9 SENSORY ORGANS: VISION AND HEARING
EYE
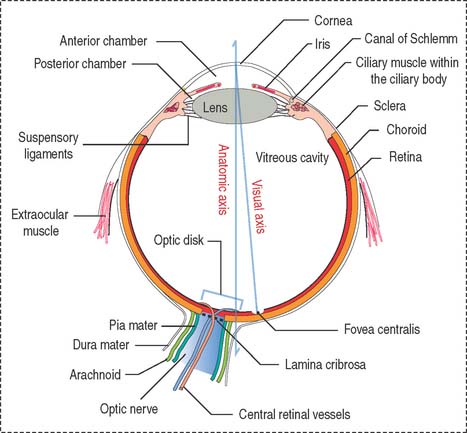
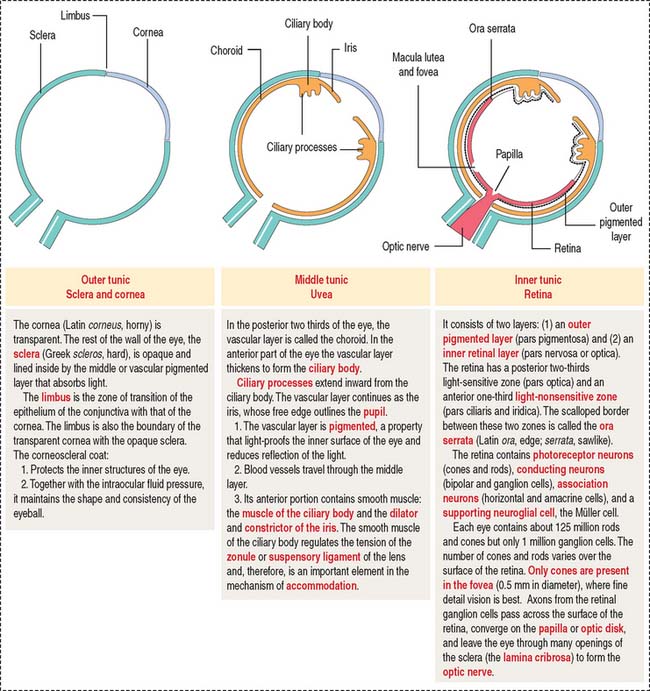
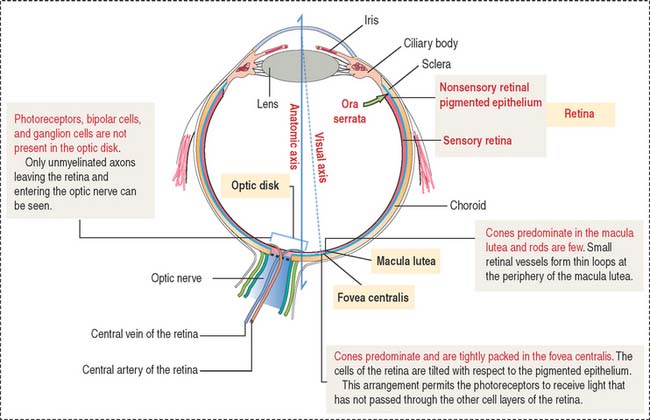
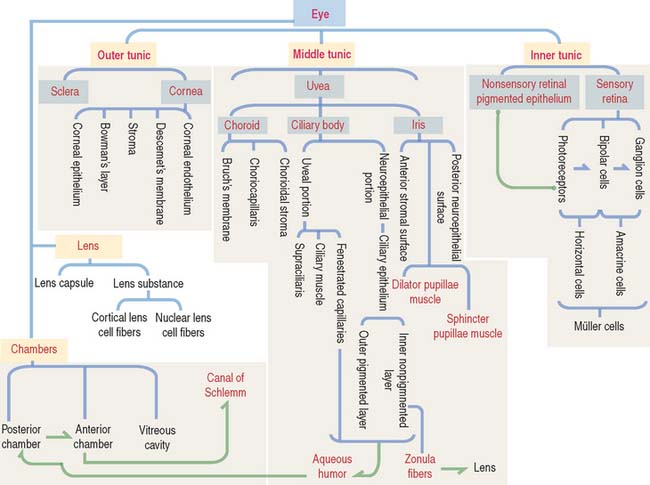
The eyeball consists of three tunics or layers which, from outside to inside, are (1) the sclera and the cornea, (2) the uvea, and (3) the retina (Figure 9-1).
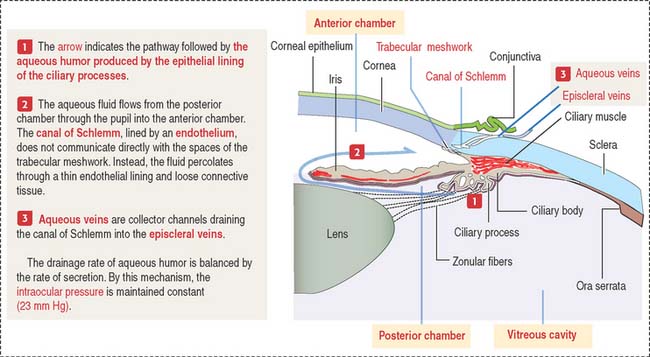
Three distinct and interconnected chambers are found inside the eyeball: the anterior chamber, the posterior chamber, and the vitreous cavity (see Box 9-A). Aqueous humor circulates from the posterior to the anterior chamber. The lens is placed in front of the vitreous cavity, which contains vitreous humor. The bony orbit, the eyelids, the conjunctiva, and the lacrimal apparatus protect the eyeball.
Box 9-A Anatomy of the eye
DEVELOPMENT OF THE EYE
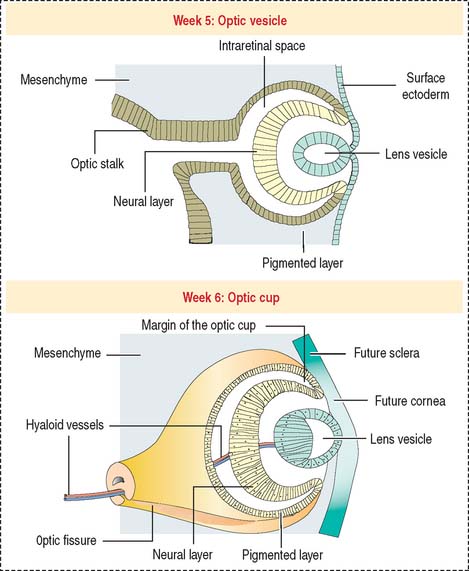
Lateral outpocketings of the right and left sides of the diencephalon give rise to two neuroepithelial optic vesicles, each remaining attached to the brain wall by a hollow optic stalk (Figure 9-2). The surface ectoderm of the head invaginates into the optical vesicle forming a lens vesicle that pinches off. Mesenchyme surrounds both the lens vesicle and the adjacent optic vesicle.
The optic vesicle invaginates and becomes a double-walled optic cup (see Figure 9-2). The optic fissure forms when the outer layer of the optic cup becomes the pigmented epithelium. Cells in the inner layer proliferate and stratify to form the neural retina. The mesenchyme extending into the invagination of the optic cup acquires a gelatinous consistency and becomes the vitreous component of the eye. The lens vesicle is kept in place by the free margins of the optic cup and the surrounding mesenchyme.
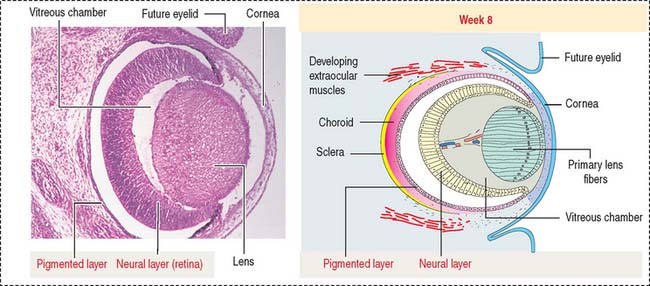
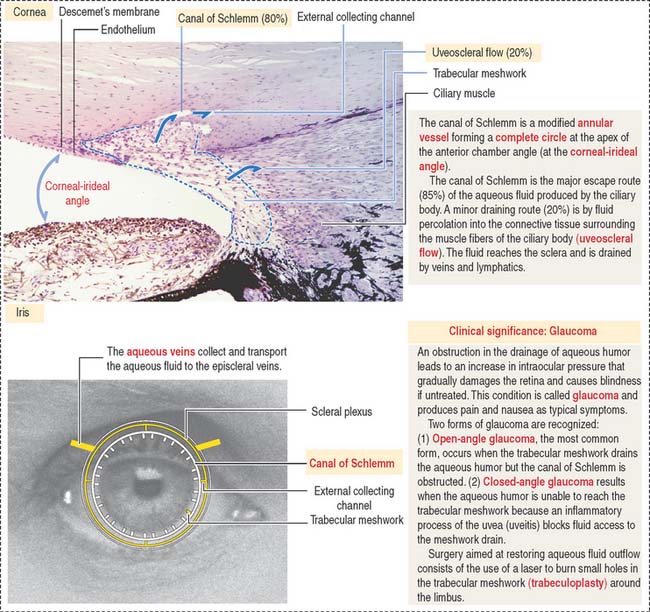
At the outer surface of the optic cup, the mesenchymal shell differentiates into the vascular choroid coat of the eye and the fibrous components of the sclera and cornea (Figure 9-3; see Box 9-B). Posterior to the lens, the vascular choroid coat forms the ciliary body, ciliary muscle, and ciliary processes. Anterior to the lens, the choroid coat forms the stroma of the iris. The ciliary processes secrete the aqueous humor that accumulates first in the posterior chamber (between the iris and lens) and then passes into the anterior chamber (between the lens and cornea) across the pupil. The aqueous humor leaves the anterior chamber by entering into the canal of Schlemm, a small vein (sinus venosus of the sclera) encircling the eye at the anterior edge of the choroid coat.
Box 9-B Development of the cornea
Around the rim of the optic cup, the inner and outer layers form the posterior epithelium of the ciliary body and iris. The sphincter and dilator pupillae muscles develop from the posterior epithelium.
OUTER TUNIC: SCLERA AND CORNEA
The sclera (Figure 9-4) is a 1.0- to 0.4-mm-thick layer of collagen and elastic fibers produced by fibroblasts. The inner side of the sclera faces the choroid, from which it is separated by a layer of loose connective tissue and an elastic tissue network known as the suprachoroid lamina. Tendons of the six extrinsic muscles of the eye are attached to the outer surface of the sclera.
Cornea
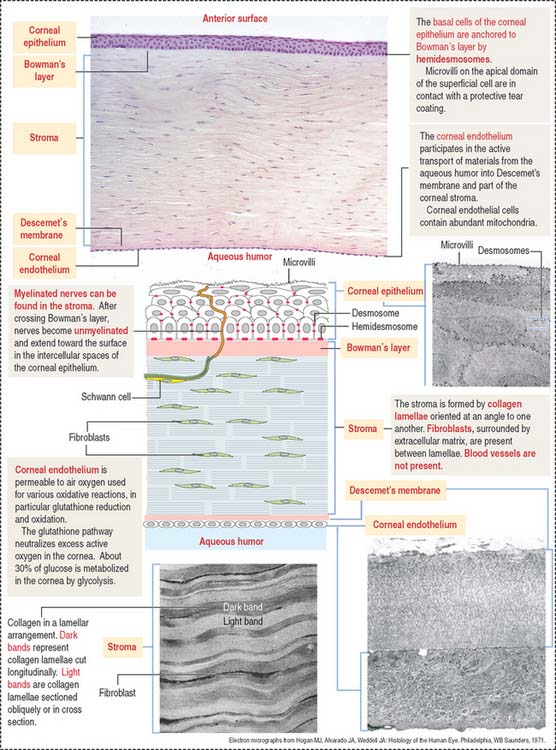
The cornea is composed of five layers (Figure 9-5):
Bowman’s layer is 6 to 9 μm thick, consists of type I collagen fibrils, and lacks elastic fibers. This layer is transparent and does not have regenerative capacity. Bowman’s layer is the anteriormost part of the corneal stroma, although differently organized. For this reason, it is designated “layer” instead of “membrane.” Bowman’s layer represents a protective barrier to trauma and bacterial invasion.
Nerves in transit to the corneal epithelium are found in the corneal stroma.
The corneal endothelium lines the posterior surface of Descemet’s membrane and faces the anterior chamber of the eye. It consists of a single layer of squamous epithelial cells, with impermeable intercellular spaces preventing influx of aqueous humor into the corneal stroma. The structural and functional integrity of the corneal endothelium is vital to the maintenance of corneal transparency (see Box 9-C).
Box 9-C Corneal transplantation
MIDDLE TUNIC: UVEA
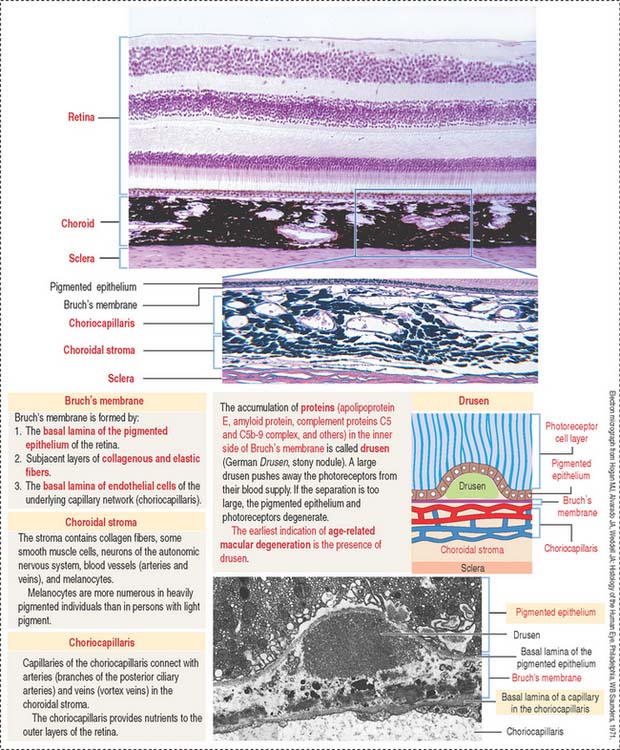
The uvea forms the pigmented vascularized tunic of the eye and is divided into three regions: (1) the choroid, (2) the ciliary body, and (3) the iris (see Figure 9-7) (see Box 9-D).
Box 9-D Uveal tract
The choroid consists of three layers (Figure 9-6):
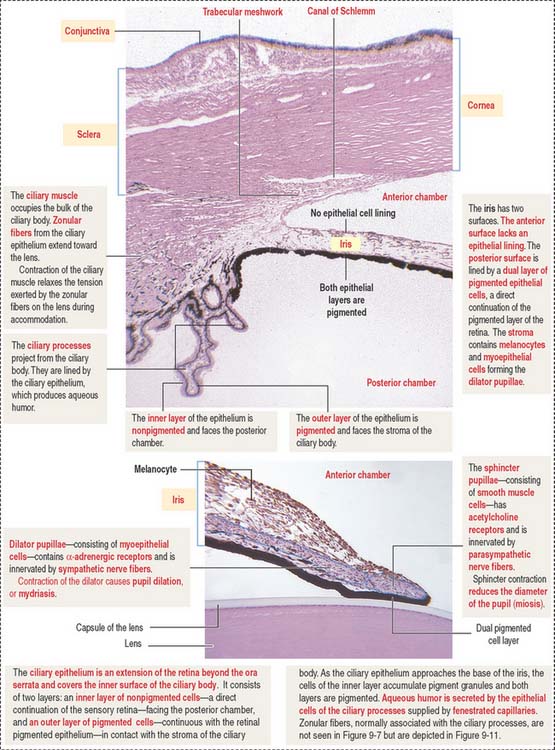
The ciliary body (Figure 9-7) is anterior to the ora serrata and represents the ventral projection of both the choroid and the retina. It is made up of two components: (1) the uveal portion and (2) the neuroepithelial portion.
The uveal portion of the ciliary body includes:
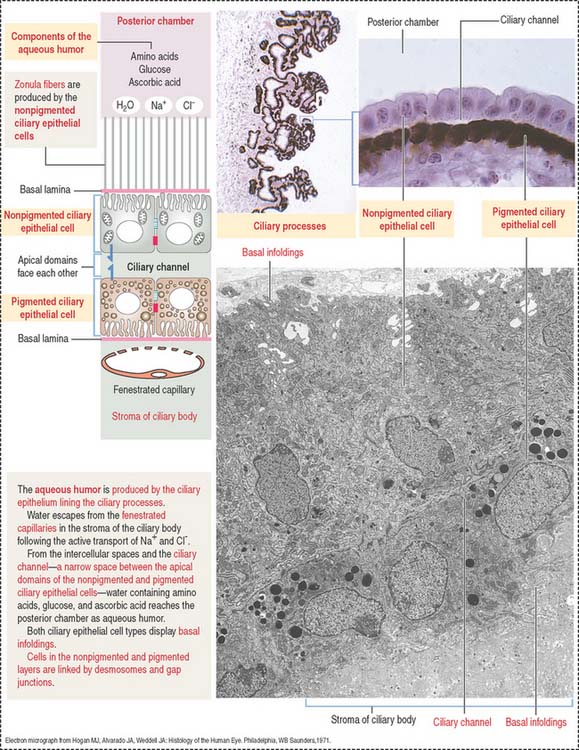
The neuroepithelial portion contributes the two layers of the ciliary epithelium:
Particular features of these two pigmented and nonpigmented epithelial cell layers are:
The anterior (outer) uveal face is of mesenchymal origin and has an irregular surface. It is formed by fibroblasts and pigmented melanocytes embedded in an extracellular matrix. The number of pigmented melanocytes determines the color of the iris. In albinos, the iris appears pink due to the abundant blood vessels. Blood vessels of the iris have a radial distribution and can adjust to changes in length in parallel to variations in the diameter of the pupil.
The posterior (inner) neuroepithelial surface consists of two layers of pigmented epithelium. The outer layer, a continuation of the pigmented layer of the ciliary epithelium, consists of myoepithelial cells that become the dilator pupillae muscle. The smooth muscle of the sphincter pupillae is located in the iris stroma around the pupil.
Three chambers of the eye
The eye contains three chambers (see Figure 9-1): (1) the anterior chamber, (2) the posterior chamber, and (3) the vitreous cavity. The vitreous is the largest component of the eye. The longest part of the optical path from the cornea to the retina is through the vitreous.
The anterior chamber occupies the space between the corneal endothelium (anterior boundary) and the anterior surface of the iris, the pupillary portion of the lens, and the base of the ciliary body (posterior boundary). The circumferential angle of the anterior chamber is occupied by the trabecular meshwork, a drainage site for the aqueous humor into the canal of Schlemm (Figures 9-9 and 9-10).
The posterior chamber (see Figure 9-9) is limited anteriorly by the posterior surface of the iris and posteriorly by the lens and the zonular fibers (suspensory ligaments of the lens). The circumferential angle is occupied by the ciliary processes, the site of aqueous humor production.
LENS
The cornea, the three chambers of the eye, and the lens are three transparent structures through which light must pass to reach the retina. Note that the refractive surface of the cornea is an interface between air and tissue and that the lens is in a fluid environment whose refractive index is higher than that of air.
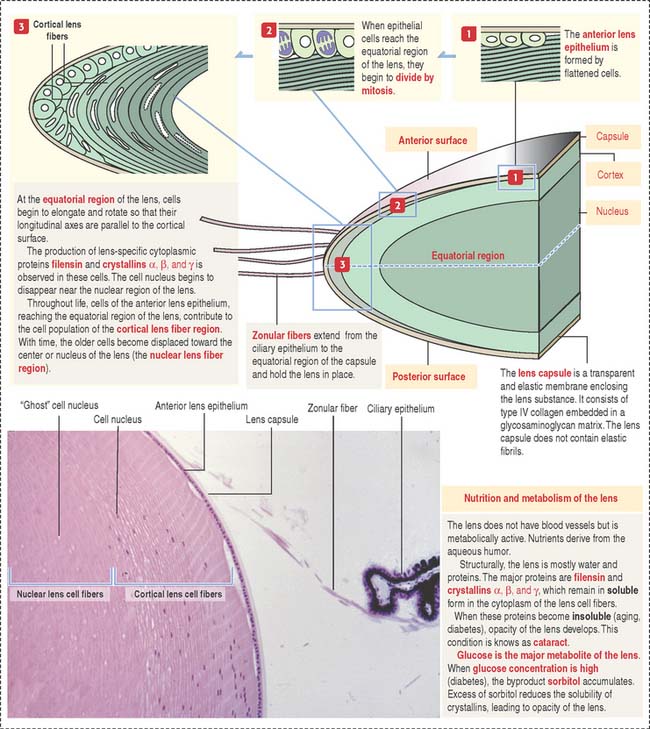
The lens is a transparent, biconvex, elastic, and avascular structure (Figure 9-11). Zonular fibers, consisting of elastin fibrils and a polysaccharide matrix, extend from the ciliary epithelium and insert at the equatorial portion of the capsule. They maintain the lens in place and, during accommodation, change the shape and optical power of the lens in response to forces exerted by the ciliary muscle. The zonular fibers support the lens “as guy wires support a tent.”
The lens capsule is a thick flexible acellular and transparent basement membrane—like structure containing type IV collagen fibrils and a glycosaminoglycan matrix. Beneath the anterior portion of the capsule is a single layer of cuboidal epithelial cells that extend posteriorly up to the equatorial region. In the cortical region of the lens, elongated and concentrically arranged cells (called cortical lens fibers) arise from the anterior epithelium at the equator region. Cortical lens fibers contain a nucleus and organelles. The nucleus and organelles eventually disappear when the cortical lens fibers approach the center of the lens—the nuclear lens fiber region.
Lens cell differentiation consists of the appearance of unique cytoskeletal proteins: (1) filensin, an intermediate filament that contains attachment sites for crystallins and (2) lens-specific proteins called crystallins (α, β, and γ). Filensin and crystallins maintain the conformation and transparency of the lens fiber cell.
Accommodation
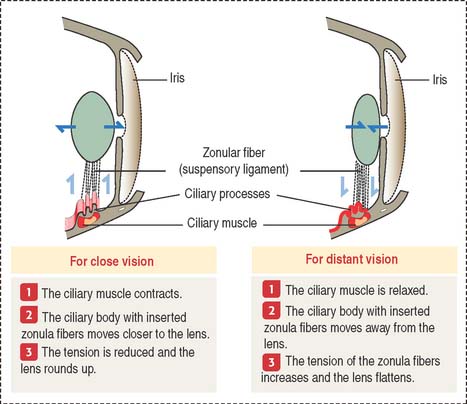
The sharpness of distant and close images focused on the retina depends on the shape of the lens (Figure 9-12). Accommodation defines the process by which the lens becomes rounder to focus the image of a nearby object on the retina and flattens when the image of a distant object is focused on the retina.
Three components contribute to the accommodation process: (1) the ciliary muscle, (2) the ciliary body, and (3) the suspensory ligaments, inserted at the equatorial region of the lens capsule.
INNER LAYER: RETINA
The retina consists of two regions (Figure 9-13): (1) the outer nonsensory retinal pigmented epithelium, and (2) the inner sensory retina (see Box 9-E).
Box 9-E Highlights of the retina
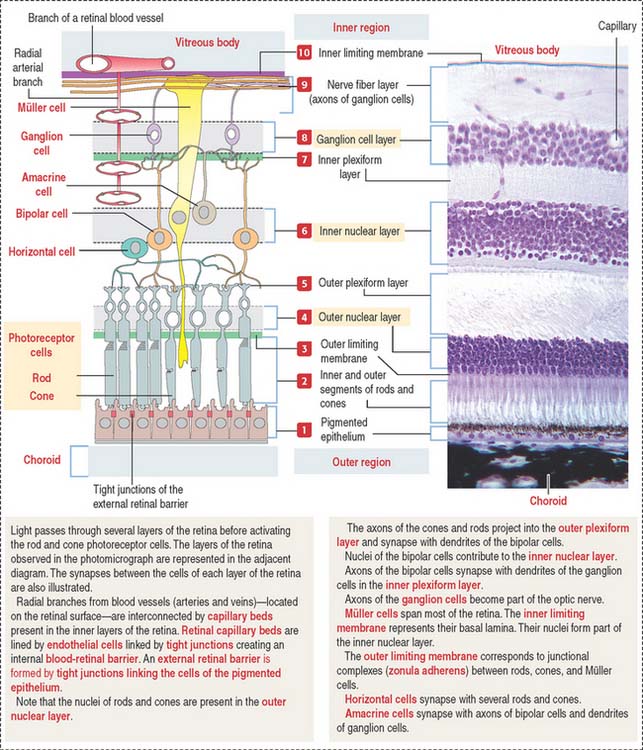
The apical domain of the cuboidal nonsensory pigmented epithelium is sealed by tight junctions to form the external retinal barrier (Figure 9-14). Granules of melanin are present in the apical cytoplasm and apical cell processes. Melanin granules absorb excess light reaching the photoreceptors.
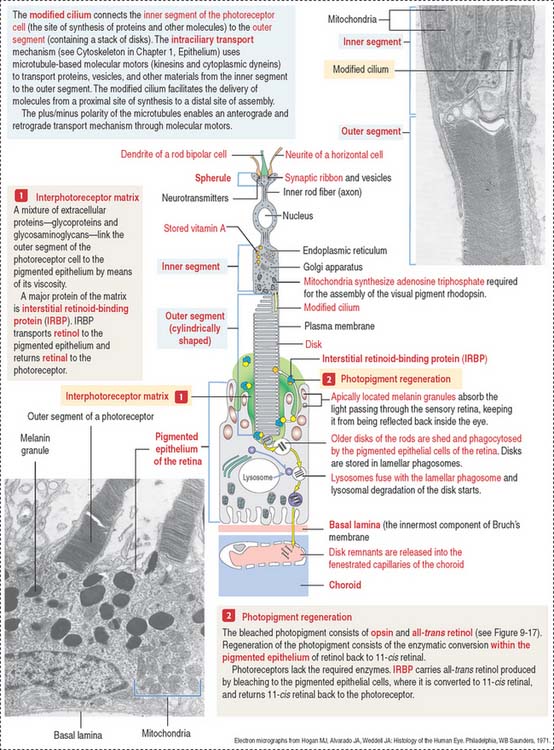
The apical surface contains microvilli that surround the outer segments of the photoreceptors (cones and rods). At this location, the sensory retina and the pigmented epithelium are attached to each other through an amorphous extracellular material, the interphotoreceptor matrix (Figure 9-15).
Clinical significance: Detachment of the retina
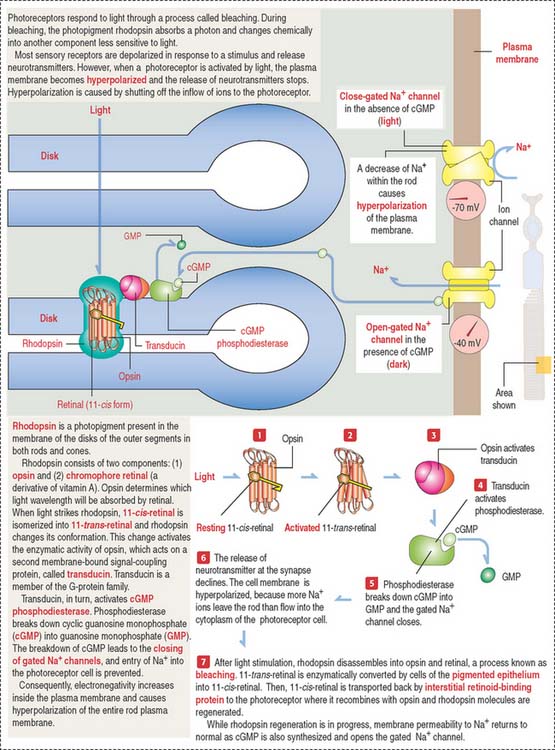
Photoreceptor neurons: Rods and cones
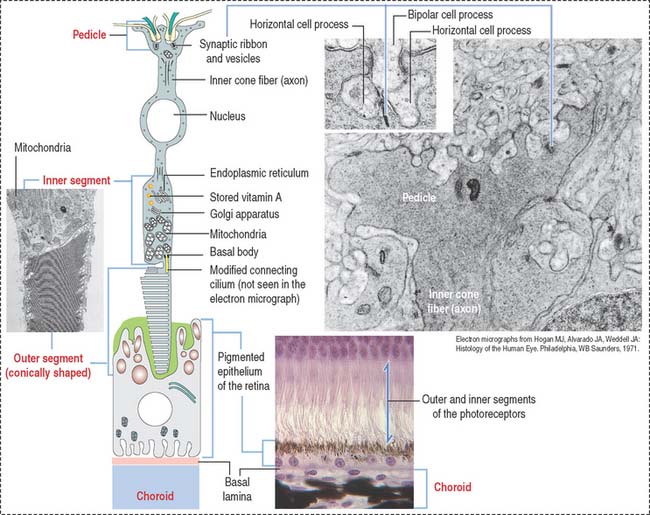
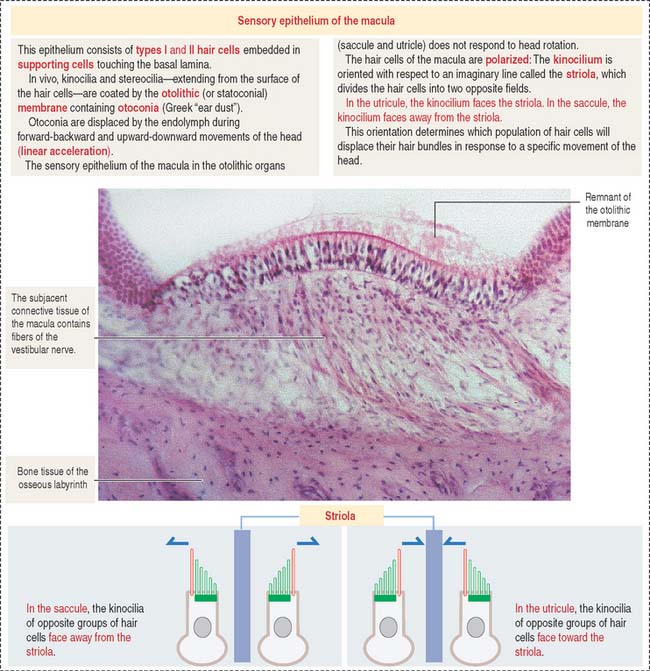
Rods (see Figure 9-15) and cones (Figure 9-16) occupy specific regions in the sensory retina. Cones are predominant in the fovea centralis and perceive color and detail. Rods are concentrated at the periphery and function in peripheral and night vision.
The various components of the disks are synthesized in the inner segment and are transported by molecular motors (kinesins and cytoplasmic dyneins) along microtubules toward the outer segment across the narrow cytoplasmic bridge containing the modified cilium. We discuss in Chapter 1, Epithelium, details of the mechanism of intraciliary transport.
There are three significant differences between rods and cones:
Box 9-F Synaptic ribbon
Box 9-G Retinitis pigmentosa
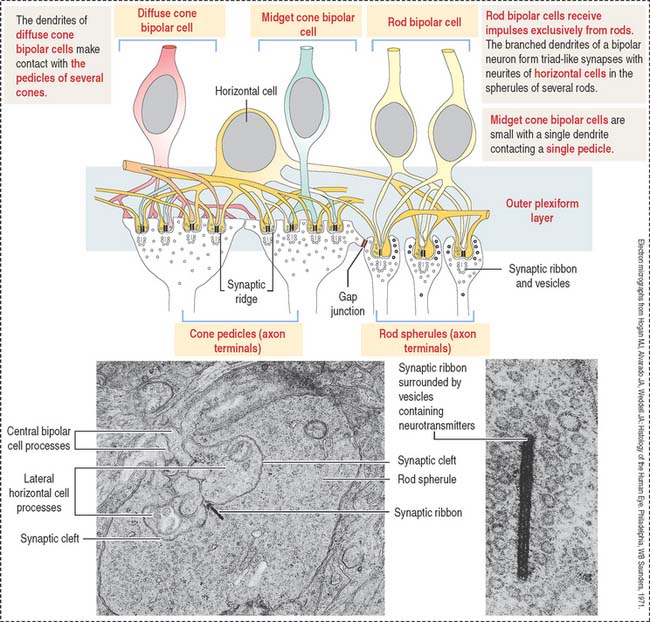
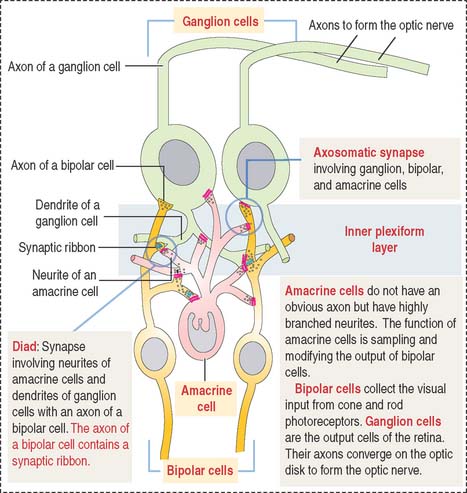
Conducting neurons: Bipolar and ganglion cells
Bipolar and ganglion cells conduct the impulse received by the photoreceptor cells.
Two major classes of bipolar cells can be distinguished (Figure 9-18):
Association neurons: Horizontal and amacrine cells
Horizontal cells give rise to neurites ending on cone pedicles. A single branching neurite synapses with both rod spherules and cone pedicles (see Figure 9-18). These neuritic synapses occur in the outer plexiform layer of the retina. This neurite and axonal distribution indicates that horizontal cells integrate cones and rods of adjacent areas of the retina.
Amacrine cells are found at the inner edge of the inner nuclear layer. They have a single neuritic process that branches to link the axonal terminals of the bipolar cells and the dendritic branches of the ganglion cells (Figure 9-19).
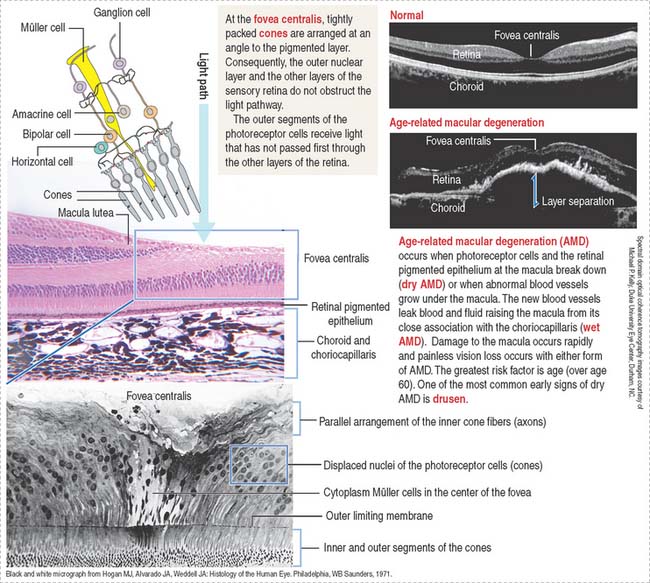
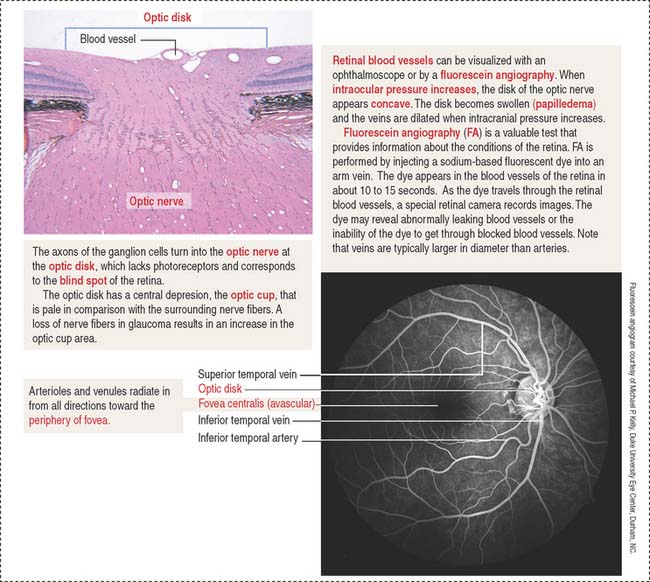
Fovea centralis and optic disk
The fovea centralis, surrounded by the macula lutea (Figures 9-20 and 9-21), is a specialized area of the retina for accurate vision under normal and dim illumination. The optic disk, which includes the optic papilla, is not suitable for vision.
The exit site from the retina of axons derived from ganglion cells is represented by the optic disk. The optic disk includes (1) the optic papilla, a protrusion formed by the axons entering the optic nerve and (2) the lamina cribrosa of the sclera, pierced by the axons of the optic nerve. Photoreceptors terminate at the edges of the optic disk, which represents the “blind spot” of the retina. The central artery and vein of the retina pass through the optic disk.
The eyelids, conjunctiva, and the lacrimal gland
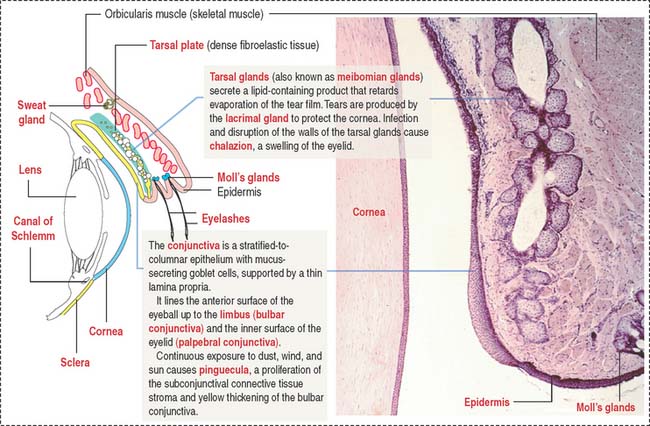
Each eyelid consists of two portions (Figure 9-22): (1) an outer cutaneous portion lined by a stratified squamous epidermis overlying a loose connective tissue dermis and skeletal muscle (orbicularis oculi muscle) and (2) an inner conjunctival portion, lined by a thin mucous membrane, the conjunctiva.
Facing the conjunctival lining is the tarsal plate, a fibroelastic dense connective tissue containing large sebaceous tarsal glands, also known as meibomian glands. Each tarsal gland opens at the margin of the eyelid. The tarsal plate is responsible for the rigidity of the eyelids.
The lacrimal gland (Figure 9-23) is a tubuloacinar serous gland with myoepithelial cells. It is organized into separate lobes with 12 to 15 independent excretory ducts. Tears enter the excretory canaliculi through the puncta and reach the nasolacrimal sac and duct to eventually drain in the inferior meatus within the nasal cavity.
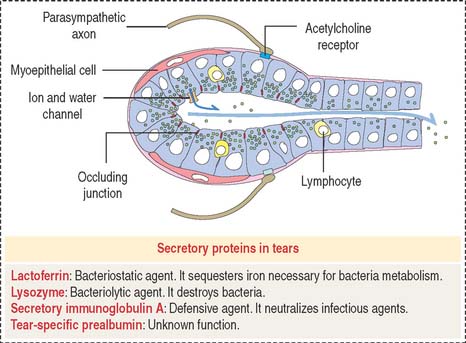
Blinking produces gentle compression of the lacrimal glands and the release of fluid. Tears keep the surface of the conjunctiva and cornea moist and rinse off dust particles. Spreading of the mucus secreted by the conjunctival epithelial cells, the oily secretion derived from the tarsal glands, and the continuous blinking of the eyelids prevent rapid evaporation of the tear film. Tears contain lysozyme, an antibacterial enzyme; lactoferrin; secretory immunoglobulin A; and tear-specific prealbumin (see Figure 9-23).
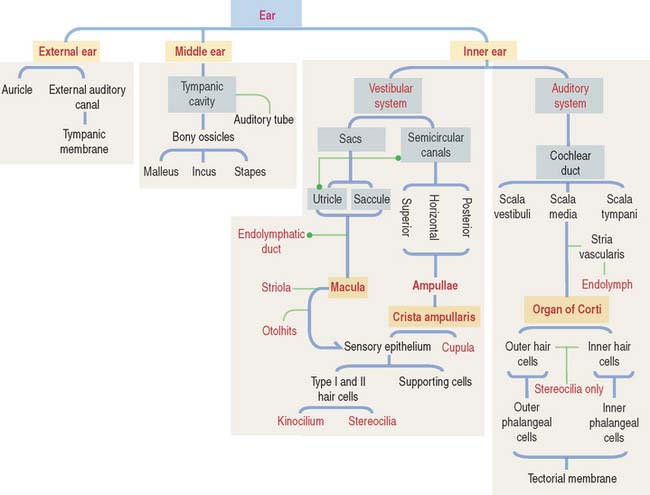
EAR
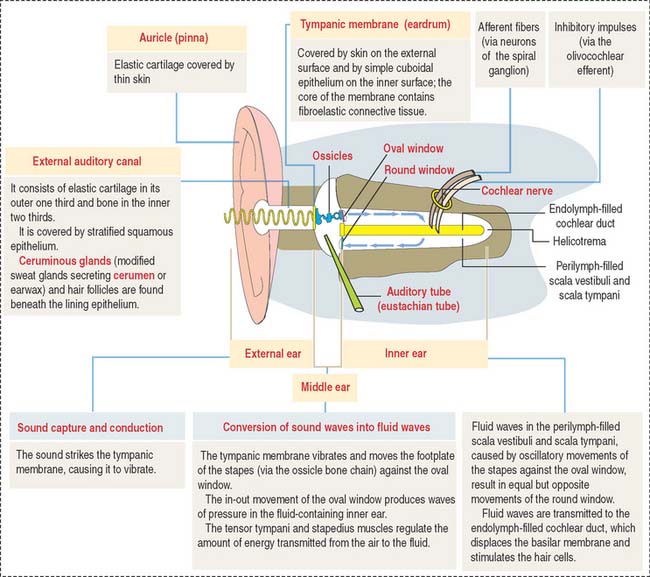
The ear consists of three components (Figure 9-24):
MIDDLE EAR
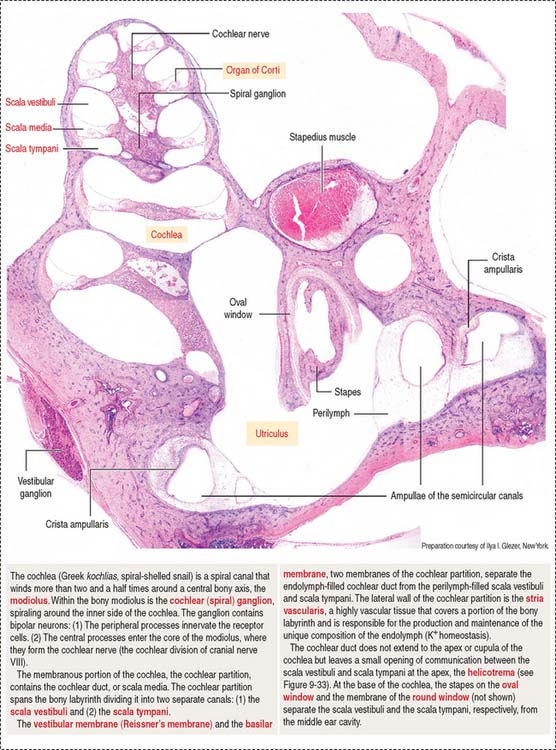
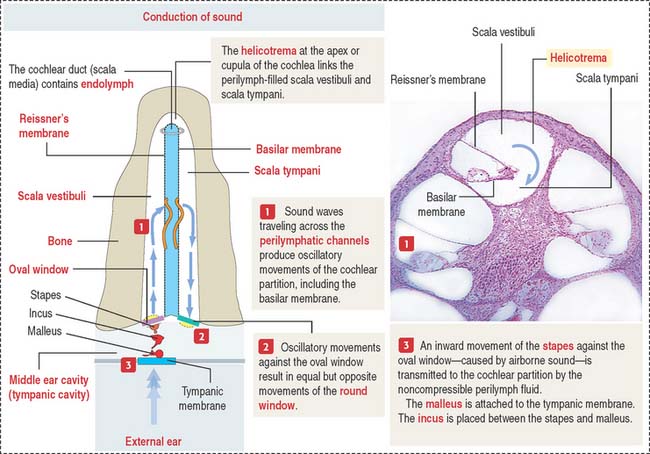
Sound transmission is carried out by the auditory or bony ossicles (malleus, incus, and stapes) organized in a chainlike fashion by interconnecting small ligaments. In this chain, the arm of the malleus is attached to the tympanic membrane at one end; at the other end, the footplate of the stapes is applied to the oval window (fenestra vestibuli), an opening of the bony labyrinth. The tensor tympani (innervated by the trigeminal nerve [cranial nerve V]) and stapedius muscles (innervated by the facial nerve [cranial nerve VII]) keep the three auditory ossicles functionally linked.
The auditory or eustachian tube links the middle ear with the nasopharynx. Adjacent to the tympanic cavity, the tube is formed by the temporal bone. Elastic cartilage continues the bony portion of the tube, which then changes into hyaline cartilage near the nasopharynx opening. A ciliated epithelium with regional variations (low columnar-to-pseudostratified near the nasopharynx) and with mucus-secreting glands lines the bony and cartilaginous segments of the tube. The role of the auditory tube is to maintain a pressure balance between the tympanic cavity and the external environment.
INNER EAR
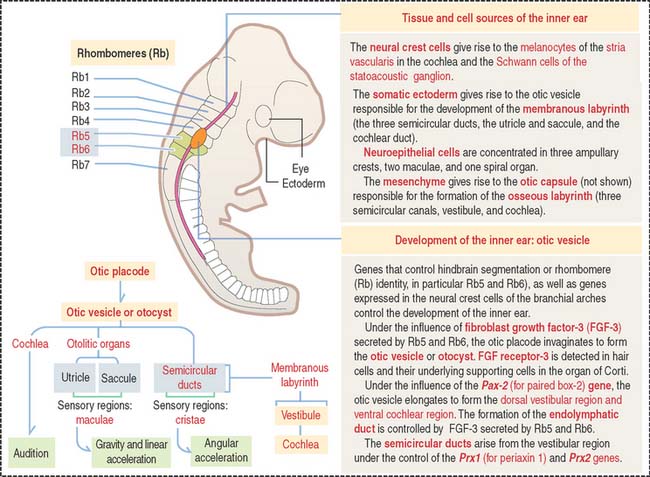
Development of the inner ear
The inner ear and associated cranial ganglion neurons derive from an otic placode on the surface of the head. The placode invaginates and forms a hollow mass of cells called the otic vesicle, or otocyst (Figure 9-25). Neural crest cells migrate out of the hindbrain and distribute around the otic vesicle. The otic vesicle elongates, forming the dorsal vestibular region and the ventral cochlear region under the influence of the Pax-2 (for paired box-2) gene. Neither the cochlea nor the spiral ganglion form in the absence of Pax-2.
Two of the semicircular ducts derive from the vestibular region and develop under the control of the Prx1 (for periaxin 1) and Prx2 genes. Note that the auditory (cochlea) and vestibular portions (semicircular canals) are under separate genetic control (Pax-2 and Prx genes, respectively).
Figure 9-25 provides the road mapping of the different portions of the inner ear derived from the otic vesicle.
Structure of the inner ear
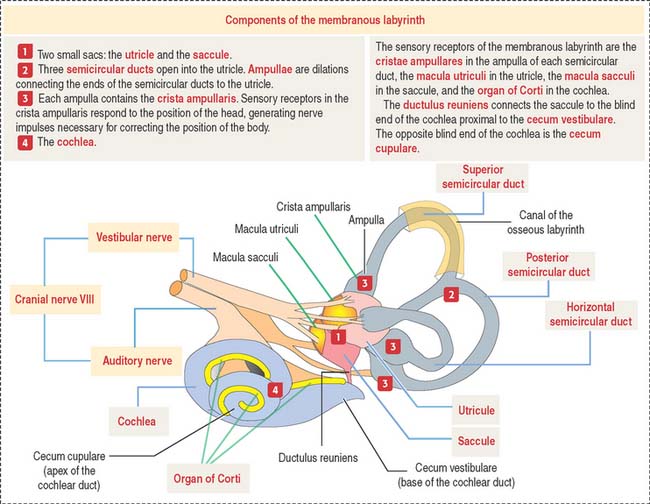
The inner ear occupies the osseous labyrinth within the petrous portion of the temporal bone. The osseous labyrinth contains the membranous labyrinth (Figure 9-26), a structure that houses both the vestibular and auditory systems.
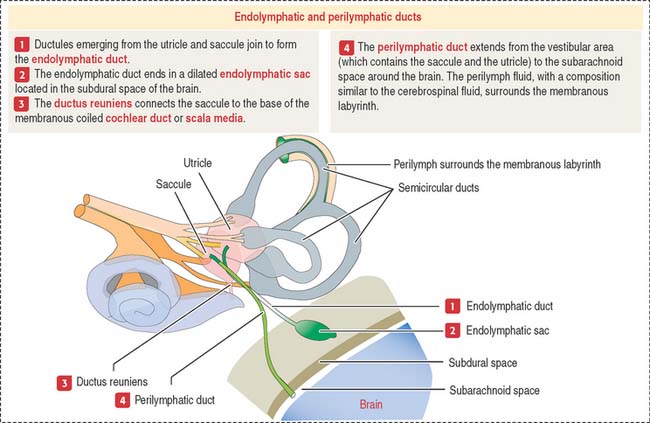
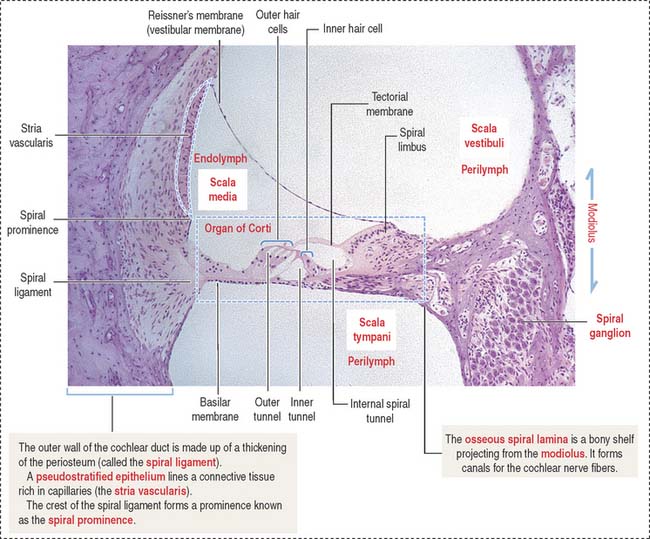
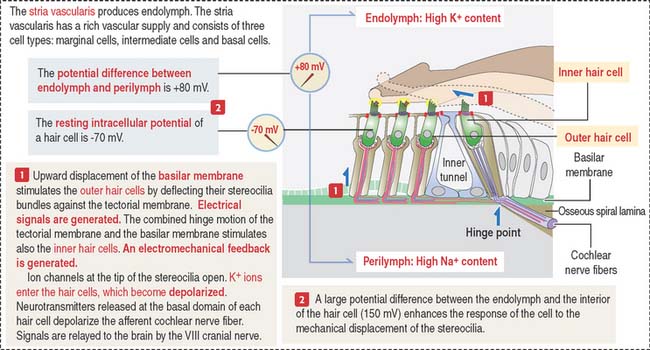
The membranous labyrinth contains endolymph, a fluid with a high concentration of K+ and a low concentration of Na+. Perilymph (with a high Na+ and low K+ content) is present between the membranous labyrinth and the walls of the osseous labyrinth (Figure 9-27).
VESTIBULAR ORGAN
Sensory cells in the vestibular organ are innervated by afferent fibers of the vestibular branch of the vestibulocochlear nerve (cranial nerve VIII). The labyrinthine artery, a branch of the anterior inferior cerebellar artery, supplies blood to the labyrinth. The stylomastoid artery supplies blood to the semicircular canals.
SEMICIRCULAR CANALS
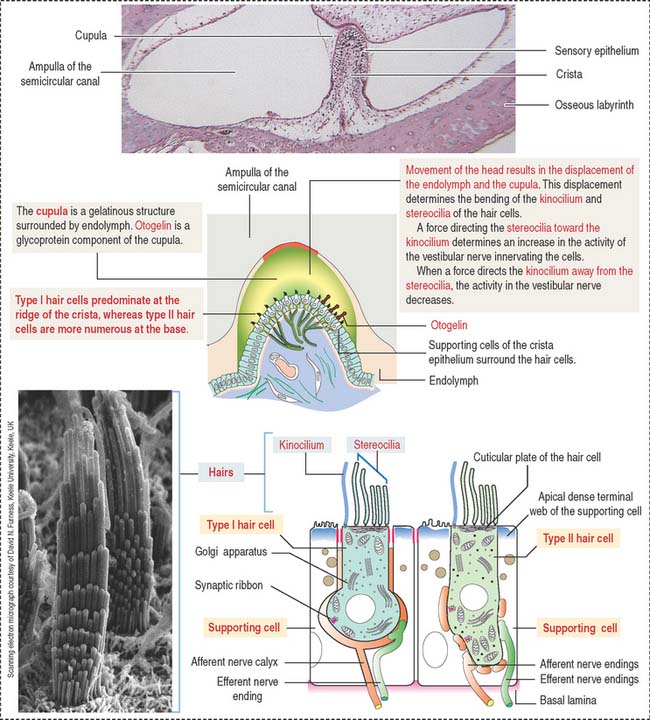
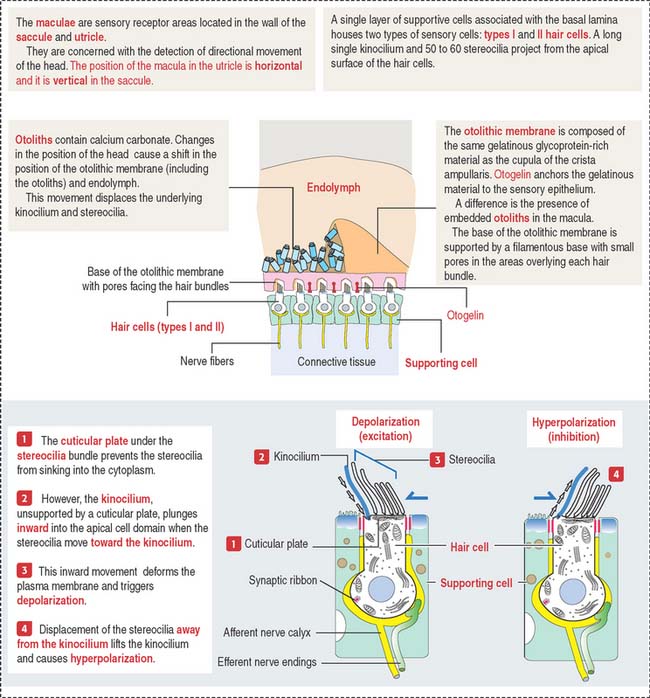
The crista ampullaris (Figure 9-28) consists of a sensory epithelium covered by a gelatinous mass called the cupula. The cupula contains otogelin, a glycoprotein anchoring the cupula to the sensory epithelium.
The sensory epithelium consists of two cell types (see Figure 9-28): (1) the hair cells and (2) the supporting cells. Like all other sensory receptors, hair cells respond to sustained stimuli by adapting and restoring their sensitivity to threshold deflections on a millisecond to sub-millisecond timescale.
The basal surface of the supporting cells is attached to a basal lamina. In contrast, the hair cells occupy a recess in the apical region of the supporting cells and do not reach the basal lamina. The apical domain of the hair cells contains 60 to 100 hairlike specialized stereocilia and a single kinocilium. Stereocilia are supported by an actin-containing cuticular plate. The free ends of both stereocilia and kinocilia are embedded in the cupula. The cupula attaches to the roof and walls of the ampulla and acts like a partition of the lumen of the ampulla (see Figure 9-28).
When the position of the cupula changes in response to movements of the endolymph, it causes displacement of the stereocilia and kinocilium of the hair cells (Figure 9-29). When stereocilia move toward the kinocilium, the plasma membrane of the hair cells depolarizes and the afferent nerve fibers are stimulated (excitation). When stereocilia are deflected away from the kinocilium, the hair cell hyperpolarizes and afferent nerve fibers are not stimulated (inhibition).
The cristae have two types of hair cells: (1) type I hair cells and (2) type II hair cells.
Both cell types are essentially similar in their internal structure, but differences exist in their shape and innervation:
Clinical significance: Ménière’s disease
Secretory cells in the membranous labyrinth and the endolymphatic sac maintain the ionic balance between endolymph and perilymph (see Figure 9-36). An increase in the volume of endolymph is the cause of Ménière’s disease, which is characterized by vertigo (illusion of rotational movement in space), nausea, positional nystagmus (involuntary rhythmic oscillation of the eyes), vomiting, and ringing in the ears (tinnitus).
OTOLITHIC ORGANS: UTRICLE AND SACCULE
The utricle and saccule display a sensory epithelium called a macula (Figure 9-30). Like the sensory epithelium of the crista ampullaris in the semicircular canals, the macula contains hair cells and supporting cells. The macula is covered by a gelatinous substance containing calcium carbonate–protein complexes forming small crystals called otoliths (see Figure 9-29). Otoliths are not present in the cupula overlying the hairs of the crista ampullaris. Small ductules derived from the utricle and saccule join to form the endolymphatic duct ending in the endolymphatic sac. The ductus reuniens links the saccule to the base of the membranous cochlear duct.
COCHLEA
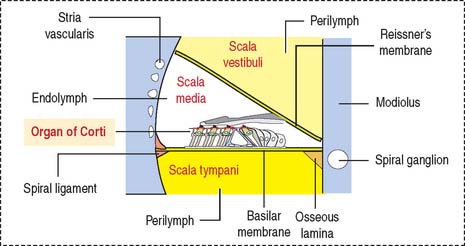
The cochlea has three spiraling chambers (Figures 9-31 to 9-33):
The scalae vestibuli and tympani are filled with perilymph and communicate at the helicotrema at the apex of the cochlea (see Figure 9-33).
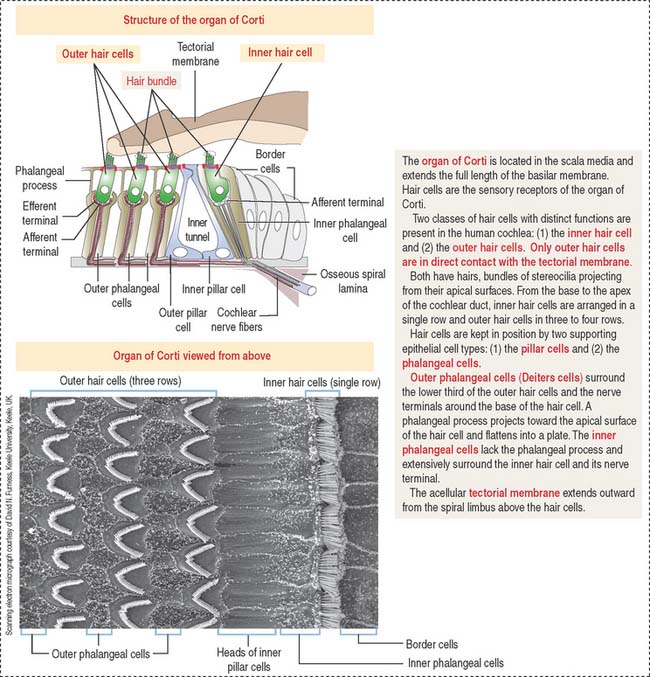
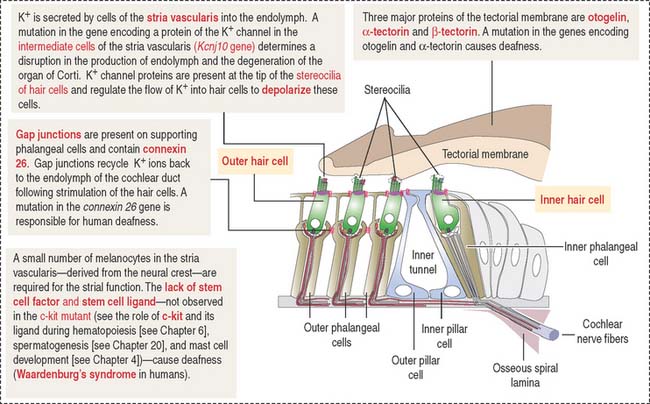
The organ of Corti (Figures 9-34 and 9-35) is the sensory epithelium of the cochlea. It is formed by (1) inner and outer hair cells; (2) supporting cells; (3) the tectorial membrane, extending outward from the spiral limbus; and (4) the inner tunnel, limited by the outer and inner pillar cells, separating inner from outer hair cells.
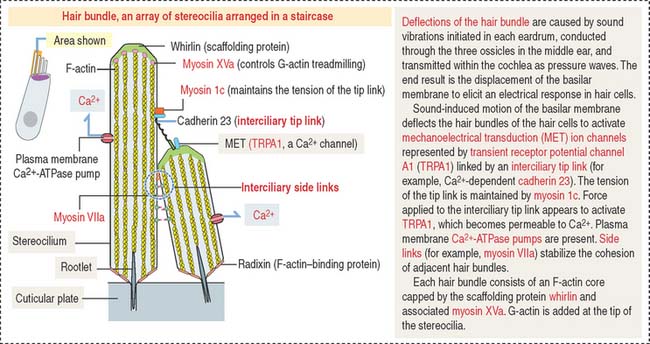
A single line of inner hair cells extends from the base to the apex of the cochlea (see Figures 9-34 and 9-35). The outer hair cells are arranged in three parallel rows, also extending from the base to the apex of the cochlea. A hair bundle, formed by 50 to 150 stereocilia in a long-to-short gradient arrangement, extends from the apical domain of each hair cell. No kinocilium is present in the hair bundle of the cochlea.
Each member of the hair bundle consists of a core of actin filaments. The tip of the actin bundle is the site where actin monomers are added under control of myosin XVa in association with the protein whirlin. Defects in myosin Va and whirlin cause abnormally short stereocilia. At the base, the actin bundle is stabilized by the protein radixin (Figure 9-36). Stereocilia within a hair bundle are interconnected by extracellular filaments (interciliary links). Side links (myosin VIIa and associated proteins) connect stereocilia along their shafts. Tip links (cadherin 23) extend from the tip of a stereocilium to the side of the taller adjacent stereocilium. The tension of the tip link is controlled by myosin 1c. Defects in interciliary links result in Usher’s syndrome, characterized by disorganization of hair bundles leading to sensorineural deafness of cochlear origin combined with retinitis pigmentosa (loss of vision).
The tectorial membrane is an extracellular matrix that contacts the stereocilia bundles of the outer hair cells. It contains α- and β-tectorin proteins and otogelin, also seen in the cupula (crista ampullaris) and otolithic membrane (maculae). As previously indicated, otogelin is essential for the anchoring of the cupula and otolithic membrane to the sensory epithelium. Conversely, otogelin appears to be dispensable for the anchoring of the tectorial membrane to the spiral limbus.
When the basilar membrane and organ of Corti are displaced, stereocilia of outer hair cells hit the tectorial membrane and depolarization of the hair cells occurs (see Figure 9-36).
Hearing process
Two factors play a significant role during the hearing process (see Figure 9-37): (1) The high concentration of K+ in the endolymph and the high concentration of Na+ in the perilymph determine an electrical potential difference. The ion concentration is regulated by the absorptive and secretory activity of the stria vascularis. (2) Fluid movement in the scala tympani induces the movement of the basilar membrane causing the taller stereocilia to be displaced by the tectorial membrane.
Clinical significance: Deafness and balance
Cytoskeletal components in the apical domain of hair cells are relatively abundant. Hair cells convert mechanical input, determined by the deflection of apical bundles of stereocilia embedded in the tectorial membrane and the otolithic membrane of the cupula, into an electromechanical input leading to synaptic transmission.
The tectorial membrane, cupula and otolithic membrane contain α-tectorin, β-tectorin and otogelin. When the α-tectorin- and otogelin-encoding genes are mutated, deafness and imbalance occur (Figure 9-38).
There are several mouse mutants with a decrease in neural crest–derived melanocytes in the stria vascularis. Although the particular role of melanocytes in the stria vascularis is not known, a mutation in the c-kit gene (encoding the stem cell factor receptor and its ligand; see Chapter 6, Blood and Hematopoiesis, for a discussion of the c-kit gene) affects the function of the stria vascularis and the mice are deaf.
Sensory Organs: Vision and Hearing
Essential concepts
Middle tunic: uvea. The uvea consists of three regions: (1) choroid, (2) ciliary body, and (3) iris.
The sensory retina consists of four cell groups: (1) photorecepor neurons (rods and cones), (2) conducting neurons (bipolar and ganglion cells), (3) association neurons (horizontal and amacrine cells), and (4) supporting neuroglia Müller cells. Cells are distributed in 10 layers summarized in Figure 9-14. There are three distinct nuclear regions: (1) the outer nuclear layer corresponds to the nuclei of the photoreceptors; (2) the inner nuclear layer corresponds to the nuclei of bipolar cells, horizontal and amacrine cells, and Müller cells; and (3) the ganglion layer contains the nuclei of the ganglion cells. The plexiform and limiting membranes represent sites of contacts among the retinal cells.
Photoreceptor cells (rods and cones) are elongated and consist of two segments: an outer segment, which contains flat membranous disks and an inner segment, the site of synthesis of various cell components. A modified cilium connects the outer and inner segments. It also provides microtubules for molecular motor proteins (kinesins and cytoplasmic dyneins) to deliver materials to the disk assembly site by the mechanism of intraciliary transport. The differences between rods and cones are the following: (1) the outer segment of the rod is cylindrical; in cones it is conical. (2) Rods terminate in a spherule; cones end in a pedicle. Both endings interact with bipolar and horizontal cells. (3) Rods contain the photopigment rhodopsin (night vision); cones contain a similar pigment, iodopsin (color vision).
Bipolar and ganglion cells are connecting neurons receiving impulses from photoreceptor cells.
The ear consists of three portions: (1) external ear, (2) middle ear, and (3) inner ear.
The inner ear occupies the osseous labyrinth, which contains the membranous labyrinth. The membranous labyrinth houses the vestibular and auditory systems. The membranous labyrinth contains endolymph (high concentration of K+ and low concentration of Na+). Perilymph (high concentration of Na+ and low concentration of K+) is present between the osseous labyrinth and the membranous labyrinth.
The modiolus, the spiraling bony axis of the cochlea, houses the spiral ganglion.