131 Sepsis and Multiple Organ System Failure in Children
 Definitions of Sepsis, Severe Sepsis, Septic Shock, and Multiple Organ Failure
Definitions of Sepsis, Severe Sepsis, Septic Shock, and Multiple Organ Failure
The 2001 International Sepsis Definitions Conference1 centered discussion on whether sepsis should continue to be defined as systemic inflammatory response syndrome plus infection or infection plus systemic inflammatory response syndrome plus signs of organ dysfunction. It was agreed that the definitions of severe sepsis remain intact. Most pediatric literature defines inclusion criteria for sepsis as hyperthermia or hypothermia, tachycardia (may be absent in the hypothermic patient), evidence of infection, and at least one of the following signs of new-onset organ dysfunction: altered mental status, hypoxemia, bounding pulses, or increased lactate. Severe sepsis is uniformly defined as sepsis and organ failure determined by various organ failure scores.2–5 Septic shock has been defined as infection with hypothermia or hyperthermia, tachycardia (may be absent with hypothermia), and altered mental status in the presence of at least one, but usually more than one, of the following: decreased peripheral pulses compared with central pulses prolonged greater than 2 seconds (cold shock) or flash capillary refill (warm shock), mottled or cool extremities (cold shock), and decreased urine output (<1 mL/kg/h). Hypotension is observed in late decompensated shock.6
The American College of Critical Care Medicine6 further defines shock according to response to therapy as fluid-refractory/dopamine-resistant, catecholamine-resistant, and refractory shock. Multiple organ failure is defined as more than one organ failure. The greater the number of concomitant organ failures, the greater the risk of mortality. Multiple organ failure generally is observed in septic shock patients who receive delayed resuscitation or inadequate source control therapies (inadequate nidus removal or ineffective antibiotic regimen). Multiple organ failure also is observed in patients with septic shock who have an underlying primary or acquired immunodeficiency that prevents timely eradication of infection and resolution of inflammation.
 Changing Outcomes and Epidemiology
Changing Outcomes and Epidemiology
The mortality rate in neonatal and pediatric severe sepsis has improved from 97% in 1963 to 9% in 1999, to 4% in 2003.7–13 Previously healthy children have better outcomes than children with chronic illness. The randomized controlled trial of bactericidal permeability-increasing protein14 for children with purpura fulminans/presumed meningococcal septic shock showed 10% mortality rates in the placebo groups. The reported outcomes in children with septic shock when using therapeutic approaches similar to those recommended in the 2002 American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Patients in Septic Shock6 show a decreasing tendency. In children with meningococcal septic shock in the United Kingdom, a 5% mortality rate was reported,15 and in the Netherlands a decreasing mortality was shown in the same patient group.16 A single-center study in the United States reported a 10% mortality rate.17 The investigators observed 0% mortality in previously healthy children but a 15% mortality rate in children with chronic illness (for the most part cancer patients). All of these children died with multiple organ failure. Ngo and colleagues18 observed a 0% mortality rate in a randomized Dengue shock fluid resuscitation trial. The US KIDS database showed a 4.2% severe sepsis mortality overall, with 2% in the previously healthy and 8% in the chronically ill child.13
Although outcomes are improving, the burden of newborn and pediatric sepsis is increasing in the United States. More children die with severe sepsis than die with cancer, with an estimated yearly healthcare cost of $4 billion in the United States for patients with this condition.12 Half are newborns, with most of these having low birth weight.9 Half of children with severe sepsis have underlying chronic illness. Neurologic and cardiovascular chronic illness is most common in infants with severe sepsis and cancer, whereas immune deficiency is most common in children with severe sepsis. Medical advances have affected etiology and epidemiology. In 1990, Jacobs and coworkers19 reported that the most common causes of septic shock in children were, in descending order, Haemophilus influenzae b, Neisseria meningitidis, and Streptococcus pneumoniae. The 1995 and 1999 U.S. estimates suggest a change. H. influenzae type b is all but nonexistent, N. meningitidis is prevalent in only a few regions of the United States, and group B Streptococcus is decreasing. The more recent use of S. pneumoniae vaccine is reducing the incidence of this infection. The Canadian government has implemented nationwide immunization in children younger than age 2 years for N. meningitidis serotype C.20 The most prevalent causes of severe sepsis and septic shock in the United States now seem to be staphylococcal and fungal infections.12 Methicillin-resistant Staphylococcus aureus (MRSA) is an emerging disease. Influenza vaccines are now universal for both endemic and pandemic forms (H1N1).
 Pathophysiology and Developmental Effects
Pathophysiology and Developmental Effects
Molecular Pathogenesis
Controlled Inflammation with Eradication of Infection
Endotoxin, mannose, and other glycoprotein moieties on the cell walls of yeast and fungi, superantigens, toxins associated with some gram-positive bacteria, mycobacteria, and viruses, also called pathogen-associated molecular patterns, activate the innate immune system after recognition by pathogen recognition receptors. The innate immune system comprises polymorphonuclear neutrophils, monocytes, and macrophages, in part through Toll-like receptors, CD14 receptors (endotoxin), and other costimulatory molecules. These innate immune cells internalize microorganisms and kill them. Monocytes and macrophages present processed antigens from these killed microorganisms to circulating T lymphocytes and coordinate the adaptive immune response. This second wave of immune response includes B-cell activation and antibody production and generation of cytotoxic T cells and natural killer cells (particularly in viral and fungal infection). Opsonization with antibodies allows more efficient recognition, killing, and clearing of microorganisms by resident macrophages in the reticuloendothelial system.21,22
Clinical Pathologic Correlates
On the basis of in vivo biochemical analyses and autopsy histology, several forms of multiple organ failure could be characterized.23–26 Thrombocytopenia-associated multiple organ failure (platelet count <100,000/µL or a 50% decrease in platelet count from baseline) was attributable to purpura fulminans and disseminated intravascular coagulation (DIC) with increased tissue factor activity in vivo and fibrin thrombi at autopsy in only 20% of patients. Of these patients, 80% showed thrombotic thrombocytopenic purpura pathophysiology with increased thrombogenic ultra-large von Willebrand factor multimers, absent von Willebrand factor cleaving protease (ADAMTS 13), increased PAI-1 activity in vivo, and platelet/fibrin thrombi at autopsy.
Coagulation System
As is generally accepted and explained in many reviews, coagulation and fibrinolysis are an integrative part of the immune system.27 There are important physiologic differences in the hemostatic system in children compared with adults. The decreased levels of several crucial coagulants and increased levels of α2-macroglobulin may contribute in part to the lower risk of thrombotic events in childhood during physiologic conditions.28,29 In pathologic conditions, these physiologic differences might lead to an earlier exhaustion of coagulation factors and DIC in infants and young children.30 ADAMTS 13 is also decreased in infancy, therefore there may be an increased susceptibility to systemic fibrin and platelet thrombosis The coagulation system is a marker of organ dysfunction in sepsis. It is associated with subsequent endothelium activation and systemic clotting and finally antifibrinolysis.
Cardiovascular System
Ceneviva and associates31 found that in contrast to adults, who predominantly have high-cardiac-output/low-vascular-resistance shock, children with fluid-refractory/inotropic-resistant shock have varied hemodynamic states, including low cardiac output/high systemic vascular resistance (60%), low cardiac output/low vascular resistance (20%), and high cardiac output/low vascular resistance (20%), which can change with time and depend on age. In contrast to adults, death from shock is most commonly associated with progressive cardiac failure, not vascular failure. Infants and children frequently are insensitive to dopamine or dobutamine and respond to epinephrine (cold shock) or norepinephrine (warm shock).31–33 Newborns are different as well. Adults can double their heart rate to improve cardiac output, but newborns cannot. Newborns, although tachycardic, depend on increased vascular tone to maintain blood pressure. Persistent pulmonary hypertension and right ventricular failure also complicate newborn septic shock.34,35
 Predisposing Factors and Prevention Strategies
Predisposing Factors and Prevention Strategies
Among the community-acquired causes of sepsis, N. meningitidis has a diverse clinical picture, ranging from a self-limiting bacteremia to meningitis to a severe rapidly fatal sepsis. After invasion of the bloodstream by the bacteria, three main cascade pathways are activated: the complement system, the inflammatory response, and the coagulation and fibrinolysis pathway. These pathways do not act independently but are able to interact with each other. Genetic polymorphisms among components of these pathways have been shown to be involved in the susceptibility, severity, and outcome of meningococcal disease. Knowledge of genetic variations associated with susceptibility to and severity of meningococcal infection has been reviewed.36
Complement deficiencies and defects in sensing or opsonophagocytic pathways, such as the rare Toll-like receptor 4 single nucleotide polymorphisms and combinations of inefficient variants of Fcγ-receptors, seem to have the most important role in genetically established susceptibility. The most recent and largest study on susceptibility is a genome-wide analysis of DNA from 1600 children with meningococcal sepsis. This study showed the significant influence of genetic variants in the complement factor H in the susceptibility.37 Effect on severity has repeatedly been reported for FcγRIIa and PAI-1 polymorphisms. Angiotensin-converting enzyme is associated with a proinflammatory response. The absence of a 284-base pair marker in the angiotensin-converting enzyme gene (D allele) is associated with higher circulating angiotensin-converting enzyme activity compared with the presence of this marker (I allele). The DD genotype is associated with increased disease severity, and although not significant, a twofold increase in mortality rate has been reported. Outcome effects have been confirmed for single nucleotide polymorphisms in properdin deficiencies, PAI-1 and combination of the −511C/T single nucleotide polymorphisms in IL-1β, and +2018C/T single nucleotide polymorphisms in IL RN. Conflicting results are reported for the effect of the −308G/A promoter polymorphism in TNF. These differences may reflect discrepancies in group definitions among studies or the influence of additional single nucleotide polymorphisms in the TNF promoter, which can form haplotypes representing different cytokine production capacity. For several single-nucleotide polymorphisms, the potential effect on susceptibility, severity, or outcome has not yet been confirmed in an independent study.
 Diagnostic Approach and Scoring Systems
Diagnostic Approach and Scoring Systems
Several prognostic factors have been related to severity and nonsurvival, as follows:
 Therapy
Therapy
Early Recognition and Goal-Directed Therapy to Improve Outcome
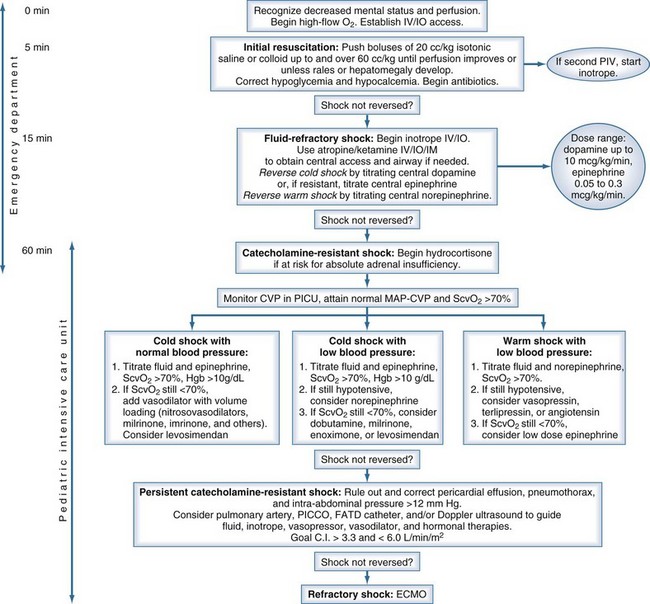
Early recognition, adequate resuscitation, appropriate therapeutic response, removal of the nidus of infection, and effective antibiotic therapy are crucial to optimal outcome.45,46 In June 2007, the American College of Critical Care Medicine published its evidence-based Clinical Practice Parameters for Hemodynamic Support of Newborns and Children with Septic Shock, based in part on the concept that early recognition and resuscitation improve outcome (Figure 131-1). The major new recommendations include the use of inotropes through a peripheral intravenous (IV) or intraosseous catheter until a central catheter is available, and administration of antibiotics in the first hour.
Immediate Resuscitation (First Hour)
Airway and Breathing
Newborns and children usually have an adequate airway, but mechanical ventilation is required in 80% in shock. Intubation should be performed according to pediatric advanced life support and Neonatal Resuscitation Program guidelines on the basis of clinical diagnosis of respiratory distress or hemodynamic instability, not blood gas analysis. Volume resuscitation and the use of the non–cardiac depressant drug ketamine as an induction agent are recommended to prevent worsening positive-pressure ventilation–associated hypotension. It is clinical practice to intubate pediatric patients in an early stage of the disease, generally when they need more than 60 mL/kg of fluid resuscitation.15
Volume Resuscitation
Virtually all children with shock require aggressive volume resuscitation10,47,48; this should be given as 20 mL/kg boluses of normal saline or colloid as IV pushes to a total of 60 mL/kg in the first 10 to 20 minutes. If the liver edge becomes palpable, rales are heard, or the perfusion pressure (mean arterial pressure—central venous pressure) narrows, more fluid is not advised. Some children have required 200 mL/kg in the first hour. Many clinicians use crystalloid as the first fluid and follow with colloid if this is unsuccessful. Serum glucose should be checked because hypoglycemia can have devastating neurologic consequences. Glucose should be administered rapidly in this condition.
Cardiovascular Therapy
Children in shock can present with low cardiac output and high systemic vascular resistance, high cardiac output and low systemic vascular resistance, or low cardiac output and low systemic vascular resistance.31 Depending on which situation exists, inotropic support should be started in the case of fluid-refractory shock or a combination of an inotrope with a vasopressor or a vasodilator. Dopamine or dobutamine is probably the first choice of support for a pediatric patient with hypotension refractory to fluid resuscitation. The choice of vasoactive agent is determined by the clinical examination. Dobutamine-refractory or dopamine-refractory shock often can be reversed with epinephrine or norepinephrine infusion.31 Pediatric patients requiring inotropic support are in a state of low cardiac output, not high cardiac output. The use of vasodilators can reverse shock in pediatric patients who remain hypodynamic with a high systemic vascular resistance state, despite fluid resuscitation and implementation of inotropic support. Nitrosovasodilators (nitroprusside or nitroglycerin have a short half-life) are used as first-line therapy for children with epinephrine-resistant low cardiac output and elevated systemic vascular resistance shock.
Adrenal Insufficiency
Lack of response to epinephrine (cold shock) or norepinephrine (warm shock) can be caused by adrenal insufficiency or thyroid deficiency.49–51 Children at risk for this condition (e.g., purpura fulminans, prior steroid exposure, central nervous system disease) should be treated with hydrocortisone. The proper dose has been poorly investigated and ranges from a stress dose (2 mg/kg) to a shock dose (50 mg/kg of hydrocortisone) followed by the same dose over 24 hours. Which dose is better in catecholamine-resistant shock has not been determined.
Antibiotics
Antibiotics and antifungal therapies should be administered according to age, setting, and resistance patterns (empirical therapy) after proper cultures have been performed. The emergence of resistant organisms mandates that antibiotics be specific to regional practice. Some investigators advocate antibiotic cycling in the ICU.52 Although survival from sepsis and septic shock can occur only if the infection is eradicated, administration of antibiotics should never supersede or postpone volume and cardiovascular resuscitation.
Stabilization of Sepsis and Septic Shock (After First Hour of Resuscitation)
Cardiovascular
The first hour of resuscitation is directed toward restoration of normal perfusion pressure; however, ensuing therapies should be directed toward obtaining normal central venous oxygen saturation. Children with persistent warm shock can respond to more volume and norepinephrine. In selected children with norepinephrine-resistant shock, vasopressin (at physiologic dose) or angiotensin can bypass alpha receptor desensitization and restore vascular tone; however, this can increase afterload and decrease cardiac output.53–55 In a large study in pediatric patients with vasodilatory shock (majority being post cardiac surgery), vasopressin was useful, with limitations regarding its adverse effects on the renal system and platelet counts.56 Children with cold shock and normal blood pressure respond to afterload reduction and volume loading.31,56 When pediatric patients remain in a normotensive low-cardiac-output and high-vascular-resistance state despite epinephrine and nitrosovasodilator therapy, the use of milrinone (if liver dysfunction is present) or amrinone (if renal dysfunction is present) should be strongly considered.57 These type III phosphodiesterase inhibitors can bypass β-adrenergic receptor desensitization.57–59 Children with cold shock and hypotension are most worrisome. They can respond to more volume and epinephrine. Neonates and children with pulmonary hypertension and right ventricular failure can respond to inhaled NO.60 These therapies should be titrated to obtain a superior vena cava oxygen saturation above 70%.61
Extracorporeal membrane oxygenation is an effective therapy in refractory neonatal shock (80% survival) and should be considered as a possible therapy in refractory pediatric shock (50% survival).62,63 This success is likely due to the fact that refractory shock in newborns and children is usually cardiac, not vascular, failure. Adults with refractory shock from Hantavirus (a low-cardiac-output/high-vascular-resistance state) have similar extracorporeal membrane oxygenation outcomes to newborns with refractory shock.64
Respiratory
Lung “protection” ventilation strategies reduced mortality rates in adults with acute respiratory distress syndrome (many who had sepsis).65 Effective tidal volumes of 6 mL/kg are a reasonable compromise when ventilating septic children with acute respiratory distress syndrome. Positive end-expiratory pressure protects against volutrauma by maintaining functional residual capacity and optimal compliance. Optimal positive end-expiratory pressure can be determined using partial pressure of oxygen in arterial blood–to–inspired oxygen fraction ratio or compliance.
Renal Failure
Blood flow to the kidney is autoregulated by preglomerular and postglomerular constriction and dilation. The ability of the preglomerular arterioles to dilate is impaired during endotoxemia and cirrhosis. Blood flow to the kidney depends on perfusion pressure (measured as mean arterial pressure—central venous pressure or, in the case of abdominal compartment syndrome, mean arterial pressure—intraabdominal pressure) in children with sepsis.66 Perfusion pressure should be maintained with volume, inotropes, and in some cases vasopressor therapies. Creatinine clearance should be measured daily to assess function. Diuretics are recommended to prevent fluid overload. Patients with myoglobinuria or uric aciduria should be treated with mannitol, alkalinization, and allopurinol (uric aciduria). Severe oliguria or anuria despite diuretics should be managed with daily or continuous hemofiltration/hemodialysis or peritoneal dialysis.
Purpura Fulminans and Disseminated Intravascular Coagulation
DIC is recognized clinically as a prolonged prothrombin time/partial thromboplastin time, reduced fibrinogen, increased fibrin degradation products or D-dimers and thrombocytopenia.41,42 When patients present with purpura fulminans/DIC, with genetic proclivity (thrombophilias), or with rapidly growing organisms (meningococcus), the process is deadly unless reversed. Tissue factor is exposed by endothelial injury and released into the bloodstream. If tissue factor is unmatched by tissue factor pathway inhibitor, it activates factor VII–mediated coagulation. Ongoing coagulation consumes clotting factors (including fibrinogen), antithrombotic factors (antithrombin III and protein C), and platelets; this leads to a state of massive clotting and bleeding. Therapeutic strategies must restore a homeostatic milieu by removing or inhibiting tissue factor activity and replacing anticoagulant factors, procoagulant factors, and platelets. If systemic clotting is limb-threatening or life-threatening, fibrinolytic therapies may be required for reperfusion. Debate continues on whether specific therapies (e.g., antithrombin III, protein C, heparin, activated protein C, tissue plasminogen activator), nonspecific therapies (fresh frozen plasma and platelet replacement or plasma exchange), or a combination of both (plasma exchange plus antithrombin III, protein C, or activated protein C with tissue plasminogen activator added for limb-threatening or life-threatening thrombosis) is best. An activated protein C trial initiated in pediatric septic shock, in which patients at risk of bleeding (low platelet counts) or receiving heparin-based continuous venovenous hemofiltration were excluded, showed no benefit of treatment compared to placebo.67 Some investigators think that patients with meningococcemia cannot activate protein C,68 whereas others have shown that these children can activate protein C.68 So far there is no evidence for benefit of either product. Studies using intensive plasma exchange therapy appears to be of possible benefit because plasma exchange reverses both fibrin and platelet-vWF multimer-mediated thrombosis.69–71
Nutrition, Electrolytes, Endocrine, and Metabolism
It is debated whether one should feed patients enterally when in shock; however, there is agreement the enteral route is best when shock resolves. Total parenteral nutrition should be considered in patients not tolerating enteral feeds and “calories given” directed to “calories expended” if a metabolic monitor is available. If a monitor is not available, calorie needs can be overestimated when using classic formulas in critically ill children. Hypoglycemia should be rigorously avoided and treated. Hypoglycemia is associated with devastating neurologic outcomes. Strict control of hyperglycemia with insulin infusion substantially reduced mortality in a pediatric ICU by reducing deaths from multiple-organ dysfunction syndrome/multiple organ failure.72 In general, infants are at risk for developing hypoglycemia when they depend on IV fluids; a glucose intake of 4 to 6 mg/kg/min or maintenance fluid intake with glucose 10% and sodium chloride 0.45% is advised.
Immune Modulation
Children who cannot kill invading organisms die from sepsis. Primary and acquired immunodeficiency states must be treated. Children with chronic granulomatous disease require white blood cell transfusions and interferon. Patients with hypogammaglobulinemia require treatment with IV immunoglobulin. Granulocyte-macrophage colony-stimulating factor was shown in a randomized controlled trial to improve survival in newborn neutropenic septic shock.73,74 Transplant and nontransplant patients who develop septic shock while receiving immune suppression die unless the immune suppressants are rapidly tapered. Polyclonal IV immunoglobulin has been reported to reduce mortality rate and is a promising adjuvant in the treatment of sepsis and septic shock. All the trials have been small in children, however, and the totality of the evidence is insufficient to support a robust conclusion of benefit. Adjunctive therapy with monoclonal IV immunoglobulin is experimental.75
Drug Dosing
Decreased cytochrome P450 activity not only is manifest in impaired steroid synthesis, but also impaired drug metabolism is present in children with sepsis, septic shock, or multiple organ failure.23 Patients with multiple organ failure are at particular risk of toxicity with drugs that are metabolized by the cytochrome P450 system. Renal function also is impaired. Creatinine clearance–directed drug dosing of renally eliminated drugs is necessary in these patients. Drugs should be administered according to pharmacodynamic and pharmacokinetic goals.
 Multicenter Randomized Controlled Trials for Pediatric Septic Shock
Multicenter Randomized Controlled Trials for Pediatric Septic Shock
Two studies were completed examining the role of endotoxin-neutralizing therapies in children with presumed meningococcal purpura fulminans/shock. Derkx and colleagues76 reported a 25% reduction in mortality rate with the HA-1A antibody, and Giroir and others14,77 reported a 25% reduction in mortality rate with rhBPI. Both studies were underpowered. Nadel repeated the Activated Protein C trial in children with septic shock and observed no benefit of DrotAA in children with severe sepsis; serious bleeding events were similar between groups and the overall safety profile acceptable, except in children younger than 60 days.78 It is unknown whether this was due to developmental differences or greater use of plasma products in children compared to adults. deOliveira and colleagues observed a greater than threefold reduction in mortality when using ACCM-PALS therapies directed to RA/SVC or RA/IVC oxygen saturations over 70%.61 The intervention arm received more fluids, blood, and inotrope/vasodilators than the nonintervention arm. In two trials, neither vasopressin nor terlipressin were effective in improving outcomes in refractory vasodilated shock.79–80
Key Points
Carcillo JA, Fields AI, American College of Critical Care Medicine Task Force Committee Members. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365-1378.
Emonts M, Hazelzet JA, de Groot R, Hermans PW. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect Dis. 2003;3:565-577.
Leteurtre S, Martinot A, Duhamel A, Gauvin F, Grandbastien B, Nam TV, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. 1999;19:399-410.
Pollard AJ, Britto J, Nadel S, DeMunter C, Habibi P, Levin M. Emergency management of meningococcal disease. Arch Dis Child. 1999;80:290-296.
An overview of the acute treatment of pediatric meningococcal sepsis is presented.
Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695-701.
1 Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530-538.
2 Wilkinson JD, Pollack MM, Glass NL, et al. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111:324-328.
3 Proulx F, Gauthier M, Nadeau D, et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025-1031.
4 Doughty L, Carcillo JA, Kaplan S, et al. Plasma nitrite and nitrate concentrations and multiple organ failure in pediatric sepsis. Crit Care Med. 1998;26:157-162.
5 Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: Use of two strategies. Med Decis Making. 1999;19:399-410.
6 Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365-1378.
7 DuPont HL, Spink WW. Infections due to gram-negative organisms: An analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958-1969. Medicine (Baltimore). 1969;48:307-332.
8 Pollack MM, Fields AI, Ruttimann UE. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit Care Med. 1985;13:454-459.
9 Stoll BJ, Holman RC, Schuchat A. Decline in sepsis-associated neonatal and infant deaths in the United States, 1979 through 1994. Pediatrics. 1998;102:e18.
10 Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242-1245.
11 Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
12 Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695-701.
13 Odetola FO, Gebremanian A. Freed GL patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494.
14 Giroir BP, Scannon PJ, Levin M. Bactericidal/permeability-increasing protein—lessons learned from the phase III, randomized, clinical trial of rBPI21 for adjunctive treatment of children with severe meningococcemia. Crit Care Med. 2001;29:S130-S135.
15 Pollard AJ, Britto J, Nadel S, et al. Emergency management of meningococcal disease. Arch Dis Child. 1999;80:290-296.
16 Maat M, Buysse CM, Emonts M, et al. Improved survival of children with sepsis and purpura: effects of age, gender, and era. Crit Care. 2007;11(5):172.
17 Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4:333-337.
18 Ngo NT, Cao XT, Kneen R, et al. Acute management of dengue shock syndrome: A randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis. 2001;32:204-213.
19 Jacobs RF, Sowell MK, Moss MM, et al. Septic shock in children: Bacterial etiologies and temporal relationships. Pediatr Infect Dis J. 1990;9:196-200.
20 MacLennan JM, Shackley F, Heath PT, et al. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: A randomized controlled trial. JAMA. 2000;283:2795-2801.
21 Delves PJ, Roitt IM. The immune system: First of two parts. N Engl J Med. 2000;343:37-49.
22 Delves PJ, Roitt IM. The immune system: Second of two parts. N Engl J Med. 2000;343:108-117.
23 Carcillo JA, Doughty L, Kofos D, et al. Cytochrome P450-mediated drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003;29:980-984.
24 Doughty L, Clark RS, Kaplan SS, et al. sFas and sFas ligand and pediatric sepsis-induced multiple organ failure syndrome. Pediatr Res. 2002;52:922-927.
25 Green J, Doughty L, Kaplan SS, et al. The tissue factor and plasminogen activator inhibitor type-1 response in pediatric sepsis-induced multiple organ failure. Thromb Haemost. 2002;87:218-223.
26 Nguyen T, Hall M, Han Y, et al. Microvascular thrombosis in pediatric multiple organ failure: Is it a therapeutic target? Pediatr Crit Care Med. 2001;2:187-196.
27 Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765-3777.
28 Andrew M, Vegh P, Johnston M, et al. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998-2005.
29 Andrew M. Developmental hemostasis: Relevance to hemostatic problems during childhood. Semin Thromb Hemost. 1995;21:341-356.
30 Hazelzet JA, Risseeuw-Appel IM, Kornelisse RF, et al. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemost. 1996;76:932-938.
31 Ceneviva G, Paschall JA, Maffei F, et al. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19.
32 Simma B, Fritz MG, Trawoger R, et al. Changes in left ventricular function in shocked newborns. Intensive Care Med. 1997;23:982-986.
33 Mercier JC, Beaufils F, Hartmann JF, et al. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16:27-33.
34 Gill AB, Weindling AM. Echocardiographic assessment of cardiac function in shocked very low birthweight infants. Arch Dis Child. 1993;68:17-21.
35 Sosa G, Milstein JM, Bennett SH. Escherichia colt endotoxin depresses left ventricular contractility in neonatal lambs. Pediatr Res. 1994;35:62-67.
36 Emonts M, Hazelzet JA, de Groot R, et al. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect Dis. 2003;3:565-577.
37 Davila S, Wright VJ, Khor CC, et al. Genome wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet. 2010. Aug Epub ahead of print
38 Marcin JP, Pollack MM, Patel KM, et al. Decision support issues using a physiology based score. Intensive Care Med. 1998;24:1299-1304.
39 Kornelisse RF, Hazelzet JA, Hop WC, et al. Meningococcal septic shock in children: Clinical and laboratory features, outcome, and development of a prognostic score. Clin Infect Dis. 1997;25:640-646.
40 Riordan FA, Marzouk O, Thomson AP, et al. Prospective validation of the Glasgow Meningococcal Septicaemia Prognostic Score: Comparison with other scoring methods. Eur J Pediatr. 2002;161:531-537.
41 Leclerc F, Hazelzet J, Jude B, et al. Protein C and S deficiency in severe infectious purpura of children: A collaborative study of 40 cases. Intensive Care Med. 1992;18:202-205.
42 Taylor FBJr, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327-1330.
43 Leteutre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192-197.
44 Shime N, Kageyama K, Ashida H, et al. Application of modified sequential organ failure assessment score in children after cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:463-468.
45 Booy R, Habibi P, Nadel S, et al. Reduction in case fatality rate from meningococcal disease associated with improved healthcare delivery. Arch Dis Child. 2001;85:386-390.
46 Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
47 Lambert HJ, Baylis PH, Coulthard MG. Central-peripheral temperature difference, blood pressure, and arginine vasopressin in preterm neonates undergoing volume expansion. Arch Dis Child Fetal Neonatal Educ. 1998;78:F43-F45.
48 Pladys P, Wodey E, Betremieux P, et al. Effects of volume expansion on cardiac output in the preterm infant. Acta Paediatr. 1997;86:1241-1245.
49 Hatherill M, Tibby SM, Hilliard T, et al. Adrenal insufficiency in septic shock. Arch Dis Child. 1999;80:51-55.
50 Joosten KF, de Kleijn ED, Westerterp M, et al. Endocrine and metabolic responses in children with meningococcal sepsis: Striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab. 2000;85:3746-3753.
51 De Kleijn ED, Joosten KF, Van Rijn B, et al. Low serum cortisol in combination with high adrenocorticotrophic hormone concentrations are associated with poor outcome in children with severe meningococcal disease. Pediatr Infect Dis. 2002;21:330-336.
52 Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med. 2001;134:298-314.
53 Yunge M, Petros A. Angiotensin for septic shock unresponsive to noradrenaline. Arch Dis Child. 2000;82:388-389.
54 Rosenzweig EB, Starc TJ, Chen JM, et al. Intravenous arginine vasopressin in children with vasodilatory shock after cardiac surgery. Circulation. 1999;100:II-182-II-186.
55 Leclerc F, Walter-Nicolet E, Leteurtre S, et al. Admission plasma vasopressin levels in children with meningococcal septic shock. Intensive Care Med. 2003;29:1339-1344.
56 Benitz WE, Rhine WD, Van Meurs KP, et al. Nitrovasodilator therapy for severe respiratory distress syndrome. J Perinatol. 1996;16:443-448.
57 Irazuzta JE, Pretzlaff RK, Rowin ME. Amrinone in pediatric refractory septic shock: An open-label pharmacodynamic study. Pediatr Crit Care Med. 2001;2:24-28.
58 Barton P, Garcia J, Kouatli A, et al. Hemodynamic effects of i.v. milrinone lactate in pediatric patients with septic shock: A prospective, double-blinded, randomized, placebo-controlled, interventional study. Chest. 1996;109:1302-1312.
59 Lindsay CA, Barton P, Lawless S, et al. Pharmacokinetics and pharmacodynamics of milrinone lactate in pediatric patients with septic shock. J Pediatr. 1998;132:329-334.
60 Lonnqvist PA. Inhaled nitric oxide in newborn and paediatric patients with pulmonary hypertension and moderate to severe impaired oxygenation: Effects of doses of 3-100 parts per million. Intensive Care Med. 1997;23:773-779.
61 de Oliveira CF, de Oliveira DS, Gottschald AF, et al. ACCM/PALS hemodynamic support guidelines for pediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34(6):1065-1075.
62 Goldman AP, Kerr SJ, Butt W, et al. Extracorporeal support for intractable cardiorespiratory failure due to meningococcal disease. Lancet. 1997;349:466-469.
63 Meyer DM, Jessen ME. Results of extracorporeal membrane oxygenation in children with sepsis. The Extracorporeal Life Support Organization. Ann Thorac Surg. 1997;63:756-761.
64 Crowley MR, Katz RW, Kessler R, et al. Successful treatment of adults with severe Hantavirus pulmonary syndrome with extracorporeal membrane oxygenation. Crit Care Med. 1998;26:409-414.
65 The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
66 Greenhalgh DG, Warden GD. The importance of intra-abdominal pressure measurements in burned children. J Trauma. 1994;36:685-690.
67 De Kleijn ED, De Groot R, Hack CE, et al. Activation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: A randomized, double-blinded, placebo-controlled, dose-finding study. Crit Care Med. 2003;31:1839-1847.
68 Faust SN, Levin M, Harrison OB, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345:408-416.
69 Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis therapy in severe sepsis and septic shock a prospective randomized controlled trial. Intensive Care Med. 2002;28(10):1484-1492.
70 Darmon M, Azoulay E, Thiery G, et al. Time course of organ dysfunction in thrombotic microangiopathy patients receiving either plasma infusion or plasma exchange. Crit Care Med. 2006;34(8):2127-2133.
71 Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange therapy increases ADAMTS 13 and reverses organ dysfunction in children with thrombocytopenia associated multiple organ failure. Crit Care Med. 2008;36(10):2878-2887.
72 Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in pediatric intensive care; a prospective randomized controlled study. Lancet. 2009;373(9663):547-556.
73 Schaison G, Eden OB, Henze G, et al. Recommendations on the use of colony-stimulating factors in children: Conclusions of a European panel. Eur J Pediatr. 1998;157:955-966.
74 Trindade E, Maton P, Reding R, et al. Use of granulocyte macrophage colony stimulating factor in children after orthotopic liver transplantation. J Hepatol. 1998;28:1054-1057.
75 Alejandria MM, Lansang MA, Dans LF, et al. Intravenous immunoglobulin for treating sepsis and septic shock. Cochrane Database Syst Rev 2002;CD001090.
76 Derkx B, Wittes J, McCloskey R. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. European Pediatric Meningococcal Septic Shock Trial Study Group. Clin Infect Dis. 1999;28:770-777.
77 Levin M, Quint PA, Goldstein B, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356:961-967.
78 Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;10(9564):836-843. 369
79 Choong K, Bohn D, Fraser DD, et al. Vasopressin in pediatric vasodilatory shock: a multicentric randomized controlled trial. Am J Resp Crit Care Med. 2009;180(7):632-639.
80 Yildizdas D, Yapicioglu H, Celik D, Sertdemir Y, Alham E. Terlipressin as a rescue therapy for catecholamine resistant septic shock in children. Intensive Care Med. 2008;34(3):511-517.