186 Sedatives and Hypnotics
 Rationale for Sedative Use in the Intensive Care Unit
Rationale for Sedative Use in the Intensive Care Unit
Medications are commonly administered to critically ill patients to diminish fundamental activities of the central nervous system (CNS) such as wakefulness, memory, and control of voluntary muscle contraction, and to minimize unpleasant symptoms such as dyspnea, pain, anxiety, and fear. Medications are most often used for these purposes in the management of mechanically ventilated patients; they are more likely to receive sedative-analgesics, and in higher doses, than nonintubated patients.1 Paradoxically, most intensive care unit (ICU) patients who receive these potent CNS-active medications are not suffering from acute neurologic diagnoses such as stroke, seizure, or infection. Therefore this chapter focuses on sedative use in critically ill patients who may have toxic-metabolic encephalopathy (e.g., delirium) or no CNS abnormalities at all.
The expression “sedation” or “sedative medications” encompasses elements of sedation, hypnosis, amnesia, analgesia, and muscle relaxation. These words have discrete but related meanings. Sedatives create a state of calmness or lack of excitability without necessarily decreasing awareness. Hypnotics and general anesthetics induce sleep or, more precisely, create the appearance of sleep by reducing the level of consciousness, arousability, or awareness. Amnestics impede new memory formation, whereas analgesics reduce the symptom of pain by peripheral or central mechanisms. Excessive skeletal muscle contraction or motor activity is a major manifestation of agitation which, along with level of consciousness, is the primary observable behavior measured by many sedation scales.2 Antipsychotics and neuroleptics ameliorate disorganized thinking and inappropriate behavior. Most “sedative” medications have clinical effects in several of these categories. For instance, a drug may have both sedative and hypnotic properties, or both analgesic and hypnotic effects, or antipsychotic and sedative effects. Although no one sedative has a completely specific effect, medications typically have greater effects in one of the categories, and the thoughtful intensivist can prescribe medication combinations that maximize desired effects while minimizing unwanted effects. Importantly, given in higher doses, almost all the medications described in this chapter decrease the level of consciousness and reduce unwanted skeletal muscle activity.
 Goals of Sedation for Patients in Intensive Care Units
Goals of Sedation for Patients in Intensive Care Units
Table 186-1 lists 15 indications for administration of sedative and analgesic medications to critically ill patients. The clinician should mentally compare the number of possible indications for use of sedative-analgesics in ICU patients with an analogous list for other common ICU medications. For example, antibiotics have two indications, to prevent or treat infections, and gastric acid–reducing medications have two indications, to prevent or treat gastrointestinal bleeding and improve symptoms of esophageal reflux. Much of the art of sedating ICU patients lies in determining which of the many indications applies to the individual patient on a given day and communicating that rationale to other caregivers.
TABLE 186-1 Indications for Administering Sedative-Analgesic Medications to Critically Ill Patients
| Indication | Comment |
|---|---|
| Minimize ventilator dyssynchrony | Poor synchrony may lead to hypoxemia and dyspnea and is distressing to caregivers. Ventilator adjustment may improve synchrony without medications. |
| Reduce dyspnea associated with severe acute respiratory failure | Reducing minute ventilation to avoid barotrauma can cause severe dyspnea. Tachypnea with short expiratory times can lead to increased auto-PEEP and hypotension. |
| Increase tolerance of intubation | A translaryngeal endotracheal tube can cause pain, gagging, and reflexive biting. |
| Reduce anxiety | Acute severe illness possibly leading to disability or death may produce unwanted psychological distress. |
| Reduce recall of ICU symptoms | Recall of distressing symptoms such as severe dyspnea, terror, restraint, or pain can have long-term psychological consequences.32 |
| Reduce stress response and oxygen consumption | Reducing unwanted motor activity or respiratory effort can decrease total-body oxygen consumption by 15%.87 |
| Reduce elevated intracranial pressure | Coughing, straining, or excessive ventilator dyssynchrony can cause dangerous spikes in intracranial pressure. |
| Reduce pain | Surgical or traumatic wounds, catheter and tube placement, and immobilization usually cause pain. |
| Prevent removal of life-support technology | Removal of an endotracheal tube or vascular catheter can cause death within minutes. |
| Induce sleep | ICU patients often have abnormal chronobiology cycles associated with delirium and impaired immune function. Commonly used sedatives have not been shown to restore normal sleep brainwave patterns. |
| Increase efficiency of patient care delivery | Constant visual observation and verbal and tactile patient reassurance may not be possible in understaffed units. |
| Protect caregivers from violent behaviors | Confused patients can violently assault caregivers. |
| Adjunct during pharmacologic paralysis | Awareness during pharmacologic paralysis is inhumane and can have long-term psychological consequences. |
| Treat delirium | Antipsychotics may reduce disorganized thought processes or behavior while the underlying cause of the delirium is treated. |
| Family considerations | Repeatedly observing the distress of a loved one can cause anguish in family members, who may request that additional sedatives be given to the patient.27 |
ICU, intensive care unit; PEEP, positive end-expiratory pressure.
 Epidemiology of Sedative Use in the Intensive Care Unit
Epidemiology of Sedative Use in the Intensive Care Unit
Two-thirds of patients requiring mechanical ventilation receive sedative medications.3,4 International practice surveys show that BZDs or propofol are the most common sedatives selected and are often combined with opioids, although the choice of opioid (e.g., morphine, fentanyl, or sufentanil) varies among countries and institutions. A study of 174 ICUs in 2007 reported that over 50% of ventilated patients received intravenous (IV) sedation: 82% received propofol, 31% received a BZD, and 4% received dexmedetomidine. Intravenous opiates were used more commonly with BZDs (70.1%) than with propofol (23.9%).5 Continuous infusion therapy was associated with a markedly prolonged duration of mechanical ventilation and longer time to achieve important weaning landmarks.4,6 In a clinical trial that enrolled patients with adult respiratory distress syndrome, sedatives were administered during 70% of ICU patient-days.7 Contrary to clinicians’ expectations, two studies showed that ventilating patients with small tidal volumes to avoid ventilator-induced lung injury was not associated with an increase in sedative exposure.8,9 Despite general practice surveys showing widespread use of sedatives, there are hospitals that have successfully managed ventilated patients with a “minimal-to-no-sedation” policy.10
What are the clinical consequences of widespread use of potent sedatives? Because there are numerous causes of decreased consciousness in critically ill patients, it is difficult to estimate the independent effect of sedative medications on patients’ clinical status. In one study, one-third of subjects were in an unarousable or deeply sedated state, one-third were in a state of moderate to light sedation, and one-third were in an alert and calm state.11 The correlation between sedation level and amount of sedative medication received during the 8 hours before the assessment was weak (r = −0.13 to −0.32) across different medication classes. These results suggest several mutually compatible possibilities: (1) factors (e.g., organ failure-associated encephalopathy) other than medications influence sedation scale measurements, (2) the pharmacologic effects of sedatives accumulate over days rather than hours, and/or (3) dose-response relationships are nonlinear. Another study showed that mechanically ventilated patients were unarousable to tactile stimulation 32% of the time, yet were rated by their nurses as “oversedated” less than 3% of the time.12
Conditions Requiring Sedation
Determining the specific reasons for administration of sedative medications is problematic in clinical studies, but the question can be approached by determining the prevalence of the syndromes, symptoms, or behaviors that may lead to sedative intervention. Some 20% to 60% of patients recall having significant pain during their ICU stay.13–15 Therefore caregivers should consider pain as the most likely cause when patients show signs of distress or agitation. Delirium was objectively diagnosed in 83% of ICU patients at some time during their illness.16 However, ICU delirium is often hypoactive, manifested as inattention rather than agitation, and therefore may not lead to sedative administration. Because the expected effects of sedative medications can mimic symptoms of delirium (e.g., inattention, confusion, fluctuating level of consciousness), studies that link administration of sedatives such as BZDs to persistent delirium should be interpreted cautiously.17
According to one study, agitated behavior, as documented by nursing notes, occurred in 71% of ICU patients, and two-thirds of the episodes were judged as being severe or dangerous. In this study, caregivers often identified three or more factors they believed contributed to the agitated episode.18 However, another study of mechanically ventilated patients detected agitation in less than 5% of 1833 separate assessments.11 The low prevalence of agitation in this study may have occurred because agitation was assessed only during a narrow time interval. These results suggest caregivers intervene quickly when patients are agitated, even if the underlying cause or causes are difficult to identify. Caregivers probably respond quickly because agitation is so visibly apparent and is associated with numerous adverse clinical events.
Anxiety during the acute illness is commonly recalled by ICU survivors,14 although a sample of 192 awake mechanically ventilated ICU patients reported a mean anxiety level during intubation that was only slightly higher than that of nonintubated patients assessed on a general medical-surgical ward.19 These results imply that caregivers should not assume that all mechanically ventilated patients require treatment with anxiolytic medications.
ICU patients recall sleep disruption as a major problem during their ICU stay. Polysomnograms demonstrated that because of frequent arousals and severely fragmented sleep architecture, only 40% of critically ill patients exhibited even brief periods of normal rapid eye movement (REM) sleep.20 The other 60% of patients, who also as a group received more sedative medications, showed no evidence of electrophysiologic sleep, but rather had electroencephalograms (EEGs) consistent with diffuse encephalopathy and coma. Sleep deprivation has been associated with a decrease in quality-of-life measures and increased incidence of complications such as neurocognitive dysfunction and delirium.21 Environmental interventions to improve sleep quality (e.g., noise and light abatement) have not been successful in improving EEG-documented sleep.22 Pharmacologic interventions such as increasing propofol infusion rates at night can generate a diurnal pattern of patient arousability, but there is no evidence that propofol or any other widely used ICU sedative creates restful physiologic sleep for ICU patients.23 In a small trial using wrist actigraphy to estimate sleep quality, nighttime administration of melatonin improved sleep in ICU patients with respiratory failure.24 Newer nonbenzodiazepine hypnotic agents such as zolpidem, zopiclone, and gaboxadol have not been studied in critically ill ICU patients to determine whether they improve disordered sleep or improve outcomes.21 The rationale for administration of additional sedation at night is often conceptualized as “resting” patients in preparation for weaning trials in the morning. However, there are few data to support this concept, and one study showed that the reintubation rate was greater among patients with lower sedation scores (e.g., greater sedation) during the shift interval before the planned extubation.11
Dyspnea is an important symptom to consider, because many ICU patients have respiratory failure requiring mechanical ventilation.25 Dyspnea is a complex symptom that arises from both acute and chronic cardiopulmonary conditions but also from constraints imposed by mechanical ventilators. Excessively small tidal volumes, short expiratory times, or slow inspiratory flow rates can worsen dyspnea and lead to potentially injurious ventilator dyssynchrony. A ventilatory mode that allows spontaneous respiratory efforts throughout the respiratory cycle was found to decrease sedation requirements in patients with acute respiratory distress syndrome (ARDS).26 Opiates are considered first-line medications to relieve dyspnea. However, in patients with communication difficulties, caregivers cannot easily determine whether a little dyspnea is causing a lot of anxiety (in which case BZDs are preferred) or a lot of dyspnea is causing a little anxiety (in which case opiates are preferred).27 ICU personnel may choose to use continuous-infusion opiate therapy for almost all ventilated patients, reasoning that most critically ill patients are dyspneic or in pain or both.28
Although detailed investigations are lacking, the severity of respiratory failure is likely positively associated with aggregate dosing of sedatives. However, the number of ICU patient-days with severe respiratory failure (e.g., high positive end-expiratory pressure, high inspired oxygen fraction, prone positioning) represents a minority of all patient ventilator days. For example, among patients with acute respiratory failure due to exacerbation of chronic obstructive pulmonary disease or ARDS, 40% of time on the ventilator was spent in the weaning phase.29 Similarly, one-third of all ventilated patients examined during a single cross-sectional time point were in the weaning phase.25 Therefore, as patients’ respiratory support requirements lessen, sedation also should be weaned. When patients become more alert, caregivers may have heightened concern for inadvertent removal of life-support technology. Although sedatives or restraints offer no guarantee against “treatment interference,”30 fewer than 2% of ventilated patients had unexpected extubations that required reintubation.29
Sedatives, especially BZDs, may be given to induce anterograde amnesia of the presumably psychologically stressful ICU experience.31 This indication is supported by results from an observational study of ARDS survivors. In this study, patients who recalled a greater number of traumatic experiences from the period when they were in the ICU were more likely to develop persistent symptoms of posttraumatic stress disorder years later.32 On the other hand, no one knows the quantity of sedative medication in each class that is required to reliably ensure complete amnesia. In a study of 149 patients, there was no relationship between aggregate sedative dose during mechanical ventilation and patients’ recall of the ICU experience 2 months later.33 In general, intensivists must balance the proven benefits of administering fewer sedative medications (by daily stopping of sedative infusions or use of sedation protocols) against the uncertain adverse effects of unpleasant symptom recall. Indeed, the data suggest that recall of delusional memories (often exacerbated by sedatives) is associated with greater post-ICU psychopathology than is patient recall of unpleasant but real memories.34
 Pharmacology and Clinical Use of Sedatives Commonly Administered in the Intensive Care Unit
Pharmacology and Clinical Use of Sedatives Commonly Administered in the Intensive Care Unit
The intensity of sedation required for patients can vary markedly throughout their ICU stay, depending on the course of their disease, the external environment, and the time of day. The ideal sedative possesses a rapid onset of action, is convenient to administer and titrate, produces effective and reproducible sedation to the desired clinical goal, and is free of hemodynamic, cardiac, or respiratory side effects. To simplify extended infusion in the critically ill patient, the ideal sedative also should exhibit linear pharmacokinetics with no clinically significant protein binding or drug interactions. Drug clearance in renal and hepatic impairment should be clearly characterized, and the ideal sedative would not be cleared by dialysis. Finally, the ideal sedative would permit rapid and predictable recovery after discontinuation, with no long-term adverse effects. Although new sedative agents have been added to the armamentarium in recent years, this optimal group of characteristics has yet to be formulated in a single agent. Therapy with more than one drug is often used to optimize sedation in critically ill patients, and some combination of a BZD or propofol with an opioid analgesic is the most commonly employed regimen.5,35–37
Opioid Analgesics
Although opioid analgesics are recognized as the drug class most frequently prescribed for pain management, opioids also have a role in management of anxiety. Unrecognized or inadequately treated pain from pathology or ICU procedures can create anxiety in 20% to 60% of patients.13–15 Patients who are unable to communicate the source of their distress may suffer from persistent pain. For this reason, early and systematic scrutiny for the presence of pain is crucial to effective management in the visibly anxious ICU patient.
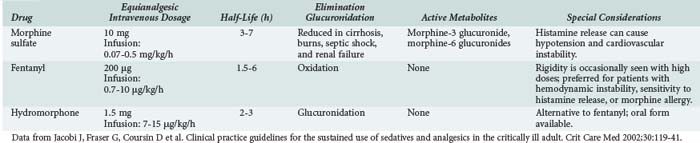
Analgesic agents recommended for use in critically ill patients by the 2002 American College of Chest Physicians/Society of Critical Care Medicine/American Society of Health System Pharmacists Clinical Practice Guidelines38 (hereafter referred to as the Practice Guidelines) are described in Table 186-2. Differences in analgesic potency, response, and recovery time are associated with the pharmacokinetic properties of each drug as well as their mu and kappa receptor-binding affinity in the CNS. In addition to sedation and analgesia, opioids can produce respiratory depression, constipation, urinary retention, nausea, and confusion. Combined use of opioids and BZDs results in synergistic effects that permit dosage reduction, which may reduce adverse effects and drug accumulation. For patients with chronic pain or previous use of opioids, increased dose requirements due to tolerance should be considered. Use of the opioid antagonist, naloxone, as a reversal agent is not recommended routinely after prolonged opioid analgesia because of the risk of withdrawal symptoms and the potential to induce cardiac arrhythmias.38
Several analgesics are not recommended for critically ill patients. Meperidine has an active metabolite, normeperidine, which causes CNS excitation associated with delirium and seizures. Because the active metabolite is excreted by the kidneys, patients with renal insufficiency are at high risk for adverse effects. Opioid antagonist-agonists (e.g., nalbuphine, butorphanol, buprenorphine) can reverse the desirable effects of other opiate agents and are not recommended for routine use in the ICU. Nonsteroidal antiinflammatory analgesics offer few advantages for the critically ill and can cause gastrointestinal bleeding, bleeding due to platelet inhibition, and renal insufficiency.38 Alfentanil, sufentanil, and remifentanil are fentanyl derivatives with higher potency and/or shorter half-lives than fentanyl, but comparative data evaluating these agents for sedation in the ICU are scarce, and the drugs are more expensive than fentanyl.37,39
Benzodiazepines
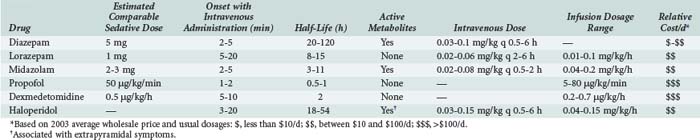
The anxiolytic, amnestic, anticonvulsant, and muscle-relaxing effects of BZDs are mediated through GABAA binding sites on neuronal γ-aminobutyric acid (GABA) receptors. After binding to the receptor site, BZDs facilitate the GABA-mediated increase in chloride conductance with subsequent membrane hyperpolarization and inhibition of neuronal impulses. The amnestic properties of these drugs correlate with their GABA agonist activity in the limbic system.40 BZD binding is stereospecific and saturable, and the potency of an individual BZD agent correlates with its receptor affinity. Other ligands act as antagonists (e.g., flumazenil) or inverse agonists. Inverse agonists reduce the efficiency of GABA interaction with the receptor, causing CNS stimulation; drugs with these properties are in development.41 Table 186-3 describes the comparative pharmacology of selected BZDs and other ICU sedatives.
Both acute and chronic tolerance to BZDs (associated with decreased receptor activity) has been described. In ICU patients, acute tolerance can occur after just 24 hours.41 Paradoxical reactions have also been associated with BZDs, most commonly in the elderly and in patients with a history of preexisting CNS disease, substance abuse, or psychiatric disease. Patients who develop a paradoxical reaction to a BZD should be switched to a medication in another drug class, such as propofol, dexmedetomidine, or haloperidol.
A key protein in the primary metabolic pathway for diazepam, the CYP subfamily enzyme, CYP2C19, is genetically polymorphic. Isoenzymes of CYP2C19 that are present in 3% to 5% of Caucasians and African Americans and 12% to 100% of Asian ethnic groups are associated with a significant decrease in diazepam metabolism. Therefore on occasion, a patient treated with diazepam may experience unexpectedly prolonged sedation.42 Some drugs commonly used in critically ill patients, such as amiodarone, fluconazole, omeprazole, and valproic acid, also inhibit CYP2C19 activity. In contrast, cigarette smoking induces hepatic microsomal enzymes. This effect increases the clearance of diazepam and other BZDs.43 For these reasons, the clinical response to diazepam is often unpredictable in critically ill patients.
Lorazepam has been a preferred agent for ICU sedation in many critical care units since its approval in 1977; lorazepam is recommeded for long-term (>48 hours) ICU sedation in the 2002 Practice Guidelines. Because lorazepam undergoes hepatic glucuronidation to inactive metabolites, its pharmacokinetic parameters are not altered significantly in elderly or critically ill patients, except in those with severe renal or hepatic failure. Lorazepam is the least lipophilic of the injectable BZDs; therefore it crosses the blood-brain barrier slowly, resulting in a delayed onset of action (5-20 minutes) and a longer duration of action, with an elimination half-life of 10 to 20 hours.44 After chronic dosing, accumulation of lorazepam and prolonged sedation are less likely than with diazepam. Lorazepam is also 5 to 6 times more potent than diazepam, and the amnestic effect of lorazepam has a longer duration than an equivalent diazepam dose. Lorazepam can be given by intramuscular injection.41
Lorazepam is formulated in 18% polyethylene glycol (PEG) and 2% benzyl alcohol in propylene glycol (PG) for injection. Although usual lorazepam doses deliver only minute amounts of PEG and PG, long-term sedation with high doses can lead to patients receiving substantial doses of PEG and PG. Both the PEG45 and the PG46,47 components of the vehicle have been associated with development of lactic acidosis, hyperosmolar coma, and reversible nephrotoxicity with high doses or lengthy infusions. Although the dosages implicated have not been prospectively defined, lorazepam doses exceeding 18 mg/h for longer than 4 weeks, or 25 mg/h for hours to days, should be avoided.38 Because of its poor solubility, precipitation can occur when lorazepam is administered by continuous infusion. On the basis of manufacturer information and clinical recommendations, the manufacturer’s vial concentration (either 2 or 4 mg/mL) should be diluted 1 : 1 with 5% dextrose injection in a glass container, not in polyvinyl chloride bags.48
Midazolam, a short-acting, water-soluble BZD prodrug, is approximately 3 times more potent than diazepam. After self-converting to a lipid-soluble form by closure of the diazepine ring at physiologic pH values in the bloodstream, midazolam rapidly enters the CNS to produce sedation within 2 to 5 minutes. This property makes midazolam ideal for patients who require immediate control of anxiety or agitation.38 Initial dosages recommended are 2 to 5 mg IV every 5 to 15 minutes. The drug quickly redistributes to peripheral tissues, and effects dissipate if a continuous infusion is not initiated. When infused over days for chronic sedation, the mean elimination half-life of 10 hours may increase to 30 hours as peripheral tissue stores release accumulated midazolam. The pharmacodynamic effects of BZDs often do not correspond well with reported elimination half-lives.49 In comparing the clinical sedation recovery rate (time to wakefulness) for midazolam versus diazepam, 8 trials reported a faster recovery rate from diazepam, 19 trials reported no difference in sedative recovery time, and only 1 trial demonstrated a faster recovery with midazolam.38
Midazolam is metabolized by the CYP3A4 isoenzyme to an active metabolite, α-hydroxymidazolam, which has 60% of the potency of the parent drug. α-Hydroxymidazolam is quickly biotransformed to its conjugated salt, α-hydroxymidazolam glucuronide (10% potency), which does not significantly contribute to the sedative properties of midazolam except when it accumulates in renal failure. Inhibitors of CYP3A4, such as macrolide antibiotics, diltiazem, propofol, and fluconazole, reduce the metabolism of midazolam and prolong its sedative actions.41 The combined effects of drug interactions, altered protein binding, fluid shifts, altered hepatic metabolism, and renal failure can result in prolonged elimination and an unpredictable time to awakening after discontinuation of midazolam when the drug is used for longer than 48 to 72 hours. For these reasons, the 2002 Practice Guidelines recommend midazolam for short-term use only.38
Several randomized controlled studies have compared BZD sedatives in critically ill patients. Two unmasked studies in mixed populations of ICU patients reported no difference between midazolam and lorazepam in time until sedation or in time until return to baseline mental status.49,50 In contrast, a double-masked randomized comparison of lorazepam versus midazolam, using a target-controlled IV infusion titrated to maintain a moderate level of sedation for 12 to 72 hours, reported a delayed emergence from sedation with lorazepam.44 Other longer-term studies suggest that lorazepam is easier to titrate to the desired sedation level than midazolam.51
Because lorazepam is equally effective and produces less hypotension, it is the BZD recommended in the 2002 Practice Guidelines for most ICU patients; it is administered either by continuous infusion or by intermittent IV dosing (1-4 mg every 2-6 hours).38
BZDs, particularly midazolam and diazepam, can cause respiratory depression and hypotension due to vasodilation when administered in large doses. If these effects require rapid reversal, flumazenil may be used to antagonize BZD agonists at the GABA receptor binding site. Flumazenil administered IV in doses of 0.2 to 1 mg reverses the sedative and amnestic effects of BZDs immediately. Flumazenil is metabolized rapidly, with a half-life of 1 hour but a clinical duration of effect often less than 30 minutes; therefore, situations requiring prolonged antagonism may necessitate a continuous flumazenil infusion. Diagnostically, flumazenil has been used to differentiate between BZD-induced unresponsiveness and other forms of CNS pathology. Flumazenil is relatively contraindicated in patients with known BZD dependence and chronic use, because acute withdrawal symptoms and seizures have been reported in these patients.41
Propofol and Fospropofol
Propofol (2,6-diisopropylphenol) was initially introduced in 1982 as an induction agent for general anesthesia. Over the past 20 years, several other useful indications have been identified for this agent. In addition to being an anxiolytic/sedative/hypnotic, propofol has antiemetic, antipruritic, anticonvulsant, bronchodilatory, muscle relaxant, and possibly antiinflammatory and antiplatelet effects.52 Propofol has been shown to improve outcome in patients with traumatic brain injury, possibly because of decreases in cerebral metabolism and intracranial pressure.53 Its anxiolytic properties are thought to result from activation of GABAA receptors within the CNS. Unlike BZDs, propofol does not exert synergistic sedative effects when administered with opioids, and propofol may not produce an amnestic effect equivalent to that of BZDs.54 Because of its high lipophilicity and short half-life, propofol has a rapid onset of action (1-2 minutes) and a short duration of action (10-15 minutes). For patients receiving propofol infusions for longer than 72 hours, the wake-up time can extend to 30 to 60 minutes. The pharmacokinetic profile of propofol is best described by a three-compartment model with an elimination half-life of 30 to 60 minutes. Propofol has a volume of distribution of 600 to 800 L, suggesting that the drug is rapidly cleared from the central compartment into fatty tissues, and elimination is not appreciably altered by hepatic or renal failure. For these reasons, an IV infusion of propofol can be predictably titrated from light sedation to a deeper hypnotic state for patients who require varying levels of sedation throughout the day. Simply stopping the infusion can reverse the sedative effects, usually within 1 hour and often within 15 minutes. The 2002 Practice Guidelines recommend propofol as the sedative of choice when rapid awakening is important.38
Propofol is available as a 1% oil-in-water emulsion that provides 1.1 kcal/mL from fat. To reduce the possibility of fat overload and hypertriglyceridemia in critically ill patients, the lipid contribution from a propofol infusion should be counted as a calorie source in the daily nutritional plan. Patients receiving propofol infusions for longer than 2 days should have their serum triglycerides monitored.38
Reports of infections in patients receiving propofol prompted the addition of 0.005% ethylenediaminetetraacetic acid (EDTA) to retard bacterial growth. A generic propofol formulation (Gensia Sicor Pharmaceuticals, Irvine, California) is also available that contains sodium metabisulfite (0.025%) as a preservative and has a lower pH than the EDTA formulation; individuals who are sensitive to sulfites should not receive this product. Although the U.S. Food and Drug Administration (FDA) considers these products to be bioequivalent and interchangeable (i.e., AB rated), reports suggest that the generic emulsion is less stable physiochemically and more conducive to microbial growth.40,55 Owing to quality problems with these products, Fresenius Propoven 1% (APP Pharmaceuticals, Schaumburg, Illinois [a company of the Fresenius Kabi Group, Bad Homburg, Germany]) was imported into the United States in 2009 to address a shortage of propofol. Fresenius propofol contains no antimicrobial retardant; each vial is a single-use container that should be discarded after 6 hours. Caregivers should adhere to strict aseptic technique and administer propofol through a dedicated IV line to avoid drug incompatibility problems. Nurses should change the bottles and tubing every 12 hours to minimize the risk of bacterial contamination.56
Propofol infusion syndrome (PRIS) is a rare but potentially lethal complication manifested by severe metabolic acidosis, rhabdomyolysis, renal failure, dysrhythmias, and cardiac arrest.57,58 Because of this risk, propofol is not recommended by the FDA for prolonged sedation of pediatric patients, and it should be used cautiously in adults who develop unexplained metabolic acidosis or cardiac arrhythmias. Caution should be used when propofol is infused for more than 48 hours at dosages above 5 mg/kg/h, particularly in patients with neurologic or inflammatory illnesses.59 Alternative sedative agents should be considered for patients receiving high-dose propofol and for those who require vasopressors or cardiac inotropes.38
Comparing the quality of short-term (<24 hours) sedation of cardiac surgery patients, two trials favored propofol over midazolam, and seven reported no difference. Time to extubation after sedative cessation was shorter for patients receiving propofol than for those receiving midazolam in five of eight studies, but the overall duration of mechanical ventilation was equivalent in six of seven studies. In surgical or mixed medical/surgical ICUs, three of six trials reported that the quality of sedation was better with propofol, whereas the other three trials found no difference. Time to extubation was less with propofol than with midazolam in all studies assessing this endpoint.60,61 Hypotension was more frequent with propofol.54,62
Fourteen surgical or mixed medical/surgical ICU studies have compared the use of sedative drugs for longer than 24 hours. The quality of sedation was comparable between propofol and midazolam in half of the studies. Midazolam was preferred over propofol in one study, and propofol was superior in two studies. In all four trials reporting time to extubation, the group receiving propofol was extubated sooner after sedation cessation than the midazolam group.54,60,61,63 Therefore, based on the best scientific evidence, propofol is at least as effective as midazolam in sedation quality and is associated with a shorter time to extubation for patients receiving short- or long-term sedation. Propofol is also associated with more hypotension and higher drug costs than midazolam.
Fospropofol (Lusedra) is a water-soluble prodrug of propofol that was recently approved for monitored anesthesia care sedation in adult patients for diagnostic or therapeutic procedures. Because fospropofol is hydrolyzed by circulating alkaline phosphatases to propofol, formaldehyde, and phosphate, its time to onset is prolonged (4-13 minutes). The most common side effects (>20%) reported are paresthesias and pruritus; these adverse effects are seen at all dosage ranges and are thought to be the result of the phosphate ester component. Because clinical experience with fospropofol is limited to studies in relatively healthy patients undergoing colonoscopy or bronchoscopy, the safety and efficacy of long-term infusions in critically ill patients is unknown. Publication of studies in coronary artery surgery and in mechanically ventilated patients may clarify the role of this non–lipid-based sedative-hypnotic agent in the ICU.64
Central α2-Adrenoreceptor Agonists
DEX offers several advantages as a sedative in the ICU. First, DEX does not cause significant respiratory depression, and it may be the ideal choice for patients nearing extubation who still require light sedation. DEX has a rapid distribution phase (6 minutes) and an elimination half-life of 2 hours. These pharmacokinetic properties permit easy dose titration in response to fluctuating sedative needs. Another advantage is the low level of sedation that can be achieved with DEX. Patients appear comfortably sedated while undisturbed but can easily be awakened.40 Current research is evaluating the feasibility and benefits of patient-controlled sedation with DEX.65
A trial comparing DEX with propofol infusion found equivalent sedation, no difference in arterial pressure, and a similar time interval from cessation of sedation infusion to extubation. Patients in the DEX group required less adjunctive opioid analgesia than patients receiving propofol, and patients receiving DEX were easily aroused for evaluation.61 Another study also documented a reduction in morphine doses by 50% when patients were treated with DEX.40 However, in short-term studies of mild to moderate sedation in healthy volunteers, DEX did not demonstrate analgesic effects against heat or electrically generated pain.66
Recent comparative studies of DEX versus BZD infusions have identified several advantages to selecting DEX for ICU sedation. The Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) trial enrolled medical and surgical ICU patients. Patients randomized to DEX exhibited 4 more days alive without delirium and coma than the patients who received lorazepam. The DEX patient group also spent more time at the targeted level of sedation. These benefits were attained with comparable pharmacy, ICU, and hospital costs.67 In the Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) study, there was no difference between the drugs in time at targeted sedation level, but the DEX-treated patients spent less time on the ventilator and experienced less delirium than patients who received midazolam. In both groups, sedatives were titrated to comparable levels of light sedation.68
When amnesia is crucial, DEX should be combined with low doses of a BZD. DEX also has been used successfully to ameliorate the hyperadrenergic state of drug withdrawal following cessation of alcohol, illicit drug, or long-term sedative-analgesic use in the ICU.69
Haloperidol
High doses of haloperidol (>400 mg/d) have been associated with QTC prolongation and an increased risk of ventricular arrhythmias, including torsades de pointes; therefore, patients receiving haloperidol should be electrocardiographically monitored. Extrapyramidal symptoms can occur that require neuroleptic discontinuation and treatment with diphenhydramine or benztropine.38 Newer atypical antipsychotics (e.g., risperidone, quetiapine, ziprasidone) offer potential safety advantages with fewer cardiovascular and extrapyramidal effects than haloperidol. Further research is needed to make firm recommendations on the comparative efficacy or safety of antipsychotic therapy for delirium.
 Optimizing Sedation at the Bedside
Optimizing Sedation at the Bedside
Most “sedation scales” are observer-rated assessments of level of consciousness and agitation.2 More comprehensive scales may assess additional domains such as pain, anxiety, or ventilator synchrony, but multidomain instruments can become unwieldy if documentation requirements are excessive. Scales can report domain scores separately or combine two domains in a single choice scale, thereby assuming the activity in one domain precludes activity in the other domain, which is not always the case. For instance, agitation can occur in the presence of decreased level of consciousness. Most consciousness scales use a graded stimulation protocol to obtain a standard patient response such as eye opening. Rating agitation is more ambiguous because patient behaviors (e.g., excessive motor activity, pulling at tubes, striking at staff) are variably graded on intensity, frequency, or probability that the agitation will cause immediate adverse consequences. How “agitated” is an otherwise calm patient who is slowly pulling on his or her endotracheal tube? Sedation scales such as the modified Ramsay sedation scale (RSS), Richmond Agitation and Sedation Scale (RASS), Sedation Agitation Scale (SAS), and Motor Activity and Assessment Scale (MAAS) are similarly constructed and scored, have excellent inter-rater reliability, and have been validated by correlation with other scales, physiologic variables, or medication exposure. The Vancouver Interaction and Calmness Scale (VICS) differs from other sedation scales because it is a summated rating scale that reports two domain scores separately.70 As such, VICS is more responsive to subtle changes in a patient’s condition, but it takes more time to complete. The clinical benefit of documenting or targeting very precisely defined sedation states is unknown and may be impractical. In 15 clinical trials characterized by close attention to achieving and maintaining a predetermined sedation target (usually with the RSS), patients were at the sedation target, on average, only 68% of the time.61
Even if sedation effects are reliably measured, determining the optimal sedation state of an ICU patient is based more on clinical opinion than scientific evidence. Titration of medications to achieve a condition such as “lightly asleep but easily arousable” or “calm and cooperative” appears sensible, but a survey of intensivists asked to choose an appropriate sedation level for a patient with severe hypoxemia yielded a remarkably wide range of responses from unresponsive to awake.71 Sedation targets for clinical trials are also highly variable; in 19 trials using the 6-level RSS, the target sedation level was defined variously as 3, 5, 2-3, 2-4, 2-5, 3-4, or 4-5.61
Monitoring cortical electrical activity to indicate sedation intensity has long been a goal of intensivists. Multichannel EEG monitoring is the gold standard for evaluating cortical activity, but interpretation remains predominantly qualitative and requires specialized training. Researchers have developed numerous signal-processing algorithms to convert limited EEG data into simpler quantitative output. The Bispectral Index (BIS) algorithm has been one of the most widely studied and yields a score of 0 (isoelectric, no cortical function) to 100 (fully awake). Initially developed to assess the depth of hypnosis during short-term general anesthesia, BIS is also used to monitor long-term ICU sedation.72 However, studies have identified problems that have slowed the acceptance of this promising technology. First, spuriously high readings (e.g., readings indicating greater wakefulness than actually exists) can result from muscle activity in nonparalyzed patients.73,74 Although new electronic filters suppress myographic signals, there can be substantial variability of output even in stable pharmacologically paralyzed patients.75 Clinicians using BIS should assess trends in BIS output and integrate other clinical data before making an intervention. Second, there is little evidence that BIS monitoring of general ICU patients has advantages over routine sedation assessment using observer-rated scales. BIS technology is superior in specialized situations (e.g., pharmacologically-induced muscle paralysis) when stimulus-response sedation assessment is inadequate. For instance, medicating to a BIS score lower than about 60 makes awareness and recall unlikely. Similarly, sedation scales cannot score below a “floor” level in which patients exhibit no motor response to painful stimuli, but BIS can distinguish between levels of deep sedation. For instance, BIS scores of 55 and 35, respectively, in two patients who both score at the lowest level of a standard sedation scale suggest that the latter patient has greater suppression of cortical activity. If there is no clinical reason for maintaining the patient at 35, sedatives could be decreased to allow the BIS to rise. This process of identifying excessively sedated patients might shorten wake-up time and lead to faster weaning; however, this putative advantage has not yet been demonstrated in large ICU studies. Third, the BIS algorithm was designed to correlate with hypnosis (arousability) and recall, but it is not a pain, dyspnea, or anxiety monitor. Postoperative studies have shown that opiate-induced hypnosis can occur before analgesic effects, and seemingly sedated patients can have significant pain when awakened.76
Until recently, clinical research in ICU sedation has focused on investigating changes in acute physiology after medication administration or conducting head-to-head medication trials. Several studies suggest, however, that the method of administering sedatives is as important as the specific drug given to patients. In one study, duration of mechanical ventilation was decreased by more than 50% in ventilated patients who were sedated with a protocol that linked medication dosing to a specified sedation level, compared to patients who were treated without a sedation protocol.77 The marked decrease in ventilator time was attributable to the protocol, which decreased infusion rates when patients were at target sedation level (RSS 3), thereby minimizing the time during which patients were receiving continuous medication infusions. In another study, a protocol used continuous infusions (midazolam or propofol) but stopped the infusions daily, restarting them (at half the rate) only after patients became awake.28 Compared with a group that did not have this “stop” intervention, the experimental group used less midazolam but similar amounts of propofol. Nevertheless, in both midazolam and propofol subgroups, the daily interruption of infusions increased the number of days patients were awake, decreased the duration of mechanical ventilation by 2.4 days, and decreased the number of diagnostic tests performed to assess abnormal mental status. Since 2000, numerous studies using randomized or quasi-experimental designs have shown that algorithm-directed sedation is safe and leads to desirable reductions in ventilator time. These studies also support the view that algorithm-directed sedation often decreases ICU length of stay. Furthermore, when administration of sedatives is carried out using an algorithmic approach, patients are usually more awake yet comfortable. Combining sedative protocols with ventilator weaning protocols gives patients, on average, three extra days alive and off mechanical ventilation compared to patients assigned to non-protocolized sedation.78
Patients with prolonged ICU stays may be treated with high doses of sedative-analgesic medications for weeks. There is growing evidence that tolerance to opiates, BZDs, and propofol can develop in less than 1 week and that abstinence or withdrawal symptoms can occur if sedative doses are reduced too rapidly.79 Withdrawal symptoms of anxiety, agitation, gastrointestinal dysfunction, and tachycardia are nonspecific, and intubated patients have difficulty communicating symptoms to caregivers. Because of the altered pharmacokinetics for many drugs in critically ill patients and the difficulty in identifying withdrawal syndromes, there are few data to guide clinicians for prescribing tapering regimens when withdrawal symptoms are suspected. Logical interventions for patients who have been on prolonged courses of sedative medications include converting continuous infusions to scheduled doses, using longer-acting medications within the same pharmacologic class, reducing the total daily dose by 10% per day, changing the IV route to enteral, and adding an α2-adrenoreceptor agonist such as clonidine.80
 Pharmacoeconomics of Sedatives Used in the Intensive Care Unit
Pharmacoeconomics of Sedatives Used in the Intensive Care Unit
New drugs cost more than available generic formulations. One comparison of lorazepam, midazolam, and propofol in critically ill trauma patients found lorazepam to be the best choice for continuous sedation, based on 1995 acquisition costs; however, lorazepam acquisition costs were lower because of an available generic formulation.81 Because the time to extubation was shorter with propofol than with midazolam, overall costs were lower with propofol in a Spanish study, even though propofol acquisition costs were three times higher than those for midazolam.63 Other investigators have compared quality of sedation, safety, and costs of propofol versus midazolam during short-to-medium and long-term sedation, with similar results. Propofol provides comparable sedation safety and efficacy at lower healthcare costs due to earlier extubation and shorter ICU stays.60 Propofol and midazolam are now both available generically at a lower cost.
Economic analysis must consider dynamic pricing of drugs within the United States and throughout the world. Acquisition costs of drugs represent only one piece of the decision-making process. In the analysis of sedative agents, adequacy of sedation, time to extubation, and time to ICU discharge are important endpoints. Preventable adverse drug effects (e.g., delirium) and increased use of diagnostic and therapeutic resources also affect total hospital costs. The SEDCOM study showed that DEX and midazolam are equally effective for attaining the targeted sedation level, but DEX-treated patients spent less time on the ventilator, developed less tachycardia and hypertension, and experienced less delirium.68 A recent pharmacoeconomic analysis of the SEDCOM study evaluated the post-randomization cost of ICU care and found that sedation with DEX reduced ICU care costs ($9679 in cost savings) compared to sedation with midazolam.82 Another economic evaluation reported overall lower costs ($6378 in cost savings) and a greater number of ventilator-free days when continuous propofol was used for sedation compared to intermittent lorazepam in the setting of daily sedative interruption.83 Health-related quality of life and post-ICU long-term consequences of ICU sedation should be incorporated into future pharmacoeconomic analysis.
Implementation of clinical practice guidelines can improve outcomes and lower costs. One institution reduced direct drug costs, ventilator time, and length of stay after implementing interdisciplinary sedation guidelines.84 The ABC trial that paired spontaneous awakening trials (e.g., daily interruption of sedatives) with spontaneous breathing trials reported better outcomes (fewer ventilator days, earlier discharge from ICU, and lower mortality) with the intensive protocols than standard practice.78 Evidence-based protocols and practice guidelines should be accompanied by interdisciplinary collaboration and education to ensure that they are positioned as guides, not rigid rules that replace clinical judgment.
 Toxic Ingestion of Sedative-Hypnotics
Toxic Ingestion of Sedative-Hypnotics
Of the sedative medications discussed in this chapter, BZDs and opiates are most likely to be involved in toxic ingestions, either accidental or intended. Patients with overdoses from either medication class can present with stupor or coma with hypotension (usually mild and responsive to fluid boluses) and hypotonia. Pupil size may be helpful: pupils are pinpoint in cases of opiate ingestion, midsize in cases of BZD toxicity. Toxicity is usually short lived and completely reversible unless complications such as anoxic encephalopathy or aspiration pneumonia occur. General principles of toxic ingestion management are paramount: assume the patient has a polydrug ingestion until conclusive data are obtained; ensure adequate ventilation and airway protection; avoid gastric lavage unless the time of ingestion is very recent; and give activated charcoal for oral ingestions.85
Flumazenil is a specific antidote for BZD toxicity. A patient’s symptoms should improve within a minute after a bolus administration of 0.2 mg and subsequent 0.3-mg doses every 30 seconds. Administration of flumazenil to patients receiving chronic BZD therapy may precipitate an unpleasant acute withdrawal syndrome and (theoretically) increase the risk of seizures.85 However, no seizures were observed after flumazenil treatment in 110 patients with suspected BZD overdose, including many patients with polydrug ingestions (e.g., co-ingestion of tricyclic antidepressants).86
Key Points
Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
Sessler CN, Pedram S. Protocolized and target-based sedation and analgesia in the ICU. Crit Care Clin. 2009;25:489-513.
Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial); a randomized controlled trial. Lancet. 2008;371:126-134.
Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients. JAMA. 2009;301:489-499.
1 Sun X, Weissman C. The use of analgesics and sedatives in critically ill patients: physicians’ orders versus medications administered. Heart Lung. 1994;23(2):169-176.
2 De Jonghe B, et al. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med. 2000;26(3):275-285.
3 Arroliga A, Frutos-Vivar F, Hall J, Esteban A, Apezteguia C, Soto L, et al. Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest. 2005;128:496-506.
4 Arroliga AC, et al. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2008;36(4):1083-1088.
5 Wunsch H, et al. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009;37(12):3031-3039.
6 Kollef MH, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541-548.
7 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301-1308.
8 Kahn JM, Andersson L, Karir V, Polissar NL, Neff MJ, Rubenfeld GD. Low tidal volume ventilation does not increase sedation use in patients with acute lung injury. Crit Care Med. 2005;33:766-771.
9 Wolthuis E, Veelo DP, Choi G, Determann R, Korevaar J, Spronk P, et al. Mechanical ventilation with lower tidal volumes does not influence the prescription of opioids or sedatives. Critical Care. 2007;11(4):R77.
10 Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475-480.
11 Ely EW, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289(22):2983-2991.
12 Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Crit Care Med. 2007;35(2):393-401.
13 Bergbom-Engberg I, Haljamae H. Assessment of patients’ experience of discomforts during respirator therapy. Crit Care Med. 1989;17(10):1068-1072.
14 Rotondi AJ, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746-752.
15 Stein-Parbury J, McKinley S. Patients’ experiences of being in an intensive care unit: a select literature review. Am J Crit Care. 2000;9(1):20-27.
16 Ely EW, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
17 Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-26.
18 Fraser GL, et al. Frequency, severity, and treatment of agitation in young versus elderly patients in the ICU. Pharmacotherapy. 2000;20(1):75-82.
19 Chlan L, Savik K, Weinert C. Development of a shortened state anxiety scale from the Spielberger State-Trait Anxiety Inventory (STAI) for patients receiving mechanical ventilatory support. J Nurs Meas. 2003;11(3):283-293.
20 Cooper AB, et al. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117(3):809-818.
21 Friese RS. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Crit Care Med. 2008;36(3):697-705.
22 Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001;7(1):21-27.
23 McLeod G, et al. Use of 2% propofol to produce diurnal sedation in critically ill patients. Intensive Care Med. 1997;23(4):428-434.
24 Shilo L, et al. Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int. 2000;17(1):71-76.
25 Esteban A, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161(5):1450-1458.
26 Sydow M, et al. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med. 1994;149(6):1550-1556.
27 Weinert CR, Chlan L, Gross C. Sedating critically ill patients: factors affecting nurses’ delivery of sedative therapy. Am J Crit Care. 2001;10(3):156-165. quiz 166-7
28 Kress JP, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471-1477.
29 Esteban A, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345-355.
30 Happ MB. Treatment interference in acutely and critically ill adults. Am J Crit Care. 1998;7(3):224-235.
31 Wagner BK, O’Hara DA, Hammond JS. Drugs for amnesia in the ICU. Am J Crit Care. 1997;6(3):192-201. quiz 202-3
32 Schelling G, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26(4):651-659.
33 Weinert C, Sprenkle M. Post-ICU consequences of patient wakefulmess and sedative exposure during mechanical ventilation. Intensive Care Med. 2008;34:82-90.
34 Jones C, et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29(3):573-580.
35 Dasta JF, Fuhrman TM, McCandles C. Patterns of prescribing and administering drugs for agitation and pain in patients in a surgical intensive care unit. Crit Care Med. 1994;22(6):974-980.
36 Watling SM, Dasta JF, Seidl EC. Sedatives, analgesics, and paralytics in the ICU. Ann Pharmacother. 1997;31(2):148-153.
37 Soliman HM, Melot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth. 2001;87(2):186-192.
38 Jacobi J, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119-141.
39 Clotz MA, Nahata MC. Clinical uses of fentanyl, sufentanil, and alfentanil. Clin Pharm. 1991;10(8):581-593.
40 Dasta JF. ICU sedative pharmacology update: A review of commonly used and emerging agents. Crit Care Med. 2002;30:S104-S110.
41 Young CC, Prielipp RC. Benzodiazepines in the intensive care unit. Crit Care Clin. 2001;17(4):843-862.
42 Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52(4):349-355.
43 Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15(6):469-494.
44 Barr J, et al. A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95(2):286-298.
45 Laine GA, et al. Polyethylene glycol nephrotoxicity secondary to prolonged high-dose intravenous lorazepam. Ann Pharmacother. 1995;29(11):1110-1114.
46 Cawley MJ. Short-term lorazepam infusion and concern for propylene glycol toxicity: case report and review. Pharmacotherapy. 2001;21(9):1140-1144.
47 Seay RE, Graves PJ, Wilkin MK. Comment: possible toxicity from propylene glycol in lorazepam infusion. Ann Pharmacother. 1997;31(5):647-648.
48 Volles DF. More on usability of lorazepam admixtures for continuous infusion. Am J Health Syst Pharm. 1996;53(22):2753-2754.
49 Pohlman AS, Simpson KP, Hall JB. Continuous intravenous infusions of lorazepam versus midazolam for sedation during mechanical ventilatory support: a prospective, randomized study. Crit Care Med. 1994;22(8):1241-1247.
50 Cernaianu AC, et al. Lorazepam and midazolam in the intensive care unit: a randomized, prospective, multicenter study of hemodynamics, oxygen transport, efficacy, and cost. Crit Care Med. 1996;24(2):222-228.
51 Swart EL, et al. Continuous infusion of lorazepam versus medazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27(8):1461-1465.
52 Borgeat A, Wilder-Smith OH, Suter PM. The nonhypnotic therapeutic applications of propofol. Anesthesiology. 1994;80(3):642-656.
53 Kelly DF, et al. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. J Neurosurg. 1999;90(6):1042-1052.
54 Weinbroum AA, et al. Midazolam versus propofol for long-term sedation in the ICU: a randomized prospective comparison. Intensive Care Med. 1997;23(12):1258-1263.
55 Redhead HM, Jones CB, Bobey DG. Pharmaceutical and antimicrobial differences between propofol emulsion products. Am J Health Syst Pharm. 2000;57(12):1174. 1176-7
56 Pelletier A. Urgent: Propofol Update. Professional. H. Schaumburg, IL: APP Pharmaceuticals; 2010:1-5.
57 Ernest D, French C. Propofol infusion syndrome–report of an adult fatality. Anaesth Intensive Care. 2003;31(3):316-319.
58 Perrier ND, Baerga-Varela Y, Murray MJ. Death related to propofol use in an adult patient. Crit Care Med. 2000;28(8):3071-3074.
59 Vasile B, et al. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29(9):1417-1425.
60 Carrasco G, et al. Propofol vs midazolam in short-, medium-, and long-term sedation of critically ill patients. A cost-benefit analysis. Chest. 1993;103(2):557-564.
61 Ostermann ME, et al. Sedation in the intensive care unit: a systematic review. JAMA. 2000;283(11):1451-1459.
62 Wahr JA, et al. Cardiovascular responses during sedation after coronary revascularization. Incidence of myocardial ischemia and hemodynamic episodes with propofol versus midazolam. Institutions of the McSPI Research Group. Anesthesiology. 1996;84(6):1350-1360.
63 Barrientos-Vega R, et al. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997;25(1):33-40.
64 Moore GD, Walker AM, MacLaren R. Fospropofol: a new sedative-hypnotic agent for monitored anesthesia care. Ann Pharmacother. 2009;43(11):1802-1808.
65 Chlan LL, Weinert CR, Skaar DJ, et al. Patient-controlled sedation: a novel approach to management of sedative therapy with mechanically ventilated patients. Chest. 2010;138(5):1045-1053.
66 Angst MS, et al. Comparative analgesic and mental effects of increasing plasma concentrations of dexmedetomidine and alfentanil in humans. Anesthesiology. 2004;101(3):744-752.
67 Pandharipande PP, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644-2653.
68 Riker RR, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489-499.
69 Maccioli GA. Dexmedetomidine to facilitate drug withdrawal. Anesthesiology. 2003;98(2):575-577.
70 de Lemos J, Tweeddale M, Chittock D. Measuring quality of sedation in adult mechanically ventilated critically ill patients. the Vancouver Interaction and Calmness Scale. Sedation Focus Group. J Clin Epidemiol. 2000;53(9):908-919.
71 Christensen BV, Thunedborg LP. Use of sedatives, analgesics and neuromuscular blocking agents in Danish ICUs 1996/97. A national survey. Intensive Care Med. 1999;25(2):186-191.
72 Simmons LE, et al. Assessing sedation during intensive care unit mechanical ventilation with the Bispectral Index and the Sedation-Agitation Scale. Crit Care Med. 1999;27(8):1499-1504.
73 Riker RR, et al. Validating the Sedation-Agitation Scale with the Bispectral Index and Visual Analog Scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27(5):853-858.
74 Vivien B, et al. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99(1):9-17.
75 Dasta JF, et al. Bispectral Index in the intensive care setting. Crit Care Med. 2003;31(3):998. author reply 998-9
76 Paqueron X, et al. Is morphine-induced sedation synonymous with analgesia during intravenous morphine titration? Br J Anaesth. 2002;89(5):697-701.
77 Brook AD, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609-2615.
78 Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126-134.
79 Cammarano WB, et al. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med. 1998;26(4):676-684.
80 Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28(6):2122-2132.
81 McCollam JS, et al. Continuous infusions of lorazepam, midazolam, and propofol for sedation of the critically ill surgery trauma patient: a prospective, randomized comparison. Crit Care Med. 1999;27(11):2454-2458.
82 Dasta JF, et al. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38(2):497-503.
83 Cox CE, et al. Economic evaluation of propofol and lorazepam for critically ill patients undergoing mechanical ventilation. Crit Care Med. 2008;36(3):706-714.
84 Mascia MF, Koch M, Medicis JJ. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000;28(7):2300-2306.
85 Mokhlesi B, et al. Adult toxicology in critical care: Part II: specific poisonings. Chest. 2003;123(3):897-922.
86 Weinbroum A, et al. Use of flumazenil in the treatment of drug overdose: a double-blind and open clinical study in 110 patients. Crit Care Med. 1996;24(2):199-206.
87 Kress JP, et al. Sedation of critically ill patients during mechanical ventilation. A comparison of propofol and midazolam. Am J Respir Crit Care Med. 1996;153(3):1012-1018.