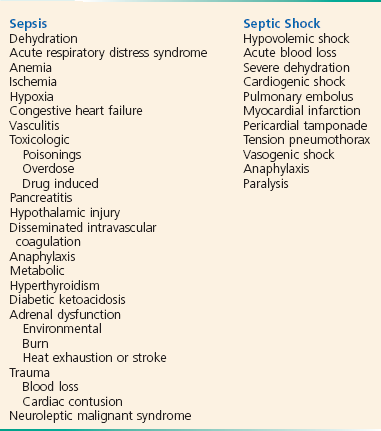
Sepsis Syndromes
Perspective
In 1992, the American College of Chest Physicians/Society of Critical Care Medicine issued a consensus statement to establish uniform criteria defining the sepsis syndromes.1 For the first time, this allowed a common nomenclature for disease classification and systematic comparisons across studies of septic patients. Systemic inflammatory response syndrome (SIRS) is defined as two or more of the following: tachycardia, tachypnea, hyperthermia or hypothermia, high or low white blood cell count, or bandemia; sepsis is the combination of infection plus SIRS; severe sepsis is sepsis plus organ dysfunction; and septic shock is severe sepsis plus hypotension, defined as a systolic blood pressure below 90 mm Hg, not responsive to a fluid challenge (Box 138-1). The importance of this nomenclature is to provide clinicians and researchers with a common classification. Efforts to validate this classification scheme in the emergency department (ED) population have demonstrated that the term sepsis, when it is characterized by fulfilling of the SIRS criteria alone, is perhaps overly sensitive and nonspecific and does not convey an increased mortality risk, although it should prompt further evaluation.2 However, organ dysfunction and shock were shown to be significant predictors of adverse outcome. Newer efforts have proposed the PIRO approach, which may help better understand and prognosticate the severity of illness.3,4 Assessment of predisposing conditions, infection source, response of the host, and organ dysfunction is proposed to help improve classification. The clinician should approach the septic patient with goals to determine who they are (e.g., underlying comorbidities), what infection they have, how they are responding, and where/if organ dysfunction is present.
Bacteremia is often present, but positive cultures are not obligatory in the diagnosis of sepsis. In several prospective studies, only 17 to 27% of patients with sepsis, 25 to 53% of patients with severe sepsis, and 69% of patients with septic shock actually had positive blood cultures.5–7 Culture-negative and culture-positive septic populations have similar outcomes in patients with similar severity of illness.8 Pneumonia, abdominal abscess with viscus perforation, and pyelonephritis are common primary causes of sepsis. Gram-positive organisms account for 25 to 50% of infections, gram-negative organisms for 30 to 60%, and fungi for 2 to 10%. The distribution varies with the study and, more important, with host factors such as the status of the host immune system, age of the patient, recent hospitalizations, and presence of indwelling vascular catheters.
The health status of the host is a crucial risk factor in the development and progression of sepsis.9 Elders and those with multiple comorbidities are overwhelmed more easily by systemic infection. Chemotherapy-induced neutropenia, acquired immunodeficiency syndrome, and steroid dependency increase susceptibility to sepsis. Increased use of indwelling devices such as intravascular catheters, prosthetic devices, and endotracheal tubes contributes to the risk of systemic infection and sepsis.
Epidemiology
Sepsis is now the tenth most common cause of death in the United States.10 It is estimated that 571,000 cases of severe sepsis present to U.S. EDs each year.11 Mortality rates from sepsis are estimated between 20 and 50%.12–14 Sepsis accounts for 4 of 1000 ED visits in the United States.14 The incidence of sepsis as a reason for hospitalization is rising disproportionately among elders compared with the young (Fig. 138-1). The incidence of sepsis in patients younger than 65 years is less than 5 in 100,000, whereas it is 26 in 100,000 in those 65 years or older.12 The cost of caring for septic patients is estimated to be $17 billion per year in the United States. Hospital discharge data have shown that the incidence of sepsis is increasing as identification improves and the population ages. Estimates have suggested that the incidence will rise 1.5% per annum or more. The number of ED visits for sepsis has risen proportionally with the rise in ED volume during the past 15 years.14 Recent research has confirmed the long-held belief that respiratory and genitourinary infections are the most common causes of sepsis.11
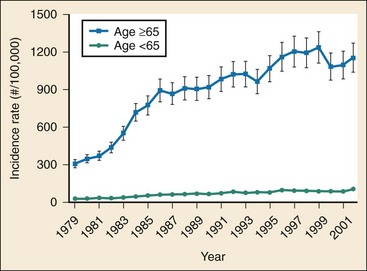
Figure 138-1 Incidence of disease in young and old over time.
Principles of Disease
Sepsis results from the complex interaction of detection molecules, signaling molecules, and numerous inflammatory and coagulation mediators in response to infection. More than 30% of patients with sepsis do not have a definite microbiologic diagnosis.15 Although our understanding of the pathophysiologic process of sepsis has evolved in the past two decades, it remains incomplete.16 The initial host response is to mobilize inflammatory cells, particularly neutrophils and macrophages, to the site of infection. These inflammatory cells then release circulating molecules, including cytokines, which trigger a cascade of other inflammatory mediators that result in a coordinated host response. Synthesis of the components of the cascade is increased at many steps along the pathway. If these mediators are not appropriately regulated, sepsis will occur. In the setting of ongoing toxin release, a persistent inflammatory response occurs with ongoing mediator activation, cellular hypoxia, tissue injury, shock, multiorgan failure, and potentially death.
Mediators of Sepsis
Host response and pathogen characteristics are both important in the pathogenesis of sepsis. More than 100 discrete markers have been identified and attributed to the sepsis cascade, but the true culprits have not been clearly identified.1,17 A pathogen is sensed by pattern recognition receptors, most notably toll-like receptors, located on the surface of the white blood cell.16 The resulting host-pathogen interaction activates the inflammatory and coagulation cascades.18–21 The subsequent inflammatory signaling occurs through cytokines, chemokines, and other soluble mediators, including increased circulating levels of the interleukins IL-1, IL-6, and IL-8 and tumor necrosis factor alpha (TNF-α). Activation of the clotting cascade results in increased D-dimer levels and decreased circulating levels of protein C.22
Instability in vascular tone is becoming increasingly important in understanding of the pathophysiologic mechanism of sepsis. Vasopressin, also known as antidiuretic hormone, is a naturally occurring hormone that is essential for cardiovascular stability. It is produced as a prohormone in the hypothalamus. The hormone is stored in the pituitary gland and released in response to stressors such as pain, hypoxia, hypovolemia, and hyperosmolality.23 In severe sepsis, there is a brief rise in circulating vasopressin levels followed by a prolonged and severe suppression. This pattern of secretion is different from other forms of shock, in which vasopressin levels remain elevated. Vasopressin has numerous physiologic effects, including vasoconstriction of the systemic vasculature, osmoregulation, and maintenance of normovolemia.
Nitric oxide (NO) is a gas that has an important role in septic shock, regulating vascular tone by an indirect effect on smooth muscle cells. NO also contributes to platelet adhesion, insulin secretion, neurotransmission, tissue injury, and inflammation and cytotoxicity. Its half-life is short (6-10 seconds), and it easily diffuses into cells. Although its mechanisms of action are not well understood, it seems to be a key mediator of sepsis. Animal data show that nitric oxide synthase, the enzyme that produces NO, is upregulated in cases of sepsis.24 Enhanced NO production is thought to contribute to the profound vasodilation found in patients in septic shock.
Organ System Dysfunction
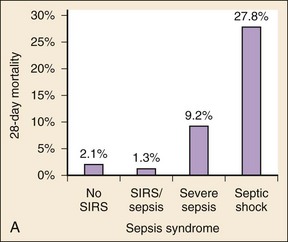
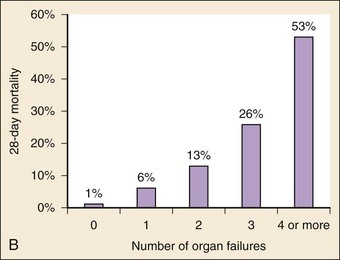
The organ dysfunction that results from sepsis is central to the pathogenesis of the disease. A 3000-patient ED-based study demonstrated that organ dysfunction with septic shock portends increasingly worse outcomes.2 Patients with suspected infection alone had a mortality rate of 2.1%, whereas the presence of SIRS criteria and suspected infection had a mortality rate of only 1.3% (Fig. 138-2A). However, the mortality rate was 9% for those patients with severe sepsis (sepsis plus organ dysfunction) and 28% for those with septic shock. The risk of death from sepsis approximately doubles for each organ that fails. The mortality rate for patients with no organ dysfunctions was 1%; rates for dysfunction of a single organ, two organs, three organs, and four or more organs were 6%, 13%, 26%, and 53%, respectively (Fig. 138-2B).
Neurologic
Patients with sepsis often display neurologic impairment manifested by altered mental status and lethargy, commonly referred to as septic encephalopathy. The incidence has been reported between 10 and 70%. The mortality rate in patients with septic encephalopathy is higher than that in septic patients without significant neurologic involvement. One prospective case series showed that a Glasgow Coma Scale score of less than 13 correlated with an increase in mortality rate from 20 to 50%.25 Although the pathophysiologic process has not been clearly defined, contributing factors include direct bacterial invasion, endotoxemia, altered cerebral perfusion or metabolism, metabolic derangements, multiorgan system failure, and iatrogenic injury. In addition, impaired renal or hepatic function in the absence of overt organ failure has been shown to correlate with encephalopathy.
Cardiovascular
Profound cardiovascular dysfunction is common with sepsis. The cardiovascular dysfunction and failure arise from both direct myocardial depression and distributive shock. Gram-negative, gram-positive, and killed organisms can cause myocardial depression. The direct insults of the toxic mediators as well as the mobilization of host mediators of sepsis produce a distributive shock. Early in sepsis, a hyperdynamic state develops, characterized by increased cardiac output and decreased systemic vascular resistance.26 Although the cardiac output is increased, it is at the expense of ventricular dilation and decreased ejection fraction. Aggressive fluid resuscitation usually increases preload and, secondarily, ejection fraction, thereby improving the cardiac index, even late in shock. Much of the cardiovascular compromise from septic shock is reversible, and normal cardiovascular function usually returns within 10 days.
Pulmonary
Sepsis produces a highly catabolic state and places significant demands on the respiratory system. At the same time, airway resistance is increased and muscle function is impaired. Irrespective of whether pneumonia is the cause of sepsis, the common pulmonary endpoint is acute respiratory distress syndrome (ARDS). ARDS is defined clinically (Box 138-2) and correlates with the pathologic finding of diffuse alveolar damage. The development of ARDS occurs 4 to 24 hours after radiographic abnormalities develop. Because of alveolar-capillary membrane damage, fluid accumulates in the alveoli. Rather than being a diffuse disease, ARDS is a heterogeneous process that results in interspersed damaged and normal alveoli.
Endocrine
An absolute or relative adrenal insufficiency is common in sepsis.27 Depending on the balance of circulating cytokines, augmentation or suppression of the hypothalamic-pituitary axis is possible. IL-1 and IL-6 both activate the hypothalamic-pituitary-adrenal axis. TNF-α and corticostatin depress pituitary function. Other factors that may contribute to adrenal insufficiency in sepsis include decreased blood flow to the adrenal cortex, decreased pituitary function, and decreased pituitary secretion of adrenocorticotropic hormone due to severe stress. As a result of these interactions, the hypothalamic thermoregulatory mechanism may be reset, and temperature lability may develop.
Hematologic
Protein C has been identified as an important modulator of both inflammation and coagulation in patients with sepsis. Impairment of the protein C–dependent anticoagulation pathway is critical to the development of the thrombotic complications of sepsis.28 In healthy humans, protein C is activated by a combination of thrombin and thrombomodulin. The activation of protein C results in downregulation of many portions of the coagulation cascade, including release of tissue factor, inactivation of factor VIIIa and factor Va, and stimulation of fibrinolysis.29 It is possible that protein C activation in early sepsis is impaired because of an inflammatory cytokine–mediated downregulation of thrombomodulin. As a result, a consumptive coagulopathy ensues. This leads to increased fibrin deposition and a resulting upregulation of the fibrinolytic pathway as identified by low plasma levels of the fibrinolytic proteins and increased fibrin split products. This sequence of events leads to consumption of coagulation factors and DIC. In late sepsis, the fibrinolytic system is suppressed.
Novel Concepts
In recent years, there has been increasing evidence that genetics are a risk factor for outcome of sepsis.30,31 The impact of genetics on future treatment modalities for sepsis remains unclear, but the prospect of customized genetic therapy for sepsis is a promising early development.
Clinical Features
The septic patient manifests signs of systemic infection through tachycardia, tachypnea, hyperthermia or hypothermia, and, if severe, hypotension. A septic patient will often have flushed skin with warm, well-perfused extremities secondary to the early vasodilation and hyperdynamic state. Alternatively, the severely hypoperfused patient with an advanced shock state may appear mottled and cyanotic. Very early in the patient’s presentation, vital sign changes such as tachycardia and tachypnea may be the only early indicators of sepsis. If the patient is in shock, a rapid assessment that excludes other causes, such as hypovolemic or cardiogenic shock, is essential to the proper initial treatment. A complete detailed clinical examination will help the physician determine the cause of the shock state (see Chapter 6). The aforementioned are “classic” signs; however, these findings may not be manifested in a septic patient, and signs and symptoms may be subtle.
The respiratory system is the most common source of infection in the septic patient. A history of a productive cough, fevers, chills, upper respiratory symptoms, and throat and ear pain should be sought. Both the presence of pneumonia and the findings of tachypnea or hypoxia have been found to be predictors of death in patients with sepsis.32 Physical examination should also include detailed evaluation for focal infection, such as exudative tonsillitis, sinus tenderness, tympanic membrane injection, and crackles or dullness on lung auscultation. Also, pharyngeal thrush should be noticed as a potential marker of an immunocompromised state.
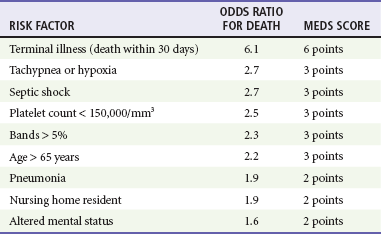
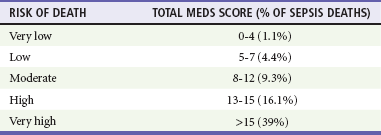
Clinicians must identify the severity of illness in patients with infection and initiate aggressive resuscitation for those patients with the potential of becoming critically ill. Although a patient may meet SIRS criteria, this alone has little predictive value in determining the severity of illness and mortality.2 The Mortality in Emergency Department Sepsis (MEDS) score is a method to risk stratify ED patients with sepsis.33 The MEDS prediction rule assigns point values to specific clinical characteristics (Table 138-1). The total score can be used to assess risk of death. Thus the greater the number of risk factors, the more likely a patient is to die during the hospitalization.
Diagnostic Strategies
Chemistry
An elevated lactate level is associated with inadequate perfusion, shock, and poorer prognosis. One ED-based study showed a progression in mortality rate with increasing venous lactate level: a lactate level of 0 to 2.5 mg/dL was associated with a 5% mortality rate; a lactate level of 2.5 to 4.0 mg/dL, 9% mortality; and a lactate level greater than 4 mg/dL, 28% mortality.34 An arterial blood gas assessment may be helpful in identifying and classifying acid-base disturbances. Metabolic acidosis suggests inadequate tissue perfusion. Liver function tests can be used to identify liver failure or dysfunction. An elevated bilirubin level may suggest the gallbladder as a cause of sepsis. An elevated lipase level may represent pancreatitis as the cause of noninfectious SIRS.
Microbiology
Proper blood, sputum, urine, cerebrospinal fluid, and other tissue culture samples are important in guiding therapy. Although the results of culture are not helpful in the initial management, culture samples should be obtained before or soon after the administration of antibiotics in the patient with sepsis syndrome. The initiation of antibiotic therapy should not be delayed while waiting for culture samples to be obtained. The literature suggests that the yield of initial blood cultures is low (5-10%), but this is probably an artifact of the lack of reliable discriminatory guidelines for obtaining blood culture samples in the ED.6 Among patients with clinical sepsis, only 30 to 60% of patients will have positive cultures.5,7
Special Procedures
The comprehensive protocol of early goal-directed therapy published by Rivers and associates35 showed that a resuscitation strategy guided by the central venous pressure (CVP), an arterial line, and a central venous catheter with continuous central venous oxygen saturation (ScvO2) monitoring capability can reduce mortality in patients with sepsis. However, whether each of these components is required is unclear. A CVP line may guide fluid resuscitation, with a low CVP indicating the need for continued fluid repletion. The ScvO2 monitor measures venous blood saturation, which is a marker of tissue metabolism and extraction. It has been suggested that intermittent monitoring of ScvO2 may suffice, although there are no firm data to support this. The goal is to ensure proper tissue oxygenation, which is routinely achieved by adjustment of oxygen delivery, keeping the ScvO2 greater than 70%. An arterial line can be useful for close monitoring of hypotensive patients, especially when one or more vasopressors are being titrated to maintain an adequate blood pressure; however, no evidence exists to show that it is mandatory. Finally, the Swan-Ganz catheter is not a mandatory part of the sepsis management, although the physiologic measurements may be useful in identifying the cause of shock and guiding fluid and inotropic therapy. Low systemic vascular resistance and high cardiac output are most commonly associated with sepsis, although this may vary with the stage of shock and the individual patient. There are data to suggest that the use of Swan-Ganz catheters does not have a substantial impact on mortality.36
Differential Considerations
The sepsis syndromes represent a spectrum of disease and clinical presentations. SIRS is defined by hemodynamic and laboratory parameters alone, whereas sepsis must include a suspected infectious cause. Often, noninfectious sources can cause a syndrome that mimics sepsis; thus one must keep in mind a broad differential diagnosis when approaching these patients (Box 138-3). A detailed history and physical examination are the first steps in narrowing the differential diagnosis to identify the true source.
Management
Early detection and aggressive management can significantly reduce the mortality from sepsis. The primary goal remains antimicrobial therapy and maintenance of adequate tissue oxygenation and perfusion, but the means by which this is achieved has changed substantially in the past years. With the advent of early goal-directed therapy and therapies such as drotrecogin alfa targeting the cascade leading to septic shock,35,37 there is increasing evidence that the natural history of sepsis can be altered. Initial resuscitation, including appropriate airway management, intravenous access, oxygen, early and appropriate antibiotics, and fluid resuscitation, remains the foundation on which new efforts may be applied.
In the study by Rivers and associates, a protocol for early and aggressive care was used to guide resuscitation in the ED.35 This randomized, double-blind, placebo-controlled study showed a 16% mortality reduction in patients with severe sepsis and septic shock. The protocol measures targeted “goals” and uses a resuscitation algorithm to facilitate an aggressive and early resuscitation. The use of this strategy has been endorsed by the Surviving Sepsis Campaign, an 11-organization international consensus panel.38 The recommendation is for the use of goal-directed therapy for all patients who have (1) suspected infection, (2) two or more SIRS criteria, and (3) evidence of hypoperfusion signified by either a systolic blood pressure of less than 90 mm Hg after 20 to 30 mL/kg fluid challenge or a lactic acid level greater than 4 mmol/dL (Fig. 138-3). The theory behind the protocol is to normalize preload and pressure and to prevent tissue hypoxia by matching oxygen delivery with consumption.
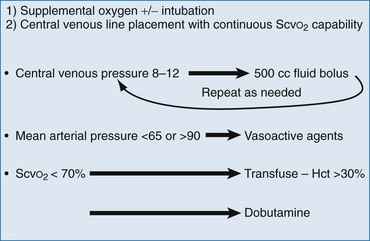
Figure 138-3 Summary of goal-directed therapy protocol.
Oxygen Delivery
The initial management of all critically ill patients should include supplemental oxygen with a goal to maintain a peripheral oxygen saturation of 95% (or the patient’s baseline oxygen saturation in the case of preexisting underlying lung disease). Once preload and pressure are normalized, the next focus is on oxygen delivery. A mixed venous saturation or assessment of lactate clearance can be used to guide this aspect of resuscitation. Organ hypoperfusion is a result of global and distributive changes in both systemic blood flow and the microvasculature. As a result of marrow suppression and dilution with crystalloid fluids, many patients with sepsis have a hemoglobin between 8 and 10 g/dL after initial resuscitation. A hematocrit between 27 and 30% has been reported to be most effective,39 although other authorities have challenged this dogma.40
The therapeutic goal is to maintain a mixed venous saturation (ScvO2) greater than 70% or to reduce lactate level by at least 10%. As oxygen demand due to sepsis increases, the ScvO2 starts to fall below 70%. This should be corrected by first ensuring that preload and pressure are adequate.41 Next, the arterial oxygen saturation should be optimized with non-rebreather oxygen delivery or intubation, as needed. Once these are optimized, one should ensure that the patient has adequate oxygen-carrying capacity by transfusion of the patient to a hematocrit greater than 30%. If this is normalized and the patient is not tachycardic, dobutamine can be added to increase cardiac contractility and to increase cardiac output, resulting in an overall improved oxygen delivery. The protocol should be run continuously with an effort toward normalization of the parameters during the entire resuscitation (Table 138-2).
Table 138-2
Management Recommendations for Hemodynamic Support
Basic Principles
Admission to intensive care unit
Arterial cannulation in patients with shock, although insertion may not be practical in the emergency department
Resuscitation to target tissue perfusion
Central venous or pulmonary arterial catheter placement to assess cardiac filling pressures
Fluid Resuscitation
Fluids are the primary modality to resuscitation*
Colloids and crystalloids are equally effective*
Invasive monitoring for patients not responding to initial resuscitation with target pulmonary capillary wedge pressure of 12 to 15 mm Hg†
Hemoglobin concentrations should be maintained above 8 to 10 g/dL†
Vasopressor Therapy
Norepinephrine is the first-line agent in shock unresponsive to aggressive fluid resuscitation‡
Dopamine and phenylephrine are alternatives
Epinephrine is reserved for refractory hypotension†
Routine low-dose (≤5 µg/kg/min) dopamine is not recommended‡
Vasopressin may be considered in refractory shock not responsive to aggressive fluid resuscitation and high-dose conventional vasopressors§
Inotropic Therapy
Dobutamine is the first choice for patients with refractory low cardiac index§
Dobutamine may improve cardiac index and organ perfusion, but empirical use is not recommended†
Vasopressors and inotropes can be titrated separately to maintain mean arterial pressure and cardiac output†
Epinephrine and dopamine can be used as inotropes, but splanchnic perfusion may be compromised*
*Supported by small randomized trials with uncertain results.
†Supported by at least one contemporaneous nonrandomized trial.
‡Supported by one randomized, controlled trial with clear results.
§Supported by nonrandomized trials with historical controls and expert opinion or case series.
Data from Hollenberg S, et al: Practice parameters for hemodynamic support of sepsis in adult patients with sepsis. Crit Care Med 27:639, 1999.
Respiratory Support
Altered mental status is common in patients in septic shock, and they may require rapid airway protection. Patients with a respiratory rate of more than 30 breaths/minute are likely to develop respiratory collapse, irrespective of arterial oxygenation. In 1999, Wheeler and Gordon reported that 85% of patients with “severe sepsis” required mechanical ventilation during their hospital course.42 Because patients with impending respiratory failure use a disproportionately large amount of energy for the muscles of respiration, improved oxygen delivery to other organs is achieved by mechanical ventilation, sedation, and paralysis. Although there are no clear intubation guidelines, hypercapnia, persistent hypoxemia, airway compromise, and profound acidosis are valid indicators.
In addition to airway protection, intubation and mechanical ventilatory support provide positive-pressure ventilation. The pattern of injury in ARDS is such that normal lung parenchyma is adjacent to affected tissue. Therefore, increased airway pressures are required to maintain normal oxygen delivery. Current recommendations are to maintain transalveolar pressures (measured as plateau pressures) below 35 cm H2O because increased pressures are associated with ventilator-induced lung injury. Maintenance of relatively low transalveolar pressure with increasing end-expiratory pressure is an effective way to increase arterial oxygen delivery.43 The ARDSNet trial established the benefit of low tidal volumes in acute lung injury (6 mL/kg), which has translated to a recommended approach of lower tidal volumes in mechanically intubated patients to prevent subsequent iatrogenic lung damage.44
Cardiovascular Support
Patients with sepsis often require large volumes of intravenous fluid to maintain adequate perfusion. The primary reasons for this intravascular hypovolemia are venodilation and diffuse capillary leak. Initial therapy for adults with septic shock should generally be 2 L of isotonic crystalloid. As much as 6 to 10 L of crystalloid may be required in the first 24 hours. Early goal-directed therapy has been shown to be an effective way to guide fluid resuscitation and should be considered in all cases of septic shock. In less critically ill patients, fluid replacement should be titrated to clinical parameters such as heart rate, blood pressure, change in mental status, capillary refill, cool skin, and adequate urine output (0.5-1 mL/kg/hr). Normal (0.9%) saline and lactated Ringer’s solution are equally effective and neither worsens lactic acidosis. Colloids are as effective as crystalloids, but they are more expensive. Efforts to identify ways to measure regional perfusion more directly, such as direct measurement of splanchnic blood flow, have been proposed. Even in the absence of global hypoxia and impaired tissue perfusion, there is evidence that regional hypoperfusion and ischemia exist.45 Although further study is necessary, therapy guided toward maintenance of splanchnic perfusion may decrease the rate of mortality.
Vasopressors
If appropriate fluid resuscitation has failed, vasopressor support may be required (Table 138-3). Only in cases of profound hypotension should vasopressors be started before adequate fluid resuscitation. Use of mean arterial pressure alone as an indicator of overall efficacy of therapeutic intervention is not helpful. A mean arterial pressure of 65 mm Hg has been recommended in otherwise healthy, normovolemic adult patients but must be correlated with other indicators of adequate perfusion, such as mental status and urine output. Patients with previously uncontrolled hypertension may require mean arterial pressures of 75 mm Hg or even higher.
Table 138-3
| DRUG | DOSAGE |
| Dobutamine | 5-15 µg/kg/min |
| Dopamine | 2-20 µg/kg/min |
| Epinephrine | 5-20 µg/min |
| Norepinephrine | 5-20 µg/min |
| Phenylephrine | 2-20 µg/min |
The 2008 Surviving Sepsis Campaign guidelines provide consensus recommendations for treatment of septic shock.38 Fluid resuscitation remains the fundamental treatment option and should be the initial treatment. Norepinephrine should be used as the initial vasopressor, with the addition of dopamine or vasopressin to norepinephrine as a reasonable adjunct. Vasopressin alone has been shown to be ineffective compared with norepinephrine in the initial treatment of refractory septic shock.46 Epinephrine should be considered a supplemental agent if dopamine or norepinephrine is ineffective. Dobutamine should be used as the primary inotropic agent if myocardial dysfunction is evident.38
Norepinephrine.: Norepinephrine is a mixed alpha- and beta-agonist with minimal beta2 activity and primarily functions to increase cardiac output and systemic vascular resistance. In a large study examining patients with shock from multiple causes, norepinephrine was shown to have fewer adverse events (particularly arrhythmias) compared with dopamine, which had a higher mortality rate in those patients with cardiogenic shock.47 In another meta-analysis, norepinephrine was shown to be superior to dopamine in both in-hospital and 28-day mortality.48
Dopamine.: Dopamine is also often used for septic shock that is unresponsive to adequate volume expansion. Dopamine is the immediate precursor of norepinephrine and epinephrine. It is primarily an alpha, beta1, and dopaminergic agonist. Although low doses alone are not effective, they may be effective in combination with other agents. “Renal-dose” dopamine has not been shown to reduce mortality or to decrease dialysis dependence and should not be used.49,50 Dosages higher than 20 µg/kg/min may produce significant vasoconstriction. Persistent tachycardia, decreased partial pressure of arterial oxygen, and increased pulmonary artery occlusion pressure are common side effects of dopamine use. Dopamine has been shown to increase oxygen delivery better than other catecholamines do. On the basis of its mechanism of action, it is most useful in patients who have depressed cardiac output.
Phenylephrine.: Phenylephrine is a selective alpha1-agonist, increasing systemic vascular resistance without significant changes in cardiac output. It can produce a reflexive bradycardia or suppression in cardiac output. A single small study has shown that phenylephrine is effective in restoring perfusion in patients with septic shock refractory to dopamine or dobutamine.51 Another small study demonstrated that phenylephrine is less effective than norepinephrine in treatment of hypotension in septic patients; however, there was no difference in other measured hemodynamic parameters, including oxygen delivery.52 Phenylephrine does not impair cardiac and renal function and may be a good choice when significant tachyarrhythmia limits the use of other agents.
Epinephrine.: Epinephrine is a potent mixed alpha- and beta-agonist. Epinephrine infusion is also associated with increased oxygen consumption, increased systemic lactate concentrations, and decreased splanchnic blood flow. The rise in lactate is short term, and there is no evidence regarding its long-term effects. As a result of all of the possible adverse effects of epinephrine, it is currently recommended only for those patients who are unresponsive to other vasopressors.
Vasopressin
Vasopressin is a naturally occurring nonapeptide that is synthesized as a large prohormone in the hypothalamus. In states of septic shock, there is an early surge of vasopressin followed by a profound drop in circulating vasopressin levels.23 This is the foundation for use of vasopressin as an adjunct therapy for patients with severe sepsis. Vasopressin should not be used as the sole initial therapy for refractory septic shock. In a well-designed randomized trial, investigators demonstrated no change in mortality for patients with severe sepsis when vasopressin was added to catecholamine vasopressors.46
Inotropic Agents
Dobutamine is a mixed alpha- and beta-agonist. In dosage ranges from 2 to 28 µg/kg/min, cardiac index is increased at the expense of heart rate. In addition, decreased splanchnic blood flow is common. Dobutamine should be used in patients with depressed cardiac index and persistent hypoperfusion in spite of adequate volume expansion and other vasopressor agents. In patients undergoing formal early goal-directed therapy, when preload, perfusion pressure, and oxygen-carrying capacity have been normalized and a low ScvO2 persists, dobutamine is used to increase cardiac output and oxygen delivery. One study suggested that survival in sepsis is associated with the patient’s increase in stroke volume in response to dobutamine.53
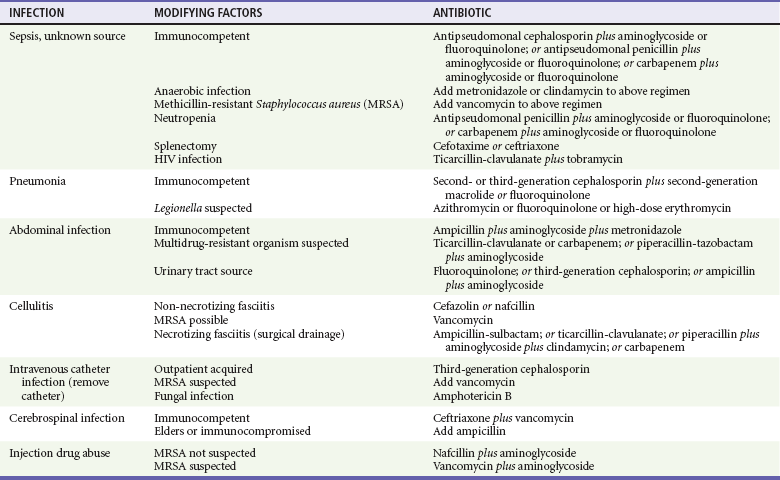
Antibiotics
Early and appropriate antibiotic therapy should target the nidus of infection; this reduces mortality, perhaps by as much as 30 to 50%.54 If the patient’s condition permits, appropriate culture specimens should be obtained before the administration of broad-spectrum antibiotics (Table 138-4). Surgically correctable conditions, such as intra-abdominal abscesses, perforated viscus, retained products of conception, or retained foreign body (such as a tampon), should be treated concurrently.
Table 138-4
Novel Therapies
After years of unsuccessful, large-scale, multicenter trials of novel therapeutic agents for sepsis, recombinant activated protein C (APC) became the first therapy to be approved by the Food and Drug Administration (FDA) for the treatment of sepsis. In the initial trial, APC was shown to improve survival by 6% in patients with severe sepsis.29 However, based on subsequent conflicting data in postmarket studies, a confirmatory trial was conducted. In the 1680 patient trial of patients with septic shock, the 28-day mortality rate in the activated protein C group was similar to the placebo group (26.4% versus 24.2%, p = .31).29a Additionally, there was no significant benefit in meaningful subgroups; thus, based on lack of efficacy, the drug has been withdrawn from the market and is no longer available for use.
Steroid Therapy
It has been nearly 30 years since the first treatment attempts to block inflammation in sepsis. Because sepsis involves a systemic inflammatory response, corticosteroids are a logical treatment modality as anti-inflammatory agents. Physicians have been working for decades to prove or to disprove their value. For example, Annane and colleagues performed a randomized, double-blind, placebo-controlled study of 300 patients with septic shock that demonstrated benefit when steroids were given to nonresponders in a corticotropin stimulation test.55 Among nonresponders, those treated with steroids had a 53% mortality rate, compared with 63% in the placebo group, for a 10% absolute benefit. Steroid therapy was not beneficial to those who responded to the corticotropin stimulation test. However, a large-scale multicenter validation failed to show a statistically significant improvement in mortality in both all-comers with septic shock and those with adrenal suppression.56 Steroids were more effective in reducing the amount of time patients spent hypotensive, but the rates of secondary infection increased, contributing to a null effect overall. At this time, with somewhat conflicting data, the utility of steroids in sepsis remains controversial and should be reserved for cases in which the risk of cardiovascular collapse from refractory hypotension due to adrenal suppression outweighs the risk of increased morbidity and mortality from secondary infection.55,57
References
1. Bone, R, American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874.
2. Shapiro, NI, et al. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006;48:583–590.
3. Howell, M, et al. Proof of principle: The predisposition, infection, response, organ failure sepsis staging system. Crit Care Med. 2011;39:322–327.
4. Levy, MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256.
5. Rangel-Frausto, M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). JAMA. 1995;273:117–123.
6. Bates, D, Cook, E, Goldman, L, Lee, T. Predicting bacteremia in hospitalized patients: A prospectively validated model. Ann Intern Med. 1990;113:495–500.
7. Brun-Buisson, C, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. JAMA. 1995;274:968–974.
8. Perl, T, Dvorak, L, Hwang, T, Wenzel, R. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995;274:338–345.
9. Murray, S, Bates, DW, Ngo, L, Ufberg, J, Shapiro, NI. Charlson Index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
10. Xu, J, Kochanek, K, Murphy, S, Tejada-Vera, B. Deaths: Final data for 2007. Natl Vital Stat Rep. 2010;58:1–51.
11. Wang, H, Shapiro, NI, Angus, D, Yealy, D. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928.
12. Angus, D, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310.
13. Martin, G, Mannino, D, Eaton, S, Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
14. Strehlow, M, et al. National study of emergency department visits for sepsis, 1992-2001. Ann Emerg Med. 2006;48:326.
15. Marshall, JC, Reinhart, K, Forum, IS. Biomarkers of sepsis. Crit Care Med. 2009;37:2290–2298.
16. Russell, J. Management of sepsis. N Engl J Med. 2006;355:1699–1713.
17. Ferreira, F, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754.
18. Akira, S, Uematsu, S, Takeuchi, O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
19. Schouten, M, Wiersinga, W, Levi, M, van der Poll, T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545.
20. Opal, S, Huber, C. Bench-to-bedside review: Toll-like receptors and their role in septic shock. Crit Care Med. 2002;6:125.
21. Zhao, B, Bowden, R, Stavchansky, S, Bowman, P. Human endothelial cell response to gram-negative lipopolysaccharide assessed with cDNA microarrays. Am J Physiol Cell Physiol. 2001;281:C1587.
22. Toussaint, S, Gerlach, H. Activated protein C for sepsis. N Engl J Med. 2009;361:2646–2652.
23. Holmes, CL, Walley, KR, Chittock, DR, Lehman, T, Russell, JA. The effects of vasopressin on hemodynamics and renal function in severe septic shock: A case series. Intensive Care Med. 2001;27:1416–1421.
24. Beasley, D, Schwartz, J, Brenner, B. Interleukin-1 induces prolonged L-arginine–dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991;87:602–608.
25. Eidelman, L, Putterman, D, Putterman, C, Sprung, C. The spectrum of septic encephalopathy: Definitions, etiologies, and mortalities. JAMA. 1996;275:470–473.
26. Werdan, K, et al. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol. 2009;87:266–274.
27. Lipiner-Friedman, D, et al. Adrenal function in sepsis: The retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–1018.
28. Faust, SN, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345:408–416.
29. Bernard, GR, et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001;29:2051–2059.
29a. Ranieri, VM, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055.
30. Sheu, C, et al. A large-scale genetic association study identifies different susceptibility loci of sepsis-related and non–sepsis-related ARDS. Am J Respir Crit Care Med. 2010;181:A2702.
31. Dolinay, T, et al. Gene expression profiling of sepsis patients reveals inflammasome-mediated susceptibility to acute respiratory distress syndrome development. Am J Respir Crit Care Med. 2011;183:A2896.
32. Shapiro, NI, et al. Mortality in Emergency Department Sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31:670–675.
33. Sankoff, J, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med. 2008;36:421–426.
34. Shapiro, NI, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528.
35. Rivers, E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
36. Shah, M, et al. Impact of the pulmonary artery catheter in critically ill patients: Meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–1670.
37. Bernard, GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709.
38. Dellinger, RP, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:296–327.
39. Czer, L, Shoemaker, W. Optimal hematocrit value in critically ill patients. Surg Gynecol Obstet. 1978;147:363–368.
40. Hebert, P, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417.
41. Pope, J, et al. Multicenter study of central venous oxygen saturation (ScvO2) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40–46.
42. Wheeler, A, Gordon, R. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214.
43. Dellinger, RP, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555.
44. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308.
45. De Backer, D, et al. Does hepato-splanchnic 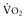 /DO2 dependency exist in critically ill septic patients? Am J Respir Crit Care Med. 1998;157:1219–1225.
/DO2 dependency exist in critically ill septic patients? Am J Respir Crit Care Med. 1998;157:1219–1225.
46. Russell, J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887.
47. De Backer, D, et al. SOAP II Investigators: Comparison of dopamine and norepinephrine in the treatment of schock. N Engl J Med. 2010;362:779–789.
48. Vasu, T, et al. Norepinephrine or dopamine for septic shock: A systematic review of randomized clinical trials. J Intensive Care Med. 2012;27:172–178.
49. Bellomo, R, Chapman, M, Finfer, S, Hickling, K, Myburgh, J. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–2143.
50. Kellum, JA, M Decker, J. Use of dopamine in acute renal failure: A meta-analysis. Crit Care Med. 2001;29:1526–1531.
51. Gregory, J, et al. Experience with phenylephrine as a component of the pharmacologic support of septic shock. Crit Care Med. 1991;19:1395–1400.
52. Morelli, A, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: A randomized, controlled trial. Crit Care. 2008;12:R143.
53. Kumar, A, et al. Cardiovascular response to dobutamine stress predicts outcome in severe sepsis and septic shock. Crit Care. 2008;12:R35.
54. Aube, H, Milan, C, Bletery, B. Risk factors for septic shock in the early management of bacteremia. Am J Med. 1992;93:283–288.
55. Annane, D, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871.
56. Sprung, CL, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124.
57. Marik, PE, Zaloga, GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31:141–145.