sterols, including beta-sitosterol and its glucoside. The lipid-soluble compounds are thought to be the major pharmacologic components. Other components of the berries are proanthocyanidins, carotenes, lipase, tannins, and sugars.
 History and Folk Use
History and Folk Use
The American Indians, and later eclectic and naturopathic physicians, used saw palmetto berries in the treatment of genitourinary tract disturbances and as a tonic to support the body nutritionally.2,3 This substance was strongly recommended as a remedy for symptoms of benign prostatic hyperplasia as early as 1919.4 It was used in men to increase the function of the testicles and relieve irritation in mucous membranes, particularly those of the genitourinary tract and prostate. Saw palmetto has been used in women with disorders of the mammary glands; long-term use was reputed to cause the breasts to enlarge slowly.2 Many herbalists have regarded saw palmetto as an aphrodisiac.1
 Pharmacology
Pharmacology
A standardized liposterolic (fat-soluble), saw palmetto berry extract has demonstrated numerous pharmacologic effects relating to its primary clinical application in the treatment of benign prostatic hyperplasia (BPH), a common disorder of the prostate gland. Saw palmetto extract affects BPH through multiple mechanisms, including inhibition of the intraprostatic conversion of testosterone to DHT and of its intracellular binding and transport, antiestrogenic, and receptor site–binding effects.5–7
Estrogen may contribute to BPH because it inhibits the hydroxylation and subsequent elimination of DHT. Serenoa appears to inhibit the activity of estrogen in the prostate. For example, in a double-blind study of 35 men with BPH, 18 were given the saw palmetto extract at 160 mg twice daily and 17 were given placebo.5 At the end of the 90-day study, the men receiving the saw palmetto extract had significantly lower cytosol and receptor values for estrogen and progesterone than the placebo group. The results imply that at least part of the efficacy of the saw palmetto extract is due to its antiestrogenic effect.
Preliminary analysis of the extract demonstrates that separate fractions are responsible for the antiandrogenic and antiestrogenic effects. Researchers in this study said, “It cannot be excluded, however, that the primary effect is antiestrogenic and that the inactivation of androgen receptors and progesterone receptors and of the 5-α-reductase activity is secondary to the estrogen receptor blockade.”7
Serenoa standardized extracts do not affect systemic levels of androgens, follicle-stimulating hormone, or luteinizing hormone in men with BPH.8 This may help explain the relatively low incidence of adverse effects of this substance in clinical trials. These findings do not, however, rule out localized effects of saw palmetto on androgen or estrogen effects in other tissues of the body.
Various locally produced growth factors also play a role in the pathogenesis of BPH, and liposterolic extracts of Serenoa block the ability of one of them, basic fibroblast growth factor, to induce prostatic hyperplasia in vitro.9 High prolactin levels may also stimulate prostate hyperplasia; Serenoa extracts interfere with this process in rats but the drug finasteride does not.10
Saw palmetto extracts exert antispasmodic effects on smooth muscle. Rat smooth muscle was originally shown to be inhibited by two Serenoa extracts owing to inhibition of calcium ion influx.11 A later study found that Serenoa extract but not pumpkin seed extract, stinging nettle root extract, or β-sitosterol consistently inhibited human α1-adrenergic receptors in vitro.12 Whether this effect is relevant clinically is still unknown.
The standardized extract has demonstrated antiedematous effects, and the polysaccharide components have been shown to have immunostimulatory effects.13,14 Serenoa extract and myristoleic acid induced apoptosis and necrosis in an androgen-sensitive human prostate cancer cell line in vitro.15
 Clinical Applications
Clinical Applications
Benign Prostatic Hyperplasia
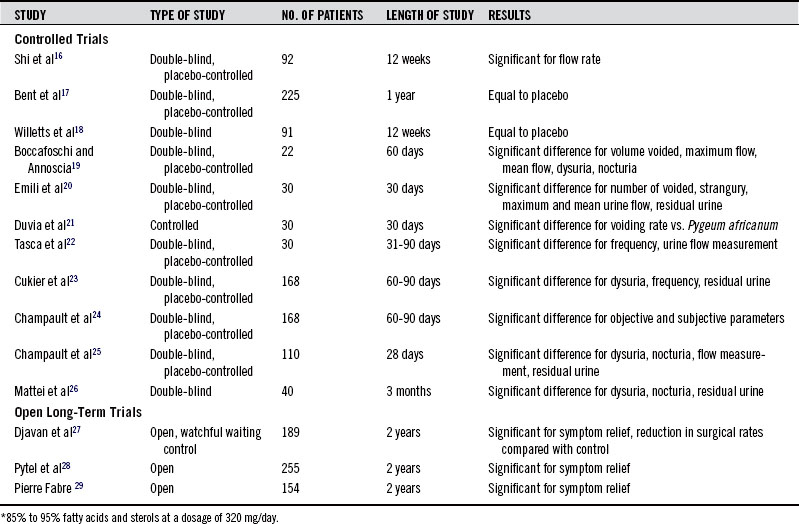
The major symptoms of BPH (increased urinary frequency, nighttime awakening to empty the bladder, and reduced force and caliber of urination; see Chapter 152), have been shown to be significantly improved by saw palmetto extract in more than a dozen double-blind placebo-controlled clinical trials (summarized in Table 122-1).16–29 At least three meta-analyses have combined data from these and other trials and concluded that, despite some limitations in trial design, Serenoa extracts have been shown to reduce symptoms of BPH and increase urine flow in comparison with placebo without affecting prostate volume.30–33 One of these analyses also concluded that Serenoa extracts are as effective as the 5-α-reductase–inhibiting drug finasteride (Proscar), with significantly fewer adverse effects.34 In the most recent meta-analysis, it was concluded that saw palmetto extracts were not superior to placebo for symptoms of BPH but were just as effective as finasteride and tamsulosin.33 However, this analysis was strongly affected by a single negative trial involving 225 men with moderate to severe BPH randomized to receive Serenoa extract 160 mg twice daily or placebo.17 After 1 year of treatment, there was no significant difference in the scores on the American Urological Association Symptom Index and the maximal urinary flow rate in men receiving 320 mg per day of the liposterolic Serenoa extract or placebo. Secondary outcome measures, including changes in prostate size, residual urinary volume after voiding, quality of life, laboratory values, and the rate of reported adverse effects also did not differ between the two groups. These results may simply indicate that treatment of moderate to severe BPH is likely unresponsive to Serenoa therapy. A large negative clinical trial of Serenoa in 369 men with mild-to-moderate BPH failed to show a benefit for up to 960 mg/day of standardized extracts compared with the placebo.33a
The single largest (n = 1,069) clinical trial of Serenoa extract compared 160 mg of Serenoa twice daily with finasteride 5 mg daily for 26 weeks.34 The two agents were equally effective at improving symptoms (by 37%-39% on average) and increasing peak urine flow (by 38%-41% on average). Although finasteride decreased prostate volume and levels of prostate-specific antigen (PSA) significantly, Serenoa extract had no such effect. Finasteride caused more sexual side effects than saw palmetto.
In a comparison double-blind trial, 542 men with BPH symptoms were randomly assigned to receive either the α blocker tamsulosin or Serenoa extract for 1 year.35 The two groups showed identical levels of improvement in symptoms. Tamsulosin was more frequently associated with ejaculatory disorders than Serenoa extract. In the subset of patients with the most severe BPH in this trial, Serenoa extract was actually statistically significantly superior to tamsulosin at relieving symptoms.36 In a separate double-blind trial involving 329 men with BPH symptoms, tamsulosin combined with placebo was just as effective as tamsulosin combined with Serenoa extract at relieving symptoms.37 Adding Serenoa extract to tamsulosin caused no alteration in adverse effects. A 6-month double-blind trial of Serenoa extract with 60 men found results very similar to those of the prior two studies, although it also demonstrated an equivalent improvement in maximum flow rate between the groups.38
Serenoa may work most effectively in combination with Urtica urens root extract. In a long-term study, the efficacy and tolerability of this combination were investigated in a prospective multicenter trial comprising elderly male patients suffering from lower urinary tract symptoms (LUTS) caused by BPH. A total of 257 patients were randomized to treatment with the combination (320 mg of Serenoa and 240 mg of U. urens extracts per day) or placebo. Following a single-blind placebo run-in phase of 2 weeks, the patients received either the study medication or placebo under double-blind conditions over 24 weeks. Double-blind treatment was followed by an open control period of 24 weeks during which all patients were given the Serenoa and U. urens combination. Outcome measures for treatment efficacy included the assessment of the patients’ LUTS by means of a self-rating questionnaire and a quality-of-life index as well as uroflow and sonographic parameters. Using the International Prostate Symptom Score (I-PSS), patients treated with the Serenoa and U. urens combination exhibited a substantially higher total score reduction after 24 weeks of double-blind treatment than patients of the placebo group (6 points vs. 4 points; P = 0.003), with a tendency in the same direction after 16 weeks. This applied to obstructive as well as to irritative symptoms and to patients with moderate or severe symptoms at baseline. Patients randomized to placebo showed a marked improvement in LUTS (as measured by the I-PSS) after being switched to the Serenoa and U. urens combination during the control period.39
Chronic Prostatitis
Several recent studies indicate that S. repens may have clinical benefit in chronic prostatitis or pelvic pain. One double-blind, randomized trial compared S. repens extract alone with a combination of S. repens, selenium, and lycopene in men with nonbacterial chronic pelvic pain.40 After 8 weeks, the two groups had equivalent improvements in symptoms, whereas only the combination product lowered PSA and levels of white blood cell in urine.
One study used a combination of S. repens extract 160 mg, Urtica dioica root extract 120 mg, quercetin 100 mg, and curcumin 200 mg once daily with the antibiotic prufloxacin 600 mg once daily in men with bacterial prostatitis.41 One fourth of the 143 subjects were randomly assigned to take prufloxacin alone. Treatment lasted 14 days. One month after treatment, patients in the combination group were dramatically more likely (88% vs. 27%) to be symptom free than the antibiotic-only group.
Male-Pattern Baldness
A combination of 400 mg S. repens extract and 100 mg beta-sitosterol daily was found effective for male-pattern baldness in a pilot double-blind placebo-controlled trial.42 The trial lasted 5 months and involved 19 men with mild to moderate baldness. Sixty percent (6 of 10) of the men in the treatment group were rated as having improved by blinded observers, whereas only 11% (1 of 9) of men in the placebo group were rated as improved. Thirty-three percent (3 of 9) of the men in the placebo group showed deterioration compared with none in the treatment group. There were no major adverse effects, although two men originally entered into the study dropped out because of adverse effects (it is not clear from the study report which group these men had been assigned to).
Dosage
The dosage for the liposterolic extract of saw palmetto berries (containing 85%-95% fatty acids and sterols) is 160 mg twice daily or 320 mg daily; the two regimens have been shown to be equally effective.43 A similar dosage using fluid extracts and tinctures would require extremely large quantities of alcohol if the liposterolic components were the primary active constituents. The eclectics used comparatively small dosages of saw palmetto in crude extracts effectively for BPH with apparent clinical success. Ellingwood4 lists a dose as 10 drops to 1 dram of specific S. repens (meaning basically a fresh berry tincture). We are unaware of any published clinical study of a crude extract.
• Crude berries: 10 g twice a day
• Liposterolic extract (standardized at 85% to 95% fatty acids and sterols): 160 mg twice a day or 320 mg once a day
The dosage of fluid extracts or tinctures is typically 3 to 5 mL two to three times per day, although there is a lack of research on this dose form.
 Toxicology
Toxicology
No significant adverse effects have been reported in clinical trials of the saw palmetto berry extract or for saw palmetto berry ingestion; rates of mild adverse effects are no different than those seen with placebo treatment.44 Mild gastrointestinal upset and erectile dysfunction (in approximately 1% of users in clinical trials)3 occasionally occur.
1. Duke J.A. CRC Handbook of medicinal herbs. Boca Raton, FL: CRC Press; 1985:. 118
2. Felter H.W., Lloyd J.U. King’s American dispensatory (1898). Portland, OR: Eclectic Medical Publications (reprint); 1983:. 1750-1752
3. Kuts-Cheraux A.W. Naturae medicina and naturopathic dispensatory. Yellow Springs, OH: Antioch Press; 1953:. 249
4. Ellingwood F. American materia medica, therapeutics and pharmacognosy. Sandy, OR: Eclectic Medical Publications, 1919, reprinted; 1998:. 457-459
5. Carilla E., Briley M., Fauran F., et al. Binding of Permixon, a new treatment for prostatic benign hyperplasia, to the cytosolic androgen receptor in the rat prostate. J Steroid Biochem. 1984;20:521–523.
6. Sultan C., Terraza A., Devillier C., et al. Inhibition of androgen metabolism and binding by a liposterolic extract of “Serenoa repens B” in human foreskin fibroblasts. J Steroid Biochem. 1984;20:515–519.
7. Di Silverio F., D’Eramo G., Lubrano C., et al. Evidence that Serenoa repens extract displays antiestrogenic activity in prostatic tissue of benign prostatic hypertrophy. Eur Urol. 1992;21:309–314.
8. Casarosa C., Cosci di Coscio M., Fratta M. Lack of effects of a liposterolic extract of Serenoa repens on plasma levels of testosterone, follicle-stimulating hormone, and luteinizing hormone. Clin Ther. 1988;10:585–588.
9. Paubert-Braquet M., Cousse H., Raynaud J.P., et al. Effect of the lipidosterolic extract of Serenoa repens (Permixon) and its major components on basic fibroblast growth factor-induced proliferation of cultures of human prostate biopsies. Eur Urol. 1998;33:340–347.
10. Van Coppenolle F., Le Bourhis X., Carpentier F., et al. Pharmacological effect of the lipidosterolic extract of Serenoa repens (Permixon) on rat prostate hyperplasia induced by hyperprolactinemia: comparison with finasteride. Prostate. 2000;43:49–58.
11. Gutierrez M., García de Boto J., Cantabrana B., et al. Mechanisms involved in the spasmolytic effect of extracts from Sabal serrulata fruit on smooth muscle. Gen Pharmacol. 1996;27:171–176.
12. Goepel M., Hecker U., Krege S., et al. Saw palmetto extracts potently and noncompetitively inhibit human alpha-1-adrenoceptors in vitro. Prostate. 1999;38:208–215.
13. Tarayre J.P., Delhon A., Lauressergues H., et al. Anti-edematous action of a hexane extract of the stone fruit of Serenoa repens Bartr. Ann Pharm Fr. 1983;41:559–570.
14. Wagner H., Proksch A. Immunostimulatory drugs of fungi and higher plants. Econ Med Plant Res. 1985;1:113–153.
15. Iguchi K., Okumura N., Usui S., et al. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate. 2001;47:59–65.
16. Shi R., Xie Q.W., Gang X., et al. Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized trial in Shanghai, China. J Urology. 2008;179:610–615.
17. Bent S., Kane C., Shinohara K., et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354(6):557–566.
18. Willetts K.E., Clements M.S., Champion S., et al. Serenoa repens extract for benign prostatic hyperplasia: a randomized clinical trial. BJU Int. 2003;92:267–270.
19. Boccafoschi C., Annoscia S. Comparison of Serenoa repens extract with placebo by controlled clinical trial in patients with prostatic adenomatosis. Urologia. 1983;50:1257–1268.
20. Emili E., Lo Cigno M., Petrone U. Clinical trial of a new drug for treating hypertrophy of the prostate (Permixon). Urologia. 1983;50:1042–1048.
21. Duvia R., Radice G.P., Galdini R. Advances in the phytotherapy of prostatic hypertrophy. Med Praxis. 1983;4:143–148.
22. Tasca A., Barulli M., Cavazzana A., et al. Treatment of obstructive symptomatology caused by prostatic adenoma with an extract of Serenoa repens: double-blind clinical study vs. placebo. Minerva Urol Nefrol. 1985;37:87–91.
23. Cukier J., Ducassou J., Le Guillou M., et al. Permixon versus placebo. C R Ther Pharmacol Clin. 1985;4:15–21.
24. Champault G., Patel J.C., Bonnard A.M. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. Br J Clin Pharmacol. 1984;18:461–462.
25. Champault G., Bonnard A.M., Cauquil J., et al. Medical treatment of prostatic adenoma. Controlled trial. PA 109 vs placebo in 110 patients. Ann Urol (Paris). 1984;18:407–410.
26. Mattei F.M., Capone M., Acconcia A. Serenoa repens extract in the medical treatment of benign prostatic hypertrophy. Urologia. 1988;55:547–552.
27. Djavan B., Fong Y.K., Chaudry A., et al. Progression delay in men with mild symptoms of bladder outlet obstruction: a comparative study of phytotherapy and watchful waiting. World J Urol. 2005;23:253–256.
28. Pytel Y.A., Vinarov A., Lopatkin N., et al. Long-term clinical and biologic effects of the lipidosterolic extract of serenoa repens in patients with symptomatic benign prostatic hyperplasia. Adv Ther. 2002;19(6):297–306.
29. Pierre Fabre. Study 95GP401, 2002. (unpublished study)
30. Boyle P., Robertson C., Lowe F., et al. Meta-analysis of clinical trials of Permixon in the treatment of symptomatic benign prostatic hyperplasia. Urology. 2000;55:533–539.
31. Wilt T.J., Ishani A., Stark G., et al. Saw palmetto extracts for treatment of benign prostatic hyperplasia: a systematic review. JAMA. 1998;280:1605–1609.
32. Boyle P., Robertson C., Lowe F., et al. Updated meta-analysis of clinical trials of Serenoa repens extract in the treatment of symptomatic benign prostatic hyperplasia. BJU Int. 2004;93(6):751–756.
33. Tacklind J., MacDonald R., Rutks I., et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev, 2009;2:CD001423.
33a. Barry M.J., Meleth S., Lee J.Y., et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306(12):1344–1351.
34. Carraro J.-C., Raynaud J.-P., Koch G., et al. Comparison of phytotherapy (Permixon (R)) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. Prostate. 1996 Oct;29(4):231–240.
35. Debruyne F., Koch G., Boyle P., et al. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: a 1-year randomized international study. Eur Urol. 2002;41:497–506.
36. Debruyne F., Boyle P., Calais Da Silva F., et al. Evaluation of the clinical benefit of Permixon an tamsulosin in severe BPH patients–-PERMAL study subset analysis. Eur Urol. 2004;45:773–780.
37. Glemain P., Coulange C., Billebaud T., et al. Tamsulosin with or without Serenoa repens in benign prostatic hyperplasia: the OCOS trial. Prog Urol. 2002;12:395–403.
38. Hizli F., Uygur C. A prospective study of the efficacy of Serenoa repens, Tamsulosin, and Serenoa repens plus Tamsulosin treatment for patients with benign prostate hyperplasia. Int Urol Nephrol. 2007;39:879–886.
39. Lopatkin N., Sivkov A., Walther C., et al. Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms: a placebo-controlled, double-blind, multicenter trial. World J Urol. 2005;23:139–141.
40. Morgia G., Mucciardi G., Galì A., et al. Treatment of chronic prostatitis/chronic pelvic pain syndrome category IIIA with Serenoa repens plus selenium and lycopene (Profluss) versus S. repens alone: an Italian randomized multicenter-controlled study. Urol Int. 2010;84(4):400–406.
41. Cai T., Mazzoli S., Bechi A., et al. Serenoa repens associated with Urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicr Agents. 33, 2009. 549-533
42. Prager N., Bickett K., French N., et al. A randomized, double-blind, placebo-controlled trial to determine in the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J Altern Complement Med. 2002;8:143–152.
43. Braeckman J., Bruhwyler J., Vandekerckhove K., et al. Efficacy and safety of the extract of Serenoa repens in the treatment of benign prostatic hyperplasia: therapeutic equivalence between twice and once daily dosage forms. Phytother Res. 1997;11:558–563.
44. Agbabiaka T.B., Pittler M.H., Wilder B., et al. Serenoa repens (saw palmetto): a systematic review of adverse events. Drug Saf. 2009;32(8):637–647.