Chapter 11 Secondary Intraparenchymal Hemorrhages
Multiple causes other than hypertension may provoke intraparenchymal hematomas. Recognizing them may be difficult, especially in the acute phase. However, some of these causes must be promptly identified to avoid devastating complications. Hemorrhages induced by excessive anticoagulation and those produced by bleeding vascular anomalies are examples of highly dangerous situations that demand immediate therapeutic intervention.
Brain imaging is particularly valuable in the distinction of secondary causes of intraparenchymal hematoma (Table 11-1). Radiological hints are frequently subtle but quite reliable. Imaging often provides the first, and sometimes only evidence that a secondary cause is responsible for the hemorrhage. Throughout this chapter we attempt to summarize useful discriminating signs as we present examples of the most common secondary causes of intraparenchymal bleeding.
TABLE 11-1 Secondary causes of intraparenchymal hemorrhage
| Cerebral amyloid angiopathy |
| Hemorrhagic transformation of ischemic infarction |
| Anticoagulation |
| Thrombolysis |
| Intrinsic coagulation disorders |
| Sympathomimetic drugs (cocaine, amphetamines, decongestants, anorectics) |
| Infective endocarditis |
| Vasculitis |
| Moyamoya disease |
| Neurosurgical and neuroendovascular procedures |
| Cerebral venous thrombosis |
| Vascular anomalies |
| Saccular aneurysms |
| Arteriovenous malformations |
| Cavernous malformations |
| Dural arteriovenous fistulas |
| Tumors (primary or metastatic) |
| Head trauma |
CEREBRAL AMYLOID ANGIOPATHY
Cerebral amyloid angiopathy (CAA) is characterized by the accumulation of amyloid β and other amyloidogenic peptides in the walls of medium and small cortical and leptomeningeal vessels leading to vascular fragility and probably dysfunction.1,2 Amyloid deposition and hemorrhage are favored by the presence of APOE 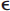 2 and
2 and 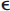 4 alleles,3 and vascular amyloid deposits are often (but not always) seen in combination with changes of Alzheimer’s disease.4 The restricted location of the deposits explains why hemorrhages are always lobar and involve the cortex of the brain (or, much less commonly, the cerebellum). It may account for more than one third of cases of lobar intracerebral hemorrhage (ICH) in the elderly population (age > 65 years).5,6 The prevalence of CAA in brain-bank studies exceeds 50% in patients over age 70,7 and it is severe in more than 10% of patients over age 80.8 Thus the incidence of CAA-related hemorrhages is likely to continue to increase substantially with the aging of the population.
4 alleles,3 and vascular amyloid deposits are often (but not always) seen in combination with changes of Alzheimer’s disease.4 The restricted location of the deposits explains why hemorrhages are always lobar and involve the cortex of the brain (or, much less commonly, the cerebellum). It may account for more than one third of cases of lobar intracerebral hemorrhage (ICH) in the elderly population (age > 65 years).5,6 The prevalence of CAA in brain-bank studies exceeds 50% in patients over age 70,7 and it is severe in more than 10% of patients over age 80.8 Thus the incidence of CAA-related hemorrhages is likely to continue to increase substantially with the aging of the population.
The definitive diagnosis of CAA requires pathological demonstration. However, in practice this is rarely feasible or justified. Criteria have been designed to make the diagnosis of possible or probable CAA based solely on clinical and radiological data.9 Possible CAA requires CT or MRI evidence of a single lobar ICH in a patient 55 years or older with no other cause for the hemorrhage. When there is clinicoradiological evidence of two or more lobar cerebral or cerebellar hemorrhages in a patient 55 years or older with no other causes for the lesions the diagnosis is probable CAA.
These criteria focus on the identification of cases of lobar ICH that can only be attributed to CAA. However, CAA may also play a role in the genesis of lobar hematomas in patients with coexistent causes. In fact, it has been shown that CAA increases the risk of intracranial bleeding in patients receiving anticoagulants10 and thrombolytic agents.11
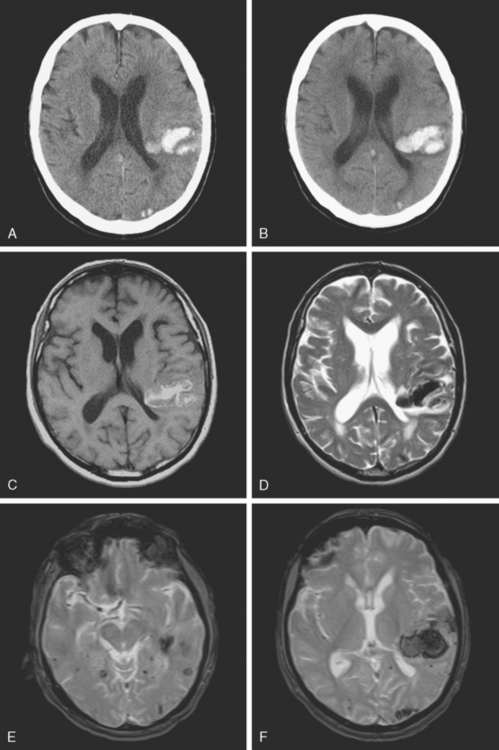
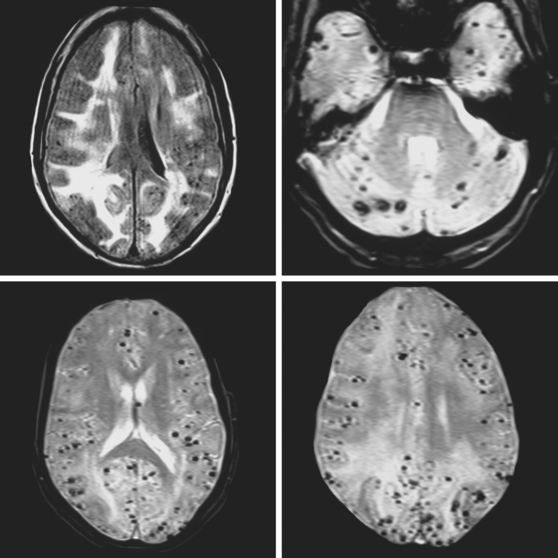
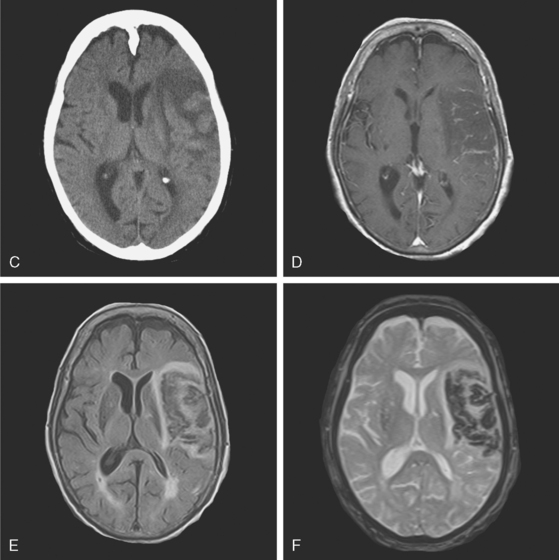
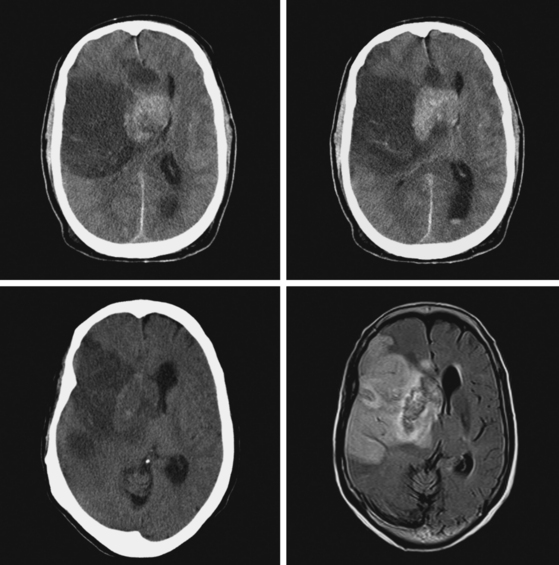
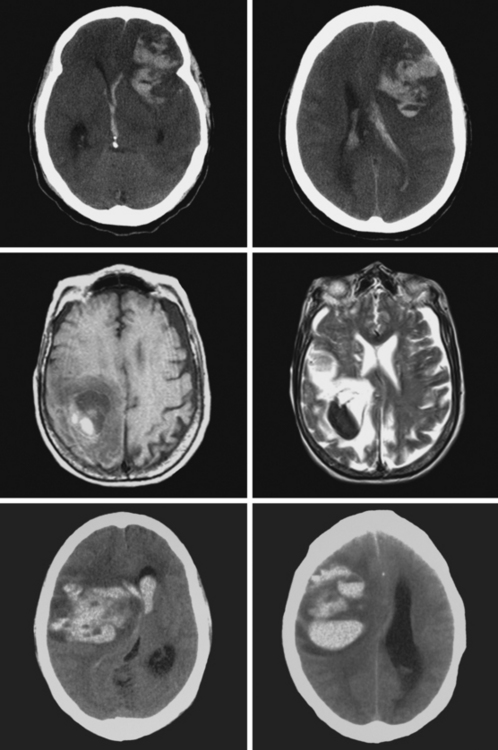
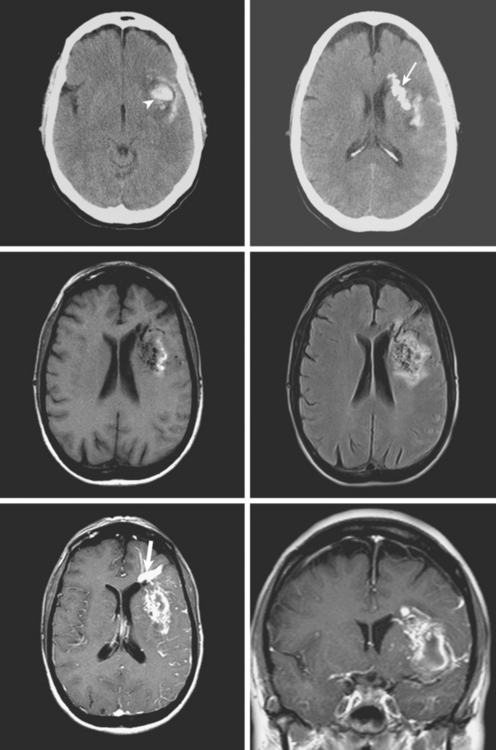
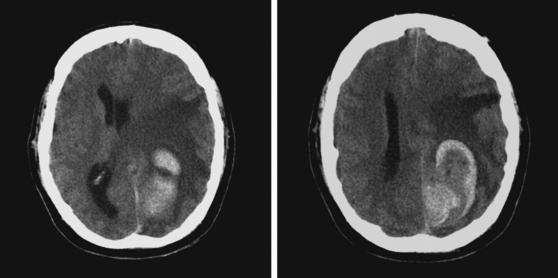
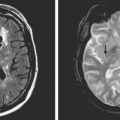
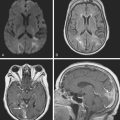
A 73-year-old man without previous known illnesses was brought to the emergency department by his son for evaluation of acute speech changes. On physical examination, the patient was fully awake and had fluent dysphasia without associated motor or sensory deficits. Head CT scan disclosed a left parietal hemorrhage of moderate volume (Figure 11-1). When specifically questioned, the patient’s son acknowledged that he had noticed subtle changes in the behavior of his father over the previous year. His memory had been declining for a longer time. Brain magnetic resonance imaging (MRI) revealed multiple cortical microhemorrhages, supporting the clinical suspicion of cerebral amyloid angiopathy. The patient developed episodes of agitation during the hospitalization and only achieved minimal recovery from his dysphasia.
HEMORRHAGIC TRANSFORMATION
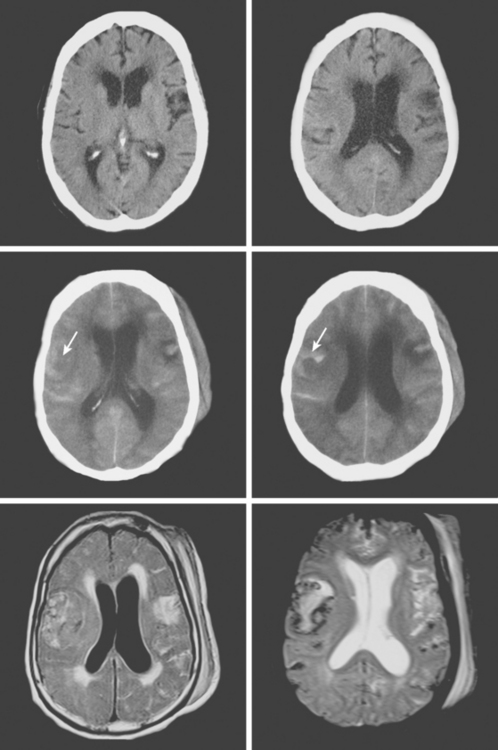
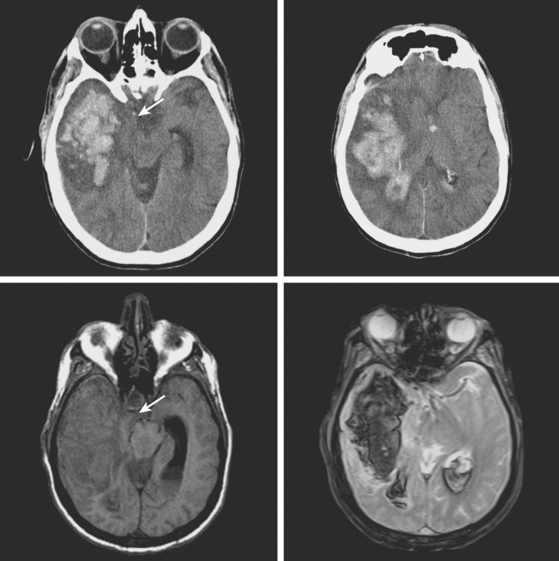
An 86-year-old woman with history of hypertension and right parietal meningioma was found unresponsive on her bed during a cruise. She was intubated and emergently transferred to our hospital for evaluation. On arrival, she was drowsy, globally aphasic, and had flaccid right hemiplegia. Her pulse was irregularly irregular, and electrocardiogram confirmed the diagnosis of atrial fibrillation. Initial CT scan is shown in Figure 11-4, A and B.
CT scan of the head and brain MRI obtained 48 hours after symptom onset showed evidence of hemorrhagic transformation (Figure 11-4, C–F). The patient had been taking aspirin before the stroke, and 325 mg of aspirin had been administered since admission. Anticoagulation had not been initiated because of the perceived high risk of hemorrhagic conversion of the ischemic infarction. On the third day after stroke onset, the patient’s neurological status deteriorated because of progression of mass effect. She responded favorably to treatment with mannitol. Her subsequent clinical course was complicated with the development of acute renal failure, congestive heart failure, bilateral pneumonia, and sepsis. She required tracheostomy and was eventually transferred to a nursing home in fair condition. Six months later, she had improved communication abilities but remained functionally dependent.
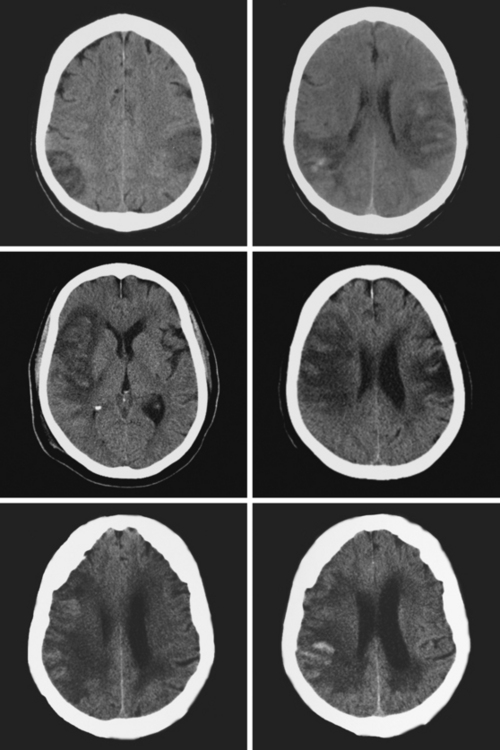
Figure 11-5 Multiples examples of hemorrhagic conversion of ischemic infarctions on head computed tomography scans.
HEMORRHAGIC COMPLICATIONS OF THROMBOLYSIS
Intracranial hemorrhage is the most feared complication of thrombolysis. Symptomatic hemorrhagic transformation occurs in 6% to 7% of patients who receive treatment with recombinant tissue plasminogen activator (rt-PA) within 3 hours of symptom onset.17,18 The rate of hemorrhage is about 10% after intra-arterial lysis.19 Hemorrhagic risks with combined intravenous–intra-arterial lysis20 and mechanical embolectomy21 also fall within this range. It tends to occur early after infusion of rt-PA and carries high mortality. However, it does not negate the value of thrombolysis in appropriate candidates for this acute intervention.
Clinical factors include older age, pretreatment hypertension, pretreatment hyperglycemia, history of diabetes mellitus, use of antiplatelets other than aspirin before stroke onset, greater stroke severity (i.e., higher score on the National Institutes of Health stroke scale) at baseline, and higher blood pressure over the first 24 hours after lysis.22–26 Radiological predictors on initial CT scan include hypodense lesion greater than one third of MCA territory and hyperdense MCA sign.22,23 MRI with diffusion-weighted imaging/perfusion-weighted imaging (DWI/PWI) may be particularly useful because very low initial apparent diffusion coefficient (ADC) values, persistent perfusion deficits on PWI, and very large areas of restricted diffusion on DWI strongly correlate with heightened risk of hemorrhagic transformation.22,27–31 Although DWI and PWI are not usually available when thrombolysis is administered within the 3-hour window, the predictive information provided by these sequences may become important if thrombolysis is considered at later times in patients with persistent radiological penumbra. Leukoaraiosis has also been associated with increased risk of ICH after thrombolysis.32 However, the presence of cerebral microhemorrhages seen on T2* sequence does not appear to influence substantially the risk of symptomatic ICH.33 Although the factors influencing the risk of hemorrhage after intra-arterial thrombolysis or multimodality therapy have been less studied, available evidence indicates that they do not differ significantly from those that determine the risk after intravenous lysis.34,35
It has been appropriately postulated that the deleterious consequences of symptomatic ICH after thrombolysis may have been overestimated by failing to consider the predicted outcome at the time of initial assessment in patients who subsequently had symptomatic ICH.36 Because outcome in many of these patients is anticipated to be poor (because of older age, more severe deficits, and larger strokes), when the predicted functional outcome is compared with the actual outcome after ICH, the number necessary to harm for intravenous thrombolysis turns out to be much smaller than commonly thought. In other words, symptomatic ICH after thrombolysis may further worsen outcome in patients with poor chances of recovery without thrombolysis, but it rarely causes devastating damage in patients with better anticipated outcome at the time of treatment.
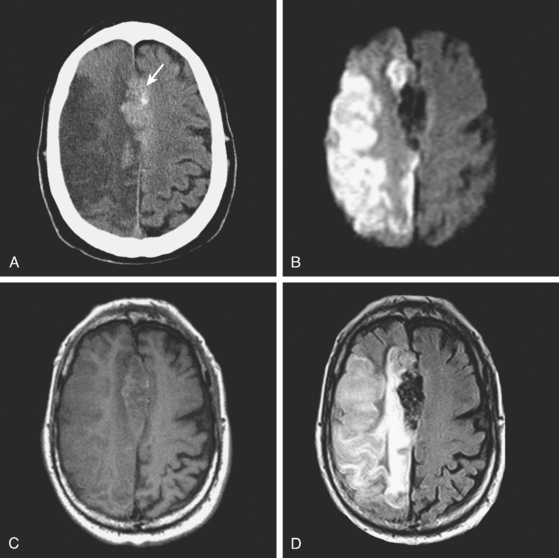
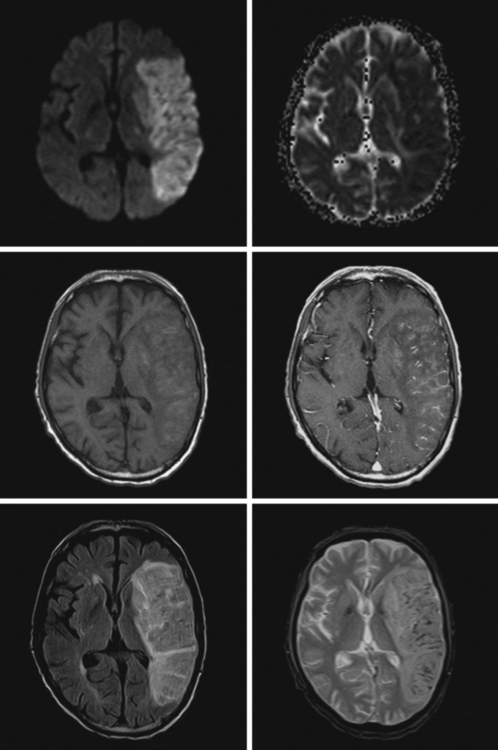
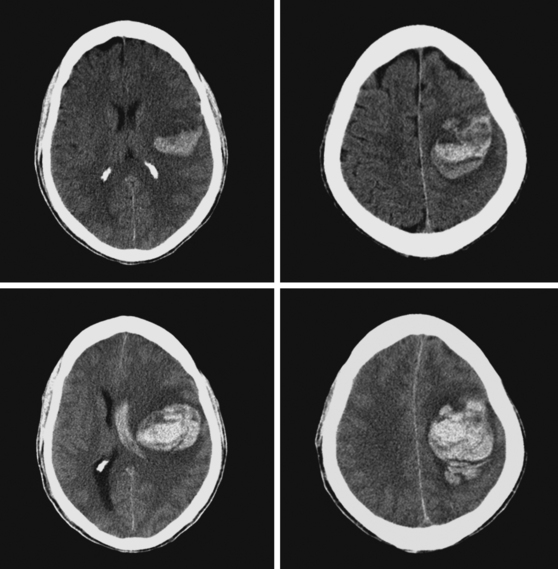
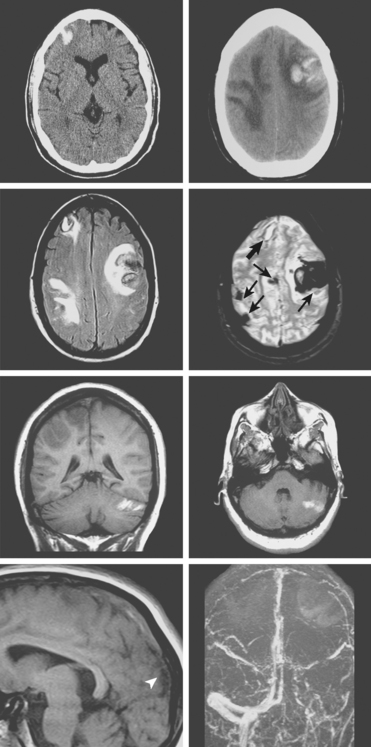
A 68-year-old man with previous history of a left hemispheric stroke presented to the emergency department with sudden onset of left-sided weakness within 2 hours of symptom onset. Examination revealed right gaze preference, left homonymous hemianopia, left hemiparesis, and partial left hemibody neglect. Initial CT scan is shown in Figure 11-10 (upper row). He had no contraindications for intravenous thrombolysis and consequently received rt-PA with the bolus given 2 hours and 45 minutes after instauration of deficits. Three hours later, the patient was noticed to be somnolent, and his weakness had worsened to the point of hemiplegia. Emergent repeat CT scan displayed intraparenchymal and subarachnoid hemorrhage (Figure 11-10, middle row). He was treated with fresh frozen plasma and cryoprecipitate. Although his deficits remained severe, he subsequently improved. Brain MRI on day 5 showed the hemorrhagic transformation of his right MCA infarction with mild regional mass effect (Figure 11-10, lower row).
ANTICOAGULATION-RELATED INTRACEREBRAL HEMORRHAGE
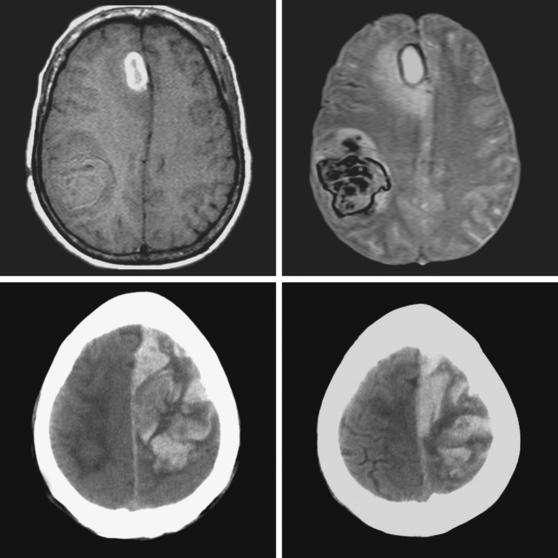
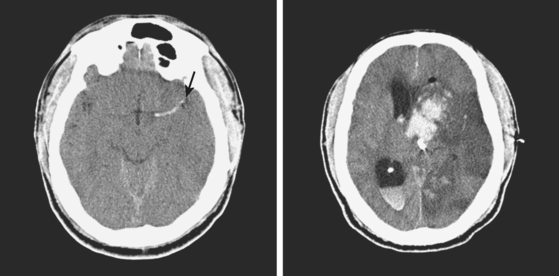
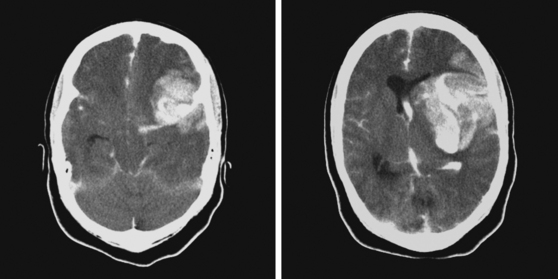
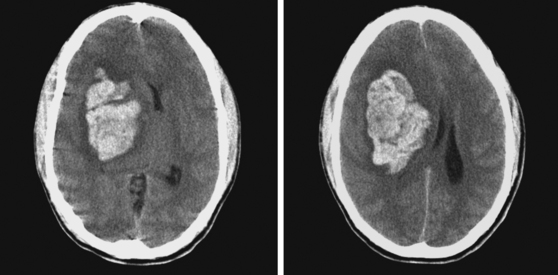
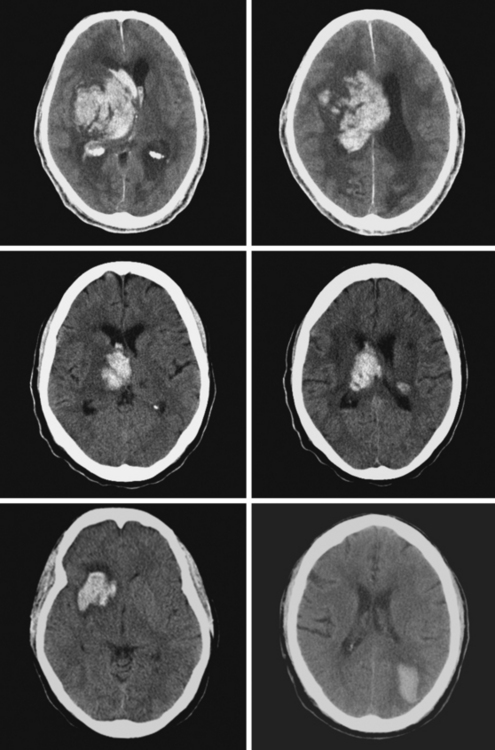
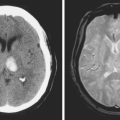
A 62-year-old man with history of atrial fibrillation, myocardial infarction, diabetes mellitus, and hypertension developed sudden dysarthria and right hemiparesis with hemisensory loss. International normalized ratio on admission was 4.2. Head CT scan is shown in Figure 11-12 (upper row). Anticoagulation was reversed with fresh frozen plasma and vitamin K. The following day, the patient was more somnolent, anarthric, and hemiplegic. Repeat CT scan revealed enlargement of the hematoma without signs of herniation. Over the next 12 hours, he became stuporous, and a third CT scan showed a 9-mm shift of the septum pellucidum (Figure 11-12, lower row). He underwent surgical evacuation of the hematoma and progressively improved after surgery. He initially required tracheostomy and percutaneous gastrostomy, but 3 months later, his tracheostomy was closed, he was eating without major difficulties, was able to communicate, and had only moderate disability from his right hemiparesis.
COAGULOPATHY
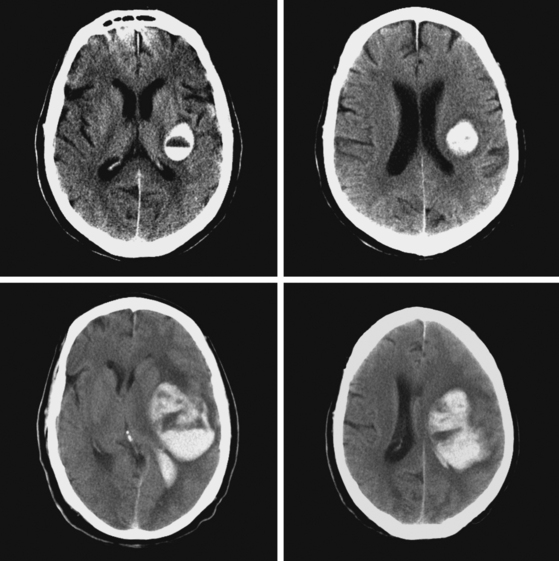
A 60-year-old man was found unresponsive by his wife and brought emergently to the emergency department. On arrival, he was stuporous, had a right fixed dilatated pupil, and exhibited no response to pain on the left limbs and withdrawal responses on the right. He had multiple purpuric lesions throughout his body and history of “easy bruising and poor clotting” according to his wife. Splinter hemorrhages were noted in his nail beds. Initial CT scan is shown in Figure 11-14. Platelet count was 8000 per mm3, and peripheral smear disclosed multiple atypical immature circulating lymphoid cells. He continued to deteriorate rapidly despite multiple platelet transfusions and was declared brain dead 18 hours later. Necropsy revealed evidence of acute myelocytic leukemia.
SUBSTANCE ABUSE
INFECTIVE ENDOCARDITIS
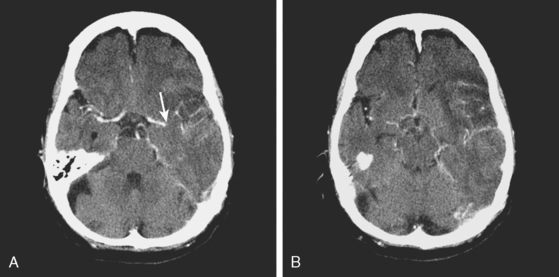
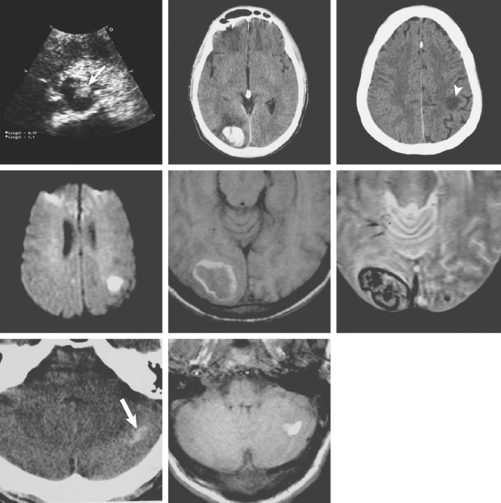
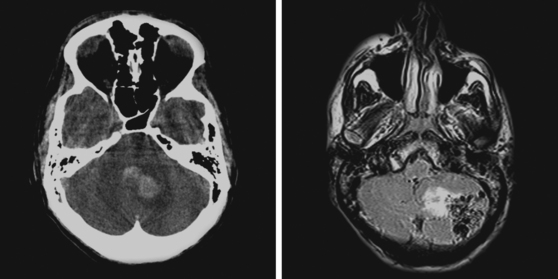
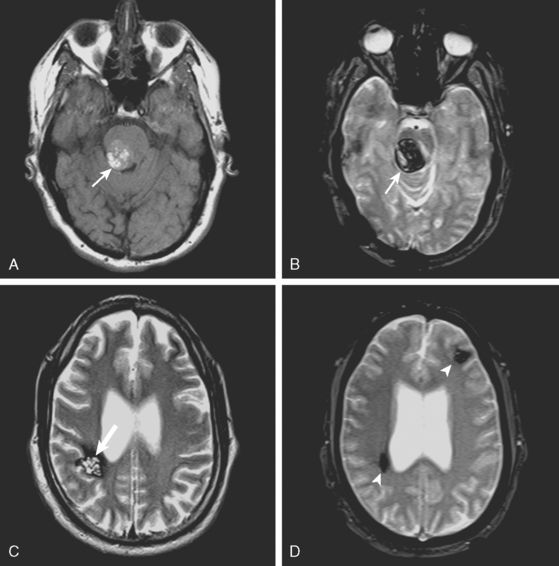
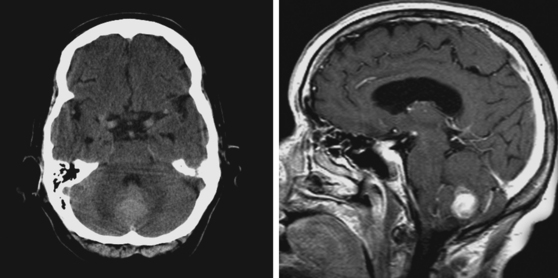
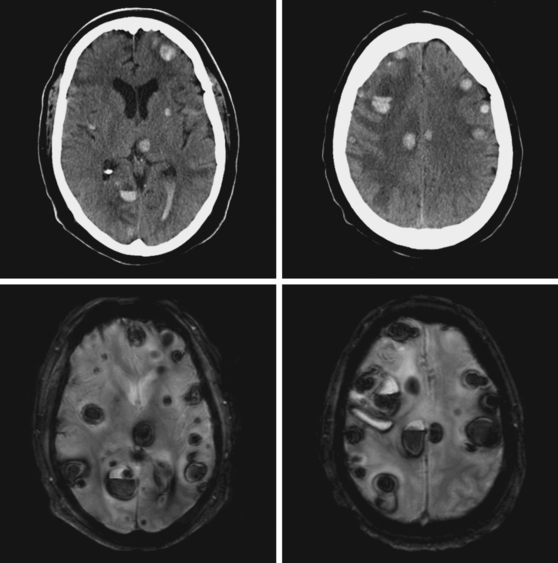
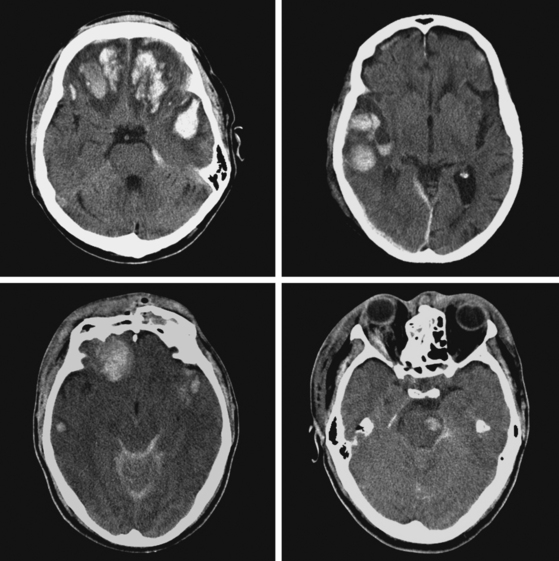
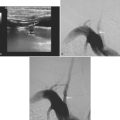
A 51-year-old man presented with complaints of fever with chills, myalgias, arthralgias, diarrhea, and profound malaise for 1 week. He used intravenous cocaine but had no documented previous illnesses. On examination, the patient appeared acutely ill and was febrile. Cardiac auscultation revealed galloped tachycardia and a diastolic cardiac murmur. Hepatosplenomegaly was apparent on abdominal auscultation. Admission blood tests showed pronounced leukocytosis, and the patient was started empirically on antibiotics after blood cultures had been obtained. In less than 24 hours, oxacillin-sensitive Staphylococcus aureus was isolated from the blood cultures. Transthoracic echocardiography demonstrated an aortic valve vegetation (Figure 11-18) with evidence of valvular regurgitation. Complaints of headaches and visual changes prompted a neurological consultation. Left homonymous visual field deficit was evident on examination. Roth spots were seen on fundoscopy. Brain imaging disclosed multiple hemorrhagic and ischemic lesions diffusely distributed in both cerebral hemispheres and the left cerebellum (Figure 11-18). Abdominal CT scan also showed embolic lesions in the liver, spleen, and both adrenal glands. The patient refused surgery but responded well to intravenous antibiotics, with no further clinical evidence of systemic embolism.
VASCULITIS
CEREBRAL VENOUS THROMBOSIS
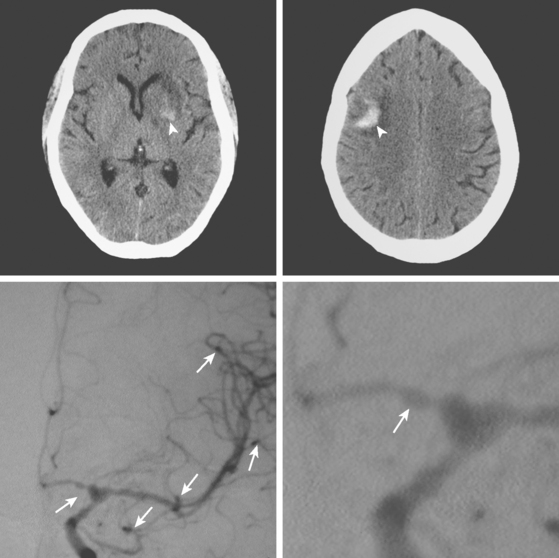
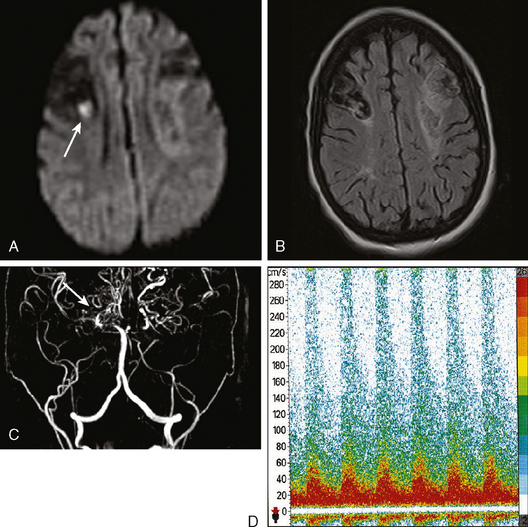
A 38-year-old woman without significant past medical history was brought to the emergency department with new onset tonic-clonic seizures. She had been complaining of increasing headaches for the previous week. On examination, the patient was confused and had right hemiparesis. Coagulation values were normal. Head CT revealed bifrontal hemorrhages with abundant edema and small right frontal subarachnoid hemorrhage as shown in Figure 11-20. Subsequent brain MRI better delineated the extent of the multiple hemorrhagic lesions, and MR venography (MRV) confirmed the suspected diagnosis of cerebral venous sinus thrombosis. She was treated with intravenous heparin and antiepileptics. Despite extensive evaluation, no evidence of an underlying hypercoagulable disorder was found. However, the patient had started to take oral contraceptive pills a few weeks before the onset of her neurological symptoms. She recovered well and was discharged to the rehabilitation unit. Four months later, she had experienced no recurrent seizures, was free of headaches, and had only minimal functional impairment.
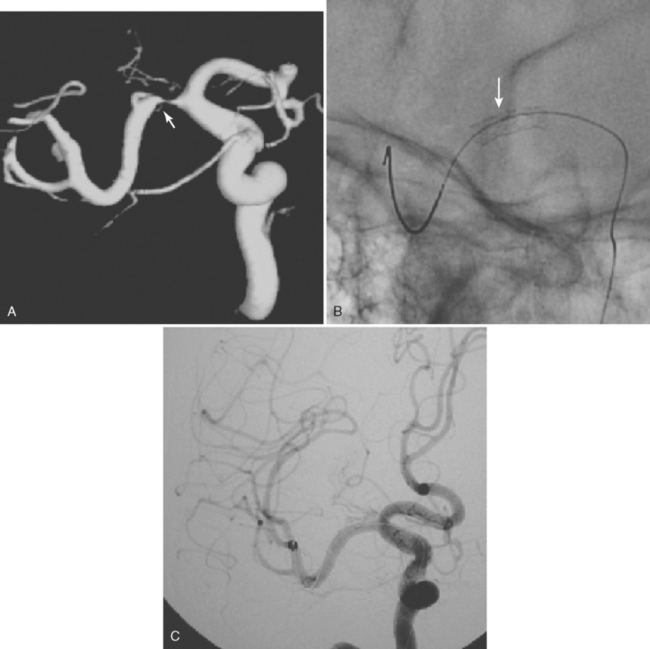
ARTERIOVENOUS MALFORMATIONS
CAVERNOUS MALFORMATION
DURAL ARTERIOVENOUS FISTULA
TUMOR
TRAUMA
1 Rensink AA, de Waal RM, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res Brain Res Rev. 2003;43:207-223.
2 Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis. 2002;13(2 Suppl):42-47.
3 O’Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240-245.
4 Olichney JM, Ellis RJ, Katzman R, Sabbagh MN, Hansen L. Types of cerebrovascular lesions associated with severe cerebral amyloid angiopathy in Alzheimer’s disease. Ann NY Acad Sci. 1997;826:493-497.
5 Badjatia N, Rosand J. Intracerebral hemorrhage. Neurologist. 2005;11:311-324.
6 Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190-1195.
7 Mastaglia FL, Byrnes ML, Johnsen RD, Kakulas BA. Prevalence of cerebral vascular amyloid-beta deposition and stroke in an aging Australian population: a postmortem study. J Clin Neurosci. 2003;10:186-189.
8 Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418-1422.
9 Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537-539.
10 Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947-951.
11 Sloan MA, Price TR, Petito CK, Randall AM, Solomon RE, Terrin ML, et al. Clinical features and pathogenesis of intracerebral hemorrhage after rt-PA and heparin therapy for acute myocardial infarction: the Thrombolysis in Myocardial Infarction (TIMI) II Pilot and Randomized Clinical Trial combined experience. Neurology. 1995;45:649-658.
12 Rosand J, Muzikansky A, Kumar A, Wisco JJ, Smith EE, Betensky RA, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005;58:459-462.
13 Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415-1420.
14 Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606-1612.
15 Kim EY, Na DG, Kim SS, Lee KH, Ryoo JW, Kim HK. Prediction of hemorrhagic transformation in acute ischemic stroke: role of diffusion-weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol. 2005;26:1050-1055.
16 Greer DM, Koroshetz WJ, Cullen S, Gonzalez RG, Lev MH. Magnetic resonance imaging improves detection of intracerebral hemorrhage over computed tomography after intra-arterial thrombolysis. Stroke. 2004;35:491-495.
17 The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587.
18 Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145-1150.
19 Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
20 The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127-2135.
21 Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006;27:1177-1182.
22 Derex L, Hermier M, Adeleine P, Pialat JB, Wiart M, Berthezene Y, et al. Clinical and imaging predictors of intracerebral haemorrhage in stroke patients treated with intravenous tissue plasminogen activator. J Neurol Neurosurg Psychiatry. 2005;76:70-75.
23 Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002;105:1679-1685.
24 Kidwell CS, Saver JL, Carneado J, Sayre J, Starkman S, Duckwiler G, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke. 2002;33:717-724.
25 Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047-2052.
26 Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1-10.
27 Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047-2052.
28 Tong DC, Adami A, Moseley ME, Marks MP. Prediction of hemorrhagic transformation following acute stroke: role of diffusion- and perfusion-weighted magnetic resonance imaging. Arch Neurol. 2001;58:587-593.
29 Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508-517.
30 Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
31 Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2007.
32 Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463-2466.
33 Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, et al. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38:2738-2744.
34 Kase CS, Furlan AJ, Wechsler LR, Higashida RT, Rowley HA, Hart RG, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603-1610.
35 Vora NA, Gupta R, Thomas AJ, Horowitz MB, Tayal AH, Hammer MD, et al. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. AJNR Am J Neuroradiol. 2007;28:1391-1394.
36 Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279-2283.
37 Thomalla G, Sobesky J, Kohrmann M, Fiebach JB, Fiehler J, Zaro WO, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38:313-318.
38 Sjoblom L, Hardemark HG, Lindgren A, Norrving B, Fahlen M, Samuelsson M, et al. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567-2574.
39 Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059-1064.
40 Kase CS, Robinson RK, Stein RW, DeWitt LD, Hier DB, Harp DL, et al. Anticoagulant-related intracerebral hemorrhage. Neurology. 1985;35:943-948.
41 Franke CL, de Jonge J, van Swieten JC, Op de Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726-730.
42 Neau JP, Couderq C, Ingrand P, Blanchon P, Gil R. Intracranial hemorrhage and oral anticoagulant treatment. Cerebrovasc Dis. 2001;11:195-200.
43 Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880-884.
44 Lee SB, Manno EM, Layton KF, Wijdicks EF. Progression of warfarin-associated intracerebral hemorrhage after INR normalization with FFP. Neurology. 2006;67:1272-1274.
45 Rabinstein AA, Wijdicks EF. Determinants of outcome in anticoagulation-associated cerebral hematoma requiring emergency evacuation. Arch Neurol. 2007;64:203-206.
46 Eyster ME, Gill FM, Blatt PM, Hilgartner MW, Ballard JO, Kinney TR. Central nervous system bleeding in hemophiliacs. Blood. 1978;51:1179-1188.
47 Groch SN, Sayre GP, Heck FJ. Cerebral hemorrhage in leukemia. Arch Neurol. 1960;2:439-451.
48 McEvoy AW, Kitchen ND, Thomas DG. Intracerebral haemorrhage and drug abuse in young adults. Br J Neurosurg. 2000;14:449-454.
49 Selmi F, Davies KG, Sharma RR, Neal JW. Intracerebral haemorrhage due to amphetamine abuse: report of two cases with underlying arteriovenous malformations. Br J Neurosurg. 1995;9:93-96.
50 Mangiardi JR, Daras M, Geller ME, Weitzner I, Tuchman AJ. Cocaine-related intracranial hemorrhage. Report of nine cases and review. Acta Neurol Scand. 1988;77:177-180.
51 Hiller F. Cerebral hemorrhage in hyperergic angiitis. J Neuropathol Exp Neurol. 1953;12:24-40.
52 Johnson RT, Richardson EP. The neurological manifestations of systemic lupus erythematosus. Medicine (Baltimore). 1968;47:337-369.
53 Salvarani C, Brown RDJr, Calamia KT, Christianson TJ, Weigand SD, Miller DV, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol. 2007;62:442-451.
54 Cloft HJ, Phillips CD, Dix JE, McNulty BC, Zagardo MT, Kallmes DF. Correlation of angiography and MR imaging in cerebral vasculitis. Acta Radiol. 1999;40:83-87.
55 Kadkhodayan Y, Alreshaid A, Moran CJ, Cross DTIII, Powers WJ, Derdeyn CP. Primary angiitis of the central nervous system at conventional angiography. Radiology. 2004;233:878-882.
56 Appenzeller S, Zeller CB, Annichino-Bizzachi JM, Costallat LT, Deus-Silva L, Voetsch B, et al. Cerebral venous thrombosis: influence of risk factors and imaging findings on prognosis. Clin Neurol Neurosurg. 2005;107:371-378.
57 Wasay M, Azeemuddin M. Neuroimaging of cerebral venous thrombosis. J Neuroimaging. 2005;15:118-128.
58 Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791-1798.
59 Stam J, De Bruijn SF, DeVeber G. Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst Rev. 2002. CD002005.
60 Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664-670.
61 Mehraein S, Schmidtke K, Villringer A, Valdueza JM, Masuhr F. Heparin treatment in cerebral sinus and venous thrombosis: patients at risk of fatal outcome. Cerebrovasc Dis. 2003;15:17-21.
62 Chaloupka JC, Mangla S, Huddle DC. Use of mechanical thrombolysis via microballoon percutaneous transluminal angioplasty for the treatment of acute dural sinus thrombosis: case presentation and technical report. Neurosurgery. 1999;45:650-656.
63 Wasay M, Bakshi R, Kojan S, Bobustuc G, Dubey N, Unwin DH. Nonrandomized comparison of local urokinase thrombolysis versus systemic heparin anticoagulation for superior sagittal sinus thrombosis. Stroke. 2001;32:2310-2317.
64 Keller E, Pangalu A, Fandino J, Konu D, Yonekawa Y. Decompressive craniectomy in severe cerebral venous and dural sinus thrombosis. Acta Neurochir Suppl. 2005;94:177-183.
65 Brown RDJr, Wiebers DO, Torner JC, O’Fallon WM. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted Country, Minnesota. J Neurosurg. 1996;85:29-32.
66 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
67 Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068.
68 Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
69 Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. 1999;10:411-417.
70 Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
71 Oertel M, Kelly DF, McArthur D, Boscardin WJ, Glenn TC, Lee JH, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109-116.
72 Servadei F, Nanni A, Nasi MT, Zappi D, Vergoni G, Giuliani G, et al. Evolving brain lesions in the first 12 hours after head injury: analysis of 37 comatose patients. Neurosurgery. 1995;37:899-906.
73 Brown CV, Weng J, Oh D, Salim A, Kasotakis G, Demetriades D, et al. Does routine serial computed tomography of the head influence management of traumatic brain injury? A prospective evaluation. J Trauma. 2004;57:939-943.
74 Becker DP, Miller JD, Ward JD, Greenberg RP, Young HF, Sakalas R. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg. 1977;47:491-502.