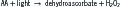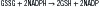Chapter 208 Senile Cataracts
 General Considerations
General Considerations
Aging-related (or senile) cataracts are discussed in this chapter; diabetes- and galactose-induced cataracts (sugar cataracts) are discussed in Chapter 161.
Cataract formation is characterized histopathologically by the following features:
• Fibrous metaplasia of the epithelium
• Liquefaction of fibers, resulting in morgagnian globule formation (drops of fluid beneath the capsule and between the lens fibers)
• Anterior subcapsular cataract: Fibrous metaplasia of lens epithelium (usually follows iritis and adherence of the iris to the lens—posterior synechiae)
• Anterior cortical cataract: Liquefaction of lens fibers occurs and morgagnian globules form in the cortex anteriorly
• Nuclear cataract: An exaggeration of the normal aging-related melding of fibers in the nucleus
• Posterior cortical cataract: Liquefaction and globular degeneration of the posterior lens cortex
• Posterior subcapsular cataract: Epithelial cells migrate posteriorly under the capsule and form large irregular nucleated cells
 Therapeutic Considerations
Therapeutic Considerations
The etiology of cataract formation is ultimately related to an inability to maintain normal homeostatic concentrations of Na+, K+, and Ca2+ within the lens. These abnormalities are apparently the result of decreased Na+,K+-ATPase activity,1–6 a defect usually due to free radical damage to some of the sulfhydryl proteins in the lens, including Na+,K+-ATPase, which contains a sulfhydryl component.
In cataract formation, the normal protective mechanisms are unable to prevent free radical damage. The lens, like many other tissues of the body, depends on adequate levels and activities of superoxide dismutase (SOD), catalase, and glutathione (GSH) as well as adequate levels of accessory antioxidants such as lutein, vitamins E and C, and selenium to help prevent damage by free radicals. Individuals with higher dietary intakes of vitamin C and E, selenium, and carotenes (especially lutein) have a much lower risk of developing cataracts.7 Several studies have shown that various nutritional supplements—multiple vitamin formulas, vitamins C and E, B vitamins (especially vitamin B12 and folic acid), and vitamin A—also offer significant protection against both nuclear and cortical cataracts.8–11 Studies conducted by the Age-Related Eye Disease Study Research Group and others indicate that a combination of these nutrients will likely produce better results than any single nutrient alone or even limited combinations of three or less nutrients in the prevention of both age-related macular degeneration and cataracts (see Chapter 187 for more information).
Antioxidants
Lutein
Lutein, the yellow-orange carotene that offers significant protection against macular degeneration, also exerts protection against cataract formation.12 Like the macula, the human lens concentrates lutein. In 1992, a prospective cohort study showed that consumption of spinach (high in lutein) was inversely related to the risk of cataracts severe enough to require extraction.13 This initial investigation was followed by three prospective studies showing that intake of lutein was inversely associated with cataract extraction (20% to 50% risk reduction).14–16 In a double-blind intervention trial, 17 patients clinically diagnosed with age-related cataracts were randomly assigned to receive dietary supplementation with lutein (15 mg), α-tocopherol (100 mg), or placebo three times a week for up to 2 years.17 Visual performance (visual acuity and glare sensitivity) improved in the lutein group, whereas there was a trend toward the maintenance of visual acuity with α-tocopherol and a decrease with placebo supplementation.
Vitamin C
A high dietary intake of vitamin C from either dietary sources or supplements has been shown to protect against cataract formation.8–1118 In addition to preventing cataracts, antioxidant nutrients like vitamin C may offer some therapeutic benefits. Several clinical studies have demonstrated that vitamin C supplementation can halt cataract progression and in some cases significantly improve vision. For example, in a study conducted in 1939, a total of 450 patients with cataracts were started on a nutritional program that included 1 g/day of vitamin C, resulting in a significant reduction in cataract development.1 Similar patients had previously required surgery within 4 years, but in the vitamin C–treated patients only a small handful needed surgery, and in most there was no evidence that the cataracts had progressed over the 11-year study period.
It appears that the daily dose of vitamin C necessary to increase the vitamin C content of the lens is 1000 mg.2 The lens of the eye and active tissue of the body require higher concentrations of vitamin C. The level of vitamin C in the blood is about 0.5 mg/dL, whereas that in the adrenal and pituitary glands is 100 times that. In the liver, spleen, and lens of the eye, the vitamin C level is increased by at least a factor of 20. In order for these concentrations to be maintained, the body must generate enormous amounts of energy to pull vitamin C out of blood against this tremendous gradient. Keeping blood vitamin C concentrations elevated helps the body concentrate vitamin C into active tissue by reducing the gradient. That is probably why such a high dose is required to raise the vitamin C content of the lens.
In another study, 450 patients with incipient cataracts were started on a nutritional program including 1 g/day of vitamin C, which led to a significant reduction in cataract development.3
In a large double-blind trial, 11,545 apparently healthy U.S. male physicians 50 years or older without a diagnosis of cataract at baseline were randomly assigned to receive 400 IU of vitamin E or placebo on alternate days and 500 mg of vitamin C or placebo daily.19 After 8 years of treatment and follow-up, there was no significant difference in cataract formation in the groups. This study may have failed to show benefit because it was below the threshold of 1 g/day of vitamin C.
Glutathione
A tripeptide composed of glycine, glutamic acid, and cysteine, GSH is found at very high concentrations in the lens. GSH plays a vital role in maintaining a healthy lens and has been postulated as a key protective factor against toxins of both intralenticular and extralenticular origin. It functions as an antioxidant, maintains reduced sulfhydryl bonds within the lens proteins, acts as a coenzyme of various enzyme systems, participates in amino acid transport with gamma-glutamyl transpeptidase, and is involved in cation transport.4 GSH levels are diminished in virtually all forms of cataracts.
Ascorbic Acid and Glutathione Interactions
Light may also cause oxidation of AA, leading to hydrogen peroxide formation as follows:
The oxidized glutathione (GSSG) serves as an inducer of the hexose monophosphate shunt, which provides the NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) necessary for reducing GSSG via riboflavin-dependent glutathione reductase, as follows:
The NADP is reduced by hexose monophosphate dehydrogenase as follows:
Selenium and Vitamin E
Selenium and vitamin E, both antioxidants, are known to function synergistically. The maintenance of proper selenium levels appears to be especially important because human lens glutathione peroxidase is selenium-dependent. Low selenium levels strongly promote cataract formation. Previous studies have shown that the selenium content in the cataractous human lens is only 15% of normal.5
A later study was conducted to better examine the role of selenium in cataract formation.6 Selenium levels in the serum, lens, and aqueous humor were determined in 48 patients with cataracts and compared with levels in matched controls. Selenium levels in the serum and aqueous humor were found to be significantly lower in the patients with cataracts (serum, 0.28 mg/mL; aqueous humor, 0.19 mg/mL) than in normal controls (serum, 0.32 mg/mL; aqueous humor, 0.31 mg/mL). However, the selenium level in the lens itself did not significantly differ between the patients with cataracts and the controls.
As previously described, vitamin E supplementation alone does not slow the progression of cataract formation.17 A double-blind study in which vitamin E was given at a dose of 500 IU daily also found that supplementation did not slow cataract formation.20 In a 7-year trial, supplementation with vitamin E (400 IU) combined with vitamin C (500 mg) and beta-carotene (15 mg) had no effect on the development or progression of cataracts.21
Superoxide Dismutase
The activity of SOD is lower in the human lens than in other tissues owing to the higher levels of ascorbate and glutathione in the lens, and a progressive decrease in SOD is encountered in cataract progression. Oral supplementation is probably of little value because it does not affect tissue SOD activity.22 Of greater value is supplementation with the trace mineral cofactors of SOD, the levels of which are greatly reduced in the cataractous lens (copper by > 90%; manganese by 50%; and zinc by > 90%).23
Tetrahydrobiopterin
Pteridine compounds are believed to play a protective role against cataract formation via the prevention of oxidation and damage by ultraviolet light. This action prevents the formation of high-molecular-weight proteins in the lens. Tetrahydrobiopterin functions as an essential coenzyme in the hydroxylation of monoamines such as phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase. Studies of human senile cataracts have demonstrated decreased levels of pteridine-synthesizing enzymes and tetrahydrobiopterin.24 Supplemental folic acid may help to compensate for this deficiency.
Other Nutritional Factors
Riboflavin
Lenticular GSH requires flavin adenine dinucleotide (FAD) as a coenzyme for GSH.25,26 Deficiency of riboflavin, the precursor of FAD, is believed to enhance cataract formation by depressing GSH activity. Although riboflavin deficiency is fairly common in the geriatric population (33%), original studies demonstrating an association between riboflavin deficiency and cataract formation were followed by studies demonstrating no such association. The patient’s riboflavin status can be determined by measuring GSH activity in red blood cells before and after stimulation with FAD.26
Amino Acids
Methionine is a component of the lenticular antioxidant enzyme methionine sulfoxide reductase and a precursor of cysteine, a component of GSH. Cysteine, along with the other amino acid precursors of GSH, has been shown to be of some aid in cataract treatment.27
Zinc, Vitamin A, and Beta-Carotene
The antioxidants zinc, vitamin A, and beta-carotene are known to be essential to normal epithelial integrity. Adequate amounts of these nutrients are vitally important to the health of the epithelial portion of the lens. In particular, beta-carotene may act as a filter, protecting against light-induced damage to the fibrous portion of the lens. Beta-carotene is the most significant of the singlet oxygen free radical scavengers and is used in treating photosensitive disorders.28 However, in long-term studies, beta-carotene supplementation (50 mg on alternate days) on its own has been found to have no impact on cataract prevention in either women or men.29,30
Melatonin
Melatonin is a very efficient free radical scavenger and antioxidant that can neutralize hydroxyl and peroxyl radicals as well as enhance endogenous and exogenous antioxidant efficiency. In animal models, melatonin has been an effective inhibitor of DNA damage, lipid peroxidation, and cataract formation. Melatonin is present at significant levels in the cell nucleus, aqueous cytosol, and lipid-rich cellular membranes.31
Dairy Products
Cataracts often develop in infants with a homozygous deficiency of either galactokinase or galactose-1-phosphate uridyl transferase and in laboratory animals fed a high-galactose diet. Abnormalities of galactose metabolism can be identified by measurements of the activity of these enzymes in red blood cells. It has been suggested that such abnormalities are an important mechanism in approximately 30% of cataracts.26 However, this mechanism of cataract formation appears to be significant only in diabetic cataract formation and is probably not relevant to senile cataract formation (for further discussion, see Chapter 161).
Heavy Metals
A number of heavy metals have been shown to have higher concentrations in both the aging lens and the cataractous lens. Although the levels are higher in the latter, the significance of this finding is unknown.23
Other elevated elements of unknown significance are bromine, cobalt, iridium, and nickel.23
Botanical Medicines
Flavonoid-Rich Extracts
Among the best may be flavonoid-rich extracts from Vaccinium myrtillus (bilberry), Vitis vinifera (grape seed), and Pinus maritima (pine bark). The occurrence of cataracts in rats can be retarded by changing their diet from a commercial laboratory chow to a “well-defined diet.”32 Preliminary research suggests that flavonoid components in the well-defined diets may be responsible for the protective effects.33
Of the flavonoid-rich extracts, bilberry anthocyanosides may offer the greatest protection. In one human study, bilberry extract plus vitamin E stopped progression of cataract formation in 97% of 50 patients with senile cortical cataracts.34
Hachimijiogan
An ancient Chinese herbal formula, Hachimijiogan, has been shown to raise the antioxidant level of the lens of the eye.35 This activity may explain its use in treatment of cataracts for hundreds of years. According to clinical research, its therapeutic effect is quite impressive in the early stages of cataract formation. In one study, 60% of the subjects receiving Hachimijiogan noted significant improvement, 20% of the group showed no progression, and only the remaining 20% displayed progression. Hachimijiogan contains the following eight herbs (per 24 g):
1. Bouton S. Vitamin C and the aging eye. Arch Intern Med. 1939;63:930–945.
2. Ringvold A., Johnsen H., Blika S. Senile cataract and ascorbic acid loading. Acta Ophthalmol (Copenh). 1985;63:277–280.
3. Atkinson D.T. Malnutrition as an etiological factor in senile cataract. Eye Ear Nose Throat Mon. 1952;31:79–83.
4. Rathbun W., Hanson S. Glutathione metabolic pathway as a scavenging system in the lens. Ophthalmic Res. 1979;11:172–176.
5. Swanson A.A., Truesdale A.W. Elemental analysis in normal and cataractous human lens tissue. Biochem Biophys Res Comm. 1971;45:1488–1496.
6. Karakucuk S., Ertugrul Mirza G., Faruk Ekinciler O. Selenium concentrations in serum, lens, and aqueous humour of patients with senile cataract. Arch Ophthalmol Scand. 1995;73:329–332.
7. Taylor A. Cataract: relationships between nutrition and oxidation. J Am Coll Nutr. 1993;12:138–146.
8. Taylor A., Jacques P.F., Chylack L.T., Jr., et al. Long-term intake of vitamins and carotenoids and odds of early age-related cortical and posterior subcapsular lens opacities. Am J Clin Nutr. 2002;75:540–549.
9. Jacques P.F., Chylack L.T., Jr., Hankinson S.E., et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch Ophthalmol. 2001;119:1009–1019.
10. Kuzniarz M., Mitchell P., Cumming R.G., et al. Use of vitamin supplements and cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2001;132:19–26.
11. Mares-Perlman J.A., Lyle B.J., Klein R., et al. Vitamin supplement use and incident cataracts in a population-based study. Arch Ophthalmol. 2000;118:1556–1563.
12. Granado F., Olmedilla B., Blanco I. Nutritional and clinical relevance of lutein in human health. Br J Nutr. 2003;90:487–502.
13. Hankinson S.E., Stampfer M.J., Seddon J.M., et al. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992;305:335–339.
14. Brown L., Rimm E.B., Seddon J.M., et al. A prospective study of carotenoid intake and risk of cataract extraction in U.S. men. Am J Clin Nutr. 1999;70:517–524.
15. Chasan-Taber L., Willett W.C., Seddon J.M., et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in U.S. women. Am J Clin Nutr. 1999;70:509–516.
16. Lyle B.J., Mares-Perlman J.A., Klein B.E., et al. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149:801–809.
17. Olmedilla B., Granado F., Blanco I., et al. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19:21–24.
18. Valero M.P., Fletcher A.E., De Stavola B.L., et al. Vitamin C is associated with reduced risk of cataract in a Mediterranean population. J Nutr. 2002;132:1299–1306.
19. Christen W.G., Glynn R.J., Sesso H.D., et al. Age-related cataract in a randomized trial of vitamins E and C in men. Arch Ophthalmol. 2010 Nov;128(11):1397–1405.
20. McNeil J.J., Robman L., Tikellis G., et al. Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology. 2004;111:75–84.
21. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–1452. Age-Related Eye Disease Study Research Group
22. Whanger P., Weswig P. Effects of selenium, chromium and antioxidants on growth, eye cataracts, plasma cholesterol and blood glucose in selenium deficient, vitamin E supplemented rats. Nutr Rep Int. 1975;12:345–358.
23. Swanson A.A., Truesdale A.W. Elemental analysis in normal and cataractous human lens tissue. Biochem Biophys Res Comm. 1971;45:1488–1496.
24. Rao G.N., Cotlier E. The enzymatic activities of GTP cyclohydrolase, sepiapterin reductase, dihydropteridine reductase and dihydrofolate reductase; and tetrahydrobiopterin content in mammalian ocular tissues and in human senile cataracts. Comp Biochem Physiol B. 1985;80B:61–66.
25. Skalka H., Prchal J. Cataracts and riboflavin deficiency. Am J Clin Nutr. 1981;34:861–863.
26. Prchal J.T., Conrad M.E., Skalka H.W. Association of pre-senile cataracts with heterozygosity for galactosemic states and riboflavin deficiency. Lancet. 1978;1:12–13.
27. Rathbun W.B. Influence on lenticular glutathione research. Ophthalmic Res. 1995;27(suppl 1):13–17.
28. Burton G.W., Ingold K.U. Beta-carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573.
29. Christen W., Glynn R., Sperduto R., et al. Age-related cataract in a randomized trial of beta-carotene in women. Ophthalmic Epidemiol. 2004 Dec;11(5):401–412.
30. Christen W.G., Manson J.E., Glynn R.J., et al. A randomized trial of beta carotene and age-related cataract in U.S. physicians. Arch Ophthalmol. 2003 Mar;121(3):372–378.
31. Reiter R.J. Oxygen radical detoxification processes during aging: the functional importance of melatonin. Aging (Milano). 1995;7:340–351.
32. Hess H.H., Knapka J.J., Newsome D.A., et al. Dietary prevention of cataracts in the pink-eyed RCS rat. Lab Anim Sci. 1985;35:47–53.
33. Pautler E.L., Maga J.A., Tengerdy C. A pharmacologically potent natural product in the bovine retina. Exp Eye Res. 1986;42:85–88.
34. Bravetti G. Preventive medical treatment of senile cataract with vitamin E and anthocyanosides: clinical evaluation. Ann Ottalmol Clin Ocul. 1989;115:109.
35. Yoshida H., Kusukawa R., Watanabe N., et al. The effects of Ba-wei-wan (Hachimijiogan) on plasma levels of high density lipoprotein-cholesterol and lipoperoxide in aged individuals. Am J Clin Med. 1985;13:71–76.