Chapter 123 Sedation and Analgesia
Patients may recall their stay in the ICU. Many patients remember having an endotracheal tube or having their lungs mechanically ventilated. Nightmares and hallucinations also have been reported.1 Either single-drug therapy or inadequate dosing may be associated with a higher incidence of recall in the patient receiving neuromuscular blocking agents. 2 In adults, delusional memories and an underlying anxiety state were predictors of the development of a posttraumatic stress disorder after sedation in the ICU.3 Delusional memories were reported much more frequently than factual memory, probably because most patients have difficulty correctly remembering the events that occur during their stay in the ICU. In pediatric patients, recall of the PICU experience also has been reported.4 More than 66% of pediatric patients remembered their stay in the PICU. Eighteen percent had bad memories, 16% remembered mechanical ventilation and anxiety, and 29% remembered pain from a procedure or movement. Overall the recollections of patients in the PICU were considered negative in approximately 15% of the patients. Sleep disturbance also was a problem.
Various scoring systems are often used to guide sedation. The most widely used is the Ramsay scale.5 The patient’s level of consciousness is classified as one of six scores (Table 123-1). The nurse at the bedside assesses the patient and then changes the sedation regimen as necessary to achieve the desired level of sedation. The ideal level of sedation varies from patient to patient, but in general, most intensive care physicians seek to maintain patients in a sleepy but easily awakened state. A Ramsay score of 2 to 3 seems to be ideal as the clinical end point for sedation. Deeper sedation should be reserved for select patients who are often younger, are receiving neuromuscular blocking agents, or have a head injury. The use of a sedation scoring system to guide sedation of surgical critical care patients has been evaluated for cost-effectiveness. Use of scoring systems has proved to save costs in the ICU.6 Because the patient can be weaned more rapidly from the ventilator through better control of the sedation level, the number of days a patient is connected to a ventilator is reduced. The COMFORT score, which is composed of eight variables (each with five categories), also has been validated for use in the PICU to assess sedation level in children. Use of this system, however, is more complicated and time consuming.7
| Level | Description |
|---|---|
| 1 | Patient awake, anxious, and agitated or restless or both |
| 2 | Patient awake, cooperative, oriented, and tranquil |
| 3 | Patient awake, responds to command only |
| 4 | Patient asleep, brisk response to light glabellar tap or loud auditory stimulus |
| 5 | Patient asleep, sluggish response to light glabellar tap or loud auditory stimulus |
| 6 | Patient asleep, no response to light glabellar tap or loud auditory stimulus |
Many scoring systems are subjective and are limited by interobserver variability. The more objective methods may be too cumbersome for routine use. A simple scoring system has been devised that is easy to use and minimizes subjectivity and observer variability.8 This system is the Brussels sedation scale. It is similar to the Ramsay scale, but the Brussels scale levels that correspond to the Ramsay scale levels 4 and 5 are better differentiated (Table 123-2).
Table 123–2 Brussels Sedation Scale
| Level | Description |
|---|---|
| 1 | Unable to be aroused |
| 2 | Responds to painful stimulation (trapezius muscle pinching) but not auditory stimulation |
| 3 | Responds to auditory stimulation |
| 4 | Awake and alert |
| 5 | Agitated |
The Bispectral Index (BIS) is a processed electroencephalogram (EEG) monitor that measures the hypnotic effects of anesthetics and sedatives. The BIS is an empirical, statistically derived measurement. The BIS monitor reports a single number from 0 to 100 that represents an integrated measure of cerebral electrical activity. The BIS has been validated as a measure of hypnosis in adults in the operating room and ICU.9 More recently it has been validated in the PICU.10 The BIS is an exciting new approach to EEG processing. It measures a state of the brain, representing the degree of alertness. It does not measure the concentration of a particular drug.11 A number of 100 on the BIS score indicates that the patient is fully awake, while a number less than 40 is suggestive of a deep hypnotic effect. A BIS value of less than 60 in surgical patients was not associated with a recall of intraoperative events.12 The use of the BIS monitor in adult surgical patients and in older pediatric patients has shown a reduction in anesthesia requirements and a shorter recovery time. The BIS monitor has been studied in several adult ICU populations. These studies have shown a correlation between the BIS score and a variety of sedation scores.13
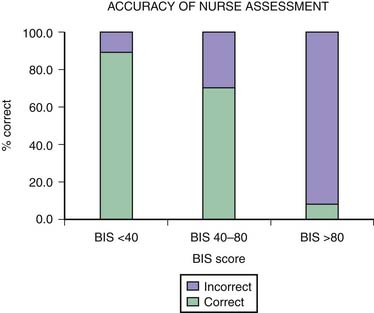
One of the main difficulties with clinical sedation scoring systems is their inability to assess depth of sedation in the patient receiving neuromuscular blocking agents (NMBAs). Patients who require NMBAs in the operating room are considered to be at increased risk of awareness during anesthesia.14 This problem also exists for the sedated patient with paralysis in the PICU. It is well known that the clinical signs of inadequate anesthesia or sedation are not reliable,15 and many other reasons account for alterations in the heart rate, blood pressure, perfusion, and pupillary responses in the PICU patient. In a study using the BIS in pediatric patients with paralysis,16 researchers found that in more than 8% of the sedation assessments in which patients were thought to be adequately sedated by the bedside nurse, their BIS scores were greater than 80 (Figure 123-1). This score reflected a significant risk of awareness. The BIS correlates well with the Ramsay scale in the sedated child and may be a useful monitor to prevent inadequate sedation in a child with paralysis.
Although the BIS monitor is used in many institutions, the question of whether its use can prevent awareness under anesthesia is still debated. A recent study enrolled 2000 adults whose anesthesia was either titrated to a BIS score of less than 60 or by the end-tidal inhalational concentration of the anesthetic agent to at least 0.7 minimum alveolar concentration (MAC).17 Postoperatively all patients were interviewed to assess their intraoperative awareness. This study found two cases of awareness in each group. The MAC values in both groups were the same. The BIS was greater than 60 in one case of awareness. Although the BIS monitor was not able to reduce this low incidence of awareness, the level of anesthesia between the groups was very similar. This study was severely underpowered to show any benefit. However, the combination of end-tidal monitoring and BIS monitoring may be helpful in reducing intraoperative awareness.
Other processed EEG sedation assessment monitors are now available. The SNAP IITM monitor18 uses a different spectrum of EEG frequency analysis. Little difference between the BIS and the SNAP IITM monitors has been shown thus far. Currently little experience has been reported with the SNAP IITM monitor in pediatric patients.
Opioids and Analgesia in the Pediatric Intensive Care Unit
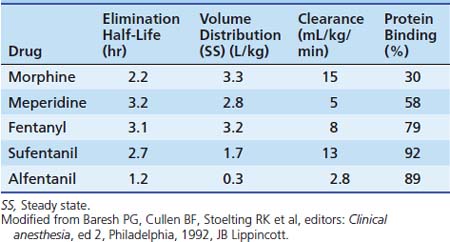
Sedation in the PICU is most commonly achieved with a mixture of opioids and benzodiazepines (BZDs). Although many synthetic and naturally occurring opioids exist, morphine is considered the agent against which others are compared. The primary source of morphine is opium obtained from the opium poppy (Papaver somniferum), which also produces alkaloids such as codeine, thebaine, papaverine, and noscapine. Opiates are substances derived from opium; the term opioid also describes substances derived from opiates (e.g., oxycodone) but also includes substances that are created synthetically but have properties that are similar to those of opiates (e.g., fentanyl and methadone) and endogenous ligands. The terms often are used interchangeably because the pharmacologic effects fall into the same category. Opioids are agonists at various opioid receptors, for which several endogenous ligands exist. There are three major classes of receptors: mu (μ), kappa (κ), and delta (δ). The opioid receptors possess the same general structure of an extracellular N-terminal region, seven transmembrane domains, and an intracellular C-terminal tail structure. Subtypes of each receptor (e.g., μ1, μ2) exist (Table 123-3), as do less well-characterized opioid receptors ε, λ, τ, and ξ.
| Subtype | Prototypic Drugs | Actions |
|---|---|---|
| Mu1 | Opiates and most opiate peptides | Supraspinal analgesia including periaqueductal gray matter, nucleus raphe magnus, and locus coeruleus |
| Prolactin release | ||
| Acetylcholine turnover in brain | ||
| Catalepsy | ||
| Mu2 | Morphine | Respiratory depression |
| Dopamine turnover in brain | ||
| Gastrointestinal tract transit | ||
| Most cardiovascular effects | ||
| Delta | Enkephalins | Spinal analgesia |
| Dopamine turnover | ||
| Kappa | Dynorphin | Spinal analgesia |
| Inhibition of antidiuretic hormone | ||
| Sedation | ||
| Sigma | N-allynormetazocine | Psychotomimetic effects |
Modified from Baresh PG, Cullen BF, Stoelting RK et al, editors: Clinical anesthesia, ed 2, Philadelphia, 1992, JB Lippincott.
Most of the therapeutic and adverse effects can be accounted for by agonist activity at the μ-receptor, which is responsible for analgesia, respiratory depression, pupillary constriction, and euphoria. At the cellular level, μ-receptor activation alters ionic permeability to K+, causing hyperpolarization and depression of excitability in the neuronal system. Associated effects on cholinergic, adrenergic, serotonergic, and dopaminergic neurotransmitter systems are seen within the central nervous system (CNS). These receptors are found at multiple sites along pain pathways including the spinal cord, midbrain, thalamus, and the cortex. At the spinal cord level, pain reflexes (nociceptive) are depressed by receptors in the substantia gelatinosa, which are mostly presynaptic and inhibit the release of substance P from C-fiber nerve terminals and account for the effectiveness of intrathecally and epidurally administered opioids. In the midbrain the analgesic effect is mediated in the periaqueductal gray matter through ascending fibers and also descending fibers that modulate the function of the dorsal horn. Acetylcholine, γ-aminobutyric acid (GABA), norepinephrine, and serotonin also are involved in these pain-modulating pathways. Peripheral opioid receptors also have been shown and can be expressed in response to inflammation.19 The intraarticular injection of morphine produces analgesia following arthroscopy through activation of opioid receptors located on white blood cells.20
The endogenous ligands for the opioid receptors are the enkephalins, endorphins, and dynorphins. They have a morphine-like effect that can be specifically antagonized by the μ-receptor antagonist naloxone. The endomorphins have potent analgesic and gastrointestinal (GI) effects. At the cellular level, they activate G proteins ([35S] GTP gamma-S binding) and inhibit calcium currents.21 Pro-opiomelanocortin is the precursor for β-endorphin (as well as adrenocorticotropic hormone and melanocyte-stimulating hormone). β-Endorphin, itself very active, also includes the amino acid sequence for met-enkephalin, although the main precursor is proenkephalin A, which contains four copies of met-enkephalin and one copy of leu-enkephalin. The met-enkephalin sequence also gives opioid activity to a number of other larger peptides. Proenkephalin B (prodynorphin) gives rise to the dynorphin series and contains three leu-enkephalin sequences. Local application of these endogenous substances to the brain provides effects that are similar to those of opiates. They do not function as analgesics because the administration of naloxone does not cause pain in the normal state. They are released during periods of sustained pain, stress, or activity to modulate physiologic pathways, including those involved with pain. Therefore they are probably important to the physiologic condition of the patient in the ICU.
Specific Opioid Agonists
Morphine
Morphine is an opiate, and its primary therapeutic actions are sedation and analgesia; anxiolysis and euphoria also may occur. These four therapeutic effects may be exploited to the benefit of the patient. These actions are mediated through the periaqueductal gray matter, the ventromedial medulla, and the spinal cord. The reduction of nociceptive reflexes occurs all over the body, even below a completely transected spinal cord. In addition to increasing the sensory threshold for pain, morphine may decrease the hurting aspect (or unpleasantness) of pain. A patient given morphine may say something such as, “I have just as much pain, but it doesn’t distress me as much.” It blunts most types and intensities of pain, although some forms of neuropathic pain are relatively resistant. The resulting analgesia may be potent enough to abolish diagnostic symptoms and signs. The sedative effects reduce higher cortical function, cause difficulty in concentration, and cause a sense of drowsiness and dream-filled sleep. Higher doses will cause a state of unconsciousness or coma. The rate of respiration is reduced with a resultant fall in minute ventilation despite an accompanying increase in depth of breathing. This effect is associated with a decreased responsiveness to carbon dioxide (CO2) and is additive to the decreased CO2 response seen during sleep. In some circumstances respiratory drive may be restricted to hypoxic stimulation of the carotid chemoreceptors; this is the most serious dose-related adverse effect of morphine. It can occur at doses used clinically for analgesia. In general, all opiates produce the same degree of respiratory depression when given in equipotent doses and for any given level of analgesia. Opioids do not have anticonvulsant properties, whereas meperidine (and its metabolite normeperidine) may lower the seizure threshold.
Another CNS effect of morphine is pupillary constriction due to a central effect on the oculomotor nucleus. Nausea results from stimulation of the chemotrigger zone; however, opioids also depress the vomiting center, so the final effect is unpredictable. Nausea and vomiting are much more frequent in ambulatory patients than in patients confined to a hospital bed. Stress-related endocrine responses can be modified by morphine. It decreases the release of several hormones including adrenocorticotropic hormone, antidiuretic hormone, prolactin, growth hormone, and epinephrine. The neuroendocrine stress response that is normally seen with trauma and surgery may be blunted. Itching may be caused by histamine release, but it also may be due to opiate receptor activation in the spinal cord.22
Morphine’s effects on smooth muscle cause constipation. It reduces the intestinal propulsion activity through its central and peripheral effects. The central effects may be mediated by the vagus nerve. The direct smooth muscle relaxation and the increased local cholinergic transmission can be partly reversed by naloxone. This decreased motility is the basis of several over-the-counter antidiarrheal preparations including diphenoxylate, a μ-agonist that does not cross the blood-brain barrier and thus acts as a peripheral opioid agonist. Morphine also causes an increase in biliary tract tone, which may cause biliary colic, as well as increased tone in the bladder detrusor muscle and vesical sphincter. Urinary retention is common with opioids and occurs in 55% of children receiving spinally administered opioid and 20% receiving intravenous (IV) opioid.23
Morphine has been studied extensively in term and preterm neonates. Glucuronidation is present in term babies and in many preterm ones. The half-life of morphine, however, is 2 hours in children, 6.5 hours in term neonates, and 9 hours in the preterm child because of reduced clearance. Volume of distribution did not vary with age.24 Morphine causes histamine release and can cause peripheral vasodilatation. Infused at analgesic doses, it has little effect on the cardiovascular system, but skin flushing is not uncommon with rapid IV administration. The histamine-releasing potential should be considered in patients with asthma, especially during an acute exacerbation, and in patients with unstable cardiovascular systems for whom safer alternatives, such as fentanyl, exist.
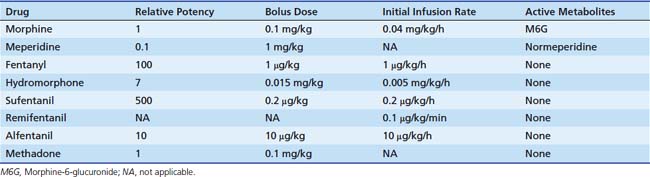
Dosing recommendations in the ICU include a bolus dose of 0.05 to 0.1 mg/kg and an infusion of 0 to 30 μg/kg/h. Fifty percent of these doses should be used if the patient is younger than 3 months of age. The pharmacokinetics of various opiates is outlined in Table 123-4. All opiates are weak bases and are moderately ionized at pH 7.4. Oral morphine is effective but undergoes hepatic first-pass metabolism, which is variable among patients. The oral dose for acute pain is two to five times the IV dose, while with long-term use the oral dose is 1.5 to 2.5 times the IV dose.
Morphine is metabolized to morphine-3-glucuronide (M3G) and M6G in the liver. M3G is the major metabolite and has little morphine-like activity, although some research has suggested that M3G may be associated with an antinociceptive effect, accounting for failure of analgesia during long-term use.25 In contrast, M6G is many times more potent than morphine itself.
Tolerance, defined as an increase in the dose required to create the same response, is a potential problem with all opiates. Tolerance is mainly limited to the depressant actions of morphine, including analgesia, respiratory depression, anxiolysis, and drowsiness. Tolerance of morphine’s inhibition of bowel motility and pupillary constriction is minimal. The mechanism of tolerance appears to involve the degree and duration of both μ- and κ-receptor occupancy. It appears more rapidly after continuous infusion, and cross-tolerance to other opiates is common, although anecdotal evidence suggests that when opioids are switched, a dose reduction may be possible because cross-tolerance sometimes appears incomplete.26 Receptor downregulation also may occur, as well as altered metabolism with an increased M3G/M6G ratio. Simultaneous blockade of N-methyl-D-aspartate receptors has been shown to be effective in reducing the development of tolerance.27 Clinical tolerance appears uncommon with an exposure of less than 3 days, but after prolonged administration, doses 10 to 20 times that which would cause respiratory arrest in nontolerant patients may be tolerated.
Meperidine
Meperidine has one tenth the potency of morphine. Compared with other common opioids, meperidine has more CNS excitatory effects including tremors, muscle spasm, myoclonus, psychiatric changes, and seizures. These effects may be due to a central serotoninergic effect.28 It is metabolized by the liver to normeperidine, which is twice as toxic as meperidine and has a longer half-life (15 hours). Normeperidine accumulation is enhanced in patients with an induced cytochrome P450 system. Meperidine has a shorter duration of action (2 to 3 hours) and has a more rapid onset because of its increased lipid solubility compared with morphine. Meperidine is unique among opioids because of its local anesthetic properties, which are capable of providing surgical spinal analgesia.29 A small dose (0.125 to 0.25 mg/kg) of meperidine may be used to treat postoperative shivering.
Fentanyl
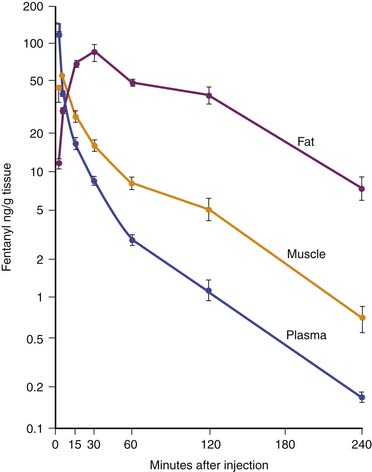
Fentanyl is one of the most commonly used opiates in the ICU. It is a synthetic derivative of meperidine without many of its unwanted side effects. It is a potent μ-agonist and is 100 times more potent than morphine. It has a rapid onset and cessation because of its high lipid solubility (Figure 123-2). Fentanyl may be administered by several routes, including IV, intramuscular (IM), transmucosal,30 and subcutaneous (SC) when venous access is inadequate. Skeletal muscle rigidity (which can occur with all synthetic opiates) is well described in the literature. It is mediated through the CNS and is an idiopathic response usually associated with a large bolus dose (≥5 μg/kg). It improves with the administration of NMBAs and is reversible with naloxone. Fentanyl has limited cardiovascular effects. Moderate bradycardia is the most common hemodynamic effect. Fentanyl does not cause histamine release. Dosing in the ICU is either by bolus (1 to 2 μg/kg) or infusion (1 to 4 μg/kg/h with higher doses as tolerance develops). The short duration of effect of a single dose of fentanyl is not due to metabolism but rather to rapid redistribution. Maximum brain concentration after a bolus is achieved within 90 seconds. Then, because of rapid redistribution, the plasma level falls by 50% in 30 minutes, and the result is a clinical duration of effect of a single dose of approximately 30 minutes. Fentanyl then accumulates in fat, where it is stored and slowly released with a longer elimination half-life of about 4 hours (longer than morphine). Marked respiratory depression occurs within 120 seconds, and a single dose of 5 μg/kg will cause apnea in 50% of patients. Also, fentanyl is metabolized by the liver to nor-fentanyl and hydroxy fentanyl derivatives, both of which are thought to be inactive. In the operating room, high-dose fentanyl is commonly used for cardiac anesthesia and for anesthetization of other unstable patients. A loading dose of 50 μg/kg, followed by 0.5 μg/kg/min, will occupy all opioid receptors and produce a state of anesthesia. Many cases of awareness with patients under anesthesia have been documented, however, even when these high doses of fentanyl were used.
Codeine
Codeine has a chemical structure and effects that are similar to those of morphine and is commonly used as an oral medication for cough suppression or mild to moderate pain relief. A large part of its effects are due to the metabolism of codeine to morphine. Ten to twenty percent of patients lack a metabolic pathway to convert codeine to morphine, which results in an unpredictable effect. Dosing is 0.5 to 1 mg/kg. Constipation is a major adverse effect, and some patients report having a vague peculiar or unpleasant feeling when they take codeine. This drug can be habit-forming. It can be given orally, IM, or rectally. Rapid IV use may result in cardiovascular collapse. Rectally administered codeine has been shown to have as rapid an onset as IM codeine, but it yields lower peak levels in children.31 Codeine has been the analgesic of choice by neurosurgeons because of the belief that pupillary signs are maintained with use of this drug. Morphine has been shown to be a more effective analgesic, however, in patients with head injuries.32
Remifentanil
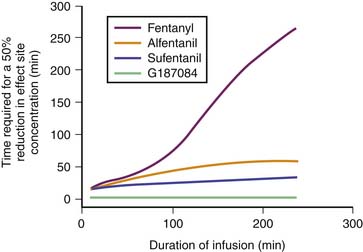
Remifentanil is one of the newest synthetic opiates available. It was designed to be metabolized by plasma esterases to provide a short half-life. It is a potent μ-agonist with mild κ and δ effects. It is substantially more potent than fentanyl. It is supplied as a white lyophilized powder that contains glycine (it should not be used for epidural or spinal analgesia). The metabolism is by nonspecific esterases not affected by pseudocholinesterase deficiency. The metabolite, a weak μ-agonist, is excreted by the kidney. The kinetics of remifentanil is different from those of most opiates used in the ICU. It has a short half-life that is due to metabolism rather than to redistribution. Therefore remifentanil has what is known as a context-sensitive half-life. The elimination half-life for remifentanil is about 8 minutes. With an infusion of remifentanil, the half-life does not increase but remains constant. With opiates such as fentanyl and alfentanil (Figure 123-3), the clinical effect half-life increases with time until it reflects the elimination half-life of between 2 and 4 hours.
Kinetics reported for neonates are similar to those reported for adults. The continuous infusion rate depends on the degree of sedation/analgesia required (0.1 to 0.5 μg/kg/min for sedation; 0.75 to 2 μg/kg/min for balanced anesthesia; 4 μg/kg/min for loss of consciousness). Remifentanil has effects on the cardiovascular system that are similar to those of fentanyl. Remifentanil causes a mild bradycardia and a slight decrease in blood pressure,33 which may be prevented with glycopyrrolate. No histamine release occurs. Remifentanil is a potent respiratory depressant. For spontaneous respiration, a low continuous infusion dose (without a bolus) should be used (0.1 μg/kg/min). Sedation can be effectively managed by continuous infusion without the need for a bolus because of the short half-life. An increase or decrease of infusion rate is rapidly reflected by a change in the degree of sedation, which is important to note. Most other opiate sedatives require bolus dosing to achieve a rapid change in effect. This type of dosing is neither appropriate nor needed for remifentanil.
Remifentanil has the usual opiate adverse effects; however, because of the short half-life, they have only brief clinical effect. Remifentanil may prove to be a safe and effective choice for PICU sedation in patients with severe renal or hepatic disease; however, the potential exists for glycine accumulation in patients with renal failure. It is an option only for those who require overnight ventilation or for those patients in whom a rapid awakening may be required for neurologic assessment. Remifentanil has been shown to reduce cerebral oxygen use and reduce cerebral blood flow if the CO2 is maintained in a normal range.34 Remifentanil is currently an expensive option and should not be considered for every patient. Also, because of its short duration, the postoperative patient may need an alternative analgesic after extubation. Rapid development of opiate tolerance with remifentanil has been described in healthy volunteers35 and also when used in the ICU setting. This rapid tolerance has also been described in postoperative scoliosis patients36; however, the increased morphine requirements described probably reflect the initial postoperative need to achieve an adequate morphine blood level rather than any acute tolerance.
Tramadol
Tramadol is an opiate analgesic that relieves pain by binding to opiate receptors and by inhibiting the reuptake in the CNS and spinal cord of norepinephrine and serotonin, two pain-modifying neurotransmitters. Tramadol does not have antiinflammatory effects. Its use is indicated in cases of moderate to severe pain.37 Despite being a narcotic-like agent, the Food and Drug Administration (FDA) has not classified tramadol as a controlled substance. Dosage (not FDA approved for patients younger than 16 years) is an initial oral dose of 1 to 2 mg/kg every 6 hours and should not exceed 6 mg/kg/day. An IV preparation is available outside of the United States. Patients with a creatinine clearance less than 30 mL/min should not receive a dose more often than once every 12 hours, with a maximum dose of 3 mg/kg/day. The dose for patients with cirrhosis or hepatic dysfunction is 1 mg/kg every 12 hours. Patients undergoing dialysis can receive their dose on the day of dialysis because only 7% of the drug is removed by the process. The adverse effects of tramadol most often involve the CNS and the GI tract. Patients may become dependent on tramadol. Abuse is possible, and it should not be given to opiate-dependent patients. Seizures have been seen in patients receiving high single oral doses of 10 mg/kg; this danger is even greater in patients with epilepsy and in anyone taking monoamine oxidase inhibitors and neuroleptic agents that lower the seizure threshold. Respiratory depression may occur if the recommended dosage is consistently exceeded or if another centrally acting depressant drug (e.g., alcohol) or an anesthetic is given concurrently. Because of the possibility of withdrawal symptoms, patients should not abruptly discontinue use of tramadol. Tramadol is not a useful drug for sedative action.
Table 123-5 provides conversion doses for some commonly used oral opiates. A summary of IV doses of different opiates is provided in Table 123-6.
| Drug | Oral | Parenteral |
|---|---|---|
| Morphine | 0.5 mg/kg q4h | 0.15 mg/kg q3h |
| Hydromorphone | 0.1 mg/kg q4h | 0.02 mg/kg q4h |
| Codeine | 4 mg/kg q3h | |
| Hydrocodone | 0.5 mg/kg q3h | |
| Oxycodone | 0.5 mg/kg q3h | |
| Meperidine | 5 μg/kg q2h | 1.5 mg/kg q2h |
| Fentanyl | 1.5 μg/kg q2h |
Opiate Antagonists
Several opiate antagonists are available. The most commonly used is naloxone, which is a specific and sensitive receptor antagonist of all opiate receptors. Dosing can be either low dose (1 μg/kg) or high dose in an emergency situation (10 μg/kg). If the drug cannot be administered intravenously, then it can be given intramuscularly, intranasally, or into the midventral surface of the tongue. When naloxone is being used for a long-acting agonist, an infusion may be necessary because its half-life is only 30 to 81 minutes (mean of 64 ± 12 minutes). In neonates, the half-life has been reported as 3.1 ± 0.5 hours; however, this prolonged effect is likely to be offset by a concomitant increase in the duration of action of the opioid for which the naloxone is given. No effect is seen in the healthy patient in the absence of administered opioids; however, in the setting of sepsis in the ICU, a vasopressor effect may occur, presumably because of an interaction with endogenous opioids released in response to stress. Nalmefene, a longer-acting antagonist, can be given through IV, IM, and SC routes. It has a redistribution half-life of 41 minutes and a terminal half-life of 10.8 hours in adults and somewhat less in children. Thus reappearance of the antagonized opioid is unlikely if it is given in an adequate dose.38
Incidental Pain Syndromes in the Pediatric Intensive Care Unit
In addition to the techniques used to sedate children to facilitate their PICU management, many children will have pain related to their underlying condition. Many different options are available for controlling pain in the pediatric patient (Box 123-1). The pharmacologic management of pain should follow the traditional World Health Organization analgesic ladder, which begins with a nonopioid analgesic such as a nonsteroidal antiinflammatory drug or acetaminophen, followed by a weak opioid such as hydrocodone added to the nonopioid, and then moves on to a strong opioid such as morphine or hydromorphone as needed. When taken orally, a sustained-release preparation is often useful once the dose requirement has been determined. The dose requirement of a strong opioid is variable in the patient taking opioids for a prolonged period, and failure to appreciate this variability is a common cause for therapeutic failure. In addition, once a dose requirement is known, the analgesic should be given to preempt pain rather than to relieve pain as required. At each level of analgesic use, the addition of adjuvant medications should be considered. Adjuvant drugs fall into six groups: antidepressant, anticonvulsant, neuroleptic, steroid, stimulant, and local anesthetic. Of the tricyclic antidepressants, nortriptyline is available in a liquid form. The tricyclic antidepressants are indicated for neuropathic pain, particularly when the patient describes a burning pain. Also useful for neuropathic pain are the anticonvulsant agents gabapentin, pregabalin, and carbamazepine. These drugs often work best when the pain is described as shooting or lancinating. Neuropathic pain may result from tumor invasion, vincristine therapy, cytomegalovirus infection, or human immunodeficiency infection. Neuroleptic drugs, including chlorpromazine and trimeprazine, may be useful in the management of nausea, anxiety, and pruritus. Steroids benefit mood, inflammation, nausea, appetite, nerve swelling/entrapment, and vasculitis. When opioid sedation is interfering with quality of life, a stimulant such as an amphetamine may restore energy and alertness while allowing ongoing analgesia from the opioid. Sometimes pain can be managed by local anesthetic, placed by peripheral nerve block, topically, or as a neuraxial block.
Patient controlled analgesia (PCA) has become the mainstay of postoperative pain relief in children because of its efficacy and safety. However, its use is limited by the child’s ability to understand how to use the PCA pump. Proxy PCA (PCA-P) has been used for younger children or those with cognitive impairment.39 The use of the PCA by the nurse or the caregiver may override the “safety net” that the PCA has. The use of PCA-P has been associated with greater need for rescue interventions; however, it is often used in sicker children. When PCA-P is used, careful evaluation and rigorous monitoring is needed.
Sickle Cell Crisis
Sickle cell disease differs from cancer and acquired immune deficiency syndrome in that intermittent episodes of severe pain occur, requiring urgent intensive treatment. A good review of this subject has been published.40 Chronic pain also may be present because of long-term tissue and bone damage from periods of ischemia during past crises, including persisting myocardial ischemia. Patients may be receiving long-acting opioids or may have had repeated exposure to opioids with past crises. Patients with a sickle cell disease crisis that involves the chest or brain are likely to be admitted to the PICU. Chest crises result from the sickling of erythrocytes in the pulmonary vasculature and result in hypoxia to the rest of the body and local lung damage. The systemic hypoxia worsens the crisis and is thus self-perpetuating. Chest radiograph changes may be late, and an associated paralytic ileus may be present. Poor pulmonary function may discourage the practitioner from using adequate opioid analgesics out of concern for worsening the hypoxia. However, it is important not to underestimate the need for pain relief and to appreciate that past opioid exposure may have resulted in tolerance to opioids. If IV access is difficult to obtain, morphine may be given subcutaneously or orally. Anecdotal success also has been reported with nebulized morphine.41
Opiate Tolerance
The use of opiate infusions in the ICU is associated with the potential for the development of tolerance or dependence.42 Iatrogenic withdrawal symptoms can occur if the opiates are discontinued abruptly. These effects have been shown to be related to the total dose and duration of fentanyl infusion. A fentanyl infusion of 5 days or a total cumulative dose of 1.6 mg/kg during the hospital stay was associated with a 50% chance of the development of narcotic withdrawal, whereas a fentanyl infusion of 9 days or longer or a total cumulative fentanyl dose of 2.5 mg/kg or more during the hospital stay had a 100% incidence of withdrawal.43 The rising plasma fentanyl levels caused by increased dosing suggested that increased metabolism or clearance was not responsible for the development of tolerance. A study of patients in a PICU to determine the degree of opiate tolerance has shown a significant increase in opiate dosing required for adequate sedation.44 The opiate infusion increased by about 80% per week for the first 3 weeks of opiate use. No difference in the rate of opiate increase was found with respect to age of the patient, postoperative status, mode of ventilation, and paralysis.
For patients considered to be at risk of withdrawal, several options are available. If circumstances allow, it is better to start the treatment for withdrawal prevention before the patient has symptoms and signs of withdrawal. Opiate withdrawal is not usually a serious medical problem; it is rarely life-threatening and is self-limited. However, treatment should be started early if possible for patient comfort. In a few circumstances the associated hypersympathetic state may not be good for the patient. The signs and symptoms of withdrawal are nonspecific, and other causes, such as infection, hypoglycemia, hypocalcemia, hyperthyroidism, and hypoxia, should be excluded. Because of the nonspecific nature of the symptoms of opiate withdrawal, several scoring systems have been described to aid with the diagnostic process. The Finnegan score is based on 31 variables and is lengthy. The Lipsitz score is shorter and easier to use than the Finnegan score. Both of these scoring systems, however, were devised for use with neonates, and several of the measurements are not appropriate for patients in the PICU. Currently no validated scoring system exists for assessing opiate withdrawal in the pediatric patient. In the limited number of articles in which opiate withdrawal in the PICU is evaluated, the authors have modified these scores (Table 123-7) in an attempt to provide an objective assessment of the patient.
| Sign/Symptom | Examples |
|---|---|
| Neurologic excitability | Sleep disturbances |
| Agitation | |
| Tremors | |
| Seizures | |
| Choreoathetoid movements | |
| GI disturbances | Vomiting |
| Diarrhea | |
| Autonomic dysfunction | |
| Hypertension (>150 mm Hg) | Tachycardia (>150 mm Hg) |
| Tachypnea (>40 beats/min) | |
| Fever (>38.58° C) | |
| Frequent yawning | |
| Sweating | |
| Goose flesh | |
| Mottling |
GI, Gastrointestinal.
Recently the assessment of a new score based on a similar set of signs and symptoms, the Withdrawal Assessment Tool,45 has been proposed. The bedside nurse reviews the patient’s chart for the previous 12 hours and performs a short assessment of the child’s level of agitation, as well as other signs/symptoms. This review is then followed by an assessment of the response to stimulus and also how quickly the child settles down after the stimulus. A score of 1 is assigned to each assessment (maximum, 12). A score of greater than 3 was associated with a greater likelihood of drug withdrawal. Although this score is new and has limited verification, it probably provides a better evaluation than just the clinician’s “bedside” opinion, especially in complex cases in which both opiate and BDZ withdrawal may occur together.
Several therapeutic options are available for the prevention and treatment of opiate withdrawal. Drugs from the same class are preferable. The FDA has approved methadone for opiate withdrawal. Other agents that may be useful include morphine, clonidine, dexmedetomidine, phenobarbital, paregoric, chlorpromazine, transdermal clonidine patch,46 and SC fentanyl. Paregoric contains morphine plus papaverine, noscapine, camphor (a CNS stimulant), ethanol (45%), benzoic acid (which competes with bilirubin-binding sites), and glycerin (which causes diarrhea). Paregoric has been used for neonatal withdrawal, but because of its composition, it may cause adverse effects. Chlorpromazine may be useful for GI adverse effects, but hypothermia and hypotension may occur. Haloperidol also may be of use, having minimal respiratory depression and no active metabolites. It also offers cardiovascular stability. Phenobarbital has been used for hyperactive behavior; however, it can cause significant CNS depression, it induces drug metabolism, and it is tolerance/dependence forming.
The convenience of the oral route, the less-frequent dosing because of its longer half-life, and the ease of calculating doses because of its equal potency to morphine make methadone attractive for use in the management of opiate withdrawal in children. However, a huge variability exists in recommendations regarding the methadone dose that should be used to prevent opiate withdrawal in children. Several factors are important in the dosing of methadone for conversion from fentanyl. After prolonged IV administration, fentanyl has a potency 100 times that of methadone; it has a metabolic half-life approximately one quarter that of methadone; and if given intravenously, it has a bioavailability 20% greater than orally administered methadone. In a study in which the effectiveness of a fentanyl-methadone conversion protocol was assessed, researchers found that giving 2.4 times the daily fentanyl dose as methadone prevented withdrawal symptoms.42 The methadone was given intravenously for 24 hours, and the fentanyl dose decreased by 50% on day 1 and by another 50% on day 2, and then it was discontinued. On day 3, the methadone was converted to oral dosing. The methadone was given intravenously initially. Because of its long half-life, oral dosing could take up to 5 days to reach a steady state. The duration of methadone requirement varied from 1 to 4 weeks, depending on the duration of opiate infusion. Methadone was being weaned by 3% to 15% per day with no signs of withdrawal. To date, there have been no published cases of respiratory arrest when methadone has been used for opiate weaning. However, it would appear prudent to initiate the conversion from fentanyl to methadone in the ICU environment in the event that problems arise and to ensure that an adequate dose is given. Once stabilized, the patient may be transferred to the floor and ultimately home, with a clearly described plan for decreasing the methadone dose over time. The weaning plan also should involve the home pediatrician so that patients have access to someone who is familiar with the process. In a follow-up of patients who had received methadone while in the ICU, 38% of patients were discharged home during the weaning process. No problems were associated with the weaning of methadone at home. Stigma regarding methadone use was not expressed by any of the parents.
The use of a clonidine patch also has been evaluated in the PICU. Clonidine has been shown to be effective in the management of nicotine, opiate, and alcohol withdrawal. It decreases sympathetic outflow from the CNS and has a synergistic effect for analgesia, both at the central and spinal level. In one report, eight patients were described after tracheal reconstructive operations. They required postoperative sedation and ventilation for 7 days, which put them at high risk for withdrawal.20 A clonidine patch was applied 12 hours before extubation, and the patients were weaned off the opiate. The dose used was approximately 6 μg/kg/day of clonidine, and the patch was left on for 7 days. One patch had to be removed because of hypotension. The patch seemed to be effective in preventing withdrawal. Use of the clonidine patch is attractive because of its noninvasive approach, which is desired. However, the use of a transdermal patch prevents titration of effect, and problems with bradycardia, hypotension, hypothermia, sedation, and dysrhythmia may occur.
A confounding issue in many publications and in the clinical management of opiate withdrawal is the potential for simultaneous BZD withdrawal. Most researchers have not been able to separate these two issues. The symptoms of BZD withdrawal differ from those of opiate withdrawal because the BZD symptoms generally include less sympathetic activation. BZD withdrawal symptoms are characterized by agitation and a movement disorder. If BZD withdrawal is a concern, low-dose lorazepam or diazepam may be added to the withdrawal management strategy (Table 123-8). A prospective study of BZD withdrawal47 following lorazepam infusion (up to 0.3 mg/kg/h) documented BZD withdrawal syndrome in approximately 25% of the children. This withdrawal occurred even when using a 6-day tapering of the lorazepam dose. All the children had been previously weaned off fentanyl infusions. No predisposing risk factors were found for BZD withdrawal with respect to BZD or opiate dosing or duration.
Rapid Opiate Detoxification
Reports have been made of rapid opiate detoxification in the ICU. These procedures have used a form of deep sedation (often with use of propofol or another anesthetic agent) to facilitate opioid withdrawal in patients addicted to the recreational use of opiates.48 The patients are given high doses of opiate antagonists to displace all opiates from the receptors and then heavy sedation is initiated to reduce the occurrence and effects of the sympathetic stimulation observed with short-term opiate withdrawal. These procedures have been safely performed in the ICU; however, there have been several reports of complications49 when these procedures were not performed with full ICU support. Currently the effectiveness and safety of 1-day opiate detoxification is still an area of debate.50 If used, however, it should be combined with an established long-term support plan to optimize long-term success.
In the PICU, deep sedation with propofol has been used to facilitate rapid opiate weaning of ventilator-dependent patients.51 The use of propofol for up to 3 days allowed a reduction of fentanyl dosing from 24 to 9 μg/kg/h (a 65% reduction). No signs or symptoms of opiate withdrawal were noted, and metabolic acidosis did not develop. Opiate antagonists were not used for this rapid weaning process. However, concern has been raised regarding the long-term administration of propofol, especially in the PICU patient, given the development of the propofol infusion syndrome.
Benzodiazepines
BZDs are among the most commonly used agents for sedation in the ICU. They augment the function of the GABA type A (GABAA) receptor at the postsynaptic membrane. This pentameric protein controls a chloride channel, the opening of which leads to an inhibitory effect due to hyperpolarization of the cell membrane.52,53 Benzodiazepines bind to BZD receptors, which in the CNS are usually found as part of the GABAA receptor, enhancing the effect of endogenous GABA.54 Peripheral BZD receptors55 are not usually associated with the GABAA receptor but are a binding site for diazepam and midazolam. These 18-kDa proteins are associated with regulation of cellular proliferation, immunomodulation, porphyrin transport, heme biosynthesis, and anion transport. In particular, they seem important in the regulation of steroid synthesis and apoptosis, and they have a significant effect on the hypothalamic-pituitary-adrenal axis.56 These latter effects may be pertinent to the physiologic care of patients in the ICU.
BZD receptors are bound by a family of endogenous peptides called endozepines, which have similar effects to the BZDs.57,58 The expression of this diazepam-binding inhibitor may be relevant to the development of dependence not only on BZDs but also on alcohol and opioids59 and may therefore be relevant in the drug dependence commonly seen in patients in the PICU who are given these agents continuously. Naturally occurring BZDs have been detected with structures similar to those used clinically.60 Subsets of GABAA receptors have been shown to have different effects. Type 1 receptors were responsible for sedation and anterograde amnesia, whereas type 2 receptors mediated anxiolysis. It may be possible to develop selective subtype receptor agonists to provide anxiolysis without sedation, amnesia, or dependence.
The general pharmacologic effects of BZDs are sedation, anxiolysis, euphoria (limbic system), reduced skeletal muscle tone (through spinal BZD receptors), anticonvulsant properties, and neuroendocrine effects. They impair acquisition and encoding of new information, providing anterograde amnesia. They do not have any analgesic properties. They have little direct effect on ICP. Their effects are dose dependent. Patient cofactors including age, concurrent disease, and any cosedation therapy influence responses to BZDs. Paradoxical reactions are reported in which agitation rather than calming is observed.61 In healthy patients, BZDs have few cardiovascular adverse effects, but in a sick, intensive care population, profound cardiovascular depression may be observed occasionally. BZDs should be used judiciously until the patient response is known.62 Midazolam has been most often associated with this effect,63 and research in dogs has shown both negative inotropy and chronotropy, especially when the sympathetic response has been abolished.64 Clinical use is largely encompassed by discussion of the pharmacologic properties of diazepam, midazolam, and lorazepam.
Specific Benzodiazepines
Diazepam
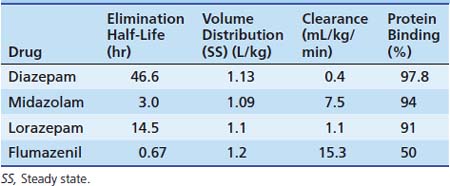
The first widely used BZD in the ICU was diazepam. Because of its low solubility in water, it is available in the IV or IM form dissolved in propylene glycol. This formulation causes a significant amount of pain and thrombophlebitis with peripheral IV use. A lipid emulsion that has fewer adverse effects is available in the United Kingdom. Diazepam is inexpensive and is effective for short-term sedation; in such cases, accumulation is less of a concern. Diazepam may be given orally because it has good absorption, but absorption tends to be erratic when it is given rectally or intramuscularly. It is highly lipid soluble with a long half-life (24 hours). Metabolism by oxidative biotransformation generates several hypnotically active metabolites with an elimination half-life that may be longer than diazepam, including oxazepam (half-life, 10 hours) and n-dimethyldiazepam (half-life, 93 hours). Delayed recovery has been reported in neonates after they received diazepam, possibly because of the long half-life of dimethyldiazepam.65 Prolongation of effects occurs in patients when clearance is reduced because of hepatic dysfunction and when metabolism is inhibited by drugs such as cimetidine and omeprazole.
Midazolam
Midazolam is an imidazobenzodiazepine. It has a short elimination half-life of 2 hours and is water soluble, which means that IV injection is nonirritating. Because of these factors, it has become popular in ICUs for sedation by infusion. Intranasally (0.2 mg/kg), midazolam has proven to be as effective at controlling febrile seizures as IV diazepam (0.3 mg/kg).66 It has extensive first-pass metabolism and provides less reliable results when given PO, although this route is often successfully used for premedication of children before general anesthesia in doses of 0.5 to 0.75 mg/kg (maximum, 20 mg). It is available in a pleasant-tasting cherry syrup and is effective in 10 to 15 minutes, providing up to 1 hour of adequate anxiolysis, although residual hangover effects may persist.67 Rectal and sublingual administration has been described.
Midazolam is about eight times more potent than diazepam, with starting dose recommendations of a bolus dose of 0.05 to 0.1 mg/kg68 and an infusion of 1 to 6 μg/kg/min. Midazolam is metabolized by the cytochrome P450 system subfamily IIIA (nifedipine oxidase), polypeptide 4 (CYP3A4),69 to hydroxymidazolam (63% potency) and hydroxymidazolam glucuronide (9% potency). Because of the high degree of protein binding (94% protein bound), the free level can be significantly changed with interactions because of the protein binding, which also may occur with heparin. Hepatic or renal failure increases the free fraction by two to three times, and its effect also can be prolonged by the accumulation of active metabolites.70 The half-life of midazolam in patients in the ICU may be prolonged compared with that in healthy patients.71 With short-term infusions (<12 hours), it retains a rapid recovery; however, with increased duration of use, the recovery becomes prolonged. Its clearance may be reduced by several commonly used ICU drugs, including calcium channel blockers, erythromycin, and triazole antifungal agents.72
Lorazepam
Lorazepam is an alternative water-soluble agent that is well absorbed after both oral and IM administration.73 It produces sedation for 4 to 8 hours after a single dose. Lorazepam has a slower onset than does midazolam. The elimination half-life is about 14 hours. Metabolism is by glucuronyl transferase, not the cytochrome P450, and there are no active metabolites. This metabolism is unaffected by cimetidine or phenobarbital, which only affects oxidative metabolic pathways. Sodium valproate may inhibit its metabolism.74 In persons with advanced liver disease, these phase II glucuronidation reactions are better preserved, and the increased half-life seen is due to increases in the volume of distribution rather than to reduced clearance. In patients with renal failure, prolonged half-life is also due to reduced protein binding because clearance is unchanged. No change in metabolism occurs with aging or critical illness. In a comparison of infusions of midazolam and lorazepam, the recovery characteristics were found to be significantly different. In patients receiving lorazepam, it took an average of 260 minutes to return to baseline, whereas in patients receiving midazolam, it took more than six times longer to return to baseline. Lorazepam may be administered by bolus (0.05 to 0.1 mg/kg every 2 to 4 hours) or by infusion (0.05 mg/kg/h). Lorazepam is slightly less expensive than is midazolam.75 It has been recommended as the BZD of choice for long-term sedation because of its more predictable recovery profile in sick patients in the ICU. Lorazepam for IV use has propylene glycol as a carrier. Risk of a metabolic lactic acidosis exists because of the metabolism of this carrier. Cases of fatal metabolic acidosis from propylene glycol have been reported in neonates taking a particular vitamin preparation. Several other potential ICU drugs may use propylene glycol as a carrier, including some IV preparations of phenytoin and phenobarbital, nitroglycerin, digoxin, and etomidate. Reports of propylene glycol toxicity in adults who received multiple propylene glycol infusions have been made.76 Care should be taken when lorazepam is infused in patients who receive these other medications. In patients in the PICU, propylene glycol levels have been shown to correlate with the dose of lorazepam received; however, no metabolic abnormalities were detected.77 Hemodialysis has been used successfully in the management of the lorazepam-associated propylene glycol toxicity.78
The metabolisms of different BZDs are intertwined with each other. Most of the agents require an oxidative process first with potentially active compounds before glucuronidation and excretion. The pharmacokinetics for different BZDs is shown in Table 123-8.
Tolerance and Dependence to the Benzodiazepines
Tolerance for and dependence on BZDs can occur as with opiates in the PICU.79 This effect is not all due to receptor number downregulation.80 Withdrawal symptoms may be avoided with a slow taper of the medication of 10% per day or by substituting a long-acting oral agent such as diazepam. Acute withdrawal symptoms may include anxiety, insomnia, nightmares, seizures, psychosis, and hyperpyrexia. A postmidazolam infusion phenomenon has been described that includes poor social interaction, decreased eye contact, and a decreased interest in the surroundings. The patient may exhibit choreoathetotic movements with dystonic posturing that can persist for 2 to 4 weeks but will resolve with no sequelae.
Flumazenil
Flumazenil is an imidazobenzodiazepine and is a specific competitive antagonist of the BZD receptor. It has no effect on other drugs such as barbiturates, ethanol, or other GABA-mimetic agents. Flumazenil reverses the hypnotic and sedative effects of BZDs. It has a half-life of approximately 1 hour after a single IV bolus. In patients with hepatic impairment, its half-life and clearance are prolonged, and a significant increase (>50%) of free drug occurs because of reduced plasma protein binding. Renal failure has little effect on the pharmacokinetics of flumazenil. It is indicated for the complete or partial reversal of the central sedative effects of BZDs. Contraindications include patients who have a known hypersensitivity to BZDs, patients with epilepsy who are receiving treatment with BZDs, and persons who have overdosed with a tricyclic antidepressant. The use of flumazenil is often associated with mild to moderate tachycardia and hypertension.
In cases of multiple drug overdose, the use of flumazenil remains controversial. It often is overused in the emergency setting without due concern for potential adverse reactions81 because of the potential toxic effects (e.g., cardiac arrhythmias or convulsions) of other psychotropic drugs ingested. The toxicity of tricyclic antidepressants becomes apparent as the effects of BZDs are antagonized. Patients should be evaluated for the signs and symptoms of a tricyclic antidepressant overdose; an electrocardiogram (ECG) may be helpful in determining the risks involved.
The dosing information for pediatric patients is limited. The initial suggested dose is 0.01 mg/kg (maximum, 0.2 mg) with incremental doses of 0.005 to 0.01 mg/kg (maximum, 0.2 mg) given every minute up to a maximum cumulative dose of 1 mg. The lower doses are suggested for sedation reversal and the higher doses for BZD overdose. Infusions at 0.05 to 0.01 mg/kg/h have been used.82 The use of flumazenil in sedated patients in the ICU should be tempered by the potential for an unrecognized BZD dependence, which would increase the risks of adverse effects. If its use is required, then a carefully titrated dose would be appropriate. The half-life of flumazenil is much shorter than that of some of the BZDs it may be counteracting (see Table 123-8). The use of an infusion may be necessary because resedation has been reported after single-bolus use.83 However, this requirement should not preclude the use of flumazenil in an ICU setting.84 Flumazenil has been used for the reversal of moderate sedation. In the pediatric population, although it was well tolerated, it was not shown to significantly reduce recovery time.85 Because flumazenil has a limited duration with the potential for resedation after discharge from medical care, an appropriate period of observation is required before discharge. A study in which researchers monitored the effects of flumazenil after sedation indicated that some of the residual effects of midazolam were still present after reversal.86 Flumazenil also has been used to treat a paradoxical midazolam reaction87 and has been shown to be effective in the management of hepatic encephalopathy or hyperammoneamia.88 A Cochrane Collaboration review of articles pertaining to flumazenil use demonstrated a short-term improvement in hepatic encephalopathy. However, no improvement in recovery or survival was documented. No serious adverse effects were noted.
Chloral Hydrate
Chloral hydrate is a widely used oral hypnotic/sedative agent. It has been used for sedation for radiographic procedures, for EEGs, and in many different health care locations. It was first synthesized in 1832 and used in 1869 as a hypnotic agent. Shortly after, reports of acute and chronic toxicity were published.89 In 1910, it was labeled as the most dangerous of hypnotics even though heroin and opium were in common use at that time. The addition of ethanol potentiates its effect (street name “Mickey Finn”). It has been used to control agitation in the intensive care nursery and to treat sleep difficulties in older patients.
Chloral hydrate is rapidly and completely absorbed from the GI tract and is immediately converted into the active component, trichloroethanol (TCE), by alcohol dehydrogenase.90 The plasma levels peaks at 30 to 60 minutes. TCE is 45% protein bound. TCE undergoes glucuronidation with some oxidation to trichloroacetate (TCA). The half-life of TCE is 8 to 12 hours, while that of TCA is 67 hours. In infants and neonates this may be increased by a magnitude of three to four. With multiple dosing, a significant potential exists for accumulation. TCA can displace bound bilirubin from albumin. Its actions include CNS depression with drowsiness and sleep in less than an hour. With an overdose, the patient falls into a deep stupor or coma, and the pupils change from contracted to dilated. At therapeutic levels, the blood pressure and respiratory rate are unaffected. Chloral hydrate has little hangover effect. It has several effects on the cardiovascular system including decreased myocardial contractility, a shortened refractory period, and an increased sensitivity of the heart to catecholamines. It also has effects on mucous membranes. Irritation can cause gastritis, nausea, and vomiting. With overdose, a severe hemorrhagic gastritis with gastric necrosis and esophagitis has been described. Chloral hydrate and ethanol interfere with one another’s metabolism through competition for alcohol dehydrogenase. Also, ethanol inhibits the conjugation of TCE, and TCE inhibits the oxidation of ethanol. Coumadin activity may be increased by chloral hydrate. Chloral hydrate is synergistic with other sedative agents. In children receiving amphetamine-based medication, chloral hydrate is contraindicated because there have been rare reports of arrhythmias. The reversal of chloral hydrate with flumazenil has been described; however, a report of ventricular tachycardia with this combination also has been made.
Signs of toxicity are usually noted within 3 hours of dosing. Paradoxical excitement also has been described in 6% of patients. There is some evidence that chloral hydrate may be genotoxic and carcinogenic. Mice studies have shown that a single-dose exposure can result in an increased risk of hepatic carcinomas and adenomas.91 Chloral hydrate overdose produces a clinical picture that is similar to acute barbiturate poisoning. Ataxia, lethargy, and coma occur within 1 to 2 hours. Also, a pearlike odor may be noted. Cardiovascular instability poses the main threat to life. Severe arrhythmias including atrial fibrillation, supraventricular tachyarrhythmia, ventricular tachyarrhythmia, torsades de pointes, and ventricular fibrillation have been described. Chronic use can cause a dependence syndrome. Also, chloral hydrate is not detectable in the blood. TCE levels are measurable, but they are not useful for clinical management, although they can be helpful for retrospective diagnosis. The management of toxicity includes evaluation and monitoring at a medical facility if an amount greater than 50 mg/kg or an unknown amount has been ingested. Two capsules may cause significant toxicity in a toddler, so there is little room for error in the history. Charcoal with intubation should be considered if significant toxicity is suspected. Standard antiarrhythmic management is often unsuccessful, although esmolol, overdrive pacing, and hemoperfusion have been tried.
Other Agents for Sedation in the PICU Patient
Butyrophenones and Phenothiazines
Haloperidol
Butyrophenones belong to the group of major tranquilizers. Haloperidol is a potent antipsychotic agent with nonspecific dopamine antagonist action. It has little effect on the cardiovascular or respiratory systems. It produces the appearance of calm with minimal hypnotic effect and reduces operant behavior (purposeful movement). The patient appears tranquil and dissociated from surroundings but is readily accessible if spoken to. Haloperidol may mask actual feelings of mental restlessness. It is a potent antiemetic agent (action at the chemotrigger zone) and has no appreciable effect on the EEG. It potentiates analgesics and other sedative agents. Compared with less potent butyrophenones, it has fewer adverse effects. Neuroleptanalgesia, a dissociative form of anesthesia, can be induced when haloperidol is combined with high-dose opiates. This anesthetic state is useful for certain cardiac and neurosurgical procedures that require cardiovascular stability and a responsive patient. It is metabolized to inactive compounds with a half-life of 15 to 25 hours. It is highly protein bound. Hepatic dysfunction increases the half-life because of reduced clearance. Adverse effects include extrapyramidal signs, although acute dystonia is rare. Prolongation of the QT interval is possible with the subsequent risk of ventricular tachycardia.92 Hepatic toxicity can occur but is rare. Haloperidol is indicated for the treatment of psychoses, Tourette’s disorder, and severe behavioral problems in children. In the PICU it is used as a treatment for agitation in patients who are often unresponsive to other more commonly used agents. It also has proved to be effective as part of a sedative withdrawal strategy. Haloperidol is available as syrup, tablets, and an IM preparation. The usual dosage for agitation in children younger than 3 years is 0.01 to 0.03 mg/kg every 4 hours. The maximum daily dose is 0.15 mg/kg/day. Two IM preparations are available: the lactate is for repeated use, and the decanoate is a slow-release monthly formulation. Although not approved by the FDA, the IM lactate form has been given intravenously without problems.
Droperidol
Droperidol is faster acting than haloperidol with a shorter duration of action and a half-life of 2 hours. It is available as an approved IV formulation. With an IV bolus, mild hypotension occurs because of mild α-adrenergic receptor blockade. Droperidol is more sedating than haloperidol and may be used as a sedation adjunct to general anesthesia. It also is used in low doses (0.05 mg/kg) as an antiemetic agent. Concerns exist about the potential for droperidol to cause prolongation of the QT interval and result in ventricular tachycardia.93
Chlorpromazine
Chlorpromazine is a weaker antipsychotic agent with general CNS depressant activity. It has an antidopaminergic effect including extrapyramidal adverse effects, lethargy, and apathy with an EEG similar to that of normal sleep. It also causes a decrease in the body’s ability to maintain temperature control, shivering is reduced, and it can be useful in patients in hypothermic-induced states. Cardiovascular effects include α-adrenergic receptor blockade with hypotension and postural hypotension, but no effect is seen on the ECG. Respiratory drive and depth are unaffected; however, some dryness of the mucosa may be noted. In the GI tract, its anticholinergic effect causes a decrease in secretions and motility. Liver effects include jaundice, which occurs in 0.5% (recurrence rate, 40%), independent of dose or duration of therapy, and is associated with a rash, fever, and eosinophilia. This syndrome has a low mortality rate and usually resolves quickly upon discontinuation of chlorpromazine. Other effects include antihistamine-like action; local analgesia; a temporary leukopenia; and, rarely, agranulocytosis. Chlorpromazine also has antiemetic properties. Indications include premedication, sedation as part of the lytic cocktail catheterization mixture number 3 (CM3),94 intractable pain, antipsychosis, treatment of hiccoughs, prevention of succinylcholine pain, and induction of hypothermia (with other active measures). Dosing (0.05-1 mg/kg every 6 hours) may be via the PO, IM, IV, or rectal routes. Chlorpromazine is metabolized both in the gut wall and by the liver. It yields more than 50 metabolites, most of which are inactive.
Neuroleptic Malignant Syndrome
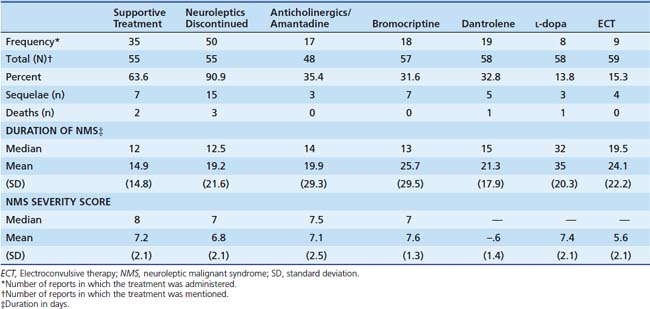
Both the butyrophenones and the phenothiazines have a rare but well-described adverse effect called the neuroleptic malignant syndrome. It is a cluster of adverse effects of antipsychotic medications first described in 1968. It involves the development of hypertonicity with autonomic instability, fever, and cognitive disturbance. The incidence is 0.5% to 1.4% of patients exposed to neuroleptic agents. The true incidence in children is unknown, however. Several different diagnostic criteria are available. Fever and rigidity present in all cases; other symptoms are shown in Box 123-2. A variety of therapies have been described (Table 123-9).
Baclofen
Recently, intrathecal baclofen (ITB) has been used with increasing frequency in children to treat spasticity. ITB was first introduced in 198495 with a pump delivery system that was available in 1992 for adults. This system allows delivery of the drug to the spinal cord and reduces the dose significantly (1% of oral requirements), limiting systemic adverse effects.96 Baclofen inhibits the release of serotonin in the brainstem. After long-term use there is accommodation of the serotonin pathways to this long-term inhibition that is consistent with the usually observed increasing doses required for ITB during the first 12 to 18 months of treatment. When this inhibition is abruptly removed, sudden excess release of serotonin occurs. Acute overload of serotonin transmission, such as an overdose of serotonin reuptake inhibitors, can result in confusion, hyperthermia, myoclonus, and autonomic instability. It also has anticholinergic and antihistamine effects that may result in drowsiness; paradoxical excitation has been reported in children. More than 25 case reports97 of ITB withdrawal have now been reported. ITB withdrawal seems to be more severe if the ITB treatment was for more than 1 year. A review of ITB pumps in 100 patients at a single center98 has shown that problems with the delivery system are fairly common. Twenty-four percent of patients experienced a problem, with a follow-up period for a maximum of 5.6 years. An average of two problems per patient was reported. Disconnection of the catheter from the implanted pump was the most common problem. Access ports on the pump seemed to increase the risk of problems (16% compared with a 2% disconnection rate); however, these ports make troubleshooting easier. Causes of difficulty with ITB delivery are shown in Box 123-3.
The ITB withdrawal syndrome is interesting because it appears to have many similarities with the neuroleptic malignant syndrome. Prolonged muscle contraction caused by rebound spasticity results in thermogenesis, hyperthermia, and rhabdomyolysis.99 Patients with ITB withdrawal often are managed initially with broad-spectrum antibiotics as if they have sepsis and multisystem organ failure, with no improvement in the clinical situation.100 This treatment results in a delay of the diagnosis of ITB withdrawal. The differential diagnosis of the hypermetabolic state is listed in Box 123-4. The symptoms of ITB withdrawal can be classified into three categories (Table 123-10). Often the first clinical signs are the development of itching and some increase in spasticity. If replacement baclofen is not given, then the symptoms may progress to a severe hypermetabolic state that can be fatal if the cause is not recognized and treated. Of 27 patients reported to the FDA, six deaths were documented.101 The management of ITB withdrawal requires early diagnosis. It involves supportive ICU care and the onset of baclofen replacement therapy as soon as possible. Box 123-5 provides a guideline for the evaluation of the patient with suspected baclofen withdrawal. A definitive diagnosis may be obtained with measurement of cerebrospinal fluid baclofen levels, but the results probably are not going to be available in the time course of treatment initiation. Although the primary aim should be to replace baclofen, rapid replacement of ITB may not be possible. The required oral baclofen replacement dose may be 50 to 100 times the intrathecal dose, and this dose often is not well tolerated by patients because of adverse effects. IV administration of a BZD should be the initial step in the treatment of baclofen withdrawal. Dantrolene has been used as an adjunct therapy for the increased spasticity.
| Designation | Description |
|---|---|
| Mild | Pruritic symptoms and increased spasticity |
| Moderate | High fever, altered mental status, seizures and profound rigidity, autonomic instability |
| Severe | Rhabdomyolysis, hepatic, renal failure, DIC brain injury, death |
DIC, Disseminated intravascular coagulation; ITB, intrathecal baclofen.
Box 123–5 Management of Suspected Baclofen Withdrawal
Modified from Kao LW, Amin Y, Kirk MA et al: Intrathecal baclofen withdrawal mimicking sepsis, J Emerg Med 24:423-427, 2003.
The use of the potent serotonin antagonist cyproheptadine has been proposed as an alternative treatment adjunct.102 It improved fever, spasticity, and itching in adult patients with gout who had ITB withdrawal. Dosages of cyproheptadine were in the range of 0.25 mg/kg/day every 6 hours, either PO or IM. In some patients the ITB withdrawal is an elective management problem due to pump removal for infection. In these patients, if a replacement pump cannot be placed, the patient needs to be observed and managed in the ICU to recognize and treat the withdrawal syndrome. The monitoring of creatine phosphokinase (CPK) levels may be helpful in managing withdrawal. In the reported cases of ITB withdrawal, CPK levels have been in the range of 1800 to more than 40,000.103 Mild elevations in CPK (300 to 500) may be an early marker of inadequate treatment.
Dexmedetomidine
Dexmedetomidine (Precedex) is a selective α2 adrenergic agonist. It has an effect at receptors in the CNS and peripheral nervous system, as well as in autonomic ganglia. Stimulation of the α2 receptor decreases the release of norepinephrine, inhibits sympathetic activity, and produces sedation, anxiolysis, and analgesia. It is 1600 times more active at the α2 receptor than at the α1 receptor and is thus eight times more selective than clonidine. It is available as a white water-soluble powder in a 100-μg vial. In adults it has a redistribution phase of 6 minutes and an elimination half-life of 2 hours. The pharmacokinetics appears to be similar in the pediatric patient, even after a 24-hour infusion.104 It is almost completely metabolized in the liver by glucuronidation and P450 pathways to inactive metabolites. In patients with renal failure the pharmacokinetics did not show any prolongation of the terminal half-life; however, these patients were sedated for longer after the infusion was terminated compared with the control group.105 The prolonged sedation may be related to reduced protein binding of this normally highly protein-bound drug (94%) and thus higher free drug levels in the patient with renal failure. In patients with hepatic dysfunction, reduced clearance has been reported. With patients in severe hepatic failure, a prolongation of the half-life almost three times longer than normal was reported.106
Dexmedetomidine has proved to be effective for sedation in the adult intensive care setting.107 Currently it is only licensed for 24 hours of sedation, although approval for more prolonged use is pending. The recommended dosage for dexmedetomidine is a loading dose of 1 μg/kg over 10 minutes followed by an infusion of 0.2 to 0.7 μg/kg/h. It appears that in pediatric patients, the higher end of the dose range is required. Doses higher than 1.5 μg/kg/h have not been shown to provide any further sedative action. Advantages of dexmedetomidine include minimal respiratory depression and predictable hemodynamic effects. Because of the reduced sympathetic activity, blood pressure and heart rate fall slightly. Clinical sedation trials have shown a decrease in heart rate of 7% and blood pressure by 10%. It has been infused before, during, and after the extubation process. Hypotension and bradycardia are more likely to occur during the loading phase, which may need to be prolonged or interrupted. Dexmedetomidine cannot be given by rapid IV bolus because hypertension may occur as a result of direct stimulation of α1-adrenergic receptors. Mild transient hypertension is sometimes noted in adults during the loading phase, although this effect was not noticed in pediatric patients. Long-term use of dexmedetomidine (160 hours) also has now been reported,108 with no evidence of accumulation. The concern about rebound hypertension after long-term α2-adrenergic agonist treatment, such as that occurring with clonidine, has not been reported; however, anecdotal reports exist regarding withdrawal phenomena, including hypertension after prolonged infusions.
Sedation from dexmedetomidine often results in a patient who is tranquil yet easily aroused. Reduced analgesic requirements have been reported with its use. The easy arousal may make it a useful agent for when repeat neurologic examinations are required. Several articles concerning the use of dexmedetomidine in the PICU have recently been published. In a retrospective review of 121 patients from a mixed medical and surgical population in the PICU, a decrease of 20% in the dose of BDZ and/or opiates was documented in 80% of the children who received dexmedetomidine.109 Bradycardia (12%) and hypotension (16%) requiring intervention was described. Another retrospective review of dexmedetomidine use (infused >36 hours) in 35 postoperative pediatric cardiac patients did not show any significant changes in cardiovascular parameters, but a reduction in the postoperative opiate requirements occurred with an equal level of sedation.109a At present, a pharmacokinetic phase 2 trial is in progress and a prospective phase 3 study is planned in the near future. These studies should help answer the questions regarding the metabolism, efficacy, and adverse effects of dexmedetomidine in the PICU population. This agent also has been safely used for a variety of noninvasive sedation procedures such as magnetic resonance imaging (MRI), and several cases have been reported of its use as an adjunct to general anesthesia for pediatric patients. It appears to be a useful agent in the management of opiate withdrawal. Furthermore, dexmedetomidine is useful for patients who are difficult to sedate, for treatment of postoperative shivering, and for postanesthesia agitation. Procedural sedation with intranasal (IN) dexmedetomidine also has been reported (dexmedetomidine, 2 μg/kg IN, along with IN sufentanil, 1 μg/kg). Twenty children sedated with IN dexmedetomidine underwent dental restorative treatment without any complications. Sedation onset took approximately 45 minutes with a recovery time of about 1 hour.
Dexmedetomidine is not without adverse effects. It is contraindicated in patients with heart block, and bradycardia has been reported in an infant treated with digoxin who received dexmedetomidine during the infusion phase.110 It also would appear prudent to avoid its use with other drugs that can reduce arteriovenous node function such as β-blockers and calcium channel blockers, as well as with patients who have severe ventricular dysfunction or hypovolemia, because reduction in sympathetic tone may cause a profound decrease in blood pressure.
Propofol
Propofol is a rapid-acting IV anesthetic agent. As a highly lipid-soluble 2,6-diisopropylphenol, it is an oil and is insoluble in water. It is formulated as a 1% aqueous emulsion (1.2% egg phosphatide, 10% soyabean oil, 2.25% glycerol) with a propofol concentration of 10 mg/mL. A water-soluble prodrug form of propofol has been released recently, although no data are available regarding its use in children. Recovery from propofol is rapid because of its short redistribution half-life (α), and it is rapidly cleared by hepatic metabolism in healthy patients after short infusions, making it ideal for short procedures. The elimination half-life is 2 hours (Table 123-11), but the half-life is context sensitive and has been reported to be between 1 and 3 days after a 10-day infusion because of significant body accumulation. The kinetics follows a three-compartment model. The dose for induction of anesthesia in children is 2.5 mg/kg to 3.5 mg/kg; higher doses are required for infants and toddlers. Anesthesia also can be maintained by an infusion. The depth of sedation/anesthesia can be easily titrated, and an infusion rate of 25 to 150 μg/kg/min usually provides adequate sedation.
As with most sedative agents, propofol has adverse effects that may be a concern for the intensivist. It often causes hypotension in the sick child, and in patients dependent on high sympathetic tone to maintain normal blood pressure, even small doses of propofol may result in a significant decrease in blood pressure. The hypotension is mainly caused by vasodilatation, and there is little direct myocardial depressant. Bradycardia also can occur upon the induction of anesthesia. Propofol increases atrial conduction time for neonatal rabbits and prolongs the refractory period. Propofol anesthesia can prevent the induction of known atrial tachycardias in the electrophysiology laboratory, and cases have been reported of conversion of atrial tachycardia to sinus rhythm upon induction of propofol anesthesia. Propofol is a potent respiratory depressant, and it has a useful depressant effect on airway reflexes, which may facilitate endotracheal intubation. The injection of propofol often causes pain, and in the alert patient, strategies to minimize this effect are useful. Most commonly, lidocaine, either mixed with the propofol or injected before the injection of propofol, will markedly reduce the pain.
Propofol sedation in the ICU has several advantages. It acts rapidly and produces an easily controllable level of sedation. Unlike the barbiturates, it provides rapid clinical recovery, even after prolonged infusion. It has antiemetic properties and can provide transient deep sedation if required for procedures. It also has been shown to facilitate sedative synergy with BZDs.111 In the adult ICU population, propofol has been compared with midazolam for long-term sedation. Both agents provide good sedation, but propofol has the advantage of being more titratable with a faster recovery.112 Despite the increased drug cost, the use of propofol can reduce overall ICU costs because of a reduction in ventilator weaning time.113
Propofol has been used in the ICU as an anticonvulsant for patients with refractory status epilepticus.114 In a comparison with pentobarbital to provide burst suppression, both drugs were equally effective. Propofol was much more rapid in effect; no difference was found in outcome or ICU support measurements or length of stay.115 In patients with raised ICP, propofol has the same effects on ICP and cerebral blood flow (CBF) and cerebral metabolic rate of oxygen as barbiturates. It also requires a similar level of hemodynamic support to maintain appropriate blood pressure and cerebral perfusion pressure (CPP). It can produce the same degree of burst suppression that may be required for uncontrolled intracranial hypertension. It also allows rapid changes in the level of sedation, to facilitate neurologic examination. In this regard it is a superior agent. As described later, however, the use of large doses of propofol in the ICU setting may be associated with worsened outcomes.
Special Issue Regarding Long-Term Infusion of Propofol
Several important problems may occur when propofol is used in the PICU. With long-term propofol infusions, a significant amount of lipid may be infused into the patient, with the same consequences as lipid infusions used for hyperalimentation. Hyperlipidemia and triglyceridemia have been reported in up to 10% of patients receiving propofol in the ICU. Pseudohyponatremia or the inability to do routine plasma electrolyte analysis has been described. It is important that the propofol calorie (20 mL/h = 528 kcal/day)116 and lipid load be included in the nutrition plan for the patient. It may be necessary to reduce enteral feeds or avoid intralipids in selected patients. With high propofol dosing, respiratory acidosis has been reported.117 The emulsion used for propofol administration is an excellent culture medium at room temperature; cases have been reported of patients with systemic infection caused by propofol during operative procedures.118 This infection is due to poor aseptic technique in the preparation and use of the propofol syringes and infusion lines. Unusual infective organisms were detected in several patients, and an epidemiological study by the Centers for Disease Control and Prevention found propofol to be the common element.119 Certain precautions should be followed when propofol is used in the PICU. The staff should be educated to the potential dangers of infection from propofol. The ampule neck should be wiped with alcohol. There are no multidose vials of propofol. Syringes should be disposed of when they are more than 6 hours old, and lines should be changed every 12 hours. Filters are available that can remove many of the potential pathogens, and they are compatible with the lipid-based propofol infusion.
A few episodes of allergy to propofol have been reported; immune reactions, involve both anaphylactic and anaphylactoid types of reactions, are estimated at 1:45,000.120 Although clinically indistinguishable, the anaphylactic response involves prior exposure to a component of the propofol suspension. Egg allergy has been considered a contraindication to its use. However, the egg phosphatide component found in propofol is not related to the major egg allergen protein ovalbumin.121 In fact, intradermal testing with propofol in 25 patients allergic to eggs was negative; therefore, current evidence suggest that anaphylaxis is not more likely to develop in patients who are allergic to eggs when they are exposed to propofol. Propofol does not release histamine and is an acceptable agent for use in patients with asthma.
Several new generic formulations of propofol are available. These formulations include different antioxidants, such as metabisulfites, which may have an increased risk of allergic reaction. However, this increased risk has not been borne out.122 They appear to be equal in efficacy and adverse effects to the propofol solution known by the brand name Diprivan. A new water-soluble prodrug, fos-propofol, has been approved by the FDA and is due for release soon. The onset of action of fos-propofol is significantly slower than for propofol (5 minutes), which may reduce the incidence of hypotension and respiratory depression. A few patients who receive propofol may have dark-green urine due to phenol metabolites; this effect is not a clinical concern.123
Propofol Infusion Syndrome
One of the most important concerns is the development of a refractory metabolic acidosis in children who had received propofol sedation in the ICU. This effect was first described in 1992 as a series of five cases124 with fatal myocardial failure in children with respiratory illnesses requiring ventilation and sedation. Five young patients from different ICUs had croup and went on to have a refractory cardiac failure, bradycardia, and acidosis. A lipemic serum had developed in all patients. They had all received propofol at an average rate of about 8 mg/kg/h for more than 70 hours.
In review, the case reports were not as simple or as complete in their reporting, with several published letters125 from physicians involved with these patients showing incomplete data in the reporting. Several other case reports of an apparently similar clinical course were then subsequently described in the literature, which was enough evidence for the Committee on Safety of Medicines in the United Kingdom to issue a warning on propofol and its use in pediatric patients. At that time the FDA could not find a causal link between propofol and the deaths in children and did not issue a warning.
This reaction to propofol came to be known as the propofol infusion syndrome (PRIS).126 It is the sudden or relatively sudden onset of a marked bradycardia resistant to treatment with a least one of the following signs: lipemia, enlarged liver, severe metabolic acidosis, or rhabdomyolysis.
A steady number of case reports of this syndrome have appeared in the literature since the initial description, as well as a couple of studies involving several hundred patients127,128 who have not shown any problem with propofol in the PICU. In these studies, lower doses of propofol (4 mg/kg/h) were used, with regular monitoring of the acid base status and triglyceride levels. “Propofol bashing” became popular.129 There are few drugs that are licensed specifically for the PICU, however, and proper trials are needed to avoid drugs being condemned as hearsay. Subsequently, a randomized, controlled trial of propofol was begun, and after its use in 327 patients, it was reviewed by the FDA.130 The study was never published, but researchers found that, despite similar pediatric risk of mortality scores, patients who had received either 1% or 2% propofol preparations had a two to three times greater risk of death compared with the control sedative group. This finding led to a letter from AstraZeneca reminding health care workers that propofol was not approved for sedation of pediatric patients.131
PRIS has also now been described in adult patients.132 These patients had similar cardiac and metabolic findings, often associated with the management of intracranial hypertension. PRIS appeared to be a higher risk if the 2% formulation was used. Patients with raised ICP require deeper levels of sedation and require higher doses of propofol; they also are receiving vasopressor support to maintain the CPP, which puts a further stress on a myocardium that is already failing.
The pathophysiologic cause of PRIS is still poorly understood, but it appears to mimic mitochondrial myopathies. Such patients are generally well until stressed. Rhabdomyolysis and cardiac and hepatic failure then develop in these patients.133 Case reports have shown some metabolic abnormalities that may be the cause of the cardiac failure and acidosis. One report describes a 10-month-old child who had the syndrome and was successfully treated with hemofiltration and plasmapheresis.134 Muscle and liver biopsy specimens showed changes consistent with a toxic insult. Analysis also showed a reduction in the cytochrome C oxidase activity in the muscle, with a normal activity in skin fibroblasts, excluding an underlying respiratory chain defect. Profound acidosis with lactic acidosis is found in different types of genetically acquired cytochrome oxidase deficiency. It was postulated that the hemofiltration removed a water-soluble metabolite of propofol that had caused a reversible reduction in the oxidase activity. A second case report135 also showed a metabolic abnormality. Elevated levels of malonylcarnitine and C5-acyl carnitine were found in a patient with PRIS. This patient was also treated successfully with hemofiltration. These findings are consistent with impaired fatty acid oxidation due to impaired entry of long chain fatty acids into the mitochondria and a failure of the respiratory chain. A review of the pathophysiologic function of the syndrome136 suggested that propofol increases the activity of malonyl coenzyme A, which inhibits the carnitine palmityl transferase I, so long chain fatty acids cannot enter the mitochondria. Propofol also uncouples oxidation, so the short and medium chain fatty acids cannot be used, even though they have entered the mitochondria and also may inhibit the respiratory chain. Low energy production leads to cardiac and peripheral muscle necrosis.
Propofol is still frequently used for procedural and short-term sedation,137 but in a recent case report, researchers describe a patient who had PRIS.138 The patient had received a propofol infusion for 15 hours at 20 mg/kg/h. After a 13-hour propofol-free period, an 8-hour infusion of propofol at 4 mg/kg/h was given, after which the patient had intractable bradycardia and acidosis. This report raises concerns about high-dose, short-term propofol use in the PICU.
In a report on the use of propofol for two cases of refractory status epilepticus, patients aged 7 and 17 years had features similar to the PRIS.139 Status epilepticus itself can result in neurologic deficit, hypoxia, rhabdomyolysis, cardiac arrhythmias, hyperthermia, metabolic acidosis, acute renal failure, and death. However, these patients received high doses of propofol (18 to 27 mg/kg/h) to achieve burst suppression for more than 48 hours. Rhabdomyolysis and cardiac failure developed in both patients. No monitoring of lipid status or acid base was performed, and propofol was used as the sole agent by practitioners with limited experience with this drug. In light of the reports now appearing in the adult neurointensive care literature with the development of a propofol infusionlike syndrome in adult neurosurgical patients,140 it would appear that propofol is not the best choice for prolonged sedation for patients with intracranial hypertension. An early indicator of the cardiac instability from PRIS may be changes in the ECG. It has been reported that the development of a right bundle-branch block with convex ST elevation was an early sign of this syndrome.141
Propofol remains a useful agent for procedural sedation in the PICU. When compared with midazolam and ketamine, propofol resulted in safe, effective sedation. The patients sedated with propofol awakened almost twice as fast; thus the efficiency of the sedation service was also improved.142 Propofol is also probably appropriate for overnight sedation, and higher doses should be avoided. It probably should not be used as a solo agent because in those cases tolerance appears to develop more rapidly. If its use is required for a prolonged period, then careful consideration should be given to its risks and benefits. A recent study showed that staff members of some PICUs are still using long-term high doses despite the potential risks involved.143 Prevention of PRIS could include adequate calorific intake. The dose and duration of propofol should be carefully managed to minimize its use. It would appear from the reports of PRIS in the neurosurgical population that the desire for rapid awakening has propagated the use of propofol coma, rather than using barbiturates. Regular monitoring of the cardiac function, ECG, and CPK are warranted; lipid profile and acid base status may help in early detection. However, these steps may not necessarily prevent mortality from the syndrome.144 Treatment should be immediate cessation of propofol. Cardiac support may be difficult because of unresponsiveness to conventional circulatory support. The use of pacing and extracorporeal membrane oxygenation has been reported. Hemodialysis or hemofiltration have been reported as having some success.
An outcome prediction table has been developed for PRIS based on more than 1000 reports from the FDA’s Medwatch program, of which 20% were pediatric patients.145 The features associated with PRIS are shown in Table 123-12. In addition to the individual features, there were several additional scores depending on a combination of features (see Table 123-12). The predicted outcome from these scores is shown in Table 123-13. These predicted outcomes were very close to the actual reported mortality of the analyzed cohort. No independent verification exists at present. However, this article does highlight the variability in features of the PRIS and accounts for the differences in the reported mortality.
Table 123–12 Features Reported for PRIS, Incidence, and Score
| Feature | % Incidence | Score |
|---|---|---|
| Cardiac | 44 | 1 |
| Hypotension | 34 | 0 |
| Rhabdomyolysis | 27 | 1 |
| Hepatic failure | 24 | 0 |
| Renal failure | 24 | 1 |
| Metabolic acidosis | 20 | 1 |
| Dyslipidemias | 5 | 0 |
| Rhabdomyolysis and hypotension | 1 | |
| Age <18 y and renal failure | –1 | |
| Rhabdomyolysis and renal failure | –1 |
Table 123–13 Predicted Outcome from PRIS
| Score | Predicted Mortality Rate (%) |
|---|---|
| 0 | 10 |
| 1 | 25 |
| 2 | 50 |
| 3 | 75 |
| 4 | 90 |
Sedation and Analgesia for Procedures
Many procedures performed on children involve pain and anxiety. In many hospitals the administration of sedation and analgesia falls to the pediatric intensive care physician.146 The pediatric intensive care physician needs to be familiar with guidelines and protocols that are used for moderate sedation outside the ICU setting. Procedural pain accounts for most of the pain experienced by children with malignancies,147 and many pediatric patients with trauma will require sedation for procedures such as correction in the emergency department of fractured limbs and lacerations.
Conscious sedation, now commonly called moderate sedation or more appropriately procedural sedation, is a medically controlled state of depressed consciousness whereby the patient remains responsive to verbal stimuli or, at most, a gentle shaking of the shoulder.148 It anticipates that protective reflexes will be maintained and that the patient retains a patent airway independently. Neither airway patency nor airway protection should be taken for granted because patients with conditions such as obstructive sleep apnea may obstruct their airway with little sedation and aspiration of food can occur even without sedation. Before moderate sedation is further explored, the insightful words spoken by Burton Epstein, in his “40th Rovenstine Lecture of the ASA” in the fall of 2002, should be considered: “The myth . . . of the achievability of a state of conscious sedation in which pediatric patients are simultaneously responsive to voice stimulus while immobile in the face of pain is just that—a myth.”149 A little consideration will reveal that for painless procedures, anxiolysis will most likely suffice, whereas for painful procedures, pharmacologic elimination of the response to pain will result in a need for general anesthesia. Between these two extremes, the use of local anesthetic agents may modify the response so as to allow potentially painful procedures to be performed during moderate sedation. Another factor to consider is the effect of variation in the level of stimulation, whereby sedation titrated to effect during a painful stimulus becomes excessive once the stimulus is completed. Thus the practitioner treads on a narrow and sometimes impossible pathway when giving moderate sedation. The state of moderate sedation is part of a continuum (Table 123-14) defined by the working groups of the American Academy of Pediatrics (AAP) and the American Society of Anesthesiology (ASA),150 which encompasses a range from anxiolysis to general anesthesia that is appropriate for surgery. This continuum is difficult to control, and staff administering moderate sedation must be able to appropriately manage any patients who enter a deeper level of sedation than that planned. The goals of sedation are shown in Box 123-6. It is helpful to think of moderate sedation as consisting of several components. A balanced sedation technique will involve amnesia, analgesia, relaxation, and inattention. Different procedures require different degrees of these components (Table 123-15).
| Designation | Description |
|---|---|
| 1 | Minimal sedation (anxiolysis) |
| 2 | Moderate sedation/analgesia (“conscious sedation”) |
| 3 | Deep sedation/analgesia |
| General anesthesia |
Types of Procedures and Preprocedure Evaluation
In many instances sedation may be beneficial in the PICU, such as for the placement of central lines and centesis tubes and during dressing changes. Sedation facilitates the procedure in uncooperative patients and allows long or uncomfortable procedures to be performed. Outside the ICU, both noninvasive and invasive radiologic examinations151,152 often require sedation (Box 123-7).
Box 123–7 Non-ICU Procedures Requiring Sedation
CT, Computed tomography; ICU, intensive care unit; TEE, transesophageal echocardiography.
All patients should be assessed before moderate sedation. Box 123-8 lists the elements that should be included in the assessment. The medical history should include evaluation of the cardiorespiratory system, any history of gastroesophageal reflux, and any previous sedation attempt that failed or any abnormal reaction to sedation. A recent asthmatic attack or respiratory tract infection, poorly controlled seizure disorder, or diabetes may require a change or postponement of the sedation plan. It is recommended that patients be classified according to the ASA preoperative patient classification (Table 123-16). In most circumstances it is generally recommended that patients in ASA class VI and some in class III are not suitable for moderate sedation.
Table 123–16 American Society of Anesthesiology Classification
| Class | Description |
|---|---|
| I | A normally healthy patient |
| II | A patient with mild or well-controlled disease state |
| III | A patient with severe or poorly controlled disease state |
| IV | A patient with severe disease state that is a constant threat to life |
| V | A moribund patient who is not expected to survive without surgery |
It is prudent to adopt the same guidelines that are used before general anesthesia is administered, in case sedation that is deeper than anticipated occurs. These guidelines are age dependent, and current recommendations are shown in Table 123-17. Despite this, less caution is often reported regarding fasting, without any apparent worsening of outcome. For example, in a survey of 450 radiology departments, 35% had no nothing by mouth (NPO) status requirement for neonates, and 17% used a 2-hour NPO status requirement for infants. For oral contrast studies, most departments sedated the patient within 1 hour of the contrast being swallowed.153 Consent should be obtained as with any medically indicated procedure or intervention. It should include discussion about the risks and benefits of the procedure and the sedation technique, as well as expectations of outcome and alternatives to the procedure and sedation.
Table 123–17 Presedation NPO Guidelines
| Solids/Nonclear Liquids∗ | Clear Liquids† | |
|---|---|---|
| Adults | 6 h | 3 h |
| Children | 6 h | 3 h |
| Neonates (<3 mo) | 4 h | 2 h |
NPO, Nothing by mouth.
∗ Milk, breast milk, pulp fruit juices.
The presedation interview for outpatients or non-ICU patients also should involve giving instructions and information to a responsible person, including postsedation instructions, a 24-hour phone contact phone number, guidelines concerning limitations of activity, and expected postsedation behavior. If moderate sedation is provided for nonscheduled patients, a review of several aspects is important (Box 123-9).
Monitoring During the Procedure
Monitoring of patients undergoing moderate sedation is an important component of safe, effective sedation. Several different recommendations have been made by the Joint Commission on Accreditation of Healthcare Organizations,154 the American Board of Anesthesiology, and the AAP. These recommendations have not yet been fully followed by persons providing sedation for children.155 A study of pediatric dentists after publication by the AAP of its new recommendations found minimal monitoring and documentation of the sedation procedure. Obtaining baseline vital signs is important. Because of the possibility of oversedation, the level of consciousness should be assessed frequently, especially during titration of effect. This level of consciousness is best assessed with the Ramsay scale (see Table 123-1). A sedation record is important for documentation of the drugs used with times and doses and for the monitored measurements that are charted on a time-based record. Monitoring should include pulse oximetry for assessment of the degree of oxygenation and heart rate. The saturation should be maintained by supplemental oxygen. Breathing can be assessed either by monitoring the respiratory rate or by capnography. The blood pressure should be checked at regular intervals during the procedure. A study of 85 pediatric patients with complications after sedation showed that most severe complications resulted from a common pathway involving respiratory depression leading to respiratory arrest, cardiac arrest, and subsequent severe neurologic devastation. The most common causes for these complications are summarized in Box 123-10. There is no particular drug that is more likely to cause problems. Polypharmacy, especially with three or more drugs, has been shown to be a risk factor for pediatric sedation complications.156 Dentists using nitrous oxide in combination with other agents appeared to have a higher incidence of problems. If long-acting drugs are used, the patient must be observed for an appropriate length of time. Several reports have been made of respiratory arrest occurring while the child was in the car seat on the way home. Any health care worker providing moderate sedation should be familiar with an emergency algorithm in case problems arise (Figure 123-4).
Many different pharmacologic options are available for moderate sedation in children. The oral route is commonly used in children; it has a slow onset time that avoids a rapid peak effect, but it also gives an unpredictable degree of sedation, which is not easy to titrate. IM administration is painful; however, it is useful in uncooperative patients. The rectal route is nearly always available and has found favor in the past for barbiturate sedation. More contemporaneously, rectal diazepam at 0.2 to 0.5 mg/kg has proved useful in the control of seizures when IV access is not available.157 Onset can be fairly slow and the duration prolonged. The IV route offers a titratable effect but also adds the danger of acute overdose. The use of intranasal sedation administration has been reported for a variety of drugs. A disposable device such as the Mucosal Aerosol Device can be used. The Mucosal Aerosol Device attaches to a Luer lock syringe and has a soft cone insert for placement in the nares. It creates a fine spray that improves drug delivery and deposition onto the nasal mucosa. Another route of administration is transmucosal administration, such as the fentanyl “lollipop” (ACTIQ). Dosing recommendations are shown in Table 123-18. One must always keep in mind that BZD-opiate or barbiturate-opiate combinations are potent causes of respiratory depression, and extra monitoring and vigilance should be used. With IM and oral medications, adequate time should elapse to allow absorption before a further dose is given to avoid accidental overdose.
| Route | Drug | Dose |
|---|---|---|
| PO | Chloral hydrate | 50–75 mg/kg (repeat 25 mg/kg) |
| Diazepam (Valium) | 0.2–0.4 mg/kg (max, 20 mg) | |
| Midazolam (Versed) | 0.5–0.75 mg/kg (max, 20 mg) | |
| IM | Pentobarbital (Nembutal) | 4–6 mg/kg (max, 100 mg) |
| Fentanyl (Sublimaze) | 1–3 μg/kg | |
| CM3 | 0.08–0.1 mL/kg (max, 2 mL) | |
| IV | Morphine | 0.1 mg/kg |
| Meperidine (Demerol) | 1–2 mg/kg (max, 75 mg) | |
| Fentanyl (Sublimaze) | 1–2 μg/kg (max, 5 μg/kg) | |
| Midazolam (Versed) | 0.05–0.1 mg/kg | |
| Diazepam (Valium) | 0.05–0.1 mg/kg |
CM3, Catheterization mixture number 3; IM, intramuscular; IV, intravenous; PO, by mouth.
As noted in Table 123-15, different procedures require different qualities of sedation. These qualities are found in the array of drugs available to the intensivist (Table 123-19). It may be useful to choose the sedative agent or agents that best fit the particular requirements for the procedure being performed.
In some circumstances the services of the anesthesia department may be useful. The anesthesia department has access to other pharmacologic agents such as propofol and nitrous oxide, as well as the inhalational agents. The ability to use a deeper level of sedation if required is easily obtained with these rapidly acting, short-acting agents. In emergency procedures where the patient’s NPO status is unsafe or unknown, patients may need to undergo intubation to protect the airway.
Anesthesiologists have the ability to perform a “needleless” sedation technique using a gas induction with anesthetic agents; an IV drip may be placed if required when the patient is asleep. This technique is especially useful for repeat procedures in oncology patients. Also, anesthesia personnel are better able to sedate patients whose illness may contraindicate routine moderate sedation protocols (Box 123-11).
Postprocedure Care and Monitoring
Moderate sedation is safe and frequently used; unconscious sedation is potentially hazardous, and patients who undergo it require careful monitoring. Hospital protocols are useful for a smoothly run, safe sedation policy.158 Staff should be appropriately trained in sedation and resuscitation basic and advanced life support. When the ASA/AAP recommendations are followed, the risks of a sedation-related complication can be reduced.159 Individual risk factors include deep sedation and the use of chloral hydrate. When all the recommendations for moderate sedation, including NPO, ASA class, avoidance of deep sedation, sedation level monitoring, and drug use were followed, the adverse event rate was zero.
Sedation for Magnetic Resonance Imaging
The PICU physician is often called upon to provide sedation for a patient in the ICU who is undergoing MRI. Also, many institutions rely on the PICU team to provide a sedation service for other inpatients or outpatients undergoing MRI. Deep sedation is often required for effective sedation of younger children undergoing an MRI scan. The same standards should be adhered to as with any other child undergoing sedation160 with respect to patient selection, monitoring, and postimaging care.
MRI is an imaging modality that is being increasingly used to aid in the diagnosis of neuroanatomic disorders. The patient is required to lie still within a small space while multiple images are obtained. MRI scanning is performed less rapidly than computed tomography (CT) scanning. Movement by the patient causes degradation of the image quality, and a change in the patient’s position may affect the homogeneity of the magnetic field, which is optimized at the beginning of the scan. Studies can take from 45 minutes to more than 2 hours, with individual sequences taking 3 to 10 minutes. The scanner is noisy and the restriction on space and movement can induce claustrophobia in some patients. The patient also may experience a slight increase in temperature. Most adults and older children (older than 6 years) are capable of lying still for the scan. With the use of earplugs and music it is well tolerated. Several groups of patients, however, may require sedation161 for the scan to be performed (Box 123-12).
Because of the large magnetic field, several unique problems162 can occur during a scan. These problems include the potential risk of the magnet causing a ferromagnetic object to move or heat up or the induction of an electric current from the radio frequency pulses and switching magnetic gradients used in generating the images. This potential risk results in a significant list of contraindications to MRI (Box 123-13). Sedating these patients also entails several risk factors. The patient is in a remote location, with limited access to and visibility of the airway. Several equipment issues exist as well (Box 123-14). The monitors used must be suitable for use in the MRI suite.163 They should be nonferromagnetic; the cables should be screened from electromagnetic interference (fiberoptic is ideal); and the signal should be filtered to avoid radio frequency interference (which interferes with image quality). Despite the specialized technology that is available, several problems remain. The ECG waveform is frequently altered, and analog information is often lacking during a scanning cycle. The ECG cables may cause burn injury, and special ECG electrodes are required to avoid burn injury. For pulse oximetry to be performed, a special probe is required. Heating of the usual probe may cause burn injury. Fiberoptic connection to the patient is best. Capnography requires long tubing, which results in a prolonged upsweep and delay in displaying real-time measurements. The respiratory rate and trends can still be useful. Any battery-powered monitor requires a nonmagnetic lithium battery. Exposure to the MRI shortens battery life. Most ICU ventilators are not MRI compatible (Servo i is available as an MRI compatible model). Some specialized MRI-safe anesthesia machines have a ventilator; however, their use should be restricted to the anesthesia staff who are familiar with the equipment. The IV poles and the equipment carts also should be nonferromagnetic. Any equipment with a transformer (e.g., syringe pumps and IV pumps) must be kept out of the magnetic field. Gas cylinders must be aluminum. The area around any magnet that generates a magnetic field stronger than 5 G should not contain any ferromagnetic items.
Specific Drugs for Sedation
Ketamine
Ketamine is a phencyclidine derivative that provides sedation and analgesia. It results in a state of dissociative (trancelike) anesthesia. It is available in a variety of different dilutions, such as 10 mg, 50 mg, and 100 mg/mL. The latter is the most useful for IM use and the preparation of infusions. For a state of general anesthesia to be induced, a dose of 2 mg/kg IV is required. Onset takes 1 to 2 minutes with anesthesia lasting 10 to 15 minutes. Lower doses may be used for sedation. Anesthesia also can be induced by the IM route with a dose of 10 mg/kg, although onset is slower (5 to 10 minutes) and duration of prolonged effect is 45 to 60 minutes. It is metabolized by the liver and excreted by the kidneys. The half-life is 3.1 hours (see Table 123-11).
The adverse effects of ketamine include hypertension, tachycardia, increased intracranial pressure, and bronchodilation. The bronchodilation is probably due to its sympathomimetic action. It is a direct myocardial depressant, but blood pressure is usually maintained by the sympathetic stimulation that ketamine causes. In critically ill patients who already are using their maximum sympathetic drive, ketamine may cause a decrease in cardiac output or even cardiac arrest. Hallucinations and other psychiatric symptoms are often reported during and after its use in adults, but they occur less frequently in children. Ketamine is a potent sialogogue, and the use of an anticholinergic agent such as glycopyrrolate may be helpful. Its use is contraindicated in patients who cannot tolerate hypertension,164 have a history of cerebrovascular hemorrhage, have psychiatric disturbances, and have raised ICP. It is a useful agent for sedation for procedures, especially if there is no IV access. It has been used in patients with status asthmaticus as an adjunct bronchodilator both in intubated and nonintubated patients at an infusion rate of 0.5 to 2 mg/kg/h. After discontinuing its use, the patient should receive BZDs to minimize the likelihood of hallucinations and be nursed in a quiet environment. Ketamine cannot be assumed to preserve pharyngeal reflexes any better than other sedatives agents, and apnea and airway obstruction can still occur.165 NPO guidelines should still be observed.
Etomidate
Etomidate is a carboxylated imidazole that is unrelated to other anesthetic agents. It is a rapidly acting IV anesthetic agent, which, like other rapid-onset anesthetic agents, partitions into the brain within one circulation time and redistributes out of the brain over the next few minutes. It is available dissolved in 30% propylene glycol as a 2 mg/mL solution. Like propofol, it causes pain on injection. The anesthetic dose is 0.2 to 0.3 mg/kg. It has a favorable adverse effect profile with minimal cardiovascular and respiratory depression. Etomidate is associated with a high incidence of nausea and vomiting after emergence from anesthesia. Its pharmacokinetics are shown in Table 123-11. The greatest disadvantage of etomidate in the intensive care setting is adrenocortical depression due to inhibition of adrenocortical mitochondrial 11-β-hydroxylase.166 This effect is present in neonates and in adults.167 The outcome of patients sedated with etomidate is worse than in those using alternative sedation, and steroid deficiency is thought to be the cause.168
Inhalational Anesthetic Agents
The inhalational agents remain the most widely used anesthetics in the operating room, although their mechanism of action is still poorly understood. The following agents are currently in clinical use: enflurane, isoflurane, sevoflurane, and desflurane. Sevoflurane and desflurane are newer agents that currently have limited use in the ICU. Isoflurane remains the most logical choice of inhalational anesthetic in the ICU based on its cost/benefit ratio. As with any drug used in the ICU, it is important to understand the pharmacology, the adverse effect profile, and, in this case, the technical aspects of delivering these agents to the patient. These drugs are all fluorinated hydrocarbons (Figure 123-5). Except for halothane, which is no longer in common clinical use, they are ethyl-methyl esters. Each agent has different physicochemical properties that are important to its properties (Table 123-20).
Adverse effects of halothane include hypotension, which is due to direct myocardial depression. It also causes bradycardia because of effects on the sinoatrial node and vagal stimulation. Cardiac arrhythmias may occur, most commonly junctional rhythm. Halothane also sensitizes the myocardium to catecholamines, especially when the patient is hypercapnic or hypoxic. Because these physiologic changes are common in the intensive care patient, the potential for serious interactions with halothane abound. Halothane is metabolized approximately 20% by the liver, and a trifluoroacetic metabolite may cause an immune-mediated fatal hepatitis.169 Isoflurane causes hypotension mainly because of vasodilation, while maintaining cardiac output. Concern has been expressed about a coronary steal phenomenon occurring in which blood is diverted from a partially obstructed coronary bed served by collateral arteries, due to vasodilation. The evidence for this phenomenon is weak, however, and ischemia is probably due to hypotension rather than a true steal phenomena. Isoflurane vapor is pungent and may cause airway irritation, coughing, and laryngospasm if the patient is not adequately sedated before its use. It is only minimally metabolized (0.2%), and a hepatitis reaction is extremely unlikely.
All of the inhalational agents cause cerebral vasodilation, which results in an increase in CBF (because of decoupling of the demand/flow ratio) and ICP. In pediatric patients with raised ICP, there was no difference among isoflurane, desflurane, and sevoflurane with respect to the increase in ICP and CBF.170 In contrast, IV anesthetics maintain the demand/flow ratio, and CBF and ICP fall, with the exception of ketamine. In addition to an effect of CBF, the arterial blood pressure typically will decrease with anesthesia, and the effect of this on the CPP must be accounted for. In one study, the effect of the decrease in arterial pressure on CPP exceeded the effect of increasing ICP by a factor of 3.171 With these potential effects on CBF, the use of isoflurane should be carefully considered in patients who have or are at risk of raised ICP. Nevertheless, isoflurane has been safely used in neuroanesthesia with controlled ventilation to a normal PaCO2 and an inspired concentration not exceeding 1 MAC.172
Isoflurane has two main applications in the PICU: sedation and the management of refractory asthma. Only a few reports have been made of long-term sedative use of isoflurane in the ICU. In an adult study, 40 patients173 who received an average of 96 MAC hours of isoflurane showed hemodynamic stability, less tachyphylaxis compared with other sedative agents, and a more rapid wean from the ventilator. No evidence was found of renal or hepatic dysfunction with serum fluorides less than 50 μmol/L. In a pediatric study, 10 patients174 who had been receiving large doses of opiates or BZDs received an average of 130 MAC hours of isoflurane. The range of use was from 1 to 30 days. Fifty percent of the patients experienced a withdrawal-like phenomenon—most commonly, those who had received more than 70 MAC hours of isoflurane. Fluoride levels also were measured, and although they were correlated with the duration of treatment, none was greater than 30 μmol/L. The highest levels were in a patient who was taking both phenytoin and phenobarbitone. Hypotension only occurred in one patient. No hepatic or renal dysfunction occurred. The withdrawal was treated with a combination of BZDs and haloperidol with good effect. Isoflurane also has been used in patients with renal dysfunction, and fluoride levels were not elevated.175 The starting dose for sedation should be 0.5%; this dose can then be titrated to effect by the ICU team. At levels above 1.5%, other sedative agents and paralytics often are not required.
Multiple case reports of the use of inhalational agents for status asthmaticus in both adults and children have been made. Because of its speed of onset and its bronchodilation effects, isoflurane is a useful adjunct to β2-adrenergic agonists. If no improvement occurs, or if unacceptable adverse effects occur, then its effects rapidly wane on discontinuation. Isoflurane is recommended for use because of its safer adverse effect profile. No reports of renal or hepatic dysfunction have been made despite its use for often prolonged periods. Hypotension seems to be more common in patients sedated with isoflurane; it is possibly related to increased intrathoracic pressure and the potential for greater preload reduction with vasodilation. Fluid boluses and occasionally vasopressors are often required. Because isoflurane is not an analgesic agent, opiates may be needed for painful or uncomfortable procedures. Also, when the patient is weaned off the isoflurane, additional sedatives will be required. The isoflurane should be started at 0.5% and titrated for effect; doses of up to 2.5% have been reported as safely used.176
The inhalational anesthetic agents are liquids at room temperature. A special delivery device called a vaporizer is required to deliver an accurate supply of the vapor. All vaporizers have several features in common. They provide a reservoir of the inhalational liquid with a level indicator and are capable of delivering a constant level of vaporization. Most newer vaporizers also have a color-coded keyed filler (e.g., purple for isoflurane and yellow for sevoflurane) that prevents the accidental filling of the vaporizer with the wrong agent. This error could result in overdosing the patient because the vaporizer calibration is drug specific. If two vaporizers are accommodated in series on the anesthesia machine back bar, then an interlocking system should be used to prevent the accidental use of both vaporizers. Otherwise, the results would be contamination of the second vaporizer by gas from the first vaporizer and an uncontrolled excess delivery of gas to the patient.
Whenever inhalational agents are used, the waste gases from the expiratory limb of the ventilator should be scavenged to avoid prolonged exposure of the health care worker to these agents. The Occupational Safety and Health Administration limits occupational exposure to 2 ppm halothane for health care workers.177 The worker is at risk of becoming sedated, and potential teratogenic effects also exist. Several large studies about prolonged exposure to these agents have not shown any increase in risks for anesthesia personnel with respect to hepatic disease, teratogenesis, spontaneous abortions, psychological difficulties, infertility, neuropathy, or bone marrow depression.178 Caution also should be taken when filling the vaporizer to avoid spilling the liquid during the process.179 Two forms of scavenging are available. A passive system involves simply a tube connected to the expiratory limb connected to the outside. This system is at risk of occlusion because of kinking or someone standing inadvertently on the tubing, which will then occlude the expiratory limb of the ventilator. An active system involves an active suction to the expiratory limb, with a safety reservoir bag in series to prevent excess suction pressure from being exposed to the patient.
The use of inhalational agents in the PICU involves the use of equipment that may be unfamiliar to pediatric intensive care physicians. Isoflurane appears to be the best choice,180 and it offers several useful advantages, including the ability to deeply sedate patients (especially those difficult to sedate) without polypharmacy.181 Although they are currently poorly defined,182 tolerance and a withdrawal-like syndrome have been described; however, they appear to occur more slowly than with other sedative agents. It may be helpful to have a set of guidelines available for isoflurane use to facilitate its use in the PICU. These guidelines could include equipment use, monitoring requirements, dosing, and treatment of complications.183 Caution should be used in patients who may have raised ICP because isoflurane may increase CBF. Isoflurane does allow for rapid arousal if neurologic examinations are required.
The Anaesthetic Conserving Device (AnaConDa) is a modified heat moisture exchanger that has been developed to allow the use of inhalational agents in the ICU without requiring high fresh gas flows or specialized ventilators.184 It is placed in the breathing circuit between the ventilator Y circuit and the endotracheal tube. The liquid anesthesia agent is injected directly into the device using a syringe pump. The device membrane allows for the inhalational agent to be taken up by the inspired gas. On expiration much of the inhalational agent is deposited on the membrane, allowing for an efficient inhalational rebreathing technique. The inspired concentration of the inhalational agent is monitored from the device using a routine gas analyzer. It has been used in several adult ICU trials as well as in a series of three children. Some problems due to excess dosing have been experienced, and the 100 mL dead space of the device, as well as resistance to gas flow, may make its use inappropriate in children. Compared with an inhalational agent vaporizer, there is no percentage inhalational agent dial. The rate on syringe injection determines the percentage of the inhalational agent. This infusion rate is set according at a pre-recommended rate. Initially the inspired concentration is low because of dilution. Differences in minute ventilation and gas flows may make this pre-recommended rate inaccurate. The injection rate needs to be titrated to the monitored percent of the inspired inhalational agent, which can increase significantly with time, especially until equilibrium is reached. The AnaConDA device is not approved for use in the United States.
Apoptosis
Whether these data are directly applicable to humans has not been established. Primate research suggests that ketamine185 induces apoptosis in vulnerable young monkeys, although an exposure to ketamine of less than 3 hours duration does not induce significant injury. To date, the studies in humans have been retrospective in design. Four studies have demonstrated conflicting findings on the effects of anesthetics on cognitive function in young children. The most impressive of these retrospective studies is from the twin registry in Denmark186 in which monozygote twins concordant or discordant for anesthetic exposure before the age of 4 years failed to demonstrate any differences in educational achievement or cognitive impairment years later. A prospective randomized study of general anesthesia versus regional anesthesia for lower abdominal surgery with long-term evaluation of cognitive function in children is currently under way (expected conclusion in 2013) and may help us to understand the risk, if any, of administering these general anesthetics to vulnerable human infants and young children.
Pharmacoeconomics
In today’s economical climate it is important to consider the cost of the different sedation options available to the pediatric intensivist (Table 123-21). Most PICUs use a low-cost sedative regimen for the bulk of the sedations required and keep the more expensive options for selected circumstances. Table 123-21 shows the different costs of some of the available agents in the PICU as well as the 24-hour cost for a child weighing 20 kg. They are presented as the cost per kilogram per hour of sedation at equipotent doses. The costs are the lowest hospital drug cost (at the Women’s and Children’s Hospital of Buffalo) for each agent in its most inexpensive form and exclude preparation, delivery, and equipment issues related to each drug. Fentanyl is inexpensive. For a 20-kg child, it costs $7.49 per day. Midazolam is now a more inexpensive option than is lorazepam. The other synthetic opiates are more expensive to use, and consideration should be given to appropriate indications for their use. A rapid recovery, however, with as quick an extubation as may be possible with remifentanil and early ICU discharge is also a considerable cost factor to be considered. Propofol (which is now available in a generic form) and ketamine are both relatively inexpensive options for ICU sedation, if they are deemed clinically appropriate. The drug costs of isoflurane and sevoflurane are comparable to those of the BZDs; however, they require the availability of a specialized delivery system, which could increase the cost. If a device were available that could deliver these agents at low flows with most of the available ICU ventilators, then inhalational sedation would be a more attractive option.
References are available online at http://www.expertconsult.com.
1. Rundshagen I., Schnabel K., Wegner C., et al. Incidence of recall, nightmares, and hallucinations during analgosedation in intensive care. Intensive Care Med. 2002;28:38-43.
2. Wagner B.K., Zavotsky K.E., Sweeney J.B., et al. Patient recall of therapeutic paralysis in a surgical critical care unit. Pharmacotherapy. 1998;18:358-363.
3. Jones C., Griffiths R.D., Humphris G., et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29:573-580.
4. Playfor S., Thomas D., Choonara I. Recollection of children following intensive care. Arch Dis Child. 2000;83:445-448.
5. Ramsay M.A., Savege T.M., Simpson B.R., et al. Controlled sedation with aphaxalone-alphadone. Br Med J. 1974;2:656-659.
6. Brattebø G., Hofoss D., Flaatten H., et al. Effect of scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. BMJ. 2002;324:1386-1389.
7. Ambuel B., Hamlett K.W., Marx C.M., et al. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol. 1992;17:95-109.
8. Detriche O., Berre J., Massaut J., et al. The Brussels sedation scale: use of a simple clinical sedation scale can avoid excessive sedation in patients undergoing mechanical ventilation in the intensive care unit,. Br J Anaesth. 1999;83:698-701.
9. Sebel P.S., Lang E., Rampil I.J., et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effects,. Anesth Analg. 1997;84:891-899.
10. Berkenbosch J.W., Fichter C.R., Tobias J.D. The correlation of the bispectral index monitor with clinical sedation scores during mechanical ventilation in the pediatric intensive care unit,. Anesth Analg. 2002;94:506-511.
11. Rosow C., Manberg P.J. Bispectral index monitoring. Anesthesiol Clin North Am. 1998;2:89-107.
12. Struys M., Versichelen L., Byttebier G., et al. Clinical usefulness of the bispectral index for titrating target effect-site concentration. Anesthesia. 1998;53:4-12.
13. Courtman S.P., Wardurgh A., Petros A.J. Comparison of the bispectral index monitor with the comfort score in assessing level of sedation of critically ill children. Intensive Care Med. 2003;29:2239-2246.
14. Moerman N., Bonke B., Oosting J. Awareness and recall during general anesthesia. Anesthesiology. 1993;79:454-464.
15. Domino K.B., Posner K.L., Caplan R.A., et al. Awareness during anesthesia. A closed claims analysis,. Anesthesiology. 1999;90:1053-1061.
16. Aneja R., Heard A.M., Fletcher J.E., et al. Sedation monitoring of children by the Bispectral Index in the pediatric intensive care unit. Pediatr Crit Care Med. 2003;4:60-64.
17. Avidan M.S., Zhang L., Burnside B.A., et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097-1108.
18. Wong C.A., Fragen R.J., Fitzgerald P., et al. A comparison of the SNAP IITM and BIS XPTM indices during sevoflurane and nitrous oxide anaesthesia at 1 and 1.5 MAC and at awakening,. Br J Anaesth. 2006;97:181-186.
19. Zhou L., Zhang Q., Stein C., et al. Contribution of opioid receptors on primary afferent versus sympathetic neurons to peripheral opioid analgesia. J Pharmacol Exp Ther. 1998;286:1000-1006.
20. Kalso E., Tramer M.R., Carroll D., et al. Pain relief from intra-articular morphine after knee surgery: a qualitative systematic review. Pain. 1997;71:127-134.
21. Zadina J.E., Martin-Schild S., Gerall A.A., et al. Endomorphins: novel endogenous mu-opiate receptor agonists in regions of high mu-opiate receptor density. Ann N Y Acad Sci. 1999;897:136-144.
22. Slappendel R., Weber E.W., Benraad B., et al. Itching after intrathecal morphine. Incidence and treatment. Eur J Anaesthesiol. 2000;17:616-621.
23. Sabbe M.B., Yaksh T.L. Pharmacology of spinal opioids. J Pain Symptom Manage. 1990;5:191-203.
24. Kart T., Christrup L.L., Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 1—Pharmacokinetics. Paediatr Anaesth. 1997;7:5-11.
25. Smith G.D., Smith M.T. Morphine-3-glucuronide: evidence to support its putative role in the development of tolerance to the antinociceptive effects of morphine in the rat. Pain. 1995;62:51-60.
26. Williams P.I., Sarginson R.E., Ratcliffe J.M. Use of methadone in the morphine-tolerant burned paediatric patient. Br J Anaesth. 1998;80:92-95.
27. Laulin J.P., Maurette P., Corcuff J.B., et al. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance,. Anesth Analg. 2002;94:1263-1269.
28. Latta K.S., Ginsberg B., Barkin R.L. Meperidine: a critical review. Am J Ther. 2002;9:53-68.
29. Kavuri S., Robalino J., Janardhan Y., et al. Low-dose intrathecal meperidine for lower limb orthopaedic surgery. Can J Anaesth. 1990;37:947-948.
30. Sharar S.R., Bratton S.L., Carrougher G.J., et al. A comparison of oral transmucosal fentanyl citrate and oral hydromorphone for inpatient pediatric burn wound care analgesia,. J Burn Care Rehabil. 1998;19:521.
31. McEwan A., Sigston P.E., Andrews K.A., et al. A comparison of rectal and intramuscular codeine phosphate in children following neurosurgery,. Paediatr Anaesth. 2000;10:189-193.
32. Goldsack C., Scuplak S.M., Smith M. A double-blind comparison of codeine and morphine for postoperative analgesia following intracranial surgery,. Anaesthesia. 1996;51:1029-1032.
33. Paris A., Scholz J., von Knobelsdorff G., et al. The effect of remifentanil on cerebral blood flow velocity,. Anesth Analg. 1998;87:569-573.
34. Reference deleted in proofs
35. Vinik R.H., Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998;86:1307-1311.
36. Crawford M.W., Hickey C., Zaarour C., et al. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662-1667.
37. Shipton E.A. Tramadol—present and future. Anaesth Intensive Care. 2000;28:363-374.
38. Rosen D.A., Morris J.L., Rosen K.R., et al. Nalmefene to prevent epidural narcotic side effects in pediatric patients: a pharmacokinetic and safety study. Pharmacotherapy. 2000;20:745-749.
39. Voepel-Lewis T., Marinkovic A., Kostrzewa A., et al. Prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth Analg. 2008;107:70-75.
40. Claster S., Vichinsky E.P. Managing sickle cell disease. BMJ. 2003;327:1151-1155.
41. Ballas S.K., Viscusi E.R., Epstein K.R. Management of acute chest wall sickle cell pain with nebulized morphine. Am J Hematol. 2004;76:190-191.
42. Siddappa R., Fletcher J.E., Heard A.M., et al. Methadone dosage for prevention of opioid withdrawal in children. Paediatr Anaesth. 2003;13:805-810.
43. Katz R., Kelly H.W., His A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994;22:763-767.
44. Joshi P., et al. Opiate use and tolerance in the PICU. Crit Care Med. 2003;A130:S526.
45. Franck L.S., Harris S.K., Soetenga D.J., et al. The Withdrawal Assessment Tool–1 (WAT–1): An assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients,. Pediatr Crit Care Med. 2008;9:573-580.
46. Deutsch E.S., Nadkarni V.M. Clonidine prophylaxis for narcotic and sedative withdrawal syndrome following laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1234-1238.
47. Dominguez K.D., Crowley M.R., Coleman D.M., et al. Withdrawal from lorazepam in critically ill children. Ann Pharmacother. 2006;40:1035-1039.
48. Kienbaum P., Scherbaum N., Thurauf N., et al. Acute detoxification of opioid-addicted patients with naloxone during propofol or methohexital anesthesia: a comparison of withdrawal symptoms, neuroendocrine, metabolic, and cardiovascular patterns. Crit Care Med. 2000;28:969-976.
49. Pfab R., Hirtl C., Zilker T. Opiate detoxification under anesthesia: no apparent benefit but suppression of thyroid hormones and risk of pulmonary and renal failure. J Toxicol Clin Toxicol. 1999;37:43-50.
50. Stephenson J. Experts debate merits of 1-day opiate detoxification under anesthesia. JAMA. 1997;277:363-364.
51. Siddappa S., et al. The use of propofol during rapid opiate weaning in the PICU,. Crit Care Med. 2002;A183:S606.
52. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2:833-839.
53. Ernst M., Brauchart D., Boresch S., et al. Comparative modeling of GABA(A) receptors: limits, insights, future developments. Neuroscience. 2003;119:933-934.
54. Rudolph U. Identification of molecular substrate for the attenuation of anxiety: a step toward the development of better anti-anxiety drugs. Sci World J. 2001;1:192-193.
55. Beurdeley-Thomas A., Miccoli L., Oudard S., et al. The peripheral benzodiazepine receptors: a review,. J Neurooncol. 2000;46:45-56.
56. Casellas P., Galiegue S., Basile A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:475-486.
57. Arvat E., Giordano R., Grottoli S., et al. Benzodiazepines and anterior pituitary function. J Endocrinol Invest. 2002;25:735-747.
58. Kolmer M., Rovio A., Alho H. The characterization of two diazepam binding inhibitor (DBI) transcripts in humans,. Biochem J. 1995;306(pt 2):327-330.
59. Ohkuma S., Katsura M., Tsujimura A. Alterations in cerebral diazepam binding inhibitor expression in drug dependence: a possible biochemical alteration common to drug dependence. Life Sci. 2001;68:1215-1222.
60. Sand P., Kavvadias D., Feineis D., et al. Naturally occurring benzodiazepines: current status of research and clinical implications. Eur Arch Psychiatry Clin Neurosci. 2000;250:194-202.
61. Robin C., Trieger N. Paradoxical reactions to benzodiazepines in intravenous sedation: a report of 2 cases and review of the literature. Anesth Prog. 2002;49:128-132.
62. Jacqz-Aigrain E., Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet. 1996;31:423-443.
63. Langlois S., Kreeft J.H., Chouinard G., et al. Midazolam: kinetics and effects on memory, sensorium, and haemodynamics. Br J Clin Pharmacol. 1987;23:273-278.
64. Saegusa K., Furukawa Y., Ogiwara Y., et al. Pharmacologic basis of responses to midazolam in the isolated, cross-perfused, canine right atrium. Anesth Analg. 1987;66:711-718.
65. Peinemann F., Daldrup T. Severe and prolonged sedation in five neonates due to persistence of active diazepam metabolites. Eur J Pediatr. 2001;160:378-381.
66. Lahat E., Goldman M., Barr J., et al. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321:83-86.
67. Cray S.H., Dixon J.L., Heard C.M., et al. Oral midazolam premedication for paediatric day case patients. Paediatr Anaesth. 1996;6:265-270.
68. Blumer J.L. Clinical pharmacology of midazolam in infants and children. Clin Pharmacokinet. 1998;35:37-47.
69. de Wildt S.N., de Hoog M., Vinks A.A., et al. Population pharmacokinetics and metabolism of midazolam in pediatric intensive care patients. Crit Care Med. 2003;31:1952-1958.
70. Bauer T.M., Ritz R., Haberthur C., et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145-147.
71. Malacrida R., Fritz M.E., Suter P.M., et al. Pharmacokinetics of midazolam administered by continuous intravenous infusion to intensive care patients. Crit Care Med. 1992;20:1123-1126.
72. Ahonen J., Olkkola K.T., Takala A., et al. Interaction between fluconazole and midazolam in intensive care patients. Acta Anaesthesiol Scand. 1999;43:509-514.
73. Kyriakopoulos A.A., Greenblatt D.J., Shader R.I. Clinical pharmacokinetics of lorazepam: a review. J Clin Psychiatry. 1978;39(10 pt 2):16-23.
74. Lee S.A., Lee J.K., Heo K. Coma probably induced by lorazepam-valproate interaction. Seizure. 2002;11:124-125.
75. Tobias J.D., Rasmussen G.E. Pain management and sedation in the pediatric intensive care unit. Pediatr Clin North Am. 1994;41:1269-1292.
76. Hayman M., Seidl E.C., Ali M., et al. Acute tubular necrosis associated with propylene glycol from concomitant administration of intravenous lorazepam and trimethoprim-sulfamethoxazole. Pharmacotherapy. 2003;23:1190-1194.
77. Chicella M., Jansen P., Parthiban A., et al. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit Care Med. 2002;30:2752-2756.
78. Parker M.G., Fraser G.L., Watson D.M., et al. Removal of propylene glycol and correction of increased osmolar gap by hemodialysis in a patient on high dose lorazepam infusion therapy. Intensive Care Med. 2002;28:81-84.
79. Shafer A. Complications of sedation with midazolam in the intensive care unit and a comparison with other sedative regimens. Crit Care Med. 1998;26:947-956.
80. Bateson A.N. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8:5-21.
81. Mathieu-Nolf M., Babe M.A., Coquelle-Couplet V., et al. Flumazenil use in an emergency department: a survey. J Toxicol Clin Toxicol. 2001;39:15-20.
82. Feld L.G., Cimino M. Pediatric dosing handbook. Hudson, OH: Lexi-Comp; 1997.
83. Winkler E., Almog S., Kriger D., et al. Use of flumazenil in the diagnosis and treatment of patients with coma of unknown etiology. Crit Care Med. 1993;21:538-542.
84. Chern C.H., Chern T.L., Wang L.M., et al. Continuous flumazenil infusion in preventing complications arising from severe benzodiazepine intoxication. Am J Emerg Med. 1998;16:238-241.
85. Peters J.M., Tolia V., Simpson P., et al. Flumazenil in children after esophagogastroduodenoscopy. Am J Gastroenterol. 1999;94:1857-1861.
86. Girdler N.M., Lyne J.P., Wallace R., et al. A randomised, controlled trial of cognitive and psychomotor recovery from midazolam sedation following reversal with oral flumazenil,. Anaesthesia. 2002;57:868-876.
87. Weinbroum A.A., Szold O., Ogorek D., et al. The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature,. Eur J Anaesthesiol. 2001;18:789-797.
88. Als-Nielsen B., Gluud L.L., Gluud C. Benzodiazepine receptor antagonists for hepatic encephalopathy (review). The Cochrane Library. 2009;3:1-40.
89. Pershad J., Palmisano P., Nichols M. Chloral hydrate: the good and the bad. Pediatr Emerg Care. 1999;15:432-433.
90. Lash L.H., Fisher J.W., Lipscomb J.C., et al. Metabolism of trichloroethylene. Environ Health Perspect. 2000;108:177-200.
91. Salmon A.G., Kizer K.W., Zeise L., et al. Potential carcinogenicity of chloral hydrate—a review. J Toxicol Clin Toxicol. 1995;33:115-121.
92. Hassaballa H.A., Balk R.A. Torsades de pointes associated with the administration of intravenous haloperidol. Am J Ther. 2003;10:58-60.
93. Kao L.W., Kirk M.A., Evers S.J., et al. Droperidol, QT prolongation, and sudden death: what is the evidence? Ann Emerg Med. 2003;41:559-560.
94. American Academy of Pediatrics Committee on Drugs. Reappraisal of lytic cocktail/Demerol, Phenergan, and Thorazine (DPT) for the sedation of children. Pediatrics. 1995;95:598-602.
95. Samson-Fang L., Gooch J., Norlin C. Intrathecal baclofen withdrawal simulating neuroleptic malignant syndrome in a child with cerebral palsy. Dev Med Child Neurol. 2000;42:561-565.
96. Kao L.W., Amin Y., Kirk M.A., et al. Intrathecal baclofen withdrawal mimicking sepsis. J Emerg Med. 2003;24:423-427.
97. Greenberg M.I., Hendrickson R.G. Baclofen withdrawal following removal of an intrathecal baclofen pump despite oral baclofen replacement. J Toxicol Clin Toxicol. 2003;41:83-85.
98. Gooch J.L., Oberg W.A., Grams B., et al. Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg. 2003;39:1-6.
99. Reeves R.K., Stolp-Smith K.A., Christopherson M.W. Hyperthermia, rhabdomyolysis, and disseminated intravascular coagulation associated with baclofen pump catheter failure. Arch Phys Med Rehabil. 1998;79:353-356.
100. Sampathkumar P., Scanlon P., Plevak D. Baclofen withdrawal presenting as multiorgan system failure. Anesth Analg. 1998;87:562-563.
101. Food and Drug Administration Safety Information and Adverse Event Reporting Program: 2002 safety alert—Lioresal (baclofen injection). Available at http://www.fda.gov/medwatch/SAFETY/2002/baclofen.htm. Accessed June 2004.
102. Meythaler J.M., Roper J.F., Brunner R.C. Cyproheptadine for intrathecal baclofen withdrawal. Arch Phys Med Rehabil. 2003;84:638-642.
103. Colachis S.C., Rea G.L. Monitoring of creatinine kinase during weaning of intrathecal baclofen and with symptoms of early withdrawal. Am J Phys Med Rehabil. 2003;82:489-492.
104. Rodarte A., Diaz S., Foley J., et al. The pharmacokinetics of dexmedetomidine in post-surgical pediatric intensive care unit patients: a preliminary study,. Anesthesiology. 2003;99:A423.
105. De Wolf A.M., Fragen R.J., Avram M.J., et al. The pharmacokinetics of dexmedetomidine in volunteers with severe renal impairment,. Anesth Analg. 2001;93:1205-1209.
106. Baughman V.L., Cunningham F., Layden T., et al. Pharmacokinetic/pharmacodynamic effects of dexmedetomidine in patients with hepatic failure. Anesth Analg. 2000;90:S391.
107. Venn R.M., Bradshaw C.J., Spencer R., et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136-1142.
108. Riker R.R., Ramsay M.A.E., Prielipp R.C., et al. Long-term dexmedetomidine infusions for ICU sedation: a pilot study. Anesthesiology. 2001;95:A383.
109. Czaja A.S., Zimmerman J.J. The use of dexmedetomidine in critically ill children,. Pediatr Crit Care Med. 2009;10:381-386.
109a. Beijan S., Valasek C., Nigro J.J. Prolonged used of dexmedetomidine in the pediatric cardiothoracic intensive care unit,. Cardiol Young. 2009;19(1):98-104.
110. Berkenbosch J.W., Tobias J.D. Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med. 2003;4:203-205.
111. Short T.G., Chui P.T. Propofol and midazolam act synergistically in combination. Br J Anaesth. 1991;67:539-545.
112. Chamorro C., de Latorre F.J., Montero A., et al. Comparative study of propofol versus midazolam in the sedation of critically ill patients: results of a prospective, randomized, multicenter trial. Crit Care Med. 1996;24:932-939.
113. Barrientos-Vega R., Mar Sanchez-Soria M., Morales-Garcia C., et al. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997;25:33-39.
114. Brown L.A., Levin G.M. Role of propofol in refractory status epilepticus. Ann Pharmacother. 1998;32:1053-1059.
115. Stecker M.M., Kramer T.H., Raps E.C., et al. Treatment of refractory status epilepticus with propofol: clinical and pharmacokinetic findings. Epilepsia. 1998;39:18-26.
116. Platt M., White D.C. Calories in sedation. Anaesthesia. 1987;42:322 AN2269.
117. Valente J.F., Anderson G.L., Branson R.D., et al. Disadvantages of prolonged propofol sedation in the critical care unit. Crit Care Med. 1994;22:710-712.
118. Bach A., Geiss H.K. Propofol and postoperative infections. N Engl J Med. 1995;30:1505-1506.
119. Bennett S.N., McNeil M.M., Bland L.A., et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med. 1995;333:147-153.
120. Laxenaire M.C., Mata-Bermejo E., Moneret-Vautrin D.A., et al. Life-threatening anaphylactoid reactions to propofol (Diprivan). Anesthesiology. 1992;77:275-280.
121. Hepner D.L., Castells M.C. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
122. Olufolabi A.J., Gan T.J., Lacassie H.J., et al. A randomized, prospective double-blind comparison of the efficacy of generic propofol (sulphite additive) with Diprivan,. Eur J Anaesthesiol. 2006;23:341-345.
123. Bodenham A., Culank L.S., Park G.R. Propofol infusion and green urine. Lancet. 1987;2:740.
124. Parke T.J., Stevens J.E., Rice A.S., et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305:613-616.
125. Propofol infusion in children. BMJ. 1992;305:952-953.
126. Bray R.J. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8:491-499.
127. Pepperman M.L., Macrae D. A comparison of propofol and other sedative use in paediatric intensive care in the United Kingdom,. Paediatr Anaesth. 1997;7:143-153.
128. Cornfield D.N., Tegtmeyer K., Nelson M.D., et al. Continuous propofol infusion in 142 critically ill children. Pediatrics. 2002;110:1177-1181.
129. Reed M.D., Blumer J.L. Propofol bashing: the time to stop is now!. Crit Care Med. 1996;24:175-176.
130. Felmet K., Nguyen T., Clark R.S., et al. The FDA warning against prolonged sedation with propofol in children remains warranted,. Pediatrics. 2003;112:1002-1003.
131. AstraZenica: Propofol, Newark, DE, March 26, 2001.
132. Kang T.M. Propofol infusion syndrome in critically ill patients. Ann Pharmacother. 2002;36:1453-1456.
133. Kam P.C.A., Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690-701.
134. Cray S.H., Robinson B.H., Cox P.N. Lactic academia and bradyarrhythmia in a child sedated with propofol. Crit Care Med. 1998;26:2087-2092.
135. Wolf A., Weir P., Segar P., et al. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet. 2001;357:606-607.
136. Vasile B., Rasulo F., Candiani A., et al. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome,. Intensive Care Med. 2003;29:1417-1425.
137. Wheeler D.S., Vaux K.K., Ponaman M.L., et al. The safe and effective use of propofol sedation in children undergoing diagnostic and therapeutic procedures: experience in a pediatric ICU and a review of the literature,. Pediatr Emerg Care. 2003;19:385-392.
138. Hlozki J., Aring C., Gillor A. Death after re-exposure to propofol in a 3-year-old child: a case report. Paediatric Anaesth. 2004;14:265-270.
139. Hanna J.P., Ramundo M.L. Rhabdomyolysis and hypoxia associated with prolonged propofol infusion in children. Neurology. 1998;50:301-303.
140. Cannon M.L., Glazier S.S., Bauman L.A. Metabolic acidosis, rhabdomyolysis, and cardiovascular collapse after prolonged propofol infusion. J Neurosurg. 1995;95:1053-1056.
141. Vernooy K., Delhaas T., Cremer O.L., et al. Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm February. 2006;3:131-137.
142. Seigler R.S., Avant M.G., Gwyn D.R., et al. A comparison of propofol and ketamine/midazolam for intravenous sedation of children,. Pediatr Crit Care Med. 2001;2:20-23.
143. Festa M., Bowra J., Schell D. Use of propofol infusion in Australian and New Zealand paediatric intensive care units. Anaesth Intensive Care. 2002;30:786-793.
144. Veldhoen E.S., Hartman B.J., van Gestel J.P.J. Monitoring biochemical parameters as an early sign of propofol infusion syndrome: false feeling of security. Pediatr Crit Care Med. 2009;10:e19-e21.
145. Fong J.J., Sylvia L., Ruthazer R., et al. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008;36:2281-2287.
146. Lowrie L., Weiss A.H., Lacombe C. The pediatric sedation unit: a mechanism for pediatric sedation,. Pediatrics. 1998;102:E30.
147. Friedman A.G., Mulhern R.K., Fairclough D., et al. Midazolam premedication for pediatric bone marrow aspiration and lumbar puncture. Med Pediatr Oncol. 1991;19:499-504.
148. American Academy of Pediatrics Committee on Drugs. Guidelines for monitoring management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89:276-281.
149. Epstein B.S. The American Society of Anesthesiologist’s efforts in developing guidelines for sedation and analgesia for nonanesthesiologists: the 40th Rovenstine Lecture,. Anesthesiology. 2003;98:1261-1268.
150. Practice guidelines for sedation and analgesia by non-anesthesiologists. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology. 1996;84:459-471.
151. Dodson B.A. Interventional neuroradiology and the anesthetic management of patients with arteriovenous malformations. In: Cotrell J.E., Smith D.S., editors. Anesthesia and neurosurgery. St Louis: Mosby, 1994.
152. Rheuban K.S., Carpenter M.A. Diagnostic cardiac catheterization, angiography, and interventional catheterization. In: Lake C.L., editor. Pediatric cardiac anesthesia. Norwalk, CT: Appleton & Lange, 1993.
153. Keeter S., Benator R.M., Weinberg S.M., et al. Sedation in pediatric CT: national survey of current practice. Radiology. 1990;175:745-752.
154. Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. St Louis: Mosby-Year Book; 1993.
155. Wilson S. A survey of the American Academy of Pediatric Dentistry membership: nitrous oxide and sedation,. Pediatr Dent. 1996;18:287-293.
156. Cote C.J., Karl H.W., Notterman D.A., et al. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633-644.
157. Dreifuss F.E., Rosman N.P., Cloyd J.C., et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures,. N Engl J Med. 1998;338:1869-1875.
158. Holzman R.S., Cullen D.J., Eichhorn J.H., et al. Guidelines for sedation by nonanesthesiologists during diagnostic and therapeutic procedures. The Risk Management Committee of the Department of Anaesthesia of Harvard Medical School,. J Clin Anesth. 1994;6:265-276.
159. Hoffman G.M., Nowakowski R., Troshynski T.J., et al. Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics. 2002;109:236-243.
160. Jorgensen N.H., Messick J.M.Jr., Gray J., et al. ASA monitoring standards and magnetic resonance imaging. Anesth Analg. 1994;79:1141-1147.
161. Heard C.M.B. Nuclear magnetic resonance imaging. In: Atlee J.L., editor. Complications in anesthesia. Philadelphia: WB Saunders, 1999.
162. Menon D.K., Peden C.J., Hall A.S., et al. Magnetic resonance for the anaesthetist. Part 1: Physical principles, applications, and safety aspects. Anaesthesia. 1992;47:240-255.
163. Peden C.J., Menon D.K., Hall A.S., et al. Magnetic resonance for the anaesthetist. Part II. Anaesthesia and monitoring in MR units. Anaesthesia. 1992;47:508-516.
164. Roy T.M., Pruitt V.L., Garner P.A., et al. The potential role of anesthesia in status asthmaticus,. J Asthma. 1992;29:73-77.
165. Smith J.A., Santer L.J. Respiratory arrest following intramuscular ketamine injection in a 4-year-old child. Ann Emerg Med. 1993;22:613-615.
166. Kenyon C.J., McNeil L.M., Fraser R. Comparison of the effects of etomidate, thiopentone and propofol on cortisol synthesis. Br J Anaesth. 1985;57:509-511.
167. Crozier T.A., Flamm C., Speer C.P., et al. Effects of etomidate on the adrenocortical and metabolic adaptation of the neonate. Br J Anaesth. 1993;70:47-53.
168. Watt I., Ledingham I.M. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia. 1984;39:973-978.
169. Clarke J.B., Thomas C., Chen M., et al. Halogenated anesthetics form liver adducts and antigens that cross-react with halothane-induced antibodies. Int Arch Allergy Immunol. 1995;108:24-32.
170. Sponheim S., Skraastad O., Helseth E., et al. Effects of 0.5 and 1.0 MAC isoflurane, sevoflurane and desflurane on intracranial and cerebral perfusion pressures in children. Acta Anaesthesiol Scand. 2003;47:932-938.
171. Petersen K.D., Landsfeldt U., Cold G.E., et al. Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: a randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329-336.
172. Fraga M., Rama-Maceiras P., Rodino S., et al. The effects of isoflurane and desflurane on intracranial pressure, cerebral perfusion pressure, and cerebral arteriovenous oxygen content difference in normocapnic patients with supratentorial brain tumors,. Anesthesiology. 2003;98:1085-1090.
173. Tanigami H., Yahagi N., Kumon K., et al. Long-term sedation with isoflurane in postoperative intensive care in cardiac surgery. Artif Organs. 1997;21:21-23.
174. Arnold J.H., Truog R.D., Rice S.A. Prolonged administration of isoflurane to pediatric patients during mechanical ventilation. Anesth Analg. 1993;76:520-526.
175. Fujino Y., Nishimura M., Nishimura S., et al. Prolonged administration of isoflurane to patients with severe renal dysfunction. Anesth Analg. 1998;86:440-441.
176. Johnston R.G., Noseworthy T.W., Friesen E.G., et al. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97:698-701.
177. U.S. Department of Labor Occupational Safety and Health Administration. Waste anesthetic gases (OSHA Fact Sheet 91–38). Washington, DC: U.S. Department of Labor Occupational Safety and Health Administration; 1991.
178. U.S. Department of Labor Occupational Safety and Health Administration: Waste anesthetic gases. Available at http://www.osha.gov/SLTC/wasteanestheticgases. Accessed June 2004.
179. Curley M.A., Molengraft J.A. Providing comfort to critically ill pediatric patients. Crit Care Nurs Clin North Am. 1995;2:267-274.
180. Parnass S.M., Feld J.M., Chamberlin W.H., et al. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66:193-195.
181. Willatts S.M., Spencer E.M. Sedation for ventilation in the critically ill. Anaesthesia. 1994;49:422-428.
182. Kong K.L., Willatts S.M. Isoflurane sedation in pediatric intensive care. Crit Care Med. 1995;23:1308-1309.
183. Wheeler D.S., Clapp C.R., Ponaman M.L., et al. Isoflurane therapy for status asthmaticus in children: a case series and protocol. Pediatr Crit Care Med. 2000;l:55-59.
184. Tobias J.D. Therapeutic applications and uses of inhalational anesthesia in the pediatric intensive care unit. Pediatr Crit Care Med. 2008;9:169-179.
185. Ikonomidou C., Bosch F., Miksa M., et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70-74.
186. Bartels M., Althoff R.R., Boomsma D.I. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genetics. 2009;2:246-253.