Seizure Disorders
Perspective
A seizure is the clinical manifestation of excessive, abnormal cortical neuron activity. The physical manifestation depends on the area of brain cortex involved and, to a lesser extent, on the specific underlying abnormality. Ictal event is the more general term used to describe the seizure or seizure-like episode. Patients who have recurring seizures without consistent provocation have epilepsy, a term that encompasses many disparate clinical syndromes. Seizures may also occur as a predictable response to certain toxic, pathophysiologic, or environmental stresses; these are reactive or secondary seizures, and patients who experience these do not have epilepsy. In the United States, 10% of people experience at least one seizure in their lifetime; the cumulative incidence of epilepsy is 3%.1
More than 1 million patients visit U.S. emergency departments (EDs) every year for seizure care.2 The evaluation of patients with seizures in the ED can be complex and difficult. A careful history must be elicited to determine the presence of ictal events that represent epilepsy, exposure to ictogenic stimuli (e.g., alcohol, cocaine), significant underlying illness (e.g., meningitis, hypoxemia, hypoglycemia, intracranial mass), or provocative stimuli (e.g., sleep deprivation in an epileptic). The physical examination focuses on the identification of focal neurologic abnormalities, systemic illness, and signs of toxic exposure. If the patient continues to experience seizure activity, airway protection and abortive therapy must be provided.3 Laboratory and radiographic evaluation are guided by historical and physical findings and may be limited or unnecessary in some cases. Finally, the appropriate disposition of a patient in the ED with a seizure or with a history of a recent seizure requires an understanding of the underlying illness, likelihood of recurrence, indications for maintenance pharmacologic therapy, and state reporting regulations.
In addition to the distinction between primary (epileptic) and secondary (reactive) seizures, many other classifications of ictal events have been proposed.4–8 Seizures are termed generalized or focal (partial) depending on the clinical manifestations. Generalized seizures result from an abnormal electrical event that simultaneously involves both cerebral hemispheres and is accompanied by loss of consciousness; in partial seizures, abnormal activity is limited to part of one cerebral hemisphere only. Generalized seizures usually are characterized by rhythmic, tonic-clonic muscle contractions, or convulsions, although nonconvulsive generalized seizures also occur. Partial seizures can be differentiated further into seizures during which cognition is maintained (simple partial) and seizures during which cognition is impaired (complex partial). The term cognition refers to involvement of at least two of five features—perception, attention, emotion, memory, and executive function—and replaces the previously used term consciousness, which is difficult to define and difficult to document.9,10 Finally, partial seizures may become generalized (partial with secondary generalization).
Inexperienced witnesses may provide histories that are insufficient for accurate categorization of seizures. However, when an accurate history is available, secondary (reactive) seizures typically are generalized, not partial, in nature. The definitive differentiation among these classifications may require electroencephalogram (EEG) recording during the seizure, sometimes in association with simultaneous video recording.11,12
Principles of Disease
The pathophysiology of seizures at the neuronal level is incompletely understood, with most of what is known coming from animal studies in which either electrical or pharmacologic stimulation is applied directly to brain cortex. To produce generalized ictus, stimuli must be simultaneously applied to both hemispheres. Some studies show the concept of recruitment, which occurs when the initiating neurons’ abnormal, increased electrical activity activates adjacent neurons and propagates until the thalamus and other subcortical structures are recruited. The clinical seizure activity typically, but not always, reflects the focus of initiation.10,13
What prompts such initiation is unclear. Proposed mechanisms include disruption of normal structure, whether congenital, maturational, or acquired (as with scar tissue), and disruption of local metabolic or biochemical function. The latter mechanism is better understood because the roles of two neurotransmitters—acetylcholine, which is excitatory to cortical neurons, and γ-aminobutyric acid (GABA), which is inhibitory—have been more fully characterized. In sensitive neurons, such as those at an ictogenic focus, subtle changes in the local concentrations of these neurotransmitters can produce sustained membrane depolarization, ultimately followed by local hyperpolarization and recruitment. Recruitment may follow contiguous paths or extend along diverse integrated circuits that are deep and cross the midline.10,13
When the ictal discharge extends below the cortex to deeper structures, the reticular activating system in the brainstem may be affected, altering consciousness. In generalized seizures, the focus often is subcortical and midline, which explains the prompt loss of consciousness and bilateral involvement. Seizures typically are self-limited; at some point, the hyperpolarization subsides and the electrical discharges from the focus terminate. This termination may be related to reflex inhibition, loss of synchrony, neuronal exhaustion, or alteration of the local balance of acetylcholine and GABA in favor of inhibition.10
The systemic manifestations of convulsive ictal activity include hypertension, tachycardia, tachypnea, and hyperglycemia from sympathetic stimulation. With more prolonged convulsions, skeletal muscle damage, lactic acidosis, and, rarely, frank rhabdomyolysis may ensue.10,14,15 Autonomic discharge and bulbar muscle involvement may result in urinary or fecal incontinence, vomiting (with significant aspiration risk), tongue biting, and airway impairment.
Clinical Features
Focal seizures in adults may be classified as simple partial or complex partial. Simple partial seizures are limited in electrical focus to one cerebral hemisphere and do not cause loss of cognition. Although the specific function of the initiating neurons determines the clinical manifestation of the ictal event (i.e., motor, somatosensory, special sensory, autonomic, or psychic), such clinical manifestations are not sufficiently specific for anatomic localization without an EEG. Typical features of simple partial seizures include focal clonic movements; paresthesias; visual, auditory, olfactory, or gustatory experiences; sweating and flushing; dysphasia; a sense of déjà vu; or a sense of unwarranted fear.10,13 Motor signs, which by definition remain ipsilateral in simple partial seizures, may spread contiguously in a stepwise fashion (Jacksonian march) as neuron recruitment occurs in the motor cortex. There is generally no postictal state after a simple partial seizure.
Complex partial seizures are ictal events that involve impairment of cognition, either at onset or evolving from focal activity. Amnesia for the ictal event is a consistent feature of complex partial seizures, although during the episode the patient may remain responsive to the surroundings. Complex partial seizures typically involve automatisms that are specific to the affected person, such as lip smacking, repeated swallowing or uttering verbal phrases, or picking at clothing. Complex partial seizures generally are associated with an aura, such as a specific smell, taste, visual hallucination, or intense emotional feeling. In contrast with those experiencing generalized seizures, these patients may continue with ongoing motor activity, such as driving an automobile, riding a bicycle, or playing a musical instrument (reactive automatisms), and they may react to their surroundings in a semi-appropriate manner.10 Partial seizures may progress rapidly to generalized seizures. A postictal state is common after complex partial seizures and may persist for hours.13
Nonconvulsive generalized seizures include absence, or petit mal, seizures; myoclonic seizures; tonic seizures; and atonic seizures. Absence seizures in adults are subclassified further as typical or atypical. Typical absence ictus is characterized by the sudden cessation of normal, conscious activity, followed by a nonconvulsive, dissociative state that persists for a few seconds to several minutes before suddenly terminating. Eye movements, blinking, or automatisms may be present. There is no aura and no postictal state. If the seizure occurs midsentence, then the patient typically will resume speaking at precisely the point of interruption without awareness of the intervening event. Absence seizures typically begin in childhood but occasionally develop in adults. Atypical absence seizures are marked by more complicated motor signs, coexistence with other forms of generalized seizures, inconsistent postictal confusion, and irregular EEG abnormalities.13
Atonic seizures are characterized by focal diminution of muscle tone (limb or head) or generalized loss of postural tone in which the head falls forward and then the body slumps to the ground (“drop attack”), usually landing buttocks first (although this can vary depending on the axis of gravity at the time of the fall). Recovery occurs immediately, and there is either no loss or an extremely brief loss of consciousness. In myoclonic-atonic seizures, a brief (less than 100 msec) myoclonic jerk of muscle groups of variable anatomy occurs before the episode of atonia.10 Because typically no postictal state is associated with these episodes, an altered level of consciousness in a patient after an atonic or myoclonic-atonic seizure should prompt an investigation for head trauma or a toxic or metabolic abnormality.
Status epilepticus is defined as serial seizure activity without interictal recovery or prolonged, continuous seizure activity. Traditionally, status epilepticus was defined as seizure activity lasting longer than 30 minutes, which is the estimated duration necessary for neuronal injury.16,17 However, because an isolated tonic-clonic seizure rarely lasts more than a few minutes, an operational definition of status epilepticus as either a continuous seizure lasting more than 5 minutes or more than two discrete seizures without intervening recovery of consciousness has been advocated.18 Although it is recognized that the underlying cause of status epilepticus is the predominant factor determining morbidity and mortality, prolonged seizure activity does cause neuronal injury and therefore warrants prompt abortive therapy. Furthermore, status epilepticus may become refractory to treatment over time.17,19,20
In some cases a patient’s first seizure episode will manifest as status epilepticus. The most common cause of status epilepticus is discontinuation of anticonvulsant medication. This situation may be compounded by barbiturate withdrawal when phenobarbital therapy is abruptly discontinued. Many other causes of status epilepticus have been documented (Box 102-1).14,16,21,22 After prolonged status epilepticus or after incomplete treatment, the patient may exhibit very subtle manifestations of continued seizure activity, such as small-amplitude twitching of the extremities or jerking of the eyes, or any visible motor activity may cease while seizure activity detectable on the EEG continues.23–25 Recognition of the latter scenario, termed nonconvulsive status epilepticus, requires a high index of clinical suspicion. Prompt treatment is essential to prevent neuronal damage; mortality approaches 30% if the seizure lasts more than an hour.26 Nonconvulsive status epilepticus may be present in more than 9% of hospitalized patients with prolonged decreased mental status.27
All classes of primary seizures may recur sporadically, randomly, or predictably. Cyclic recurrence has been reported with awakening, sleep deprivation, emotional or physical stress, alcohol, and menses, among other factors. Seizures also may be triggered by specific sensory stimuli, the most common of which is visual stimulation in the form of flashing lights, such as strobe lights, television, and video games.10,28 Seizures also can be caused by auditory, gustatory, tactile, or startle triggers that are specific to the affected person. The most common cause of recurrent primary seizures is medication noncompliance.13,29
Reactive Seizures in Adults
Seizures Caused by Metabolic Derangements
Hypoglycemia is a common metabolic cause of reactive seizures. Ictal activity can occur when the plasma glucose level is less than 45 mg/dL, although some patients may manifest neurologic disturbances even at higher levels.30 A rapid bedside glucose test is an integral part of the evaluation of the patient exhibiting seizure activity. Convulsive and nonconvulsive seizures and generalized and partial seizures all may occur during hypoglycemia.31–34 Patients at the extremes of age are particularly susceptible to glucose stress during acute illness. Hypoglycemia also may result from insulin reaction, a deliberate insulin or hypoglycemic agent overdose, alcoholism, poor nutrition, and sepsis. Hypoglycemic seizures respond to glucose therapy; anticonvulsants and benzodiazepines are unnecessary. At times, however, prolonged seizures may cause hypoglycemia. Seizures that do not cease after correction of low blood glucose deserve further evaluation and treatment.
Cation derangements, notably hypernatremia and hyponatremia, hypomagnesemia, and hypocalcemia, are other common metabolic causes of ictal activity.35,36 Hypo-osmolar and hyperosmolar states can precipitate seizures. Disorders of sodium, the primary cation in the extracellular fluid compartment and the primary determinant of serum osmolarity, are most common. Hyponatremia is the most frequently identified electrolyte disorder in hospitalized patients, and sodium levels less than 120 mEq/L often are associated with seizures.37,38 The rate at which the sodium level decreases, and not the absolute magnitude of the decrease, determines the risk for neurologic manifestation.37 Correcting hyponatremia should be undertaken slowly to avoid osmotic demyelination. If seizures are persistent, administration of hypertonic (3%) saline may be indicated.37 Hypernatremia will result in cerebral edema and seizures in the setting of rapid elevation of serum sodium to greater than 160 mEq/L or during aggressive correction of subacute hyponatremia.36,39
Hypercalcemia reduces neuronal excitability and rarely causes seizures; significant hypocalcemia (below 7.5 mEq/L), however, is associated with ictal activity. Hypocalcemia may result from hypoparathyroidism, renal failure, or acute pancreatitis and typically is associated with hypomagnesemia, which also can precipitate seizures, particularly at serum levels less than 1 mEq/L. Hypomagnesemia is seen most often as a result of poor nutrition, especially in alcoholic patients. Patients with significant hypomagnesemia or hypocalcemia are treated empirically for both disorders.35,36
Nonketotic hyperosmolar hyperglycemia can be associated with seizure activity. Partial seizures, including partial status, predominate. These seizures do not respond to anticonvulsants; rather, they are best managed with gradual correction of fluid deficits and glucose excess.40–42
Seizures may complicate the course and treatment of renal failure.43 Ictal activity occasionally complicates uremic encephalopathy, more commonly occurs as a result of acute fluid and electrolyte shifts during dialysis (dialysis disequilibrium syndrome), and can be a complication of immunosuppressive therapy after renal transplantation.
Thyroid hormones lower seizure threshold, and consequently Graves’ disease and thyrotoxicosis may occasionally manifest as seizures, including status epilepticus.44 Seizures also occur with hypoparathyroidism as a result of secondary hypocalcemia.14
Seizures Caused by Infectious Diseases
Infectious diseases can cause seizures independent of a purely febrile mechanism. These seizures generally result from primary central nervous system (CNS) infections but occasionally arise from other septic sources. The most important ictogenic infections are meningitis, encephalitis, cerebral abscess, cerebral parasitosis, and human immunodeficiency virus (HIV) disease and associated opportunistic infections, with their protean CNS manifestations. Cerebral malaria is a common cause in developing countries.45
Seizures may result from the acute inflammatory response that occurs with bacterial or viral meningitis. During the acute course of illness, 15% of patients with bacterial meningitis will have at least one seizure,46 and surviving patients have an increased risk of epilepsy.47 Viral meningoencephalitides, the most common of which are caused by the herpes simplex virus, also are associated with seizures. These seizures may be generalized or partial, often recur during the acute phase of the illness, and may persist after the illness has resolved.48
The parasitic CNS infection neurocysticercosis is relatively common in areas of the United States where there is a population of immigrants from Latin America. Seizures complicate 50 to 90% of neurocysticercosis cases.49 Latent syphilis can cause adult-onset seizures. Primary HIV disease of the CNS with its attendant infectious and mass lesion complications constitutes a significant cause of generalized and partial seizures. Common HIV-associated infections and conditions include toxoplasmosis, lymphoma, and the demyelinating infection progressive multifocal leukoencephalopathy.50 Choosing an antiepileptic drug (AED) for an HIV-infected patient with seizures is done in consultation with infectious disease and neurology specialists because of the well-recognized increase in adverse effects of AEDs and the interactions between AEDs and antiviral medications.
Seizures Caused by Drugs and Toxins
The list of substances reported to cause seizures either as an idiosyncratic side effect of therapeutic use or as a manifestation of toxic overdose is extensive.22,51 The recognition of this etiologic category is crucial in the ED setting. Seizure activity is a dire sign of toxicity and may herald the onset of life-threatening instability.
Seizures may occur after therapeutic doses of antimicrobials, cardiovascular agents, neuroleptics, and sympathomimetics.52 Seizures also may result from exposure to plant toxins, insecticides, rodenticides, and hydrocarbons. Certain over-the-counter supplements also have been associated with seizures, either alone or through adverse interactions with prescription medications.53,54 The most common drug-associated and toxin-associated seizures occur, however, in conjunction with illicit drugs, such as cocaine, amphetamines, and phencyclidine; with overdoses of anticholinergic agents, such as cyclic antidepressants and antihistamines; as a manifestation of withdrawal from ethyl alcohol and sedative-hypnotics; and with toxic levels and deliberate overdoses of diverse medications, including aspirin, theophylline, meperidine, isoniazid, lithium, and anticonvulsants, including phenytoin, carbamazepine, lamotrigine, and topiramate.51,55 Standard ED therapeutic measures are usually effective for management of toxic seizures. In some cases, specific antidotal therapy is available, such as alkalinization for cyclic antidepressant and salicylate overdoses, and pyridoxine (vitamin B6) for isoniazid overdose. Hemodialysis is the treatment of choice for serious salicylate and lithium toxicity. Dilantin administration is contraindicated in cases of ingestion because its sodium channel blocking actions can worsen the hemodynamic impact of the ingestion.3
Because of its prevalence in urban ED patient populations, cocaine toxicity warrants special mention. Seizures may occur after isolated recreational use or chronic abuse, after overdose, and in “body packers” and “body stuffers.”56 Cocaine-related seizures may be a manifestation of direct CNS toxicity or an indirect result of hypoxemia from cardiac toxicity.57 Seizures in cocaine-intoxicated patients are managed as part of the overall toxic reaction, which often includes high fever, rhabdomyolysis, and cardiac arrhythmias. A benzodiazepine is the appropriate initial therapeutic agent.
Ethyl alcohol use and abuse are additional common toxic causes of seizures; up to 40% of seizure patients in the ED have alcohol-related seizures.58 Ictal events may occur with acute inebriation but are more common during withdrawal from alcohol.59 Withdrawal seizures are typically generalized and recurrent and begin within 6 hours of cessation of or decrease in alcohol consumption. Through a phenomenon termed kindling, the risk and severity of seizures increase with each episode of withdrawal. Kindling means that with each episode of alcohol withdrawal, the seizure threshold is lower. Alcoholic patients with seizures should also be evaluated for related, concomitant ictogenic problems (e.g., hypoglycemia, electrolyte derangements, head trauma, coingestion of other toxins, pregnancy). The preferred treatment for alcohol-associated seizures is with benzodiazepines, as these drugs substitute for the GABA-enhancing effect of ethanol in the CNS.
Seizures Caused by Trauma
Post-traumatic seizures can occur acutely as a result of blunt or penetrating head trauma or as a post-traumatic sequela. Immediate post-traumatic seizures occur within 24 hours of injury. Epidural, subdural, and intracerebral hematomas and traumatic subarachnoid hemorrhages all can be acutely ictogenic, particularly as intracranial pressure rises. More often, however, the onset of seizure activity is delayed for at least several hours. Early post-traumatic seizures occur within 1 week of injury, whereas late post-traumatic seizures occur after 1 week. Immediate and early post-traumatic seizures are more common in children than in adults, and children also are more likely than adults to be presented in status epilepticus in the immediate or early post-traumatic phase.60
The severity of head injury correlates with the likelihood of post-traumatic seizures. Although seizures rarely occur after mild traumatic brain injury, the incidence approaches 30% in patients with depressed skull fracture.60,61 Antiepileptic drugs are recommended for prophylaxis against post-traumatic seizures occurring within the first 7 days after severe brain injury in adults; however, they have not been shown to be effective in preventing late post-traumatic seizures.62,63
Seizures Associated with Malignancy or Vasculitis
Seizures are a common manifestation of primary and metastatic CNS neoplasms. They also may complicate cancer treatment as a result of postsurgical scarring or chemotherapy-related electrolyte derangements, hematologic abnormalities, or immunosuppression. Although any CNS tumor can be ictogenic, low-grade and slow-growing primary neoplasms (e.g., well-differentiated gliomas and oligodendrogliomas) are implicated most commonly.64 In such cases, seizures, which most often are partial with secondary generalization, may be the initial clinical manifestation. A new-onset seizure in a patient with a non-CNS primary malignancy, such as melanoma and tumors of the lung, breast, colon, germ cells, or renal cells, prompts consideration of CNS metastasis and warrants neuroimaging.
Seizures also may be the presenting manifestation of CNS vasculitis in patients with systemic lupus erythematosus and polyarteritis nodosa and are usually complex partial seizures that give a general indication of the acute inflammatory focus. Secondary generalization may also occur.65
Seizures Caused by Strokes, Arteriovenous Malformations, Aneurysms, and Migraines
Ischemic or hemorrhagic stroke is the cause of new-onset seizures in 40 to 54% of elderly patients.66 The overall incidence of seizures with stroke ranges from 4 to 15%; more than one half occur within the first week after stroke. The incidence of epilepsy after stroke is 4 to 9%.67,68 Seizures that occur acutely with stroke are thought to result from local metabolic alterations in the CNS; these events are transient, and the seizures often are focal and self-limited. Seizures that develop later are more likely to be generalized.
Convulsive seizures occur in 6 to 26% of patients with subarachnoid hemorrhage after aneurysmal rupture; 8 to 15% of patients will experience nonconvulsive seizures.69 Seizures also occur in conjunction with unruptured cerebrovascular aneurysms and arteriovenous malformations.70 Arteriography may be required to confirm the diagnosis; unruptured arteriovenous malformations are easier to detect on an enhanced cranial computed tomography (CT) scan than are smaller, unruptured aneurysms.
Seizures also may arise in concert with vascular headaches, either coincidentally, by migrainous activation of an epileptic focus, or after vascular headache has induced cerebral infarction that becomes an epileptic focus.71
Seizures Caused by Degenerative Disease of the Central Nervous System
In approximately 3% of patients with multiple sclerosis, focal or generalized seizures develop during the course of their illness. These seizures must be differentiated from the tonic spasms that may occur in multiple sclerosis.72
CNS degeneration associated with aging, including dementia and Alzheimer’s disease, increases the risk of reactive seizures and epilepsy.66,73 The elderly also are more likely to have other ictogenic problems (e.g., stroke, brain neoplasm, toxic and metabolic disturbances, blunt head trauma from falls). Maintenance treatment of elder patients with epilepsy often is complicated by drug-drug interactions, and breakthrough seizures may result even when patients are compliant. Although the incidence of unprovoked seizures increases after age 60 years, the management of these patients must include a thorough evaluation for causes of secondary seizures.66
Gestational Seizures
Seizures associated with pregnancy are divided into two categories: gestational epilepsy, in which hormonal and metabolic changes exacerbate underlying epilepsy or adversely influence serum levels of anticonvulsants, and eclampsia or toxemia, which is a gestational hypertensive encephalopathy manifesting with seizures, hypertension, coma, proteinuria, and edema. For the former, antiepileptic therapy should be tailored by the patient’s neurologist and obstetrician to maximize seizure control and minimize the risk of teratogenic effects.74
Convulsive generalized status epilepticus in pregnancy jeopardizes both mother and fetus. The definitive treatment for eclamptic seizures is magnesium sulfate and immediate delivery of the fetus; benzodiazepines are useful adjuncts to magnesium. Simultaneous reduction in blood pressure with use of hydralazine, labetalol, or nifedipine is recommended.75 Postpartum eclampsia represents 25% of eclamptic seizures, can occur up to 8 weeks after delivery, and can be seen in women without preceding preeclampsia.76
Psychogenic Nonepileptic Seizures
Psychogenic seizures, or pseudoseizures, are functional events that may be associated with alterations in cognition, abnormal movements and behaviors, and autonomic changes. They are not the result of abnormal CNS electrical activity. Psychogenic seizures may be primarily motor and mimic convulsive generalized seizures, including refractory status epilepticus, or they may be nonconvulsive and mimic either absence or complex partial seizures. Although certain features of convulsive psychogenic seizures may suggest the diagnosis, no clinical criteria are 100% specific; simultaneous video and EEG recordings may be required to confirm the diagnosis.77,78
The ED evaluation of these patients is difficult, because epilepsy and pseudoseizures can coexist in a minority of patients.79 All but obviously functional abnormalities should be treated as true ictus pending formal neurologic evaluation. Many patients with pseudoseizures are not deliberately attempting to mislead the examining physician. The long-term treatment of patients with confirmed pseudoseizures may include direct confrontation, intensive psychotherapy, and use of a placebo.
Postictal States
Two unusual postictal manifestations may provoke particular consternation in the ED: postictal paralysis and neurogenic pulmonary edema. Postictal paralysis, or Todd’s paralysis, may follow generalized or complex partial seizures and is a focal motor deficit that may persist up to 24 hours and may be caused by transient focal cerebral hypoprofusion.80 Weakness of one extremity or a complete hemiparesis may occur; in the latter case the patient should be safely restrained to avoid falls caused by a combination of weakness and diminished responsiveness resulting from the postictal state. Todd’s paralysis is associated with a high likelihood of an underlying structural cause for the seizure.
Neurogenic pulmonary edema is a relatively common, although often subclinical, complication of any structural CNS insult, including seizure, trauma, and hemorrhage.81 Neurogenic pulmonary edema is likely caused by centrally mediated sympathetic discharge and generalized vasoconstriction, coupled with increased pulmonary capillary membrane permeability. After a seizure, neurogenic pulmonary edema can be confused clinically and radiographically with aspiration pneumonia. Otherwise unexplained hypoxia or other clinical evidence of pulmonary congestion after a seizure prompts consideration of neurogenic pulmonary edema. Significant neurogenic pulmonary edema is managed with ventilatory support, including positive end-expiratory pressure.82,83
A prolonged postictal state raises clinical suspicion for nonconvulsive status epilepticus. After prolonged tonic-clonic seizures, ongoing cerebral epileptic activity may manifest with subtle motor movements such as twitching of the digits or face. EEG is the most definitive way to confirm the diagnosis.26
Diagnostic Strategies
The essential components of the seizure evaluation in the ED are discussed in Chapter 18. An accurate and thorough history of the ictal event and information about any known or potential precipitants or exposures and the patient’s medical problems are obtained. A thorough physical examination, including a complete neurologic examination, is essential. Any identified focal neurologic deficits must be monitored for progression or resolution. Appropriate ancillary studies may be comprehensive, but if precipitants (e.g., hypoglycemia, intoxication, medication noncompliance) are known, studies may be comparatively limited. Frequently, the cause of the first seizure in an adult cannot be found in the ED.84
Although the Academy of Neurology recommends neuroimaging, by either CT or magnetic resonance imaging (MRI), for all adults with an apparently unprovoked first seizure, the usefulness of emergent imaging depends on the clinical situation.85 An emergent cranial CT scan is indicated when a serious structural lesion is suspected on clinical grounds, including presence of a new focal deficit, persistent altered mental status, fever, recent trauma, persistent headache, history of cancer, anticoagulant use, suspicion or known history of acquired immunodeficiency syndrome (AIDS), age older than 40 years, and partial-complex seizure.86 A reasonable approach is to obtain a brain CT scan on an outpatient, follow-up basis in patients who have recovered completely from the ictal event and in whom no apparent cause has been elucidated. However, if reliable follow-up care is unlikely or even questionable, the CT scan should be obtained in the ED to ensure its completion. In patients with known epilepsy and recurrent seizures, the same considerations apply, but in addition, epileptic patients with a change in seizure pattern, prolonged postictal state, or persistent abnormal mental status should undergo brain CT scan in the ED.
Children with seizures can be evaluated in a similar manner. Those with febrile seizures are not more likely than those with fever alone to have a serious bacterial infection. Children with afebrile seizures who are well appearing can generally be managed as outpatients. Blood tests and other diagnostics are indicated in children younger than 6 months, those who have had a prolonged seizure or postictal state, and those who have a preexisting metabolic disorder or suspected dehydration. Emergent neuroimaging is indicated for children with significant past medical histories or in cases of focal seizures in children younger than 3 years.87,88 Children younger than 6 months may have positive, relevant brain CT findings in 50% of cases.89
The decision to initiate anticonvulsant therapy after a single seizure depends on the cause of the seizure. Seizures caused by structural lesions, such as stroke, tumor, or head injury, are likely to recur and may warrant antiepileptic medication. However, such patients also are likely to be admitted to the hospital if the lesion is newly discovered. For patients with a single unprovoked seizure, evidence-based recommendations are that antiepileptic therapy should not be initiated; rather, the patient should be discharged with referral for neurologic consultation.84,86,90,93,94 The rationale for this approach is threefold. First, the diagnosis may be incorrect, especially if the seizure-like activity was not witnessed by experienced medical personnel. It is estimated that 20 to 25% of patients diagnosed as having seizures are eventually determined not to have seizures, with the most frequent alternative diagnoses being cardiovascular and psychopathologic.95–98 Second, the patient may not have a recurrent seizure. It is estimated that less than 50% of patients who have had a single unprovoked seizure will experience a recurrent seizure within 2 years.90,99,100 Furthermore, whereas treatment decreases the risk of early recurrent seizure, it does not affect long-term prognosis of epilepsy,90,101–103 nor does it have an impact on patient quality of life,104 with the exception of driving limitations, which are prolonged in a patient with recurrent seizure. Third, antiepileptic medications have side effects that may outweigh the benefit of treatment, especially in women of childbearing age, owing to the teratogenic risk of AEDs; in patients with liver, kidney, or hematologic disorders; and in patients already taking multiple medications.
Recurrent Seizures
The initial approach to stabilization of a patient with a known seizure disorder does not differ from that for a patient with new-onset seizure; this includes a rapid blood glucose determination and evaluation for precipitant causes. The most common cause of seizures in a patient with a diagnosed seizure disorder is noncompliance with medications.29 However, supratherapeutic and toxic levels of some anticonvulsants, such as carbamazepine, phenytoin, lamotrigine, and topiramate, can also cause seizures.105–110 Accordingly, it is prudent to check the serum drug level, if this test is available, before giving a full loading dose of anticonvulsants to patients on long-term therapy. Meanwhile, a thorough history and physical examination focus on intercurrent illness or trauma, drug or alcohol use, potential adverse drug-drug interactions with anticonvulsants, a recent change in anticonvulsant dosage regimens, and any change in ictal pattern or characteristics. Clinical indications dictate the selection of other laboratory or radiographic tests.
Differential Considerations
Even when a “seizure” is witnessed in the ED, other abnormal movements and states of consciousness can be confused with ictal activity. The most common misdiagnoses are cardiovascular (syncope) and psychogenic, but other considerations in the differential diagnosis include hyperventilation and breath-holding, certain toxic and metabolic states, transient ischemic attacks, narcolepsy, and some movement disorders.13,96,111–114
Hyperventilation syndrome can be associated with mood disturbances, paresthesias, and posturing movements of the distal extremities. Manifestations of toxic and metabolic disorders that mimic ictus include delirium tremens and alcoholic blackouts, the alteration in consciousness associated with hypoglycemia and acute intermittent porphyria, the buccolingual spasms of phencyclidine intoxication, and the tonic spasms caused by tetanus, strychnine, and camphor.13 Nonictal CNS events, such as transient ischemic attacks, transient global amnesia, and atypical migraines, may manifest in a manner similar to absence seizures and postictal states such as Todd’s paralysis. Carotid sinus hypersensitivity, which can result even from a tight necktie, may cause sudden falls that mimic drop attacks.115 Narcolepsy (recurrent irresistible daytime sleepiness), especially when it occurs with cataplexy (sudden falls), may be associated with hallucinations and abnormal movements. It can be differentiated from seizure activity by the history and response to stimulation. Movement disorders, such as hemiballismus and tics, usually are associated with other neurologic problems. Finally, dissociative states such as fugue and panic attacks can be confused with seizures. An EEG is an appropriate diagnostic option in unclear cases.
Management
In general, the first-line pharmacologic agent for treatment of any active seizure is a parenteral benzodiazepine. Because benzodiazepines directly enhance GABA-mediated neuronal inhibition, they affect clinical and electrical manifestations of seizures. Benzodiazepines are effective in terminating ictal activity in a majority of patients and have been shown to be more effective than phenytoin in terminating status epilepticus.116,117 Although phenobarbital appears to be as effective as the benzodiazepine lorazepam in terminating status epilepticus, the associated high risk of hypoventilation and hypotension limits its use as a first-line agent.117
The most commonly used benzodiazepines include diazepam, lorazepam, and midazolam (Table 102-1). All three may be used in patients of any age, and all share the following characteristics: rapid onset (seconds to minutes), relatively short duration of anticonvulsant action, a sedative effect, and the potential for hypotension and respiratory depression. Lorazepam has emerged as the drug of choice for the initial management of convulsions, because it terminates seizures rapidly (within 2 minutes) and has a longer duration of action (4-6 hours, compared with 20 minutes for diazepam), thus necessitating fewer repeat doses.118–121 For this reason, it also is the preferred agent for control of alcohol withdrawal seizures.122 Lorazepam is most effective when given intravenously but can also be administered intramuscularly, intranasally, intraosseously, and as a sublingual preparation for out-of-hospital control of seizures in children.123
Table 102-1
Medications Used in the Abortive Treatment of Status Epilepticus in the Emergency Department
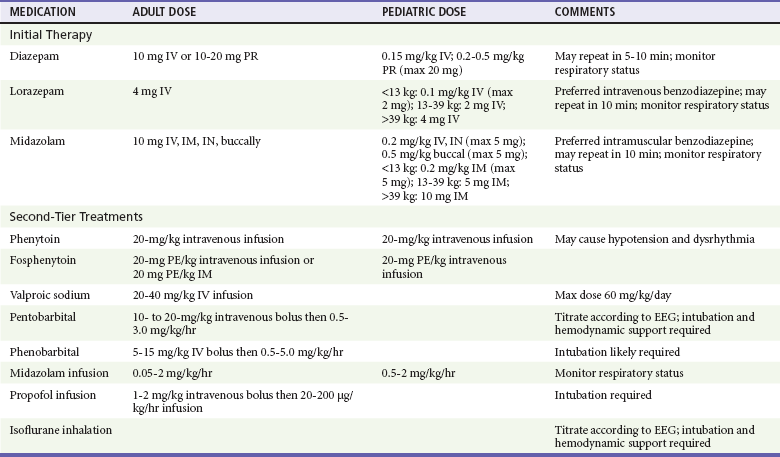
Brophy GM, et al: Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 17:3-23, 2012.
An advantage of diazepam is that it is in liquid form at room temperature and is therefore available premixed in resuscitation kits, and it can be administered quickly and without a need for reconstitution by the intravenous, endotracheal, or intraosseous route. Its onset of action with intravenous administration is within 10 to 20 seconds, but there is a 50% chance of recurrent seizure within 2 hours if diazepam is used.117 Diazepam can also be administered rectally via suppositories or a rectal gel. Rectal diazepam achieves variable success in seizure cessation, perhaps owing to the dual venous drainage of the rectum. The inferior and middle rectal veins drain into the central circulation via the common iliacs, whereas the superior rectal vein flows into the hepatic portal system. Diazepam placed too deeply into the rectum would undergo first-pass metabolism in the liver, thus limiting bioavailability and clinical effect.
Midazolam’s onset of action is within 1 minute; it is available in both intranasal and buccal formulations,124 and among the benzodiazepines it has the least cardiovascular effect.121 Midazolam is rapidly and reliably absorbed after intramuscular administration and has been shown to be as effective when given intramuscularly as intravenous lorazepam, making it a preferred medication when intravenous access has not yet been established.125 Nonintravenous midazolam is as effective as intravenous diazepam, and buccal midazolam is superior to rectal diazepam in achieving symptom control.126
Second-line abortive anticonvulsant therapy consists of phenytoin and phenobarbital. Phenytoin reduces the repetitive firing of action potentials through sodium channel blockade, thereby stabilizing neuronal membranes.10 Phenytoin neither sedates patients nor causes respiratory depression, but rapid intravenous administration of phenytoin in its propylene glycol diluent may cause hypotension and cardiac bradydysrhythmias, as well as local vascular injury, including venous thrombosis and localized tissue necrosis (purple glove syndrome).127–129 Phenytoin’s onset of action is within 10 to 30 minutes, and intravenous administration typically requires at least 20 minutes.130 The duration of action is approximately 24 hours. Continued benzodiazepine administration is appropriate until phenytoin achieves adequate brain levels. Phenytoin is not recommended in toxin-induced seizures, as it is likely to be ineffective and may be harmful in cases of theophylline or tricyclic antidepressant ingestions.3
Fosphenytoin is a water-soluble prodrug form of phenytoin, with a more physiologic pH. Its main advantages are that it is not likely to precipitate during intravenous infusion and that it also can be administered intramuscularly, although the volume required for full loading by the intramuscular route may be in the range of 20 mL or more.131 Although fosphenytoin can be infused more rapidly, the time to therapeutic concentration of the active drug is the same as for intravenous phenytoin.132 The hemodynamic advantages of fosphenytoin over intravenous phenytoin have not proven to be significant.133,134 Fosphenytoin use is most appropriate when intravenous access is not obtainable or when the intravenous line is of small gauge, as is often the case in children and elders.120
If the serum drug level of phenytoin is subtherapeutic in a patient already being treated for seizures, a loading dose can be given intravenously or orally in divided doses over 6 hours. Oral loading of phenytoin is associated with fewer adverse events than loading with either intravenous phenytoin or fosphenytoin,134 but it is inadequate when therapeutic activity is required urgently.
Phenobarbital is similar to benzodiazepines in that it binds to and enhances the inhibitory neurotransmitter GABA, thereby acting as a CNS depressant that decreases ictal and physiologic cortical electrical activity. Sedation and depression of respiratory drive and blood pressure must be anticipated, and for this reason nonsedating phenytoin is preferred.121 The onset of action of phenobarbital is within 15 to 30 minutes, and the duration of action is 48 hours.
Valproic acid administered intravenously has recently been recognized as a safe and effective treatment for seizures, especially in patients with allergies to phenytoin, the elderly, and patients with cardiorespiratory instability who might be at increased risk of adverse events from phenytoin.135–137 Valproic acid administered intravenously has been shown to be as effective as phenytoin in patients with benzodiazepine-refractory status epilepticus, with fewer cardiopulmonary side effects.138 Hyperammonemic encephalopathy after valproic acid loading has been reported and should be evaluated by obtaining the serum ammonia level in a patient who does not regain consciousness after seizure resolution.21,139 Appropriate ED dosage regimens for the benzodiazepines, phenytoin, fosphenytoin, phenobarbital, and valproic acid are listed in Table 102-1.
Eclampsia complicates 1 in 1000 deliveries in the United States and can occur antepartum (91% of cases occur after 28 weeks of gestation), during the peripartal period, and up to 8 weeks postpartum.140–142 Abortive treatment for eclamptic seizures is with magnesium sulfate, which is superior to both diazepam and phenytoin in limiting maternal mortality and preventing further seizures in eclampsia.143,144 The loading dose of magnesium sulfate is 4 to 6 g, followed by an infusion of 2 g/hr for 24 hours.75,143,144 Because hypermagnesemia may cause respiratory arrest, it is essential to monitor patients for hyporeflexia, which precedes respiratory compromise. In the uncommon event of respiratory compromise resulting from excessive neuromuscular blockade caused by magnesium sulfate, 1 g of 10% calcium gluconate is an effective reversal agent.75 Simultaneous reduction in blood pressure in the eclamptic patient, with use of hydralazine, labetalol, or nicardipine, is recommended.75
Nonpregnant patients who continue having seizures in the ED despite management with benzodiazepines, phenytoin, or phenobarbital are likely to meet the clinical criteria for refractory status epilepticus. Additional therapeutic measures include use of valproate, midazolam infusion, propofol infusion, barbiturate coma, and general inhalational anesthesia. Valproate, which increases GABA concentration, may be given intravenously in status epilepticus (see Table 102-1).23 Another alternative is the use of propofol, a nonbarbiturate anesthetic agent with hypnotic and anticonvulsant activity. Studies suggest that propofol acts at a location other than the benzodiazepine-binding site and modifies the chloride channel by a mechanism that is different from, and possibly synergistic with, those for benzodiazepines and barbiturates. Propofol usually is administered as an intravenous loading dose, followed by an infusion with continuous EEG monitoring to ensure persistent burst suppression.145,146
Barbiturate coma is effective in terminating seizures by acting as a GABA agonist, although it also suppresses all brainstem function. Neurologic consultation is advisable before induction of barbiturate coma in a patient with refractory status epilepticus. Expected consequences of barbiturate coma include respiratory arrest, myocardial depression, and hypotension. At the same time, intracranial pressure may be decreased, thus increasing cerebral perfusion. The preferred agent for barbiturate coma is pentobarbital (see Table 102-1). Patients who are treated with barbiturate coma require intubation and ventilatory support, continuous cardiac monitoring, and invasive hemodynamic monitoring. Pressors may be required to support the blood pressure.147
The role of newer AEDs, such as lacosamide and levetiracetam, in the management of status epilepticus has not yet been well established.148,149
Long-Term Management
Even when witnessed in the ED, apparent ictal activity may not be a seizure. Diagnosing a seizure is more difficult when the event resolved before the patient’s arrival at the ED and is based on witness reports. In patients diagnosed with a seizure, 20 to 25% are subsequently found to have been misdiagnosed.150,151
The risk of seizure recurrence is difficult to estimate in the ED setting. In patients with an initial unprovoked seizure, the 2-year risk of recurrence without treatment generally is considered to be less than 50%.90,99,100 The presence of EEG abnormalities suggests greater risk, but this information usually is unavailable in the ED setting. Other factors associated with an increased risk of recurrence are partial (versus generalized) ictus, status epilepticus, a history of intracranial surgery or trauma, and the presence of a persistent neurologic abnormality, such as Todd’s paralysis.
The presence of specific underlying conditions may affect the decision to institute long-term therapy. For example, it would be reasonable to initiate antiepileptic therapy for an initial seizure in an HIV-positive patient when the seizure is not a result of correctable factors such as drug toxicity or metabolic derangement.50,90 Alcohol-related seizures are notoriously unresponsive to anticonvulsants. Prophylaxis against post-traumatic seizures beyond the first week after injury probably is unnecessary,152 but the occurrence of early post-traumatic seizures should prompt at least short-term initiation of therapy.153 Furthermore, if a patient not receiving antiepileptic therapy arrives in the ED with a second seizure, initiation of treatment is warranted because of the estimated 70% risk of recurrent events.154
The side effects of anticonvulsants can be debilitating for the patient (Table 102-2). These effects should be considered before such therapy is initiated, particularly in women of reproductive age, because many anticonvulsants are teratogenic, and some precipitate failure of oral contraceptives.
Table 102-2
Important Adverse Effects and Drug-Drug Interactions of Antiepileptic Drugs
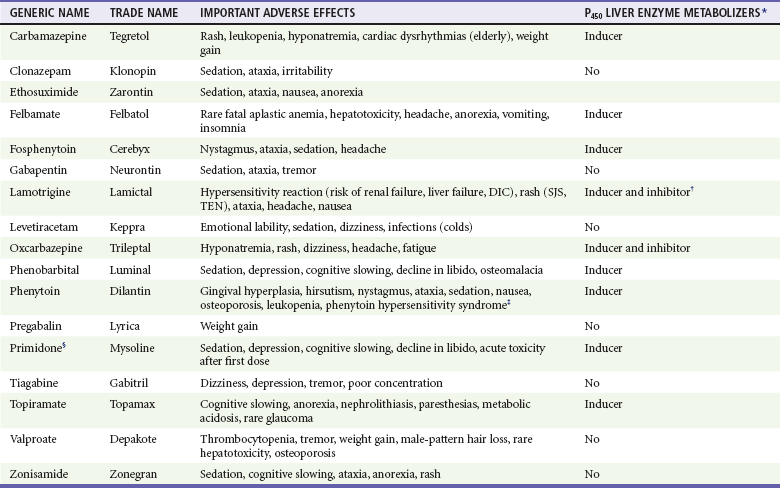
*All inducers of liver enzymes reduce the efficacy of oral contraceptives.
†Oral contraceptive pills also reduce Lamictal serum levels.
‡Phenytoin hypersensitivity syndrome includes rash, fever, hepatitis, lymphoid hyperplasia, and blood dyscrasias. Side effects of intravenous phenytoin include hypotension, arterioventricular block, and purple glove syndrome (edema, pain, and discoloration of the limb distal to the site of infusion).
§Primidone is a congener of phenobarbital.
Data from Engel J Jr, Pedley TA (eds): Epilepsy: A Comprehensive Textbook, 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 2008; Harden CL, Leppik I: Optimizing therapy of seizures in women who use oral contraceptives. Neurology 67:S56, 2006; French JA, et al: Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new onset epilepsy: Report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society [see comment]. Neurology 62:1252, 2004; and Mattson RH: Efficacy and adverse effects of established and new antiepileptic drugs. Epilepsia 36:S13, 1995.
In the absence of specific underlying conditions that increase risk of recurrence, evidence does not support the initiation of anticonvulsant therapy from the ED after a single unprovoked seizure in adults.13,101,104,155 If the seizure was provoked, the decision should be based on whether the provoking factor can be corrected; if it cannot, anticonvulsant therapy should be instituted. The anticonvulsant dosage regimen for a patient with known epilepsy should be modified only in consultation with the patient’s physician (Table 102-3).
Table 102-3
Medications Used for Long-Term Anticonvulsant Therapy in Adults
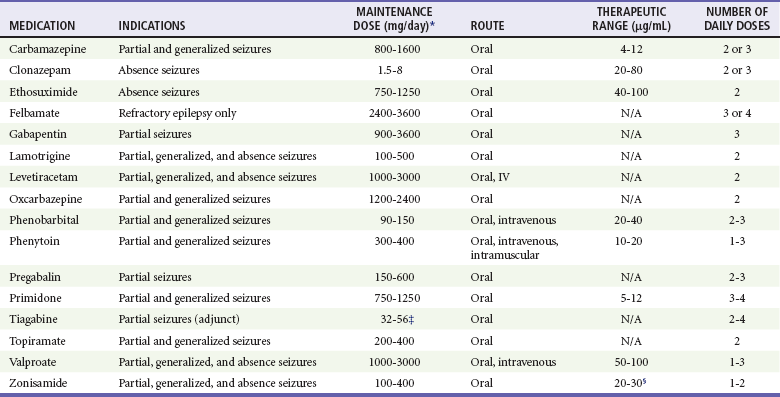
*Adjust dose for hepatic or renal disease and with use of other medications.
‡If patient is not taking enzyme-reducing antiepileptic drugs, then daily dose should be halved (16-28).
§Some studies indicate a range of 10-50 mg/L.
Data from Engel J Jr, Pedley TA (eds): Epilepsy: A Comprehensive Textbook, 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 2008; Mattson RH: Efficacy and adverse effects of established and new antiepileptic drugs. Epilepsia 36:S13, 1995; Perucca E: Clinical pharmacology and therapeutic use of the new antiepileptic drugs. Fundam Clin Pharmacol 15:405, 2001; and Drugs for Epilepsy. Treatment Guidelines from the Medical Letter 6:37, 2008.
Drug monotherapy is preferable in anticonvulsant regimens. Choosing the right drug depends on numerous factors, including the type of seizure, comorbid conditions, other medications the patient is taking, and the potential for pregnancy, and it is best determined in consultation with a neurologist, ideally after MRI neuroimaging and an EEG.85 However, if an anticonvulsant is to be initiated in the ED, then the choice is among the three medications with the strongest evidence of efficacy in the treatment of a tonic-clonic seizure of either generalized onset or partial onset with secondary generalization: carbamazepine, phenytoin, and valproate.156 It is essential to inform women of childbearing age that carbamazepine and phenytoin decrease the efficacy of oral contraceptive pills, so a second form of contraception should be used pending consultation with a neurologist. Valproate, although not altering the efficacy of oral contraceptives, carries a high risk of teratogenic effects and is therefore not an ideal first-line agent for women of childbearing years.157,158
References
1. Martindale, JL, Goldstein, JN, Pallin, DJ. Emergency department seizure epidemiology. Emerg Med Clin North Am. 2011;29:15.
2. Pallin, DJ, et al. Seizure visits in U.S. emergency departments: Epidemiology and potential disparities in care. Int J Emerg Med. 2008;1:97.
3. Sharma, AN, Hoffman, RJ. Toxin-related seizures. Emerg Med Clin North Am. 2011;29:125.
4. Benbadis, SR. Epileptic seizures and syndromes. Neurol Clin. 2001;19:251.
5. Engel, J, Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796.
6. Engel, J, Jr. ILAE classification of epilepsy syndromes. Epilepsy Res. 2006;70(Suppl 1):5.
7. Fisher, RS, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46:470.
8. Seino, M. Classification criteria of epileptic seizures and syndromes. Epilepsy Res. 2006;70(Suppl 1):27.
9. Blume, WT, et al. Glossary of descriptive terminology for ictal semiology: Report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212.
10. Engel, J, Pedley, TA. Epilepsy: A Comprehensive Textbook, 2nd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
11. Alving, J, Beniczky, S. Diagnostic usefulness and duration of the inpatient long-term video-EEG monitoring: Findings in patients extensively investigated before the monitoring. Seizure. 2009;18:470.
12. Watemberg, N, et al. Adding video recording increases the diagnostic yield of routine electroencephalograms in children with frequent paroxysmal events. Epilepsia. 2005;46:716.
13. Shneker, BF, Fountain, NB. Epilepsy. Dis Mon. 2003;49:426.
14. Goldberg, PA, Inzucchi, SE. Critical issues in endocrinology. Clin Chest Med. 2003;24:583.
15. Orringer, CE, Eustace, JC, Wunsch, CD, Gardner, LB. Natural history of lactic acidosis after grand-mal seizures. A model for the study of an anion-gap acidosis not associated with hyperkalemia. N Engl J Med. 1977;297:796.
16. Bassin, S, Smith, TL, Bleck, TP. Clinical review: Status epilepticus. Crit Care. 2002;6:137.
17. Lowenstein, DH. The management of refractory status epilepticus: An update. Epilepsia. 2006;47(Suppl 1):35.
18. Huff, JS, Fountain, NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin North Am. 2011;29:1.
19. Pellock, JM. Overview: Definitions and classifications of seizure emergencies. J Child Neurol. 2007;22(Suppl 5):9.
20. Aranda, A, et al. Generalized convulsive status epilepticus management in adults: A cohort study with evaluation of professional practice. Epilepsia. 2010;51:2159.
21. Rossetti, AO, Hurwitz, S, Logroscino, G, Bromfield, EB. Prognosis of status epilepticus: Role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77:611.
22. Thundiyil, JG, Kearney, TE, Olson, KR. Evolving epidemiology of drug-induced seizures reported to a Poison Control Center System. J Med Toxicol. 2007;3:15.
23. Lowenstein, DH, Alldredge, BK. Status epilepticus. N Engl J Med. 1998;338:970.
24. Walker, MC. Treatment of nonconvulsive status epilepticus. Int Rev Neurobiol. 2007;81:287.
25. Meierkord, H, Holtkamp, M. Nonconvulsive status epilepticus in adults: Clinical forms and treatment. Lancet Neurol. 2007;6:329.
26. Chang, AK, Shinnar, S. Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65.
27. Alroughani, R, Javidan, M, Qasem, A, Alotaibi, N. Non-convulsive status epilepticus; the rate of occurrence in a general hospital. Seizure. 2009;18:38.
28. Takahashi, T, Tsukahara, Y. Pocket Monster incident and low luminance visual stimuli: Special reference to deep red flicker stimulation. Acta Paediatr Jpn. 1998;40:631.
29. Ettinger, AB, Manjunath, R, Candrilli, SD, Davis, KL. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14:324.
30. Weiner, WJ, Shulman, LM. Emergent and Urgent Neurology, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999.
31. Monami, M, Mannucci, E, Breschi, A, Marchionni, N. Seizures as the only clinical manifestation of reactive hypoglycemia: A case report. J Endocrinol Invest. 2005;28:940.
32. Buckingham, B, et al. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. 2008;31:2110.
33. Malouf, R, Brust, JC. Hypoglycemia: Causes, neurological manifestations, and outcome. Ann Neurol. 1985;17:421.
34. Yoshino, T, et al. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci. 2012;117:347–351.
35. Castilla-Guerra, L, del Carmen Fernández-Moreno, M, López-Chozas, JM, Fernández-Bolaños, R. Electrolytes disturbances and seizures. Epilepsia. 2006;47:1990.
36. Riggs, JE. Neurologic manifestations of electrolyte disturbances. Neurol Clin. 2002;20:227.
37. Adrogué, HJ, Madias, NE. Hyponatremia. N Engl J Med. 2000;342(21):1581.
38. Halperin, ML, Bohn, D. Clinical approach to disorders of salt and water balance. Emphasis on integrative physiology. Crit Care Clin. 2002;18:249.
39. Adrogué, HJ, Madias, NE. Hypernatremia. N Engl J Med. 2000;342(20):1493.
40. Cokar, O, Aydin, B, Ozer, F. Non-ketotic hyperglycaemia presenting as epilepsia partialis continua. Seizure. 2004;13:264.
41. Kumar, S. Epilepsia partialis continua stopped by insulin. J R Soc Med. 2004;97:332.
42. Trence, DL, Hirsch, IB. Hyperglycemic crises in diabetes mellitus type 2. Endocrinol Metab Clin North Am. 2001;30:817.
43. Palmer, CA. Neurologic manifestations of renal disease. Neurol Clin. 2002;20:23.
44. Song, TJ, Kim, SJ, Kim, GS, Choi, YC, Kim, WJ. The prevalence of thyrotoxicosis related seizures. Thyroid. 2010;20:955.
45. Idro, R, et al. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297:2232.
46. van de Beek, D, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849.
47. Jagaralapudi, MKM, Sudesh, P. Bacterial meningitis and epilepsy. Epilepsia. 2008;49(Suppl 6):8S.
48. Annegers, JF, Hauser, WA, Beghi, E, Nicolosi, A, Kurland, LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407.
49. Carpio, A. Neurocysticercosis: An update. Lancet Infect Dis. 2002;2:751.
50. Kellinghaus, C, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure. 2008;17:27.
51. Wills, B, Erickson, T. Drug- and toxin-associated seizures. Med Clin North Am. 2005;89:1297.
52. Mokhlesi, B, Corbridge, T. Toxicology in the critically ill patient. Clin Chest Med. 2003;24:689.
53. Haller, CA, Meier, KH, Olson, KR. Seizures reported in association with use of dietary supplements. Clin Toxicol (Phila). 2005;43:23.
54. Kupiec, T, Raj, V. Fatal seizures due to potential herb-drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755.
55. Zimmerman, JL. Poisonings and overdoses in the intensive care unit: General and specific management issues. Crit Care Med. 2003;31:2794.
56. Shanti, CM, Lucas, CE. Cocaine and the critical care challenge. Crit Care Med. 2003;31:1851.
57. Goldfrank, LR, Flomenbaum, N. Goldfrank’s Toxicologic Emergencies, 8th ed. New York: McGraw-Hill; 2006.
58. McMicken, D, Liss, JL. Alcohol-related seizures. Emerg Med Clin North Am. 2011;29:117.
59. Hillbom, M, Pieninkeroinen, I, Leone, M. Seizures in alcohol-dependent patients: Epidemiology, pathophysiology and management. CNS Drugs. 2003;17:1013.
60. Frey, LC. Epidemiology of posttraumatic epilepsy: A critical review. Epilepsia. 2003;44(Suppl 10):11.
61. Temkin, NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44(Suppl 10):18.
62. Chang, BS, Lowenstein, DH. Practice parameter: Antiepileptic drug prophylaxis in severe traumatic brain injury: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60(1):10.
63. Schierhout, G, Roberts, I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. (4):2001.
64. Wen, PY, Schiff, D. Neurologic complications of solid tumors. Neurol Clin. 2003;21:107.
65. Nadeau, SE. Neurologic manifestations of connective tissue disease. Neurol Clin. 2002;20:151.
66. LaRoche, SM, Helmers, SL. Epilepsy in the elderly. Neurologist. 2003;9:241.
67. Camilo, O, Goldstein, LB. Seizures and epilepsy after ischemic stroke. Stroke. 2004;35:1769.
68. Labovitz, DL, Hauser, WA, Sacco, RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57:200.
69. Gilmore, E, Choi, HA, Hirsch, LJ, Claassen, J. Seizures and CNS hemorrhage: Spontaneous intracerebral and aneurysmal subarachnoid hemorrhage. Neurologist. 2010;16:165.
70. Raps, EC, et al. The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol. 1993;50:265.
71. Forderreuther, S, Henkel, A, Noachtar, S, Straube, A. Headache associated with epileptic seizures: Epidemiology and clinical characteristics. Headache. 2002;42:649.
72. Koch, M, Uyttenboogaart, M, Polman, S, De Keyser, J. Seizures in multiple sclerosis. Epilepsia. 2008;49:948.
73. Mendez, M, Lim, G. Seizures in elderly patients with dementia: Epidemiology and management. Drugs Aging. 2003;20:791.
74. Katz, JM, Pacia, SV, Devinsky, O. Current management of epilepsy and pregnancy: Fetal outcome, congenital malformations, and developmental delay. Epilepsy Behav. 2001;2:119.
75. Sibai, BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402.
76. Stead, LG. Seizures in pregnancy/eclampsia. Emerg Med Clin North Am. 2011;29:109.
77. Kerr, MP, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011;52:2133.
78. Cragar, DE, Berry, DT, Fakhoury, TA, Cibula, JE, Schmitt, FA. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev. 2002;12:31.
79. Duncan, R. Psychogenic nonepileptic seizures: Diagnosis and initial management. Expert Rev Neurother. 2010;10:1803.
80. Mathews, MS, Smith, WS, Wintermark, M, Dillon, WP, Binder, DK. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd’s paresis. Neuroradiology. 2008;50:397.
81. Lim, HB, Smith, M. Systemic complications after head injury: A clinical review. Anaesthesia. 2007;62:474.
82. Baumann, A, Audibert, G, McDonnell, J, Mertes, PM. Neurogenic pulmonary edema. Acta Anesthesiol Scand. 2007;50:447.
83. Fontes, RB, et al. Acute neurogenic pulmonary edema: Case reports and literature review. J Neurosurg Anesthesiol. 2003;15:144.
84. Jagoda, A, Gupta, K. The emergency department evaluation of the adult patient who presents with a first-time seizure. Emerg Med Clin North Am. 2011;29:41.
85. Krumholz, A, et al. Practice parameter: Evaluating an apparent unprovoked first seizure in adults (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996.
86. ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2004;43:605.
87. Hampers, LC, Spina, LA. Evaluation and management of pediatric febrile seizures in the emergency department. Emerg Med Clin North Am. 2011;29:83.
88. Sharieff, GQ, Hendry, PL. Afebrile pediatric seizures. Emerg Med Clin North Am. 2011;29:95.
89. Harden, CL, et al. Reassessment: Neuroimaging in the emergency patient presenting with seizure (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69:1772.
90. Miller, LC, Drislane, FW. Treatment strategies after a single seizure: Rationale for immediate versus deferred treatment. CNS Drugs. 2007;21:89.
91. Reference deleted in proofs.
92. Reference deleted in proofs.
93. Seneviratne, U. Management of the first seizure: An evidence based approach. Postgrad Med J. 2009;85:667.
94. Wiebe, S, Téllez-Zenteno, JF, Shapiro, M. An evidence-based approach to the first seizure. Epilepsia. 2008;49(Suppl 1):50.
95. Josephson, CB, Rahey, S, Sadler, RM. Neurocardiogenic syncope: Frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221.
96. Zaidi, A, Clough, P, Cooper, P, Scheepers, B, Fitzpatrick, AP. Misdiagnosis of epilepsy: Many seizure-like attacks have a cardiovascular cause. J Am Coll Cardiol. 2000;36:181.
97. Bergfeldt, L. Differential diagnosis of cardiogenic syncope and seizure disorders. Heart. 2003;89:353.
98. McKeon, A, Vaughan, C, Delanty, N. Seizure versus syncope. Lancet Neurol. 2006;5:293.
99. Ramos Lizana, J, et al. Seizure recurrence after a first unprovoked seizure in childhood: A prospective study. Epilepsia. 2000;41:1005.
100. Berg, AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49(Suppl 1):13.
101. Marsoni, A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: A randomised controlled trial. Lancet. 2005;365:2007.
102. Shinnar, S, Hauser, WA. Do occasional brief seizures cause detectable clinical consequences? Prog Brain Res. 2002;135:221.
103. Marson, A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: A randomised controlled trial. Lancet. 2005;365:2007.
104. Jacoby, A, Gamble, C, Doughty, J, Marson, A, Chadwick, D. Quality of life outcomes of immediate or delayed treatment of early epilepsy and single seizures. Neurology. 2007;68:1188.
105. Chua, HC, Venketasubramanian, N, Tan, CB, Tjia, H. Paradoxical seizures in phenytoin toxicity. Singapore Med J. 1999;40:276.
106. Craig, S. Phenytoin overdose complicated by prolonged intoxication and residual neurological deficits. Emerg Med Australas. 2004;16:361.
107. Lofton, AL, Klein-Schwartz, W. Evaluation of lamotrigine toxicity reported to poison centers. Ann Pharmacother. 2004;38:1811.
108. Dinnerstein, E, Jobst, BC, Williamson, PD. Lamotrigine intoxication provoking status epilepticus in an adult with localization-related epilepsy. Arch Neurol. 2007;64:1344.
109. Spiller, HA, Carlisle, RD. Status epilepticus after massive carbamazepine overdose. Clin Toxicol. 2002;40:81.
110. Brandt, C, et al. Topiramate overdose: A case report of a patient with extremely high topiramate serum concentrations and nonconvulsive status epilepticus. Epilepsia. 2010;51:1090.
111. Hindley, D, Ali, A, Robson, C. Diagnoses made in a secondary care “fits, faints, and funny turns” clinic. Arch Dis Child. 2006;91:214.
112. Macleod, S, Ferrie, C, Zuberi, SM. Symptoms of narcolepsy in children misinterpreted as epilepsy. Epileptic Disord. 2005;7:13.
113. Sethi, NK, Labar, D, Torgovnick, J. Myoclonic epilepsy masquerading as a tic disorder. Neurol Neurosurg. 2007;109:509.
114. d’Orsi, G, Demaio, V, Minervini, MG. Myoclonic status misdiagnosed as movement disorders in Rett syndrome: A video-polygraphic study. Epilepsy Behav. 2009;15:260.
115. Anpalahan, M, Gibson, S. The prevalence of neurally mediated syncope in older patients presenting with unexplained falls. Eur J Intern Med. 2012;23:48.
116. Treiman, DM. Treatment of convulsive status epilepticus. Int Rev Neurobiol. 2007;81:273.
117. Treiman, DM, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792.
118. Alldredge, BK, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631.
119. Cock, HR, Schapira, AH. A comparison of lorazepam and diazepam as initial therapy in convulsive status epilepticus. QJM. 2002;95:225.
120. Lowenstein, DH. Treatment options for status epilepticus. Curr Opin Pharmacol. 2005;5:334.
121. Prasad, K, Al-Roomi, K, Krishnan, PR, Sequeira, R. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. (4):2005.
122. D’Onofrio, G, Rathlev, NK, Ulrich, AS, Fish, SS, Freedland, ES. Lorazepam for the prevention of recurrent seizures related to alcohol. N Engl J Med. 1999;340:915.
123. Arya, R, Gulati, S, Kabra, M, Sahu, JK, Kalra, V. Intranasal versus intravenous lorazepam for control of acute seizures in children: A randomized open-label study. Epilepsia. 2011;52:788.
124. Mahmoudian, T, Zadeh, MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5:253.
125. Silbergleit, R, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591.
126. McMullan, J, Sasson, C, Pancioli, A, Silbergleit, R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: A meta-analysis. Acad Emerg Med. 2010;17:575.
127. Bhattacharjee, P, Glusac, EJ. Early histopathologic changes in purple glove syndrome. J Cutan Pathol. 2004;31:513.
128. Burneo, JG, Anandan, JV, Barkley, GL. A prospective study of the incidence of the purple glove syndrome. Epilepsia. 2001;42:1156.
129. Chokshi, R, Openshaw, J, Mehta, NN, Mohler, E, 3rd. Purple glove syndrome following intravenous phenytoin administration. Vasc Med. 2007;12:29.
130. Smith, BJ. Treatment of status epilepticus. Neurol Clin. 2001;19:347.
131. Pryor, FM, Gidal, B, Ramsay, RE, DeToledo, J, Morgan, RO. Fosphenytoin: Pharmacokinetics and tolerance of intramuscular loading doses. Epilepsia. 2001;42:245.
132. Eriksson, K, Keränen, T, Kälviäinen, R. Fosphenytoin. Expert Opin Drug Metab Toxicol. 2009;5:695.
133. Coplin, WM, et al. Randomized evaluation of adverse events and length-of-stay with routine emergency department use of phenytoin or fosphenytoin. Neurol Res. 2002;24:842.
134. Swadron, SP, et al. A comparison of phenytoin-loading techniques in the emergency department. Acad Emerg Med. 2004;11:244.
135. Limdi, NA, et al. Safety of rapid intravenous loading of valproate. Epilepsia. 2007;48:478.
136. Peters, CN, Pohlmann-Eden, B. Intravenous valproate as an innovative therapy in seizure emergency situations including status epilepticus—experience in 102 adult patients. Seizure. 2005;14:164.
137. Trinka, E. The use of valproate and new antiepileptic drugs in status epilepticus. Epilepsia. 2007;48(Suppl 8):49.
138. Agarwal, P, et al. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16:527.
139. Embacher, N, Karner, E, Wanschitz, J, Beer, R, Trinka, E. Acute encephalopathy after intravenous administration of valproate in nonconvulsive status epilepticus. Eur J Neurol. 2006;13:5.
140. Zhang, J, Meikle, S, Trumble, A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203.
141. Chames, MC, Livingston, JC, Ivester, TS, Barton, JR, Sibai, BM. Late postpartum eclampsia: A preventable disease? Am J Obstet Gynecol. 2002;186:1174.
142. Minnerup, J, et al. Late onset postpartum eclampsia: It is really never too late? A case of eclampsia 8 weeks after delivery. Stroke Res Treat. 2010;2010:798616.
143. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455.
144. Duley, L, Henderson-Smart, D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. (4):2003.
145. Parviainen, I, Uusaro, A, Kalviainen, R, Mervaala, E, Ruokonen, E. Propofol in the treatment of refractory status epilepticus. Intensive Care Med. 2006;32:1075.
146. Rossetti, AO, Reichhart, MD, Schaller, MD, Despland, PA, Bogousslavsky, J. Propofol treatment of refractory status epilepticus: A study of 31 episodes. Epilepsia. 2004;45:757.
147. Claassen, J, Hirsch, LJ, Emerson, RG, Mayer, SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: A systematic review. Epilepsia. 2002;43:146.
148. Albers, JM, et al. Intravenous lacosamide—an effective add-on treatment of refractory status epilepticus. Seizure. 2011;20:428.
149. Wheless, JW, Treiman, DM. The role of the newer antiepileptic drugs in the treatment of generalized convulsive status epilepticus. Epilepsia. 2008;49(Suppl 9):74.
150. Perrig, S, Jallon, P. Is the first seizure truly epileptic? Epilepsia. 2008;49(Suppl 1):2.
151. Benbadis, S. The differential diagnosis of epilepsy: A critical review. Epilepsy Behav. 2009;15:15.
152. Benardo, LS. Prevention of epilepsy after head trauma: Do we need new drugs or a new approach? Epilepsia. 2003;44(Suppl 10):27.
153. Beghi, E. Overview of studies to prevent posttraumatic epilepsy. Epilepsia. 2003;44(Suppl 10):21.
154. Hauser, WA, Rich, SS, Lee, JR, Annegers, JF, Anderson, VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429.
155. Kim, LG, Johnson, TL, Marson, AG, Chadwick, DW. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: Further results from the MESS trial. Lancet Neurol. 2006;5:317.
156. Glauser, T, et al. ILAE treatment guidelines: Evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094.
157. Harden, CL, Leppik, I. Optimizing therapy of seizures in women who use oral contraceptives. Neurology. 2006;67(12 Suppl 4):56.
158. Patsalos, PN, Perucca, E. Clinically important drug interactions in epilepsy: Interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2:473.







