Chapter 13 Seizures and Epilepsy in the Elderly
Introduction
In developed countries, demographic trends project continued increases in the number of older people in the population. In the United States, there were 36.8 million adults over the age of 65 in 2005.1 By 2030, the U.S. Department of Health and Human Services predicts that this figure will have increased to 71.5 million and account for roughly 20% of the population. In this older age group, seizures and epilepsy are the third most common neurological condition, behind only stroke and dementia. Although the primary goals of treatment—freedom from seizures, absence of adverse drug effects, and maintenance of a high quality of life—are the same for all patients with epilepsy, several issues specific to the elderly population need to be considered in approaching diagnosis and making treatment decisions. Diagnosis can be challenging because of the many comorbid conditions that are common in the elderly. These comorbidities, along with the medications prescribed to treat them, must be considered carefully when devising treatment strategies. Dealing with these issues will only become more challenging in the coming years, as the number of people over the age of 65 increases steadily.
Epidemiology
The incidence of both acute symptomatic seizures and unprovoked seizures or epilepsy is highest in people over the age of 65.2–4 In a British population study, nearly 25% of newly identified seizures occurred in patients 60 years of age or older.5 The incidence continues to rise with increasing age: In those older than 75, the incidence is five times that of younger adults. In the United States, about 50,000 new cases of epilepsy occur each year in this age group.6
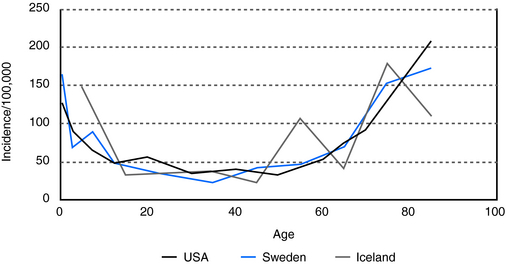
In people over 60 years of age, the prevalence of unprovoked seizures and epilepsy is at least 1%, and it is even higher, 1.2 to 1.5%, in people over 75 (Figure 13-1). This is a prevalence rate about twice that seen in younger adults.10,11 In a study of 1, 130, 155 U.S. veterans who were at least 65 years of age, 1.8% had epilepsy.12,13 In specific populations at risk, such as nursing home residents, who have significantly higher rates of comorbid conditions associated with epilepsy, including dementia and stroke, prevalence rates as high as 3 to 9% have been reported.14–16
Etiology
ACUTE SYMPTOMATIC SEIZURES
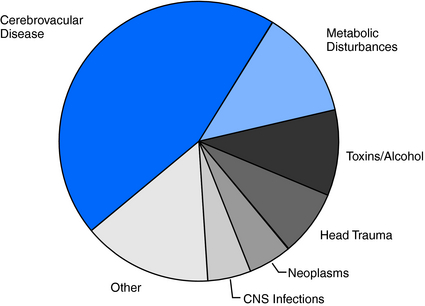
Because brain injuries caused by stroke, head injury, infections, neoplasms, and metabolic disturbances (hypoglycemia, hyponatremia, uremia) are so common in the elderly, it is not surprising that the incidence of acute symptomatic seizures is likewise high in this age group.6 Cerebrovascular disease accounts for 40% to 50% of acute symptomatic seizures, metabolic disturbances for 10 to 15%, and acute head trauma, brain infections, neoplasms and toxins/alcohol each for 5 to 10%2,6,17 (Figure 13-2).
UNPROVOKED SEIZURES AND EPILEPSY
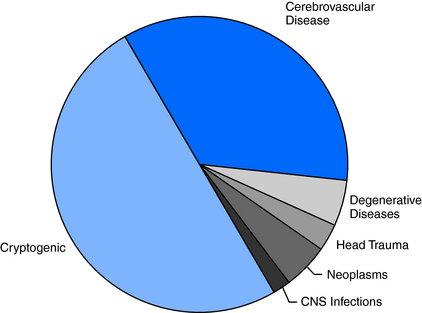
The high frequency of unprovoked seizures and epilepsy in elderly persons can also be attributed in large part to the high prevalence of a history of stroke, brain tumor, and head trauma in older age groups. Even so, about 50% of cases remain cryptogenic (Figure 13-3), although this is a significantly lower percentage than that found in younger age groups, where about 70% of cases have no identifiable cause.3,17
Cerebrovascular disease is the most frequently identified antecedent of epilepsy, accounting for 30 to 40% of all new cases.6,18 Individuals with cerebrovascular disease are 20 times more likely to develop epilepsy than the general population.19 About 15% of stroke survivors will develop unprovoked seizures within the first 5 years, and the elevated risk continues for many years thereafter.
Seizures may, in fact, be the first presentation of unrecognized cerebrovascular disease. This is especially true if previous strokes had occurred in clinically silent brain areas. In a review of new onset seizures in 4709 patients older than age 60, none of whom had a known history of cerebrovascular disease, dementia, tumor, or alcohol abuse, Cleary and colleagues found that the relative risk of stroke was nearly three times that of controls.20 Hypertension is also an independent risk factor for unprovoked seizures,21 although hypertension may be a surrogate for the progressive arterial changes associated with lacunes, other small strokes, and periventricular white matter lesions.
Although the incidence of head trauma is highest in adolescents and young adults, a relative peak of occurrence in the elderly is largely attributable to falls. Approximately one-third of people over the age of 65 living at home, and about half of nursing home residents, have at least one fall each year.22,23 Trauma with loss of consciousness is associated with a threefold increased risk of epilepsy.6 With more severe head injuries, risk is even higher. Overall, head trauma accounts for 2 to 3% of new cases of epilepsy in the elderly.
Although the incidence of central nervous system infections is highest in childhood, a second peak occurs in the elderly. Survivors of central nervous system (CNS) infections have a threefold risk of developing epilepsy, and a history of infections accounts for 2 to 3% of cases.24
Alzheimer’s and other neurodegenerative diseases are associated with a 5- to 10-fold increase in epilepsy compared to the general population.6,25 Unprovoked seizures may be seen in as many as 8 to 15% of patients with Alzheimer’s disease.25,26
Clinical Presentation
As would be expected from the etiological profile, the great majority of seizures in the elderly, more than 70%, are of partial onset.18,27,28 Even generalized tonic-clonic seizures without obvious focal features are likely to have a localized or regional onset, as it is very unusual for idiopathic generalized epilepsy syndromes to present at this age. In any event, diagnosis of epilepsy in the elderly can be difficult and may be delayed. In a recent Veteran’s Administration cooperative study of epilepsy in elderly patients, epilepsy was an initial diagnostic consideration in 73% of patients that were eventually diagnosed with epilepsy.18 In a subset of 151 patients from this study, Spitz and colleagues found that the delay from initial symptoms to diagnosis was 2.3 years, with a median time of 1 year. Only 37% of patients in this subgroup were correctly diagnosed at the time of initial evaluation. Two-thirds of patients with generalized tonic-clonic (GTC) seizures, but only one-fourth of patients with complex partial seizures, were correctly diagnosed.29
Because the lesions associated with epilepsy in elderly people can involve any area of the brain, extratemporal neocortical epilepsies are more common than in younger adults. Consequently, seizures may manifest with a wide variety of sensory, visual, cognitive, and behavioral phenomena that are frequently atypical for the physician’s experience and thus more difficult to recognize as having an epileptic basis. Classic auras are less common in the elderly, and initial manifestations of partial seizures, such as dizziness, a vague feeling related to the head, memory loss, or confusion, may be interpreted as nonspecific symptoms due to any number of possible causes. Thus, given the high frequency with which metabolic derangements, cerebrovascular events, and dementia occur in the elderly, clinical manifestation of partial seizures, as well as of postictal states, may be incorrectly attributed to these other conditions. For example, postical aphasia or hemiparesis may be diagnosed as ischemic events.30 Finally, although published data are lacking, it is said that generalized tonic-clonic seizures, the easiest type of seizure to recognize, occur with less frequency in older age groups.
Falls in the elderly are common and a frequent cause of admission to a hospital.23 However, seizures are rarely considered an important etiology, especially early in the diagnostic evaluation. More often, falls are attributed to cardiovascular, cerebrovascular, or arthritic etiologies. Confusion or memory loss, which may be ictal or postical in nature, is often first considered to be a manifestation of dementia, metabolic abnormalities (e.g., dehydration) or a head injury related to a fall.
Difficulty in diagnosis is compounded by the increase in social isolation in the elderly population. In 2006, the U.S. Department of Health and Human Services found that 30% of noninstitutionalized people over 65 years of age, totaling more than 10 million people, lived alone.1 As such, elderly patients are often brought to the hospital by emergency medical services without anyone available to provide information through direct observation of an episode.
NONEPILEPTIC PAROXYSMAL PHENOMENA
As at other ages, elderly persons can have episodic paroxysmal phenomena that mimic seizures but are nonepileptic (Table 13-1). In a study of 94 patients at least 60 years of age (mean 70 years) who were referred for video-electroencephalogram (EEG) monitoring for evaluation of paroxysmal episodes, 27 (29%) had nonepileptic events, including 13 with psychogenic seizures.31 The majority of these patients had been taking antiepileptic drugs for presumed epileptic seizures. Such findings underscore the need for video-EEG monitoring in patients whose seizures are atypical or have not responded to treatment with antiepileptic drugs (AEDs).
TABLE 13–1 Paroxysmal Phenomena That May Mimic Seizures in Elderly Patients
| Syncope |
| Transient ischemic attacks |
| Transient global amnesia |
| Migraine |
| Drop attacks |
| Myoclonus |
| Confusional episodes due to medication interactions or overmedication |
| Hypoglycemia |
| Electrolyte disturbances/dehydration |
| REM behavior disorder |
| Nonepileptic psychogenic seizures |
Risk of Recurrence
Physicians should have a low threshold for suspecting seizures in the elderly because of the increased incidence and the higher rate of seizure recurrence in this population.3,4
In patients presenting with a first unprovoked seizure, the overall risk of recurrence with at least 2 years of follow-up is about 38% (range 25 to 52%).32–35 Factors that increase the risk of recurrence include abnormal EEG findings and presence of an underlying definable etiology.
Data regarding specific effects of older age on risk of recurrence are inconclusive, and there are no population-based studies. However, when seizures can be attributed to a recognized underlying neurologic condition (that is, they are symptomatic of an acquired pathogenic mechanism), risk of recurrence is roughly double that of a cryptogenic first seizure.33 In selected populations, the recurrence rate may be especially high. For example, seizures recurred in more than 80% of a small series of patients with a remote history of stroke.36
Treatment Considerations
Although it often stated that epilepsy is more easily controlled in elderly patients than in younger ones,37,38 it is not clear that this is accurate, as no well-controlled large studies specifically address this issue. Data from two recent studies looking at tolerability of anticonvulsant drugs in the elderly showed that seizure-free rates are similar to those seen in the adult population at large. In a randomized trial that compared response in the elderly to treatment regimens using modest dosages of carbamazepine (dosage range 200 to 800 mg daily) and lamotrigine (dosage range 75 to 300 mg daily), Brodie and colleagues found that only 33% remained seizure free during the final 16 weeks of the study.39 Although a high rate of medication withdrawal likely contributed to the relatively low rate of seizure control, these findings suggest that seizures are, in fact, not necessarily easily controlled in this population. Similarly, in the VA cooperative study #428, which randomized elderly patients with new-onset epilepsy to treatment with gabapentin, lamotrigine, or carbamazepine, 53% of patients who remained on treatment were seizure free at 12 months.40 These data are not substantially different from seizure control rates in the general population, as illustrated by the study by Kwan and Brodie of 470 previously untreated patients with epilepsy, ranging in age from 9 to 93.41 They found that 47% of patients became seizure free on the first drug, and a total of 61% became seizure free with the second or third monotherapy agent.
PHARMACOKINETIC CHANGES
Absorption
Absorption of drugs depends on dissolution of their particular formulations. This, in turn, is mainly related to gastric acid secretion, which often declines in the elderly.42 In addition, to varying degrees, gastric emptying slows, intestinal transit time increases, mesenteric blood flow decreases, and the intestinal absorptive surface may decrease.43 All these changes contribute to variable and often unpredictable absorption of different drugs. Overall, this combination of factors typically results in a diminished ability to absorb antiepileptic drugs, which reduces their bioavailability.
These age-related changes in absorption can be compounded by frequent use of antacids, which can specifically impair absorption of phenytoin. In addition, gabapentin, which is absorbed via a saturable L-amino acid transporter system in the small intestine,44 may be particularly susceptible to physiological changes in gastrointestinal absorption.
Protein Binding
In healthy adults, serum albumin concentration decreases only slightly with age.45 However, in elderly patients with acute systemic and neurological illnesses, serum albumin levels can decline significantly. Suboptimal nutrition may exacerbate this. With reductions in serum albumin concentrations, the free fraction of highly protein-bound medications can increase substantially, sometimes resulting in prominent adverse effects despite little or no change in the total serum level.
AEDS that are highly protein bound include tiagabine, phenytoin, valproate, diazepam, clonazepam, clobazam, and to a lesser extent, carbamazepine.46
Hepatic Clearance
Hepatic mass and blood flow both decline with age. As a result, liver volume is about 25% lower in people age 65 compared to young adults.47 The degree to which the various hepatic enzymes change with increasing age is not known definitively.48 The majority of drugs metabolized in the liver utilize the cytochrome P450 system. With increasing age, this system is believed to decline in function, though to a variable and unpredictable degree. The hepatic glucoronidation conjugation process is thought to be less affected by age.
AEDs metabolized primarily by the cytochrome P450 system include phenytoin, phenobarbital, primidone, carbamazepine, ethosuximide, oxcarbazepine, and tiagabine. Felbamate, topiramate, valproate, and zonisamide are also partially metabolized via this pathway.46 AEDs that primarily undergo conjugation include lamotrigine, zonisamide, and valproate.46
Renal Clearance
The most consistent age-related change affecting pharmacokinetics is a decline in renal function related to reduction in renal mass and loss of glomeruli. This results in lower glomerular filtration rate (GFR) and a reduced ability to handle renally excreted medications and toxins. On average, GFR declines by about 50% between the third and eighth decades of life.49 However, this is variable, and about one-third of people may not experience declines of this degree.50 Because muscle mass, the source of serum creatinine, also decreases with age, changes in serum creatinine levels frequently do not parallel the decline in GFR. The validity of common formulas used to estimate GFR has been questioned, even in otherwise healthy elderly patients.51
AEDs that are excreted primarily by the kidney include gabapentin, levetiracetam, vigabatrin, and pregabalin. Felbamate, zonisamide, and topiramate are also partially renally excreted.46
Table 13-2 summarizes the effect of older age on AED clearance.
TABLE 13–2 Average Changes in Apparent Oral Clearance of Older and Newer AEDs in Elderly Patients (Interindividual variation may be considerable in relation to age and other factors.)
| Drug | Effect of Old Age on Drug Clearance |
|---|---|
| Carbamazepine | Decrease by 25–40%52 |
| Felbamate | Decrease by 10–20%53 |
| Gabapentin | Decrease by about 30–50%54 |
| Lamotrigine | Decrease by about 35%55 |
| Levetiracetam | Decrease by about 20–40%56 |
| Oxcarbazepine | Decrease by 25–35%57 |
| Phenobarbital | Decrease by about 20%58 |
| Phenytoin | Decrease by about 25%59 |
| Tiagabine | Decrease by about 30%60 |
| Topiramate | Decrease by 20%61 |
| Valproic acid | Decrease by about 40%62 |
| Vigabatrin | Decrease by 50–85%63 |
| Zonisamide | No data |
Reprinted with permission from Perucca E, Berlowitz D, Birnbaum A, et al. Pharmacological and clinical aspects of antiepileptic drug use in the elderly. Epilepsy Res. 2006;68[Suppl 1]:S49-S63.
PHARMACODYNAMIC CHANGES
In addition to age-related pharmokinetic changes, it is likely that actions of AEDs at the cellular level are also altered in the elderly. Although such changes have not been well characterized, the appearance of adverse central nervous system effects (e.g., drowsiness, unsteadiness) at unbound serum levels that rarely produce similar toxicity in younger adults is probably one reflection of this. As a result, it is important to anticipate a much narrower therapeutic window for AEDs in the elderly population (Figure 13-4).
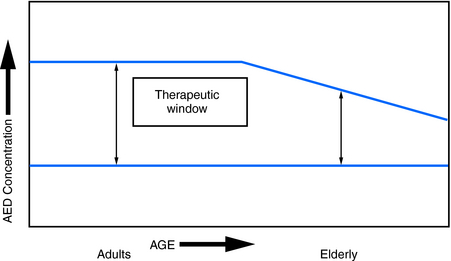
Figure 13–4 Effect of age on therapeutic ranges. The elderly typically have a narrower therapeutic window, the range between the lowest effective concentration and the maximal tolerated concentration.64,65
Reprinted with permission from Bergey GK. Initial treatment of epilepsy: special issues in treating the elderly. Neurology. 2004;63[10 Suppl 4]:S40-S48.
CHOOSING AN AED
As nearly all of the currently used AEDs have similar efficacy in treating partial and secondarily generalized seizures, choice of medication depends mainly on tolerability and the presence of comorbid conditions. Nearly 90% of elderly patients in the community take at least one prescription drug,66 and nursing home residents take an average of five.67 Serum levels of AEDs that are metabolized by the cytochrome p450 enzymes are most likely to be affected, if other medications administered concurrently inhibit this pathway. Common examples include H2-blockers (cimetidine), macrolide antibiotics (erythromycin, clarithromycin), antifungal agents (ketoconazole, fluconazole), and isoniazid. Ticlopidine increases levels of carbamazepine and phenytoin. Thus, the possibility of therapeutically significant drug interactions must be a major consideration when selecting an AED.
Detailed information concerning drug interactions can be found in an extensive review by Patsalos and Perucca.68
Older-Generation AEDs
Although phenytoin, phenobarbital, primidone, valproate, and carbamazepine are effective in localization-related epilepsy,69,70 tolerability concerns have generally displaced them as drugs of first choice in elderly patients.
Phenobarbital and primidone have significant sedative and cognitive effects and the first VA Cooperative Trial70 showed them to be much less well tolerated than carbamazepine and phenytoin. They are also potent inducers of hepatic enzymes, and this reduces the efficacy of many drugs administered concurrently to treat comorbid conditions.
Drug interactions are also common with valproate because it is both a potent inhibitor of hepatic enzymes and also highly protein bound. Occasionally, encephalopathy occurs due to hyperammonemia, which can occur in the absence of hepatic enzyme abnormalities.71
Carbamazepine is another inducer of hepatic enzymes, and thus drug interactions must be anticipated. In addition, hyponatremia may be more common and less well tolerated in elderly patients taking carbamazepine.72
Newer-Generation AEDs
In a recent VA cooperative study, patients 65 or older with newly diagnosed epilepsy were randomized to treatment with gabapentin 1500 mg per day, lamotrigine 150 mg per day, or carbamazepine 600 mg per day.40 The primary outcome measure was early termination, most often due to adverse effects, which occurred in 64.5% of patients taking carbamazepine in contrast to 51% of patients taking gabapentin and 44% of patients taking lamotrigine. Seizure-free rates and time to first seizure did not differ significantly among the three medications.
An earlier study by Brodie and colleagues, in which newly diagnosed elderly patients with epilepsy were randomized to lamotrigine or carbamazepine, had similar findings: 42% of patients on carbamazepine dropped out due to adverse events compared to only 18% of patients on lamotrigine.39 Somnolence occurred in 29% of patients on carbamazepine versus only 12% of patients on lamotrigine. It was also of interest that only 3% of patients on lamotrigine developed allergic rash compared to 19% of those taking carbamazepine.
In general, the newer generation AEDs are less sedating and overall have more favorable adverse effect profiles than the older generation AEDs. Details of these comparisons were reviewed extensively in the 2004 AAN guidelines on the efficacy and tolerability of the newer antiepileptic drugs.73
Levetiracetam also has a favorable adverse effect profile. It is nonsedating and does not produce significant cognitive dysfunction. It is renally excreted, is not highly protein bound, and does not induce hepatic enzymes. As a result, drug interactions are rare. About 7% of patients experience irritability and behavioral side effects.74 A major advantage of levetiracetam is that therapeutic efficacy can be achieved at starting dosages.
Oxcarbazepine is metabolized in the liver, but it is only a weak inducer of hepatic enzymes. Drug interactions are minimal. Like carbamazepine, hyponatremia is seen more often in elderly patients, and concomitant use of diuretics should be cautious.75
EPILEPSY SURGERY
Neurologists have generally been reluctant to consider resective surgery in elderly patients with epilepsy because of the higher incidence of associated comorbidities, as well as a perceived increase in complication rates. In addition, a long duration of epilepsy has been reported to be negative predictor for achieving long-term seizure control.76 However, in appropriate patients, one might equally argue that the same comorbid conditions and their consequent effects on drug absorption, clearance, rate of adverse effects, and interactions justify earlier consideration of potentially curative epilepsy surgery.
Reports on the efficacy of surgery in elderly patients come from a relatively small series of patients.77–79 Grivas and colleagues recently published the outcome of temporal lobe resection in 52 patients older than 50 (range 50 to 71, mean 56) years.80 At follow-up of at least 12 months (mean duration 33 months), 71% remained seizure free (Engel class 1). This seizure-free rate is virtually identical to that seen in a comparable cohort of 321 patients less than 50 years (72% seizure free) operated on in the same time period. Notably, 11 of the 52 patients were older than 60, and outcome was equally good in this subgroup. Only one patient showed no improvement in postoperative seizure control (2% Engel class 4). Surgical complications were somewhat higher (7.7%) in this older cohort, and there was also a higher rate (3.8%, 2 patients) of permanent mild neurologic morbidity, compared to younger patients. No patient had severe permanent neurologic morbidity, and there were no mortalities. These data suggest that surgery should not be excluded as a treatment option simply on the basis of age.
Epilepsy Morbidity
STATUS EPILEPTICUS
The incidence of status epilepticus in persons older than 60 years of age is at least twice, and possibly greater than 10 times, that seen in younger adults. The highest rates occur in patients older than 70.81–83 In a series of 171 elderly patients with status epilepticus, more than half (56%) had no previous history of seizures.84 Cerebrovascular disease is the most common cause, accounting for as many as 60% of cases.81,85,86 Other causes include hypoxia, metabolic derangement, alcohol, tumor, infection, trauma, and dementia. The mortality rate associated with status epilepticus is 38% in patients over 60 years of age, but nearly 50% in those over 80. In contrast, the overall mortality rate for status epilepticus is 22%.84,85 The increased mortality rates reflect the elderly population’s susceptibility to systemic illnesses (e.g., pneumonia, organ failure), as well as the often life-threatening or progressive nature of the underlying cause of the status epilepticus. Along with advancing age, etiology is a strong determinant of mortality: The highest rates occur from acute symptomatic causes, such as ischemic stroke, intracranial hemorrhage, and anoxic brain injury.87,88
There are no established protocols for treating status epilepticus that are age specific, but the same pharmacokinetic considerations discussed earlier in this chapter must be considered. Pharmacodynamic changes and the presence of comorbid conditions in elderly patients increase their susceptibility to CNS and respiratory depression from benzodiazepines and barbiturates, as well as to the cardiovascular effects of intravenous phenytoin and fosphenytoin.
BONE HEALTH, FALLS, AND FRACTURES
Patients with epilepsy have an approximately twofold increase in fractures compared to controls.89 In a study of more than 8000 community-dwelling women older than 65, those taking AEDs were 75% more likely to experience a fall.90 Although part of this increase relates to the seizures themselves causing falls, loss of balance due to the increased sensitivity of the aging brain to adverse CNS effects of AEDs also plays a role.
In adults, peak bone mineral density is attained between 20 and 30 years of age. After this, bone mineral density gradually declines, but it becomes most pronounced in women following onset of menopause. Older-generation AEDs, including phenytoin, carbamazepine, primidone, and phenobarbital, are commonly associated with altered bone metabolism and decreased bone density because they are potent inducers of the hepatic cytochrome P450 enzyme system.91 Valproate, an inhibitor of the P450 system, was initially believed not to affect bone health. However, it has recently been shown to be associated with reductions in bone mineral density. Polytherapy has a higher risk of abnormalities in bone metabolism than monotherapy. Multiple mechanisms have been postulated to support the association, but none is entirely satisfactory.92
CONCLUSIONS/RECOMMENDATIONS
We advise keeping the following principles in mind:
1. A profile of older Americans. In: U.S. Department of Health and Human Services, ed. Administration on Aging. Washington, DC: Author; 2006.
2. Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935-1984. Epilepsia. 1995;36(4):327-333.
3. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993;34(3):453-468.
4. Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorffer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005;4(10):627-634.
5. Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet. 1990;336(8726):1267-1271.
6. Hauser WA. Epidemiology of seizures and epilepsy in the elderly. In: Rowan AJ, Ramsay RE, editors. Seizures and Epilepsy in the Elderly. Boston: Butterworth-Heineman; 1997:7-20.
7. Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia. 1996;37(3):224-229.
8. Sidenvall R, Forsgren L, Blomquist HK, Heijbel J. A community-based prospective incidence study of epileptic seizures in children. Acta Paediatr. 1993;82(1):60-65.
9. Cloyd J, Hauser W, Towne A, et al. Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res. 2006;68(suppl 1):S39-S48.
10. de la, Court A, Breteler MM, Meinardi H, Hauser WA, Hofman A. Prevalence of epilepsy in the elderly: the Rotterdam Study. Epilepsia. 1996;37(2):141-147.
11. Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940-1980. Epilepsia. 1991;32(4):429-445.
12. Perucca E, Berlowitz D, Birnbaum A, et al. Pharmacological and clinical aspects of antiepileptic drug use in the elderly. Epilepsy Res. 2006;68(suppl 1):S49-S63.
13. Pugh MJ, Cramer J, Knoefel J, et al. Potentially inappropriate antiepileptic drugs for elderly patients with epilepsy. J Am Geriatr Soc. 2004;52(3):417-422.
14. Galimberti CA, Magri F, Magnani B, et al. Antiepileptic drug use and epileptic seizures in elderly nursing home residents: a survey in the province of Pavia, Northern Italy. Epilepsy Res. 2006;68(1):1-8.
15. Garrard J, Cloyd J, Gross C, et al. Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2000;55(7):M384-M392.
16. Schachter SC, Cramer GW, Thompson GD, Chaponis RJ, Mendelson MA, Lawhorne L. An evaluation of antiepileptic drug therapy in nursing facilities. J Am Geriatr Soc. 1998;46(9):1137-1141.
17. Loiseau J, Loiseau P, Duche B, Guyot M, Dartigues JF, Aublet B. A survey of epileptic disorders in southwest France: seizures in elderly patients. Ann Neurol. 1990;27(3):232-237.
18. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. 2004;62(5 suppl 2):S24-S29.
19. So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology. 1996;46(2):350-355.
20. Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet. 2004;363(9416):1184-1186.
21. Ng SK, Hauser WA, Brust JC, Susser M. Hypertension and the risk of new-onset unprovoked seizures. Neurology. 1993;43(2):425-428.
22. Rubenstein LZ, Robbins AS, Schulman BL, Rosado J, Osterweil D, Josephson KR. Falls and instability in the elderly. J Am Geriatr Soc. 1998;36(3):266-278.
23. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701-1707.
24. Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38(9):1407-1410.
25. Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46(3):727-730.
26. Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47(5):867-872.
27. Dam AM, Fuglsang-Frederiksen A, Svarre-Olsen U, Dam M. Late-onset epilepsy: etiologies, types of seizure, and value of clinical investigation, EEG, and computerized tomography scan. Epilepsia. 1985;26(3):227-231.
28. Hauser WA. Seizure disorders: the changes with age. Epilepsia. 1992;33(suppl 4):S6-S14.
29. Spitz M, Bainbridge JL, Ramsay R, et al. Observations on the delay in the diagnosis of seizures in the elderly: update 2. Epilepsia. 2002;43(suppl 7):166.
30. Godfrey JW, Roberts MA, Caird FI. Epileptic seizures in the elderly: II. Diagnostic problems. Age Ageing. 1982;11(1):29-34.
31. McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia. 2002;43(2):165-169.
32. Randomized clinical trial on the efficacy of antiepileptic drugs in reducing the risk of relapse after a first unprovoked tonic-clonic seizure. First Seizure Trial Group (FIR.S.T. Group). Neurology. 1993;43(3 Pt 1):478-483.
33. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41(7):965-972.
34. Hart YM, Sander JW, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: recurrence after a first seizure. Lancet. 1990;336(8726):1271-1274.
35. Hauser WA, Rich SS, Annegers JF, Anderson VE. Seizure recurrence after a 1st unprovoked seizure: an extended follow-up. Neurology. 1990;40(8):1163-1170.
36. Lühdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia. 1986;27(4):458-463.
37. Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13(3):277-282.
38. Lühdorf K, Jensen LK, Plesner AM. Epilepsy in the elderly: prognosis. Acta Neurol Scand. 1986;74(5):409-415.
39. Brodie MJ, Overstall PW, Giorgi L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Res. 1999;37(1):81-87.
40. Rowan AJ, Ramsay RE, Collins JF, et al. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868-1873.
41. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
42. Schmucker DL. Aging and drug disposition: an update. Pharmacol Rev. 1985;37(2):133-148.
43. Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82(1):87-96.
44. Goa KL, Sorkin EM. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46(3):409-427.
45. Campion EW, deLabry LO, Glynn RJ. The effect of age on serum albumin in healthy males: report from the Normative Aging Study. J Gerontol. 1988;43(1):M18-M20.
46. Perucca E. Pharmacokinetics. In: Engel JJr, Pedley, TA. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven Publishers; 1997:1131-1144.
47. Woodhouse KW, Wynne HA. Age-related changes in liver size and hepatic blood flow. The influence on drug metabolism in the elderly. Clin Pharmacokinet. 1988;15(5):287-294.
48. Ahronheim JC. Age-related changes in drug metabolism and action. In: Rowan AJ, Ramsay RE, editors. Seizures and Epilepsy in the Elderly. Boston: Butterworth-Heinemann; 1997:55-61.
49. Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31(2):155-163.
50. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278-285.
51. Malmrose LC, Gray SL, Pieper CF, et al. Measured versus estimated creatinine clearance in a high-functioning elderly sample: MacArthur Foundation Study of Successful Aging. J Am Geriatr Soc. 1993;41(7):715-721.
52. Battino D, Croci D, Rossini A, Messina S, Mamoli D, Perucca E. Serum carbamazepine concentrations in elderly patients: a case-matched pharmacokinetic evaluation based on therapeutic drug monitoring data. Epilepsia. 2003;44(7):923-929.
53. Richens A, Banfield CR, Salfi M, Nomeir A, Lin CC, Jensen P, et al. Single and multiple dose pharmacokinetics of felbamate in the elderly. Br J Clin Pharmacol. 1997;44(2):129-134.
54. Boyd RA, Turck D, Abel RB, Sedman AJ, Bockbrader HN. Effects of age and gender on single-dose pharmacokinetics of gabapentin. Epilepsia. 1999;40(4):474-479.
55. Posner J, Holdich T, Crome P. Comparison of lamotrigine pharmacokinetics in young and elderly healthy volunteers. J Pharm Med. 1991;1:121-128.
56. Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43(11):707-724.
57. van Heiningen PN, Eve MD, Oosterhuis B, et al. The influence of age on the pharmacokinetics of the antiepileptic agent oxcarbazepine. Clin Pharmacol Ther. 1991;50(4):410-419.
58. Messina S, Battino D, Croci D, Mamoli D, Ratti S, Perucca E. Phenobarbital pharmacokinetics in old age: a case-matched evaluation based on therapeutic drug monitoring data. Epilepsia. 2005;46(3):372-377.
59. Bachmann KA, Belloto RJJr. Differential kinetics of phenytoin in elderly patients. Drugs Aging. 1999;15(3):235-250.
60. Snel S, Jansen JA, Mengel HB, Richens A, Larsen S. The pharmacokinetics of tiagabine in healthy elderly volunteers and elderly patients with epilepsy. J Clin Pharmacol. 1997;37(11):1015-1020.
61. Doose DR, Larson KL, Natarajan J, Neto W. Comparative single-dose pharmacokinetics of topiramate in elderly versus young men and women. Epilepsia. 1998;39(suppl 6):56.
62. Perucca E, Grimaldi R, Gatti G, Pirracchio S, Crema F, Frigo GM. Pharmacokinetics of valproic acid in the elderly. Br J Clin Pharmacol. 1984;17(6):665-669.
63. Haegele KD, Huebert ND, Ebel M, Tell GP, Schechter PJ. Pharmacokinetics of vigabatrin: implications of creatinine clearance. Clin Pharmacol Ther. 1988;44(5):558-565.
64. Bergey GK. Initial treatment of epilepsy: special issues in treating the elderly. Neurology. 2004;63(10 suppl 4):S40-S48.
65. Cloyd J. Commonly used antiepileptic drugs: age-related pharmacokinetics. In: Rowan AJ, Ramsay R, editors. Seizures and Epilepsy in the Elderly. Boston: Butterworth-Heinemann; 1997:219-228.
66. Helling DK, Lemke JH, Semla TP, Wallace RB, Lipson DP, Cornoni-Huntley J. Medication use characteristics in the elderly: the Iowa 65+ Rural Health Study. J Am Geriatr Soc. 1987;35(1):4-12.
67. Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia. 1998;39(10):1083-1087.
68. Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2(8):473-481.
69. Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med. 1992;327(11):765-771.
70. Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med. 1985;313(3):145-151.
71. Zaret BS, Beckner RR, Marini AM, Wagle W, Passarelli C. Sodium valproate-induced hyperammonemia without clinical hepatic dysfunction. Neurology. 1982;32(2):206-208.
72. Van Amelsvoort T, Bakshi R, Devaux CB, Schwabe S. Hyponatremia associated with carbamazepine and oxcarbazepine therapy: a review. Epilepsia. 1994;35(1):181-188.
73. French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs. II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62(8):1261-1273.
74. White JR, Walczak TS, Leppik IE, et al. Discontinuation of levetiracetam because of behavioral side effects: a case-control study. Neurology. 2003;61(9):1218-1221.
75. Kutluay E, McCague K, D’souza J, Beydoun A. Safety and tolerability of oxcarbazepine in elderly patients with epilepsy. Epilepsy Behav. 2003;4(2):175-180.
76. Janszky J, Janszky I, Schulz R, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain. 2005;128(Pt 2):395-404.
77. Boling W, Andermann F, Reutens D, Dubeau F, Caporicci L, Olivier A. Surgery for temporal lobe epilepsy in older patients. J Neurosurg. 2001;95(2):242-248.
78. Cascino GD, Sharbrough FW, Hirschorn KA, Marsh WR. Surgery for focal epilepsy in the older patient. Neurology. 1991;41(9):1415-1417.
79. Sirven JI, Malamut BL, O’Connor MJ, Sperling MR. Temporal lobectomy outcome in older versus younger adults. Neurology. 2000;54(11):2166-2170.
80. Grivas A, Schramm J, Kral T, et al. Surgical treatment for refractory temporal lobe epilepsy in the elderly: seizure outcome and neuropsychological sequels compared with a younger cohort. Epilepsia. 2006;47(8):1364-1372.
81. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029-1035.
82. Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42(6):714-718.
83. Vignatelli L, Tonon C, D’Alessandro R. Incidence and short-term prognosis of status epilepticus in adults in Bologna, Italy. Epilepsia. 2003;44(7):964-968.
84. DeLorenzo RJ. Clinical and epidemiologic study of status epilepticus in the elderly. In: Rowan AJ, Ramsay RE, editors. Seizures and Epilepsy in the Elderly. Boston: Butterworth-Heinemann; 1997:191-205.
85. DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):316-325.
86. Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. 2002;58(7):1070-1076.
87. Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35(1):27-34.
88. Waterhouse EJ, Vaughan JK, Barnes TY, et al. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res. 1998;29(3):175-183.
89. Vestergaard P, Tigaran S, Rejnmark L, Tigaran C, Dam M, Mosekilde L. Fracture risk is increased in epilepsy. Acta Neurol Scand. 1999;99(5):269-275.
90. Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50(10):1629-1637.
91. Pack AM. The association between antiepileptic drugs and bone disease. Epilepsy Curr. 2003;3(3):91-95.
92. Fitzpatrick LA. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004;5(Suppl 2):S3-S15.