CHAPTER 94 SEPSIS, SEPTIC SHOCK, AND ITS TREATMENT
sepsis and septic shock, especially when associated with single or multiple organ dysfunction, are important causes of morbidity and mortality in trauma patients. Thus, an organized approach directed at preventing infectious complications as well as a strategy ensuring early diagnosis and treatment of infections, when they occur, are important components of trauma care. Over the last decade, progress has been made in developing such strategies, not just in trauma patients, but in all intensive care unit (ICU) patient populations. In fact, because sepsis and septic shock are such a major cause of mortality, an international group of experts from nine different societies met, and, through a Delphi-type process, developed a series of guidelines for the optimal treatment of sepsis and septic shock. These evidence-based recommendations were published in 2004 and are called the Surviving Sepsis Campaign guidelines.1
INCIDENCE
In trauma patients, injury not only predisposes to infection by promoting bacterial contamination of normally sterile tissues and spaces, but also induces the development of an immunocompromised state with infection and infection-related multiple organ failure being common causes of late death after trauma. Although the exact incidence of sepsis and septic shock in trauma patients is not fully known, the incidence of sepsis and septic shock is increasing nationally and affects an estimated 751,000 patients per year in the United States with an overall mortality in excess of 30%.2 This mortality rate is even higher in the presence of multiple organ failure, where mortality rates up to 85% have been reported. This increase in the incidence of sepsis and septic shock appears to involve trauma patients as well and can be attributed to several factors, including the aging of the population, which results in more elderly patients with significant comorbidities sustaining trauma. Additionally, advances in medical and surgical care have resulted in more severely injured patients surviving their initial injuries and thus being at risk for the subsequent development of infectious complications. Lastly, certain trauma-related variables appear to increase the risk of developing an infectious complication and these include the presence of preoperative shock, colon injuries, central nervous system (CNS) injuries, and injury to multiple organs as well as the administration of blood transfusions, due to their immunosuppressive effects.3
MECHANISM OF INFECTION
Infections in trauma patients may occur for many reasons. These include the global immunosuppressive effects of a major injury, patient-related factors, consequences of injuries to specific organs such as the intestine, as well as infections occurring after operations or due to the need for invasive monitoring or mechanical ventilation. For example, risk factors for sepsis include extremes of age and chronic underlying medical conditions, such as malnutrition, alcoholism, malignancy, and diabetes mellitus, all of which compromise the immune system.2 There is also evidence to suggest that some patients may have a genetic predisposition for the development of sepsis, as well as increased mortality when an infection is acquired.4 For example, genetic polymorphisms have been identified in the genes for TNF, IL-6, IL-10, IL-1 receptor antagonist, heat shock protein, CD14, and lipopolysaccharide binding proteins.2 There is even evidence that gender influences the risk of post-trauma sepsis with males being more susceptible than premenopausal females.5 Thus, genetic factors and to a lesser extent sex hormonal status may help explain the heterogeneity observed among patients developing infections after trauma as well as in the evaluation of new therapeutic molecules in patients with sepsis.
Another reason why infectious complications are relatively common in trauma patients is that traumatic injury itself leads to bacterial contamination of spaces that are normally sterile, while the presence of hematomas and devitalized or necrotic tissue causes an anoxic microenvironment that impairs the ability of the host’s antibacterial factors to effectively clear bacteria if these hematomas or devitalized tissue sites become infected. Clinical examples of this phenomenon are secondary infections of major liver injuries or splenic injuries treated with splenic artery embolization. Likewise, pulmonary contusions, which occur in 10%–25% of patients after blunt chest injury, are associated with impaired immune function and an increased susceptibility to infectious complications. Additionally, many of our therapeutic maneuvers potentially predispose patients to an increased risk of infection. For example, invasive monitoring lines allow bacteria colonizing the skin surface direct access to the bloodstream, while endotracheal tubes and Foley catheters also promote the development of pneumonia and urinary tract infection (UTI). In fact, the incidence of nosocomial infections in mechanically-ventilated trauma patients is relatively high and increases as the ICU length of stay increases, reaching over 50% in patients in the ICU more than 7–10 days. This increased infection rate appears to be associated with up to a three-fold increase in mortality, especially in patients with moderately severe injuries who might otherwise be expected to survive.6
DIAGNOSIS
The making of an early and accurate diagnosis of infection or septic shock is an important, but potentially difficult, aspect of the care of the trauma patient. One reason for this difficulty is that the common signs of infection, such as fever, leukocytosis, and tachycardia are relatively common in noninfected trauma patients and represent the host’s inflammatory response to injury. The fact that noninfectious septic responses are common after major trauma and can be indistinguishable from an infectious-mediated septic response therefore confounds diagnostic decision making. Furthermore, the need for ventilatory support and sedation in many of these patients further impairs the ability of the clinician to accurately question and examine the patient. Additionally, the presence of postoperative pain after procedures, such as a laparotomy, further limits the accuracy of abdominal symptoms or physical examination in the patient at risk for developing abdominal sepsis. For all of these reasons, vigilance, a high level of suspicion and knowledge of the likely sites of infection in each specific patient are required to facilitate the early diagnosis of an infection. Likewise, it is important to recognize that certain physiologic changes in the otherwise stable patient may be an early sign of a serious infection. Examples of these physiologic derangements, in addition to the standard signs of fever and tachycardia, include new onset ileus, a change in sensorium, fluid sequestration manifesting as an increasing need for fluids to maintain urine output, the development of a metabolic acidosis, or worsening of the patient’s respiratory status.
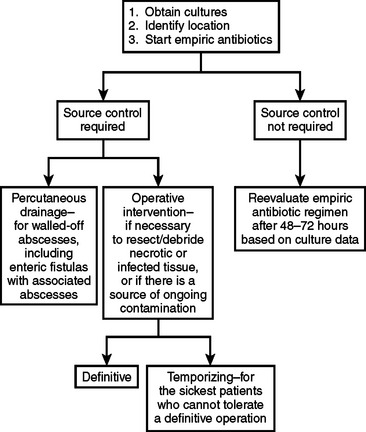
Since pneumonia, line sepsis, and infections at the sites of injury or previous operations are especially common in severely injured trauma patients, special attention should be focused on these areas in the at risk patient in whom infection is suspected. In fact, ventilator-associated pneumonia is one of the most common causes of infection-related death in the ICU and can be difficult to diagnose, as are episodes of life-threatening line sepsis. Whenever an infection is suspected, diagnostic studies to determine the causative organisms should be performed before the start of antibiotic therapy, although commencement of antibiotic therapy should not wait for diagnostic studies to be completed since the early treatment of an infection appears to reduce morbidity and mortality (Figure 1). Specifically, when infection is suspected, two sets of blood cultures should be drawn—one percutaneously, and one through any intravascular catheter more than 48 hours old. The rationale for drawing cultures from existing vascular lines is to screen for line sepsis and if the blood culture from the vascular line comes back positive earlier than the percutaneously drawn blood culture (i.e., >2 hours), this suggests that the vascular access device is a likely source of the infection.7 Additionally, as appropriate, cultures of urine, cerebrospinal fluid, and body fluids should be obtained. Bronchoscopy and bronchoalveolar lavage with quantitative cultures should be used for the diagnosis of ventilator-associated pneumonia. Radiologic imaging studies with sampling of potential sources of infection can also be helpful, especially in patients at risk of having intra-abdominal septic sites.
STAGING
An international sepsis definitions consensus conference of the American College of Chest Physicians and the Society of Critical Care Medicine attempted to define a staging system for sepsis. This was called PIRO (predisposition, insult or infection, response, and organ dysfunction). Predisposition includes premorbid conditions that influence the likelihood of infection, sepsis, morbidity, and survival. Insult or infection refers to the specific organism causing the septic response, the sensitivity pattern, and whether the infection is community acquired or nosocomial. Response attempts to quantify the clinical manifestations of the systemic inflammatory response syndrome (SIRS), by using markers such as procalcitonin, IL-6, HLA-DR, TNF, PAF, and C-reactive protein. Organ dysfunction refers to the type and number of dysfunctional organs, and whether it is reversible or irreversible.8 Organ dysfunction is judged by other scoring systems discussed elsewhere in this text, including multiple organ dysfunction syndrome (MODS) score, logistic organ dysfunction system (LODS), and sequential organ failure assessment (SOFA). Although the PIRO classification is not in wide use, it provides a conceptual framework for staging sepsis (Table 1).
| Predisposition | Premorbid conditions influencing the likelihood of infection, sepsis, morbidity, or survival (age, sex, hormonal state, genetic polymorphisms) |
| Insult/infection | Insult or organism associated with the sepsis response (type of organism, sensitivity pattern, community or nosocomial acquisition) |
| Response | Clinical manifestation of the SIRS response (procalcitonin, IL-6, TNF, C-reactive protein) |
| Organ dysfunction | Type and number of dysfunctional organs (reversible vs. irreversible dysfunction), severity of dysfunction (judged by scoring systems such as MODS, LODS, and SOFA) |
IL, Interleukin; LODS, logistic organ dysfunction score; MODS, multiple organ dysfunction score; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; TNF, tumor necrosis factor.
Adapted from Magnotti LJ, Croce MA, Fabian TC: Is ventilator-associated pneumonia in trauma patients an epiphenomenon or a cause of death? Surg Infect (Larchmt) 5:237–242, 2004.
MEDICAL AND SURGICAL MANAGEMENT
Management of sepsis due to an infectious process begins with prompt recognition that an infection is likely, determination of the probable site of infection and assessment of the severity of the physiologic derangements. Based on the source and cause of the infection, specific therapy may be limited to antibiotics or require a combination of antibiotics plus operative or interventional source control. The choice of antibiotics depends on the suspected site of infection and the organisms that commonly inhabit that location (Table 2). Antibiotic choice may have to be modified for patients likely to have an abnormal flora, such as patients who have had a previous course of antibiotics or those who have been colonized by hospital-acquired pathogens. In these circumstances, treatment is initiated with a single agent or combination of agents with the lowest possible toxicities, whose spectrum of activity is broad enough to cover all suspected pathogens. Since victims of major trauma are at increased risk of developing multiple infections, it is crucial that antibiotics are stopped as soon as possible to limit the emergence of antibiotic-resistant bacteria. There is no good evidence to suggest that antibiotic treatment should exceed 7–10 days, except in unusual circumstances. Likewise, in determining the length of antibiotic use, it is also important to differentiate between prophylaxis and therapy, especially in the trauma patient in whom injury-induced bacterial contamination may have occurred. For example, in a trauma patient undergoing laparotomy for blunt or penetrating trauma, the patient receives preoperative antibiotics (usually a second-generation cephalosporin), which are usually stopped within 24 hours postoperatively. However, if a major degree of contamination is present, as in a colon injury where the incidence of abdominal abscess formation reaches over 25%,9 the antibiotics should be continued for 3–5 days, since they serve both prophylactic (for the wound) and therapeutic roles (for the peritoneum).
Table 2 Common Organisms and Antibiotic Choice Based on Location of Suspected Infection
| Site | Common Community-Acquired Organisms and Treatment | Common Nosocomial Organisms and Treatment |
|---|---|---|
| Lung | Streptococcus pneumoniae, Haemophilus influenzae, Legionella, Chlamydia, Pneumocystic carinii | Aerobic Gram-negative bacilli |
| Macrolide and third-generation cephalosporin or levofloxacin | Cefepime or imipenem-cilastatin with aminoglycoside | |
| Abdomen | Escherichia coli, Bacteroides fragilis | Aerobic Gram-negative rods, anaerobes, Candida |
| Imipenem-cilastatin or piperacillin-tazobactam with or without aminoglycoside | Imipenem-cilastatin with or without aminoglycoside, or piperacillin-tazobactam with or without amphotericin B | |
| Skin/soft tissue | Group A Streptococcus, Staphylococcus aureus, Clostridium, polymicrobial, enteric Gram-negative rods, Pseudomonas Aeruginosa, anaerobes, Staphylococci | Staphylococcus aureus, aerobic Gram-negative rods |
| Vancomycin with or without imipenem-cilastatin or piperacillin-tazobactam | Vancomycin with cefepime | |
| Urinary tract | Escherichia coli, Klebsiella sp., Enterobacter sp., Proteus sp. | Aerobic Gram-negative rods, Enterococci |
| Ciprofloxacin with or without aminoglycoside | Vancomycin with cefepime | |
| CNS | S. pneumoniae, Neisseria meningitidis, Listeria monocytogenes, E. coli, H. influenzae | P. aeruginosa, E. coli, Klebsiella, Staphylococcus |
| Vancomycin, third-generation cephalosporin, or meropenem | Cefepime or meropenem with vancomycin |
Adapted from Balk RA: Optimum treatment of severe sepsis and septic shock: evidence in support of the recommendations. Dis Month 50:163–213, 2004.
However, even in spite of appropriate initial surgical management, postoperative intra-abdominal infections remain a major cause of morbidity and mortality in the trauma victim. Although the peritoneal cavity is relatively resistant to a bacterial challenge, the presence of adjuvant substances such as fibrin or hemoglobin allows bacteria to evade the normal host defenses and can result in intra-abdominal infection. Thus, if there is suspicion of a postoperative intra-abdominal infection, the primary goal is to determine whether the patient has intra-abdominal sepsis, and if so, to determine the necessity and timing of reoperation versus percutaneous therapy. In this situation, the most useful diagnostic modality in a stable patient is a helical abdominal computed tomography (CT) scan. Abdominal CT scan has a sensitivity of 97% and a specificity of 65% in diagnosing an intraabdominal source of sepsis.10 If there are no signs of overwhelming sepsis and the CT scan shows evidence of a localized abscess, whether in the peritoneal cavity or at the site of an injured organ, such as the liver, the intra-abdominal infection usually can be managed nonoperatively with fluid resuscitation, CT-guided drainage of the abscess and broad-spectrum antibiotics. This is especially useful in the patient who has undergone multiple abdominal operations, where relaparotomy can be difficult. Even though anastomotic leaks and breakdowns may result in severe sepsis or the formation of an enterocutaneous fistula, these patients still may be candidates for percutaneous drainage if there is no evidence of continuing contamination, if the catheter drainage route does not traverse bowel or uncontaminated organs and the infectious source is controlled by catheter drainage.11 In contrast, when these conditions can not be met, or in the patient with diffuse peritonitis, prompt reoperation to control the source of infection and provide drainage is mandatory and can be life-saving.
MORBIDITY AND COMPLICATIONS MANAGEMENT
Septic Shock
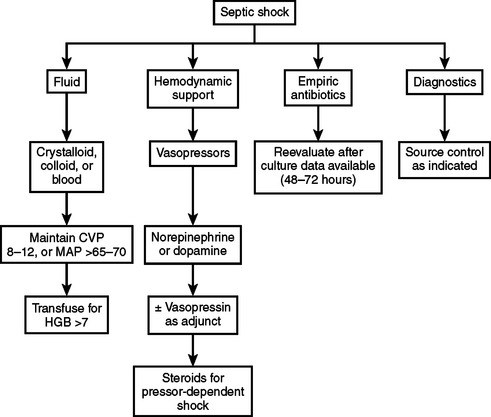
The therapeutic approach to a patient with septic shock has several components and begins with fluid administration (Figure 2). Fluids are the initial component of therapy since septic shock is characterized by excessive vasodilation and a decrease in systemic vascular resistance, although the cardiac output may be greatly increased. Septic shock is also associated with an increase in capillary permeability resulting in a decrease in intravascular volume secondary to third-spacing of fluid. In spite of the ongoing debate as to the relative value of crystalloids versus colloids, they both have similar effects on preload, stroke volume, and oxygen delivery, thereby restoring tissue perfusion to the same degree, although approximately three times more volume of crystalloid is required than colloid, because of the propensity of crystalloid to leak into the extravascular space. Despite this debate, no difference has been shown in length of stay, mortality, or pulmonary edema when comparing colloid to crystalloid.12 Consequently, it is the adequate and rapid administration of volume that appears more important than the fluid administered. To that end, it is important to try to reach certain specific physiologic goals to ensure that the amount of volume administered is adequate (Table 3). The importance of the early institution of goal-directed volume resuscitation in patients with septic shock was recently shown in a prospective, randomized, controlled trial where patients resuscitated to a central venous pressure of 8–12 (or 12–15 in mechanically ventilated patients due to the increased intrathoracic pressure), a mean arterial pressure above 65 mm Hg, a urine output above 0.5 ml/kg/hr, and a mixed venous oxygen saturation of over 70% had a significant reduction in hospital mortality (30% vs. 46%) as compared to patients treated with standard therapy.13 The major difference between the two groups was that the patients in the early goal-directed group received more volume in the first 6 hours than the standard therapy group, reinforcing the importance of early and adequate volume resuscitation.
Adapted from Dellinger RP, Carlet JM, Masur H, et al: Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873, 2004 and Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377, 2001.
The role of blood products in the resuscitation of patients with septic shock should be limited to patients with significant anemia or evidence of cardiac ischemia. There is no physiologic benefit to routine RBC transfusion in the critically ill patient to maintain a hemoglobin level above 9–10 g/dl and banked blood may actually be harmful in light of its documented immunesuppressive effects,3 and the fact that blood transfusions have been shown to be an independent predictor of MODS. In fact, the Transfusion Requirements in Critical Care Investigators (TRICC) trial demonstrated that the patients who only received blood transfusions to maintain a hemoglobin level of 7 gm/dl had a decrease in the incidence and severity of new organ dysfunction as compared to the liberal transfusion group.14
The role of vasopressors in septic shock continues to be an area of active investigation and they are the second line of therapy. Currently vasopressors are indicated when appropriate fluid resuscitation fails to restore adequate arterial pressure or organ perfusion (see Figure 2). They also should be used when life-threatening hypotension is present, even if fluid resuscitation is not complete.15 In this latter situation, vasopressors are used as a bridge to temporarily maintain blood pressure while fluid resuscitation is completed. The choice of agents includes dopamine, norepinephrine, epinephrine, phenylephrine, vasopressin, and dobutamine. Dopamine and norepinephrine are the first-line pressors in septic shock, since they have limited directed cardiac effects and there are no consistent, hard data supporting the use of one over the other.1,16 Dopamine is a precursor of epinephrine and norepinephrine with dose-dependent pharmacologic effects. At moderate doses, it stimulates beta-adrenergic receptors, and thereby increases heart rate and contractility with minimal effects on systemic vascular resistance. At high doses, the alphaadrenergic effects predominate causing vasoconstriction with increased mean arterial pressure. Norepinephrine is an alphaadrenergic agonist with some beta effects. Overall it causes vasoconstriction, so it was traditionally thought to have negative effects on splanchnic and renal perfusion. Recent studies, however, show that this is not true, and there are data to suggest that norepinephrine actually improves renal perfusion. A drawback with norepinephrine use is that there can be an adrenergic receptor down-regulation in sepsis, which may require large doses of the drug to be administered in order to achieve the desired physiologic effects. Norepinephrine increases mean arterial pressure without changing heart rate and cardiac output is either unchanged or increased, while systemic vascular resistance is increased. However, the increase in afterload observed with norepinephrine use may be problematic in those patients with underlying cardiac dysfunction.
Although epinephrine increases cardiac index, heart rate, and stroke volume, it is not a first-line treatment for septic shock because it decreases splanchnic blood flow and oxygen delivery. It has also been shown to be associated with transiently elevated lactate levels and can cause tachyarrhythmias. Phenylephrine is a potent alpha-adrenergic agonist, but is not a first-line agent because it can decrease cardiac output and heart rate as well as decrease splanchnic blood flow. Thus, in the patient who is refractory to dopamine or norepinephrine, the next option is to add vasopressin. The rationale for vasopressin use is based on small patient series where it was effective in patients with otherwise pressor-refractory septic shock.17 Physiologically, endogenous vasopressin is rapidly depleted in patients with septic shock, and it has been shown to have a permissive effect on adrenergic agents increasing their vasoconstrictive effects. Specifically, low-dose vasopressin (0.01 and 0.04 units/min) has been shown to improve mean arterial pressure, cardiac index, and left ventricular stroke work when added to norepinephrine. Currently, clinical trials are investigating the clinical efficacy of vasopressin use in conjunction with dopamine or norepinephrine to augment their vasopressor effects.
Another approach to the patient with pressor-refractory septic shock is the use of low-dose steroids. This option is based on the recent recognition that patients with vasopressor-dependent septic shock often have an inappropriately low cortisol response to the shock state and are in a state of relative adrenal insufficiency. While absolute adrenal insufficiency in the septic state is rare (0%–3%), relative adrenal insufficiency is present in 50%–75% of patients in septic shock, and several recent studies have shown that low-dose corticosteroids improve survival in septic shock.18–20 There is some controversy in defining adrenal insufficiency in patients with shock, with most series using the corticotropin stimulation test to establish the diagnosis of relative adrenal insufficiency. In this test, a baseline cortisol level is drawn, and the patient is then stimulated with 250 micrograms of corticotropin. Cortisol levels are drawn 30 and 60 minutes after administration of the corticotropin. An absolute incremental increase of less than 9 micrograms/dl 30–60 minutes after stimulation indicates relative adrenal insufficiency. Random cortisol levels of less than 25 microgram/dl have also been used as a marker for relative adrenal insufficiency in septic patients. Furthermore, since a subgroup of patients who were not found to have adrenal insufficiency responded to low-dose steroid therapy, it is unclear at this time whether the use of low-dose corticosteroids should be limited to patients with relative adrenal insufficiency or given to all patients with vasopressordependent septic shock, in light of the beneficial hemodynamic effects. On the other hand, there are no data to support the use of corticosteroids in septic patients without shock.
Practically speaking, empiric steroid therapy is generally begun as soon as the diagnostic tests are complete and blood has been obtained for cortisol measurements. Since the results of these tests may not return for more than 24 hours, it is also possible to use the patient’s response in deciding whether the steroid therapy should be continued. The preferred glucocorticoid in patients with septic shock is hydrocortisone because it is the most closely related to the active cortisol molecule, and consequently does not rely on metabolic transformation in order to directly replace cortisol. In addition, it is the only steroid with intrinsic mineralocorticoid activity for those with absolute primary adrenal insufficiency. Several studies recommend daily dosages of 200–300 mg of hydrocortisone given in divided doses (q6–q8 hours) administered for 5–7 days or longer. There is no established protocol for cessation of steroid therapy, and since a rebound effect has been noted with abrupt cessation of steroid treatment, the dose is generally tapered over a few days. The European Corticus trial recommends halving the steroid dosage over a 3-day period with complete cessation after 6 days. Restarting of therapy is recommended if shock reoccurs during weaning of the steroid.20
The use of recombinant human activated protein C (rhAPC) in the treatment of septic shock is another potential option based on a large clinical trial showing that it improved survival in patients with severe sepsis, some of whom had septic shock.21 In this phase III trial, termed the PROWESS study, rhAPC reduced the absolute 28-day mortality by 6% in patients with severe sepsis and evidence of organ failure in two or more organs (19% relative risk reduction). However, due to its ability to cause severe bleeding complications, it is contraindicated in patients with head injury, an increased risk of lifethreatening bleeding, and with epidural catheters or recent active bleeding. Thus, although rhAPC is the only immunomodulatory treatment shown to date to improve survival in patients with a high risk of death (Apache II score above 25 or multiple organ failure), little information is available on its effectiveness in trauma patients. Furthermore, in contrast to the PROWESS study, no 28-day mortality benefit was shown in septic patients with a low risk of death (Apache II scores <25 or single organ failure); however, it doubled the risk of the development of a bleeding complication.22 Thus, it appears that the use of rhAPC may not be appropriate for many trauma patients, but it should be considered in selected patients.
Multiple Organ Dysfunction Syndrome
Since infections can contribute to the development of MODS as well as increase mortality, several key concepts must be kept in mind to limit as well as to treat infection-related organ dysfunction. For example, sepsis is one of the most common clinical precursors to ARDS. Tachypnea and hyperventilation are frequent manifestations of severe sepsis, and increased work of breathing and abnormalities of oxygenation often make oxygen delivery difficult. Thus, one of the primary goals in severe sepsis is to ensure adequate oxygen delivery and oxygenation; however, the timing of mechanical ventilation is controversial. Clearly, patients with clinically evident acute lung injury or ARDS should be intubated early; however, the benefits of early ventilatory support should be weighed against the risks of ventilator-associated complications. In mechanically ventilated patients, the goal is to keep the oxygen saturation above 88%–95% while keeping the FiO2 less than 0.6. Lung-protective strategies for improved outcome include volume-cycled ventilation in the assist control mode, with a low tidal volume (6 ml/kg of body weight). In the ARDSNET trial, low tidal volume strategies produced significantly decreased in-hospital mortality with an increase in ventilator-free and organ failure–free days.23 In this trial, plateau pressures were kept below 30 cm of water, and tidal volumes were approximately 6 ml/kg; however, if necessary, tidal volumes were dropped to 4 ml/kg and permissive hypercapnia was allowed to achieve this goal. Permissive hypercapnia has not been shown to be detrimental, except in patients with elevated intracranial pressures. Once intubated, the only preventive strategy for decreasing the incidence of ventilator-associated pneumonia is semirecumbent positioning.24 Patients may be laid flat for procedures, hemodynamic measurements, or episodes of hypotension, but the remainder of the time they should be semirecumbent. In addition, sedation and neuromuscular blockade should be frequently evaluated. Neuromuscular blockade should be avoided due to prolonged paralysis and skeletal muscle weakness. If necessary, depth of blockade with train-of-four monitoring should be used. Sedation should also be monitored with sedation scales and daily periods of lightening or interrupting continuous sedation, which has been shown to decrease the length of mechanical ventilation and ICU stays.25
Sepsis can also lead to cardiac dysfunction and associated myocardial depression, manifested as both diastolic dysfunction and a decreased cardiac response to catecholamines. This causes a decreased ejection fraction despite an increased cardiac index. This reversible biventricular myocardial dysfunction has been attributed to TNF-alpha, IL-1, or nitric oxide. Hypovolemia also exacerbates cardiac dysfunction and thus must be avoided. During this period of myocardial depression, the ventricles dilate and the ejection fraction is decreased. Even in the presence of normal or increased cardiac output, cardiac function is not always adequate to provide sufficient oxygen to the tissues to meet metabolic needs. Thus, a goal of therapy is to maintain oxygen delivery at sufficient levels to allow optimization of oxygen consumption at the tissue level. Consequently, if there is evidence of inadequate systemic oxygen delivery associated with a low cardiac output despite adequate fluid therapy, then the use of inotropes is indicated with dobutamine being the agent of choice.1 On the other hand, attempts to drive oxygen delivery to supranormal levels should not be done, since this strategy has been shown to increase rather than decrease mortality.26
While renal dysfunction is common in the setting of severe sepsis or septic shock, renal failure requiring renal replacement therapy occurs in less than 5% of patients with severe sepsis. The development of acute renal failure requiring replacement therapy, either hemodialysis or continuous venovenous hemofiltration, in severe sepsis is associated with an increased risk of death. Although it has been hypothesized that plasma exchange may be beneficial in removing molecules in the bloodstream that initiate or propagate the sepsis cascade, there are no compelling data to support the efficacy of this approach. When acute renal failure occurs in the septic patient, it is generally a consequence of inadequate volume resuscitation, the use of nephrotoxic drugs or a prolonged period of shock. Thus, prevention of renal failure is primarily aimed at maintenance of effective renal perfusion, including volume administration and cardiovascular support. In contrast, multiple studies have shown that there is no role for “renal-dose” dopamine in the prevention or treatment of acute renal failure.27 Since the mortality rate of nonoliguric renal failure is about half that of oliguric renal failure, once renal failure becomes manifest it is important to attempt to convert oliguric to nonoliguric renal failure. Therapeutic strategies that can be used include volume administration to maintain pulmonary arterial wedge pressure of 15–18 and the administration of mannitol or high-dose continuous loop diuretics to convert nonoliguric to oliguric renal failure.28 In addition, in patients with renal failure, drug dosing is modified and levels of drugs such as aminoglycosides are monitored. Lastly, endogenously produced nephrotoxic substances such as myoglobin must be cleared, and continued attempts are made to control the infectious process.
Since the early studies performed by Moore et al. in 1989 documenting that early enteral nutrition is associated with a reduction in infectious complications and length of stay in trauma patients,29 many other studies have validated the concept that early enteral feeding reduces infection rates in trauma as well as other ICU patient populations. Not only is there evidence that enteral nutrition is beneficial, but there is also evidence from several clinical trials that total parenteral nutrition (TPN) may be harmful as illustrated in the VA cooperative perioperative trial of 395 malnourished surgical patients.30 In this study, the TPN-treated patients had an infection rate that was almost 2.5-fold higher than the saline controls (14% vs. 6%), thereby highlighting the potential risk of parenteral nutrition. The mechanisms by which enteral nutrition appears to exert its beneficial effects appears to be multifactorial, and is related to the ability of enteral nutrition, in contrast to TPN, to directly feed the gut as well as the rest of the body.31 More recent studies using newer enteral diets containing higher levels of various nutrients, such as glutamine, arginine, fiber, or omega-fatty acids, have documented that early enteral nutrition is associated not just with less infectious complications but also with a reduction in organ dysfunction.31 When choosing an enteral formula, the following nutritional guidelines for patients with sepsis should be considered: (1) caloric intake 25–30 kcal/kg of lean (not total) body weight, (2) protein 1.3–2.0 g/kg/day, (3) glucose 30%–70% of total nonprotein calories, and (4) lipids 15%–30% of total nonprotein calories.
The reason why TPN was associated with an increased incidence of infectious complications was initially unclear when these clinical TPN studies were performed; however, it has now become clear that hyperglycemia is a major risk factor in critically ill patients and TPN was commonly associated with hyperglycemia. Since then, another component of metabolic management that has been shown to improve survival in ICU patients is tight glucose control, where the blood glucose is maintained at 90–110 mg/dl.32 Consequently, the nutritional support of the septic as well as the nonseptic trauma patient includes enteral alimentation as well as the liberal use of insulin to avoid hyperglycemia.
CONCLUSIONS
The mortality associated with sepsis and septic shock remains high, ranging from 13% to 50% and as high as 85% when complicated by multiple organ failure. After recovery from severe sepsis, mortality is higher during the first year of follow-up.13 The challenge remains to prevent infections and limit the development of SIRS and MODS. Although our understanding of the pathophysiologic changes that occur in septic shock continues to increase, we are just beginning to employ novel therapeutic strategies with a significant survival benefit.
1 Dellinger RP, Carlet JM, Masur H, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
2 Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
3 Hebert PC, McDonald BJ, Tinmouth A. Clinical consequences of anemia and red cell transfusion in the critically ill. Crit Care Clin. 2004;20:225-235.
4 Holmes CL, Russell JA, Walley KR. Genetic polymorphisms in sepsis and septic shock. Chest. 2003;124:1103-1115.
5 Ananthakrishnan P, Deitch EA. Gut origin sepsis and MODS: the role of sex hormones in modulating intestinal and distant organ injury. XX vs XY. 2003;1:108-117.
6 Magnotti LJ, Croce MA, Fabian TC. Is ventilator-associated pneumonia in trauma patients an epiphenomenon or a cause of death? Surg Infect (Larchmt). 2004;5:237-242.
7 Blot F, Schmidt E, Nitenberg G, et al. Earlier positivity of central venous versus peripheral blood cultures is highly predictive of catheter-related sepsis. J Clin Microbiol. 1998;36:105-109.
8 Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. International Sepsis Definitions Conference 2001. Crit Care Med. 2003;31:1250-1256.
9 Miller PR, Fabian TC, Croce MA, Magnotti LF, Pritchard E, Minard G, Stewart RM. Improving outcomes following penetrating colon wounds: application of a clinical pathway. Ann Surg. 2002;23:775-781.
10 Velmahos GC, Kamel E, Berne TV, Yassa N, Ramicone E, Song Z, Demetriades D. Abdominal computed tomography for the diagnosis of intraabdominal sepsis in critically injured patients: fishing in murky waters. Arch Surg. 1999;134:831-836.
11 Men S, Akhan O, Koroglu M. Percutaneous drainage of abdominal abscess. Eur J Radiol. 2002;43:204-218.
12 Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomized trials. BMJ. 1998:961-964.
13 Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
14 Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, et al. A multi-center, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-417.
15 Vincent JL. Hemodynamic support in septic shock. Intensive Care Med. 2001;27:S80-S92.
16 Steel A, Bihari D. Choice of catecholamine: does it matter? Curr Opin Crit Care. 2000;6:347-353.
17 Beale RJ, Hollenberg SM, Vincent JL, Parrillo JE. Vasopressor and inotropic support in septic shock: an evidence-based review. Crit Care Med. 2004;32:S455-S465.
18 Annane D, Sebille V, Charpienter C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
19 Keh D, Boehnke T, Weber-Carstens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blinded, randomized, placebo-controlled crossover study. Am J Respir Crit Care Med. 2003;167:512-520.
20 Keh D, Sprung CL. Use of corticosteroid therapy in patients with sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32:S527-S533.
21 Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Recombinant human protein C worldwide evaluation in severe sepsis (PROWESS) study group: efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
22 Abraham E, Laterre PF, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332-1341.
23 The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
24 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomized trial. Lancet. 1999;354:1851-1858.
25 Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272-1276.
26 Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med. 1995;333:1025-1032.
27 Bellomo R, Chapman M, Finfer S, et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomized trial. Lancet. 2000;356:2139-2143.
28 Shilliday IR, Quinn KJ, Allison ME. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant. 1997;12:2592-2596.
29 Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major torso trauma reduced septic morbidity. J Trauma. 1989;29:916-923.
30 VA Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525-532.
31 Deitch EA, Sambol JT. The gut-origin hypothesis of MODS. In: Deitch EA, Vincent JL, Windsor A, editors. Sepsis and Multiple Organ Dysfunction: A Multidisciplinary Approach. Philadelphia: WB Saunders; 2002:105-116.
32 Van der Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359-1367.
33 Balk RA. Optimum treatment of severe sepsis and septic shock: evidence in support of the recommendations. Dis Month. 2004;50:163-213.