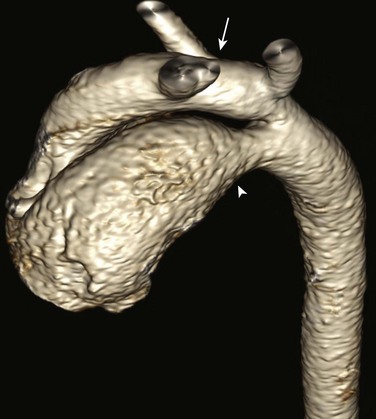
Septal Defects
Atrial Septal Defect
Overview: An atrial septal defect (ASD) is a defect in the atrial septum that allows communication between the right and left atria. Isolated ASDs account for 6% to 10% of all cases of congenital heart disease.
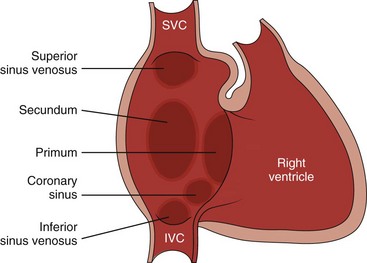
Etiology: Two primary types of ASDs occur and are classified by their relationship to the fossa ovalis (e-Fig. 73-1). Secundum defects (which comprise 80% to 90% of all ASDs) occur in the region of the fossa ovalis. Although a patent foramen ovale is in a similar location, it usually is not considered a septal defect but is the remnant of the normal interatrial communication present during fetal life and is present in 27% to 34% of the general population (Fig. 73-2).1,2 Ostium primum defects occur caudal to the fossa ovalis at the base of the atrial septum, are usually large defects, and almost always are associated with other types of structural heart disease. Additionally, two defects—a sinus venosus septal defect and an unroofed coronary sinus—do not involve the atrial septum but are physiologically equivalent to an ASD because they allow blood to shunt from the left atrium to the right atrium.3,4 Sinus venosus septal defects are located posterior to the fossa ovalis and occur as a result of a deficiency in the sinus venosus septum, which separates the right pulmonary veins from the superior vena cava and from the posterior aspect of the right atrium. This defect is usually located in the wall between the posterior and inferior border of the superior vena cava and the right atrium, and it is commonly associated with anomalous drainage of the right upper, middle, or lower pulmonary veins draining to either the right atrium or superior vena cava (see Chapter 72).5 An unroofed coronary sinus is rare and occurs as a result of a partial or complete absence of the wall between the inferior left atrium and the roof of the coronary sinus. An unroofed coronary sinus generally is associated with drainage of a left superior vena cava to the coronary sinus or left atrium.6
Pathophysiology and Clinical Presentation: Small secundum ASDs may close spontaneously7; however, primum ASDs, sinus venosus septal defects, and coronary sinus defects generally do not decrease in size. Shunt volume is related to the size of the defect, right and left heart compliance, and pulmonary vascular resistance. With larger shunt volumes, right atrial, right ventricular, and pulmonary artery sizes increase. Over time, pulmonary hypertension may develop.
Most infants and young children with ASDs are asymptomatic. ASDs usually are detected at about 6 months of age,8 often during evaluation for a murmur or as an incidental finding on a chest radiograph. Older children with moderate to large ASDs may have symptoms of fatigue and dyspnea. In addition to pulmonary hypertension, older children with ASDs may have atrial tachyarrhythmias or paradoxical strokes, a risk that increases with age.9–12
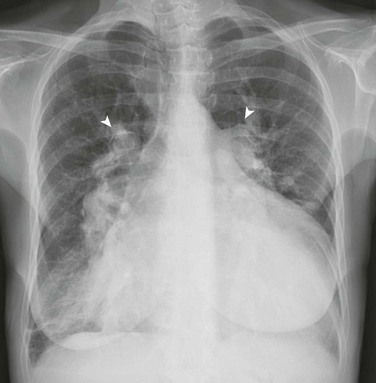
Imaging: Chest radiography in the neonate usually shows that the heart is normal in size and pulmonary flow is normal. Findings in infancy and childhood include mild cardiomegaly related to right atrial and right ventricular enlargement (Fig. 73-3). The left atrium is not enlarged, which distinguishes an ASD from other left-to-right shunt lesions. Usually main pulmonary artery enlargement and increased pulmonary vascularity is found if the pulmonary to systemic flow ratio is greater than two to one. If the patient has significant pulmonary hypertension, enlarged central pulmonary arteries and peripheral pulmonary arterial vessel tapering may be seen (e-Fig. 73-4).
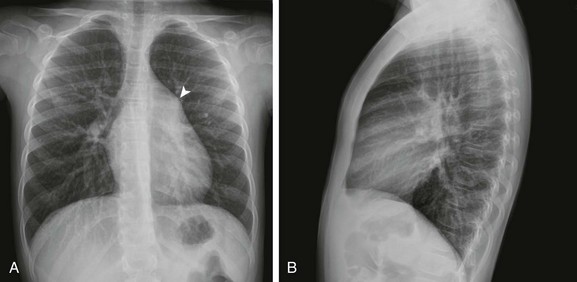
Figure 73-3 Atrial septal defect, secundum type, in a 7-year-old.
Frontal (A) and lateral (B) views of the chest show mild cardiomegaly, a prominent pulmonary artery (arrowhead), and increased pulmonary vascularity without left atrial dilation.
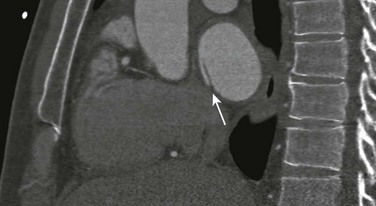
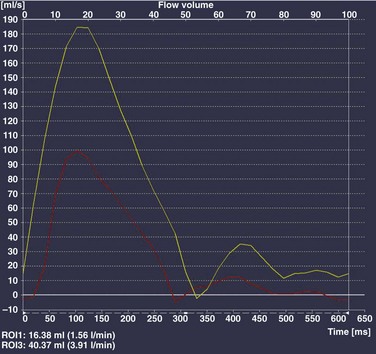
Echocardiography is the imaging modality of choice to determine the location and direction of flow across the defect, to evaluate atrial and ventricular chamber size and ventricular function, and to assess for associated cardiovascular abnormalities. Magnetic resonance imaging (MRI) or computed tomography (CT) also may be used for evaluation of the atrial septum in cases of poor acoustic windows13 or to evaluate the pulmonary veins in patients with suspected sinus venosus defects (Videos 73-1 to 73-3). Left and right ventricular size and quantitative systolic function can be assessed, and right ventricular volume overload is detected as diastolic septal flattening or diastolic bowing of the septum from right to left with severe volume overload. Comparison of right and left ventricular stroke volumes can be used to calculate the ratio of pulmonary to systemic arterial flow (Qp : Qs). Right ventricular pressure is assessed by evaluating the degree of tricuspid regurgitation and septal systolic position. Systolic septal flattening is indicative of elevated right ventricular pressure. Phase contrast MRI can be used to estimate the size of the ASD and to determine the direction and amount of shunting at the atrial level by calculation of Qp : Qs (e-Fig. 73-5).14
Treatment: Closure should be performed in childhood to avoid complications of arrhythmia, right ventricular dysfunction, pulmonary hypertension, and paradoxical embolus.15 ASD closure can be performed surgically or via transcatheter closure; the latter procedure largely has become primary therapy for anatomically favorable secundum defects. After closure, children with ASDs have an excellent prognosis.
Atrioventricular Septal Defect
Overview: Atrioventricular septal defects (AVSDs) account for 4% of all cases of congenital heart disease.16 Most patients with complete AVSD have Down syndrome.17 AVSD also is associated with the visceral heterotaxy syndromes.18,19
Etiology: AVSD results from abnormal development of the embryologic endocardial cushions and produces a spectrum of disease (Fig. 73-6). In its mild form or in partial AVSD, a crescent-shaped defect is found in the inferior portion of the atrial septum immediately adjacent to the atrioventricular (AV) valve, along with an associated “cleft” mitral valve with separate mitral and tricuspid valve orifices. Persons with the complete form have an ostium primum, a large inlet ventricular septal defect (VSD) beneath the plane of the AV valves, and a single or common AV valve, with variable leaflet size, location, and morphology. Most importantly, the common valve has two components that “bridge” the ventricular septum and may form attachments to the septal surface and/or both the right and left sides of the heart.20 In most cases, the common AV valve is shared equally between the right and left ventricles, but the valve orifice may be unequally shared and may favor either the right or left ventricle.19
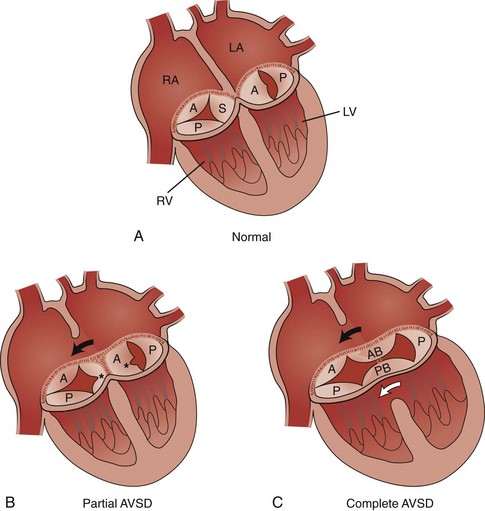
Figure 73-6 Diagrams illustrating the spectrum of atrioventricular septal defect (AVSD).
A, The normal tricuspid valve has three leaflets, anterior (A), posterior (P), and septal (S), and the normal mitral valve has two leaflets, anterior (A) and posterior (P). B, Partial AVSD showing the clefts (asterisks) in the septal leaflet of the tricuspid valve, the anterior leaflet of the mitral valve, and the ostium primum atrial septal defect (ASD) (arrow). C, Complete AVSD showing the common atrioventricular valve with the anterior bridging (AB) and posterior bridging (PB) leaflets, the ostium primum ASD (black arrow), and the inlet ventricular septal defect (white arrow). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (Modified from Park MK. Left-to-right shunt lesions. In Park MK, editor: Pediatric cardiology for practitioners, St. Louis: Mosby Elsevier; 2002.)
Pathophysiology and Clinical Presentation: With complete AVSD, the amount of left-to-right shunting is generally large but is related to the size of the ASD and VSD, right and left heart compliance, and pulmonary vascular resistance. The shunting may be interatrial or interventricular. The cleft mitral valve can lead to significant mitral insufficiency and can exacerbate congestive heart failure.
Infants with complete AVSD present with tachypnea, tachycardia, and signs of congestive heart failure as pulmonary resistance falls during the newborn period. Patients with partial AVSD may be asymptomatic as infants and young children, although they may have symptoms earlier in life if significant associated mitral valve regurgitation is present.21
Imaging: The amount of left-to-right shunting is reflected on the chest radiograph and may reflect the physiology of either the ASD or VSD or both. With complete AVSD, findings include moderate to marked cardiomegaly with right atrial and right ventricular enlargement and increased pulmonary vascularity (Fig. 73-7). Left atrial enlargement may be seen if associated mitral insufficiency is present. Children with large left-to-right shunts commonly have lung hyperinflation, which may be related to an increase in airway resistance from enlarged pulmonary arteries and veins or to an increase in lung volume related to the increase in blood volume.
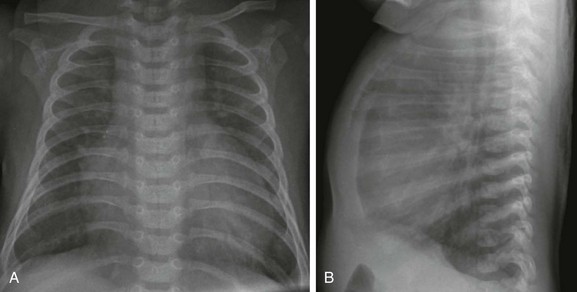
Figure 73-7 Atrioventricular septal defect in a 6-week-old.
Frontal (A) and lateral (B) views of the chest show moderate cardiomegaly with enlargement of the right atrium and ventricle, increased pulmonary vascularity, and lung hyperinflation.
Echocardiography is the primary modality for evaluation of AVSD, but MRI can be used as an adjunct in complex cases, especially when an accurate assessment of right and left ventricular size is needed. Components to evaluate include the ostium primum portion of the atrial septum and the inlet portion of the ventricular septum, the AV valve leaflet morphology and attachments, the papillary muscle architecture, the level and direction of shunting, ventricle size and systolic function, and the presence of outflow tract obstruction or other cardiovascular anomalies (Fig. 73-8).
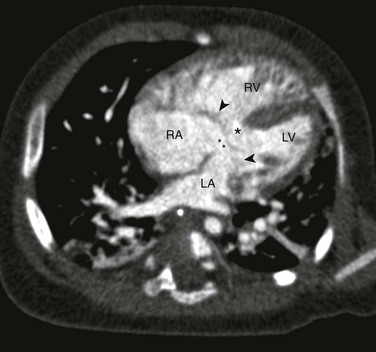
Figure 73-8 Complete atrioventricular septal defect (AVSD).
Four-chamber computed tomography view in an infant with a balanced AVSD shows the ostium primum defect (double asterisks), inlet ventricular septal defect (single asterisk), and common atrioventricular valve (arrowheads) that bridges the right and left sides of the heart. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Treatment: Patients with partial AVSD generally undergo complete surgical repair between 1 and 4 years of age. For patients with complete AVSD, early repair between 2 and 4 months of life is recommended to avoid the development of significant pulmonary hypertension.19 Surgical goals include ASD and VSD closure with creation of two patent and competent AV valves and preservation of the conduction system. Long-term survival after repair for partial and complete AVSD survival is excellent.17,22–24
Ventricular Septal Defect
Overview: Isolated VSD accounts for approximately 20% of all cases of congenital heart disease, with an incidence of approximately two per 1000 live births.25 Isolated VSDs are slightly more common in girls than in boys.26,27 VSD may be isolated or part of a complex congenital heart malformation.28–30
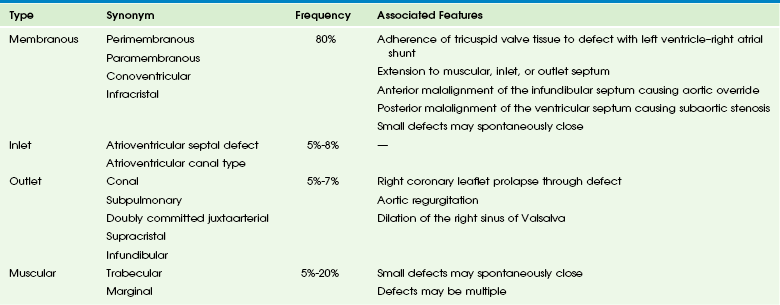
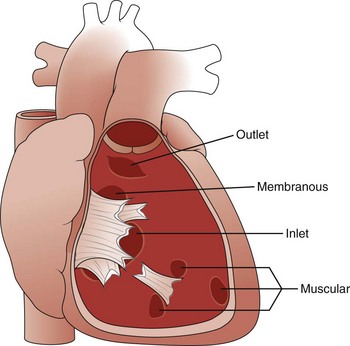
Etiology: The ventricular septum can be divided into four components, and VSDs may involve one or more of these components (Table 73-1). The inlet septum extends from the tricuspid annulus to the septal attachments of the tensor apparatus of the tricuspid valve. AVSD is associated with a defect in this portion of the septum. The muscular septum involves the trabeculated portion of the right ventricle and extends from the tricuspid valve attachments to the apex of the ventricle and up to the septal band. Single or multiple muscular defects can be present in this portion of the septum. The outlet or conal portion of the ventricular septum separates the ventricular outflow tracts and, when viewed from the right ventricle, extends from the septal band to the pulmonary valve. The thin, membranous septum lies adjacent to the anteroseptal commissure of the tricuspid valve on the right side of the heart and to the right and noncoronary cusps of the aortic valve on the left side. Approximately 80% of VSDs involve the area around the membranous septum (e-Fig. 73-9).31,32
Pathophysiology and Clinical Presentation: The physiologic effect of a VSD is determined by its size, right and left heart compliance, and pulmonary vascular resistance. Small defects offer high resistance to flow, and large defects offer low resistance to flow. Large defects subject the pulmonary vasculature to high flow and high pressure, which frequently results in pulmonary hypertension.
Symptoms vary depending on the size of the VSD and the degree of left-to-right shunting. At birth, pulmonary resistance is high, limiting left-to-right shunting. As pulmonary resistance falls during early infancy, left-to-right shunting increases. When shunting becomes significant, failure to thrive, dyspnea, and congestive heart failure may be seen. Irreversible pulmonary hypertension can develop within 2 to 3 years and frequently is seen in young adults.29
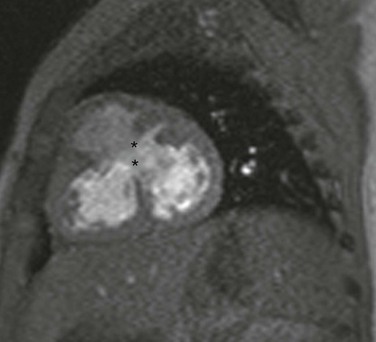
Imaging: Chest radiograph findings vary depending on the size of the VSD. A chest radiograph appears normal in a person with a small VSD. Common findings with moderate to large VSDs include cardiomegaly with enlargement of the left atrium, left ventricle, and pulmonary arteries and increased pulmonary blood flow (Fig. 73-10). Congestive heart failure occurs frequently in infants with a moderate or large defect. Pulmonary hypertension may be evident in older children or young adults.
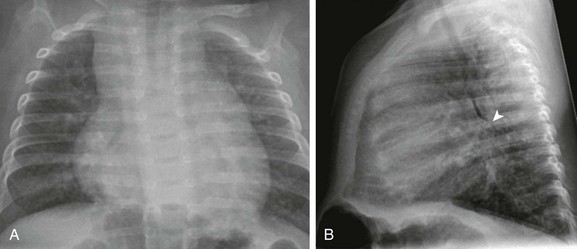
Figure 73-10 Ventricular septal defect in a 3-month-old.
Frontal (A) and lateral (B) views of the chest show right atrial, right ventricular, and left atrial enlargement with posterior displacement of the left main stem bronchus (arrowhead in B) and increased pulmonary vascularity.
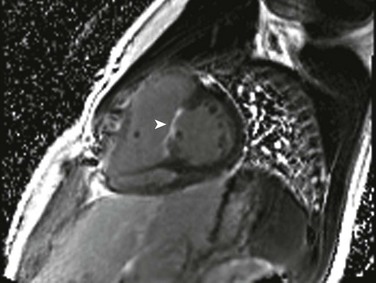
Echocardiography is the imaging method of choice for evaluation of VSDs. Imaging goals include addressing the size, number, and location of VSDs and the degree of left-to-right or right-to-left shunting. Estimates of right ventricular and pulmonary artery pressures and right and left heart volumes are obtained. The tricuspid and aortic valves are evaluated for possible tethering of valve tissue into the borders of the defect. MRI and CT are useful for noninvasive evaluation of VSDs and hemodynamic consequences, with a reported 90% accuracy in detection (Fig. 73-11).33,34 Phase contrast MRI measurements in the aorta and pulmonary artery or a comparison of right and left ventricular stroke volumes can be used to evaluate Qp : Qs, and qualitative assessment of the septal position can be used as a measure of ventricular volume and/or pressure overload.
Treatment: During the first year of life, 80% to 90% of small (<0.5 cm) VSDs become smaller or completely close.27 Operative correction is preferable before 6 months of age in patients with large VSDs to decrease the chance of having irreversible pulmonary hypertension develop. Consideration for early closure of outlet defects is recommended to prevent development of aortic sinus prolapse and progressive valve regurgitation. The results of operative closure are excellent, with a very low risk of morbidity or mortality. VSDs are closed most frequently via a right atrial approach, but transpulmonary artery and transaortic approaches also are used. Transcatheter device closure is an alternative to surgery for selected single or multiple VSDs, usually in the muscular septum, and also has been used intraoperatively (e-Fig. 73-12).
Patent Ductus Arteriosus
Overview: Patent ductus arteriosus (PDA) accounts for approximately 10% of all cases of congenital heart disease.35 Approximately 20% to 30% of premature infants have a PDA, and the incidence increases with increased prematurity.36 Complex congenital heart disease may include PDA with ductal dependent pulmonary or systemic blood flow.
Etiology and Pathophysiology: PDA represents the persistence of the embryologic sixth aortic arch, which most commonly connects the left pulmonary artery with the descending aorta just beyond the origin of the left subclavian artery (see Chapter 62). With a right arch, the ductus arteriosus may be on the right. Prostaglandins maintain ductal patency during fetal life. At birth, increased blood oxygen concentration causes functional constriction of the duct, usually within hours after birth. Ductal closure is delayed in premature infants with respiratory distress and hypoxia, because the immature ductal tissue is less sensitive to oxygen-mediated constriction. In a majority of cases of PDA in term infants, the etiology is unknown.37–39
Clinical Presentation: The amount of left-to-right shunting depends on the length and diameter of the duct and the degree of pulmonary hypertension. Pulmonary hypertension may develop if a large PDA is left untreated. In premature infants without significant lung disease, a PDA may become clinically apparent 24 to 72 hours after birth. Congestive heart failure occurs if the shunt is large. In premature infants with significant lung disease, the prevalence of PDA is greater than 80%. Screening echocardiography may detect a PDA in these infants before clinical manifestations occur. A term infant with a small PDA is generally asymptomatic, and the PDA often is detected because of the presence of a murmur. Term infants with moderate to large PDAs may display poor feeding, irritability, and failure to thrive and may have congestive heart failure.40
Imaging: Pulmonary edema and cardiomegaly may be seen as signs of a PDA on a chest radiograph. In premature infants who exhibit lung infiltrates after the first few days of life, particularly with an increase in heart size, PDA should be considered. Chest radiographs of term infants with significant shunting show increased pulmonary blood flow and cardiomegaly (e-Fig. 73-13).
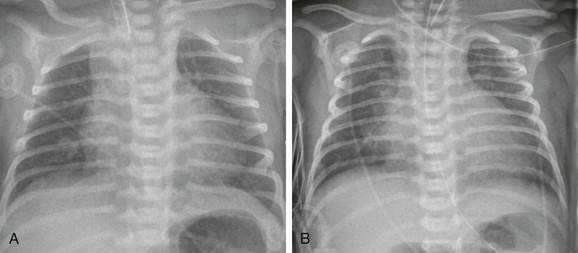
e-Figure 73-13 Sequential chest radiographs in a newborn with a patent ductus arteriosus (PDA).
A, Frontal chest radiograph on day 2 of life shows top normal heart size and normal pulmonary vascularity. B, A chest radiograph on day 8 of life shows an increase in heart size and increased pulmonary vascularity consistent with a PDA diagnosed by echocardiography.
Echocardiography is the standard imaging technique for evaluating PDA. Goals of imaging are to measure the PDA size and diameter, assess the amount of left-to-right or right-to-left shunting and the degree of pulmonary hypertension, and identify possible complicating factors such as a ductal aneurysm. CT or MRI may be used in conjunction with echocardiography to define anatomy in complicated cases and to determine Qp : Qs (Fig. 73-14).41,42
Treatment: Indomethacin and/or ibuprofen therapy often is successful in achieving PDA closure in premature infants, followed by surgical ligation if medical therapy fails. Nonsurgical transcatheter closure is the method of choice for small to moderate PDAs in most term infants.43,44 Larger PDAs in term infants generally are managed surgically. Patient prognosis is excellent if ductal closure is performed between 6 and 24 months of age.45
Aortopulmonary Window
Aortopulmonary window is rare and accounts for 0.2% cases of congenital heart disease.46–49 Aortopulmonary window is the result of incomplete aortopulmonary septation, and as a result, communication occurs between the ascending aorta and pulmonary artery. This defect creates a large, high-pressure, left-to-right shunt that usually is apparent clinically in the first weeks of life and is similar to a large PDA in pathophysiology.
Imaging: Chest radiograph findings include cardiomegaly with left atrial and left ventricular enlargement, increased pulmonary blood flow, and prominence of the ascending aorta and pulmonary artery related to the left-to-right shunt at the level of the great vessels. Imaging is performed to evaluate the anatomy of the aortopulmonary window and its relationship to the aortic and pulmonary valves, to define the coronary anatomy, to estimate pulmonary pressure and ventricular function, to assess Qp : Qs, and to evaluate for other cardiovascular defects. Differentiation from truncus arteriosus is critical and is accomplished by the identification of two separate semilunar valves (Fig. 73-15).
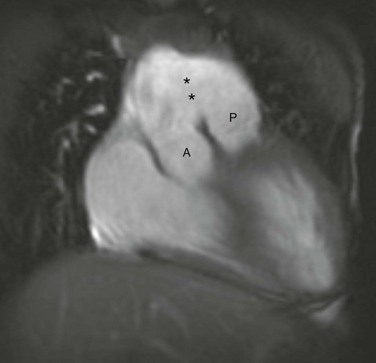
Figure 73-15 Aortopulmonary window.
A coronal steady-state free-precession magnetic resonance image shows separate origins of the aortic root (A) and pulmonary trunk (P) with a large communication (asterisks) between the ascending aorta and the pulmonary trunk. (Courtesy Deborah L. Thompson, MD, Dalhousie University.)
Rojas, CA, El-Sherief, A, Medina, HM, et al. Embryology and developmental defects of the interatrial septum. AJR Am J Roentgenol. 2010;195(5):1100–1104.
Van Praagh, R, Geva, T, Kreutzer, J. Ventricular septal defects: how shall we describe, name and classify them? J Am Coll Cardiol. 1989;14(5):1298–1299.
Wang, ZJ, Reddy, GP, Gotway, MB, et al. Cardiovascular shunts: MR imaging evaluation. Radiographics. 2003;23(Spec No):S181–S194.
References
1. Hagen, PT, Scholz, DG, Edwards, WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59(1):17–20.
2. Schneider, B, Zienkiewicz, T, Jansen, V, et al. Diagnosis of patent foramen ovale by transesophageal echocardiography and correlation with autopsy findings. Am J Cardiol. 1996;77(14):1202–1209.
3. Attenhofer Jost, CH, Connolly, HM, Danielson, GK, et al. Sinus venosus atrial septal defect: long-term postoperative outcome for 115 patients. Circulation. 2005;112(13):1953–1958.
4. Rojas, CA, El-Sherief, A, Medina, HM, et al. Embryology and developmental defects of the interatrial septum. AJR Am J Roentgenol. 2010;195(5):1100–1104.
5. Van Praagh, S, Carrera, ME, Sanders, SP, et al. Sinus venosus defects: unroofing of the right pulmonary veins—anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994;128(2):365–379.
6. Raghib, G, Ruttenberg, HD, Anderson, RC, et al. Termination of left superior vena cava in left atrium, atrial septal defect, and absence of coronary sinus; a developmental complex. Circulation. 1965;31:906–918.
7. Hanslik, A, Pospisil, U, Salzer-Muhar, U, et al. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. Pediatrics. 2006;118(4):1560–1565.
8. Porter, C-BJ, Edwards, JE, Atrial septal defects. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult, 7th ed. Moss, A, Allen, H, eds. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult, Philadelphia, Lippincott Williams & Wilkins, 2008;vol 2.
9. Berger, F, Vogel, M, Kramer, A, et al. Incidence of atrial flutter/fibrillation in adults with atrial septal defect before and after surgery. Ann Thorac Surg. 1999;68(1):75–78.
10. Gatzoulis, MA, Freeman, MA, Siu, SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340(11):839–846.
11. Shah, D, Azhar, M, Oakley, CM, et al. Natural history of secundum atrial septal defect in adults after medical or surgical treatment: a historical prospective study. Br Heart J. 1994;71(3):224–227. [discussion 228].
12. Tikoff, G, Schmidt, AM, Hecht, HH. Atrial fibrillation in atrial septal defect. Arch Intern Med. 1968;121(5):402–405.
13. Holmvang, G, Palacios, IF, Vlahakes, GJ, et al. Imaging and sizing of atrial septal defects by magnetic resonance. Circulation. 1995;92(12):3473–3480.
14. Powell, AJ, Tsai-Goodman, B, Prakash, A, et al. Comparison between phase-velocity cine magnetic resonance imaging and invasive oximetry for quantification of atrial shunts. Am J Cardiol. 2003;91(12):1523–1525. [A1529].
15. Backer, CL, Mavroudis, C. Atrial septal defect, partial anomalous pulmonary venous connection, and scimitar syndrome. In Mavroudis C, Backer CL, eds.: Pediatric cardiac surgery, 3rd ed, Philadelphia: Mosby, 2003.
16. Backer, CL, Mavroudis, C. Atrioventricular canal defects. In Mavroudis C, Backer CL, eds.: Pediatric cardiac surgery, 3rd ed, Philadelphia: Mosby, 2003.
17. Backer, CL, Mavroudis, C, Alboliras, ET, et al. Repair of complete atrioventricular canal defects: results with the two-patch technique. Ann Thorac Surg. 1995;60(3):530–537.
18. Digilio, MC, Marino, B, Toscano, A, et al. Atrioventricular canal defect without Down syndrome: a heterogeneous malformation. Am J Med Genet. 1999;85(2):140–146.
19. Shuhaiber, JH, Ho, SY, Rigby, M, et al. Current options and outcomes for the management of atrioventricular septal defect. Eur J Cardiothorac Surg. 2009;35(5):891–900.
20. Rastelli, GC, Ongley, PA, Kirklin, JW, et al. Surgical repair of the complete form of persistent common atrioventricular canal. J Thorac Cardiovasc Surg. 1968;55(3):299–308.
21. Clapp, S, Perry, BL, Farooki, ZQ, et al. Down’s syndrome, complete atrioventricular canal, and pulmonary vascular obstructive disease. J Thorac Cardiovasc Surg. 1990;100(1):115–121.
22. Bogers, AJ, Akkersdijk, GP, de Jong, PL, et al. Results of primary two-patch repair of complete atrioventricular septal defect. Eur J Cardiothorac Surg. 2000;18(4):473–479.
23. El-Najdawi, EK, Driscoll, DJ, Puga, FJ, et al. Operation for partial atrioventricular septal defect: a forty-year review. J Thorac Cardiovasc Surg. 2000;119(5):880–889. [discussion 889-890].
24. Stellin, G, Vida, VL, Milanesi, O, et al. Surgical treatment of complete A-V canal defects in children before 3 months of age. Eur J Cardiothorac Surg. 2003;23(2):187–193.
25. Wells, WJ, Lindesmith, GG. Ventricular septal defect. In: Arciniegas E, ed. Pediatric cardiac surgery. Chicago: Year Book Medical Publishers, 1985.
26. Mavroudis, C, Backer, CL, Jacobs, JP. Ventricular septal defect. In Mavroudis C, Backer CL, eds.: Pediatric cardiac surgery, 3rd ed, Philadelphia: Mosby, 2003.
27. Garne, E. Atrial and ventricular septal defects—epidemiology and spontaneous closure. J Matern Fetal Neonatal Med. 2006;19(5):271–276.
28. Marino, B, Papa, M, Guccione, P, et al. Ventricular septal defect in Down syndrome. Anatomic types and associated malformations. Am J Dis Child. 1990;144(5):544–545.
29. McDaniel, NL, Gutgesell, HP, Ventricular septal defects. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult, 7th ed. Moss, A, Allen, H, eds. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult, Philadelphia, Lippincott Williams & Wilkins, 2008;vol 2.
30. Musewe, NN, Alexander, DJ, Teshima, I, et al. Echocardiographic evaluation of the spectrum of cardiac anomalies associated with trisomy 13 and trisomy 18. J Am Coll Cardiol. 1990;15(3):673–677.
31. Soto, B, Ceballos, R, Kirklin, JW. Ventricular septal defects: a surgical viewpoint. J Am Coll Cardiol. 1989;14(5):1291–1297.
32. Van Praagh, R, Geva, T, Kreutzer, J. Ventricular septal defects: how shall we describe, name and classify them? J Am Coll Cardiol. 1989;14(5):1298–1299.
33. Didier, D, Higgins, CB. Identification and localization of ventricular septal defect by gated magnetic resonance imaging. Am J Cardiol. 1986;57(15):1363–1368.
34. Rajiah, P, Kanne, JP. Computed tomography of septal defects. J Cardiovasc Comput Tomogr. 2010;4(4):231–245.
35. Mitchell, SC, Korones, SB, Berendes, HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43(3):323–332.
36. Siassi, B, Blanco, C, Cabal, LA, et al. Incidence and clinical features of patent ductus arteriosus in low-birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics. 1976;57(3):347–351.
37. Gittenberger-de Groot, AC. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. 1977;39(6):610–618.
38. Gittenberger-de Groot, AC, Strengers, JL. Histopathology of the arterial duct (ductus arteriosus) with and without treatment with prostaglandin E1. Int J Cardiol. 1988;19(2):153–166.
39. Gittenberger-de Groot, AC, Strengers, JL, Mentink, M, et al. Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol. 1985;6(2):394–404.
40. Moore, P, Brook, MM, Heymann, MA, Patent ductus arteriosus and aortopulmonary window. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult, 7th ed. Moss, A, Allen, H, eds. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult, Philadelphia, Lippincott Williams & Wilkins, 2008;vol 2.
41. Goitein, O, Fuhrman, CR, Lacomis, JM. Incidental finding on MDCT of patent ductus arteriosus: use of CT and MRI to assess clinical importance. AJR Am J Roentgenol. 2005;184(6):1924–1931.
42. Wang, ZJ, Reddy, GP, Gotway, MB, et al. Cardiovascular shunts: MR imaging evaluation. Radiographics. 2003;23(Spec No):S181–S194.
43. Dyamenahalli, U, Smallhorn, JF, Geva, T, et al. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J Am Coll Cardiol. 2000;36(1):262–269.
44. Butera, G, De Rosa, G, Chessa, M, et al. Transcatheter closure of persistent ductus arteriosus with the Amplatzer duct occluder in very young symptomatic children. Heart. 2004;90(12):1467–1470.
45. Hillman, ND, Mavroudis, C, Backer, CL. Patent ductus arterious. In Mavroudis C, Backer CL, eds.: Pediatric cardiac surgery, 3rd ed, Philadelphia: Mosby, 2003.
46. Backer, CL, Mavroudis, C. Surgical management of aortopulmonary window: a 40-year experience. Eur J Cardiothorac Surg. 2002;21(5):773–779.
47. Hew, CC, Bacha, EA, Zurakowski, D, et al. Optimal surgical approach for repair of aortopulmonary window. Cardiol Young. 2001;11(4):385–390.
48. Tkebuchava, T, von Segesser, LK, Vogt, PR, et al. Congenital aortopulumonary window: diagnosis, surgical technique and long-term results. Eur J Cardiothorac Surg. 1997;11(2):293–297.
49. van Son, JA, Puga, FJ, Danielson, GK, et al. Aortopulmonary window: factors associated with early and late success after surgical treatment. Mayo Clin Proc. 1993;68(2):128–133.