47 Sedation for Diagnostic and Therapeutic Procedures Outside the Operating Room
THE USE OF SEDATION and analgesia for diagnostic and therapeutic procedures performed by anesthesiologists and nonanesthesiologists outside the operating room (OR) continues to dramatically increase. Millions of such procedures are performed each year throughout the world. Great progress has been made in understanding the need for and the risks associated with sedation and analgesia outside the OR; the mere restraint of a child for a frightening and/or painful procedure is difficult to justify.1–4 Indeed, the time-honored techniques of immobilization by physical restraint or sedation with archaic medications, such as the “lytic cocktail” (meperidine, promethazine, and chlorpromazine), are unacceptable and were made possible only because children were easily overpowered, not routinely asked if they were in pain, and were unable to withdraw consent. Furthermore, many of these techniques were inefficient and failed to provide the conditions necessary to complete required procedures. Sedation/analgesia for painful procedures performed outside the OR (e.g., bone marrow aspiration, lumbar puncture, repair of minor surgical wounds, insertion of arterial or venous catheters, burn dressing changes, fracture reduction, bronchoscopy, and endoscopy) requires the same attention to detail as for procedures performed in the OR, because sedation levels needed often achieve the state of deep sedation or general anesthesia, particularly in children 6 years of age and younger. Nonpainful procedures often require sedation, immobility, and sometimes breath-holding. Children undergoing diagnostic studies (e.g., computed tomography [CT], magnetic resonance imaging [MRI], positron emission tomography, electroencephalography [EEG], electromyography), or who require high doses of ionizing radiation, also require deep levels of sedation or general anesthesia because they must remain absolutely motionless.5 Given these requirements, a team approach is required to provide appropriate care for the child and to provide optimal conditions for the procedure.
Young and developmentally delayed children, are often unable to remain motionless for even short periods of time. Even some older children and adults may be unable to enter the confined space and often frightening environment of a diagnostic imaging scanner. The fear and anxiety associated with procedures are difficult to control and may be exacerbated by parental anxiety, separation from parents, and the pain (or anticipation of pain) from the procedure. Although distraction, guided imagery, and the use of videos and music have clear and documented benefit, they are often not enough to efficiently and successfully complete procedures.6–10
The pharmacologic armamentarium for sedation for diagnostic and therapeutic procedures has greatly expanded to include drugs classified as sedatives, as well as those classified as general anesthetics. Who uses these drugs and what qualifications are needed by those who provide such sedation is controversial.11 The demand for safe efficient sedation and analgesia outside the OR comes from many sources, including hospital administrators, insurance companies, and medical specialists. Failed sedations for diagnostic or therapeutic procedures are no longer acceptable, as this increases costs and frustrates parents12; however, this manpower need has far outstripped the available supply of anesthesiologists. This led to the demand to create sedation services for diagnostic or therapeutic procedures by nonanesthesiologists (i.e., nurse practitioners, pediatricians, emergency medicine physicians, intensivists, and dentists) who often use sedative agents, including general anesthetics. The development of pediatric sedation services has taken many directions. In a 2005 survey of pediatric sedation practice in 116 children’s hospitals in the United States and Canada,13 only 50% of the hospitals had a formal pediatric sedation service. Anesthesiologists were the sole sedation providers in only 26% of institutions. A consortium of pediatric hospitals heavily involved in sedation noted that anesthesiologists were involved in 19% of sedation procedures.14 Even when looking at the use of drugs such as propofol, which is considered a “general anesthetic,” this group noted anesthesiologists involvement only 10% of the time.15 This chapter will focus on the current definitions of sedation for diagnostic and therapeutic procedures and the goals, risks, and guidelines for creation of safe conditions for children who require sedation for procedures outside the OR.
As anesthesiologists we need to ask—why should we care? We believe that we provide the best, most efficient, and safest conditions for procedures, whether they are painful or not and whether they are in the OR or not. However, there simply aren’t enough anesthesia care providers to meet the ever-increasing demand. Further, after years of training and the ability to provide anesthesia for “big” cases (e.g., liver transplants, craniofacial reconstruction, trauma, etc.), we need to ask ourselves whether we are “overtrained” to care for children who require sedation or anesthesia for procedures outside of the OR, or whether it can be safely done by others with less training. Federal regulatory agencies, hospital administrations, and professional societies have recognized the expertise of anesthesiologists to provide sedation and have required us to lead, teach, and be responsible for oversight and credentialing of all sedation providers. We must “grab this tiger by its tail.”12,16
Definition of Levels of Sedation
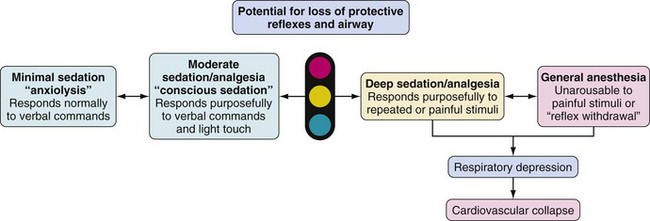
Several organizations have created guidelines and definitions of sedation for diagnostic and therapeutic procedures in children.17–20 The definitions of the American Academy of Pediatrics (AAP), the American Society of Anesthesiologists (ASA), The Joint Commission (previously called the Joint Commission on Accreditation of Healthcare Organizations), and the American Academy of Pediatric Dentistry (AAPD) are the most frequently cited and agreed-upon position statements.18,19,21–23 These organizations defined sedation and analgesia for procedures as a continuum of consciousness to unconsciousness, ranging from minimal sedation (anxiolysis) to moderate sedation (previously, “conscious sedation”) to deep sedation and general anesthesia (Fig. 47-1). Note that a child’s depth of sedation may easily pass from a light level all the way to general anesthesia.24
A clear understanding of the definition of sedation is mandatory to recognize when the child has progressed to a deeper level of sedation than anticipated (i.e., from moderate sedation to deep sedation or from deep sedation to general anesthesia). Recognition of this transition allows escalation of monitoring and care to avoid complications. The AAP, ASA, and The Joint Commission formalized and defined the concepts of minimal sedation, moderate sedation, deep sedation, and general anesthesia. The definitions that follow are taken from The Joint Commission (2010)25,26 and are in agreement with AAP, AAPD, and ASA definitions18,19,21:
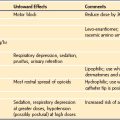
 Minimal sedation (anxiolysis): A drug-induced state during which patients respond normally to verbal commands. Although cognitive function and coordination may be impaired, ventilatory and cardiovascular functions are unaffected (Video 47-1)
Minimal sedation (anxiolysis): A drug-induced state during which patients respond normally to verbal commands. Although cognitive function and coordination may be impaired, ventilatory and cardiovascular functions are unaffected (Video 47-1)![]() .
.
 Moderate sedation (previously called “conscious sedation” or sedation/analgesia): A drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate. Cardiovascular function is usually maintained (Video 47-2)
Moderate sedation (previously called “conscious sedation” or sedation/analgesia): A drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate. Cardiovascular function is usually maintained (Video 47-2)![]() .
.
 Deep sedation: A drug-induced depression of consciousness during which patients cannot be easily aroused but respond purposefully after repeated or painful stimuli (note: reflex withdrawal from a painful stimulus is not considered a purposeful response). The ability to independently maintain ventilatory function may be impaired. Patients may require assistance in maintaining a patent airway and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained (Video 47-3)
Deep sedation: A drug-induced depression of consciousness during which patients cannot be easily aroused but respond purposefully after repeated or painful stimuli (note: reflex withdrawal from a painful stimulus is not considered a purposeful response). The ability to independently maintain ventilatory function may be impaired. Patients may require assistance in maintaining a patent airway and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained (Video 47-3)![]() .
.
 General anesthesia: A drug-induced loss of consciousness during which patients are not arousable, even to painful stimuli. The ability to independently maintain ventilatory function is often impaired. Patients often require assistance in maintaining a patent airway, and positive-pressure ventilation may be required because of depressed spontaneous ventilation or drug-induced depression of neuromuscular function. Cardiovascular function may be impaired (Video 47-4)
General anesthesia: A drug-induced loss of consciousness during which patients are not arousable, even to painful stimuli. The ability to independently maintain ventilatory function is often impaired. Patients often require assistance in maintaining a patent airway, and positive-pressure ventilation may be required because of depressed spontaneous ventilation or drug-induced depression of neuromuscular function. Cardiovascular function may be impaired (Video 47-4)![]() .
.
Painful procedures or nonpainful procedures requiring complete immobility (e.g., diagnostic imaging or radiation therapy) cannot be performed in a child who is moderately (“consciously”) sedated. Most pediatric procedures require deep sedation. The myth that a state of moderate sedation in which children are conscious and simultaneously responsive to voice command while immobile in the face of pain is just that, a myth.27
Assessing the depth of sedation based on the child’s responses to stimulation is illustrated in Figure 47-2. This is a difficult challenge in children and depends on their verbal abilities, age, level of maturity, and underlying condition. A common problem with classifying a child as either moderately or deeply sedated is to misinterpret any movement in response to touch as “purposeful” and therefore a sign of “moderate sedation.” A child who is moderately sedated should respond to touch or firm rubbing by an appropriate response, such as saying “ouch,” pushing your hand away, and pulling up the covers. An inappropriate response, such as nonpurposeful movement, is a sign that the child has progressed to a deeper sedation level, which should lead to an escalation of care because respiratory depression may occur.28–31 Similarly, children who are deeply sedated should respond purposefully to painful stimuli. Reflex withdrawal, a nonpurposeful response or no response to painful stimuli, is a sign that the child has progressed to a level of anesthesia and should be cared for using guidelines and personnel for general anesthesia. Children may quickly move from one level of sedation to another. A study in a large pediatric hospital showed that the goal of either moderate or deep sedation was only attained in 50% to 75% of patients. An awake state was noted in 12% to 28% of children, and a level consistent with general anesthesia was observed in up to 35% of children.32 The need for accurate assessment of the sedated child has brought about several scoring systems.
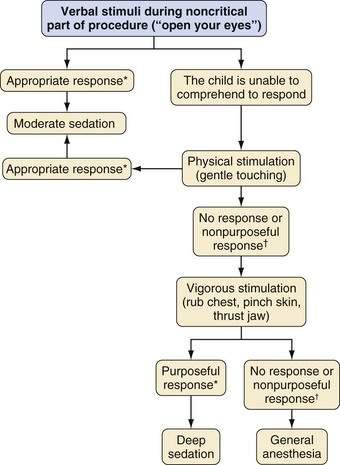
*Purposeful: opens eyes, talks back, pushes you out of the way.
†Nonpurposeful: winces, shrugs shoulders, nonspecific withdrawal to pain.
The Ramsay scale was described by Ramsay and colleagues33 in 1974 for the purpose of monitoring sedation with alphaxalone-alphadolone (Table 47-1). It continues to be the most widely used scale for assessing and monitoring sedation in daily practice, as well as in clinical research. It spans the continuum of sedation but does not clearly separate purposeful from nonpurposeful responses. The Ramsey scale has been modified to more clearly coincide with the AAP and Joint Commission guidelines (Table 47-2).34 A score of 2 to 3 is anxiolysis, 4 to 5 is moderate sedation, 6 is deep sedation, and 7 to 8 is general anesthesia.
| Level | Characteristics |
|---|---|
| 1 | Patient awake, anxious, agitated, or restless |
| 2 | Patient awake, cooperative, orientated, and tranquil |
| 3 | Patient drowsy, with response to commands |
| 4 | Patient asleep, brisk response to glabella tap or loud auditory stimulus |
| 5 | Patient asleep, sluggish response to stimulus |
| 6 | Patient has no response to firm nail-bed pressure or other noxious stimuli |
TABLE 47-2 Modified Ramsay Sedation Scale
| Score | Characteristics |
|---|---|
| 1 | Awake and alert, minimal or no cognitive impairment |
| 2* | Awake but tranquil, purposeful responses to verbal commands at conversation level |
| 3* | Appears asleep, purposeful responses to verbal commands at conversation level |
| 4† | Appears asleep, purposeful responses to verbal commands but at louder than usual conversation level or requiring light glabellar tap |
| 5† | Asleep, sluggish purposeful responses only to loud verbal commands or strong glabellar tap |
| 6‡ | Asleep, sluggish purposeful responses only to painful stimuli |
| 7§ | Asleep, reflex withdrawal to painful stimuli only (no purposeful responses) |
| 8§ | Unresponsive to external stimuli, including pain |
The Observer’s Assessment of Alertness/Sedation (OAA/S) scale35 is often cited as a scale for sedation. It is scored as follows: 1, no response to shaking; 2, responds to mild prodding; 3, responds to name called loudly; 4, lethargic response to name; and 5, readily responds to name. However, its inability to clearly categorize deep levels of sedation and lack of a clear differentiation between purposeful and nonpurposeful responses limit its usefulness. The University of Michigan Sedation Scale (UMSS)36 is an assessment tool that has been shown to be valid when compared with the OAA/S scale and other scales of sedation (Table 47-3). It readily separates patients into the sedation categories defined by the AAP, ASA, and Joint Commission.
TABLE 47-3 University of Michigan Sedation Scale (UMSS)
| Score | Characteristics |
|---|---|
| 0 | Awake and alert |
| 1 | Minimally sedated: tired/sleepy, appropriate response to verbal conversation and/or sound |
| 2 | Moderately sedated: somnolent/sleeping, easily aroused with light tactile stimulation or a simple verbal command |
| 3 | Deeply sedated: deep sleep, arousable only with significant physical stimulation |
| 4 | Unarousable |
These subjective, responsiveness-based assessment tools all have the disadvantage of intermittently stimulating the child during the procedure and thereby undoing the very reason that sedation was given in the first place! Poking or prodding the child to determine their depth of sedation defeats the purpose of sedation in many situations, e.g., infants, developmentally delayed, or procedures requiring immobility. None of the guidelines require this type of assessment. These responsiveness-based definitions are meant to provide a roadmap for safety, with the understanding that a nonresponding child requires an increased level of vigilance and the provider must have the skills to rescue should an adverse respiratory or cardiac event occur.12 Concerns regarding these types of assessment tools have led to a drive by some to revise the sedation continuum and to use other monitoring to assess level of sedation.37
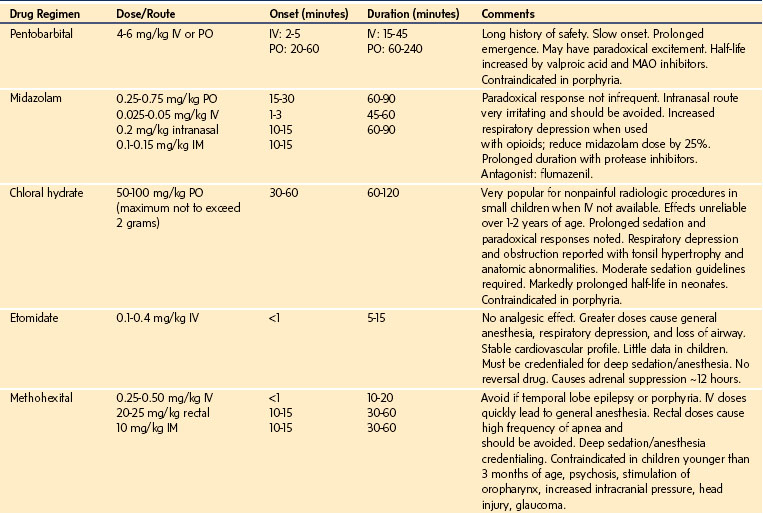
To address the problem of defining the depth of sedation or anesthesia, a nonstimulating continuous-assessment tool using a processed EEG, as in the bispectral index (BIS) monitor, has been extensively explored. The BIS was derived from empirically estimating processed EEG parameters that best predicted OAA/S scale levels in adult volunteers receiving a wide variety of anesthetics, analgesics, and sedatives.38 BIS values in adults range from 100 to 95, awake; 95 to 70, light to moderate sedation; 70 to 60, deep sedation with low probability of explicit recall; and 60 to 40, general anesthesia.39 Unfortunately, the EEG in children varies markedly with age and drug, precluding accurate measures by the processed EEG.40,41 Attempts to correlate BIS values with the depth of sedation in children have had varying success. In healthy children between 1 and 12 years of age who were sedated with chloral hydrate, meperidine and promethazine, midazolam, fentanyl, or pentobarbital, the BIS reading correlated well with the UMSS scores.42 In a similar study with children between birth and 18 years of age who received inhalational anesthetics, chloral hydrate, midazolam, and fentanyl,43 the BIS readings validated the sedation scores, although the data identified several important limitations when distinguishing moderate from deep sedation. For example, the researchers concluded that a BIS of 80 is the optimal cutoff for a diagnosis of deep sedation although this value is inconsistent with that found in the adult literature. In children recovering from propofol and remifentanil anesthesia, there was a marked overlap between moderate and deep sedation using both the BIS and the composite A-line autoregressive index (auditory EEG).44,45 From a safety perspective, it is worrisome that the BIS underestimates the depth of sedation with certain medications, such as chloral hydrate.45,46 During pentobarbital sedation, the BIS had “limited ability to distinguish between moderate and deep sedation levels in children.”47
Furthermore BIS values are not age specific,48,49 may differ from one side of the head to the other, are diminished in developmentally delayed children,50 may increase with larger doses of ketamine because of ketamine-induced central excitation,51,52 and are unaffected even by high-dose dexmedetomidine.53 Therefore the overlap between levels of sedation, the few large well-controlled studies in children, the overall low quality of BIS data in some studies, as well as a lack of information for different age-groups and specific drugs, precludes validation of the BIS for use in sedated children.40 In addition, the use of BIS-type monitoring is not feasible for many procedures, such as MRI, CT scans, or those involving the mouth and airway (endoscopy, dental, bronchoscopy), because the monitor is either not compatible, creates artifacts, is easily dislodged, or is in the way of the proceduralist.
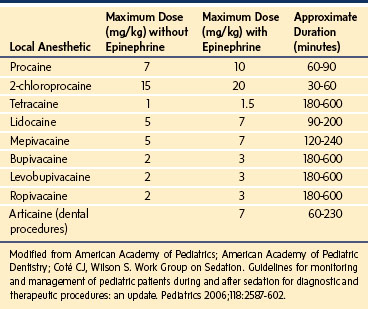
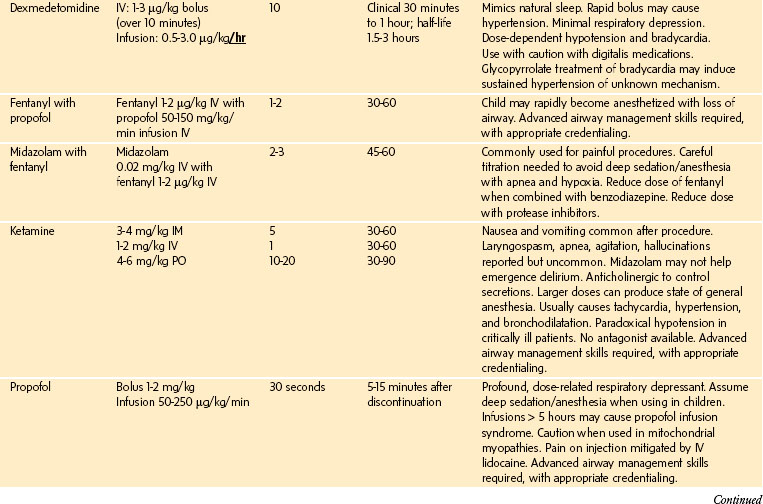
The definitions and tools discussed previously attempt to assess sedation and decrease risk by quantitating the response to stimulation or EEG activity. These tools, however, do not directly measure the risk of loss of control of the airway, which is the primary cause of morbidity and mortality during sedation.54,55 The implication is that there is a direct quantifiable correlation between response to touch or painful stimulation and the ability to control the airway. Thus a moderately sedated child who can respond to light touch can protect his or her airway and a deeply sedated child who can respond appropriately only to pain may not be able to protect his or her airway. The important assessment of the child is not the response to stimulation, but the ability to protect the airway. Furthermore, different sedative drugs have different effects on analgesia separate from obtundation of the airway. Propofol is not a profound analgesic but has profound effects on the airway.56 Conversely, the sedative dexmedetomidine may provide profound sedation with little depression of respiratory function. Ketamine produces intense analgesia and most children maintain a patent airway and adequate respiratory effort (Table 47-4).57
TABLE 47-4 Examples of Sedative Drugs That Have Varying Effects on Response to Pain and Airway Protection
| Response to Pain Does Not Predict Airway Maintenance All Drugs Are Different |
||
| Response to Pain | Airway Maintenance | |
| Fentanyl | ↓↓ | ↓ |
| Propofol | ± | ↓↓ |
| Ketamine | ↓↓ | ± |
| Dexmedetomidine | ↓ | + |
↓↓↓, Large decrease in response to pain or large effect on airway patency; ↓, some decrease in response to pain or some effect on airway patency; ±, minimal to no effect on response to pain or effect on airway patency; +, no effect on airway patency.
Until recently, a quantifiable metric to assess control of the airway in sedated children was not available.58 Studies on the changes of airway dimensions under different anesthetic and sedative agents, the effect of different positions on the upper airway,59–61 and upper airway collapsibility with different concentrations of sedative drugs provide a more meaningful assessment of airway risk and protection. The profound effect of propofol-induced collapse of the upper airway can be partially mitigated by reducing the dose and including supplemental drugs, such as midazolam and nalbuphine.62,63
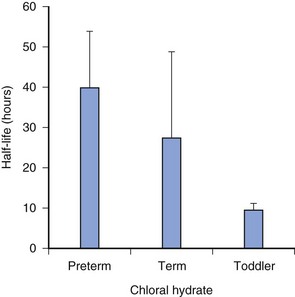
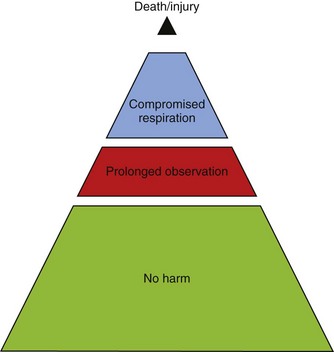
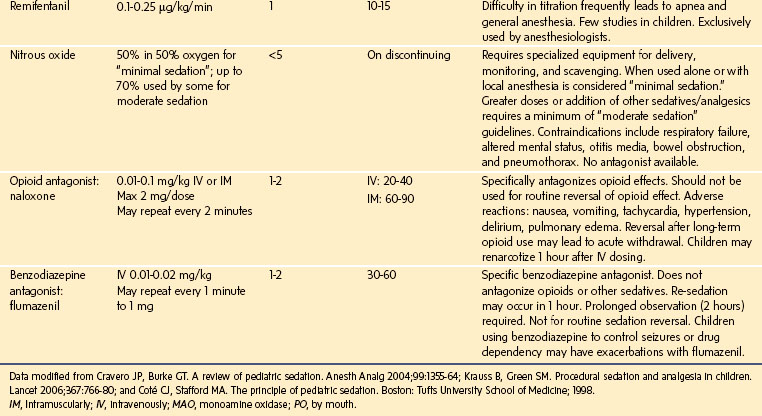
Assessing sedation must also focus on appropriate discharge readiness. Adverse effects, including death, have occurred after premature discharge following sedation for a procedure.55 These events have occurred most often when a long-lasting (long half-life) sedative, such as chloral hydrate, has been given (Fig. 47-3). This can result in the child being unable to spontaneously clear his or her airway.54 A simple modified “maintenance of wakefulness” score (infants had to stay awake for at least 20 minutes in a quiet environment before discharge) ensured that more than 90% of children had returned to baseline, compared with only 55% of children assessed as “street ready” according to usual hospital discharge criteria.44
Goals of Sedation
The goals of pediatric sedation have not changed over the years and can be summarized as follows18,31:
 Guard the child’s safety and welfare. The individual who performs the procedure cannot safely monitor the child as well. A second individual must monitor the child, administer drugs, and record vital signs.
Guard the child’s safety and welfare. The individual who performs the procedure cannot safely monitor the child as well. A second individual must monitor the child, administer drugs, and record vital signs.
 Minimize physical discomfort and pain.
Minimize physical discomfort and pain.
 Control anxiety, minimize psychological trauma, and maximize the potential for amnesia.
Control anxiety, minimize psychological trauma, and maximize the potential for amnesia.
 Control behavior and/or movement, to allow the safe completion of the procedure.
Control behavior and/or movement, to allow the safe completion of the procedure.
 Rapidly return of the child to a state in which he or she is safe for discharge from medical supervision, as determined by recognized criteria.
Rapidly return of the child to a state in which he or she is safe for discharge from medical supervision, as determined by recognized criteria.
 Create an optimal and efficient system that is not a burden to the health care providers
Create an optimal and efficient system that is not a burden to the health care providers
Risks and Complications Associated with Sedation
A recent area of concern that was not previously recognized in providing safe sedation or anesthesia to small children is whether drugs used for sedation or anesthesia are neurotoxic to children64–71; this topic is covered in detail in Chapter 23. More important than the theoretical concern regarding potential neurotoxicity of sedating medications is the more practical risk assessment regarding both inadequate and excessive sedation. Sedation centers in the United States are less likely to offer sedation for painful procedures than similar centers in Europe. Thirty percent of U.S. respondents to a mail survey reported performing bone marrow biopsies in children without significant sedation more than 50% of the time, as compared with 0% of European centers!72 When 1140 children (mean age 2.96 ± 3.7 years) were sedated by nonanesthesiologists who followed AAP guidelines for procedures,73 approximately 13% received inadequate sedation.
The psychological consequences of inadequate sedation have not been specifically studied but can be inferred from children’s responses to other stressful situations. Inadequate preoperative sedation is clearly linked to increased anxiety in children and their families. Of children undergoing a stressful anesthesia induction, 54% have postoperative maladaptive behaviors for up to 2 weeks after the experience.74 The incidence of these behaviors decreases with the use of appropriate preoperative sedation. Similar sequelae have been reported after repeated invasive procedures in pediatric intensive care units (ICUs).75 Although the number of children who developed posttraumatic stress syndrome because of no sedation or less than optimal sedation during diagnostic and therapeutic procedures is unknown, one can assume that it would be similar to that found in the study situations.
There have been many approaches to assess the risk involved in oversedation of children. One approach is to review sedation-related deaths and critical incidents to find common causes. In 2000, a now classic retrospective review was undertaken of 95 cases of sedation-related deaths and critical incidents derived from the U.S. Food and Drug Administration’s (FDA) adverse drug event reporting system, the U.S. Pharmacopeia, and a survey of pediatric specialists.54 Although a number of the incidents cited in this study occurred over 20 years ago, the findings remain applicable today. The analysis revealed that, rather than being related to a specific medication, the overwhelming majority of critical events were preventable and caused by operator error or lack of robust rescue systems. Drug interactions were the most common factor, followed by drug overdose, inadequate monitoring, inadequate cardiopulmonary resuscitation skills, inadequate evaluation before sedation, and premature discharge from medical supervision. The report emphasizes that most sedation complications are related to adverse respiratory events (80%); a majority progressed to cardiac arrest, indicating a lack of rescue skills of the practitioners. A disproportionately large number of severe complications and deaths occurred when sedation was performed in offices outside of hospitals, pointing to a lack of trained personnel available for rescue. There was no relationship with class of drug or route of administration, although there was a positive correlation when three or more sedating medications were administered.55 Indeed, the combination of a sedative, hypnotic, and an opioid is well recognized as a precursor to significant respiratory depression.
There are many underpowered reports with relatively few patients (n <200) receiving a variety of sedative medications in a variety of settings declaring successful completion of the procedures and no fatalities.76–86 Most studies describe adequate conditions and successful completion with no severe outcomes, but with varying frequencies of transient airway obstruction or desaturation. Conclusions often state that the particular technique and sedation providers are safe and effective. An example is a study that prospectively followed 1140 children (mean age 2.96 ± 3.7 years) sedated for procedures by nonanesthesiologists following AAP guidelines and using a quality-assurance tool.73 Neither death nor sedation-induced crises occurred, although they reported a 5.3% incidence of respiratory events, including one in which a child developed apnea. Given that the expected incidence of a sedation-induced crisis should be on the order of one in tens of thousands, it is not surprising that the majority of similar studies are underpowered to report a critical event.87
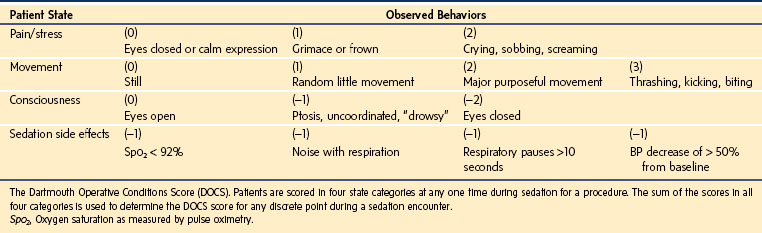
Another approach to assessing safety is to carefully observe relatively small groups of children and track minute-to-minute changes in their level of sedation. This has been accomplished by the creation of the Dartmouth Operative Conditions Scale (DOCS) (Table 47-5).88 This scale was derived from observing 110 videos of pediatric sedation outside the OR. The DOCS quantifies the child’s “state” based on observable behavior. Continuously applying this scale allows for careful tracking of level of sedation, effectiveness of sedation, uncontrolled adverse effects, and the timing of induction of sedation and recovery without poking or prodding the child to determine their depth of sedation. These data can help to quantify the quality of sedation and best practices.
Simulations have been used to carefully examine rare adverse effects in pediatric sedation and ability to rescue.89,90 In a simulated scenario of a sedation complication, a standard reproducible event was introduced in which physiologic variables degraded with inappropriate interventions and improved when effective treatment was introduced. The simulation was distributed throughout the hospital and used with different sedation provider teams (e.g., postanesthesia care unit, emergency department [ED], radiology, and sedation unit). The event was videotaped and scored based on deviations from best practice; it quantifies episodes of hypoventilation, apnea, hypoxia, and cardiovascular collapse. Hypoxia and hypotension lasted 90 seconds in the pediatric sedation unit and 360 seconds in the ED and radiology setting.91 Such studies demonstrate that prospective and retrospective analysis of simulated but rare adverse events can identify areas within the hospital that are in need of improving their clinical skills, and have a positive impact on patient safety.
There has been a recent effort to capture information from the large numbers of pediatric sedation procedures necessary to understand the nature and frequency of adverse reactions to sedation. The Pediatric Sedation Research Consortium (PSRC) comprises a group of over 30 institutions in North America who share prospective data on pediatric sedation.14 Analysis of the first 30,000 records identified demographics, procedures, sedation techniques, outcomes, and adverse events: (1) there were no deaths and only one cardiac arrest reported; (2) unanticipated admission occurred infrequently, 1 per 1500 sedations; (3) vomiting occurred 1 per 200 sedations, including one aspiration; (4) stridor, laryngospasm, wheezing, and apnea occurred in 1 of 400 procedures; and (5) airway or ventilatory manipulations occurred in 1 time per 200 sedations.91 Risk factors included age younger than 3 months, ASA physical status 3 or greater, and multiple drug combinations for sedation. The PSRC has also reviewed the incidence of adverse events in 49,386 propofol sedation and/or anesthesia encounters for procedures outside of the OR.15 Propofol was delivered by multiple providers (anesthesiologists, intensivists, emergency medicine, pediatricians, and radiologists). No deaths were recorded. CPR was required in 2 children (anesthesiologists were not involved in their care) and aspiration occurred in 4 children. Desaturation of less than 90% for more than 30 seconds occurred 154 times per 10,000 sedations/anesthesias; central apnea or obstruction occurred 575 times per 10,000. Stridor, laryngospasm, excessive secretions, and vomiting occurred 50, 96, 341, and 49 times per 10,000, respectively. The most prominent type of complication was related to the airway and occurred 1 out of 65 sedations; 1 in 70 children required airway rescue. Unexpected admissions occurred 7.1 times per 10,000 sedations. When all possible adverse events are considered, anesthesiologists report fewer events than nonanesthesiologists, with an odds ratio of 1.38 (95% confidence interval [CI] of 1.21 to 1.57, P < 0.001).15 No difference, however, was noted when outcomes were limited only to major pulmonary complications. The authors concluded that propofol sedation and/or anesthesia when given under these strict organized conditions, i.e., following the AAP sedation guideline, with very well-trained providers, can result in effective sedation with a minimal incidence of severe adverse events. The safety of this practice is contingent on the ability to quickly and safely manage less serious events. In a prospective cohort study of 131,751 procedural sedations92 in radiology (62%), hematology–oncology (11%), and minor medical or surgical procedures (16%), in which sedation included primarily propofol (60%), midazolam (25%), and others provided by anesthesiologists, emergency physicians, intensivists, pediatricians, and “other” (pediatric residents, radiologists, dentists, surgeons, and nurses), the rate of major complications per 10,000 sedations (and 95% CIs) were: anesthesiologist 7.6 (4.6 to 12.8), emergency physician 7.8 (5.5 to 11.2), intensivist 9.6 (7.3 to 12.6), pediatrician 12.4 (6.9 to 20.4) and “other” 10.2 (5.1 to 18.3). There were no statistical differences between any providers before or after potential confounding variables (age, ASA status, fasting status, propofol use). A significant limitation of the study was the selection bias that was introduced because not all sedation areas within each hospital reported data and the providers were not randomized. The study did not track whether high-risk patients were referred to practitioners outside of their sedation practice (i.e., the anesthesia service). Only major complications and not minor but dangerous complications (i.e., desaturation, hypoventilation, and need for airway support) were monitored. The participants in these hospitals are highly motivated, and application of these data outside of the consortium requires rigorous evaluation of safeguards and training. The ASA thinks that anesthesiologist participation in all deep sedation is the best means to achieve the safest care. The ASA acknowledges that nonanesthesiologists administer or supervise the administration of deep sedation and has created guidelines to help anesthesiologists provide leadership in this area.93,94
Guidelines
The first sedation guidelines were published in 1985 from the Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics.95 These guidelines provided the first framework to improve safety for children requiring sedation for a procedure. The guidelines emphasized systems issues, such as the need for informed consent, appropriate fasting before sedation, frequent measurement and charting of vital signs, the availability of age- and size-appropriate equipment, the use of continuous physiologic monitoring, the need for basic life support skills, as well as proper recovery and discharge procedures. The guidelines stated that an independent observer whose only responsibility was to monitor the child was required for deep sedation. Advanced airway and resuscitation skills were encouraged but not required. In 1992, the guidelines were revised96 emphasizing that a child could readily progress from one level of sedation to another and that the practitioner should be prepared to increase vigilance and monitoring as indicated. In 2002, the same Committee on Drugs of the AAP updated and amended the guidelines.97 It eliminated the use of the confusing term conscious sedation and replaced it with the term moderate sedation. It also emphasized the application of these guidelines outside the hospital, in recognition of the relatively high complication rate in non–hospital-based settings. The ASA guidelines in use today were updated in 2002 and, in many respects, modeled after the AAP guideline.98 These guidelines were systematically developed by a task force of 10 members. The task force reviewed the strength of evidence and made recommendations that include the following:
 Patient Evaluation: Clinicians should be familiar with the sedation-related aspects of the patient’s medical history. These include (1) abnormalities of major organ systems, (2) previous adverse effects with sedation and general anesthesia, (3) drug allergies, current medications, and drug interactions, (4) time and nature of oral intake, and (5) history of tobacco, alcohol, or substance abuse. A focused physical examination, including vital signs, auscultation of the heart and lungs, and evaluation of the airway, is recommended.
Patient Evaluation: Clinicians should be familiar with the sedation-related aspects of the patient’s medical history. These include (1) abnormalities of major organ systems, (2) previous adverse effects with sedation and general anesthesia, (3) drug allergies, current medications, and drug interactions, (4) time and nature of oral intake, and (5) history of tobacco, alcohol, or substance abuse. A focused physical examination, including vital signs, auscultation of the heart and lungs, and evaluation of the airway, is recommended.
 Preprocedural Preparation: Patients should be informed of and agree to sedation, including its risks, benefits, limitations, and alternatives. Sufficient time should elapse before a procedure to allow gastric emptying in elective patients. Minimum fasting periods of 2 hours (clear liquids), 4 hours (breast milk), and 6 hours (infant formula, nonhuman milk, and light meal), are recommended for healthy patients. If urgent, emergent, or other situations impair gastric emptying, the potential for pulmonary aspiration of gastric contents must be considered in determining the target level of sedation, delay, or intubation.
Preprocedural Preparation: Patients should be informed of and agree to sedation, including its risks, benefits, limitations, and alternatives. Sufficient time should elapse before a procedure to allow gastric emptying in elective patients. Minimum fasting periods of 2 hours (clear liquids), 4 hours (breast milk), and 6 hours (infant formula, nonhuman milk, and light meal), are recommended for healthy patients. If urgent, emergent, or other situations impair gastric emptying, the potential for pulmonary aspiration of gastric contents must be considered in determining the target level of sedation, delay, or intubation.
 Monitoring Level of Consciousness: Monitoring of verbal commands should be routine during moderate sedation, with the exception of young children and mentally impaired, uncooperative patients, or when the response would be detrimental. During deep sedation the response to a more profound stimulus should be sought to be sure the patient has not drifted into general anesthesia.
Monitoring Level of Consciousness: Monitoring of verbal commands should be routine during moderate sedation, with the exception of young children and mentally impaired, uncooperative patients, or when the response would be detrimental. During deep sedation the response to a more profound stimulus should be sought to be sure the patient has not drifted into general anesthesia.
 Physiologic Monitoring: All patients undergoing sedation/analgesia should be monitored by pulse oximetry with appropriate alarms. In addition, ventilatory function should be continually monitored by observation or auscultation. Monitoring of end-tidal carbon dioxide (etco2) should be considered for all patients receiving deep sedation and for patients whose ventilation cannot be directly observed during moderate sedation. Multiple studies have confirmed the usefulness in etco2 monitoring especially during deep sedation. Although not currently required, adverse events can be anticipated and corrected by using etco2 monitoring.99 It should be noted that ASA standards for basic monitoring have been amended to include etco2 monitoring for all sedated patients beginning in 2012.99,100 When possible, blood pressure (BP) should be determined before sedation/analgesia is initiated. Once sedation/analgesia is established, BP should be measured at regular intervals during the procedure, unless such monitoring interferes with the procedure (e.g., pediatric MRI, in which stimulation from the BP cuff could arouse an appropriately sedated child). Electrocardiographic (ECG) monitoring should be used in all children during deep sedation, and during moderate sedation in those with significant cardiovascular disease or those who are undergoing procedures in which dysrhythmias are anticipated.
Physiologic Monitoring: All patients undergoing sedation/analgesia should be monitored by pulse oximetry with appropriate alarms. In addition, ventilatory function should be continually monitored by observation or auscultation. Monitoring of end-tidal carbon dioxide (etco2) should be considered for all patients receiving deep sedation and for patients whose ventilation cannot be directly observed during moderate sedation. Multiple studies have confirmed the usefulness in etco2 monitoring especially during deep sedation. Although not currently required, adverse events can be anticipated and corrected by using etco2 monitoring.99 It should be noted that ASA standards for basic monitoring have been amended to include etco2 monitoring for all sedated patients beginning in 2012.99,100 When possible, blood pressure (BP) should be determined before sedation/analgesia is initiated. Once sedation/analgesia is established, BP should be measured at regular intervals during the procedure, unless such monitoring interferes with the procedure (e.g., pediatric MRI, in which stimulation from the BP cuff could arouse an appropriately sedated child). Electrocardiographic (ECG) monitoring should be used in all children during deep sedation, and during moderate sedation in those with significant cardiovascular disease or those who are undergoing procedures in which dysrhythmias are anticipated.
 Recording of Monitored Parameters: For both moderate and deep sedation, the child’s level of consciousness, ventilatory and oxygenation status, and hemodynamic variables should be assessed and recorded at a frequency that depends on the type and amount of medication administered, the length of the procedure, and the general condition of the patient. At a minimum, this should be (1) before the beginning of the procedure; (2) after administration of sedative/analgesic agents; (3) at regular intervals during the procedure; (4) during initial recovery; and (5) just before discharge. If recording is performed automatically, device alarms should be set to alert the care team to critical changes in patient status. (Children’s National Medical Center [CNMC] hospital policy requires recording of vital signs every 15 minutes and every 5 minutes for moderate and deep sedation, respectively.)
Recording of Monitored Parameters: For both moderate and deep sedation, the child’s level of consciousness, ventilatory and oxygenation status, and hemodynamic variables should be assessed and recorded at a frequency that depends on the type and amount of medication administered, the length of the procedure, and the general condition of the patient. At a minimum, this should be (1) before the beginning of the procedure; (2) after administration of sedative/analgesic agents; (3) at regular intervals during the procedure; (4) during initial recovery; and (5) just before discharge. If recording is performed automatically, device alarms should be set to alert the care team to critical changes in patient status. (Children’s National Medical Center [CNMC] hospital policy requires recording of vital signs every 15 minutes and every 5 minutes for moderate and deep sedation, respectively.)
 Availability of an Individual Responsible for Patient Monitoring: A designated individual, other than the practitioner performing the procedure should be present to monitor the child throughout procedures performed with sedation/analgesia. During deep sedation, this individual should have no other responsibilities. However, during moderate sedation, this individual may assist with minor, interruptible tasks once the patient’s level of sedation/analgesia and vital signs have stabilized, provided that adequate monitoring for the child’s level of sedation is maintained.
Availability of an Individual Responsible for Patient Monitoring: A designated individual, other than the practitioner performing the procedure should be present to monitor the child throughout procedures performed with sedation/analgesia. During deep sedation, this individual should have no other responsibilities. However, during moderate sedation, this individual may assist with minor, interruptible tasks once the patient’s level of sedation/analgesia and vital signs have stabilized, provided that adequate monitoring for the child’s level of sedation is maintained.
 Training of Personnel: Individuals responsible for children who receive sedation/analgesia should understand the pharmacology of the medications that are administered, as well as the role of pharmacologic antagonists for opioids and benzodiazepines. Individuals who monitor children who receive sedation/analgesia should be able to recognize the associated complications. At least one individual capable of establishing a patent airway and positive-pressure ventilation, as well as a means for summoning additional assistance, should be present whenever sedation/analgesia is administered. It is recommended (although not specifically stated) that an individual with advanced life support skills be immediately available (within 5 minutes) for moderate sedation; such an individual must be within the procedure room for deep sedation.
Training of Personnel: Individuals responsible for children who receive sedation/analgesia should understand the pharmacology of the medications that are administered, as well as the role of pharmacologic antagonists for opioids and benzodiazepines. Individuals who monitor children who receive sedation/analgesia should be able to recognize the associated complications. At least one individual capable of establishing a patent airway and positive-pressure ventilation, as well as a means for summoning additional assistance, should be present whenever sedation/analgesia is administered. It is recommended (although not specifically stated) that an individual with advanced life support skills be immediately available (within 5 minutes) for moderate sedation; such an individual must be within the procedure room for deep sedation.
 Availability of Emergency Equipment: Pharmacologic antagonists, as well as appropriately sized equipment for establishing a patent airway and providing positive-pressure ventilation with supplemental oxygen, should be present whenever sedation/analgesia is administered. Suction, advanced airway equipment, and resuscitation medications should be immediately available and in good working order. A functional defibrillator should be immediately available whenever deep sedation is administered and when moderate sedation is administered to those with mild or severe cardiovascular disease.
Availability of Emergency Equipment: Pharmacologic antagonists, as well as appropriately sized equipment for establishing a patent airway and providing positive-pressure ventilation with supplemental oxygen, should be present whenever sedation/analgesia is administered. Suction, advanced airway equipment, and resuscitation medications should be immediately available and in good working order. A functional defibrillator should be immediately available whenever deep sedation is administered and when moderate sedation is administered to those with mild or severe cardiovascular disease.
 Use of Supplemental Oxygen: Equipment to administer supplemental oxygen should be present when sedation/analgesia is administered. Supplemental oxygen should be considered for moderate sedation and should be administered during deep sedation unless specifically contraindicated for a particular child or procedure. If hypoxemia is anticipated or develops during sedation/analgesia, supplemental oxygen should be administered.
Use of Supplemental Oxygen: Equipment to administer supplemental oxygen should be present when sedation/analgesia is administered. Supplemental oxygen should be considered for moderate sedation and should be administered during deep sedation unless specifically contraindicated for a particular child or procedure. If hypoxemia is anticipated or develops during sedation/analgesia, supplemental oxygen should be administered.
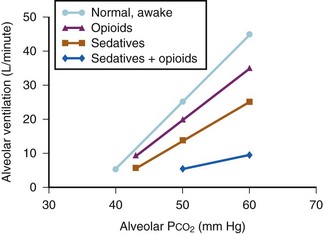
 Combinations of Sedative/Analgesic Agents: Combinations of sedative and analgesic agents may be administered as indicated for the procedure being performed and the condition of the child. Ideally, each component should be administered individually to achieve the desired effect (e.g., additional analgesic medication to relieve pain; additional sedative medication to decrease awareness or anxiety). The propensity for combinations of sedative and analgesic agents to cause respiratory depression and airway obstruction emphasizes the need to appropriately reduce the dose of each component, as well as the need to continually monitor respiratory function (Fig. 47-4).
Combinations of Sedative/Analgesic Agents: Combinations of sedative and analgesic agents may be administered as indicated for the procedure being performed and the condition of the child. Ideally, each component should be administered individually to achieve the desired effect (e.g., additional analgesic medication to relieve pain; additional sedative medication to decrease awareness or anxiety). The propensity for combinations of sedative and analgesic agents to cause respiratory depression and airway obstruction emphasizes the need to appropriately reduce the dose of each component, as well as the need to continually monitor respiratory function (Fig. 47-4).
 Titration of Intravenous Sedative/Analgesic Medications: Intravenous (IV) sedative/analgesic drugs should be given in small, incremental doses that are titrated to the desired end points of analgesia and sedation. Sufficient time must elapse between doses to allow the effect of each dose to be assessed before subsequent drug administration. When drugs are administered by non-IV routes (e.g., oral, rectal, intramuscular [IM], transmucosal), allowance should be made for the time required for drug absorption before supplementation is considered. Because absorption may be unpredictable, administration of repeat doses of oral medications to supplement sedation/analgesia is not recommended.
Titration of Intravenous Sedative/Analgesic Medications: Intravenous (IV) sedative/analgesic drugs should be given in small, incremental doses that are titrated to the desired end points of analgesia and sedation. Sufficient time must elapse between doses to allow the effect of each dose to be assessed before subsequent drug administration. When drugs are administered by non-IV routes (e.g., oral, rectal, intramuscular [IM], transmucosal), allowance should be made for the time required for drug absorption before supplementation is considered. Because absorption may be unpredictable, administration of repeat doses of oral medications to supplement sedation/analgesia is not recommended.
 General Anesthetic Induction Agents Used for Sedation/Analgesia (Propofol, Methohexital, Ketamine): Even if moderate sedation is intended, children who are sedated with either propofol or methohexital by any route should receive care consistent with that required for deep sedation. Accordingly, practitioners who administer these drugs should be qualified to rescue the children from any level of sedation, including general anesthesia. Children who are sedated with ketamine should be cared for in a manner consistent with the level of sedation that is achieved.
General Anesthetic Induction Agents Used for Sedation/Analgesia (Propofol, Methohexital, Ketamine): Even if moderate sedation is intended, children who are sedated with either propofol or methohexital by any route should receive care consistent with that required for deep sedation. Accordingly, practitioners who administer these drugs should be qualified to rescue the children from any level of sedation, including general anesthesia. Children who are sedated with ketamine should be cared for in a manner consistent with the level of sedation that is achieved.
 Recovery Care: After sedation for diagnostic and therapeutic procedures, the children should be observed in an appropriately staffed and equipped area until they are near their baseline level of consciousness and are no longer at increased risk for cardiorespiratory depression. Oxygenation should be monitored periodically until they are no longer at risk for hypoxemia. Ventilation and circulation should be monitored at regular intervals until the children are suitable for discharge. Discharge criteria should be designed to minimize the risk of central nervous system (CNS) or cardiorespiratory depression after discharge from observation by trained personnel.
Recovery Care: After sedation for diagnostic and therapeutic procedures, the children should be observed in an appropriately staffed and equipped area until they are near their baseline level of consciousness and are no longer at increased risk for cardiorespiratory depression. Oxygenation should be monitored periodically until they are no longer at risk for hypoxemia. Ventilation and circulation should be monitored at regular intervals until the children are suitable for discharge. Discharge criteria should be designed to minimize the risk of central nervous system (CNS) or cardiorespiratory depression after discharge from observation by trained personnel.
 Consultation and Availability of an Anesthesiologist: Whenever possible, appropriate medical specialists should be consulted before sedating children with significant underlying conditions. The choice of specialists depends on the nature of the underlying condition and the urgency of the situation. For severely compromised or medically unstable children (e.g., anticipated difficult airway, severe obstructive pulmonary disease, or congestive heart failure), or if it is likely that sedation to the point of unresponsiveness will be necessary to obtain adequate conditions, practitioners who are not trained in the administration of general anesthesia should consult an anesthesiologist (Table 47-6).
Consultation and Availability of an Anesthesiologist: Whenever possible, appropriate medical specialists should be consulted before sedating children with significant underlying conditions. The choice of specialists depends on the nature of the underlying condition and the urgency of the situation. For severely compromised or medically unstable children (e.g., anticipated difficult airway, severe obstructive pulmonary disease, or congestive heart failure), or if it is likely that sedation to the point of unresponsiveness will be necessary to obtain adequate conditions, practitioners who are not trained in the administration of general anesthesia should consult an anesthesiologist (Table 47-6).
TABLE 47-6 Guidelines for the Consultation of an Anesthesiologist
2. Procedures requiring deep sedation in patients with a full stomach
ASA, American Society of Anesthesiologists; BMI, body mass index.
The above ASA guideline pertains to all age-groups. The AAP’s most recent guideline update was published in 2006.18 In general, their updated guideline reinforces the ASA guideline. The AAP updated revision retains the same definitions of the continuum of sedation that are identical to the ASA and Joint Commission definitions. The AAP guidelines emphasize a systematic approach to sedation that includes:
 No administration of sedative medications without the safety net of medical supervision (i.e., no sedative medications given at home)
No administration of sedative medications without the safety net of medical supervision (i.e., no sedative medications given at home)
 Careful presedation evaluation to include review of pertinent medical and surgical conditions
Careful presedation evaluation to include review of pertinent medical and surgical conditions
 Careful history for ingestion of nutraceuticals and other medications that may alter drug metabolism and prolong sedation
Careful history for ingestion of nutraceuticals and other medications that may alter drug metabolism and prolong sedation
 A “time out” should be performed before sedation
A “time out” should be performed before sedation
 Appropriate fasting guidelines for elective and urgent procedures. There should be a balance between the depth of sedation and the risk for those who are unable to fast because of the urgent nature of the procedure
Appropriate fasting guidelines for elective and urgent procedures. There should be a balance between the depth of sedation and the risk for those who are unable to fast because of the urgent nature of the procedure
 Focused airway examination with particular attention to anatomic airway abnormalities and enlarged tonsils
Focused airway examination with particular attention to anatomic airway abnormalities and enlarged tonsils
 Understanding of the pharmacologic and PD effects of sedation medications and drug interactions
Understanding of the pharmacologic and PD effects of sedation medications and drug interactions
 Appropriate training and skills in airway management to allow for rescue. Deep sedation requires training in pediatric advanced life support
Appropriate training and skills in airway management to allow for rescue. Deep sedation requires training in pediatric advanced life support
 Immediate availability of size- and age-appropriate airway, monitoring, and resuscitation equipment
Immediate availability of size- and age-appropriate airway, monitoring, and resuscitation equipment
 Appropriate medications and reversal agents
Appropriate medications and reversal agents
 Sufficient numbers of sedation providers to carry out the procedure and monitor the child
Sufficient numbers of sedation providers to carry out the procedure and monitor the child
 Appropriate physiologic monitoring during and after the procedure; use of capnography is encouraged
Appropriate physiologic monitoring during and after the procedure; use of capnography is encouraged
 Appropriate recovery personnel, monitoring, and discharge criteria with return to baseline condition before discharge
Appropriate recovery personnel, monitoring, and discharge criteria with return to baseline condition before discharge
 Prolonged observation in a step-down unit of children who have been sedated with drugs known to have a long half-life (e.g., chloral hydrate, “lytic cocktail” [see later discussion])
Prolonged observation in a step-down unit of children who have been sedated with drugs known to have a long half-life (e.g., chloral hydrate, “lytic cocktail” [see later discussion])
 Continuous quality improvement to track common markers of potential safety issues, such as desaturation events, airway obstructions, laryngospasm, unplanned hospital admission, unsatisfactory sedation, and medication errors
Continuous quality improvement to track common markers of potential safety issues, such as desaturation events, airway obstructions, laryngospasm, unplanned hospital admission, unsatisfactory sedation, and medication errors
 Use of simulators to practice how to manage rare adverse events
Use of simulators to practice how to manage rare adverse events
 Assume all children younger than 6 years of age to be deeply sedated from the beginning of the sedation process
Assume all children younger than 6 years of age to be deeply sedated from the beginning of the sedation process
Other organizations, including the American College of Emergency Physicians (ACEP), published their own sedation guidelines.101–105 The latter guidelines are distinguishable from the AAP and ASA guidelines in several respects, including the definition of the continuum of sedation. The ACEP guideline does not use the terms “moderate” or “deep” sedation but rather the term “procedural sedation.” It is defined “as a technique of administering sedatives, analgesics, dissociative agents, alone or in combination to induce a state that allows the child to tolerate unpleasant procedures while maintaining cardiopulmonary function.” It is intended to result in a depressed level of consciousness but one that allows the child to “independently and continuously” control their own airway. Unfortunately, the lack of uniform agreement on definitions and overlap of “procedural sedation” into “deep sedation” and “anesthesia” has led to considerable confusion and debate among practitioners. In 2006 the ASA asserted that “privileges to administer deep sedation should be granted only to practitioners who are qualified to administer general anesthesia.”93,94 Intensivists and emergency room physicians took umbrage with the ASA “usurping” control over their sedation practice.11 They pointed out that their specialties have been using drugs, such as propofol and ketamine, for procedural sedation with minimal complications at levels that are considered deep sedation or anesthesia for many years,106,107 and that they resent an outside organization imposing guidelines on their practices. In 2010 the ASA acknowledged that Medicare regulations permit some non–anesthesia practitioners to administer deep sedation and created guidelines to help hospital directors of anesthesia services grant privileges to such individuals.93 The guidelines include: formal training in administration of deep sedation during residency training (within 2 years) or an Accreditation Council for Graduate Medical Education accredited training program; experience and competency in managing sedated patients; clinical experience with more than 35 patients; knowledge of ASA guidelines; advanced cardiac life support training; and quality assurance tracking. Separate privileging is required for the care of children. The exact nature of these requirements are not specified, but are over and above baseline competencies and dependent on the hospital’s director of anesthesia services.
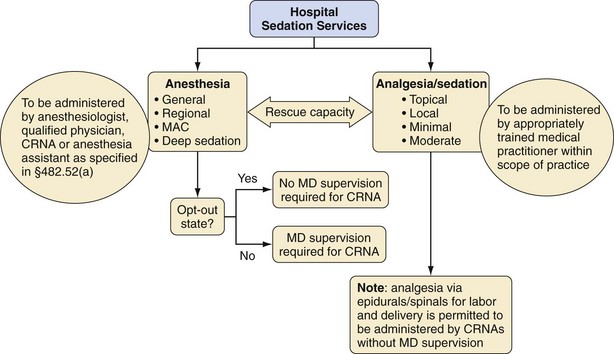
It is mandatory that sedation policies used in hospitals conform to Joint Commission standards that have been derived from the Department of Health & Human Services (DHHS) Centers for Medicare & Medicaid Services (CMS). In the 1990s, the Joint Commission recognized that there is considerable variation in the level of care provided to sedated children, depending on where the sedation is administered, even within one institution. They mandated that the standard of care for all sedated patients be uniform throughout any one institution Thus institutionally standardized documentation (e.g., medical history, physical status, and record-keeping during the procedure and the recovery from the procedure), fasting guidelines, and informed consent procedures are mandatory for all patients undergoing sedation, regardless of the nature, duration, patient’s history, and location of the procedure. Similarly, the sedation personnel, monitoring equipment, and recovery facilities must be standardized or uniform within an institution. All of this must be part of an active quality improvement process. Hospitals risk losing federal funding if they fail to comply. In 2004, 18% of hospitals were found noncompliant in planning the administration of moderate or deep sedation.108 In the most recent Joint Commission regulations, 2010,25 the leadership and responsibilities for the delivery of deep sedation fall on the shoulders of the anesthesia department (Fig. 47-5).
Specific standards for sedation are mentioned in Provision of Care, Treatment and Services (PC.03.01.01, .03, .05 .07), Improving Organization Performance (PI.01.01.01), and records (RC.02.01.03).23 These standards address moderate and deep sedation, as well as anesthesia services. The standards require that each hospital develop specific appropriate protocols for patients receiving sedation. These protocols must be consistent with professional standards and address the following:
1. Qualified individuals in sufficient numbers to perform and monitor patients during and after the procedure. A registered nurse must supervise perioperative nursing care.
2. Competency-based education, training, and experience in evaluating patients. These must include the following:
3. Appropriate equipment for care and resuscitation
4. The following must occur before moderate or deep sedation:
5. Appropriate monitoring of vital signs during and after the procedure, including, but not limited to, heart rate and oxygenation using pulse oximetry, respiratory frequency and adequacy of pulmonary ventilation, monitoring of BP at regular levels, and cardiac monitoring (by ECG or use of a continuous cardiac monitoring device) in patients with significant cardiovascular disease or when dysrhythmias are anticipated or detected
6. Documentation of care before, during, and after the procedure
7. Monitoring of outcomes. In particular, analysis of data is performed on adverse events or patterns of adverse events during moderate or deep sedation.
Implementation of Sedation Guidelines
The implementation of a successful institution-wide policy involves organization, education, record keeping, enforcement, and continuing quality improvement.109 A sedation committee must be carefully organized and involves many departments, practitioners, and geographic areas within the institution. The goal of the committee is to create a sedation policy that can facilitate patient care without placing undue burden on practitioners. Ideally, the committee should be composed of representatives from at least one and preferably two to three sedation practitioner services (e.g., an endoscopist, intensivist, dentist, surgeon, or emergency medicine physician) and departments: anesthesiology, nursing, pharmacy, hospital administration, and—very importantly—risk management. The responsibilities of the sedation committee include the creation of hospital- or institution-wide sedation policies, determination of hospital- or institution-wide personnel and equipment needs, creation of educational programs, monitoring of sedation problems, modification of policies as needed, and granting sedation privileges.
The department of anesthesiology (chairperson or their designee) must play a central role in formulating policy, educating nonanesthesiology sedation practitioners, acting as consultants for potentially complex cases, particularly those patients with a difficult airway, and determining when sedation by a nonanesthesiologist is or is not appropriate. The department of anesthesiology should approve sedation flow sheets and records, and be involved, along with the committee and the institution’s risk management department, in periodic review of the records and compliance with documentation and institutional policies and procedures. A member of the department of anesthesiology is responsible for the process of continuous quality improvement. Continuous quality improvement is needed to review complications, incident reports, and sedation flow sheets to ensure compliance with policy and recommend changes to the sedation committee. Finally, sedation and analgesia require a treatment plan. The department of anesthesiology must play a leading role in determining which sedatives, hypnotics, general anesthetics, and analgesics can be safely used alone and in combination in each institution. Several drugs in particular can easily produce deep sedation or general anesthesia, airway obstruction, an unprotected airway, and cardiorespiratory collapse, namely, methohexital, thiopental, nitrous oxide (when combined with other sedating medications), ketamine, propofol, and remifentanil. Which drugs can be used and by whom should be made on an institution-by-institution basis. Education is vital to maintain safety. An institution-wide ongoing educational program on sedation, emphasizing physician (and dentist) responsibility, nursing responsibility, guidelines, and the pharmacology of drugs, should be given frequently enough to train the staff and to accommodate staff turnover (usually one to two times a year). Teaching modules, videos, handouts, simulations, and hands-on supervision have been used to supplement such programs.87 One institution (CNMC) uses a computerized teaching module that includes a review of hospital policy, equipment, personnel, pharmacology of drugs used, and rescue from deeper levels of sedation (see Children’s Exchange [CHEX] course module). A quiz must be successfully completed at the end of the computerized teaching module. The module is part of the orientation and hospital privileging procedure for each physician and nurse, and must be successfully completed before they can administer sedation; the module must be reviewed and successfully completed every 2 years. Staff privileges also require training for Basic Life Support or equivalent for those providing moderate sedation and Pediatric Advanced Life Support or equivalent for those administering deep sedation. Each division chief or section supervisor must attest to the practitioner’s competency to provide the desired level of sedation.
Specialty organizations are also becoming involved in training of sedation providers. The Society for Pediatric Sedation provides an extensive “primer” on pediatric sedation on its web site at www.pedsedation.org. This organization also provides a credentialing course for pediatric sedation that uses a test of knowledge based on its written materials (see previous) and the completion of a full day “Sedation Provider Course,” which includes lectures, interactive sessions, and human patient simulation sessions (developed from data relating to pediatric sedation adverse events) to teach and test core competencies.
Education should also emphasize the limits of sedation and appropriate referral to an anesthesiologist. Of particular concern is upper airway obstruction that would likely become worse with the administration of sedatives (Table 47-6).54 Tonsil and adenoid hypertrophy is common in children aged 2 to 12 years, and is associated with loud snoring or obstructive sleep apnea. Obstructive sleep apnea occurs in 1% to 3% of all children, in up to 12% of adolescents, is threefold more frequent in African American children and fivefold more frequent in obese children.110 Parents will frequently report that their child snores loudly and then “stops breathing.” These children are at increased risk for airway obstruction and should be referred to an airway specialist (anesthesiologist, pediatric intensivist, pediatric emergency medicine specialist) if sedation will be required for their procedures. They may also require postsedation overnight admission and monitoring (see also Chapters 4 and 31).98,111–113 Children with developmental disabilities should raise concern for sedation because they have a high incidence of airway obstruction during sedation and anesthesia.114 Problems for which consultation with an anesthesiologist or other expert is suggested are listed in Table 47-6.
The performance improvement administrative part of sedation implementation is the final piece of the puzzle. Compliance can be monitored by the medical and dental staff office, the department of nursing, as well as a committee charged with the responsibility of continuing quality improvement. This committee should fall under the purview of risk management. Nursing and medical staff offices should monitor compliance with educational certification for appropriate credentialing. The medical staff office should report to the department chairman and nursing office a list of individuals who need to be recertified in sedation. It is the responsibility of the department chairman and nursing supervisors to secure individual staff compliance. Finally, variance reports should be generated when sedation policy is not followed or when a critical incident occurs. The appropriate institutional committee reviews the incident and informs the sedation committee. Educational and corrective action should take place as quickly as possible. This committee should not be viewed as “sedation police,” but rather as a resource to objectively and unemotionally review critical sedation-related events. The committee can then determine where the “system” broke down, to define what went wrong and why (e.g., inadequate history taking combined with sedation, inadequate monitoring combined with delay in problem recognition, or inadequate recovery procedures combined with re-sedation at home). Tracking common problems, such as desaturation events, the need for bag-mask ventilation, apnea, unplanned hospital admissions, unsatisfactory sedation, and so on, can provide direction for developing changes in policy that will prevent more severe but much less frequent events from occurring (Fig. 47-6). This type of critical incident analysis allows recommendations to be made for future prevention.115–117 An example of a successful hospital sedation policy is that of the CNMC (see Appendix 47-1).
Documentation
Although differing departmental procedures, practice requirements, patient needs, and location limitations produce unique problems, a unified systematic approach to sedation creates a safety net that will protect children while providing sedation/analgesia for procedures. A single sedation flow sheet that is used wherever sedation is given within the institution will allow for uniformity of care and compliance with sedation policy. The universal sedation flow sheet allows for nurses to rotate to different areas and feel familiar with sedation requirements in different locations. The sedation flow sheet should be organized such that the practitioners have a practical guide to the sedation policy. Following and completing the sedation flow sheet will ensure compliance with hospital policy (E-Fig 47-1). CNMC is in the process of converting to electronic records. As such we have an opportunity to move to an electronic paperless sedation document, which can “pull in” data from other databases and automatically add vital signs (Video 47-5)![]() . Our first draft will be available through this book. We still use the paper form when we are in areas that are remote or that do not have electronic record keeping (e.g., MRI, offsite locations) (see E-Fig 47-1). The flow sheet (both paper and electronic) is organized into Presedation, During Sedation, and Postsedation responsibilities of the registered nurse and licensed independent practitioner (physician or dentist) providing sedation. The electronic version has various dropdown menus which allows the practitioner to access rating scale information and automatically enter vital signs (see Video 47-5).
. Our first draft will be available through this book. We still use the paper form when we are in areas that are remote or that do not have electronic record keeping (e.g., MRI, offsite locations) (see E-Fig 47-1). The flow sheet (both paper and electronic) is organized into Presedation, During Sedation, and Postsedation responsibilities of the registered nurse and licensed independent practitioner (physician or dentist) providing sedation. The electronic version has various dropdown menus which allows the practitioner to access rating scale information and automatically enter vital signs (see Video 47-5).
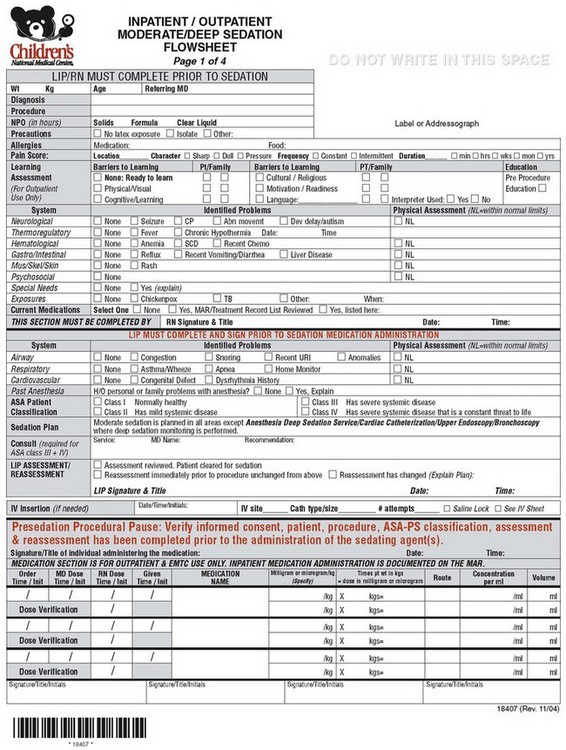
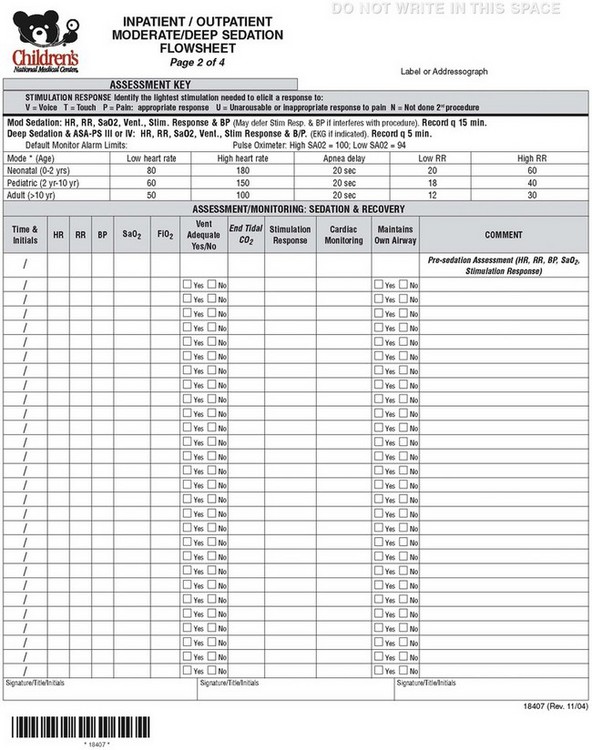
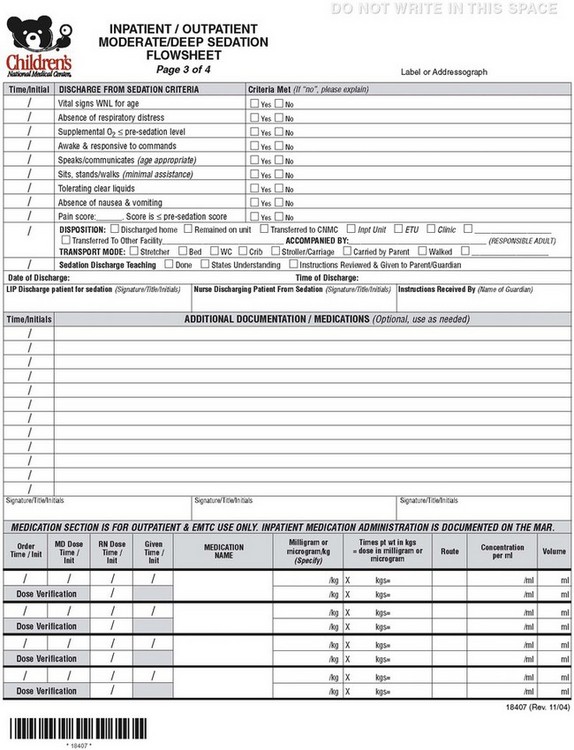

E-FIGURE 47-1 Sample hospital sedation flow sheet (four pages).
(Courtesy Children’s National Medical Center, Washington, D.C.)
 Presedation responsibilities: During this time, the responsibilities of the registered nurse include evaluation of the child to include learning assessment, NPO (nothing by mouth) status, allergies, current medications, and review of medical problems. The licensed independent practitioner verifies this information with emphasis on airway assessment. A sedation plan is identified and informed consent is obtained. The licensed independent practitioner assigns an ASA physical status and reassesses the child immediately before the procedure.
Presedation responsibilities: During this time, the responsibilities of the registered nurse include evaluation of the child to include learning assessment, NPO (nothing by mouth) status, allergies, current medications, and review of medical problems. The licensed independent practitioner verifies this information with emphasis on airway assessment. A sedation plan is identified and informed consent is obtained. The licensed independent practitioner assigns an ASA physical status and reassesses the child immediately before the procedure.
 Presedation pause: A “time out” is performed immediately before sedation medications are given. The “time out” includes a process of verbal confirmation of the patient and procedure identification, verification of informed consent, as well as ASA physical status and confirmation of the procedure and site of the procedure. No medications will be administered without completion of the “time out.” One advantage of the electronic form is that it forces the licensed independent practitioner to verify “time out” before it allows further access to the rest of the sedation flow sheet.
Presedation pause: A “time out” is performed immediately before sedation medications are given. The “time out” includes a process of verbal confirmation of the patient and procedure identification, verification of informed consent, as well as ASA physical status and confirmation of the procedure and site of the procedure. No medications will be administered without completion of the “time out.” One advantage of the electronic form is that it forces the licensed independent practitioner to verify “time out” before it allows further access to the rest of the sedation flow sheet.
 During sedation: The time-based flow sheet identifies the type of stimulation needed to elicit a response, as well as what monitors and how frequently vital signs should be recorded for both moderate and deep sedation. A summary of monitoring, documentation, personnel, and equipment during sedation is given in Table 47-7.
During sedation: The time-based flow sheet identifies the type of stimulation needed to elicit a response, as well as what monitors and how frequently vital signs should be recorded for both moderate and deep sedation. A summary of monitoring, documentation, personnel, and equipment during sedation is given in Table 47-7.
 Postsedation: The criteria for discharge from the sedation area, pain score, and disposition of the child, as well as discharge teaching are specified. This includes documentation and signature by the licensed independent practitioner and nurse. Space is given for additional documentation and medications given.
Postsedation: The criteria for discharge from the sedation area, pain score, and disposition of the child, as well as discharge teaching are specified. This includes documentation and signature by the licensed independent practitioner and nurse. Space is given for additional documentation and medications given.
TABLE 47-7 Recommended Intensity of Monitoring, Documentation, Personnel, and Equipment for Different Levels of Sedation
| Moderate Sedation | Deep Sedation | |
|---|---|---|
| Monitoring |
ECG, Electrocardiography; etco2, end-tidal carbon dioxide.
Individual departments may have special needs or situations that require addenda to the hospital sedation flow sheet. In addition, individual departments may find it helpful to create information sheets on presedation assessment, telephone interviews, and postsedation follow-up. The radiology–nursing database that consists of presedation telephone information, presedation instructions, NPO guidelines, health history, and postexamination telephone call information is shown in E-Figures 47-2 and 47-3. Samples of presedation and postsedation instruction sheets for pediatric dentistry are shown in E-Figures 47-4 and 47-5, respectively. These forms are available in Spanish and are explained via interpreters if necessary.


E-FIGURE 47-2 Sample department of radiology/nursing database patient sedation phone call and assessment form.
(Courtesy Children’s National Medical Center, Washington, D.C.) PIV, Peripheral IV.

E-FIGURE 47-3 Sample diagnostic imaging and radiology discharge teaching information sheet for parents or guardians of treated children.
(Courtesy Children’s National Medical Center, Washington, D.C.)
Specific Sedation Techniques
A sedation treatment plan analyzing the requirements for analgesics, anxiolytics, or both is necessary for each child and will vary depending on the procedure and the anxiety of the child and family. Psychological techniques to allay anxiety (cuddling, parental support, warm blankets, a gentle reassuring voice, and hypnosis) are extraordinarily useful adjuncts to the sedation plan.6–10
Although the FDA is responsible for approval of the use of drugs, for a variety of reasons many of the drugs used for sedation and analgesia in children are not approved by the FDA for use in young children (e.g., fentanyl less than 2 years; morphine less than 12 years, bupivacaine less than 12 years, propofol less than 2 months, and dexmedetomidine less than 18 years of age). Although midazolam is approved even in preterm infants, the reversal agent flumazenil is not approved in children younger than 1 year of age! The lack of “approval” by the FDA does not imply that a drug should not be used; rather, it only means that the manufacturer never carried out the appropriate studies to gain FDA approval.28,118–121 A number of legislative changes are intended to improve drug research in children and improve drug labeling; however, some of the drugs are no longer under patent protection and there is no motivation for drug companies to study their use in children.122–124 It is hoped that the continuing legislative efforts will improve drug labeling for children; see Chapter 6 for a more complete discussion of this issue.
It is important to review the child’s presedation medications. Particular note of the use of protease inhibitors (used by many patients with human immunodeficiency virus infection) must be sought. These protease inhibitors (e.g., nelfinavir, ritonavir, saquinavir) are potent inhibitors of the cytochrome P-450 CYP3A metabolic pathway. This pathway is responsible the metabolism of many sedatives, including midazolam, and may markedly prolong duration of action and may lead to life-threatening respiratory depression. Erythromycin and some calcium channel blockers may also inhibit the cytochrome system and delay metabolism of midazolam.125–127 Dexmedetomidine should not be combined with digoxin in infants because severe bradycardia has been reported.128 Another concern for the practitioner is the widespread use of herbal medicines that can enhance or shorten sedative medication activity.129–133 Herbal medicines (e.g., St. John’s wort or echinacea) may alter drug PK through inhibition of the cytochrome P450 system, resulting in prolonged drug effect and altered (increased or decreased) blood drug concentrations. Kava may increase the effects of sedatives, and valerian may itself produce sedation (see also E-Table 4-4).134
Local Anesthetics
Local anesthetics play a critical role in analgesia for painful procedures and greatly reduce requirements for systemic opioids. Unlike most drugs used in medicine, local anesthetics must be physically deposited at their site of action to produce their effect. Cessation of action often depends on the rapidity of removal of drug from this site. Systemic toxicity is related to how much and how rapidly local anesthetics are absorbed into the blood. Thus small amounts of local anesthetics can be toxic if injected intraarterially or IV. On the other hand very large amounts of local anesthetics can be safely administered in vascular-poor structures like fat. For most blocks and local skin infiltration, epinephrine (1 : 200,000 [5 µg/mL]) is used as a vasoconstrictor to lengthen the duration of blockade, decrease bleeding, and reduce systemic toxicity by decreasing vascular uptake. Local anesthetics should only be prepared in labeled syringes immediately before use. They should never be prepared before a procedure starts because of the possibility of inadvertent and catastrophic injection of the local anesthetic into an IV catheter or infusion. Finally, no more than the maximum allowable dose (mg/kg) should be prepared in the syringe to minimize the risk of an accidental overdose (Table 47-8). Epinephrine-containing local anesthetics can cause tissue ischemia and are contraindicated in end-arterial areas (digits, ear, and penis), as well as in Bier blocks. Local anesthetics can obtund airway reflexes when sprayed in the mouth for bronchoscopy or gastrointestinal endoscopy.
Specific blocks used in the sedated child include subcutaneous (SC) infiltration, field blocks, and IV regional anesthesia. A discussion of these topics and treatment of local anesthetic toxicity is found in Chapters 41 and 42. Topical administration of local anesthetics is useful in the sedated patient. EMLA cream is a eutectic mixture of local anesthetics (lidocaine 2.5% and prilocaine 2.5%) (AstraZeneca Pharmaceutical LP, Wilmington, Del.). When placed on the skin for 60 minutes,135,136 it is useful for reducing the pain of skin incision, IV cannula insertions, lumbar punctures, and circumcision.137–139 Absorption of large amounts of prilocaine can cause methemoglobinemia. It should be applied only to normal intact skin in appropriate doses31 (i.e., for children weighing less than 10 kg, apply to a maximum of approximately 100 cm2 surface area; for those 10 to 20 kg, apply to a maximum of approximately 600 cm2 surface area; and for those more than 20 kg, apply to a maximum of approximately 2000 cm2 surface area). The duration of action is 1 to 2 hours after the cream is removed. Adverse reactions include erythema, itching, rash, and methemoglobinemia. It also causes blanching of the skin, which can make IV access difficult. It is contraindicated in children younger than 1 month of age, in children with congenital or idiopathic methemoglobinemia, or in infants receiving methemoglobinemia-inducing drugs (e.g., phenytoin, phenobarbital, acetaminophen, and sulfonamides).
ELA-Max and LMX4 (Ferndale Healthcare, Ferndale, Mich.) are topical 4% liposomal lidocaine solutions whose effects occur in 30 minutes.140 The S-Caine patch (ZARS, Inc., Salt Lake City) is a eutectic mixture of 70 mg of lidocaine and 70 mg tetracaine in a bioadhesive layer that contains a heating element. The 20-minute application is effective in lessening pain from venipuncture procedures.141
Anxiolytics and Sedatives
Chloral hydrate is one of the most widely used sedatives in neonates and children younger than 3 years of age (Table 47-9).142–145 It is used as a sedative to facilitate nonpainful diagnostic procedures, such as EEG, CT, or MRI.144 It is rapidly and completely absorbed when given orally. Rectal administration is erratically absorbed and therefore not recommended. The onset of sedation is 30 to 60 minutes, and the usual clinical duration is 1 hour. Although it has a long safety record, it can cause respiratory depression as a result of airway obstruction. Deaths have been associated with its use alone and when combined with other sedating medications.54,113,146–149 One large series showed a 0.6% incidence of respiratory depression, especially at larger doses (75 to 100 mg/kg).150 Its effect is primarily mediated by the active metabolite trichloroethanol, which is formed by the liver and erythrocytes.151 Trichloroethanol has a half-life of 10 hours in toddlers, 18 hours in term infants, and 40 hours in preterm infants (see Fig. 47-3).152 The prolonged effects of chloral hydrate warrant a longer period of postsedation observation; chloral hydrate can also cause adverse effects after discharge, which include motor imbalance (31%), gastrointestinal effects (23%), agitation (19%), and restlessness (14%). Agitation and restlessness lasted more than 6 hours in more than one-third of children who experienced these effects, 5% of whom did not return to baseline activity for 2 days after the procedure!44,153 The unpredictable onset and active metabolites dictate that this drug (as well as all sedatives for sedation) is given only in facilities capable of resuscitation (AAP guidelines) and that discharge occurs when sedation is clearly lessening and the child meets discharge criteria. Airway obstruction and death occurred after chloral hydrate sedation in a child who was in a child’s car seat in the back of a car.54 Despite being restricted in some countries as a result of potential carcinogenicity, in the United States the AAP states that there is insufficient evidence to avoid single doses of chloral hydrate.154
The benzodiazepines are commonly used in pediatric sedation. They are anxiolytic, amnestic, sedative hypnotics with anticonvulsant activities but without analgesic properties. Their high lipid solubility at physiologic pH accounts for the rapid CNS effects. As opposed to diazepam, midazolam is delivered in a water-soluble form (pH 3.5), which markedly decreases the incidence of pain on injection and thrombophlebitis.151 However, the resulting decrease in fat solubility markedly delays transport into the CNS (peak EEG effect 4.8 minutes for midazolam vs. 1.6 minutes for diazepam; Fig. 47-7).155,156 Benzodiazepines exert their effects by occupying the benzodiazepine receptor that modulates γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain. The clearance of benzodiazepines is decreased in neonates and also by liver enzyme inhibition that occurs during concomitant use of erythromycin, cimetidine, or protease inhibitors.126
The sedated child usually becomes compliant but does not lose consciousness. Children frequently move, and another agent, such as an opioid, may be necessary if the child must not move to successfully accomplish the procedure. Although many children initially act disinhibited and “drunk” after small doses of benzodiazepines, some have a true paradoxical response.157 Paradoxical responses, such as combativeness, disorientation, hyperexcitability, and agitation may appear in 1% to 15% of treated children.158 It is prudent to switch to a different sedative drug in these children, because increasing the dose of benzodiazepines may lead to severe agitation followed by unconsciousness and respiratory compromise. The benzodiazepines have the advantage of antegrade amnesia in a significant number of children.159,160 The markedly prolonged and variable elimination half-life and active metabolite of diazepam (desmethyldiazepam) make midazolam a superior sedative drug in children, particularly infants.161–166 The time to peak effect after IV administration of midazolam is 2 to 4 minutes, and its duration is 45 to 60 minutes. Midazolam can be given IV, intranasally, sublingually, orally, or rectally (see Table 47-9). It is the only drug in this class approved for neonates. A manufactured oral cherry-flavored form is available. The oral route has become very popular and is well tolerated. The oral route can lead to variable effects, dependent on gastric emptying and first-pass hepatic metabolism. Nasal administration causes nasopharyngeal burning and should be avoided.167,168 Rectal administration is usually well tolerated in children who have not been toilet trained (younger than approximately 1 year of age), but absorption may be irregular owing to many factors, including superior versus inferior hemorrhoidal vein absorption within the rectum. Benzodiazepines produce mild respiratory depression and upper airway obstruction.169–171 Even small doses of oral midazolam (0.3 mg/kg) that cause minimal sedation were associated with a 6.5% decrease in functional residual capacity and a 7.4% increase in respiratory resistance.172,172
Respiratory depression may become severe in compromised children or in children with tonsil hypertrophy. Benzodiazepines must be given according to appropriate guidelines.18,56,74 The combination of benzodiazepines and opioids is particularly troubling because they can produce a “super additive effect” on respiratory depression where the total depressant effect from the combination of drugs is much greater than the sum of their anticipated individual effects.173,174
Flumazenil is a specific benzodiazepine-receptor antagonist and will rapidly reverse the sedative and respiratory effects of benzodiazepines.175–179 It is the first specific reversal agent for benzodiazepines and rapidly reverses CNS-induced unconsciousness, respiratory depression, sedation, amnesia, and psychomotor dysfunction. Children who take benzodiazepines for seizures or drug dependency may have those symptoms rapidly recur if flumazenil is given.180 It should also be used with caution in children with benzodiazepine dependence, increased intracranial pressure, and in those taking drugs that decrease the seizure threshold (e.g., cyclosporine, theophylline, and lithium). The recommended dose of flumazenil is 10 µg/kg up to 0.2 mg every minute to a maximum cumulative dose of 1 mg IV. Antagonism begins within 1 to 2 minutes and lasts approximately 1 hour. Because re-sedation after 1 hour may occur, any child who receives flumazenil must be carefully monitored for at least 2 hours. Repeat flumazenil may be necessary. It should be noted that flumazenil will not antagonize respiratory depression caused by opioids.181 Flumazenil should not be administered for the routine reversal of the sedative effects of benzodiazepines, but reserved for reversal of respiratory depression.
Barbiturates
Pentobarbital is the most commonly used intermediate-acting barbiturate for sedation. It has no analgesic effect and produces sedation, hypnosis, and amnesia. It has a long history of use during radiologic procedures. Sedation starts in 3 to 5 minutes and peaks in 10 minutes. Studies have shown a low incidence of respiratory obstruction and transient desaturation, as well as hypotension.182,183 The barbiturates tend to make children more sensitive to pain and should be combined with analgesics when used during painful procedures. Pentobarbital’s relatively long duration of action (1 to 2 hours or greater) and slow recovery sometimes leaves children in a prolonged disinhibited state that necessitates prolonged recovery and restraint143; pentobarbital “rage” occasionally occurs. The alkaline pH of barbiturates can lead to local erythema and thrombophlebitis if subcutaneous infiltration occurs. Newer, shorter-acting, faster recovery drugs are quickly replacing pentobarbital (see Table 47-9).
Opioids
Opioid analgesics are rarely used alone for diagnostic and therapeutic procedures in children. These potent analgesics are important during painful diagnostic and therapeutic procedures. They bind with four primary opioid receptor types (µ, κ, δ, and σ) that are located in the brain, spinal cord, and periphery. Once opioids bind to the receptor, they trigger the production of signaling proteins (such as G proteins) whose end effects are modulation of pain neurotransmission.184 The µ1 subgroup is responsible for supraspinal analgesia and the development of dependence. The µ2 and κ receptors are responsible for sedation. Although the opioids provide some sedation, the sedation produced is usually inadequate, therefore requiring combination with a sedative. The “super-additive” respiratory depressant effects of the combination of opioids and sedatives must be reemphasized (see Fig. 47-1).173,185 This is especially true in infants and children with upper airway obstruction (e.g., tonsil and/or adenoid hypertrophy, trisomy 21, mucopolysaccharidosis). In addition, infants younger than 3 months of age and children who were born preterm have very large patient-to-patient drug metabolism variability.186 Other serious effects of opioids include bradycardia, hypotension, seizures, and opioid-induced glottic and/or chest wall rigidity.
Morphine may be considered for painful procedures (lasting more than 1 hour) or when the child will also be in pain after the procedure. The duration of action is 3 to 5 hours after IV administration.186 Morphine may be given orally (0.2 to 0.5 mg/kg), IV (0.05 to 0.1 mg/kg [maximum 0.3 mg/kg]), or IM (0.1 to 0.2 mg/kg). Rectal administration may cause delayed respiratory depression from erratic absorption and should be abandoned.187 Time to peak effect for oral, IV, or IM administration is 60 minutes, 3 to 5 minutes, and 10 to 30 minutes, respectively. Its slow onset and prolonged duration have caused it to be replaced by shorter-acting opioids when used for sedation and analgesia for procedures.
Meperidine (Demerol) was also once used for sedation for procedures. Its clinical duration of action is 2 to 4 hours.31 In addition to respiratory depression, the active metabolite of meperidine (normeperidine) may cause seizures, particularly after repeated doses of meperidine. Other adverse reactions include delirium, nausea, vomiting, urinary retention, pruritus, smooth muscle spasm, and hypotension (histamine induced). Special considerations include avoiding its use in children taking monoamine oxidase inhibitors (effects can include serotonin-induced hyperpyrexia and cardiovascular instability). CNS toxicity may occur in children taking tricyclic antidepressants and phenothiazines. Children taking phenytoin (Dilantin) may have a reduced analgesic effect. Reversal of meperidine-induced respiratory depression by antagonists may precipitate seizures caused by normeperidine. Because of these undesirable effects and prolonged duration, meperidine is not recommended for use as an analgesic or sedative in children.
DPT “lytic cocktail” is meperidine (Demerol [25 mg/mL]), promethazine (Phenergan [6.25 mg/mL]), and chlorpromazine (Thorazine [6.25 mg/mL]). Its long sedation duration (7 to 19 hours), hypotension, seizures, extrapyramidal reactions, and severe, prolonged, life-threatening respiratory depression143,188–191 have caused its use to be abandoned.189–195
IV fentanyl is a potent, pure opioid (i.e., approximately 50 to 100 times more potent than morphine) with no amnesic properties. Its high lipid solubility allows for onset within 30 seconds and a peak effect at 2 to 3 minutes. It has a brief clinical duration of 20 to 40 minutes when given in small doses, owing to its rapid redistribution to skeletal muscle, fat, and other inactive sites. Unlike morphine, it has no active metabolites. The clearance of fentanyl is decreased and its half-life increased in preterm and term infants compared with older infants.196 It is fully antagonized by naloxone. Fentanyl is frequently used with a short-acting anxiolytic (midazolam). IV doses usually start at 0.5 to 1 µg/kg and are titrated every 5 minutes to effect but not to exceed 5 µg/kg.197 Doses must be given in small aliquots and carefully titrated to avoid chest wall and glottic rigidity. When carefully titrated and appropriately monitored, fentanyl has few adverse effects. Chest wall rigidity is a centrally mediated idiosyncratic reaction that can interfere with respiratory function. The mechanism of action is partially modulated by GABA pathways at the spinal level. Chest wall rigidity can be reversed with either naloxone or muscle relaxants. The rigidity is quite rare and not described after these small doses were given in boluses for sedation in neonates.198–200 Other adverse reactions from fentanyl include bradycardia, dysphoria, delirium, nausea, vomiting, pruritus, urinary retention, hypotension, and smooth muscle spasm. Close postprocedural observation is required because respiratory depression can outlast analgesia (see Table 47-9).
Remifentanil an ultrashort-acting, rapid-acting, extremely potent, lipophilic opioid that is metabolized by plasma cholinesterase and must be administered as a continuous infusion. It has a context-sensitive half-life of 3 to 6 minutes that is independent of the duration of infusion. Remifentanil, when used as a sole agent in adults in the ED, caused a 17% incidence of respiratory complications that required bag mask ventilation or the placement of an laryngeal mask airway.201 This suggests a need to clearly understand the PK and PD when used outside of the OR.201
Remifentanil has been used for intraoperative and procedural sedation by anesthesiologists and in intubated children in the ICU.202–207 It has been suggested that children may develop opioid tolerance to this drug after several hours of infusion.208 Remifentanil is associated with a substantial incidence of apnea, hypoxemia, chest wall and glottic rigidity, and should not be used as a sole agent for pediatric sedation (see Table 47-9).204,206,209
Opioid antagonists specifically reverse the respiratory and analgesic effects of opioids, and should be readily available when opioids are used (see Table 47-9). Naloxone (Narcan) is the most commonly used antagonist.210 Opioid antagonists should not be used to routinely reverse the sedative effects of opioids, but reserved for reversal of respiratory depression or respiratory arrest. Naloxone may be given IV, IM, or SC.211 The initial dose for respiratory depression is 0.01 mg/kg titrated to effect every 2 to 3 minutes. A dose of 10 to 100 µg/kg up to 2 mg may be required for respiratory arrest. Adverse reactions from reversal of analgesia include nausea, vomiting, tachycardia, hypertension, delirium, and pulmonary edema.212–214 Children on long-term opioid therapy should be given opioid reversal agents in low doses and with extreme caution, because withdrawal seizures and delirium may occur. Children given naloxone may have recurrence of opioid effects after 1 hour. If naloxone is used, the child should be observed for a minimum of 2 hours. Repeat naloxone may be necessary. To avoid recrudescence of respiratory depression, some clinicians administer the same dose that was effective IV as an IM injection. Nalmefene (Revex) has a greater half-life (approximately 10 hours) than naloxone.215 Although experience in children is limited, it has been shown to accelerate recovery from sedation.216 Because of its relatively long half-life, it outlasts the effects of fentanyl and negates the treatment of pain with opioids for several hours.
α2-Adrenoceptor Agonist: Dexmedetomidine
Dexmedetomidine is the most recent addition to the list of sedative drugs used in pediatrics. Unlike other sedatives, it is an imidazole α2 agonist that is similar to clonidine but with an even greater α2– to α1-adrenoceptor specificity ratio of 1600 to 1. Clearance is predominantly by glucuronide conjugation, as well as by CYP2A6 action in the liver. The drug is highly lipid soluble and quickly crosses the blood-brain barrier. Its CNS effect is to stimulate receptors in the medullary vasomotor center, which decreases sympathetic tone. It also stimulates central parasympathetic outflow and decreases sympathetic outflow from the locus ceruleus of the brainstem. The decreased outflow from the locus ceruleus allows for increased activity of the inhibitory GABA neurons, which cause sedation and analgesia.217 Dexmedetomidine is approved for sedation of ventilated adult patients in the ICU, and for procedural sedation, but as of 2012, it is not approved for use in children younger than 18 years of age. In addition to IV use, it has been administered via the buccal and IM routes in adults.218 When administered in clinical doses, it causes limited effects on ventilation in adults and may mimic natural rapid-eye-movement sleep.219–221 Because rapid IV boluses can cause transient systemic hypertension, the initial loading dose must be given over 10 minutes, followed by an infusion. When given in the recommended fashion, it decreases BP and heart rate in a dose-dependent manner. It should be used with caution in children with preexisting bradycardia, atrioventricular conduction defects, hypotension, and decreased cardiac output.222,223 It has been associated with severe bradycardia in infants receiving digitalis.128 The minimal effects on ventilation have led to a surge in its use in children.224–227
Sedation after IV administration has a relatively rapid onset of 10 min and a half-life of 1.5 to 3 hours. It is unique among sedatives in that it mimics natural sleep. Its minimal effect on respiration has led to its use alone in nonpainful procedures (e.g., EEG, CT, and MRI), in children with Down syndrome, and children with obstructive sleep apnea. It has also been used in combination with ketamine and propofol for painful procedures and syndromes (e.g., cardiac catheterizations and complex regional pain syndrome)228,229; however, caution is advised in children with known congenital heart disease, as it depresses sinus and atrioventricular node function and may pose an increased risk for children prone to bradycardia.228–232 It has also been used as a novel approach to control the adverse effects of opioid and sedative withdrawal in children in the ICU.233–235 The minimal respiratory effects of dexmedetomidine has been demonstrated both anatomically and physiologically in healthy children. Analyzed by MRI, the dose-response effects of dexmedetomidine (1 to 3 µg/kg/min) on upper airway morphology in children without obstructive sleep apnea236,237 demonstrated minimal changes in upper airway dimensions in several planes, with no clinical evidence of airway obstruction. Infants and children 5 months to 16 years of age who were sedated with dexmedetomidine for nonpainful procedures (e.g., MRI, MRI plus EEGs, and nuclear medicine procedures) showed minimal effects on respiration.57 Boluses of 0.5 to 1.0 µg/kg dexmedetomidine were infused over 5 to 10 minutes, followed by an infusion of 0.5 to 1.0 µg/kg/hr. Heart rate, BP, and respiratory rate decreased but remained within normal limits for age. The etco2 exceeded 50 mm Hg in only 7 of 404 measurements. Maximum etco2 was 52 mm Hg; mean recovery time was 84 minutes. The minimal effect of dexmedetomidine on respirations has also been verified in children with obstructive sleep apnea.236,237
Initial studies demonstrated that standard doses of dexmedetomidine could not consistently provide optimal conditions for sedation for these procedures. Some clinicians increased the initial loading dose and maintenance infusion dose of dexmedetomidine, whereas others supplemented the original dosing regimen with midazolam 0.1 mg/kg, in search of a successful regimen for sedation for radiologic investigations. In the case of sedation for MRI,238 a loading dose of 3 µg/kg administered over 10 minutes IV followed by an infusion of 2 µg/kg/hr minimized the need for rescue with pentobarbital (2.4%). Mean arterial pressure was maintained in all children and oxygen saturation remained greater than 95% in all children. However, most importantly, in 30 of the 747 (4%) children, the heart rate decreased to less than the 20th percentile for age. Heart rates in the 1- to 3-year-old and 3- to 6-year-old age-groups were as low as the 30 to 40 beats per minute, although no adverse effects were noted, nor were any treatments required. Although not apparently harmful in this cohort, we do not recommend use of such large doses of dexmedetomidine, because heart rates of 30 to 40 beats per minute are well below the acceptable range of normal heart rates in children! Caution should also be used when administering glycopyrrolate to treat the bradycardia from dexmedetomidine in children, because severe and sustained hypertension after traditional doses of 5 µg/kg have occurred (e.g., BP 161/113 in a 3-year-old); the mechanism of this unusual response remains elusive.239 An alternative approach240 included a loading dose of 1 µg/kg dexmedetomidine over 10 minutes followed by an infusion of 0.5 µg/kg/min plus a 0.1 mg/kg bolus of midazolam after a sevoflurane inhalational induction for IV placement. With this regimen, all MRI scans were completed successfully, with only small changes in etco2 (maximum 57 mm Hg). Hemodynamically, systemic BP and heart rate were maintained, with the lowest systolic BP being 70 mm Hg and the lowest heart rate 64 beats per minute in 3 children. This regimen was compared with propofol at 300 µg/kg/min for 10 minutes and a dose of 250 µg/kg/min thereafter, with equivalent hemodynamic responses and no complications.
General Anesthetics
Ketamine has been available since the 1960s. It is one of the few sedatives that produce both amnesia and analgesia. It is structurally similar to phencyclidine and exerts its dissociative properties by interacting with the limbic and thalamic systems. Additional mechanisms postulated are antagonism of N-methyl-d-aspartate (NMDA) receptors and agonism of opioid subgroups.151 The clinical appearance is that of a child who has open eyes (usually with horizontal nystagmus) but does not respond to pain, the so called “dissociative” state. Ketamine preserves cardiovascular function and exerts limited effects on respiratory mechanics, allowing spontaneous respirations in most children.241 Although it is certainly not a new drug, ketamine has recently experienced a resurgence in popularity, particularly in the ED for procedural sedation. Some emergency medicine physicians suggest that ketamine not be considered an anesthetic, but rather a “dissociative sedation” drug with unique properties that warrant their own separate monitoring guidelines.242 New clinical practice guidelines for EDs have been written which emphasize this position107; however, given the fact that airway obstruction, apnea, and laryngospasm occur with some regularity (approximately 1% to 2%), we strongly disagree with attempts to reclassify the drug as a means of side-stepping the AAP sedation guidelines.107,243–245
IM and IV ketamine (with and without midazolam) are frequently used for closed fracture reductions and other painful minor procedures in the ED (see Table 47-9).245–247 In a review of 1022 children sedated with ketamine in the ED, there were no instances of aspiration, and airway reflexes were preserved, although complications included the need for airway management (0.7%), laryngospasm (0.4%), and apnea or respiratory depression (0.3%). Unpleasant reactions were rare and were not improved by prophylactic benzodiazepines.104 In a more recent review of 4252 children, respiratory complications occurred in 2.4%, with serious airway events occurring more frequently: of this 2.4%, laryngospasm occurred in 28%, hypoxia in 79%, and apnea in 10%.99,248 The risks in children younger than 2 years during ketamine with midazolam (62% of children) and morphine with midazolam (16% of children) showed a 6% incidence of complications in which all but one incident were related to ketamine with midazolam. Most were considered minor in severity, although one child required tracheal intubation.249 Ketamine (1 to 3 mg/kg) has also been studied in children undergoing gastroendoscopy procedures, with transient laryngospasm occurring in 8.2%, emesis in 4.1%, emergence agitation in 2.4%, partial airway obstruction in 1.3%, apnea and respiratory depression in 0.5%, and excessive salivation in 0.3%.83 The analgesic effects of ketamine make it very appealing for use during burn dressing changes. In one study, 4.9% of the sedations resulted in adverse outcomes, of which 2.9% required an intervention. Eight events were related to the airway.250 Ketamine sedation/analgesia has a very long history of safety, perhaps having advantage over other sedation regimens, although these studies indicate that potentially life-threatening events can and do occur during ketamine sedation. Ketamine is also associated with nonpurposeful motion, which limits its usefulness when immobility is necessary (e.g., use during CT).
Typical starting doses251 of ketamine are 1 to 2 mg/kg IM, 0.25 to 1.0 mg/kg IV, and 4 to 6 mg/kg orally.252–254 The onset after IM injection is 2 to 5 minutes, with a peak at ∼20 minutes; duration can be 30 to 120 minutes. Onset after IV administration occurs in less than 1 minute, with a peak effect in several minutes and duration of action of approximately 15 minutes. Oral doses of 4 to 6 mg/kg are usually combined with atropine and have an effect in 30 minutes and last up to 120 minutes.31 Larger doses or supplementation with other sedatives or opioids may produce deep sedation or general anesthesia. We advise that ketamine be administered with an antisialagogue (atropine, 0.02 mg/kg, or glycopyrrolate, 0.01 mg/kg) because copious secretions from ketamine alone may induce laryngospasm.255
Etomidate is a carboxylated imidazole that is primarily used as an induction agent for anesthesia. Its mechanism is thought to be potentiation of GABA inhibitory neurotransmission via alteration of chloride conductance. Loss of consciousness occurs in 15 to 20 seconds, and recovery results from redistribution and occurs in 5 to 10 minutes. It is hydrolyzed in the liver to inactive metabolites and excreted (90%) in the urine.151 Etomidate produces sedation and anesthesia, anxiolysis, and amnesia similar to the barbiturates and propofol. Its major advantage is its lack of adverse cardiovascular effects. Etomidate has been used in adults and children for procedural sedation, although the end point of sedation is not well described and often is a state of general anesthesia.79 Using data from the Pediatric Sedation Research Consortium, etomidate has been compared with pentobarbital for CT sedation. Adverse events were more common with pentobarbital (4.5%) than etomidate (0.9%); one child who received etomidate experienced apnea.256 Etomidate with fentanyl has been compared to ketamine with midazolam for reduction of limb fractures in children in the ED. Etomidate with fentanyl had a quicker recovery time but was less effective in reducing observed patient distress.257 Transient adrenal suppression can occur after multiple doses and has been described after single-dose administration in septic patients.258–262
Propofol is an anesthetic that is widely used for pediatric sedation and anesthesia. It is an isopropyl phenol derivative whose mechanism of action appears to be activation of the sodium channel of the β1 subunit of GABA receptors, thereby enhancing inhibitory transmission.263 Its onset is within 30 seconds. It is highly lipid soluble and is provided in a lipid solution that is the same as the lipid component in total parenteral solutions. The lipid solubility makes the drug effect diminish extremely quickly (5 to 15 minutes).264 It has no analgesic properties, but it does have antiemetic and antipruritic properties. Although small doses of propofol (25 to 50 µg/kg/min) can provide moderate sedation in adults, deep sedation and airway obstruction quickly occurs in some children at greater rates of administration. Dosing in adults for sedation is recommended at 25 to 200 µg/kg/min, whereas children, particularly infants and those with cognitive impairment, require larger doses (150 to 250 μg/kg/min). Propofol is also a profound respiratory depressant and can cause apnea. Respiratory complications have been reported in 8% to 30% of children.265 It is generally best administered by titration with an infusion pump. Other adverse reactions include increased salivary and tracheobronchial secretions, myoclonic movements, anaphylactic reactions, and bacterial contamination. Pain on injection can be lessened by several strategies, although the two most effective strategies are pretreatment with nitrous oxide by inhalation, or a mini–Bier block with 1 mg/kg lidocaine applied for 1 minute.266,267 Hypotension is mild and usually not clinically significant. Cases of fatal metabolic acidosis, myocardial failure, and lipemic serum have been reported in children who received propofol infusions for more than 48 hours at doses exceeding 5 mg/kg/hr (83 μg/kg/min), with sporadic cases, which may have been exacerbated by an underlying mitochondrial disorder, of the syndrome appearing after only 5 to 6 hours in some cases.268–273 Propofol should only be administered by practitioners with advanced sedation training and airway skills, because the propofol-sedated child will always be deeply sedated.
The use of all sedatives drugs in children continues to grow. All providers must be aware of the potency and risks of sedative drugs. There are many anecdotes and reviews with small numbers of incidents that attest to the risks of propofol. This is especially true when sedatives with general anesthetic potential are used by health care providers who are not specifically trained to use them in children. Propofol sedation frequently causes anesthesia with an inability to maintain and protect the airway. In a study of propofol sedation (2.5 to 3 mg/kg IV loading dose with an infusion up to 200 µg/kg/min) in 105 painful procedures in the pediatric ICU, 21% of the children required airway repositioning, 17% had apnea, 5% had hypotension, and 45% had events that required intervention.86 In the ED, propofol was administered to 113 children in a dose (4.5 mg/kg in addition to fentanyl 1 to 2 µg/kg)274 sufficient to ensure the children were motionless during the reduction of fractures. The net effect was an incidence of desaturation of 21%, laryngospasm in 1%, and a supplemental oxygen requirement in 25%. A study of sedation by nonanesthesiologists for bone marrow procedures, lumbar punctures, and esophagoscopies titrated propofol to a target of “not arousable”; 21 children 27 weeks to 18 years of age were studied. Only after a BIS score of 45 or less was 100% adequate sedation accomplished. Propofol total doses were 520 µg/kg/min for these short procedures.275 These responses to stimulation, effects on the airway, doses of propofol, and BIS levels are beyond the range of deep sedation, and more in keeping with the definition of general anesthesia, with the inherent risk of loss of the airway. More recent studies that reviewed the use of propofol by ED physicians specifically trained in sedation and performed in highly monitored environments, show that propofol can be used with much less severe adverse outcomes (and much smaller doses) than previously reported.15,92,106,276 The debate about who is best to provide sedation with all drugs and especially propofol will continue. The key to safety is training and environment.
The risks associated with propofol were brought into the international spotlight with the death of Michael Jackson in 2009. Michael Jackson’s physician who administered the propofol was tried for homicide and found guilty of involuntary manslaughter. This was not the first reported case of homicide using propofol.277
Fospropofol (Lusedra) is a water-soluble prodrug of propofol. Fospropofol is hydrolyzed by plasma alkaline phosphatase to propofol, formaldehyde, and phosphate.278,279 As fospropofol must first undergo dephosphorylation to propofol, time to onset of action is delayed (4 to 8 minutes).280,281 Because of its slower onset, it was proposed as a safe alternative to propofol with fewer restrictions on its administration. In December 2008, the FDA qualified fospropofol to be administered only by persons trained in the administration of general anesthesia (i.e., the same wording as propofol). Currently, there are no data on fospropofol use in children. The slow onset, adverse effect of perineal burning, and formaldehyde and formate byproducts may make this drug less appealing for sedation and only useful during general anesthesia.278,282 One possible advantage for this preparation may be for long-term sedation because it is free of any fatty acids and thus may avoid possible propofol infusion syndrome. Further studies are needed, particularly because preliminary PK data were flawed.283
There has been increasing use of a combination of ketamine (10 mg/mL) with propofol (10 mg/mL), so called “ketofol,” for painful procedures in the ED and for oncology patients.284–289 This combination is administered in incremental doses of 0.5 mg/kg of each drug at approximately 1-minute intervals. The limited number of prospective studies at this point preclude assessment of safety and efficacy. A literature review found insufficient evidence to recommend routine use of this combinaiton.288 A prospective study of adults found one patient in each group to require positive pressure ventilation.285
Methohexital is a short-acting oxybarbiturate that is rapidly metabolized and redistributed, and has a rapid recovery (see Table 47-9).290 Induction of anesthesia occurs with IV doses of 1 to 2 mg/kg. Apnea, hiccups, and methohexital-induced seizures in children with temporal lobe epilepsy have been reported.291 Methohexital has been used IM in doses of 8 to 10 mg/kg but has a slow onset292 and is not generally recommended. Rectal methohexital (20 to 25 mg/kg [from a 100 mg/mL solution]) can induce deep sedation in 7 to 11 minutes293 with a duration of action of about 30 to 45 minutes.294 Absorption through the rectal route is erratic.295 Children given methohexital (1 mg/kg) completed their head CT more quickly and needed less total sedation monitoring than those given pentobarbital (2 mg/kg).296 The variability of absorption, tendency to deep sedation, and airway problems have decreased the use of methohexital in favor of newer drugs. This medication is rarely used and should be used only by individuals with advanced airway skills, because upper airway obstruction and apnea may readily occur.297
Nitrous oxide (N2O) is a potent inhalation analgesic with a peak effect in 3 to 5 minutes and very rapid return to baseline when discontinued (see Table 47-9). A premixed tank of no more than 50% N2O is available (Entonox). Administration of N2O can be used for “minimal sedation” under the following AAP guidelines: (1) only ASA physical status I or II patients; (2) only 50% nitrous oxide or less is used; (3) inhalation equipment must have the capacity to deliver 100% oxygen and never less than 25% oxygen; and (4) a calibrated oxygen analyzer must be used. The child is able to maintain verbal communication throughout the procedure. Although N2O in 50% concentration with oxygen usually produces “minimal” sedation, the addition of any sedatives or hypnotic may rapidly produce a deeper level of sedation and require increased monitoring and vigilance.148,169 Nitrous oxide is frequently used by dentists for sedation. A recent questionnaire showed that all respondents performed dental treatment as a trainee, to patients who were under sedation, and the majority used nitrous oxide inhalation sedation.298,299 Extensive guidelines, following the ASA and AAP definitions and guidelines, have been written by the American Academy of Pediatric Dentistry that detail the accepted use of nitrous oxide.299 Adverse reactions, drug interactions, and special concerns are listed in Table 47-9.
Coté CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: a critical incident analysis of contributory factors. Pediatrics. 2000;105:805–814.
Cravero JP, Beach M, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric sedation/anesthesia for procedures with propofol outside the operating room: report from the Pediatric Sedation Research Consortium. Anesthesia Analgesia. 2009;108:795–804.
Cravero JP, Blike GT, Beach M, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;118:1087.
1 Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987;1:62–66.
2 Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9.
3 Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology. 1990;73:661–670.
4 Grunau RV, Whitfield MF, Petrie JH, Fryer EL. Early pain experience, child and family factors, as precursors of somatization: a prospective study of extremely premature and fullterm children. Pain. 1994;56:353–359.
5 Yaster M, Krane EJ, Kaplan RF, Coté CJ, Lappe DG. Pediatric pain management and sedation handbook, 1st ed. St Louis: Mosby-Year Book; 1997.
6 Newton JT, Shah S, Patel H, Sturmey P. Non-pharmacological approaches to behaviour management in children. Dent Update. 2003;30:194–199.
7 Shaw AJ, Welbury RR. The use of hypnosis in a sedation clinic for dental extractions in children: report of 20 cases. ASDC J Dent Child. 1996;63:418–420.
8 Aitken JC, Wilson S, Coury D, Moursi AM. The effect of music distraction on pain, anxiety and behavior in pediatric dental patients. Pediatr Dent. 2002;24:114–118.
9 Peretz B, Bimstein E. The use of imagery suggestions during administration of local anesthetic in pediatric dental patients. ASDC J Dent Child. 2000;67:263–267.
10 Langley P. Guided imagery: a review of effectiveness in the care of children. Paediatr Nurs. 1999;11:18–21.
11 Green SM, Krauss B. Who owns deep sedation? Ann Emerg Med. 2011;57:470–474.
12 Coté CJ, Cravero JP. Brave new world: do we need it, do we want it, can we afford it? Pediatr Anesth. 2011;21:919–923.
13 Lalwani K, Michel M. Pediatric sedation in North American children’s hospitals: a survey of anesthesia providers. Paediatr Anaesth. 2005;15:209–213.
14 Cravero JP, Blike GT, Beach M, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the pediatric sedation research consortium. Pediatrics. 2006;118:1087–1096.
15 Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795–804.
16 Coté CJ. Round and round we go: sedation—what is it, who does it, and have we made things safer for children? Paediatr Anaesth. 2008;18:3–8.
17 Scottish Intercollegiate Guidelines Network. SIGN Guideline 58: safe sedation of children undergoing diagnostic and therapeutic procedures. Paediatr Anaesth. 2008;18:11–12.
18 Coté CJ, Wilson S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587–2602.
19 American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017.
20 American Academy on Pediatrics, American Academy on Pediatric Dentistry. Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatr Dent. 2008;30:143–159.
21 American Academy of Pediatrics, American Academy of Pediatric Dentistry, Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Adopted 2006 www.aapd.org/policies/
22 Joint Commission on Accreditation of Healthcare Organizations. Comprehensive accreditation manual for hospitals (CAMH): the official handbook. Oakbrook Terrace, Ill.: Joint Commission Resources; 2005. p. PC41-3
23 Joint Commission Resources Staff. The Joint Commission comprehensive accreditation manual. E-ed www.jointcommission.org, 2011.
24 Dial S, Silver P, Bock K, Sagy M. Pediatric sedation for procedures titrated to a desired degree of immobility results in unpredictable depth of sedation. Pediatr Emerg Care. 2001;17:414–420.
25 Centers for Medicare & Medicaid Services. CMS Manual System Publication 100-07. State operations manual. Rev. 1, May 21 www.cms.gov, 2004.
26 McEwan J, Giridharan W, Clarke RW, Shears P. Paediatric acute epiglottitis: not a disappearing entity. Int J Pediatr Otorhinolaryngol. 2003;67:317–321.
27 Coté CJ. “Conscious sedation”: time for this oxymoron to go away!. J Pediatr. 2001;139:15–17.
28 Coté CJ. Sedation for the pediatric patient. A review. Pediatr Clin North Am. 1994;41:31–58.
29 Coté CJ. Monitoring guidelines: do they make a difference? AJR Am J Roentgenol. 1995;165:910–912.
30 Coté CJ. Sedation protocols–why so many variations? Pediatrics. 1994;94:281–283.
31 Coté CJ, Strafford MA. The principles of pediatric sedation. Boston: Tufts University School of Medicine; 1998.
32 Proulx J. Respiratory monitoring: arterial blood gas analysis, pulse oximetry, and end-tidal carbon dioxide analysis 38. Clin Tech Small Anim Pract. 1999;14:227–230.
33 Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659.
34 Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43:247–255.
35 Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251.
36 Malviya S, Voepel-Lewis T, Tait AR, et al. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS). Br J Anaesth. 2002;88:241–245.
37 Green SM, Mason KP. Stratification of sedation risk—a challenge to the sedation continuum. Paediatr Anaesth. 2011;21:924–931.
38 Kerssens C, Sebel PS. To BIS or not to BIS? That is the question. Anesth Analg. 2006;102:380–382.
39 Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology. 2000;93:1336–1344.
40 Shields CH, Creamer KM. Validation of the BIS monitor during conscious sedation and deep sedation in children. Anesth Analg. 2004;98:277–278.
41 Eeg-Olofsson O, Petersen I, Sellden U. The development of the electroencephalogram in normal children from the age of 1 through 15 years. Paroxysmal activity. Neuropadiatrie. 1971;2:375–404.
42 Sadhasivam S, Ganesh A, Robison A, Kaye R, Watcha MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. 2006;102:383–388.
43 Malviya S, Voepel-Lewis T, Tait AR. A comparison of observational and objective measures to differentiate depth of sedation in children from birth to 18 years of age. Anesth Analg. 2006;102:389–394.
44 Malviya S, Voepel-Lewis T, Ludomirsky A, Marshall J, Tait AR. Can we improve the assessment of discharge readiness?: a comparative study of observational and objective measures of depth of sedation in children. Anesthesiology. 2004;100:218–224.
45 Blusse van Oud-Alblas HJ, Peters JW, de Leeuw TG, et al. A comparison in adolescents of composite auditory evoked potential index and bispectral index during propofol-remifentanil anesthesia for scoliosis surgery with intraoperative wake-up test. Anesth Analg. 2008;107:1683–1688.
46 Shields CH, Styadi-Park G, McCown MY, Creamer KM. Clinical utility of the bispectral index score when compared to the University of Michigan Sedation Scale in assessing the depth of outpatient pediatric sedation. Clin Pediatr (Phila). 2005;44:229–236.
47 Mason KP, Michna E, Zurakowski D, et al. Value of bispectral index monitor in differentiating between moderate and deep Ramsay Sedation Scores in children. Paediatr Anaesth. 2006;16:1226–1231.
48 Jeleazcov C, Schmidt J, Schmitz B, Becke K, Albrecht S. EEG variables as measures of arousal during propofol anaesthesia for general surgery in children: rational selection and age dependence. Br J Anaesth. 2007;99:845–854.
49 Malviya S, Voepel-Lewis T, Tait AR, et al. Effect of age and sedative agent on the accuracy of bispectral index in detecting depth of sedation in children. Pediatrics. 2007;120:e461–e470.
50 Valkenburg AJ, de Leeuw TG, Tibboel D, Weber F. Lower bispectral index values in children who are intellectually disabled. Anesth Analg. 2009;109:1428–1433.
51 Hans P, Dewandre PY, Brichant JF, Bonhomme V. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–340.
52 Overly FL, Wright RO, Connor FA, Jr., et al. Bispectral analysis during pediatric procedural sedation. Pediatr Emerg Care. 2005;21:6–11.
53 Mason KP, O’Mahony E, Zurakowski D, Libenson MH. Effects of dexmedetomidine sedation on the EEG in children. Paediatr Anaesth. 2009;19:1175–1183.
54 Coté CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: A critical incident analysis of contributory factors. Pediatrics. 2000;105:805–814.
55 Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633–644.
56 Sundman E, Witt H, Sandin R, et al. Pharyngeal function and airway protection during subhypnotic concentrations of propofol, isoflurane, and sevoflurane: volunteers examined by pharyngeal videoradiography and simultaneous manometry. Anesthesiology. 2001;95:1125–1132.
57 Berkenbosch JW, Wankum PC, Tobias JD. Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children. Pediatr Crit Care Med. 2005;6:435–439.
58 Litman RS. Upper airway collapsibility: an emerging paradigm for measuring the safety of anesthetic and sedative agents. Anesthesiology. 2005;103:453–454.
59 Litman RS, Weissend EE, Shrier DA, Ward DS. Morphologic changes in the upper airway of children during awakening from propofol administration. Anesthesiology. 2002;96:607–611.
60 Litman RS, Wake N, Chan LM, et al. Effect of lateral positioning on upper airway size and morphology in sedated children. Anesthesiology. 2005;103:484–488.
61 Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–477.
62 Crawford MW, Rohan D, Macgowan CK, Yoo SJ, Macpherson BA. Effect of propofol anesthesia and continuous positive airway pressure on upper airway size and configuration in infants. Anesthesiology. 2006;105:45–50.
63 Machata AM, Kabon B, Willschke H, Prayer D, Marhofer P. Upper airway size and configuration during propofol-based sedation for magnetic resonance imaging: an analysis of 138 infants and children. Paediatr Anaesth. 2010;20:994–1000.
64 Sun LS, Li G, Dimaggio C, et al. Anesthesia and neurodevelopment in children: time for an answer? Anesthesiology. 2008;109:757–761.
65 Durieux M, Davis PJ. The Safety of Key Inhaled and Intravenous Drugs in Pediatrics (SAFEKIDS): an update. Anesth Analg. 2010;110:1265–1267.
66 Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–253.
67 Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804.
68 Hudson AE, Hemmings HC, Jr. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–37.
69 Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085.
70 Hansen TG, Henneberg SW. Neurotoxicity of anesthetic agents and the developing brain in rodents and primates: the time has come to focus on human beings. Anesthesiology. 2010;113:1244–1245.
71 Hansen TG, Flick R. Anesthetic effects on the developing brain: insights from epidemiology. Anesthesiology. 2009;110:1–3.
72 Kanto J, Klotz U. Pharmacokinetic implications for the clinical use of atropine, scopolamine and glycopyrrolate. Acta Anaesthesiol Scand. 1988;32:69–78.
73 Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85:1207–1213.
74 Kain ZN, Mayes LC, Wang SM, Caramico LA, Hofstadter MB. Parental presence during induction of anesthesia versus sedative premedication: which intervention is more effective? Anesthesiology. 1998;89:1147–1156.
75 Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23:133–144.
76 D’Agostino J, Terndrup TE. Chloral hydrate versus midazolam for sedation of children for neuroimaging: a randomized clinical trial. Pediatr Emerg Care. 2000;16:1–4.
77 Havel CJ, Jr. Strait RT, Hennes H: A clinical trial of propofol vs midazolam for procedural sedation in a pediatric emergency department. Acad Emerg Med. 1999;6:989–997.
78 Dachs RJ, Innes GM. Intravenous ketamine sedation of pediatric patients in the emergency department. Ann Emerg Med. 1997;29:146–150.
79 Dickinson R, Singer AJ, Carrion W. Etomidate for pediatric sedation prior to fracture reduction. Acad Emerg Med. 2001;8:74–77.
80 Freyer DR, Schwanda AE, Sanfilippo DJ, et al. Intravenous methohexital for brief sedation of pediatric oncology outpatients: physiologic and behavioral responses. Pediatrics. 1997;99:E8.
81 Auden SM, Sobczyk WL, Solinger RE, Goldsmith LJ. Oral ketamine/midazolam is superior to intramuscular meperidine, promethazine, and chlorpromazine for pediatric cardiac catheterization. Anesth Analg. 2000;90:299–305.
82 Hertzog JH, Dalton HJ, Anderson BD, et al. Prospective evaluation of propofol anesthesia in the pediatric intensive care unit for elective oncology procedures in ambulatory and hospitalized children. Pediatrics. 2000;106:742–747.
83 Green SM, Klooster M, Harris T, Lynch EL, Rothrock SG. Ketamine sedation for pediatric gastroenterology procedures. J Pediatr Gastroenterol Nutr. 2001;32:26–33.
84 Pomeranz ES, Chudnofsky CR, Deegan TJ, et al. Rectal methohexital sedation for computed tomography imaging of stable pediatric emergency department patients. Pediatrics. 2000;105:1110–1114.
85 McDowall RH, Scher CS, Barst SM. Total intravenous anesthesia for children undergoing brief diagnostic or therapeutic procedures. J Clin Anesth. 1995;7:273–280.
86 Vardi A, Salem Y, Padeh S, Paret G, Barzilay Z. Is propofol safe for procedural sedation in children? A prospective evaluation of propofol versus ketamine in pediatric critical care. Crit Care Med. 2002;30:1231–1236.
87 Cravero JP, Blike GT. Review of pediatric sedation. Anesth Analg. 2004;99:1355–1364.
88 Cravero JP, Blike GT, Surgenor SD, Jensen J. Development and validation of the Dartmouth Operative Conditions Scale. Anesth Analg. 2005;100:1614–1621.
89 Blike GT, Christoffersen K, Cravero JP, Andeweg SK, Jensen J. A method for measuring system safety and latent errors associated with pediatric procedural sedation. Anesth Analg. 2005;101:48–58. table
90 Blike GT, Cravero JP. Use of standardized simulated events as a provocative test for medical system rescue capabilities. Adv Patient Safety. 2005;4:193–210.
91 Cravero JP, Blike GT. Pediatric anesthesia in the nonoperating room setting. Curr Opin Anaesthesiol. 2006;19:443–449.
92 Couloures KG, Beach M, Cravero JP, Monroe KK, Hertzog JH. Impact of provider specialty on pediatric procedural sedation complication rates. Pediatrics. 2011;127:e1154–e1160.
93 Committee of Origin. Ad Hoc on Non-Anesthesiologist Privileging. Statement on granting privileges for deep sedation to non-anesthesiologist sedation practitioners. 2010. Available at www.asahq.org/~/../Advisory_Non-anesthesiologist_Privileging.ashx (accessed Sept 27 2012).
94 Committee of Origin. Ad Hoc Committee on Credentialing. Statement on granting privileges for administration of moderate sedation to practitioners who are not anesthesia professionals. 10-18-2006. Available at www.asahq.org/~/../Advisory_Non-anesthesiologist_Privileging.ashx (accessed Sept 27 2012).
95 Committee on Drugs, Section on Anesthesiology, American Academy of Pediatrics. Guidelines for the elective use of conscious sedation, deep sedation, and general anesthesia in pediatric patients. Pediatrics. 1985;76:317–321.
96 Committee on Drugs American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89:1110–1115.
97 Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: addendum. Pediatrics. 2002;110:836–838.
98 Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.
99 Metzner J, Domino KB. Risks of anesthesia or sedation outside the operating room: the role of the anesthesia care provider. Curr Opin Anaesthesiol. 2010;23:523–531.
100 Committee of Origin. Standards and Practice Parameters. Standards for basic anesthetic monitoring. 2010. Available at http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx (accessed 27 Sept 2012).
101 American Academy of Pediatric Dentistry. Guidelines for the elective use of pharmacologic conscious sedation and deep sedation in pediatric dental patients. Pediatr Dent. 1993;15:297–299.
102 American College of Emergency Physicians. Clinical policy for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 1998;31:663–677.
103 Godwin SA, Caro DA, Wolf SJ, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45:177–196.
104 Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–780.
105 Sacchetti A, Cravero J. Sedation in the emergency department. Pediatr Ann. 2005;34:617–622.
106 Mallory MD, Baxter AL, Yanosky DJ, Cravero JP. Emergency physician-administered propofol sedation: a report on 25,433 sedations from the pediatric sedation research consortium. Ann Emerg Med. 2011;57:462–468.
107 Green SM, Roback MG, Kennedy RM, Krauss B. Clinical Practice Guideline for Emergency Department Ketamine Dissociative Sedation: 2011 Update. Ann Emerg Med. 2011.
108 Joint Commission on Accreditation of Healthcare Organizations. Joint Commission: The Source, planning the administration of moderate or deep sedation. 3(10). Oakbrook Terrace, Ill.: Joint Commission on Accreditation of Healthcare Organizations; 2005.
109 Kaplan RF, Yang CI. Sedation and analgesia in pediatric patients for procedures outside the operating room. Anesthesiol Clin North America. 2002;20:181–194.
110 Schwengel DA, Sterni LM, Tunkel DE, Heitmiller ES. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109:60–75.
111 Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–712.
112 Fishbaugh DF, Wilson S, Preisch JW, Weaver JM, 2nd. Relationship of tonsil size on an airway blockage maneuver in children during sedation. Pediatr Dent. 1997;19:277–281.
113 Biban P, Baraldi E, Pettenazzo A, Filippone M, Zacchello F. Adverse effect of chloral hydrate in two young children with obstructive sleep apnea. Pediatrics. 1993;92:461–463.
114 Kannikeswaran N, Mahajan PV, Sethuraman U, Groebe A, Chen X. Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Paediatr Anaesth. 2009;19:250–256.
115 Flanagan JF. The critical incident technique. Psychol Bull. 1954;51:327–358.
116 Bogner MS, ed. Human error in medicine. Hillsdale, N.J.: Lawrence Erlbaum Associates, 1994.
117 Moray N. Error reduction as a systems problem. In: Bogner MS, ed. Human error in medicine. Hillsdale, N.J.: Lawrence Erlbaum Associates; 1994:67–91.
118 Committee on Drugs American Academy of Pediatrics. Unapproved uses of approved drugs: the physician, the package insert, and the FDA. Pediatrics. 1996;98:143–145.
119 Coté CJ, Kauffman RE, Troendle GJ, Lambert GH. Is the “therapeutic orphan” about to be adopted? Pediatrics. 1996;98:118–123.
120 Coté CJ. Unapproved uses of approved drugs. Paediatr Anaesth. 1997;7:91–92.
121 Coté CJ. “Off-label” use of drugs in pediatric anesthesia: legal, clinical, and policy considerations. Curr Op Anaesth. 1999;12:325–327.
122 Department of Health and Human Services, Food and Drug Administration. Specific requirements on content and format of labeling for human prescription drugs; revisions of the “Pediatric Use” subsection in the labeling; final rule. Federal Registrar, 64240-64250. 1994. 21 CFR Part 201. 12-13-1994.
123 Food and Drug Administration Modernization and Accountability Act. Pub. L. 105-15, Section 111 of (21 U.S.C. 355A). 1997.
124 Schultheis LW, Mathis LL, Roca RA, et al. Pediatric drug development in anesthesiology: an FDA perspective. Anesth Analg. 2006;103:49–51.
125 Olkkola KT, Aranko K, Luurila H, et al. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305.
126 Hiller A, Olkkola KT, Isohanni P, Saarnivaara L. Unconsciousness associated with midazolam and erythromycin. Br J Anaesth. 1990;65:826–828.
127 Backman JT, Olkkola KT, Aranko K, Himberg JJ, Neuvonen PJ. Dose of midazolam should be reduced during diltiazem and verapamil treatments. Br J Clin Pharmacol. 1994;37:221–225.
128 Berkenbosch JW, Tobias JD. Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med. 2003;4:203–205.
129 Gorski JC, Huang SM, Pinto A, et al. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100.
130 Hall SD, Wang Z, Huang SM, et al. The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74:525–535.
131 Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290:1500–1504.
132 Wang Z, Gorski JC, Hamman MA, et al. The effects of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70:317–326.
133 Xie HG, Kim RB. St John’s wort-associated drug interactions: short-term inhibition and long-term induction? Clin Pharmacol Ther. 2005;78:19–24.
134 Abebe W. Herbal medication: potential for adverse interactions with analgesic drugs. J Clin Pharm Ther. 2002;27:391–401.
135 Bjerring P, Arendt-Nielsen L. Depth and duration of skin analgesia to needle insertion after topical application of EMLA cream. Br J Anaesth. 1990;64:173–177.
136 Gajraj NM, Pennant JH, Watcha MF. Eutectic mixture of local anesthetics (EMLA) cream. Anesth Analg. 1994;78:574–583.
137 Taddio A, Nulman I, Goldbach M, Ipp M, Koren G. Use of lidocaine-prilocaine cream for vaccination pain in infants. J Pediatr. 1994;124:643–648.
138 Wolf SI, Shier JM, Lampl KL, Schwartz R. EMLA cream for painless skin testing: a preliminary report. Ann Allerg. 1994;73:40–42.
139 Benini F, Johnston CC, Faucher D, Aranda JV. Topical anesthesia during circumcision in newborn infants. JAMA. 1993;270:850–853.
140 Eichenfield LF, Funk A, Fallon-Friedlander S, Cunningham BB. A clinical study to evaluate the efficacy of ELA-Max (4% liposomal lidocaine) as compared with eutectic mixture of local anesthetics cream for pain reduction of venipuncture in children. Pediatrics. 2002;109:1093–1099.
141 Sethna NF, Verghese ST, Hannallah RS, et al. A randomized controlled trial to evaluate S-Caine patch for reducing pain associated with vascular access in children. Anesthesiology. 2005;102:403–408.
142 Temme JB, Anderson JC, Matecko S. Sedation of children for CT and MRI scanning. Radiol Technol. 1990;61:283–285.
143 Cook BA, Bass JW, Nomizu S, Alexander ME. Sedation of children for technical procedures: current standard of practice. Clin Pediatr (Phila). 1992;31:137–142.
144 Greenberg SB, Faerber EN, Aspinall CL, Adams RC. High-dose chloral hydrate sedation for children undergoing MR imaging: safety and efficacy in relation to age. AJR Am J Roentgenol. 1993;161:639–641.
145 Greenberg SB, Faerber EN, Aspinall CL. High dose chloral hydrate sedation for children undergoing CT. J Comput Assist Tomogr. 1991;15:467–469.
146 Vade A, Sukhani R, Dolenga M, Habisohn-Schuck C. Chloral hydrate sedation in children undergoing CT and MR imaging: safety as judged by American Academy of Pediatrics (AAP) Guidelines. Am J Rad. 1995;165:905–909.
147 Moore PA, Mickey EA, Hargreaves JA, Needleman HL. Sedation in pediatric dentistry: a practical assessment procedure. J Am Dent Assoc. 1984;109:564–569.
148 Litman RS, Kottra JA, Verga KA, Berkowitz RJ, Ward DS. Chloral hydrate sedation: the additive sedative and respiratory depressant effects of nitrous oxide. Anesth Analg. 1998;86:724–728.
149 Wilson S. Chloral hydrate and its effects on multiple physiological parameters in young children: a dose-response study. Pediatr Dent. 1992;14:171–177.
150 Olson DM, Sheehan MG, Thompson W, Hall PT, Hahn J. Sedation of children for electroencephalograms. Pediatrics. 2001;108:163–165.
151 Maxwell LG, Tobias JD, Cravero JP, Malviya S. Adverse effects of sedatives in children. Expert Opin Drug Saf. 2003;2:167–194.
152 Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DK, Kasian GF. Chloral hydrate disposition following single-dose administration to critically ill neonates and children. Dev Pharmacol Ther. 1991;16:71–77.
153 Malviya S, Voepel-Lewis T, Prochaska G, Tait AR. Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics. 2000;105:E42.
154 American Academy of Pediatrics Committee on Drugs and Committee on Environmental Health. Use of chloral hydrate for sedation in children. Pediatrics. 1993;92:471–473.
155 Buhrer M, Maitre PO, Hung O, Stanski DR. Electroencephalographic effects of benzodiazepines. I. Choosing an electroencephalographic parameter to measure the effect of midazolam on the central nervous system. Clin Pharmacol Ther. 1990;48:544–554.
156 Buhrer M, Maitre PO, Crevoisier C, Stanski DR. Electroencephalographic effects of benzodiazepines. II. Pharmacodynamic modeling of the electroencephalographic effects of midazolam and diazepam. Clin Pharmacol Ther. 1990;48:555–567.
157 Doyle WL, Perrin L. Emergence delirium in a child given oral midazolam for conscious sedation. Ann Emerg Med. 1994;24:1173–1175.
158 Golparvar M, Saghaei M, Sajedi P, Razavi SS. Paradoxical reaction following intravenous midazolam premedication in pediatric patients—a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth. 2004;14:924–930.
159 Payne KA, Coetzee AR, Mattheyse FJ. Midazolam and amnesia in pediatric premedication. Acta Anaesthesio Belg. 1991;42:101–105.
160 Twersky RS, Hartung J, Berger BJ, McClain J, Beaton C. Midazolam enhances anterograde but not retrograde amnesia in pediatric patients. Anesthesiology. 1993;78:51–55.
161 Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Investig. 1975;55:347–359.
162 Mandelli M, Tognoni G, Garattini S. Clinical pharmacokinetics of diazepam. Clin Pharmacokinet. 1978;3:72–91.
163 Greenblatt DJ, Allen MD, Harmatz JS, Shader RI. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27:301–312.
164 Burtin P, Jacqz-Aigrain E, Girard P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther. 1994;56:615–625.
165 Jacqz-Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet. 1996;31:423–443.
166 Jacqz-Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol. 1992;42:329–332.
167 Karl HW, Keifer AT, Rosenberger JL, Larach MG, Ruffle JM. Comparison of the safety and efficacy of intranasal midazolam or sufentanil for preinduction of anesthesia in pediatric patients. Anesthesiology. 1992;76:209–215.
168 Connors K, Terndrup TE. Nasal versus oral midazolam for sedation of anxious children undergoing laceration repair. Ann Emerg Med. 1994;24:1074–1079.
169 Litman RS, Berkowitz RJ, Ward DS. Levels of consciousness and ventilatory parameters in young children during sedation with oral midazolam and nitrous oxide. Arch Pediatr Adolesc Med. 1996;150:671–675.
170 Alexander CM, Gross JB. Sedative doses of midazolam depress hypoxic ventilatory responses in humans. Anesth Analg. 1988;67:377–382.
171 Drummond GB. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br J Anaesth. 1996;76:663–667.
172 von Ungern-Sternberg BS, Erb TO, Habre W, Sly PD, Hantos Z. The impact of oral premedication with midazolam on respiratory function in children. Anesth Analg. 2009;108:1771–1776.
173 Yaster M, Nichols DG, Deshpande JK, Wetzel RC. Midazolam-fentanyl intravenous sedation in children: case report of respiratory arrest. Pediatrics. 1990;86:463–467.
174 Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938–945.
175 Blouin RT, Conard PF, Perreault S, Gross JB. The effect of flumazenil on midazolam-induced depression of the ventilatory response to hypoxia during isohypercarbia. Anesthesiology. 1993;78:635–641.
176 Flogel CM, Ward DS, Wada DR, Ritter JW. The effects of large-dose flumazenil on midazolam-induced ventilatory depression. Anesth Analg. 1993;77:1207–1214.
177 Flumazenil study group. Reversal of central nervous system effects by flumazenil after intravenous conscious sedation with midazolam: report of a multicenter study. Clin Ther. 1992;14:861–877.
178 Jones RDM, Lawson AD, Andrew LJ, Gunawardene WMS, Bacon-Shone J. Antagonism of the hypnotic effect of midazolam in children: a randomized, double-blind study of placebo and flumazenil administered after midazolam-induced anaesthesia. Br J Anaesth. 1991;66:660–666.
179 Sugarman JM, Paul RI. Flumazenil: a review. Pediatr Emerg Care. 1994;10:37–43.
180 McDuffee AT, Tobias JD. Seizure after flumazenil administration in a pediatric patient. Pediatr Emerg Care. 1995;11:186–187.
181 Weinbrum A, Geller E. The respiratory effects of reversing midazolam sedation with flumazenil in the presence or absence of narcotics. Acta Anaesthesiol Scand Suppl. 1990;92:65–69.
182 Strain JD, Harvey LA, Foley LC, Campbell JB. Intravenously administered pentobarbital sodium for sedation on pediatric CT. Radiology. 1986;161:105–108.
183 Moro-Sutherland DM, Algren JT, Louis PT, Kozinetz CA, Shook JE. Comparison of intravenous midazolam with pentobarbital for sedation for head computed tomography imaging. Acad Emerg Med. 2000;7:1370–1375.
184 Standifer KM, Pasternak GW. G proteins and opioid receptor-mediated signalling. Cell Signal. 1997;9:237–248.
185 Neidhart P, Burgener MC, Schwieger I, Suter PM. Chest wall rigidity during fentanyl- and midazolam-fentanyl induction: ventilatory and haemodynamic effects. Acta Anaesthesiol Scand. 1989;33:1–5.
186 Olkkola KT, Hamunen K, Maunuksela EL. Clinical pharmacokinetics and pharmacodynamics of opioid analgesics in infants and children. Clin Pharmacokinet. 1995;28:385–404.
187 Gourlay GK, Boas RA. Fatal outcome with use of rectal morphine for postoperative pain control in an infant. Br Med J. 1992;304:766–767.
188 Acute Pain Management Guideline Panel. Acute pain management: operative or medical procedures and trauma. Clinical practice guideline. Rockville, Md.: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; 1992.
189 Terndrup TE, Cantor RM, Madden CM. Intramuscular meperidine, promethazine, and chlorpromazine: analysis of use and complications in 487 pediatric emergency department patients. Ann Emerg Med. 1989;18:528–533.
190 Terndrup TE, Dire DJ, Madden CM, et al. A prospective analysis of intramuscular meperidine, promethazine, and chlorpromazine in pediatric emergency department patients. Ann Emerg Med. 1991;20:31–35.
191 Snodgrass WR, Dodge WF. Lytic/“DPT” cocktail: time for rational and safe alternatives. Pediatr Clin North Am. 1989;36:1285–1291.
192 Yeung PK, Hubbard JW, Korchinski ED, Midha KK. Pharmacokinetics of chlorpromazine and key metabolites. Eur J Clin Pharmacol. 1993;45:563–569.
193 Loo JC, Midha KK, McGilveray IJ. Pharmacokinetics of chlorpromazine in normal volunteers. Commun Psychopharmacol. 1980;4:121–129.
194 Terndrup TE, Dire DJ, Madden CM, Gavula D, Cantor RM. Comparison of intramuscular meperidine and promethazine with and without chlorpromazine: a randomized, prospective, double-blind trial. Ann Emerg Med. 1993;22:206–211.
195 American Academy of Pediatrics, Committee on Drugs. Reappraisal of lytic cocktail/demerol, phenergan, and thorazine (DPT) for the sedation of children. Pediatrics. 1995;95:598–602.
196 Greeley WJ, de Bruijn NP. Changes in sufentanil pharmacokinetics within the neonatal period. Anesth Analg. 1988;67:86–90.
197 Kennedy RM, Porter FL, Miller JP, Jaffe DM. Comparison of fentanyl/midazolam with ketamine/midazolam for pediatric orthopedic emergencies. Pediatrics. 1998;102:956–963.
198 Billmire DA, Neale HW, Gregory RO. Use of i.v. fentanyl in the outpatient treatment of pediatric facial trauma. J Trauma. 1985;25:1079–1080.
199 Fahnenstich H, Steffan J, Kau N, Bartmann P. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000;28:836–839.
200 Muller P, Vogtmann C. Three cases with different presentation of fentanyl-induced muscle rigidity–a rare problem in intensive care of neonates. Am J Perinatol. 2000;17:23–26.
201 Phillips WJ, Halpin J, Jones J, McKenzie K. Remifentanil for procedural sedation in the emergency department. Ann Emerg Med. 2009;53:163.
202 Bauman LA, Cannon ML, McCloskey J, et al. Unconscious sedation in children: a prospective multi-arm clinical trial. Paediatr Anaesth. 2002;12:674–679.
203 Berkenbosch JW, Graff GR, Stark JM, Ner Z, Tobias JD. Use of a remifentanil-propofol mixture for pediatric flexible fiberoptic bronchoscopy sedation. Paediatr Anaesth. 2004;14:941–946.
204 Litman RS. Conscious sedation with remifentanil during painful medical procedures. J Pain Symptom Manage. 2000;19:468–471.
205 Akinci SB, Kanbak M, Guler A, Aypar U. Remifentanil versus fentanyl for short-term analgesia-based sedation in mechanically ventilated postoperative children. Paediatr Anaesth. 2005;15:870–878.
206 Litman RS. Conscious sedation with remifentanil and midazolam during brief painful procedures in children. Arch Pediatr Adolesc Med. 1999;153:1085–1088.
207 Dahaba AA, Lischnig U, Kronthaler R, et al. Bispectral-index-guided versus clinically guided remifentanil/propofol analgesia/sedation for interventional radiological procedures: an observer-blinded randomized study. Anesth Analg. 2006;103:378–384. table
208 Crawford MW, Hickey C, Zaarour C, Howard A, Naser B. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662–1667.
209 Rosow C. Remifentanil: A unique opioid analgesic. Anesthesiology. 1993;79:875–876.
210 Perry HE, Shannon MW. Diagnosis and management of opioid- and benzodiazepine-induced comatose overdose in children. Curr Opin Pediatr. 1996;8:243–247.
211 AAP Committee on Drugs. Naloxone dosage and route of administration for infants and children: addendum to emergency drug doses for infants and children. Pediatrics. 1990;86:484–485.
212 Prough DS, Roy R, Bumgarner J, Shannon G. Acute pulmonary edema in healthy teenagers following conservative doses of intravenous naloxone. Anesthesiology. 1984;60:485–486.
213 Chamberlain JM, Klein BL. A comprehensive review of naloxone for the emergency physician. Am J Emerg Med. 1994;12:650–660.
214 Bowdle TA. Clinical pharmacology of antagonists of narcotic-induced respiratory depression. A brief review. Acute Care. 1988;12:70–76.
215 Glass PS, Jhaveri RM, Smith LR. Comparison of potency and duration of action of nalmefene and naloxone. Anesth Analg. 1994;78:536–541.
216 Chumpa A, Kaplan RL, Burns MM, Shannon MW. Nalmefene for elective reversal of procedural sedation in children. Am J Emerg Med. 2001;19:545–548.
217 Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705.
218 Karaaslan D, Peker TT, Alaca A, et al. Comparison of buccal and intramuscular dexmedetomidine premedication for arthroscopic knee surgery. J Clin Anesth. 2006;18:589–593.
219 Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–1076.
220 Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–1133.
221 Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–308.
222 Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70.
223 Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–1142.
224 Mason KP. The pediatric sedation service: who is appropriate to sedate, which medications should I use, who should prescribe the drugs, how do I bill? Pediatr Radiol. 2008;38:S218–S224.
225 Gozal D, Mason KP. Pediatric sedation: a global challenge. Int J Pediatr. 2010;2010:701257.
226 Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–131.
227 Su F, Hammer GB. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10:55–66.
228 Koruk S, Mizrak A, Gul R, et al. Dexmedetomidine-ketamine and midazolam-ketamine combinations for sedation in pediatric patients undergoing extracorporeal shock wave lithotripsy: a randomized prospective study. J Anesth. 2010;24:858–863.
229 Koruk S, Mizrak A, Kaya UB, et al. Propofol/dexmedetomidine and propofol/ketamine combinations for anesthesia in pediatric patients undergoing transcatheter atrial septal defect closure: a prospective randomized study. Clin Ther. 2010;32:701–709.
230 Nama S, Meenan DR, Fritz WT. The use of sub-anesthetic intravenous ketamine and adjuvant dexmedetomidine when treating acute pain from CRPS. Pain Physician. 2010;13:365–368.
231 Hammer GB, Drover DR, Cao H, et al. The effects of dexmedetomidine on cardiac electrophysiology in children. Anesth Analg. 2008;106:79–83. table
232 Hammer GB. Con: dexmedetomidine should not be used for infants and children during cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:152–154.
233 Finkel JC, Elrefai A. The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant. Anesth Analg. 2004;98:1658–1659. table
234 Finkel JC, Johnson YJ, Quezado ZM. The use of dexmedetomidine to facilitate acute discontinuation of opioids after cardiac transplantation in children. Crit Care Med. 2005;33:2110–2112.
235 Tobias JD. Dexmedetomidine to treat opioid withdrawal in infants following prolonged sedation in the pediatric ICU. J Opioid Manag. 2006;2:201–205.
236 Mahmoud M, Gunter J, Donnelly LF, et al. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745–753.
237 Mahmoud M, Radhakrishman R, Gunter J, et al. Effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children. Paediatr Anaesth. 2010;20:506–515.
238 Mason KP, Zurakowski D, Zgleszewski SE, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403–411.
239 Mason KP, Zgleszewski S, Forman RE, Stark C, Dinardo JA. An exaggerated hypertensive response to glycopyrrolate therapy for bradycardia associated with high-dose dexmedetomidine. Anesth Analg. 2009;108:906–908.
240 Heard C, Burrows F, Johnson K, et al. A comparison of dexmedetomidine-midazolam with propofol for maintenance of anesthesia in children undergoing magnetic resonance imaging. Anesth Analg. 2008;107:1832–1839.
241 Tobias JD. End-tidal carbon dioxide monitoring during sedation with a combination of midazolam and ketamine for children undergoing painful, invasive procedures. Pediatr Emerg Care. 1999;15:173–175.
242 Green SM, Krauss B. Procedural sedation terminology: moving beyond “conscious sedation”. Ann Emerg Med. 2002;39:433–435.
243 Green SM, Roback MG, Krauss B. Laryngospasm during emergency department ketamine sedation: a case-control study. Pediatr Emerg Care. 2010;26:798–802.
244 Green SM, Roback MG, Krauss B, et al. Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:158–168.
245 Green SM, Rothrock SG, Harris T, et al. Intravenous ketamine for pediatric sedation in the emergency department: safety profile with 156 cases. Acad Emerg Med. 1998;5:971–976.
246 Hosey MT. UK National Clinical Guidelines in Paediatric Dentistry. Managing anxious children: the use of conscious sedation in paediatric dentistry. Int J Paediatr Dent. 2002;12:359–372.
247 Kennedy RM, Luhmann JD. Pharmacological management of pain and anxiety during emergency procedures in children. Paediatr Drugs. 2001;3:337–354.
248 Melendez E, Bachur R. Serious adverse events during procedural sedation with ketamine. Pediatr Emerg Care. 2009;25:325–328.
249 Misra S, Mahajan PV, Chen X, Kannikeswaran N. Safety of procedural sedation and analgesia in children less than 2 years of age in a pediatric emergency department. Int J Emerg Med. 2008;1:173–177.
250 Owens VF, Palmieri TL, Comroe CM, et al. Ketamine: a safe and effective agent for painful procedures in the pediatric burn patient. J Burn Care Res. 2006;27:211–216.
251 Hannallah RS, Patel RI. Low-dose intramuscular ketamine for anesthesia pre-induction in young children undergoing brief outpatient procedures. Anesthesiology. 1989;70:598–600.
252 Gutstein HB, Johnson KL, Heard MB, Gregory GA. Oral ketamine preanesthetic medication in children. Anesthesiology. 1992;76:28–33.
253 Tobias JD, Phipps S, Smith B, Mulhern RK. Oral ketamine premedication to alleviate the distress of invasive procedures in pediatric oncology patients. Pediatrics. 1992;90:537–541.
254 Reinemer HC, Wilson CF, Webb MD. A comparison of two oral ketamine-diazepam regimens for sedating anxious pediatric dental patients. Pediatr Dent. 1996;18:294–300.
255 Gingrich BK. Difficulties encountered in a comparative study of orally administered midazolam and ketamine. Anesthesiology. 1994;80:1414–1415.
256 Baxter AL, Mallory MD, Spandorfer PR, et al. Etomidate versus pentobarbital for computed tomography sedations: report from the Pediatric Sedation Research Consortium. Pediatr Emerg Care. 2007;23:690–695.
257 Lee-Jayaram JJ, Green A, Siembieda J, et al. Ketamine/midazolam versus etomidate/fentanyl: procedural sedation for pediatric orthopedic reductions. Pediatr Emerg Care. 2010;26:408–412.
258 Bloomfield R, Noble DW. Etomidate, pharmacological adrenalectomy and the critically ill: a matter of vital importance. Crit Care. 2006;10:161.
259 Dorr HG, Kuhnle U, Holthausen H, Bidlingmaier F, Knorr D. Etomidate: a selective adrenocortical 11 beta-hydroxylase inhibitor. Klin Wochenschr. 1984;62:1011–1013.
260 Pizarro CF, Troster EJ, Damiani D, Carcillo JA. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med. 2005;33:855–859.
261 Sacchetti A, Stander E, Ferguson N, Maniar G, Valko P. Pediatric Procedural Sedation in the Community Emergency Department: results from the ProSCED registry. Pediatr Emerg Care. 2007;23:218–222.
262 Schenarts CL, Burton JH, Riker RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1–7.
263 Reves JG, Glass PS, Lubarsky DA, McEvoy MD. Intravenous nonopioid anesthetics. In: Miller RD, Fleisher LA, Johns RA, et al, eds. Miller’s anesthesia. 6th ed. Philadelphia: Elsevier; 2005:317–378.
264 Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334–341.
265 Green SM, Krauss B. Propofol in emergency medicine: pushing the sedation frontier. Ann Emerg Med. 2003;42:792–797.
266 Jalota L, Kalira V, George E, et al. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110.
267 Picard P, Tramer MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90:963–969.
268 Parke TJ, Stevens JE, Rice AS, et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305:613–616.
269 Hanna JP, Ramundo ML. Rhabdomyolysis and hypoxia associated with prolonged propofol infusion in children. Neurology. 1998;50:301–303.
270 Strickland RA, Murray MJ. Fatal metabolic acidosis in a pediatric patient receiving an infusion of propofol in the intensive care unit: is there a relationship? Crit Care Med. 1995;23:405–409.
271 Fudickar A, Bein B, Tonner PH. Propofol infusion syndrome in anaesthesia and intensive care medicine. Curr Opin Anaesthesiol. 2006;19:404–410.
272 Koch M, De BD, Vincent JL. Lactic acidosis: an early marker of propofol infusion syndrome? Intensive Care Med. 2004;30:522.
273 Haase R, Sauer H, Eichler G. Lactic acidosis following short-term propofol infusion may be an early warning of propofol infusion syndrome. J Neurosurg Anesthesiol. 2005;17:122–123.
274 Godambe SA, Elliot V, Matheny D, Pershad J. Comparison of propofol/fentanyl versus ketamine/midazolam for brief orthopedic procedural sedation in a pediatric emergency department. Pediatrics. 2003;112:116–123.
275 Powers KS, Nazarian EB, Tapyrik SA, et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics. 2005;115:1666–1674.
276 Green SM. Propofol in emergency medicine: further evidence of safety. Emerg Med Australas. 2007;19:389–393.
277 Kirby RR, Colaw JM, Douglas MM. Death from propofol: accident, suicide, or murder? Anesth Analg. 2009;108:1182–1184.
278 Fechner J, Schwilden H, Schuttler J. Pharmacokinetics and pharmacodynamics of GPI 15715 or fospropofol (Aquavan injection)—a water-soluble propofol prodrug. Handb Exp Pharmacol. 2008:253–266.
279 Schywalsky M, Ihmsen H, Tzabazis A, et al. Pharmacokinetics and pharmacodynamics of the new propofol prodrug GPI 15715 in rats. Eur J Anaesthesiol. 2003;20:182–190.
280 Lubarsky DA, Candiotti K, Harris E. Understanding modes of moderate sedation during gastrointestinal procedures: a current review of the literature. J Clin Anesth. 2007;19:397–404.
281 Fechner J, Ihmsen H, Hatterscheid D, et al. Comparative pharmacokinetics and pharmacodynamics of the new propofol prodrug GPI 15715 and propofol emulsion. Anesthesiology. 2004;101:626–639.
282 Candiotti KA, Gan TJ, Young C, et al. A randomized, open-label study of the safety and tolerability of fospropofol for patients requiring intubation and mechanical ventilation in the intensive care unit. Anesth Analg. 2011;113:550–556.
283 Struys MM, Fechner J, Schuttler J, Schwilden H. Erroneously published fospropofol pharmacokinetic-pharmacodynamic data and retraction of the affected publications. Anesthesiology. 2010;112:1056–1057.
284 Andolfatto G, Abu-Laban RB, Zed PJ, et al. Ketamine-propofol combination (ketofol) versus propofol alone for emergency department procedural sedation and analgesia: a randomized double-blind trial. Ann Emerg Med. 2012;59:504–512. e1-2
285 Nejati A, Moharari RS, Ashraf H, Labaf A, Golshani K. Ketamine/propofol versus midazolam/fentanyl for procedural sedation and analgesia in the emergency department: a randomized, prospective, double-blind trial. Acad Emerg Med. 2011;18:800–806.
286 da Silva PS, de Aguiar VE, Waisberg DR, Passos RM, Park MV. Use of ketofol for procedural sedation and analgesia in children with hematological diseases. Pediatr Int. 2011;53:62–67.
287 Andolfatto G, Willman E. A prospective case series of pediatric procedural sedation and analgesia in the emergency department using single-syringe ketamine-propofol combination (ketofol). Acad Emerg Med. 2010;17:194–201.
288 Loh G, Dalen D. Low-dose ketamine in addition to propofol for procedural sedation and analgesia in the emergency department. Ann Pharmacother. 2007;41:485–492.
289 Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30.
290 Bjorkman S, Gabrielsson J, Quaynor H, Corbey M. Pharmacokinetics of i.v. and rectal methohexitone in children. Br J Anaesth. 1987;59:1541–1547.
291 Rockoff MA, Goudsouzian NG. Seizures induced by methohexital. Anesthesiology. 1981;54:333–335.
292 Jeffries G. Radiotherapy and children’s anaesthesia. Anaesthesia. 1988;43:416–417.
293 Manuli MA, Davies L. Rectal methohexital for sedation of children during imaging procedures. AJR Am J Roentgenol. 1993;160:577–580.
294 Liu LMP, Goudsouzian NG, Liu P. Rectal methohexital premedication in children: a dose comparison study. Anesthesiology. 1980;53:343–345.
295 Liu LM, Gaudreault P, Friedman PA, Goudsouzian NG, Liu PL. Methohexital plasma concentrations in children following rectal administration. Anesthesiology. 1985;62:567–570.
296 Chun TH, Amanullah S, Karishma-Bahl D, et al. Comparison of methohexital and pentobarbital as sedative agents for pediatric emergency department patients for computed tomography. Pediatr Emerg Care. 2009;25:648–650.
297 Daniels AL, Coté CJ, Polaner DM. Continuous oxygen saturation monitoring following rectal methohexitone induction in paediatric patients. Can J Anaesth. 1992;39:27–30.
298 Woolley SM, Hingston EJ, Shah J, Chadwick BL. Paediatric conscious sedation: views and experience of specialists in paediatric dentistry. Br Dent J. 2009;207:E11.
299 Policy on the use of deep sedation and general anesthesia in the pediatric dental office. Pediatr Dent. 2008;30:66–67.