CHAPTER 63 Selective Amygdalohippocampectomy
Historical Considerations
Over the course of the past century, two main factors have driven the surgical approach to epilepsy. First, an appreciation of the central role of the mesial basal temporal structures in many cases of human epilepsy has been gained from clinical observation and neuropathologic, electrophysiologic, and radiologic studies.1 Second and in parallel has been accumulating evidence from numerous surgical series demonstrating that temporal lobe resection, in particular, resection of mesial temporal structures, leads to an 80% to 90% rate of freedom from seizures in patients with medically intractable temporal lobe epilepsy.1–7
A number of surgical techniques have evolved in the process. The standard temporal resection procedure has been en bloc anterior temporal lobectomy (ATL) involving anterior neocortical resection of up to 4.5 to 6.5 cm, depending on whether the dominant temporal lobe is being operated on, and mesial temporal resection encompassing the amygdala and at least 3 cm of hippocampus. This approach was originally advocated by Falconer and colleagues in 19558 and is still used today.
Selective surgical approaches to the amygdala and hippocampus evolved as evidence increasingly indicated a critical role for these structures in epileptogenesis, and methods were sought to minimize collateral surgical injury to important temporal neocortical structures. Niemeyer was the first to describe selective transcortical transventricular amygdalohippocampectomy (STTAH) in 1958.9 A direct approach to the mesial temporal structures via the middle temporal gyrus was used, an approach that Olivier would later refine with the assistance of frameless stereotaxy.1
Other surgeons have approached the mesial temporal structures via less direct paths. Weiser, Yasargil, and their coworkers reported a transsylvian approach to the medial temporal structures that had the theoretical advantage of complete avoidance of neocortical injury; however, the approach was technically more demanding and placed critical vascular structures at risk.10,11 Hori and associates described a subtemporal selective approach and reported fewer neuropsychological sequelae as an advantage.12,13
With the recent confirmation that temporal lobectomy is superior to the best medical therapy for patients with intractable temporal lobe epilepsy,7 focus has now shifted toward understanding what is the best surgical approach to achieve this end. Selective amygdalohippocampectomy (SAH) represents an approach to the mesiobasal temporal structures that is as effective as ATL in selected patients, has the potential to minimize neocortical injury and its neurological sequelae, and is an approach that may be used by most neurosurgeons.
Surgical Rationale for Selective Amygdalohippocampectomy
Seizure outcome after epilepsy surgery has been noted to depend largely on diagnostic and clinical variables that have an impact on patient selection.3,6 The efficacy of selective approaches versus traditional en bloc ATL with mesial resection for seizure control has been examined in a number of noncontrolled series and found to be comparable in most,3,5 but not all reports,2 and this variability may reflect differences in the types of patients selected for surgery. Indeed, even in studies that have claimed the highest rates of freedom from seizures with the selective approach,1 it is evident that strict attention to preoperative selection is important.
In the absence of surgical complications such as stroke or infection, neuropsychological complications, particularly memory loss, are the major potential morbidity after all temporal resections. It is argued that these complications must arise from resection of functioning normal tissue and are therefore less likely to occur in patients undergoing more selective resection. Although confounding variables such as the dominance of the resected side, the absence of ipsilateral hippocampal sclerosis, the focality of the epileptic source, the presence of intact memory, and methodologic differences in neuropsychological testing make direct comparisons difficult, there is accumulating evidence that individually tailored or selective approaches may have a more favorable cognitive outcome than standard resections3,5,14,15; however, not all studies have shown such results.
Preoperative Evaluation and Decision Making
Patients under consideration for epilepsy surgery have medically refractory epilepsy. Although the definition of medical intractability may vary, data from Kwan and Brodie and from others suggest that these patients can be identified early, perhaps after as few as two failed trials of antiepileptic drugs.16
Clinically significant postoperative memory loss is more likely in patients with left (dominant) temporal lobe epilepsy, intact preoperative verbal memory, normal magnetic resonance imaging (MRI) results, bilateral hippocampal abnormalities, and later age at seizure onset.3,17 Assessment of these factors guides preoperative counseling regarding the risk for postoperative memory deficits. These risks are tempered by the hazards associated with not operating (some patients with medically treated refractory temporal lobe epilepsy have accelerated memory loss) and by the knowledge that some cognitive skills can stabilize or improve after successful surgery.
Selective Transcortical Transventricular Amygdalohippocampectomy—Operative Procedure and Avoidance of Complications
All patients undergo preoperative stereotactic brain MRI with fiducial scalp markers in place. Images are transferred to a Stealth Workstation (Medtronic, Minneapolis, MN), and the surgical entry point and trajectory are planned so that the most direct route is taken to the ipsilateral temporal horn from the middle temporal gyrus, approximately 3 cm from the tip of the temporal lobe (Fig. 63-1).
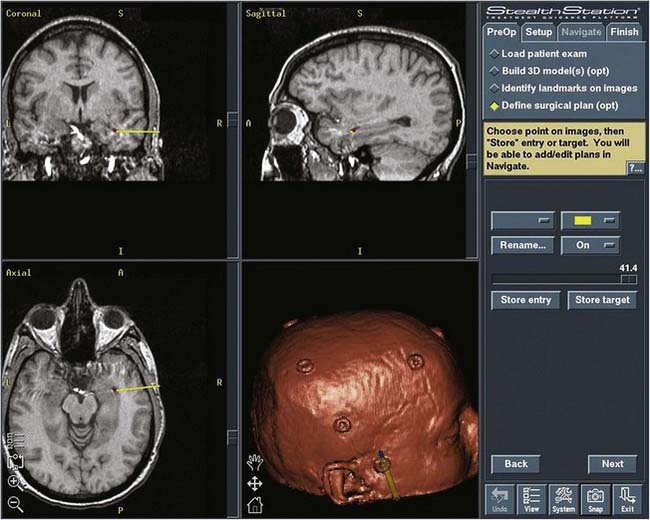
FIGURE 63-1 Image from the Stealth Workstation showing the plan for the entry point and trajectory to the temporal horn.
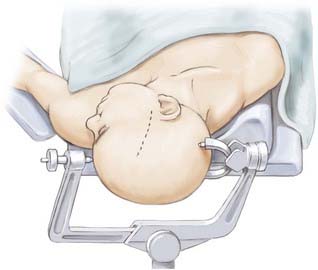
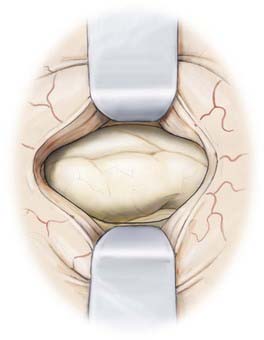
The patient undergoes routine general anesthesia and endotracheal intubation with insertion of a urinary catheter and administration of antibiotics. Mannitol is not used routinely. The patient is positioned supine with the head rotated 90 degrees to the opposite side, parallel to the floor with the head held in a three-pin fixateur. Attention to level positioning of the head is important to achieve a true lateral trajectory. Fiducials are registered and the planned cortical entry point marked on the scalp along with the intended bone flap and scalp incision (Fig. 63-2). The scalp is then prepared, draped, and infiltrated with 1% lidocaine and a 0.25% bupivacaine mixture with epinephrine.
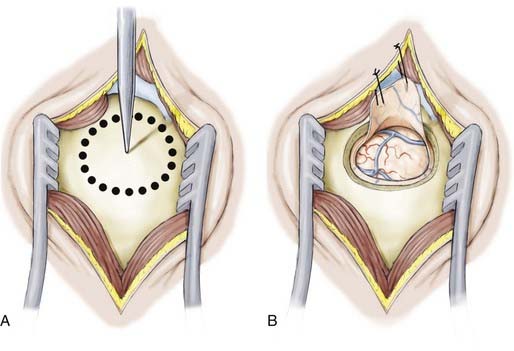
The linear scalp incision is opened, the temporalis fascia sharply incised, and the longitudinal fibers of the temporalis dissected from the periosteum and retracted laterally. The neuronavigation system is then used to direct the position of the temporal craniotomy, which invariably reaches the floor of the middle cranial fossa. After the dura is exposed and the surface vessels controlled with diathermy, the dura is opened and reflected inferiorly (Fig. 63-3).
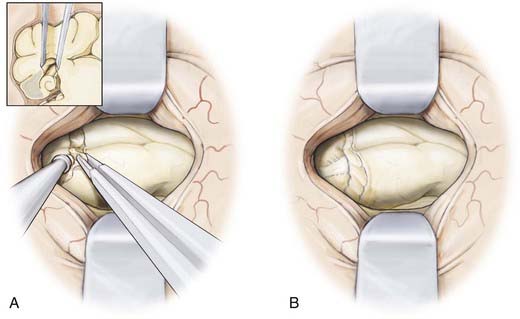
The neuronavigation system is used to estimate the distance from the tip of the temporal lobe, which is confirmed manually, and to select the cortical entry site on a portion of the middle temporal gyrus that is free of significant cortical vessels. For the dominant temporal lobe, we select an entry point no farther than 3.5 cm from the temporal tip and plan for a cortical incision 1.5 to 2 cm in length (Fig. 63-4). With the use of bipolar cautery, the cortical surface is opened and an endopial dissection is performed toward the temporal horn guided at all times by the image guidance system (Fig. 63-5).
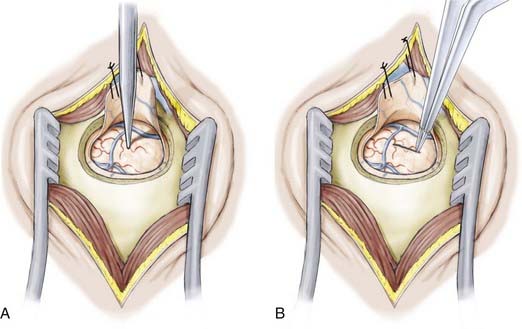
Once the temporal horn is entered, two self-retaining brain retractors are placed to optimize visualization of the intraventricular anatomy (Fig. 63-6). The following structures should be identified to aid navigation: the medial prominence of the hippocampus; the collateral eminence, which overlies the collateral sulcus separating the parahippocampal and occipitotemporal gyri; and the choroid plexus.
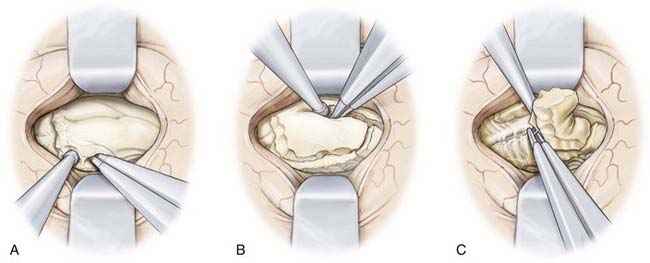
Resection of the mesial temporal lobe structures is commenced by entering the parahippocampal gyrus located ventral to the hippocampus (Fig. 63-7). The resection begins at the pial surface of the most ventrolateral portion of the gyrus and then progresses medially toward the uncus. After this part of the resection is complete, it possible to visualize the edge of the tentorium and the contents of the basal cisterns and be sufficiently oriented to perform the amygdalectomy safely. The navigation system is used throughout the procedure. The mesial pial border must be strictly preserved to avoid injury to the posterior cerebral artery and third nerve. These structures can be visualized through the preserved pial membrane.
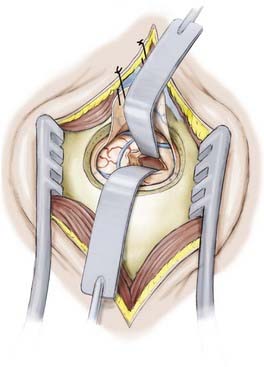
After the anterior portion of the parahippocampal gyrus and the amygdala have been removed, attention is directed toward the hippocampus (Fig. 63-8). Here, identification of the choroid plexus and choroidal fissure guides the resection by defining the superomedial limit of resection. The hippocampus is dissected first from this border starting anteriorly from the posterior limit of the amygdalectomy and extending posteriorly to the level of the tectal plate. Again, frameless stereotaxy is used to ensure the completeness of resection from the uncus to the posterior of the hippocampus. At the posterior limit of the planned resection the hippocampal tail is removed and the lateral margin of the hippocampus is then dissected down the pia of the middle cranial fossa to completely isolate the structure. The body of the hippocampus can then be removed piecemeal via a suction technique on either side of the hippocampal sulcus. While removing the hippocampus and separating it from the hippocampal sulcus, it may be necessary to coagulate small hippocampal vessels, but great care should be taken to preserve small branches of the anterior choroidal artery and posterior cerebral artery in this vicinity.
The resection cavity is then inspected and hemostasis is achieved (Fig. 63-9). The brain retractors are removed and the dura is closed in watertight fashion. The bone flap is secured with miniplates, the temporalis muscle is reapproximated, and the scalp is closed in layers with absorbable suture.
Outcome
There are no randomized controlled trials comparing SAH with ATL. Hence, the existing nonrandomized comparative studies suffer from potential selection bias, and many compare groups of patients who are not comparable with each other.3,5,18 Nonetheless, the overwhelming finding from these studies is therapeutic equivalence; both SAH and ATL lead to high rates of freedom from seizures in carefully selected patients. Many studies restricted SAH to a highly selected group of patients with hippocampal sclerosis as the sole pathology. Others have reported excellent outcomes with SAH in patients with lesions identified on preoperative imaging studies, as well as those with normal MRI studies.19 It is clear, however, that surgical outcomes suffer if the patient selection process deviates from the strict criteria that are used to identify patients with unilateral mesial temporal lobe epilepsy who may benefit from conventional temporal lobe resection surgery.20 Although there is little dispute that patients with unilateral hippocampal sclerosis are excellent candidates for SAH, the characteristics of other patients who might benefit from this procedure have yet to be fully defined.
Neuropsychological outcomes in most,3,5,12,13,17,21–23 but not all24–26 studies favor SAH over ATL. Nonetheless, this remains a subject of controversy. Many test measures examining postoperative neurological function are similar between the two surgical groups; however, several studies have reported superior outcomes with SAH in terms of verbal memory, attention, and some composite measures of neuropsychological outcome.3,5 However, it is clear from large, well-conducted trials that patients undergoing left (dominant) SAH are not immune to substantial verbal memory impairment and that the more selective procedure is not a panacea.17,21 Careful preoperative neuropsychological assessment and counseling remain critical.
A large, randomized, controlled comparison of transsylvian and transcortical SAH demonstrated no differences in seizure-free outcomes or most neuropsychological measures between the two surgical groups. However one performance measure (verbal fluency) was superior in the transcortical group.2 Hori and colleagues have argued that cognitive sequelae are minimized by a subtemporal approach,12,13 but this remains to be demonstrated convincingly.
Abosch A, Bernasconi N, Boling W, et al. Factors predictive of suboptimal seizure control following selective amygdalohippocampectomy. J Neurosurg. 2002;97:1142.
Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446.
Bate H, Eldridge P, Varma T, et al. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14:90.
Clusmann H, Schramm J, Kral T, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131.
Falconer MA, Meyer A, Hill D, et al. Treatment of temporal-lobe epilepsy by temporal lobectomy; a survey of findings and results. Lancet. 1955;268:827.
Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia. 2004;45:960.
Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87.
Goldstein LH, Polkey CE. Short-term cognitive changes after unilateral temporal lobectomy or unilateral amygdalo-hippocampectomy for the relief of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1993;56:135.
Hori T, Yamane F, Ochiai T, et al. Selective subtemporal amygdalohippocampectomy for refractory temporal lobe epilepsy: operative and neuropsychological outcomes. J Neurosurg. 2007;106:134.
Hori T, Yamane F, Ochiai T, et al. Subtemporal amygdalohippocampectomy prevents verbal memory impairment in the language-dominant hemisphere. Stereotact Funct Neurosurg. 2003;80:18.
Jones-Gotman M, Zatorre RJ, Olivier A, et al. Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia. 1997;35:963.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314.
Lacruz ME, Alarcon G, Akanuma N, et al. Neuropsychological effects associated with temporal lobectomy and amygdalohippocampectomy depending on Wada test failure. J Neurol Neurosurg Psychiatry. 2004;75:600.
Mittal S, Montes JL, Farmer JP, et al. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg. 2005;103:401.
Morino M, Uda T, Naito K, et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95.
Niemeyer P. The transventricular amygdalohippocampectomy in temporal lobe epilepsy. In: Baldwin M, Bailey P, editors. Temporal Lobe Epilepsy. Springfield, IL: Charles C Thomas; 1958:461.
Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Can J Neurol Sci. 2000;27(suppl 1):S68.
Paglioli E, Palmini A, Portuguez M, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104:70.
Pauli E, Pickel S, Schulemann H, et al. Neuropsychologic findings depending on the type of the resection in temporal lobe epilepsy. Adv Neurol. 1999;81:371.
Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51:465.
Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311.
Wieser HG. Selective amygdalohippocampectomy: indications and follow-up. Can J Neurol Sci. 1991;18:617.
Wieser HG, Ortega M, Friedman A, et al. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:751.
Wieser HG, Siegel AM, Yasargil GM. The Zurich amygdalo-hippocampectomy series: a short up-date. Acta Neurochir Suppl. 1990;50:122.
Wolf HK, Campos MG, Zentner J, et al. Surgical pathology of temporal lobe epilepsy. Experience with 216 cases. J Neuropathol Exp Neurol. 1993;52:499.
Yasargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93.
1 Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Can J Neurol Sci. 2000;27(suppl 1):S68.
2 Bate H, Eldridge P, Varma T, et al. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14:90.
3 Clusmann H, Schramm J, Kral T, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131.
4 Mittal S, Montes JL, Farmer JP, et al. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg. 2005;103:401.
5 Paglioli E, Palmini A, Portuguez M, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104:70.
6 Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51:465.
7 Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311.
8 Falconer MA, Meyer A, Hill D, et al. Treatment of temporal-lobe epilepsy by temporal lobectomy; a survey of findings and results. Lancet. 1955;268:827.
9 Niemeyer P. The transventricular amygdalohippocampectomy in temporal lobe epilepsy. In: Baldwin M, Bailey P, editors. Temporal Lobe Epilepsy. Springfield, IL: Charles C Thomas; 1958:461.
10 Wieser HG, Siegel AM, Yasargil GM. The Zurich amygdalo-hippocampectomy series: a short up-date. Acta Neurochir Suppl. 1990;50:122.
11 Yasargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93.
12 Hori T, Yamane F, Ochiai T, et al. Subtemporal amygdalohippocampectomy prevents verbal memory impairment in the language-dominant hemisphere. Stereotact Funct Neurosurg. 2003;80:18.
13 Hori T, Yamane F, Ochiai T, et al. Selective subtemporal amygdalohippocampectomy for refractory temporal lobe epilepsy: operative and neuropsychological outcomes. J Neurosurg. 2007;106:134.
14 Lacruz ME, Alarcon G, Akanuma N, et al. Neuropsychological effects associated with temporal lobectomy and amygdalohippocampectomy depending on Wada test failure. J Neurol Neurosurg Psychiatry. 2004;75:600.
15 Morino M, Uda T, Naito K, et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95.
16 Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314.
17 Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87.
18 Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446.
19 Wieser HG, Ortega M, Friedman A, et al. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:751.
20 Abosch A, Bernasconi N, Boling W, et al. Factors predictive of suboptimal seizure control following selective amygdalohippocampectomy. J Neurosurg. 2002;97:1142.
21 Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia. 2004;45:960.
22 Pauli E, Pickel S, Schulemann H, et al. Neuropsychologic findings depending on the type of the resection in temporal lobe epilepsy. Adv Neurol. 1999;81:371.
23 Wieser HG. Selective amygdalohippocampectomy: indications and follow-up. Can J Neurol Sci. 1991;18:617.
24 Goldstein LH, Polkey CE. Short-term cognitive changes after unilateral temporal lobectomy or unilateral amygdalo-hippocampectomy for the relief of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1993;56:135.
25 Jones-Gotman M, Zatorre RJ, Olivier A, et al. Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia. 1997;35:963.
26 Wolf HK, Campos MG, Zentner J, et al. Surgical pathology of temporal lobe epilepsy. Experience with 216 cases. J Neuropathol Exp Neurol. 1993;52:499.