8 Secondary Glaucoma
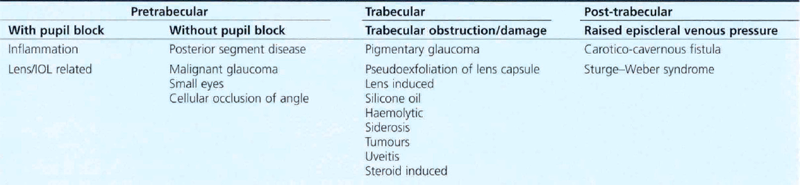
PRETRABECULAR OUTFLOW OBSTRUCTION
SECONDARY ANGLE CLOSURE WITH PUPIL BLOCK
Possible reasons for pupil block are:
1. Posterior synechiae from inflammation in the anterior segment
2. Occlusion of the pupil in pseudophakic and aphakic eyes by the implant or vitreous gel
Inflammation in the anterior segment
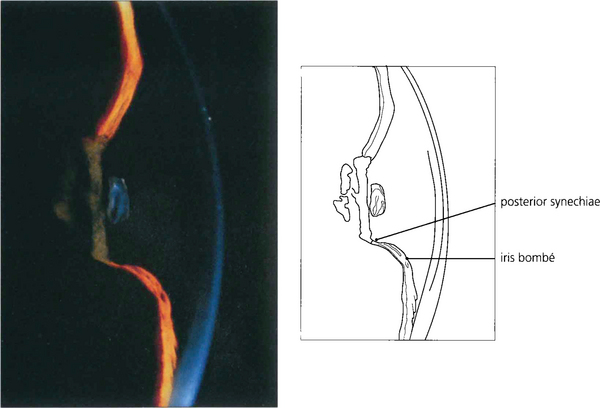
Fig. 8.1 In this patient with uveitis pupil block due to seclusion of the pupil is evident from the forward convexity of the iris resulting in a shallow peripheral anterior chamber, whereas the central chamber remains deep. The deep central anterior chamber distinguishes pupil block from malignant glaucoma with forward movement of the iris–lens diaphragm secondary to posterior segment pathology or pupil block from intumescent cataract.
Pupil block in pseudophakic and aphakic eyes
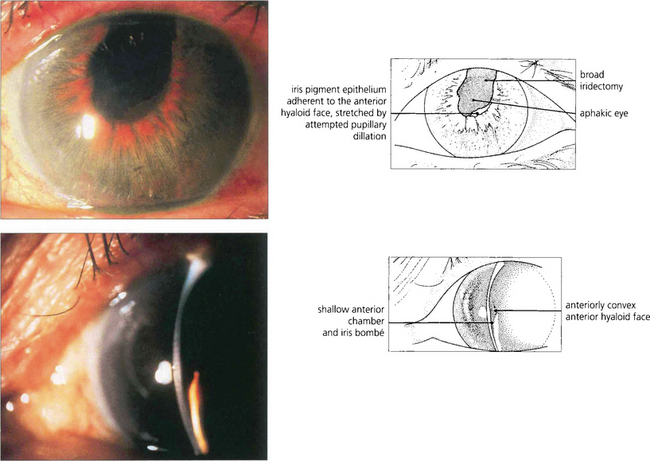
Fig. 8.3 In this patient there is pupil block from posterior synechiae to the vitreous face in an aphakic eye. The slit-lamp image shows peripheral shallowing of the anterior chamber. The extent to which the gel prolapses into the anterior chamber determines the central depth.
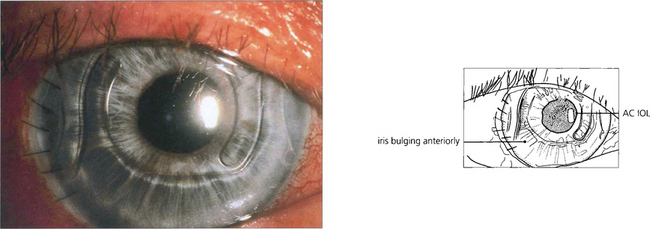
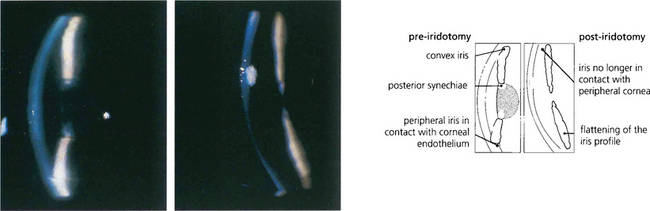
Fig. 8.4 Anterior chamber intraocular lens implantation without peripheral iridectomy is likely to cause pupil block. In this case the iris can be seen bulging forwards around the implant. Although the angle can often be reopened by a laser iridotomy this readily reoccludes with vitreous and so does not reliably prevent recurrence. Surgical iridectomy is definitive but difficult to achieve through a corneal phacoemulsification wound unless it is placed temporally. Temporal iridectomies may cause glare or monocular diplopia, however, and so surgical entry by a separate superior corneal incision is preferable.
Forward movement of the anterior lens surface
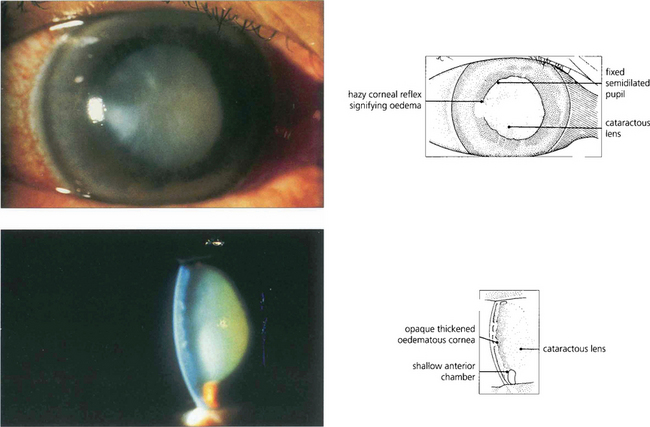
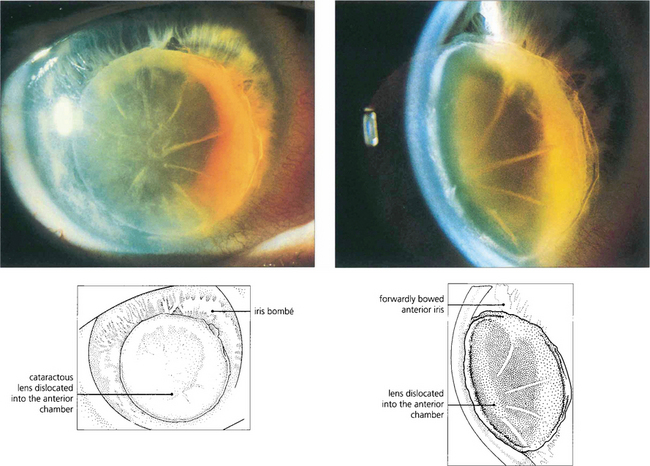
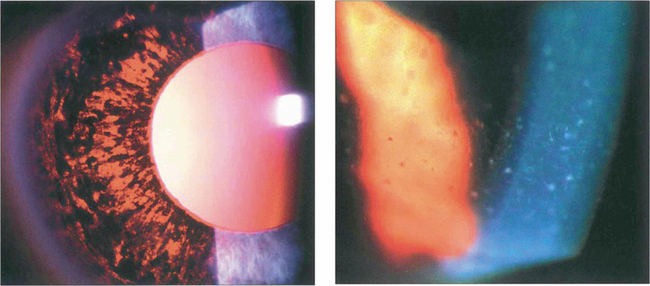
Fig. 8.6 Acute angle closure due to an intumescent cataractous lens. The eye is red with a hazy view of the anterior segment from corneal oedema, with a fixed irregular semidilated pupil from iris infarction. The slit image shows the corneal oedema and a very shallow anterior chamber. Some uveitis may be present because of ischaemia, and this must be differentiated from the larger accumulations of lens material and macrophages seen with phacolytic glaucoma (see Fig. 8.49).
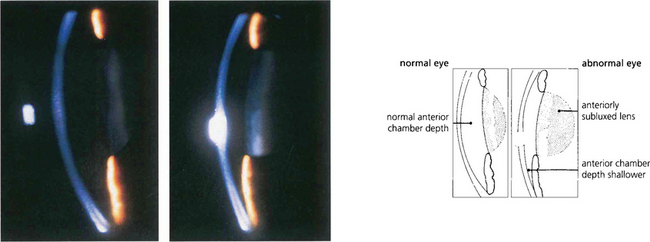
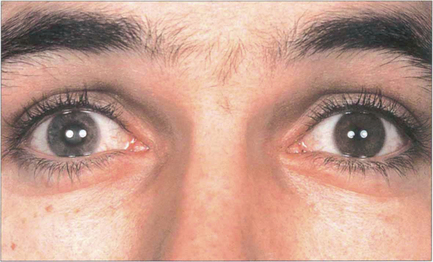
Fig. 8.7 Slit-image photography of the left and right eye of this patient demonstrates a subluxed lens in the left eye. The anterior chamber is shallow and further examination shows that the anterior lens surface is closer to the posterior corneal surface inferiorly than superiorly occluding the pupil and producing pupillary block.
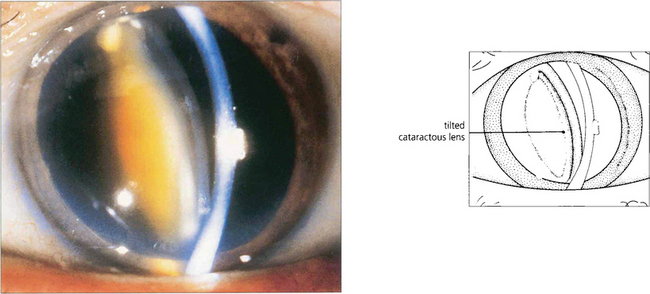
Fig. 8.8 Occasionally pupillary dilatation allows a subluxed lens to swing into the pupillary plane and block communication between the posterior and anterior chamber. Careful positioning of the patient and the use of miotics usually allow the lens to be repositioned safely until definitive treatment can be arranged which in this case is removal of the lens.
SECONDARY ANGLE CLOSURE WITHOUT PUPIL BLOCK
Angle closure may occur without pupil block in four ways:
1. Changes in the posterior segment that push the lens–iris diaphragm forwards
2. Changes in the anterior segment that result in loss of the anterior chamber and iris–trabecular contact with synechial closure
4. Cellular proliferation within the angle of the anterior chamber resulting in iris–trabecular adhesions.
Angle closure from changes in the posterior segment
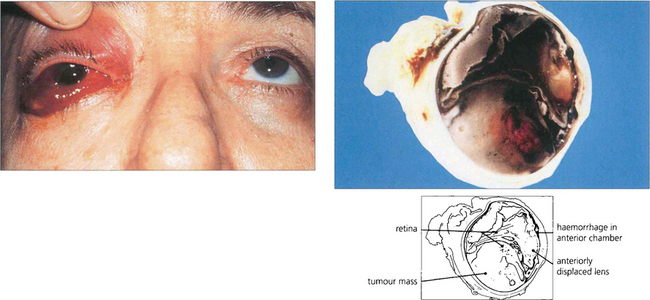
Fig. 8.10 A patient with a long-standing blind eye presented with recent onset of pain, conjunctival oedema and anterior chamber haemorrhage. Posterior segment ultrasonographic examination showed a large choroidal melanoma. Hemisection of the enucleated eye demonstrates a large haemorrhagic choroidal melanoma together with an anteriorly displaced lens and loss of the anterior chamber.
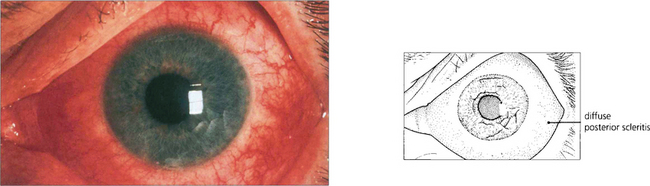
Fig. 8.11 Posterior scleritis may be associated with an annular choroidal effusion that causes the ciliary body to rotate forward about the scleral spur and the iris–lens diaphragm to move forward to produce angle-closure glaucoma. Ciliary body detachment may result in a normal or low IOP, even in the presence of angle closure. This photograph shows a red eye with diffuse anterior and posterior scleritis, although many of these eyes are completely white with inflammation limited to the posterior sclera alone.
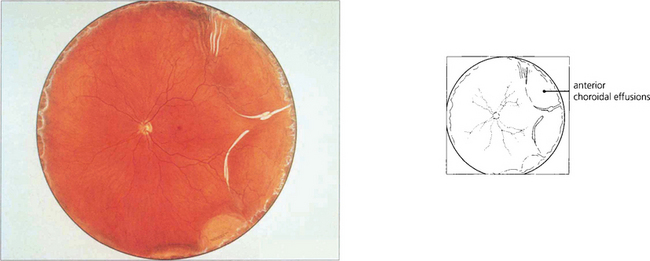
Fig. 8.12 A fundus painting of the same patient reveals an annular choroidal effusion (see also Ch. 5).
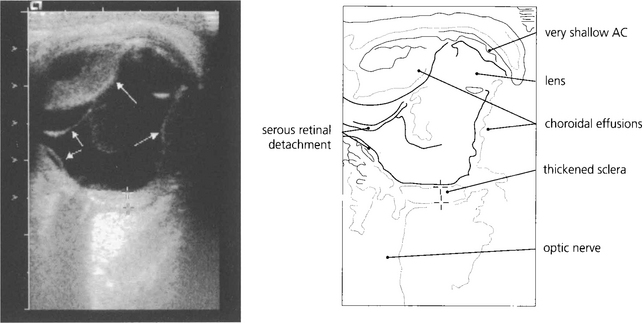
Fig. 8.13 In less obvious cases than the case illustrated, such effusions are easily missed unless the peripheral fundus is inspected carefully under full mydriasis. B-scan ultrasonography is very useful.
By courtesy of Ms M Restori.
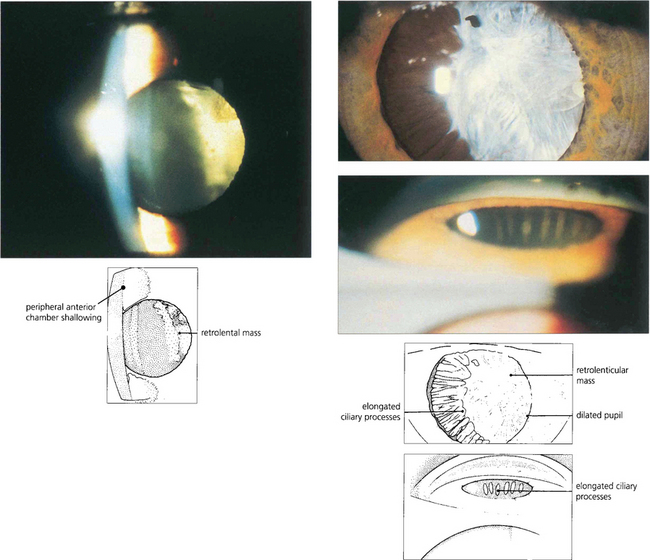
Fig. 8.14 Persistent hyperplastic primary vitreous can produce a contracting fibrotic retrolental mass with forward rotation of the ciliary body and lens–iris diaphragm pushing the iris forwards to occlude the angle. The anterior chamber is often shallow in these eyes making angle occlusion more likely. (Top left) This slit-image photograph shows a shallow anterior chamber and retrolental mass. (Top right) Following mydriasis elongated ciliary processes can be seen being pulled into the mass. (Bottom right) Gonioscopy demonstrates traction and elongation of the ciliary processes.
Malignant glaucoma (ciliolenticular block or aqueous misdirection syndrome)
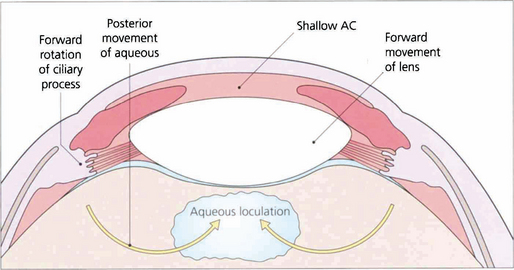
Fig. 8.15 This diagram illustrates how aqueous passes posteriorly and then pushes the lens–iris diaphragm forwards.

Fig. 8.16 These two slit-lamp photographs of the anterior segments of each eye of a patient demonstrate a shallow anterior chamber in the right eye (left), whereas the left eye (right) has virtually no anterior chamber. The left eye had recently undergone a trabeculectomy followed by loss of the anterior chamber from malignant glaucoma. It is worth using topical atropine in higher-risk eyes at the end of filtration surgery to prevent this.
Synechial closure of the angle
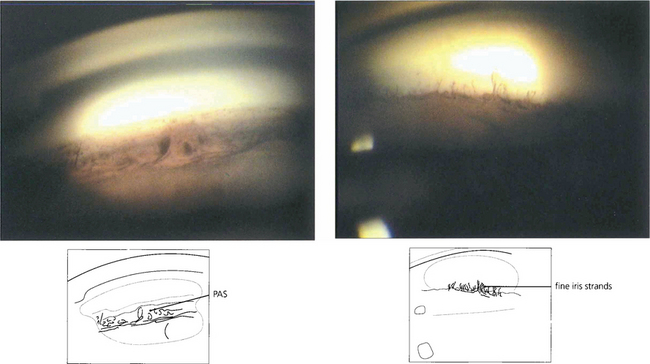
Fig. 8.17 PAS (left) should be distinguished from fine strands from the anterior iris surface to the trabecular meshwork which may be seen in normal eyes (right).
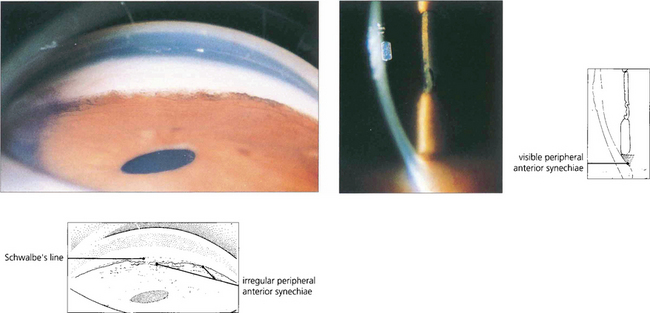
Fig. 8.18 Slit-image and gonioscopic photographs demonstrate the development of PAS in chronic uveitis. Although often confined to the inferior angle such synechiae can extend circumferentially. With sarcoidosis, trabecular granulomas occasionally form as focal lesions around the circumference of the angle and, if untreated, may produce small areas of PAS (see Ch 10).
Small eyes
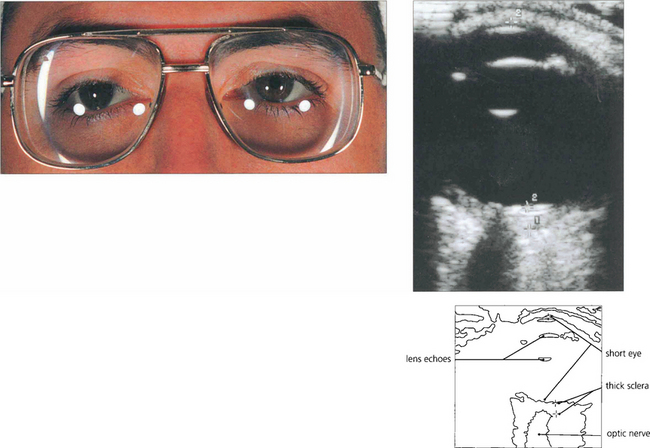
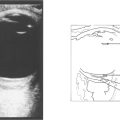
Fig. 8.20 Nanophthalmic eyes have an axial length of less than 20 mm, a small corneal diameter and a normal-sized crystalline lens that is disproportionally large in a small eye. High hyperopia is the norm. They are predisposed to angle closure glaucoma in early to middle age because of crowding of the angle. In this patient B-scan ultrasonography confirms that the axial length is short (15.25 mm) and posterior sclera thicker than average (1.94 mm). Congenital syphilis and mucopolysaccharidoses also predispose to small eyes and increased risk of angle closure glaucoma.
By courtesy of Ms M Restori.
Cellular proliferation with angle closure
• neovascularization (e.g. rubeotic glaucoma)
• endothelium (e.g. iridocorneal endothelial (ICE) syndromes)
• epithelium (e.g. epithelialization of the anterior chamber).
Neovascularization (rubeosis) of the iris and angle with accompanying fibrous tissue formation is the most common type of cellular proliferation causing angle occlusion. It is seen most frequently in diabetic patients or in eyes with an ischaemic central retinal vein occlusion (see Ch. 14). Iris neovascularization may occur less commonly with uveitis, ocular arterial insufficiency, long-standing retinal detachment, intraocular tumours or radiation retinopathy. In all of these conditions the stimulus for neovascularization appears to be retinal hypoxia with the release of vascular endothelial growth factor (VEGF) which stimulates new vessel growth on the iris and in the angle. New vessels appear initially around the pupil margin and in the angle. Contraction of the fibrovascular tissue on the anterior iris surface produces the fixed dilated pupil with ectropion uveae seen in the late stages of the condition. New vessels in the angle are usually followed by PAS formation, outflow obstruction and raised IOP follow.
Rubeotic glaucoma
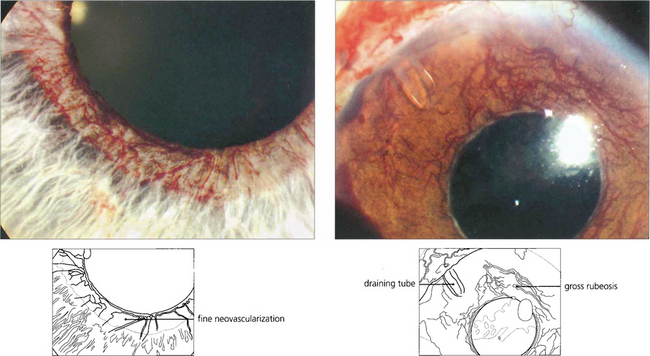
Fig. 8.21 In the early stages (left), neovascularization is seen around the pupil margin as a fine vascular network that does not have a normal anatomical distribution. In an advanced case (right) there is florid neovascularization, corneal oedema and aqueous flare. Pain in these eyes is often due to a combination of inflammation and raised IOP; both can be treated to some degree by topical mydriatics and steroids. Trabeculectomy is rarely successful in such eyes but enucleation may often be avoided by transscleral diode cyclophotocoagulation (cyclodiode) or implantation of an aqueous shunt.
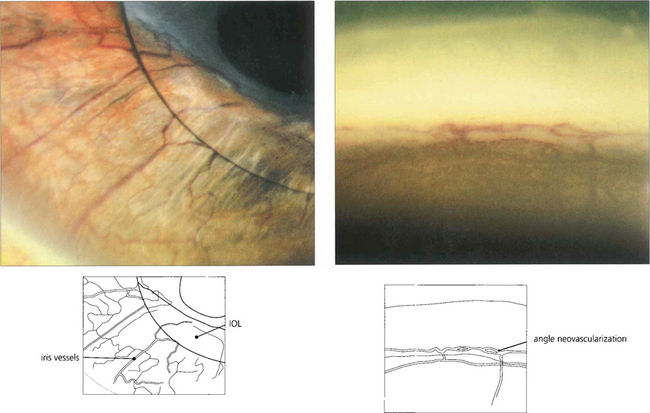
Fig. 8.22 Differentiating neovascularization from dilated iris vessels can sometimes be difficult. (Left) Radial iris vessels might be confused with normal vessels, but gonioscopic examination reveals typical circumferential neovascularization in the angle (right).
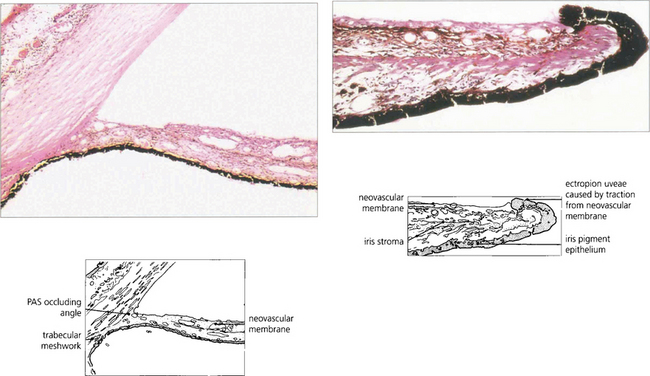
Fig. 8.23 Histological examination of the iris in neovascular glaucoma demonstrates a neovascular membrane on the anterior iris surface pulling the iris pigment epithelium on to the anterior surface (ectropion uveae). The angle is occluded by a neovascular membrane with endothelial slide from the corneal surface creating angle closure.
The iridocorneal endothelial (ICE) syndromes
Essential iris atrophy, Chandler’s syndrome and the iris–naevus (Cogan–Reese) syndrome all result from a primary disorder of the corneal endothelium and are different morphological aspects of the same disease process. ICE syndrome is unilateral and sporadic; it occurs in early adulthood and is more common in women. The basic defect appears to be a loss of cell contact inhibition with corneal endothelial cell proliferation and formation of a Desçemet’s-like membrane spreading across the angle and on to the anterior iris surface (see Ch. 6). A demarcation line may be seen on the cornea between normal and abnormal endothelium. Herpes simplex infection has been implicated in the aetiology but antiviral therapy does not appear to influence the disease process.
Chandler’s syndrome is characterized by corneal endothelial cell decompensation and glaucoma producing oedema and early presentation with haloes. The degree of corneal oedema is usually disproportionate to the level of IOP although it is often improved by reducing the IOP. The endothelium that has been completely replaced assumes a ‘hammered silver’ appearance (see Ch. 6). PAS and corectopia (eccentric pupil) are not major features. The increase in IOP does not necessarily correlate with the degree of synechial closure as an open angle may still be occluded by a subclinical ICE membrane.
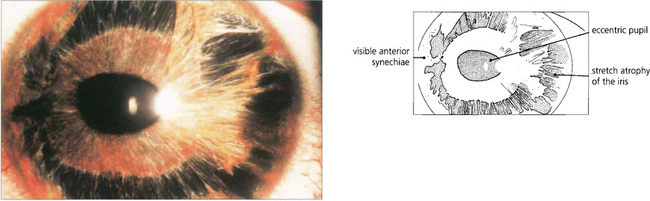
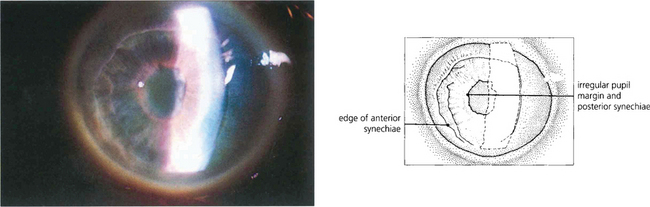
Fig. 8.24 ‘Essential iris atrophy’ is characterized by unilateral glaucoma, corectopia (eccentric pupil), pseudopolycoria and PAS. Migration of endothelium on to the iris produces chronic traction at that point and stretching and eventual hole formation on the opposite side of the iris (pseudopolycoria).
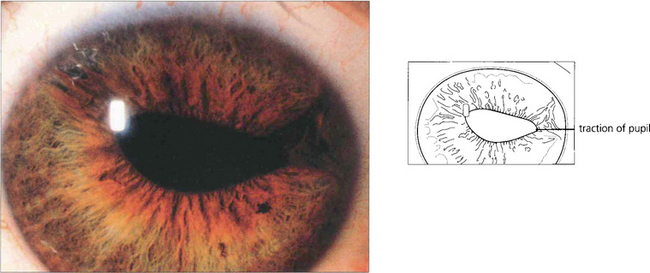
Fig. 8.25 In this patient the prominent feature is the pupillary distortion by an ICE membrane. Less obvious is the iris stromal thinning in the opposite quadrant.
By courtesy of Dr A J W Huang.
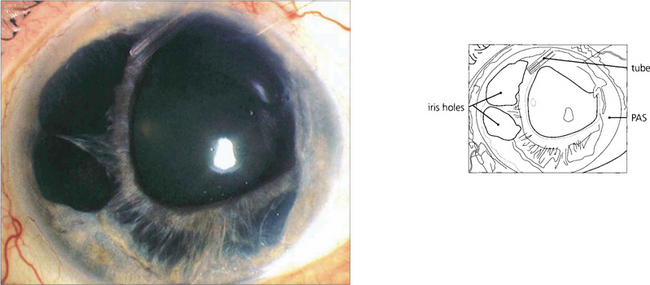
Fig. 8.26 This left eye with more advanced disease and intractable glaucoma has had a drainage device implanted. There is marked nasal pseudopolycoria due to the ICE membrane drawing the iris temporally.
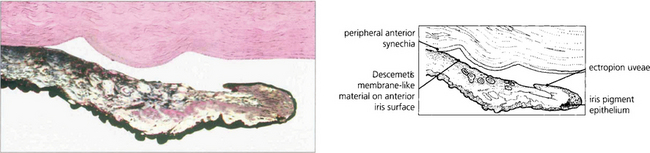
Fig. 8.27 Histological appearance shows a surface membrane on the iris stroma with ectropion uveae and angle closure. The glaucoma is usually much worse than would be anticipated by the gonioscopic appearances, reflecting subclinical changes in the angle elsewhere.
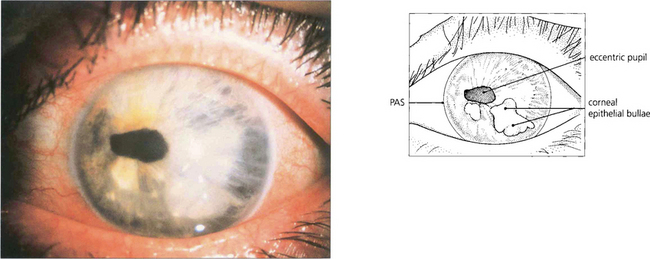
Fig. 8.28 This patient with Chandler’s syndrome shows a bullous keratopathy (a prominent feature of the syndrome) together with corectopia and iris atrophy (both of which are less marked than in essential iris atrophy). PAS tend to be less extensive than in essential iris atrophy although the IOP may still be raised.
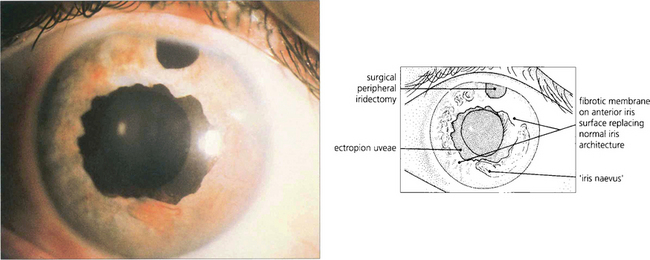
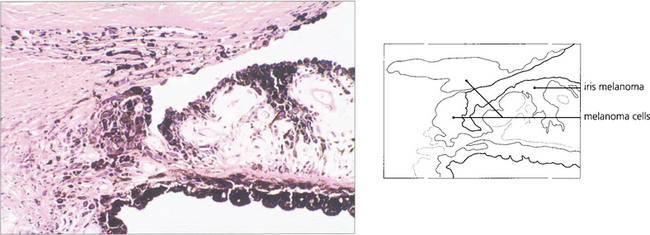
Fig. 8.29 In the iris–naevus syndrome, iris atrophy is less obvious than in the typical case of essential iris atrophy but there is more corneal oedema. The anterior surface of the iris is covered by a sheet of Desçemet’s membrane-like material through which normal nodules of iris tissue protrude. These have been mistaken for iris melanoma resulting in enucleation. Ectropion uveae is common. In this photograph note the ectropion uveae together with loss of the usual appearance of the anterior iris stroma.
Epithelialization of the anterior chamber
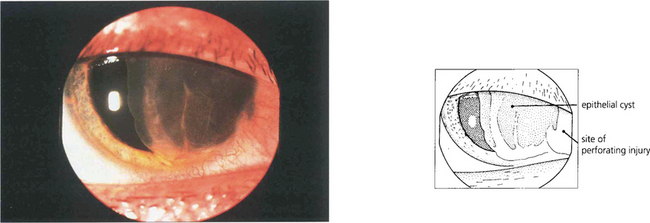
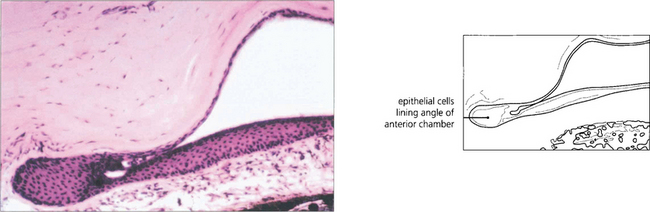
Fig. 8.30 In this example a large epithelial anterior chamber cyst has developed after a traumatic perforation at the limbus. Surgical excision of this type of cyst carries a high risk of converting a self-limiting cyst to a more malign sheet-like ingrowth.
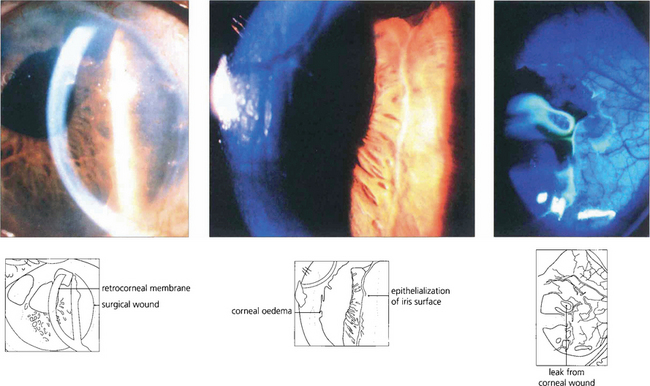
Fig. 8.31 In this patient sheet-like epithelial downgrowth has occurred after a secondary intraocular lens implantation to produce a retrocorneal membrane (left) and a membrane on the anterior iris surface (centre). A positive Seidal test is seen at the limbus (right).
By courtesy of Dr W W Culbertson, Bascom Palmer Eye Institute, University of Miami School of Medicine.
TRABECULAR OUTFLOW OBSTRUCTION: SECONDARY OPEN ANGLE GLAUCOMA
Trabecular meshwork function may be reduced by:
ANGLE INJURY
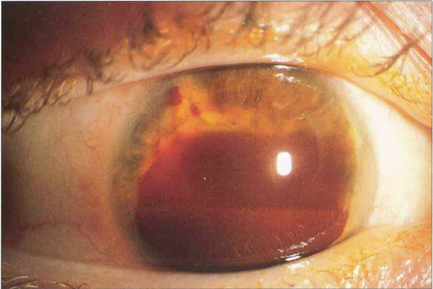
Fig. 8.33 Traumatic hyphema seldom results in significant IOP increase in Caucasian eyes but IOP can rise dramatically in black patients with sickle cell trait as the erythrocytes sickle in the anterior chamber due to metabolic acidosis and clear very slowly. It is worth remembering that angle recession is common in these patients who may later develop secondary open-angle glaucoma.
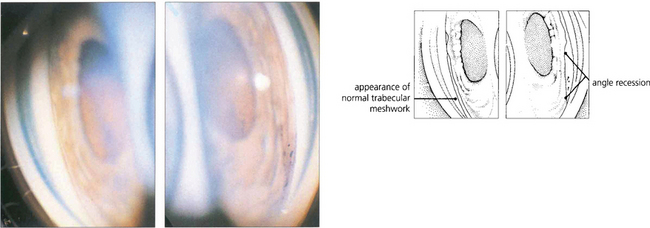
Fig. 8.34 Gonioscopy in angle recession can demonstrate widening of the ciliary band (iris root) or occasionally, following a complete tear of ciliary muscle, widening of the angle together with exposure of a white strip of sclera. These goniophotographs show the angle in both the normal (left) and the involved (right) eye.
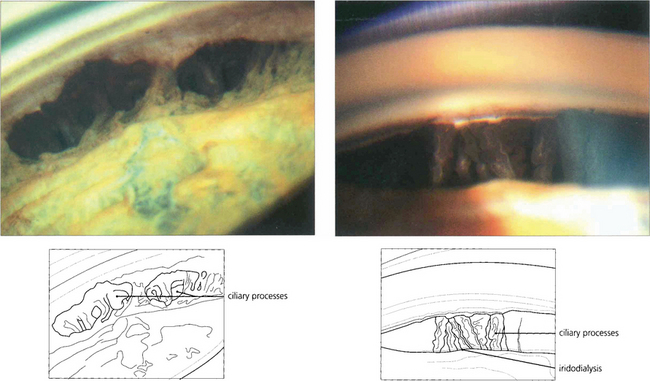
Fig. 8.35 (Left) Angle recession is combined with full-thickness tears in the peripheral iris, through which the ciliary body is seen. (Right) A segment of iris is completely separated from its root resulting in an iridodialysis and exposure of the ciliary processes.
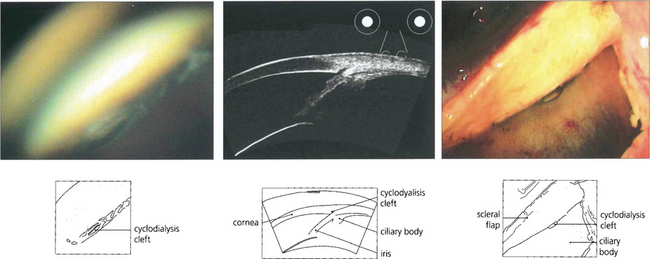
Fig. 8.36 If the ciliary band (and hence ciliary body) detaches from sclera, a pathway (cyclodialysis cleft) is opened for aqueous to flow from the anterior chamber to the suprachoroidal space. (Left) This results in chronic hypotony, often with maculopathy, and loss of vision. Spontaneous closure of a cleft is not infrequent and if this occurs IOP may then rise precipitously. (Middle) Cyclodialysis clefts can sometimes be difficult to see; gonioscopy and high resolution ultrasonic biomicroscopy is extremely useful in identifying their presence.
By courtesy of Prof D Reinstein. Chronic hypotony requires surgical closure of the cleft. (Right) Cyclodialysis cleft viewed during surgery from the suprachoroidal space.
TRABECULAR MESHWORK OCCLUSION BY CELLS OR OTHER MATERIAL
Pigmentary glaucoma
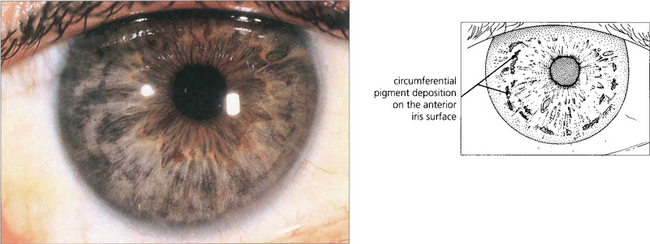
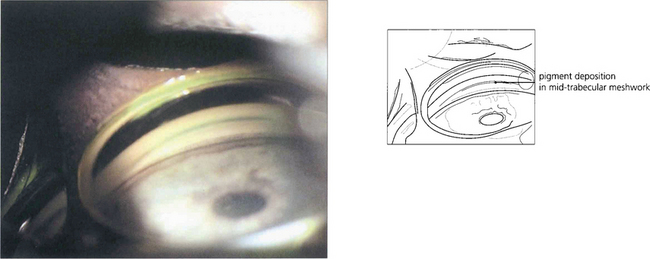
Fig. 8.37 In this patient extensive pigment deposition rather like dust particles, is seen on the anterior iris surface lying in the wrinkles formed by iris contraction.
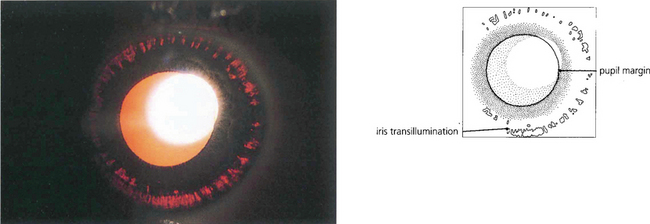
Fig. 8.38 Retroillumination of the iris of the patient in Fig. 8.37 shows multiple slit-like defects in the pigment epithelium of the peripheral iris. Care must be taken in diagnosing pigmentary glaucoma as a number of other conditions also cause peripheral iris transillumination defects.
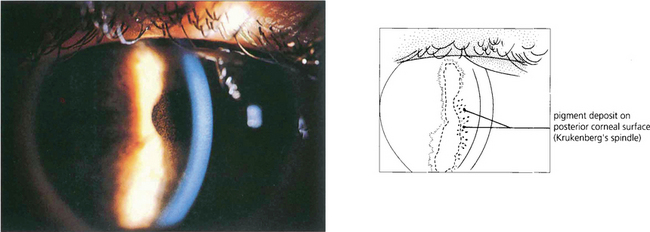
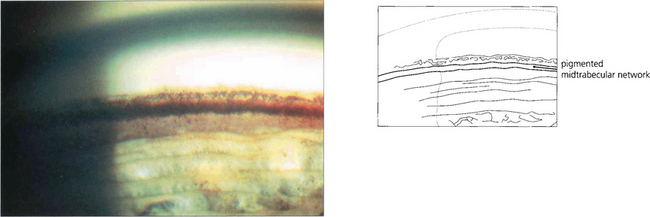
Fig. 8.39 Krukenberg’s spindle is the term given to pigment deposition on the posterior corneal surface. The pigment is usually deposited in a vertical band.
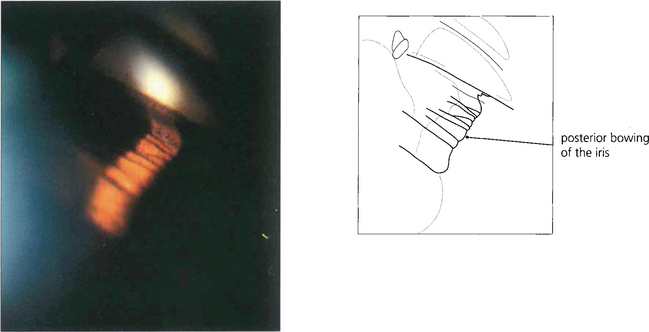
Fig. 8.41 The likely mechanism of pigmentary dispersion is abrasion of the peripheral iris pigment epithelium by the anterior zonules which come into contact during episodes of reverse pupil block induced by accommodation in some young myopes. This results in backward bowing of the peripheral iris.
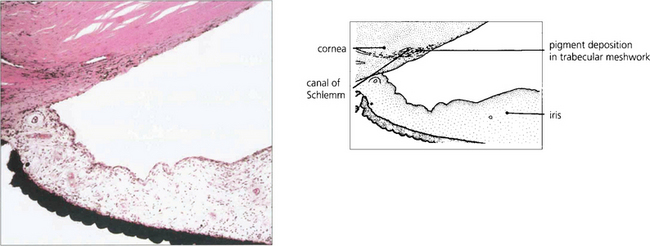
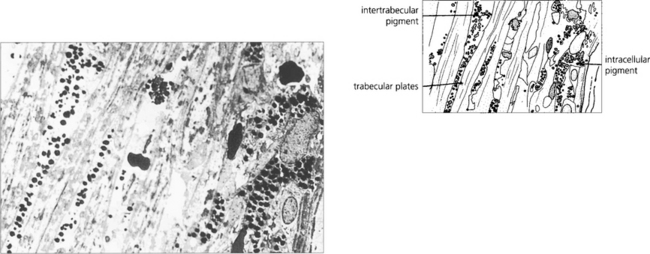
Fig. 8.42 Histological section showing extensive pigment accumulation in the angle. Pigment granules lie freely between the trabecular plates and are phagocytosed within the endothelium or by macrophages. Loss of trabecular endothelial cells results with collapse of normal trabecular meshwork architecture.
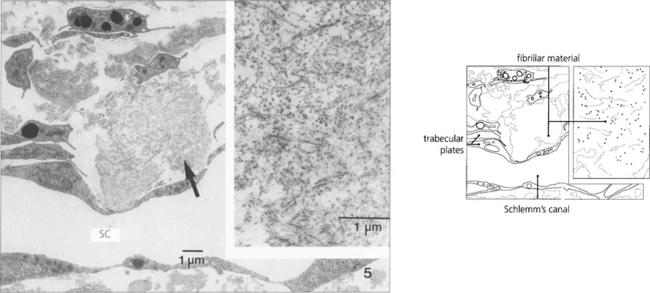
Pseudoexfoliation glaucoma (exfoliation syndrome)
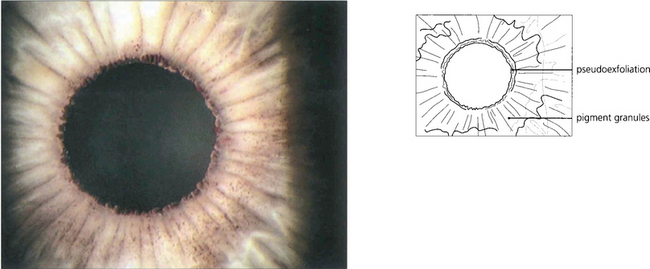
Fig. 8.44 In pseudoexfoliation, exfoliated material abraded by the iris from the anterior lens surface collects on the pupil margin where it looks like dandruff. Pigment is also rubbed off the posterior surface of the peripupillary iris and is deposited on the anterior iris surface and in the iridocorneal angle. This eye also shows widespread pigment deposition on the anterior iris surface.
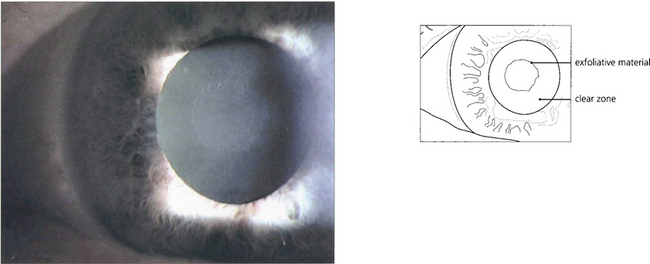
Fig. 8.45 Pupillary abrasion creates a characteristic zone free of pseudoexfoliative material. With further mydriasis the abnormal material can be seen on the anterior lens surface.
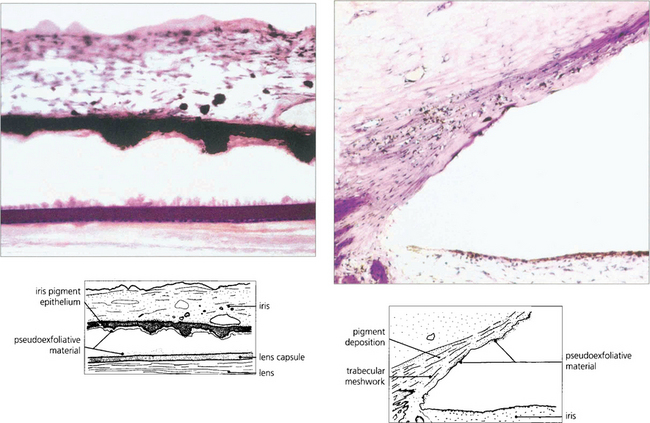
Fig. 8.47 Histological appearance shows eosinophilic pseudoexfoliative material on the anterior lens capsule and posterior surface of the iris. The angle contains more pigment than usual together with the fibrillar material caught up in the meshwork. This material can also be found histologically around the conjunctival vessels.
Lens-induced glaucoma (phacolytic glaucoma)
The lens capsule of a hypermature cataract may leak denatured cortical material. This is particularly common with Morgagnian cataracts which should be removed to forestall this complication (see Ch. 11). Leakage in turn excites a macrophage response; the macrophages, gorged with lens material accumulate and clog the trabecular meshwork causing a secondary open-angle glaucoma known as phacolytic glaucoma. Much more common today is ‘lens fragment-related glaucoma’ in which retained fragments of lens nucleus after cataract surgery cause increased IOP after surgery. Retained fragments should be carefully looked for in these eyes with gonioscopy especially in the inferior angle where they may be missed and offending lens matter should be removed.
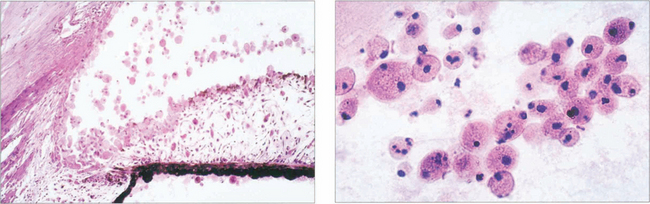
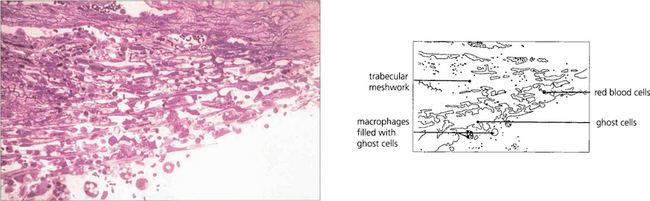
Fig. 8.49 Histological examination demonstrates lens-filled macrophages obstructing the angle and trabecular meshwork. Low magnification (left) and higher magnification (right) of lens-filled macrophages. Clinically these are seen as large accumulations floating in the anterior chamber of an eye with a mature cataractous lens and acute glaucoma, an open angle and a deep anterior chamber.
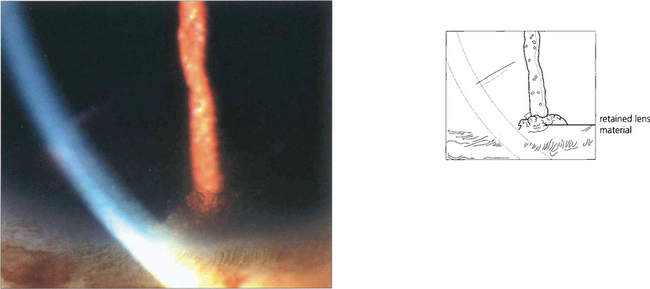
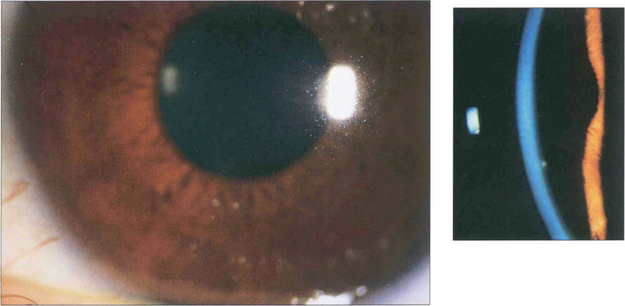
Fig. 8.50 Retained fragments of lens nucleus or cortex from complicated cataract surgery can cause an intractable increase in IOP and recurrent inflammation. Here, a fragment of cortex is visible in the inferior angle, even without gonioscopy. Removal of retained cortex usually improves control of both the IOP and intraocular inflammation.
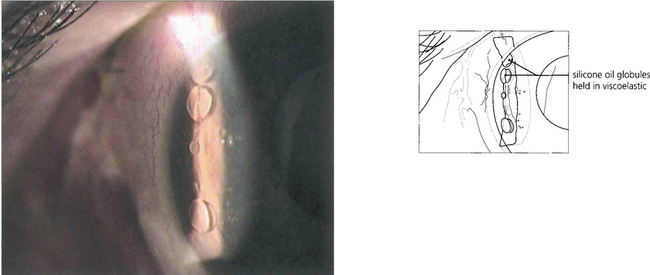
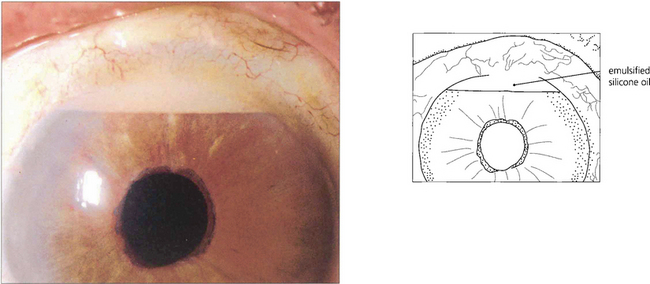
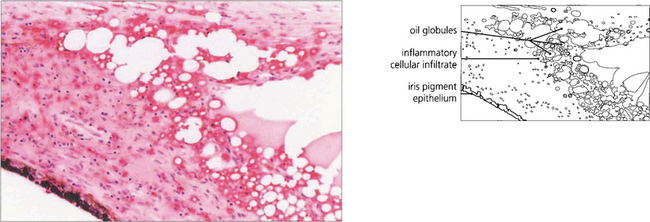
Fig. 8.51 This anterior chamber contains both viscoelastic and silicone oil after combined cataract and vitreoretinal surgery. In the upright position the silicone oil is prevented from rising to the top of the anterior chamber by the retained viscoelastic. Here, it is retained viscoelastic that is causing the increased IOP after vitreoretinal surgery; its removal by anterior chamber washout should improve IOP control.
Siderosis
Tumour infiltration
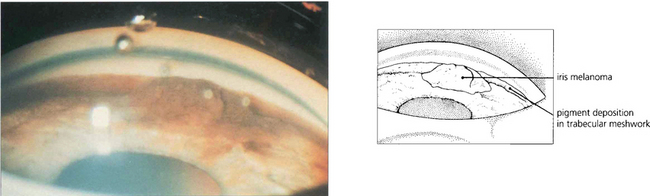
About 5 per cent of eyes with tumours develop glaucoma either as a result of angle closure from forward movement of the lens–iris diaphragm (see Fig. 8.10), neovascularization or invasion of the angle by tumour causing secondary open-angle glaucoma. Less commonly, a malignant melanoma arising from the iris root and ciliary body may invade the angle directly to produce glaucoma. On very rare occasions ‘melanomalytic’ glaucoma arises from trabecular obstruction by macrophages engorged with melanin from a necrotic tumour.
UVEITIC GLAUCOMA
Uveitis has already been seen as a cause of pupil block (see Fig. 8.1). In addition it may cause chronic angle-closure glaucoma from PAS (see Figs 8.17 and 8.18). Outflow facility may also be reduced in uveitic eyes as a result of obstruction of an open angle by inflammatory cellular debris or direct involvement of the trabecular meshwork in the inflammatory process (trabeculitis). Two specific uveitic syndromes, the Posner–Schlossman syndrome and Fuchs’ heterochromic cyclitis, are also particularly associated with open-angle glaucoma. Finally, topical steroid treatment may induce an open-angle glaucoma. Table 8.2 lists the mechanisms of IOP disturbance in patients with uveitis.
Fuchs’ heterochromic cyclitis (see also Ch. 10)
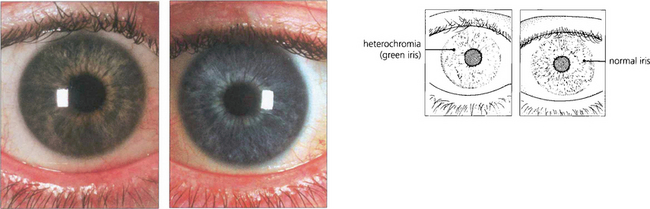
This chronic nongranulomatous uveitis is almost always unilateral. Patients usually present complaining of floaters but later vision is reduced as a result of cataract. Iris atrophy (the affected iris has anterior stromal atrophy and a lighter colour) and iris nodules, fine widespread keratic precipitates, cataract, glaucoma and occasionally a fine neovascularization of the angle are all seen in established cases (see Ch. 10). Neither anterior nor posterior synechiae develop. The glaucoma responds to conventional medical or surgical treatment and the eye responds well to cataract surgery should it be indicated. The uveitis responds poorly to steroids.
Steroid-induced glaucoma
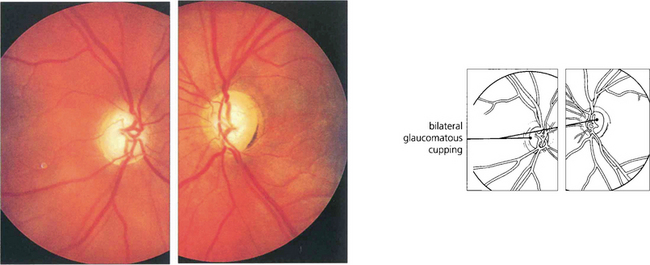
Fig. 8.62 Glaucomatous cupping, more advanced on the left than in the right, is seen in this patient who had used topical steroids without supervision for treatment of mild ocular irritation associated with contact lens wear.
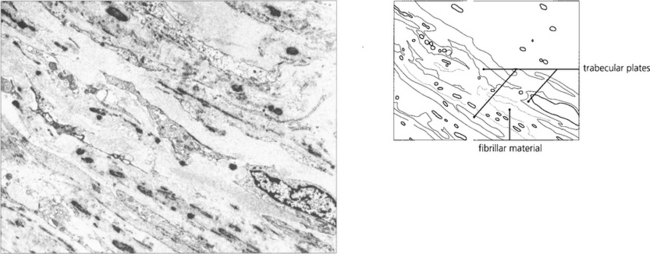
Fig. 8.63 Electron micrograph of the corneal scleral meshwork in a patient with steroid-induced glaucoma showing the intertrabecular spaces filled with fibrillar material. In some areas the trabecular lamellae are not completely covered with endothelial cells.
Courtesy of Professor E Lutjen-Drecoll.
POST-TRABECULAR OUTFLOW OBSTRUCTION: RAISED EPISCLERAL VENOUS PRESSURE
With fistulae between the carotid artery and cavernous sinus where the shunt is usually of high flow, the diagnosis is sometimes obvious from the dramatic neuro-ophthalmic signs (see Ch. 20). However, coexistent ocular ischaemia complicates this picture and even in the presence of rubeosis iridis (which is not infrequently present in these eyes) the IOP may be low. In contrast, arteriovenous communications within the dural vessels are frequently of a low-flow type; these patients present with red eyes, arterialized conjunctival vessels and glaucoma without the other overt signs of bruits or proptosis. Apart from glaucoma, these dural shunts usually have a benign prognosis and may resolve spontaneously. The glaucoma may vary in severity; patients with high IOP respond poorly to medical treatment.
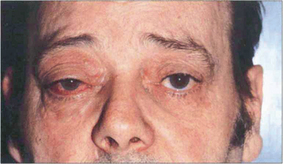
Fig. 8.64 This patient presented with a red right eye with raised IOP. A helpful diagnostic sign of a caroticocavenous fistula is an increased pulse amplitude due to the A–V shunt which is observed when performing applanation tonometry.
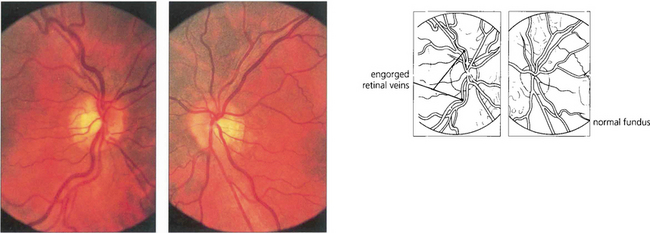
Fig. 8.66 Retinal veins are engorged in the right eye although at this stage the optic disc does not show glaucomatous changes. The IOP in the left eye had an increased pulse amplitude. The patient was found to have a dural caroticocavernous fistula that was treated by embolization.
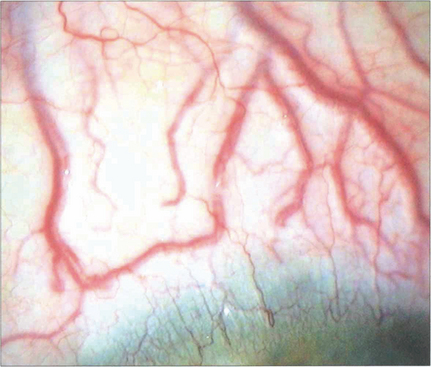
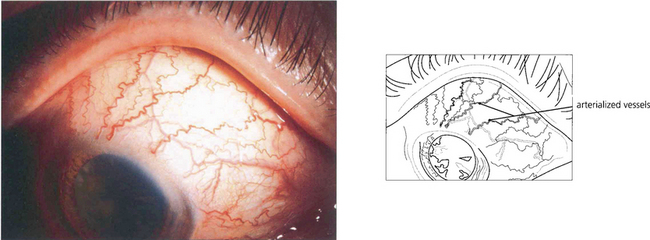
Fig. 8.67 Grossly arterialized vessels are seen in another patient with a long-standing dural fistula.
STURGE–WEBER SYNDROME
The Sturge–Weber syndrome presents with facial and intracranial angiomas of variable degree. Glaucoma occurs in at least 30 per cent of patients and possibly as high as 70 per cent. It is said to be more common when the upper lid is involved. About 50 per cent of patients present with buphthalmos within the first 2 years of life but there is a lifelong risk of glaucoma. Unilateral or marked asymmetrical ocular involvement is typical. The condition responds poorly to medical treatment and surgery for buphthalmos is commonly required in infancy, childhood or early adult life. Filtration surgery may be extremely hazardous. If a coexistent choroidal haemangioma is found or suspected (see Ch. 9), surgery should be performed under hypotensive anaesthesia to minimize the risk of intraoperative choroidal haemorrhage. The dissection of the scleral flap may be prejudiced by angiomatous changes in the sclera; meticulous haemostasis is required.
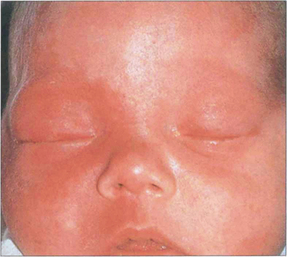
Fig. 8.68 This baby has a facial angioma involving the upper lid and maxillary area. Both eyes were buphthalmic but the right eye was worse than the left. The child later developed severe epilepsy from an intracranial angioma.
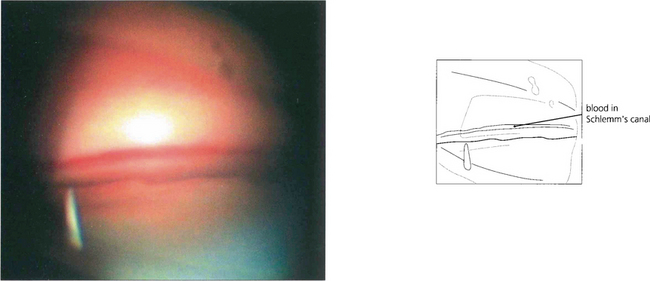
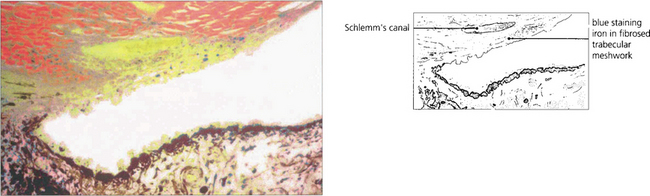
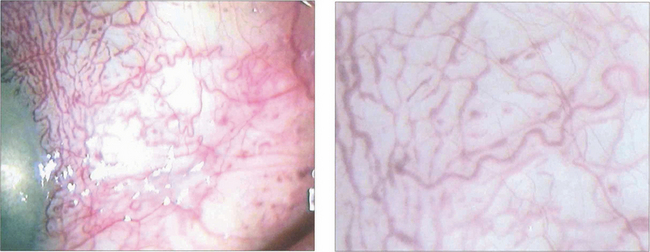
Fig. 8.70 Goniophotographs show blood in Schlemm’s canal because the normally outwardly directed pressure gradient is reversed by the raised episcleral venous pressure.
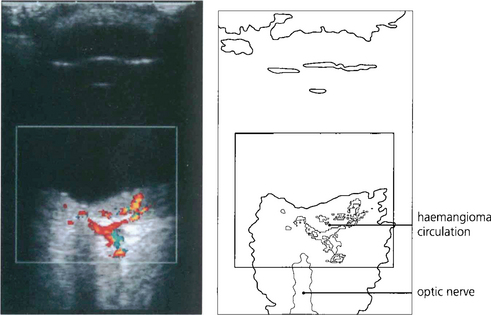
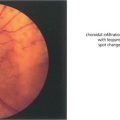
Fig. 8.71 When planning surgery it is important to look for choroidal involvement as these can bleed and cause devasting haemorrhage when the eye is opened. This colour Doppler B-scan ultrasonographic image demonstrates blood flow within a choroidal haemangioma in the region of the posterior pole.
By courtesy of Ms M Restori.