Sedative Hypnotics
Barbiturates
Barbiturates are discussed in do-it-yourself suicide manuals and were implicated in the high-profile deaths of Marilyn Monroe, Jimi Hendrix, Abbie Hoffman, and Margaux Hemingway as well as in the mass suicide of 39 members of the Heaven’s Gate cult in 1997. Although barbiturates are still useful for seizure disorders, they rarely are prescribed as sedatives, with the availability of safer alternatives, such as benzodiazepines. Mortality from barbiturate poisoning declined from approximately 1500 deaths per year in the 1950s to only two fatalities in 2009.1
Principles of Disease
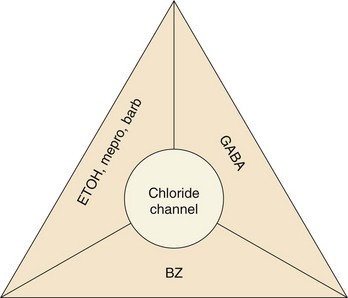
The GABAA receptor is a protein complex found on postsynaptic membranes in the CNS. Structurally, it consists of several distinct receptor sites surrounding a chloride ion (Cl−) channel (Fig. 165-1). GABA opens the chloride channel. The resulting flow of Cl− into the cell increases the negative resting potential, hyperpolarizing and stabilizing the membrane. There are separate receptor sites for barbiturates and for benzodiazepines and a third site that binds GABA, ethanol, and meprobamate. Although barbiturates and ethanol can directly increase Cl− conductance, benzodiazepines require the presence of GABA to affect Cl− flow, which may account for the relative safety of benzodiazepines in comparison with barbiturates.
Barbiturates are classified according to their onset and duration of action (Box 165-1): ultra-short acting (onset immediate after intravenous dose, duration minutes), short acting (onset 10-15 minutes after oral dose, duration 6-8 hours), intermediate acting (onset 45-60 minutes, duration 10-12 hours), and long acting (onset 1 hour, duration 10-12 hours). Only long-acting preparations have anticonvulsant effects in doses that do not cause sedation. Short- and intermediate-acting preparations are almost completely metabolized to inactive metabolites in the liver, whereas 25% of a phenobarbital (long-acting) dose is excreted unchanged through the kidney. Because phenobarbital is a weak acid (pKa 7.2), alkalinization of the urine will increase the amount of drug present in ionized form, minimizing tubular reabsorption and increasing drug clearance. Short- and intermediate-acting barbiturates are not significantly affected by pH changes in this range.
Management
Gastrointestinal Decontamination and Enhanced Elimination
Gastric emptying by lavage is not indicated. For large overdoses, there is evidence that clearance of phenobarbital is markedly increased with multidose activated charcoal (MDAC).2 One dosage for MDAC is 25 g every 2 hours in an adult; the pediatric dose is 0.5 g/kg every 2 hours. If vomiting occurs, a smaller dose or antiemetics should be used. MDAC can also be administered slowly through a nasogastric tube. Contraindications to MDAC include an unprotected airway and gastrointestinal obstruction or perforation. Decreased peristalsis can result in constipation with MDAC and is a relative contraindication to MDAC.2 Although MDAC may shorten the duration of the intoxication, there is no evidence for improved outcome over supportive care, and supportive care without administration of activated charcoal is also an acceptable approach.
Although alkalinization of the urine with sodium bicarbonate has been recommended in the past, a nonrandomized study suggested that MDAC alone is most effective at increasing the drug’s clearance.3 The authors of that study hypothesize that alkalinization may interfere with the ability of the drug to diffuse across intestinal mucosa from the blood into the gut. A recent comprehensive review concluded that there is no role for urine alkalinization in acute barbiturate poisoning.4
Hemodialysis is rarely needed but may increase clearance of phenobarbital in the presence of renal or cardiac failure, acid-base or electrolyte abnormalities, unstable cardiorespiratory status, or inadequate response to less invasive measures. Because phenobarbital is 40 to 60% protein bound, hemoperfusion was advocated over hemodialysis; however, newer high-efficiency dialyzers using high blood flow rates provide drug clearance greater than that achieved by hemoperfusion.5 Unfortunately, there are insufficient data to determine the true risk-benefit ratio of hemodialysis in acute barbiturate overdose.4
Benzodiazepines
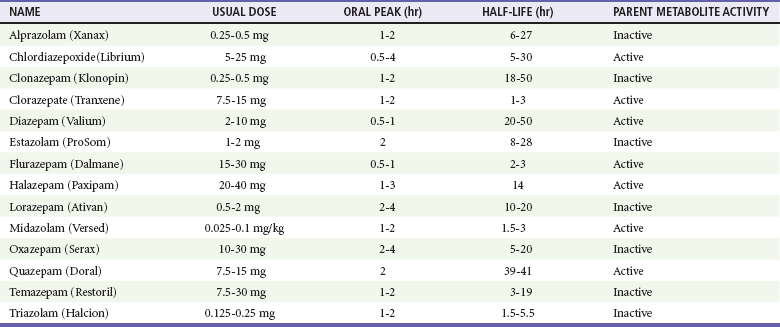
Benzodiazepines remain among the most widely prescribed class of drugs (Table 165-1) and are the most common prescription drugs used in suicide attempts. Fortunately, most benzodiazepine overdoses follow a relatively benign clinical course. Children make up 10% of benzodiazepine overdose cases.
Pharmacokinetics
Benzodiazepines are rapidly absorbed orally. Intramuscular use of chlordiazepoxide and diazepam is limited by erratic absorption, but lorazepam and midazolam are predictably absorbed after intramuscular injection. After absorption, benzodiazepines distribute readily and rapidly penetrate the blood-brain barrier. In plasma, benzodiazepines are highly protein bound.6
All benzodiazepines are metabolized in the liver. Oxazepam, temazepam, and lorazepam are directly conjugated to an inactive, water-soluble glucuronide metabolite that is excreted by the kidney. Other benzodiazepines must first be converted by the hepatic cytochrome P450 system. Chlordiazepoxide, diazepam, flurazepam, and clorazepate are metabolized to active derivatives that are then slowly conjugated and excreted. The long elimination half-lives of these intermediates can cause accumulation in the body with repeated dosing. Triazolam, alprazolam, and midazolam are converted to hydroxylated intermediates that are active, but because they are so rapidly conjugated and excreted, they do not contribute significantly to the drug’s overall effect.6
Cytochrome P450 processes may be significantly impaired in elderly patients or those with liver disease, leading to prolonged elimination of some benzodiazepines. Coingestion of drugs that also undergo cytochrome P450 metabolism (e.g., cimetidine, ethanol) also prolongs the half-lives of these benzodiazepines, but the clinical significance of these interactions is unclear.7
Clinical Features
CNS depression is common in patients with benzodiazepine poisoning and ranges from mild drowsiness to coma. Respiratory depression is due mainly to upper airway obstruction and increased upper airway resistance from loss of muscle tone rather than central apnea. Significant respiratory depression is rare but can be seen with large oral overdoses or during intravenous conscious sedation, particularly when the benzodiazepine is combined with an opioid such as fentanyl.7 Hypotension is uncommon. Other potential complications include aspiration pneumonia and pressure necrosis of skin and muscles.
Prolonged or high-dose infusions of certain benzodiazepine preparations have been associated with the development of lactic acidosis. Metabolism of the propylene glycol diluent in diazepam and lorazepam intravenous solutions by alcohol dehydrogenase produces lactate, which can accumulate and cause acidosis severe enough to require intervention. Patients with renal or hepatic insufficiency are at increased risk for this complication.8
Diagnostic Strategies
Any patient with altered mental status should have a blood glucose level rapidly determined. Qualitative immunoassays for benzodiazepines in urine are available but do not aid management decisions. Most of these tests detect only benzodiazepines that are metabolized to oxazepam glucuronide; therefore, clonazepam, lorazepam, midazolam, and alprazolam are not detected on many urine drug screens.9 Serum drug concentrations are not routinely available and do not correlate with clinical severity. A lack of alcohol odor or a negative breathalyzer or blood ethanol test result suggests benzodiazepine or another sedative as a possible cause.
The benzodiazepine antagonist flumazenil should not be routinely administered to patients with coma of unknown origin or suspected benzodiazepine overdose, either for diagnostic or for therapeutic purposes.10 Any possibility of concomitant tricyclic overdose contraindicates flumazenil use.
Management
Initial stabilization, including endotracheal intubation, should not be delayed by the administration of an antidote. Most benzodiazepine overdoses can be managed expectantly. Activated charcoal is generally not beneficial in overdose.11 MDAC, hemodialysis, and whole-bowel irrigation are not effective in benzodiazepine overdose.
Antidote
Flumazenil, a nonspecific competitive antagonist of the benzodiazepine receptor, can reverse benzodiazepine-induced sedation after general anesthesia, procedural sedation, and confirmed benzodiazepine overdose, but it is not recommended for the routine reversal of sedative overdose in the ED. Although theoretic benefits of flumazenil use include cost savings and avoidance of procedures and tests such as endotracheal intubation and lumbar puncture, several studies have not been able to demonstrate an actual benefit.12 Seizures and cardiac dysrhythmias can occur after flumazenil administration, and fatalities have been reported. Flumazenil use can precipitate acute withdrawal in patients who are dependent on benzodiazepines. Similarly, this antidote is hazardous when it is given to patients who have coingested seizure-causing drugs (such as cocaine or a tricyclic antidepressant) because of loss of the benzodiazepine’s protective anticonvulsant properties. Coingestants that cause dysrhythmias, such as carbamazepine and chloral hydrate, may increase the likelihood of cardiac effects. Other risk factors are summarized in Box 165-2. One study found that 12% of patients receiving flumazenil after known pure or mixed benzodiazepine overdose actually had a contraindication to its use.13
In summary, benzodiazepine overdose requires primarily supportive care (including, in some cases, intubation). Flumazenil may precipitate seizures or acute withdrawal and should be used only in highly selected cases, such as small children with accidental poisoning or for accidental overdose of benzodiazepines during procedural sedation. When flumazenil is used, careful monitoring is necessary because of the risk for recurrent respiratory depression or resedation. Use of flumazenil has not consistently altered outcome, complication rate, number of costly procedures performed, or duration of hospital stay in ED patients.13
Benzodiazepine Withdrawal Syndrome
Abrupt discontinuation of a benzodiazepine in a chronic user results in a characteristic constellation of symptoms similar to ethanol withdrawal (Box 165-3). Risk for withdrawal is a function of both the dose of benzodiazepine and the duration of its use. Continuous treatment for more than 4 months is generally required before a patient is at risk for withdrawal. With abrupt discontinuation of a benzodiazepine, the most severe withdrawal symptoms are expected within several days to a week.14 Use of flumazenil can precipitate immediate withdrawal symptoms. Treatment of withdrawal consists of restarting of benzodiazepines.
Flunitrazepam
Flunitrazepam (Rohypnol) has been used in Europe, Asia, and Latin America for insomnia and preoperative sedation since 1975. Although it has never been manufactured or sold in the United States, flunitrazepam has been suggested in many “date rape” incidents. Flunitrazepam has been an active agent in the illicit drug market, where it is used to alter the effects of other drugs, including heroin and cocaine.15
Flunitrazepam has 10 times more affinity than diazepam for certain benzodiazepine receptors. CNS depression occurs within 30 minutes. The drug is most frequently ingested with alcohol, producing additional disinhibition and amnesia. Despite marked CNS depression, patients can usually be aroused with noxious stimuli. The half-life of the drug is 16 to 35 hours, but coma can be prolonged for up to 48 hours.15 Flunitrazepam is easily obtained outside the United States. The drug is not detected on routine urine drug screens, but if it is needed as evidence, urine should be refrigerated or frozen and the local or state police crime laboratory contacted to arrange specific testing. Metabolites of flunitrazepam are detected in the urine up to 72 hours after exposure.
Buspirone
Buspirone has several advantages over benzodiazepines. The drug causes minimal CNS depression, even in combination with ethanol. Dosage adjustment is not needed for elderly patients. A withdrawal state after discontinuation has not been reported. Only one case of isolated buspirone overdose has been published. That patient was lethargic and had a tonic-clonic seizure but recovered fully.16
Zolpidem and Zaleplon
Zolpidem (Ambien) and zaleplon (Sonata) differ in structure from both the benzodiazepines and buspirone, and neither is detected on a benzodiazepine toxicology screen. They act selectively at a specific benzodiazepine receptor, producing sedation without many of the side effects seen with benzodiazepines. They have modest anxiolytic, muscle relaxant, and anticonvulsant properties. Significant drug interactions are rare. Compared with zolpidem, zaleplon causes less memory loss and sedation at therapeutic doses and is more rapidly eliminated.17 Transient visual disturbances, transient global amnesia, and hallucinations can occur in patients with normal levels of consciousness with both zolpidem and zaleplon.17–19 Abuse of zolpidem is limited by vomiting, which may occur after a supratherapeutic dose. Both zolpidem and zaleplon are rapidly eliminated and lack active metabolites.20 In 2005, a controlled-release formulation of zolpidem (Ambien CR) became available. The dual-layered tablet releases an immediate dose of zolpidem, followed by a slow, extended release from the inner layer to maintain plasma zolpidem concentrations. Clinical experience thus far suggests that overdoses with the controlled-release formulation mirror those of the immediate-release preparation, with only small differences in the likelihood of drowsiness, hallucinations, and ataxia.21
Patients with zolpidem overdose do well with supportive care alone. Fatalities from isolated zolpidem overdose are rare. All published cases have involved individuals found dead at home and have been associated with coingestants, particularly other sedative-hypnotics or antipsychotics.22 Drowsiness is by far the most common symptom. Coma and respiratory failure are rare, despite overdoses of up to 40 times the normal dose, although intubation may be required, particularly if there are coingestants. Zolpidem overdose in children follows a similarly benign course. Drowsiness, ataxia, and hallucinations generally resolve within 10 hours.23
Overdose information for zaleplon is limited. In one case series, patients had CNS depression and mild hypotension. Arousal was temporally associated with flumazenil use in one patient.24 The only published fatality involved a mixed drug overdose including unknown quantities of zaleplon and butalbital, with postmortem serum zaleplon concentration 40 times greater than therapeutic.25 Adverse effects with therapeutic use include headache, anterograde amnesia, and transient visual hallucinations.20 The blue-green discoloration of gastric contents, mouth, and urine after zaleplon overdose is attributed to the indigo carmine dye present in zaleplon’s capsule shell.26
Eszopiclone
Eszopiclone (Lunesta) has been marketed in the United States since 2005 for treatment of insomnia. It is the S-isomer of racemic zopiclone, which has been used for decades outside the United States. Eszopiclone has a structure unrelated to that of benzodiazepines, barbiturates, zolpidem, and zaleplon.27–30
The mechanism of eszopiclone’s action is not completely described but may involve a specific GABAA receptor close to or coupled with the benzodiazepine receptor.27–29 Eszopiclone is rapidly absorbed, with a peak serum level at 1 hour and a half-life of 6 hours. It is metabolized in the liver to minimally active metabolites. The usual bedtime dose is 3 mg. It is recommended that elderly patients and those with hepatic insufficiency be treated with a lower (1 mg) dose.
Adverse effects with therapeutic use of eszopiclone include drowsiness, dizziness, dry mouth, unpleasant taste, nausea, and vomiting. Auditory and visual hallucinations have been reported.31 Experience with eszopiclone overdose is limited. The key to treatment is good supportive care. CNS depression may be prolonged and pronounced in elderly patients.32 A retrospective case review described 525 eszopiclone ingestions, but 259 of these patients had also ingested other drugs or chemicals.28 The ingestions involved eszopiclone doses up to 210 mg and had mild to moderate symptoms at most. Two deaths occurred, both involving significant coingestants. A single case report described a 52-year-old man who had coronary vasospasm and a ventricular fibrillation arrest after ingestion of 45 to 60 mg of eszopiclone.30 However, the arrest occurred approximately 20 hours after ingestion, and it is unclear what role, if any, eszopiclone played in causing the arrest.
Chloral Hydrate
Chloral hydrate has a low therapeutic ratio and can produce significant, potentially fatal toxicity. Whereas chloral hydrate use is rare today, it is still occasionally prescribed as a sedative in the elderly and for sedation in children undergoing medical procedures. The hypnotic oral adult dose is 0.5 to 1.0 g. The toxic oral dose in adults is approximately 10 g and may be as little as 1.5 g in a child.33
The toxic effects of chloral hydrate include CNS depression, gastrointestinal irritation, cardiovascular instability, hepatitis, and proteinuria.34,35 The primary active metabolite of chloral hydrate, trichloroethanol, has a barbiturate-like effect on GABAA receptors and is responsible for most of the CNS depression seen with significant overdose.
Chloral hydrate is rapidly absorbed from the gastrointestinal tract and almost immediately metabolized to trichloroethanol by the enzyme alcohol dehydrogenase. Onset of action is 20 to 30 minutes.36 Trichloroethanol is long acting, and its half-life can be significantly prolonged after overdose as metabolic pathways become saturated.
Clinical Features
Chloral hydrate toxicity causes CNS and respiratory depression, gastrointestinal irritation, cardiovascular instability, and dysrhythmias.34,35 The combination of deep coma and cardiac dysrhythmia without hypoxia is characteristic of severe cases.
Mild chloral hydrate toxicity can mimic ethanol or barbiturate overdose, with drowsiness, ataxia, and lethargy. A pear-like odor to the patient’s breath or gastric contents may suggest the diagnosis. More severe toxicity includes miosis, muscle flaccidity, diminished deep tendon reflexes, hypoventilation, hypotension, and hypothermia.33 Chloral hydrate is corrosive and causes nausea, vomiting, esophagitis, hemorrhagic gastritis, and, more rarely, gastrointestinal perforation or necrosis.33 Transient hepatic or renal dysfunction can also occur.
Dysrhythmias from chloral hydrate toxicity can be fatal. Chloral hydrate decreases myocardial contractility, shortens the cardiac refractory period, and increases the sensitivity of myocardium to catecholamines. Dysrhythmias include atrial fibrillation, supraventricular tachycardia, ventricular tachycardia, multifocal premature ventricular contractions, torsades de pointes, ventricular fibrillation, and asystole.36 Hypotension results from inhibition of central neurovascular regulatory centers and impaired myocardial contractility.
Management
The key to management is support of cardiorespiratory function. Intubation may be required for airway protection or to support ventilation and oxygenation. Avoid naloxone or flumazenil, which may precipitate ventricular dysrhythmias.33 Because chloral hydrate, like other chlorinated hydrocarbons, sensitizes myocardium to catecholamines, epinephrine and norepinephrine should also be avoided. Standard antidysrhythmic agents, such as lidocaine, do not appear effective against chloral hydrate–induced cardiac ectopy. The treatment of choice is a beta-blocker.37 Intravenous propranolol can be given in adult doses of 0.5 mg until ectopy is suppressed, followed by an infusion of 1 to 2 mg/hr, titrated to a heart rate of 80 to 100 beats/minute. A short-acting agent such as esmolol can also be used. Torsades de pointes should be treated with intravenous magnesium or overdrive pacing. Type I antidysrhythmic agents such as quinidine should be avoided. Unstable patients not responding to conservative therapy can be treated with hemoperfusion or hemodialysis.33
Over-the-Counter Sleep AIDS
Clinical Features
Impaired consciousness is the most frequent finding with diphenhydramine overdose. Somnolence, psychotic behavior, and agitation are common. Seizures can occur. Anticholinergic effects may be apparent, as noted in Chapter 150. Cardiovascular effects include sodium-channel blockade and wide-complex tachycardia.38 Apart from a lower incidence of psychosis, doxylamine has toxicity similar to that of diphenhydramine. Seizures and rhabdomyolysis may occur with severe toxicity. Serious cardiotoxicity is rare. Doxylamine toxicity has been reported to cause false-positive results on some immunoassay-based drug screens for methadone and phencylcidine.39
Management
Management of mild to moderate toxicity from OTC sleep aid overdose is generally supportive. Specific details of anticholinergic toxicity are discussed in Chapter 150.
γ-Hydroxybutyrate
Originally synthesized in the 1960s as an anesthetic, γ-hydroxybutyrate (GHB) was later discovered to be a naturally occurring metabolite of GABA. Since 1970, GHB has been used to treat narcolepsy, alcohol addiction, and opiate withdrawal.40
A 1977 report that GHB may enhance effects of steroids and increase release of growth hormone resulted in marketing of the agent as a “natural” aid for increasing muscle mass. Numerous reports of adverse effects followed. In 1989, the U.S. Food and Drug Administration (FDA) called for a voluntary withdrawal of the drug from store shelves. Its sale and manufacture were banned in 1990; however, illicit use of GHB increased, along with its precursors γ-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) (Box 165-4).41 The Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000 made GHB a schedule I controlled substance. In 2005, GHB was approved by the FDA for the treatment of narcolepsy under the trade name Xyrem (sodium oxybate, 0.5 mg/mL) as a schedule III drug.
GHB remains a popular drug of abuse.42 Some individuals take GHB for its purported muscle-building and fat-burning actions, others for its psychoactive effects. The drug’s euphoria-producing properties make it popular at “raves” (large, crowded youth parties with energetic dancing to rhythmic music for many hours).43 Self-treatment of insomnia with GHB has been reported and can cause dependence.44 CNS depression, amnesia, and disinhibition caused by mixing of GHB with ethanol make this combination a potential agent in “date rape” situations.45,46 A recent review of 226 GHB-associated deaths revealed that calls for medical assistance were often delayed or absent because of the false belief that victims need only “sleep off” their intoxications. Death occurs most often in the prehospital setting, both directly and by increasing the risk for fatal accidents. In this series, GHB was the sole intoxicant in 35% of deaths, underlining the lethal potential of the drug and its congeners.47
Chemical precursors to GHB are also commonly abused. GBL is rapidly converted to GHB by plasma lactonases. 1,4-BD is metabolized to γ-hydroxybutyraldehyde by the enzyme alcohol dehydrogenase and then to GHB by aldehyde dehydrogenase.48,49
Principles of Disease
GHB binds to specific GHB receptors and at high concentrations to GABAB receptors.50 The complex interaction between these two receptors may explain the sometimes paradoxical manifestations of GHB toxicity of somnolence alternating with agitation. Through its action on the GABAB receptor, GHB decreases release of dopamine.51,52
As underground laboratories often synthesize liquid GHB by mixing and heating butyrolactone and sodium hydroxide, careless preparation can result in residual unreacted base, causing significant caustic injury when the liquid is ingested.41
GBL is an industrial solvent that is rapidly absorbed after ingestion and metabolized within minutes to GHB by peripheral and hepatic lactonases.41 Before conversion to GHB, GBL itself is inactive and has no sedating effects.40 It produces a clinical syndrome similar to that of GHB ingestion, but its effects are greater and more prolonged. In fact, after ingestion, GBL is more efficient at delivery of GHB to the CNS than GHB itself is.53 GBL is available under a number of street names (Box 165-5).
1,4-BD is converted after ingestion to GHB by the enzyme alcohol dehydrogenase.49 Like GBL, it is used as an industrial solvent. Unlike GBL, 1,4-BD itself has sedative-hypnotic effects. Clinical findings are similar to those of GHB. When 1,4-BD and ethanol are ingested together, ethanol acts as a competitive inhibitor of alcohol dehydrogenase, so the toxic effects of 1,4-BD are delayed and prolonged, and the risk of death is increased.49 1,4-BD is available under a number of street names (Box 165-6).
In 2007, a children’s toy marketed under the names Aqua Dots and Bindeez Beads was contaminated when 1,4-BD was substituted for a more expensive industrial solvent during the manufacturing process. The toy consisted of tiny brightly colored spheres that were readily ingested by toddlers, causing decreased levels of consciousness, coma, or apparent seizures.54
Clinical Features
Diagnosis of GHB intoxication is based on the history and clinical course. Rapid recovery from coma, or periods of agitation alternating with periods of decreased level of consciousness, is characteristic. Hypothermia may occur.43 In the presence of coma, bradycardia with or without hypotension may be seen and can respond to stimulation alone.43 Miosis with or without nystagmus may be seen. Emesis occurs in 50% of cases.43 Apparent seizure activity may actually represent random myoclonic movements of the face and extremities. Severity is dependent on the dose and the concurrent use of alcohol or other psychoactive drugs.44
Diagnostic Strategies
GHB is not detected on urine toxicology screens. If laboratory confirmation is required, specimens must be collected early and sent for gas chromatography–mass spectroscopy. The drug may be detected in urine up to 12 hours after ingestion.55
Management
Because of the high incidence of emesis with GHB overdose, intubation for airway protection should be seriously considered in patients with significant CNS depression. Bradycardia unresponsive to stimulation can be treated with atropine. Treatment of isolated GHB ingestion is supportive. Although some authors suggest physostigmine to reverse GHB-induced coma, the efficacy and safety of this intervention have not been demonstrated, and physostigmine is not indicated or recommended.48,56,57
Withdrawal
Patients who suddenly stop GHB or its precursors after chronic, frequent use can experience a severe and potentially life-threatening withdrawal syndrome.50,58,59 Because of the short half-life of GHB, symptoms of withdrawal begin within several hours of the last dose. The typical patient will have been using these products for weeks or years, every 1 to 3 hours around-the-clock, to avoid withdrawal symptoms.
Initial treatment begins with high-dose benzodiazepines. However, GHB withdrawal may involve depleted levels of GABA.58 Because the effect of benzodiazepines requires the presence of GABA, they may not be effective in control of GHB withdrawal. Barbiturates, such as pentobarbital, which do not need GABA to be effective, are often required in cases of severe intoxication.60
These patients often require intensive care admission for aggressive sedation and to monitor fluctuating vital signs. Rhabdomyolysis and severe hyperthermia should be ruled out. Deaths have been reported, sometimes many days after presentation and after apparent improvement.58
References
1. Bronstein, AC, et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila). 2010;48:979.
2. American Academy of Clinical Toxicology; European Association of Poison Centres and Clinical Toxicologists. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. J Toxicol Clin Toxicol. 1999;37:731.
3. Ebid, AI, et al. Pharmacokinetics of phenobarbital during certain enhanced elimination modalities to evaluate their clinical efficacy in management of drug overdose. Ther Drug Monit. 2001;23:209.
4. Roberts, DM, et al. Enhanced elimination in acute barbiturate poisoning—a systematic review. Clin Toxicol (Phila). 2011;49:2.
5. Palmer, BF. Effectiveness of hemodialysis in the extracorporeal therapy of phenobarbital overdose. Am J Kidney Dis. 2000;36:640.
6. Chouinard, G, et al. Metabolism of anxiolytics and hypnotics: Benzodiazepines, buspirone, zopiclone, and zolpidem. Cell Mol Neurobiol. 1999;19:533.
7. Tanaka, E. Clinically significant pharmacokinetic drug interactions with benzodiazepines. J Clin Pharm Ther. 1999;24:347.
8. Zosel, A, et al. Severe lactic acidosis after an iatrogenic propylene glycol overdose. Pharmacotherapy. 2010;30:219.
9. Perry, HE, Shannon, MW. Diagnosis and management of opioid- and benzodiazepine-induced comatose overdose in children. Curr Opin Pediatr. 1996;8:243.
10. Goldfrank, LG. Flumazenil: A pharmacologic antidote with limited medical toxicology utility, or … an antidote in search of an overdose. Acad Emerg Med. 1997;4:935.
11. American Academy of Clinical Toxicology; European Association of Poison Centres and Clinical Toxicologists. Position statement: Single-dose activated charcoal. J Toxicol Clin Toxicol. 1997;35:721.
12. Seger, DL. Flumazenil—treatment or toxin? J Toxicol Clin Toxicol. 2004;42:209.
13. Mathieu-Nolf, M, et al. Flumazenil use in an emergency department: A survey. J Toxicol Clin Toxicol. 2001;39:15.
14. Molle, HJ. Effectiveness and safety of benzodiazepines. J Clin Psychopharmacol. 1999;19:2.
15. Waltzman, ML. Flunitrazepam: A review of “roofies.”. Pediatr Emerg Care. 1999;15:59.
16. Catalano, G, et al. Seizures associated with buspirone overdose: Case report and literature review. Clin Neuropharmacol. 1998;21:347.
17. Drover, DR. Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives: Zaleplon, zolpidem and zopiclone. Clin Pharmacokinet. 2004;43:227.
18. Tsai, MJ, et al. A novel clinical pattern of visual hallucinations after zolpidem use. J Toxicol Clin Toxicol. 2003;41:869.
19. Tsai, MY, et al. Transient global amnesia–like episode due to mistaken intake of zolpidem: Drug safety concern in the elderly. J Patient Saf. 2009;5:32.
20. Weitzel, KW, et al. Zaleplon: A pyrazolopyrimidine sedative-hypnotic agent for the treatment of insomnia. Clin Ther. 2000;22:1254.
21. Forrester, MB. Immediate- and controlled-release zolpidem ingestions reported to Texas poison centers. Hum Exp Toxicol. 2009;28:505.
22. Gock, SB, et al. Acute zolpidem overdose: Report of two cases. J Anal Toxicol. 1999;23:559.
23. Kurta, D, et al. Zolpidem (Ambien): A pediatric case series. J Toxicol Clin Toxicol. 1997;35:453.
24. Hojer, J, et al. Zaleplon-induced coma and bluish-green urine: Possible antidotal effect by flumazenil. J Toxicol Clin Toxicol. 2002;40:571.
25. Moore, KA, et al. Mixed drug intoxication involving zaleplon (“Sonata”). Forensic Sci Int. 2003;134:120.
26. Louis, CJ, et al. A case of zaleplon overdose. Clin Toxicol (Phila). 2008;46:782.
27. Eszopiclone. Drugs. 2005;6:111.
28. Forrester, MB. Eszopiclone ingestions reported to Texas poison control centers, 2005-2006. Hum Exp Toxicol. 2007;26:795.
29. Halas, CJ. Eszopiclone. Am J Health Syst Pharm. 2006;63:41.
30. Miller, AH, et al. Lunesta overdose: ST-elevation coronary vasospasm, troponemia, and ventricular fibrillation arrest. Am J Emerg Med. 2006;24:741.
31. Duggal, HS. New-onset transient hallucinations possibly due to eszopiclone: A case study. Prim Care Companion J Clin Psychiatry. 2007;9:468.
32. Lovett, B, et al. Case report: Prolonged coma after eszopiclone overdose. Am J Emerg Med. 2007;25:735.e5–735.e6.
33. Stracciolini, A. Chloral hydrate. Clin Toxicol Rev. 1998;21:1.
34. Laurent, Y, et al. Electrocardiographic changes with segmental akinesia after chloral hydrate overdose. J Emerg Med. 2006;30:179.
35. Lin, YC, et al. Severe esophageal burn following chloral hydrate overdose in an infant. J Formos Med Assoc. 2006;105:235.
36. Sing, K, et al. Chloral hydrate toxicity from oral and intravenous administration. J Toxicol Clin Toxicol. 1996;34:101.
37. Zahedi, A, et al. Successful treatment of chloral hydrate cardiac toxicity with propranolol. Am J Emerg Med. 1999;17:490.
38. Jang, DH, et al. Status epilepticus and wide-complex tachycardia secondary to diphenhydramine overdose. Clin Toxicol (Phila). 2010;48:945.
39. Syed, H, et al. Doxylamine toxicity: seizure, rhabdomyolysis and false positive urine drug screen for methadone. BMJ Case Rep. 2009.
40. Wong, CG, et al. γ-Hydroxybutyric acid: Neurobiology and toxicology of a recreational drug. Toxicol Rev. 2004;23:3.
41. Palmer, RB. γ-Butyrolactone and 1,4-butanediol: Abused analogues of γ-hydroxybutyrate. Toxicol Rev. 2004;23:21.
42. Miro, O, et al. Trends in illicit drug emergencies: The emerging role of gamma-hydroxybutyrate. J Toxicol Clin Toxicol. 2002;40:129.
43. Chin, RL, et al. Clinical course of gamma-hydroxybutyrate overdose. Ann Emerg Med. 1998;31:716.
44. Galloway, GP, et al. Gamma-hydroxybutyrate: An emerging drug of abuse that causes physical dependence. Addiction. 1997;92:89.
45. Liechti, ME. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006;81:323.
46. Thai, D, et al. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J Clin Psychopharmacol. 2006;26:524.
47. Zvosec, DL, et al. Case series of 226 γ-hydroxybutyrate-associated deaths: Lethal toxicity and trauma. Am J Emerg Med. 2011;29:319.
48. Mason, PE, Kerns, WP. Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med. 2002;9:730.
49. Zvosek, DL, et al. Adverse events, including death, associated with the use of 1,4-butanediol. N Engl J Med. 2001;344:87.
50. Wong, C, et al. From the street to the brain: Neurobiology of the recreational drug γ-hydroxybutyric acid. Trends Pharmacol Sci. 2004;25:29.
51. Zvosek, DL, et al. Agitation is common in γ-hydroxybutyrate toxicity. Am J Emerg Med. 2005;23:316.
52. Gable, RS. Acute toxic effects of club drugs. J Psychoactive Drugs. 2004;36:303.
53. Graeme, KA. New drugs of abuse. Emerg Med Clin North Am. 2000;18:626.
54. Gunja, N, et al. γ-Hydroxybutyrate poisoning from toy beads. Med J Aust. 2008;188:54.
55. Smith, KM, et al. Club drugs: Methylenedioxymethamphetamine, flunitrazepam, ketamine hydrochloride, and γ-hydroxybutyrate. Am J Health Syst Pharm. 2002;59:1067.
56. Traub, SJ, et al. Physostigmine as a treatment for gamma-hydroxybutyrate toxicity: A review. J Toxicol Clin Toxicol. 2002;40:781.
57. Zvosec, DL, et al. Physostigmine for gamma-hydroxybutyrate coma: Inefficacy, adverse events, and review. Clin Toxicol (Phila). 2007;45:281.
58. Dyer, J, et al. Gamma-hydroxybutyrate withdrawal syndrome. Ann Emerg Med. 2001;37:147.
59. Schneir, AB, et al. A case of withdrawal from the GHB precursors gamma-butyrolactone and 1,4-butanediol. J Emerg Med. 2001;21:31.
60. Sivilotti, ML, et al. Pentobarbital for severe gamma-butyrolactone withdrawal. Ann Emerg Med. 2001;38:660.