Sensation
What is sensation?
Sensory receptors
The somatosensory system reacts to diverse stimuli from different sensory receptors, thermoreceptors (temperature), mechanoreceptors (physical distortion) and chemoreceptors (chemicals). Each sensory modality has a unique sensory receptor which is activated when a specific stimulus, within its receptive field, reaches a threshold intensity. This receptive field may be altered as a result of pathology when it can contribute to the experience of pain (S3.29) and abnormal sensation. The density of receptors is relevant to the ability to locate a stimulus (awareness) and to distinguish between two stimuli (discrimination).
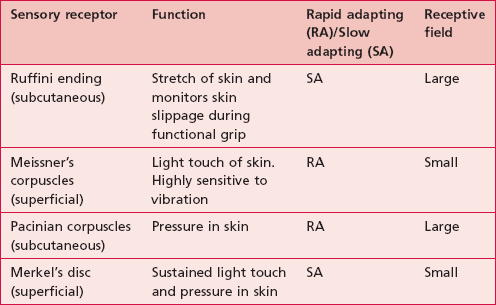
The duration of a sensory experience is related to a further characteristic of the sensory receptor termed ‘adaptation’. A rapidly adapting receptor responds to change and is therefore activated at the onset and termination of the stimulus only. A slowly adapting receptor responds continuously to a persistent stimulus. This should be borne in mind when assessing particular modalities. Figure 23.1 shows the main somatosensory receptors and Table 23.1 identifies the functional characteristics of these receptors.
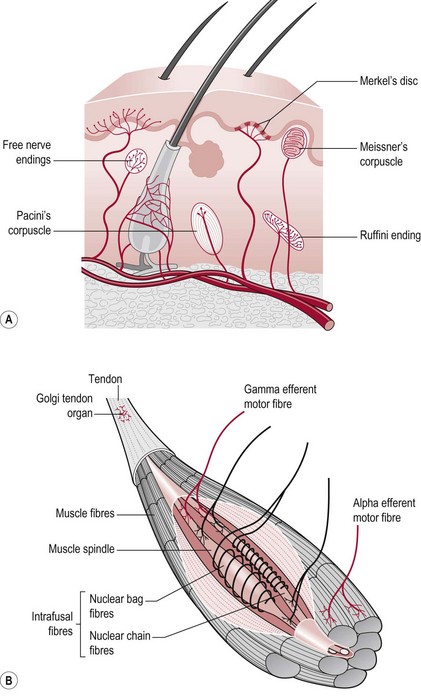
Figure 23.1 Sensory receptors of the skin and muscle.
Sensory pathway
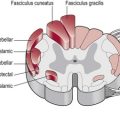
This afferent information from sensory receptors in the periphery is conducted along afferent neurons to the spinal cord (S2.13) and transmitted via the ascending tracts (S2.15) to the thalamus (S2.9), brain stem (S2.10) and higher centres (S2.7). Sensory input is systematically mapped on the contralateral primary somatosensory cortex (parietal lobe), then processed and integrated with other relevant information by the somatosensory association area (parietal lobe), basal ganglia (S2.11) and cerebellum (S2.12) before being acted upon.
Sensory modalities
Table 23.2 shows a summary of the functional anatomy related to the modalities described below.
Tactile
In terms of touch a hierarchy of sensory function was proposed by Fess (1990). The lowest level is the ability to identify a single stimulus (awareness). The hierarchy proceeds with the ability to distinguish between two stimuli (discrimination), the ability to differentiate different characteristics or strength of one stimulus (quantification) and the highest level is the ability to recognize objects by touch alone (recognition). This hierarchy suggests that if the higher levels are intact, the lower levels do not need to be assessed.
Light touch
The sensation of light touch allows us to be aware of tactile stimuli, including situations as innocuous as the clothes we wear, but also has a role in warning us of impending damage. Following a neurological lesion, a patient may become hypersensitive to touch, when even clothes may initiate a sensory experience and an inappropriate motor response. This altered sensation may also be perceived as pain, paraesthesia or dysaesthesia (S3.29). In a scenario where a patient becomes hyposensitive to light touch, the lack of awareness of limbs particularly, may result in soft tissue injury.
Nociception
Pain (S3.29)
Pain serves to notify the nervous system about tissue damage that has occurred. Although pain can be elicited without tissue damage (neurogenic pain) (S3.29), nociceptive pain will remain the focus here. The sensory receptors sensitive to nociceptive stimuli are free nerve endings, however not all noxious stimuli is perceived as pain because the response to a stimuli is highly subjective. The free nerve endings are much less specialized than other receptors and may respond to mechanical, thermal or chemical stimuli.
Proprioception
Our sense of proprioception is complex and results from integration of information from several sources, visual (S2.10, S3.27), vestibular (S2.10) and somatosensory systems. In this text, proprioception is defined as consisting of three sub-modalities (Riemann and Lephart 2002a,b); the ability to sense:
• Position sense (body parts and their relationship to each other)
Historically, joint receptors have been considered to have an important role in proprioception, however recent studies have shown that this is not the case in most joints (Proske and Gandevia 2009). In fact, the major sensory receptors involved in position and movement sense are muscle spindles and stretch receptors in the skin (Ruffini endings). The skin receptors have a greater role in circumstances where the controlling muscle is distant to the joint being moved (e.g. fingers) (Collins et al. 2005) and in the case of two joint muscles (Sturnieks et al. 2007). Ruffini endings have been found to be accurate within 2° in relation to change of joint angle in the fingers and deficits contribute to altered timing and force of grip in stroke (Blennerhassett et al. 2007).
Why do I need to assess sensation?
• Inability to interact with the environment
• Inability to produce skilled movement (Campbell 2000)
• Significant reduction in functional ability (Fang et al. 2003, Tyson et al. 2008) and quality of life (Forsaa et al. 2008)
• Production of central neuropathic pain (S3.29) (Widerstrom-Noga et al. 2002).
The incidence of sensory deficit is also high in this patient population. In Parkinson’s disease (PD), there is a growing body of evidence that suggests that non-motor symptoms such as abnormal sensation may actually precede the motor deficits by which it is presently diagnosed. Between 50% and 100% of people with PD have sensory deficits (Rahman et al. 2008) with a decrease in two-point discrimination and proprioception highlighted (Zia et al. 2000). This is attributed to an impairment of sensorimotor processing by the basal ganglia (S2.11) (Juri et al. 2009).
In CVA and multiple sclerosis (MS) the lesion may directly involve the somatosensory system or the processing centres and therefore may result in a dysfunction of any of the somatosensory modalities. The incidence is up to 60% in CVA (Schabrun and Hillier 2009) with tactile deficits most common and considered a poor prognostic indicator for recovery (Tyson et al. 2008).
How do I assess sensation?
The primary modalities that should be part of a neurological assessment include light touch, deep touch/pressure, temperature, pain and proprioception (Cassiopeia and Okun 2002). In order to save time, clinicians may opt to assess pain or temperature (anterior spinothalamic tract), light touch or deep touch (dorsal columns) and proprioception.
Tactile – light touch
Therapist
1. Decide on the distribution of testing
• Peripheral nervous system (PNS) lesion testing dermatomes (S3.24) (Fig. 23.2) or peripheral nerve distribution (Fig. 23.3) will give the therapist the most useful information.
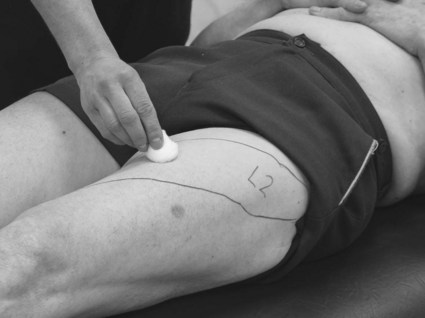
Figure 23.2 Testing a dermatome for light touch.
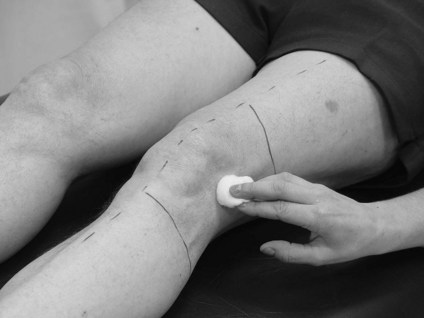
Figure 23.3 Testing a peripheral nerve for light touch.
• Central nervous system (CNS) lesion. Any sensory loss will be due to damage affecting the sensory homunculus and therefore is likely to correlate to body segments. Therefore, testing the distribution of peripheral nerves or dermatomes is neither necessary nor relevant. Testing the area of skin related to different aspects (medial, lateral, anterior, posterior) of particular body segments is more accurate in terms of the pathophysiology and provides clear landmarks by which the patient can report localization of the stimuli. For example, front of the elbow (Fig. 23.4), back of the wrist, inside of the knee and outside of the ankle.
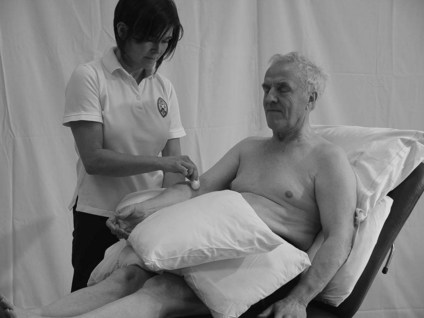
Figure 23.4 Testing segmental distribution for light touch.
2. Explain and demonstrate the procedure to the patient with their eyes open.
3. Test an area of skin considered to be normal to ensure the patient understands what is expected.
4. Ask the patient to close their eyes.
5. Using a cotton wool ball, touch the area of skin being assessed (Figs 23.2–23.4). A brief dab will activate the rapid adapting Meissner’s corpuscles, whereas a sustained application of the cotton wool will stimulate the slow adapting Merkel’s disc.
6. Compare the same area of skin on the other side.
7. The therapist should encourage the patient not to try and guess if they are not sure about accuracy. With this in mind the therapist is advised to randomize the testing points in terms of the body segment tested and the timing of the test.
8. Ask the patient to respond as follows:
Say ‘yes’ every time you feel me touch you. This evaluates sensory awareness. A correct ‘yes’ response, indicates that the sensory pathway itself is intact. If there is no response, the area is likely to be anaesthetic (lacks sensation). This could indicate dysfunction of either the peripheral or central nervous systems.
9. Further questioning at the same test point may allow the therapist to establish more detail which will assist clinical reasoning later:
Does it feel normal? No, it feels strange. This could be peripheral or central neuropathic pain and needs further exploration (S3.29).
Does it feel the same as the other side? No. What is different? Which side feels normal? This could indicate an ipsilateral peripheral nervous system lesion or a contralateral central nervous system lesion.
Tell me where I am touching you This tests the ability to localize (identify the location of) the sensation. Inability to localize the stimuli implicates a lesion of the primary somatosensory cortex (parietal lobe).
10. Record any loss or change in normal sensation.
11. To ensure nothing is missed, the procedure should be repeated to include the whole body (limbs and trunk) bilaterally. However, a recent study by Busse and Tyson (2009) recommended that in CVA, if one site on a limb was assessed as either completely absent or intact, no further testing of that limb was required. However, if the segment was assessed as partially impaired, the whole limb should be tested.
Two-point discrimination
A specialist tool provided with the Rivermead Assessment of Somatosensory Performance (Fig. 23.5) provides a standardized tool for testing, however if this is unavailable, two paper clips can be used.
1. The therapist should hold the tool against the patient’s skin with constant gentle pressure over the palmar surface of the hand and fingers.
2. Randomly apply one point or two points of the tool (5 mm, 4 mm, 3 mm apart), starting with the greater distance first. The normal range for two-point discrimination on the finger pads is 2–4 mm and for the palm 8–15 mm.
3. The patient is asked to report whether they feel either one point or two points.
4. Record the distances at which the patient is able to discriminate two points. This is easily documented on a diagram of the hand.
Tactile – deep touch/pressure
The therapist should follow the assessment procedure as for light touch but should use the blunt end of a neurotip as the instrument for producing the stimulus of pressure (Fig. 23.6) or a pressure pen, which gives a better standardization of application (Fig. 23.6). As the Pacinian corpuscles are rapid adapting receptors the neurotip should be placed on and off rather than be applied continuously.
Pain (pin prick)
The therapist should follow the assessment procedure as for light touch but should use the sharp end of a neurotip as the instrument for producing the stimulus of pain (Fig. 23.7). Note: Be very careful not to pierce the skin! Using the tip at a 45° angle is advised.
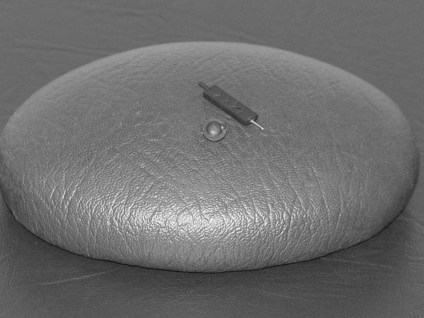
Figure 23.7 Tool for testing pain sensation (pin prick).
Temperature (heat and non-noxious cold)
The therapist should follow the assessment procedure as for light touch but should use thermo controlled test tubes, one cold (6–10°C) and one hot (44–48°C) as the instruments for producing the stimulus of heat and non-noxious cold (Fig. 23.8). This procedure should test for temperature awareness of both heat and cold separately and also the ability to discriminate between the two (is this hot or cold?).

Figure 23.8 Tool for testing temperature.
Proprioception
Mirroring
Therapist
1. Explain and demonstrate the procedure.
2. Ask the patient to close their eyes.
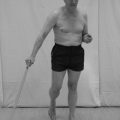
3. The therapist places and holds one limb into a set position (Fig. 23.9).
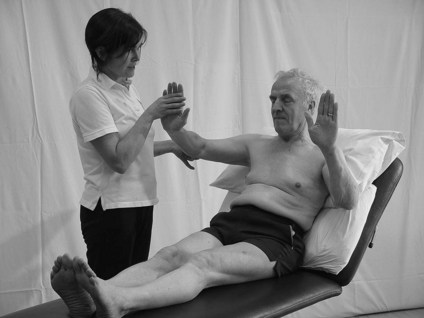
Figure 23.9 Testing for proprioception (mirroring).
4. The patient is requested to copy the position with the opposite limb and the therapist makes a judgement on the accuracy of the response (Fig. 23.9).
5. An inaccurate response may indicate a deficit of joint position sense.
However, there are several shortcomings with this method:
• It requires a certain level of motor ability in the limb being moved by the patient, therefore motor ability is being assessed simultaneously.
• In the case of conditions which present with bilateral dysfunction, such as multiple sclerosis, the test may be inaccurate and invalid as accurate assessment is not possible.
• It simultaneously assesses multiple joints.
• It allows for the assessment of position sense but not movement sense.
Specific joint position sense and movement sense testing
Therapist
1. Explain and demonstrate the procedure to the patient.
2. Establishing a communication system in layman’s terms is vital to the success of this method. For example, if the positions to be assessed are elbow flexion and extension, the terms used by the patient could be ‘bent’ (for flexion) and ‘straight’ (for extension). This should be clarified and agreed to prior to testing each joint.
3. Ask the patient to close their eyes.
4. The therapist places the joint being assessed in the extreme of one position (e.g. full elbow flexion) and holds it still (Fig. 23.10).
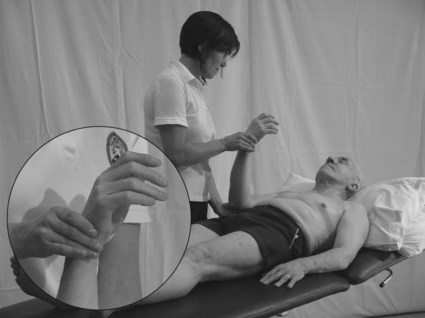
Figure 23.10 Testing for proprioception (individual joints).
5. The patient is asked to indicate the joint position (e.g. if the elbow is bent or straight).
6. This procedure should be repeated for both extremes of the same movement (e.g. full elbow flexion and extension).
7. If a deficit is suspected, additional detailed information may be gathered to assist in analysis of the problem:
• Gross position sense. The therapist makes large changes in the position. The patient being asked is the elbow ‘more bent’ or ‘more straight’?
• Fine position sense. The therapist makes small changes in the position and asks the patient is the elbow ‘more bent’ or ‘more straight’?
8. Movement sense. The therapist should move the body segment and simultaneously ask the patient is the elbow ‘bending’ or ‘straightening’? This should also be tested using large and small ranges of movement.
9. The procedure should then be repeated for all joints and at least one direction of movement at each joint.
Recording
A body chart provides a clear and time efficient way of representing most of the sensory findings:
It is usual to record only the existing deficits, which are marked on the corresponding position of the body chart. A key indicating the symbols used to identify different modalities is advisable. Awareness, discrimination and quantification can be added as appropriate, as long as the chart remains legible. See the body chart for pain (S3.29) as an example.
Analysis
Sensory deficits may have wide ranging implications for the neurologically impaired patient from potential soft tissue damage because of lack of awareness, poor balance and falls and reduced fine finger function. Therefore, it is important for the therapist to understand the specific sensory deficit of the patient and the implications it may be having for movement and function. There is also some evidence to support the success of sensory retraining following CVA (Schabrun and Hillier 2009) and therefore accuracy in assessment is paramount.
Outcome measures
Research
• KinCom (IsoKinetic International, Harrison, TN) can be used to quantify proprioception.
• American Spinal Injury Association score (ASIA). ASIA produces a score based on a coding system (0 = absent; 1 = abnormal; 2 = normal) (Bromley 2006). This system is widely used in spinal cord injuries where the dermatome distribution is tested but has less relevance in conditions such as CVA, multiple sclerosis and Parkinson’s disease, where testing in this specific distribution is not necessary.
• Rivermead Assessment of Somatosensory Performance (RASP). The RASP provides a standardized quantifiable assessment of somatosensory impairments. A score is calculated for specified regions of the body and it has been validated for the CVA population (Winward et al. 2002). Precise instructions are included with the tool.
References and Further Reading
Blennerhassett, JM, Matyas, TA, Carey, LM. Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabilitation and Neural Repair. 2007; 213:263–272.
Bromley, I. Tetraplegia and paraplegia: a guide for physiotherapists, ed 6. Edinburgh: Churchill Livingstone; 2006.
Busse, M, Tyson, SF. How many body locations need to be tested when assessing sensation after stroke? An investigation of redundancy in the Rivermead Assessment of Somatosensory Performance. Clinical Rehabilitation. 2009; 231:91–95.
Campbell, M. Rehabilitation for traumatic brain injury: physical therapy practice in context. Edinburgh: Churchill Livingstone; 2000.
Cassiopeia, F, Okun, MS. Origins of the sensory examination in neurology. Seminars in Neurology. 2002; 22:399–408.
Collins, DF, Refshauge, KM, Todd, G, et al. Cutaneous receptors contribute to kinesthesia at the index finger elbow and knee. Journal of Neurophysiology. 2005; 94:1699–1706.
Fang, Y, Chen, X, Li, H, et al. A study on additional early physiotherapy after stroke and factors affecting functional recovery. Clinical Rehabilitation. 2003; 176:608–617.
Fess, EE. Assessment of the upper extremity: instrumentation criteria. Occupational Therapy Practice. 1990; 1:1–11.
Forsaa, EB, Larsen, JP, Wentzel-Larsen, T, et al. Predictors and course of health related quality of life in Parkinson’s disease. Movement Disorders. 2008; 23:1420–1427.
Juri, C, Rodriguez-Oroz, M, Obeso, JA. The pathophysiological basis of sensory disturbances in Parkinson’s disease. Journal of Neurologic Science. 2009; 289:60–65.
Kandel, ER, Schwartz, JH, Jessell, TM. Principles of neural science, ed 4. New York: McGraw-Hill; 2000.
Proske, U, Gandevia, SC. The kinesthetic senses. Journal of Physiology. 2009; 587:4139–4146.
Rahman, S, Griffin, HJ, Quinn, NP. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Movement Disorders. 2008; 23:1428–1434.
Riemann, BL, Lephart, SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. Journal of Athletic Training. 2002; 37:71–79.
Riemann, BL, Lephart, SM. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. Journal of Athletic Training. 2002; 37:80–84.
Schabrun, SM, Hillier, S. Evidence for the retraining of sensation after stroke: a systematic review. Clinical Rehabilitation. 2009; 231:27–39.
Smith, JL, Crawford, M, Proske, U, et al. Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. Journal of Applied Physiology. 2009; 106:950–958.
Sturnieks, DL, Wright, JR, Fitzpatrick, RC. Detection of simultaneous movement at two human arm joints. Journal of Physiology. 2007; 585:833–842.
Tyson, SF, Hanley, M, Chillala, J, et al. Sensory loss in hospital-admitted people with stroke: characteristics associated factors and relationship with function. Neurorehabilitation and Neural Repair. 2008; 222:166–172.
Widerstrom-Noga, EG, Duncan, R, Felipe-Cuervo, E, et al. Assessment of the impact of pain and impairments associated with spinal cord injuries. Archives of Physical Medicine and Rehabilitation. 2002; 83:395–404.
Winward, CE, Halligan, PW, Wade, DT. The Rivermead Assessment of Somatosensory Performance RASP: standardization and reliability data. Clinical Rehabilitation. 2002; 16:523–533.
Zia, S, Cody, F, O’Boyle, D. Joint position sense is impaired in Parkinson’s disease. Annals of Neurology. 2000; 47:218–228.