CHAPTER 42 Secondary Bone Tumors
INTRODUCTION
Approximately 10% of all cancer patients develop clinically significant spinal metastases.1 Metastatic spine tumors are 40 times more frequent than all primary bone tumors combined.2 In autopsy series, vertebral body metastases were found in over one-third of patients dying of cancer.3 The most common cancers to metastasize to the spine are breast, lung, prostate, and renal carcinomas (Table 42.1). Lymphoid cancers, including lymphoma and myeloma, are systemic diseases and common sources of spinal involvement. However, many authors do not consider these lesions true spinal metastases, and they are not included in many clinical series. When lymphoma and myeloma are included, they represent 8% and 5% of secondary spine tumors, respectively.4
Table 42.1 The Most Common Primary Cancers that Metastasize to the Spine4,11
| Primary Malignancy | Percentage of all Metastatic Spine Lesions |
|---|---|
| Breast | 21–30 |
| Lung | 13–19 |
| Prostate | 7–10 |
| Renal | 6–12 |
| Gastrointestinal | 4–7 |
| Thyroid | 5 |
| Various other cancers | 9 |
| Cancers of unknown origin/primary | 10–15 |
Although spinal metastases can occur in all age groups, the risk of metastatic spread to the spine coincides with the relatively high cancer risk period of 40–65 years of age.5 The average time between diagnosis of primary cancer and occurrence of spinal metastases varies widely (lung: 4 months; prostate: 22 months; breast: 86 months).6 Like most metastatic disease, metastatic spine tumors are rare in children. Exceptions to this are Ewing’s sarcoma and osteosarcoma (from other skeletal sites), neuroblastoma, and rhabdomyosarcoma.7
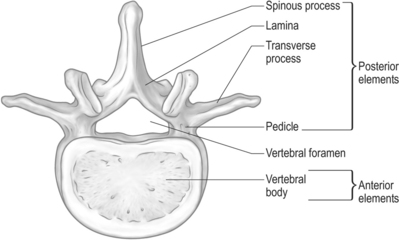
The spine can be divided into anterior elements and posterior elements (Fig. 42.1). Anterior elements consist of the vertebral body, and the posterior elements include the remainder of the vertebra (pedicles, transverse processes, laminae, and spinous process). Metastases involving the spine are located in the bony vertebral column 85% of the time,8 and the anterior elements of the spine are 20 times more likely to be involved than the posterior elements.9 Other metastases involving the spine may be located in the paravertebral region and less often in the epidural space.
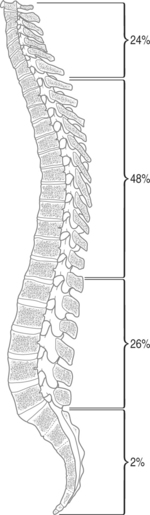
The thoracic spine is most frequently invaded by metastases, followed by cervical, then lumbar segments.10 It has been suggested that the distribution of metastases to the spine is roughly proportional to the height of each segment, with the cervical spine hosting 24% of metastases, thoracic 48%, lumbar 26%, and sacral 2% (Fig. 42.2).11
Early diagnosis is critical in both primary and secondary tumors of the spine. Unlike primary tumors, the early diagnosis and treatment of secondary tumors will not prevent metastatic disease. However, much of the significant morbidity related to spinal metastases can be lessened with early intervention. For instance, the best predictor of neurologic outcome after radiotherapy is the neurologic function prior to treatment; patients with severe neurologic deficit before radiation are unlikely to improve.12 In addition, patients undergoing surgery for neurologic symptoms had much better outcomes if they were ambulatory prior to surgery.13 Neoplastic paraplegia not only reduces a patient’s quality of life, it results in decreased life expectancy and a large economic cost to society.
The primary treatment goals in metastatic spine disease are to preserve/improve quality of life, alleviate pain, preserve/improve neurologic function, prevent/correct spinal instability, and optimize local tumor control as well as treatment of primary malignancy. Treatment options include various medications, external bracing, chemotherapy, radiation therapy, vertebroplasty/kyphoplasty, radiofrequency ablation, embolization, and surgery. Due to the many goals and modes of therapy, treatment of a patient with spinal metastatic disease requires a multidisciplinary approach, which often includes a pain specialist, medical oncologist, interventional radiologist, spine surgeon, physical medicine/rehabilitation specialist, social worker, and hospice care.
EXPLANATION OF ANATOMIC LOCATION
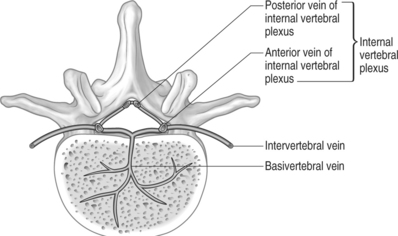
It is thought that the highly vascular, sinusoidal nature of the red marrow within vertebral bodies makes them particularly susceptible to seeding of metastatic cells. Moreover, retrograde venous flow into the internal vertebral plexus (Batson’s plexus)14 has historically been implicated in the spread of metastases to the spine. The internal vertebral plexus, a network of valveless veins in the vertebral canal, travels outside the dura from the foramen magnum to the coccyx. Due to the lack of valves, a rise in intrathoracic or intra-abdominal pressure can cause venous blood from the azygous system and the pelvic venous plexus to enter the internal vertebral plexus, allowing seeding of metastatic emboli from various organs. Drainage of the breast through the azygous vein and the prostate through the pelvic venous plexus predisposes the spine to metastatic processes from these areas. Drainage of the lung through the pulmonary vein, and colon through the portal system, tend to result in more diffuse embolic patterns.15 Oeppen et al. published a case report of a patient with renal vein involvement of renal cell carcinoma with spinal metastases that centered on the basivertebral veins at three contiguous levels in the low thoracic spine. Magnetic resonance imaging (MRI) demonstrated tumor in the intervertebral veins, which link the azygous system to the internal vertebral plexus.16 This case was highly suggestive of vertebral metastases due to retrograde venous spread through the internal vertebral plexus. Oge et al. described a case of a broken pacemaker lead tip migrating from the common iliac vein to a vein within the internal vertebral plexus at the level of L5.17 Due to the lack of valves and lengthy nature of the internal vertebral plexus, metastatic lesions occur at multiple noncontiguous levels in approximately 25% of cases.18 Some authors disagree with the Batson’s plexus theory and instead believe that arterial hematogenous spread to the marrow of the vertebral body results in the characteristic growth of tumors in the vertebral body, which eventually grow to directly or indirectly impinge the spinal cord (Fig. 42.3).19
CLINICAL PRESENTATION
Pain
As in primary spinal tumors, the first indication of spinal metastases is most often due to the pain produced by these tumors. Back pain is so common, and is typically such an early symptom of spinal metastases, that it may lead to recognition of a previously undiagnosed primary malignancy, such as lung carcinoma or prostatic cancer. Back pain is the first symptom of a spinal metastasis in 90–97% of cases.11,13 The presence of back or neck pain in a child with a cancer is caused by metastatic disease in approximately half of patients, and of these patients with spinal metastatic disease, spinal cord compression is present in approximately one-third.20 Pain often precedes other neurologic symptoms by weeks or months. In 42 patients undergoing surgery for metastatic disease of the spine, the median time from onset of back pain to appearance of neurological signs was 7 months with a range of 0–72 months.21 In addition, back pain may be present before a radiographic lesion can be detected.
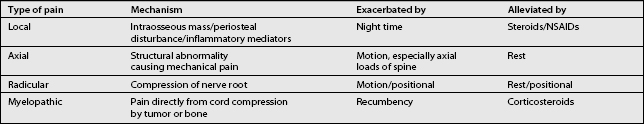
Metastatic disease to the spine can manifest in back pain in various ways and is often multifactorial (Table 42.2). When possible, it is important to determine the mechanism of back pain because the treatment may vary depending on the mechanism. Local pain is produced by an intraosseous mass effect of the tumor or local stretching/distortion of the periosteum due to tumor destruction. In addition, this pain may be produced and exacerbated by inflammatory mediators. Local pain is persistent, often worse at night, and not typically affected by movement. Low-dose steroids (decadron 12 mg daily) often relieve the pain. In addition, local pain is often relieved by treatment of the underlying tumor with radiation or surgery.22 Axial pain, which is mechanical in nature, evolves from a structural abnormality of the spine and may indicate instability. Axial pain may be produced by axial loads on the spine; therefore, in such cases, it is exacerbated by motion and alleviated by rest. Radicular pain may develop from nerve root compression by tumor epidural extension and is worse with motion. It is often positional, alleviated by one position and exacerbated by another. Cauda equina syndrome, caused by compression of the nerve roots below the conus medularis, may exhibit lumbar and sacral radicular pain as well as paresthesias and weakness. Symptoms are often asymmetric. Some patients may develop a combination of radiculopathy and axial pain resulting from instability and neuroforaminal compression. Myelopathic pain is due to direct compression of the spinal cord either by tumor or bone. Recumbency often makes myelopathic pain worse; this is thought to be due to the distention of the internal venous plexus. Steroids often reduce myelopathic pain by reducing vasogenic edema.
Neurologic impairments
Neurologic manifestations other than pain often begin with radiculopathy, followed later by myelopathy due to spinal cord compression.22 Progression of symptoms can be gradual, but acute deterioration may occur as a result of spinal instability. Acutely worsening symptoms in patients with spinal metastases requires emergent attention. Along with pain, radiculopathy in the cervical and lumbar regions causes weakness in the arms and legs, respectively. Radiculopathy due to lesions in the thoracic spine may cause pain in a band-like pattern in the corresponding dermatomes along the thorax and abdomen. Objective sensory loss is rare when a single nerve root is involved due to the overlap from neighboring roots. Cauda equina syndrome may present with loss of sensation in the buttocks and legs, unilateral or asymmetric leg weakness, hypotonia, decreased reflexes, early bladder and bowel incontinence, and lumbosacral radicular pain.
Myelopathy, which occurs in 20% of adult patients with spinal metastases,23 often begins as hyperreflexia below the level of the compression. This can progress to weakness, proprioceptive sensory loss, loss of pain and temperature sensation, urinary and fecal incontinence, impotence, and even paralysis. Eighty percent of patients with spinal cord compression will have weakness or paralysis.24 Impaired proprioception, sphincter function,24 and ability to ambulate25 indicate more serious neurologic damage when affected, and they are less likely to be recovered with treatment. In addition, patients tend to underestimate the loss of bladder and bowel control and sometimes discount them as symptoms of other medical problems, such as prostatic hypertrophy or side effects of chemotherapy.
Other symptoms
As with any suspected malignancy, constitutional symptoms such as fatigue, fever, and unexpected weight loss must be included in a careful review of symptoms. If suspicious of a metastatic lesion in the spine, the physician should inquire about symptoms of possible primary cancers. A family history of cancer may also be helpful in elucidating the diagnosis of a patient with a suspected metastatic lesion. Red flags that suggest malignancy of the spine are presented in Table 42.3.
Table 42.3 Elements of the Presentation that are Worrisome for Spinal Malignancy
| Red Flags |
|---|
| History of prior malignancy |
| Back pain worse at night/pain that wakes patient from sleep |
| Consistent progression of pain |
| Pain unchanged during rest or activity |
| Acute neurologic deterioration |
| Presence of a mass |
| Presence of constitutional symptoms |
PHYSICAL EXAMINATION
The examination in a patient with possible metastatic disease to the spine is similar to that in a patient with a suspected primary tumor (see previous chapter). A complete examination of the spine and its neurologic function should be performed on any patient with a suspected spine tumor. One may cautiously attempt to elicit Lhermitte’s sign. Lhermitte’s sign is defined as a subjective electric shock-like sensation traveling down the spinal column and through the upper and lower limbs that occurs with neck flexion. This finding was encountered in 15% of patients with symptomatic spinal metastases, and all lesions associated with this finding were in the thoracic spine.26 In addition, careful examination of the neck, breasts, lungs, abdomen, and prostate can often reveal a potential source for metastatic spinal tumors. Identification of lymphadenopathy in the cervical, axillary, and inguinal lymph nodes can suggest leukemia, lymphoma, or other systemic malignancy. A rectal exam, including stool guaiac and assessment of sensory, motor, and reflex components, may be informative. Careful palpation of the extremities, rib cage, and iliac crests for painful areas can alert the examiner to other sites of bony metastatic disease.
WORK-UP
Laboratory studies
The laboratory work-up in a patient with a suspected tumor of the spine can be involved, especially if an undiagnosed primary malignancy is suspected. A complete blood count (CBC) with a differential is important when working up any suspected malignancy. Elevated erythrocyte sedimentation rates (ESR) and C-reactive protein (CRP) levels signal that an inflammatory process is involved, but cannot consistently differentiate an infectious process from a malignancy. Lactate dehydrogenase (LDH) levels can be elevated in sarcomas, and LDH isoenzymes 2 and 3 can suggest a diagnosis of lymphoma.27 In order to check for liver cancer, alpha fetoprotein (AFP) levels are often obtained in patients with hepatitis C or those who are heavy drinkers. Carcinoembryonic antigen (CEA) is a marker of adenocarcinomas such as colonic, rectal, pancreatic, gastric, and breast.28 Prostate specific antigen (PSA) levels can help diagnose prostate cancer. A thyroid panel can help eliminate the suspicion of a rare thyroid primary, and parathyroid hormone (PTH) can be ordered to look for hyperparathyroidism. An elevated PTH level may lead to diagnosis of a brown tumor of the spine, which can be mistaken for metastatic disease. The diagnosis of multiple myeloma can be confirmed by the identification of monoclonal proteins in the serum or urine via serum protein electrophoresis (SPEP) or urine protein electrophoresis (UPEP); however, up to 3% of patients may have negative serum and urine electrophoresis.4 A chemistry panel can be used to assess kidney function and allows calcium and phosphate levels to be followed to detect and avoid the development of malignant hypercalcemia associated with metastatic lysis of bone. An elevated alkaline phosphatase level can also provide evidence for a neoplastic bone disease.
X-ray
The sensitivity is low for early metastatic involvement of the spine; however, plain films should be obtained initially, as in the work-up of primary tumors of the spine. Of patients with spinal metastases that underwent autopsy, 48% had no visible lesions on plain films, and 26% had negative X-rays despite gross involvement by tumor.3 The high false-negative rate can be partly attributed to the amount of cancellous bone (50%)29 that must be destroyed before becoming radiographically evident. Paraspinal tumors invading through the neural foramen may produce no radiographic abnormality. Therefore, the work-up of a spinal tumor does not end with a negative plain film. Despite low sensitivity, plain films are inexpensive and can offer information not provided by MRI and other imaging modalities.
Bone scan
Bone scanning (skeletal scintigraphy) utilizes a disphosphonate compound, tagged with technetium 99m, which, after intravenous injection becomes incorporated into bone by osteoblastic activity. Bone scanning provides images of the entire body in a fairly short period of time. It is a fairly sensitive technique for the detection of bone metastases and can detect these lesions earlier than plain films; however, its one weakness is low specificity. Bone scans demonstrate areas of osteoblastic activity, and the radionuclide accumulates at sites of fracture, infection, degenerative disease, bone metastases, and benign tumors such as some hemangiomas and fibrous dysplasia. The pattern of uptake is frequently helpful in deciding if uptake is likely to represent metastatic disease. False-negative bone scans are often due to destructive activity that exceeds reactive or blastic activity, as in multiple myeloma, aggressive tumors, and in tumors which are confined to the medullary cavity and do not affect the cortex.30 Also, paraspinal tumors that invade the epidural space through the intervertebral foramen are often missed on bone scan.
Myelography (conventional and CT-myelography)
Myelography is an invasive procedure with inherent risks. Before MRI, conventional myelography was the gold standard for detection of cord compression and intrinsic cord lesions, but it has been largely replaced by MRI scanning, and by CT-myelography when MRI is contraindicated. Myelography may fail to reveal secondary sites of epidural spinal cord compression and has been shown to be less sensitive in diagnosing spinal tumors than MRI.31 CT-myelography, like conventional myelography, involves the instillation of contrast into the dural sac, but the amount of contrast used is much less due to the enhanced ability of CT to depict subtle contrast differences. By employing various window settings for the images, details of the paraspinal structures, bone, and dural sac contents are well demonstrated. Both conventional and CT-myelography may be used when metallic fixation devices have been placed in and around the spine and MRI is unable to provide adequate images. This problem is becoming less frequent with the increased use of titanium spinal hardware.
Magnetic resonance imaging
Magnetic resonance imaging detects spinal and paraspinal pathology better than any other imaging technique. It reliably depicts changes in the water content of structures, and thus most pathology, before changes in gross architecture occur. Pathology is detected by employing imaging sequences that emphasize various components of tissues such as fat, fluid, and vascularity. MRI is the only noninvasive technique able to visualize pathology within the spinal cord and clearly depicts the degree of cord compression, as well as the process causing the compression. MRI defines lesions in the vertebrae as well as disc pathology and is the best method to diagnose discitis and paraspinal infections. MRI is also more reliable than other techniques in separating benign compression fractures from pathologic fractures of the vertebral bodies. This distinction is made by analyzing signal intensity changes in the bone and paraspinal space as well as by evaluating the shape of the vertebrae and integrity of the cortical margins. MRI reveals bone metastases earlier than bone scintigraphy and depicts foci of osteolytic and osteoblastic activity equally. Most bone metastases are readily detected without the use of gadolinium-based intravenous contrast (most are easily demonstrated on T1-weighted and fat-suppressed T2-weighted images without contrast).32 Contrast actually may obscure metastases to bone as enhancement may cause the signal in the lesion to increase to that of normal bone marrow on T1-weighted scans.33
Positron emission tomography
The most common radiotracer used in clinical positron emission tomography (PET) imaging is fluorine-18-fluoro-2-D-deoxyglucose (18F-FDG), which accumulates in areas of high glycolysis and membrane transport of glucose, both known to be increased in malignant tissue. Unlike the agent used in bone scanning, 18F-FDG may detect bone marrow-occupying lesions before cortical involvement occurs, thus detecting bone metastases before they can be found on bone scans. Sclerotic metastases, however, as found in some breast and prostate cancers, are less likely to be detected by PET as these lesions have lower glycolytic rates and are less cellular than lytic metastases.34 18F-FDG is not specific for tumors and may accumulate at sites of infection but is less likely to be detected at sites of degenerative change than technetium 99m, the agent used in bone scans. Therefore, it is somewhat more specific for tumors. PET also demonstrates metastases in soft tissue throughout the body, resulting in additional diagnostic value. In addition to detecting spine tumors, PET may also be useful in distinguishing malignant lesions from benign.
Biopsy
Types of biopsy
As discussed in the preceding chapter, there are two types of biopsy commonly used for spinal lesions: percutaneous, guided biopsy and open, surgical biopsy. Both fluoroscopic-guided and CT-guided percutaneous biopsies can be utilized, and both are effective. The accuracy of CT makes it superior when dealing with small, deep-seated lesions, especially in the cervical and thoracic regions.37 CT allows better selection of the optimal location to sample tissue. For lesions visible via fluoroscopic monitoring, fluoroscopic-guided biopsy offers real-time positioning of the needle. Open biopsy maximizes tissue retrieval, providing the highest diagnostic success rate; however, it is typically reserved for failed percutaneous biopsies due to the increased morbidity of the open procedure and greater risk of wound contamination with tumor. Regardless of which method is used, the goal is to obtain an adequate amount of tissue while minimizing complications.
Biopsy success rate
Accurate diagnosis of tumorous and nontumorous lesions using CT-guided biopsy is achieved greater than 90% of the time.37–39 In lesions with central necrosis, the ability to obtain the correct diagnosis may be enhanced by obtaining tissue from the periphery of the lesion. In paucicellular aspirates, a cell block can be prepared or additional tissue, such as a core biopsy, can be obtained. If histology yields only peripheral blood in an obviously destructive mass, biopsy can be repeated, by directing the needle/device at a slightly different area of the lesion.39 If indicated, corticosteroids should only be administered after biopsy due to their lytic effect on certain tumors, including leukemia. This lytic effect can lead to a nondiagnostic biopsy.
Percutaneous biopsy of solitary lesions
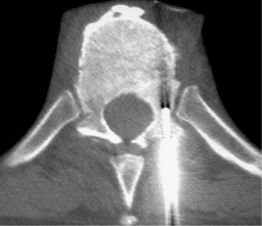
The approach to the percutaneous biopsy of a solitary spinal lesion is fairly straightforward. Usually, the approach involves the shortest path to the lesion that does not place vital structures at risk. For biopsies of the spine, this typically involves a posterior approach; however, in the cervical spine, anterolateral approaches are often used. Since most metastatic lesions are found in the vertebral body, a posterior transpedicular approach is often used. The transpedicular approach, shown in Figure 42.4, helps to avoid vital structures while minimizing the amount of tissue susceptible to tumor contamination of the needle tract. Virtually any lesion within the vertebral body of cervical, thoracic, or lumbar vertebrae can by accessed via this approach.40 Lesions located in the posterior elements are typically biopsied with a direct approach.
Percutaneous biopsy when multiple lesions are present
When multiple lesions are present in the spine, biopsy of one lesion may be satisfactory if the lesions appear to be from the same malignancy. Several factors play a role in choosing which lesion to biopsy: size of the lesion, radiologic morphology, location along the spinal column, and location within the vertebra. Often, the largest lesion is easier to biopsy and more tissue is available to aid in the histologic diagnosis. The biopsy of the most aggressive lesion may demonstrate malignant cells that may be less abundant or not present in more benign-appearing lesions. Lumbar lesions are often easier to biopsy with the transpedicular approach because of the large size of lumbar pedicles when compared to those in the thoracic and cervical levels. Also, it is often desirable to biopsy a lesion that requires the least amount of bone disruption in order to cause the least amount of structural damage to the vertebra, which may already be compromised by tumor destruction.
Complications of biopsy
Biopsies of potentially tumorous lesions should be well planned. It is well known that inadequate or inappropriate open biopsies adversely affect outcome. Complications arising in these unsound biopsies include disability due to more complex resection, loss of function, local recurrence, and death.41 The surgeon who performs the definitive surgical procedures, if further surgery becomes necessary, should ideally perform the open biopsy. This ensures that the subsequent surgery can be performed using the optimal incision and approach, while excising the biopsy incision and tract. This also helps to eliminate unnecessary and improperly performed open biopsies.
Complications of percutaneous needle biopsy include bleeding, infection, neurologic compromise, fracture, biopsy tract contamination, and death, although serious complications are rare. There may be risk of tumor contamination of the biopsy tract.42 The needle tract may be excised if a subsequent surgery is indicated, although this procedure is somewhat controversial. Whenever possible, guided biopsies should be done at the same institution where definitive surgical treatment will occur. Typically, pathologists at the larger referral centers will be more experienced with uncommon primary and secondary malignant tissues obtained from the spine and will typically review specimens despite previous histologic diagnosis from outside institutions. Also, a team approach between the interventional radiologist and the treating surgeon is more likely to produce a favorable result.
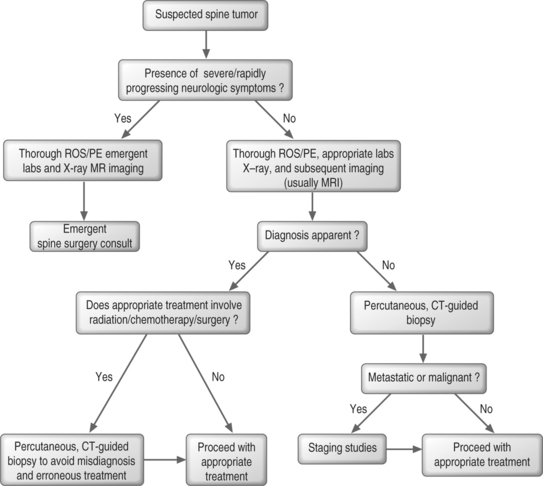
Algorithmic approach to work-up
When a spine tumor is suspected in a patient with severe or rapidly progressing neurologic symptoms, a thorough review of systems (ROS) and physical examination (PE) should be performed and laboratory tests and imaging, usually MRI and radiographs, should be obtained promptly (Fig. 42.5). A spine surgeon should be consulted as soon as possible. In the absence of severe or rapidly deteriorating symptoms, a thorough ROS and PE are again necessary. Laboratory tests should be selected and performed based on the working differential diagnosis. Plain films should be obtained and are usually followed up with more sophisticated imaging. If the diagnosis is apparent at this point in time, appropriate treatment may be initiated. However, if treatment involves radiation, chemotherapy, or surgery, tissue diagnosis is typically required. If the diagnosis is in question, percutaneous CT-guided biopsy should be performed. Appropriate treatment may be initiated if the diagnosis is benign, but staging studies, such as CT of the chest, abdomen, and pelvis, may be appropriate before initiating treatment if the lesion is a metastatic or primary malignant lesion.
PRIMARY MALIGNANCIES THAT METASTASIZE TO THE SPINE
Breast
Breast cancer has a high propensity for bone. Typical skeletal sites include vertebral bodies, pelvis, proximal femur, and humerus. One autopsy study demonstrated that approximately 75% of breast cancer patients developed metastases to spine.3 A portion of the venous drainage from the breast empties into the thoracic portion of the azygous system, which accounts for thoracic location of most spine metastases. Lumbar involvement is not uncommon in breast cancer. One study found the L2 vertebra most commonly involved, followed closely by T9.43
Breast metastases are often osteolytic; however, some can be of mixed type, and less frequently purely sclerotic.44,45 Breast tumors that metastasize to bone are more frequently estrogen receptor-positive and well differentiated than those that metastasize to the lungs or liver.4 In general, breast metastases are relatively sensitive to radiation therapy. However, radiotherapy may be more effective on poorly differentiated, rapidly growing lesions when compared to well-differentiated, slower growing lesions of bone.
Lung
Lung carcinoma commonly metastasizes to liver, skeleton, bone marrow, and brain.46,47 In one autopsy study approximately 45% of patients dying of lung cancer developed metastases to spine.3 The most common location for spine metastatic disease was the lower thoracic region, with T12 involved in 10 of 15 cases examined in one study.43 Metastatic spine lesions due to lung cancer most often result in lytic lesions. Small cell lung carcinoma has the best prognosis and longest survival. Lung carcinomas are generally considered to be intermediately responsive to radiation. Small cell carcinoma is more responsive than other forms to radiation, as well as to chemotherapy. Given the limited survival of most patients with lung cancer, and the responsiveness of small cell carcinoma to radiation and chemotherapy, most are not candidates for operative treatment.4
Prostate
Prostate cancer and metastases of prostate cancer to the spine are common. In autopsy studies prostate cancer is found in 24–46% of men over the age of 50 years.48 One autopsy study demonstrated that approximately 90% of prostate cancer patients dying of their disease developed metastases to spine.3 Prostatic spinal metastases are most often located in the lumbar spine.
Skeletal prostate metastases are typically osteoblastic, and sclerotic lesions in the vertebral bodies and pelvis are most common. Although skeletal metastases are typically osteoblastic (80%), a mixed osteoblastic/osteolytic pattern is not uncommon (12%.)49 An osteolytic pattern represents 4% of these skeletal metastases49 and is typically found in poorly differentiated tumors (combined Gleason’s score of 9 to 10).50 Expression of prostate-specific antigen and prostate-specific acid phosphatase, which can be identified immunohistochemically, is a helpful feature in diagnosis, although some poorly differentiated prostate carcinomas may be negative for both. Treated prostate carcinoma can be very inconspicuous, mimicking non-neoplastic cells such as histiocytes, in which case epithelial markers such as keratin can aid in diagnosis.50
Because prostate metastatic lesions in the spine are usually blastic, pathologic fractures and neurologic involvement are relatively rare.51 The prognosis for life expectancy when cord compression occurs is better than that of most metastatic diseases. Treatment of prostate cancer includes hormonal therapy, radiation, chemotherapy, and surgery. The need for surgical intervention for spinal metastases is not as common as for tumors of other origins.
Renal
Renal cell carcinoma is the fourth most common type of metastatic spinal tumor. By the time renal carcinoma is diagnosed, it has often reached advanced stages. These carcinomas are known to metastasize to unusual sites such as the eye, skin, tongue, heart, and breast. Eighty percent of patients with renal cell carcinoma will eventually develop metastases.52 In one autopsy study, approximately 30% of patients dying of renal cancer had developed metastases to spine.3
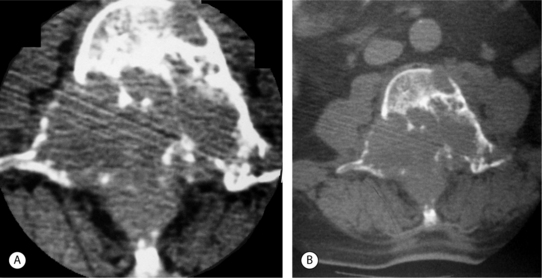
Renal cell carcinoma most often produces lytic lesions.53 The margins are generally indistinct and aggressive lesions expand into the surrounding soft tissues (Fig. 42.6).4 A small proportion of renal cell carcinomas dedifferentiate into pleomorphic sarcomatoid carcinoma and the sarcomatoid elements may be the only components present in spine metastases. These lesions may have features like fibrosarcoma and malignant fibrous histiocytoma and can be misdiagnosed as primary bone lesions. Gross pathologic examination shows the majority of renal cell carcinomas to be very hypervascular.
To date, chemotherapy has been shown to be ineffective in the treatment of renal metastases to the spine. Although radiation treatment is often used, the tumor is relatively radioresistant. While median survival times are short (generally 6–9 months),54 the clinical course of metastatic renal carcinoma is variable. Survival of patients with renal cell carcinoma and spinal metastases is most dependent on the pathologic characteristics of the primary tumor, followed by severity of neurologic deficit and presence of other metastases.52 Those with predominantly osseous metastases fare better than those with other organ involvement. Renal cell carcinoma more commonly causes neurologic deficiencies than other spinal metastases,52 and surgery is relatively common for these spinal lesions. Preoperative arterial embolization is often necessary to diminish intraoperative blood loss, which can be extensive.52,55 In a study by Sundaresan et al., 90% of patients with cord compression causing neurologic compromise showed neurologic improvement after surgery.55 In another study, 88% of patients had partial or complete relief of pain, with 64% of the bedridden patients able to walk after surgery.52
Gastrointestinal
Most carcinomas of the gastrointestinal tract are highly aggressive lesions with a high propensity for metastasis.56 The skeletal sites most frequently involved are the spine, ribs, pelvis, and femur. Patients dying of gastrointestinal tract tumors, including those of the pancreas and liver, have histological evidence of spine metastases 25% of the time.3 Gastric carcinomas are more prone to develop metastases to the spine than carcinomas of the colon. Colorectal cancers, which are unusual sources of spinal metastases, favor the lumbar spine.57 Spine metastases are often late findings in colorectal cancer and often generate lytic lesions.53
Thyroid
The behavior of thyroid carcinomas varies widely from indolent, well-differentiated tumors to highly malignant, poorly differentiated carcinomas. Well-differentiated carcinomas, especially follicular, have a unique propensity to metastasize to bone. After metastases to the neck lymph nodes, the skeleton is the next most frequent metastatic location.50 In one autopsy study, approximately 40% of patients dying of thyroid cancer had metastases to spine.3
Radiographically, thyroid carcinomas typically appear as destructive, lytic lesions in bone. The lesions are usually poorly demarcated, and it is unusual for these tumors to demonstrate a periosteal reaction.4 Histologically, thyroid carcinoma is usually easily recognized in bone; however, immunohistochemical staining for thyroglobulin in follicular and papillary carcinomas and calcitonin in medullary carcinomas may be helpful.50
Lymphoma
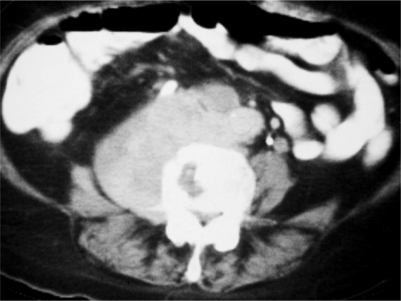
In one study, 29% of patients that died of lymphoma had evidence of spinal metastases.3 The typical route of metastasis for lymphoma is by direct, contiguous spread from the retroperitoneum to the paraspinous and epidural spaces via the neural foramina. Neurologic symptoms are usually due to spinal cord compression; however, direct infiltration of the spinal cord and nerve roots may also occur. On MRI, metastases to the spine usually show vertebral body involvement accompanying the paraspinous soft tissue mass. This can also be seen in CT with soft tissue settings (Fig. 42.7). The vertebral body lesion usually has lower signal intensity than marrow on T1-weighted images. This low signal abnormality may be subtle in children; it may be difficult to detect in older patients with heterogeneous marrow signal. On T2-weighted MRI, these tumors exhibit high signal intensities.58 For a patient with confirmed systemic lymphoma and newly diagnosed epidural metastases, local radiation therapy using 30–40 Gy is the treatment of choice.
Multiple myeloma
Multiple myeloma and solitary myeloma are often considered separate entities due to their significant differences. Solitary myeloma, which is addressed in the previous chapter, is a rare disease that occasionally occurs in the spine. Multiple myeloma is more common, with an incidence of approximately 35 per million and is found most commonly in patients over 40 years of age. Multiple myeloma involves the uncontrolled proliferation of malignant plasma cells and their products. An elevated level of IgG monoclonal light chains is most frequently found followed by IgA and IgD monoclonal light chains. Multiple myeloma is most commonly found in bones that contain hematopoietic marrow with the majority of lesions found in the axial skeleton. Fifty-five percent of patients that died of myeloma in one study had evidence of spinal involvement.3 Regarding location within the spinal column, no one vertebral level seemed to be preferentially involved.43
The majority of multiple myeloma lesions in the skeleton are lytic, and sporadic areas of sclerosis may be present. As mentioned previously, bone scans may be negative. The typical appearance of untreated multiple myeloma on MRI is similar to that of other metastatic lesions that replace fatty bone marrow. These lesions have relatively low T1-weighted signal intensity and high T2-weighted signal intensity when compared to normal bone marrow. The usual pattern of involvement is diffuse marrow replacement in multiple vertebral bodies, often with multiple well-circumscribed lesions.58
Patients with multiple myeloma fare poorly, with a median survival of 28 months. In cases with spinal metastases the prognosis is worse, with 76% of patients deceased within 1 year.59 In the case of disseminated myeloma, chemotherapy should be initiated. The treatment of choice in multiple myeloma is radiation, bisphosphonate therapy, and if necessary, bracing. Because of the sensitivity of this cancer to radiation and the poor survival rate, surgery is not often indicated. If indicated, surgery is often difficult to perform due to multilevel involvement and severe osteopenia seen in these patients.4
MANAGEMENT
General medical treatment
Bisphosphonates
Bisphosphonates are drugs that inhibit osteoclastic activity, suppressing bone resorption. The most common bisphosphonate used in cancer patients is pamidronate. Used in conjunction with systemic chemotherapy, pamidronate has been shown to decrease or delay pathologic fractures due to bone metastases in breast cancer60 and multiple myeloma61 patients.
Corticosteroids
The use of corticosteroids in the treatment of spinal cord compression in secondary tumors of the spine is similar to their use in primary tumors, discussed in the previous chapter. Corticosteroids, by reducing the vasogenic edema of acute spinal cord compression, stabilize or improve neurologic status and relieve pain in some patients. Due to the low mineralocorticoid activity, low cost, and use in clinical trials, dexamethasone is commonly used. The optimal dose used to treat acute spinal cord compression is controversial. One randomized, controlled trial showed that 96 mg/day of dexamethasone in patients with epidural spinal cord compression provided a significantly higher percentage of patients who were still ambulatory at long-term follow-up.62 One retrospective study comparing 16 and 96 mg/day doses demonstrated a significantly higher incidence of both serious and nonserious side effects with the higher dose.63 This study also showed no difference in efficacy between the two doses; therefore, the recommended dose for symptomatic patients is a 10 mg i.v. bolus followed by 16 mg/day administered four times daily. The larger dose of 96 mg/day should only be administered to patients with rapidly progressing neurologic deficits.1
Steroids are recommended for neurologic compromise of acute onset. However, caution must be taken in a patient with an undiagnosed spinal mass with regards to corticosteroid treatment. One must not deliver steroids prior to biopsy because of the oncolytic effect for certain tumors, such as lymphoma.62 Other complications of corticosteroid treatment include metabolic abnormalities, GI bleeding/perforation, iatrogenic adrenal insufficiency after discontinuation of steroids, osteoporosis, osteonecrosis, and psychosis. Postoperative infection and wound breakdown are also increased with corticosteroid use.
Pain management
Management of pain in patients with spinal metastases often begins with a trial of nonsteroidal antiinflammatory drugs (NSAIDs). NSAIDS should be discontinued prior to surgery to avoid the potential for excessive blood loss. Although nonsteroidal antiinflammatory agents may provide pain control, patients with cord compression usually require opiates for adequate pain relief. Opiates, along with autonomic dysfunction and limited mobility, can cause constipation. Therefore, patients may need an aggressive bowel treatment regimen to prevent constipation and resulting pain during straining.65 As mentioned previously, steroids may alleviate pain due to cord compression. A pain management consultation may be helpful in patients with spinal metastases.
Bracing
External spinal bracing performs two functions: alleviating pain and preventing or halting vertebral collapse. In so doing, bracing can help prevent neurologic involvement in those patients with intact neurologic function while they receive medical treatment. Patients can be treated with radiation and bracing alone if there is no neurologic deficit, minimal compression fracture, no significant kyphosis, and no bony retropulsion compromising the canal. Moreover, the patient who has a short life expectancy can be treated in this fashion. The Halo vest was studied in patients with metastatic prostate cancer to the cervical spine. Good maintenance of neurologic function was achieved; however, the average patient wore the vest for approximately one-third of his or her remaining life.66 Some recommend the use of an antiflexion device, such as the Jewitt brace, for lesions between T7 and L2, or a molded lumbosacral orthosis corset for lesions of the lower lumbar spine.4 Spinal bracing in patients with neurologic deficits has not been carefully studied and should be considered only in patients with pain who cannot receive standard management or those with pain refractory to the standard management. Bracing is not for every nonoperative patient. Bracing should only be used in those who will likely benefit from them due to their ability to decrease quality of life.
Tumor-specific medical treatment
Chemotherapy
The use of chemotherapy as the primary treatment for some vertebral metastases from systemic diseases such as myeloma and lymphoma can be successful. Chemotherapy can reduce the size of spinal lesions in these cancers and may eliminate the need for surgery. Adjuvant chemotherapy in preoperative and postoperative settings has an important role in the treatment of chemosensitive tumors such as Ewing’s sarcoma, osteosarcoma, and lymphoma.22
Chemotherapy is more effective for certain tumor types, whereas others remain without significant response. For those tumors that are sensitive, the issue becomes one of responsiveness rather than sensitivity alone. The most important variables in cases of neural compression secondary to spinal metastases are the time required for a measurable response to drug therapy and the duration of that response. Possible complications of chemotherapy vary depending on the chemotherapeutic agent used, but typically include immunosuppression, delayed wound healing, and perioperative wound infections.13
Radiation therapy
Radiation is the mainstay of spinal metastatic treatment unless the tumor is radioresistant and progressive, causes instability of the spine or bony compression of the cord/cauda equina, or causes significant spinal cord or cauda equina dysfunction. Similar to chemotherapy, radiation has a variable effect among tumor types. Prostate, lymphoid, and breast are the most sensitive to radiation therapy. Lung and thyroid are intermediately responsive; GI, melanoma, and renal are typically radioresistant lesions.13,54
Radiation therapy leads to resolution of back pain in most patients. Pretreatment neurologic function is the strongest predictor of post-treatment neurologic function.65 In one study, a significant difference was found in the duration of response between patients with radiosensitive malignancies and those with radioresistant malignancies (11 months versus 3 months).67 This study also showed median survival varied significantly between patients with radiosensitive malignancies and those with radioresponsive malignancies.
The standard radiotherapy treatment for palliation of spinal metastases is daily 3 Gy fractions with a total dose of 30 Gy.65 Spinal cord and cauda equina tolerance to radiotherapy is the limiting factor in significantly raising the dose to greater levels to achieve higher rates of local control.22 Advances in radiotherapy, including intraoperative radiation therapy (IORT), three-dimensional conformal radiation therapy (3D-CRT), and intensity-modulated radiation therapy (IMRT) are being developed and studied.22 Such approaches may permit the delivery of higher doses of radiation to target tissue, while allowing the dose delivered to the spinal cord to remain within acceptable limits.
Minimally invasive procedures
Radiofrequency ablation
Radiofrequency (RF) ablation has received increasing attention as a promising technique in the treatment of malignant tumors.68,69,70 One study of patients with unresectable spine metastases demonstrated significant pain relief and reduction of disability with RF ablation guided with CT and fluoroscopy.71 Neurologic function was preserved or stabilized in the vast majority of these patients. However, RF treatment is not advisable for most spinal lesions because of the proximity to nerves within the spinal canal, lateral recesses, and neural foramina. For safety, the electrode should be at least 1 cm away from major nerves.72 The use of RF ablation for treating osteoid osteomas of the spine has been documented and is discussed in the previous chapter.
Embolization
Embolization is often performed preoperatively for hypervascular tumors, including renal cell and thyroid carcinomas. Preoperative embolization is safe and effective and can make complete resection possible in a previously unresectable tumor by reducing tumor size and reducing intraoperative blood loss.73 The average intraoperative blood loss in 51 patients with hypervascular metastatic spinal neoplasms (30 patients with renal cell carcinoma) treated with preoperative embolization was 2586 mL. Intraoperative blood loss for tumors with near-total or total embolization was largely related to unembolized epidural veins.74 Embolization has also been used for palliation of pain and reduction of tumor volume in renal metastases to the skeleton75 and spine.76 Chemoembolization, the administration of selective arterial chemotherapy at the time of embolization, has been shown to significantly reduce pain in bony pelvic and spinal metastases.77
Embolization has been successfully used as a primary treatment for sacral giant cell tumors78,79 as well as an effective preoperative surgical adjuvant in the treatment of aneurysmal bone cysts of the spine. Treatment of these primary tumors is discussed in the previous chapter.
Vertebroplasty and kyphoplasty
Percutaneous vertebroplasty using polymethyl methacrylate (PMMA) has been used in the treatment of benign compression fractures since the late 1980s. Although this does not expand collapsed vertebrae, it has demonstrated utility in restoring mechanical stability and decreasing pain in patients with collapsed vertebrae. Some authors have reported the use of vertebroplasty in treating local and axial pain due to vertebral body metastases.80–82 The relief of pain in vertebral metastases is less likely to be successful compared to benign compression fractures.80 This may be due in part to the multifactorial nature of metastatic spine pain. However, one study demonstrated that seven out of eight patients with metastatic disease had no further vertebral compression, and spinal canal compromise was prevented.80 Epidural extension of PMMA more often complicates cases of vertebral metastases when compared to benign compression fractures.81 This may produce or dramatically worsen neurologic impairments. Complications due to extravasation of PMMA can be significantly reduced when caution is observed and excellent imaging conditions are maintained in key steps of the procedure. The use of high-viscosity cement, relatively small injection volumes, and use of kyphoplasty in selected cases can reduce cement leakage-related complications even further.82 Other complications of vertebroplasty include pulmonary embolism due to injection of PMMA into the venous system of the vertebral body, infection, cement toxicity, and adjacent vertebral compression.
Although vertebroplasty alleviates pain and restores mechanical stability, the inability to restore vertebral body height is a limitation. Kyphoplasty uses an inflatable bone tamp, which is placed in the vertebral body and inflated to restore vertebral height, although the extent of height restoration is debatable. PMMA is then injected similarly to vertebroplasty. The use of kyphoplasty for vertebral metastases has not been extensively studied, but one study of 32 kyphoplasties and 65 vertebroplasties (56 patients) revealed complete pain relief in 84% of procedures and no instances of worsening of pain.82 Asymptomatic cement leakage occurred during vertebroplasty 9.2% of the time, and no cement extravasation occurred during kyphoplasty. Precise indications for the use of vertebroplasty and kyphoplasty in the treatment of metastatic spine tumors are evolving; however, vertebroplasty and kyphoplasty are safe and feasible in well-selected patients with pain refractory to standard treatment.82 In the case of cortical disruption, especially in the posterior wall, or vertebral height loss below one-third of the original, vertebroplasty and kyphoplasty are technically difficult. Contraindications to vertebroplasty and kyphoplasty include neural compression, infection, elevated WBC count of uncertain etiology, known allergic reactions to PMMA, and pregnancy.83 Significant coagulopathy and severe cardiopulmonary disease are relative contraindications.
Surgery
The role for surgery in the treatment of spinal metastases has changed significantly over the last 30 years. Because metastatic lesions usually involve vertebral bodies, neural elements are more often compressed from the ventral side. Laminectomy alone, however, is a posterior decompressive procedure. Early on, several comparative studies showed no difference in outcomes between external photon beam radiation therapy and laminectomy53,84 and numerous investigators found a high incidence of neurologic worsening after laminectomy;85,86 therefore, radiation therapy became the first-line therapy. The evolution of instrumentation allowing rigid segmental spine stabilization and the development of more aggressive surgical approaches have made more appropriate surgical options available. In addition, the life expectancy of patients with cancer is increasing, with many living longer with their disease due to more aggressive medical therapy and fewer side effects. As these patients survive longer, there is an increasing probability that they will develop metastases to the spine that will become symptomatic, negatively affect quality of life, and lead to death. Improved surgical options and a better understanding of patient life expectancy, coupled with the desire of patients to seek all available interventions for survival, are allowing surgery to play an increasing role in the treatment of spine metastases. The spine surgeon should consider these factors in deciding when to intervene and should do so within the framework of the overall management provided by the oncology team.
Surgical contraindications
Contraindications to surgery include quadriplegia without a reasonable chance to restore neurologic function, short life expectancy less than 4 months, diffuse involvement of the spine with multiple sites of cord compression, absolute neutrophil count <1500, platelet count <50 000, abnormal coagulation, and poor pulmonary function tests (PFTs). The subject of life expectancy is controversial and varies from 4 weeks to 4 months.87,88,89 Therefore, the decision to operate in this range should be carefully considered, and the spine surgeon should be reasonably certain the patient’s quality of life would be improved enough to justify the pain, life disruption, hospitalization, and recovery.
Operative stabilization
Historically, the vast majority of surgical patients underwent laminectomy for tumor removal and subsequently had worsening pain, neurologic deterioration, and progressive deformity. It is now more universally accepted to perform anterior approaches, and spine surgeons are increasingly trained to apply recent advanced techniques of instrumentation using pedicle and lateral mass screws, rods, plates, and vertebral body replacement devices such as cages. Bone graft stabilization should be augmented with a plate or rod construct, but a metallic cage device is preferred if postoperative radiation will be used since there is a 50% nonunion rate with bone graft with the use of postoperative radiation.90 However, there are times when metallic cages are not long enough to achieve stabilization and bone graft or PMMA will have to suffice. Although PMMA generates substantial heat with polymerization, there is no risk of cord damage from the heat.91 Postoperative radiation should be postponed for 1 month to maximize wound healing.
OUTCOMES
In a review of the recent literature, an average of 60% of patients regained ambulatory function after surgical treatment.92 Surgical decompression and stabilization has been shown to improve pain and overall well-being.93 Patients with spinal metastases who were treated with anterior, posterior, or combined decompression and stabilization demonstrated a mean survival time of 16 months after surgery. Seventy-five percent of patients maintained neural function, while 20% improved.94
With newer stabilization techniques and devices, spine surgeons are more confident in aggressively removing the metastatic tumors and restoring stability while limiting the extension over multiple segments. As such, surgical outcomes are much better and patients are living longer and more enjoyable lives.
The information listed in Table 42.4 is helpful when consulting a spine surgeon; however, one should not delay consultation to obtain test results if the patient has significant or rapidly deteriorating symptoms.
Table 42.4 Information that may be Helpful when Consulting a Spine Surgeon
| Location of lesion within spine | Pain evaluation |
| Solitary or multiple spinal levels | Approximate life expectancy |
| Thought to be primary or secondary | Presence of any contraindications for surgery |
| Presence of instability | Patient comorbidities |
| Neurologic status | History of prior treatment |
ALGORITHMIC APPROACH TO TREATMENT
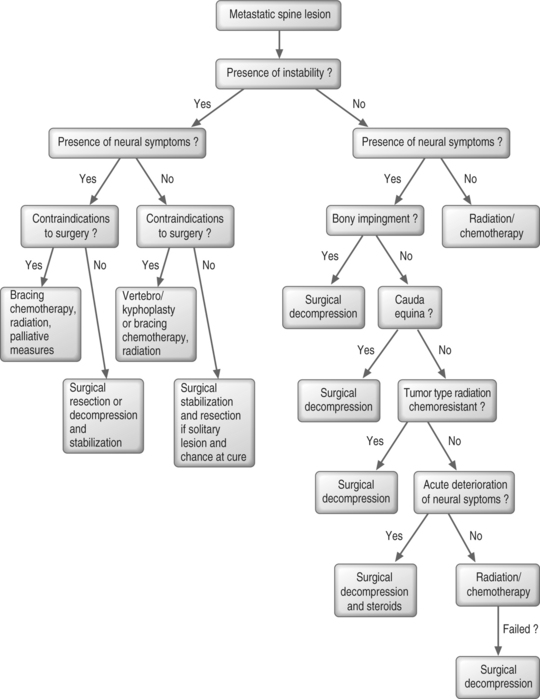
In regards to treatment of patients with histologically identified spine metastases (Fig. 42.8), one should first consider if the patient has spinal instability. If the patient has instability of the spine, surgical stabilization is necessary. If neural symptoms, in addition to instability, are present due to compression of the cord, cauda equina, or nerve roots, decompression or en bloc resection should be performed in addition to the stabilization procedure. In a solitary spine lesion, resection may be considered even in the absence of neural symptoms, if resection is feasible and if the patient has a chance of cure. If surgical intervention is contraindicated, bracing and chemotherapy or radiation therapy along with palliative measures may be the best treatment. In the absence of neurologic compression, which is a contraindication, vertebroplasty or kyphoplasty may be a treatment option for instability when more invasive surgery is contraindicated.
Beyond the treatment of instability, the role of surgery is not well defined. Considerable differences of opinion exist. However, the recommendations presented here are based on the literature and the experiences of physicians at the authors’ institution. Ultimately, the medical oncologist and surgeon must weigh the morbidity and risks of surgery against the patient’s life expectancy and quality of life.
If the patient has a stable spine and no bony impingement, the presence and characteristics of neural symptoms should dictate treatment. A patient with neurologic compromise in the absence of instability or bony impingement typically responds to radiotherapy alone.95 If the patient experiences acute deterioration, some authors suggest that radiation and/or chemotherapy treatment can be augmented by systemic corticosteroids.12,96,97 However, we recommend surgical decompression for acute deterioration if the patient’s condition permits. In the absence of acute deterioration, surgery is indicated for patients who have persistent pain or neurologic symptoms despite appropriate treatment with radiation and/or chemotherapy, keeping in mind that radiation, chemotherapy, and steroids contribute significantly to subsequent surgical morbidity.98,99 Due to the increased complications of surgery after radiation and chemotherapy, surgery should be considered as a primary treatment option in patients with tumor types that are characteristically radiation and chemotherapy resistant. This is particularly true in patients with rapidly progressive neurologic symptoms. Surgical treatment is also indicated for cauda equina syndrome, a surgical emergency. Generally, in patients without neurologic symptoms, radiation and/or chemotherapy are the primary treatment modalities. Vertebroplasty may be performed if a compression fracture is causing significant pain.
Whenever there is doubt about the origin of a metastasis, a biopsy should be performed. This can typically be done without surgical intervention via CT- or MRI-guided biopsy. However, if a surgical biopsy is required and the patient is at risk of fracture, vertebral collapse, or neurologic compromise, surgical stabilization with or without decompression may be performed at the time of surgical biopsy.100
Patients in whom instability is likely to develop despite radiotherapy may undergo surgical stabilization before starting radiotherapy to maintain spinal alignment and to minimize wound complications.100 For patients who present with complete paraplegia, it is recommended that surgical decompression and fusion be considered with caution, although, surgical decompression and fusion may be appropriate if a surgical procedure is required for diagnostic purposes.101
1 Walsh GL, Gokaslan ZL, McCutcheon IE, et al. Anterior approaches to the thoracic spine in patients with cancer: indications and results. Ann Thorac Surg. 1997;64(6):1611-1618.
2 Harrington KD. Metastatic disease of the spine. In: Harrington KD, editor. Orthopaedic management of metastatic bone disease. St. Louis: CV Mosby; 1988:309-383.
3 Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the imposters. Spine. 1990;15(1):1-4.
4 Heiner JP, Kinsella TJ, Zdeblick TA. Management of metastatic disease to the musculoskeletal system. St. Louis: Karen Berger, 2002.
5 Black Giannakopoulos G, Nair S. Spinal epidural tumors. In: Wikins RH, editor. Neurosurgery. New York: McGraw-Hill; 1996:1791-1804.
6 Onimus M, Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5(6):407-411.
7 Klein SL, Sanford RA, Muhlbauer MS. Pediatric spinal epidural metastases. J Neurosurg. 1991;74(1):70-75.
8 Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
9 Brihaye J, Ectors P, Lemort M, et al. The management of spinal epidural metastases. Adv Tech Stand Neurosurg. 1988;122:166.
10 Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89(4):599-609.
11 Nazarian S. Place de la chirurgie dans le traitement des metastases du rachis. Rev Chir Orthop suppl III. 1997;83:109-174.
12 Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
13 Nazzaro JM. Metstatic spinal lesions. In: Benzel E, editor. Spine surgery: techniques, complication avoidance, and management. New York: Churchill Livingstone; 1999:679-695.
14 Batson O. The role of vertebral veins in metastatic processes. Ann Intern Med. 1942;16:38-45.
15 Wetzel FT. Spine. In: Simon MA, Springfield D, editors. Surgery for bone and soft-tissue tumors. Philadelphia: Lippincott-Raven; 1998:649-670.
16 Oeppen RS, Tung K. Retrograde venous invasion causing vertebral metastases in renal cell carcinoma. Br J Radiol. 2001;74(884):759-761.
17 Oge HK, Aydin S, Cagavi F, et al. Migration of pacemaker lead into the spinal venous plexus: case report with special reference to Batson’s theory of spinal metastasis. Acta Neurochir (Wien). 2001;143(4):413-416.
18 O’Rourke T, George CB, Redmond J3rd, et al. Spinal computed tomography and computed tomographic metrizamide myelography in the early diagnosis of metastatic disease. J Clin Oncol. 1986;4(4):576-583.
19 Arguello F, Baggs RB, Duerst RE, et al. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
20 Antunes NL. Back and neck pain in children with cancer. Pediatr Neurol. 2002;27(1):46-48.
21 Hatrick NC, Lucas JD, Timothy AR, et al. The surgical treatment of metastatic disease of the spine. Radiother Oncol. 2000;56(3):335-339.
22 Bilsky MH, Lis E, Raizer J, et al. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459-469.
23 Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine. 1985;10(1):19-20.
24 Healey JH, Brown HK. Complications of bone metastases: surgical management. Cancer. 2000;88(12 Suppl):2940-2951.
25 Sorensen S, Borgesen SE, Rohde K, et al. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer. 1990;65(7):1502-1508.
26 Perrin R. Metastatic tumors of the axial spine. Curr Opin Oncol. 1992;4:525-532.
27 Dumontet C, Drai J, Bienvenu J, et al. Profiles and prognostic values of LDH isoenzymes in patients with non-Hodgkin’s lymphoma. Leukemia. 1999;13(5):811-817.
28 Sarobe P, Huarte E, Lasarte JJ, et al. Carcinoembryonic antigen as a target to induce anti-tumor immune responses. Curr Cancer Drug Targets. 2004;4(5):443-454.
29 Schiff D. Clinical features and diagnosis of epidural spinal cord compression, including cauda equina syndrome. UpToDate Online 2003.
30 Duncker CM, Carrio I, Berna L, et al. Radioimmune imaging of bone marrow in patients with suspected bone metastases from primary breast cancer. J Nucl Med. 1990;31(9):1450-1455.
31 Carmody RF, Yang PJ, Seeley GW, et al. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
32 Sevick RJ. Cervical spine tumors. Neuroimaging Clin N Am. 1995;5(3):385-400.
33 Sze G, Krol G, Zimmerman RD, et al. Malignant extradural spinal tumors: MR imaging with Gd-DTPA. Radiology. 1988;167(1):217-223.
34 Hiraga T, Mundy GR, Yoneda T. Bone metastases-morphology. In: Rubins R, Mundy GR, editors. Cancer and the skeleton. London: Martin Danitz; 2000:65-74.
35 Bohdiewicz PJ, Wong CY, Kondas D, et al. High predictive value of F-18 FDG PET patterns of the spine for metastases or benign lesions with good agreement between readers. Clin Nucl Med. 2003;28(12):966-970.
36 Nair N, Ali A, Green AA, et al. Response of osteosarcoma to chemotherapy. Evaluation with F-18 FDG-PET scans. Clin Positron Imaging. 2000;3(2):79-83.
37 Babu NV, Titus VT, Chittaranjan S, et al. Computed tomographically guided biopsy of the spine. Spine. 1994;19(21):2436-2442.
38 Minart D, Vallee JN, Cormier E, et al. Percutaneous coaxial transpedicular biopsy of vertebral body lesions during vertebroplasty. Neuroradiology. 2001;43(5):409-412.
39 Ozsarlak O, De Schepper AM, Wang X, et al. CT-guided percutaneous needle biopsy in spine lesions. JBR-BTR. 2003;86(5):294-296.
40 Hadjipavlou AG, Kontakis GM, Gaitanis JN, et al. Effectiveness and pitfalls of percutaneous transpedicle biopsy of the spine. Clin Orthop. 2003;411:54-60.
41 Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78(5):656-663.
42 Schwartz HS, Spengler DM. Needle tract recurrences after closed biopsy for sarcoma: three cases and review of the literature. Ann Surg Oncol. 1997;4(3):228-236.
43 Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer. 1975;36(2):590-594.
44 Mootoosamy IM, Anchor SC, Dacie JE. Expanding osteolytic bone metastases from carcinoma of the breast: an unusual appearance. Skeletal Radiol. 1985;14(3):188-190.
45 Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994;32(1):73-84.
46 Matthews M. Lung cancer: Natural history, prognosis, and therapy. In: Israel L, editor. Problems in morphology and behavior of bronchopulmonary malignant disease. New York: Academic Press; 1976:23-62.
47 Naidich TP, Doundoulakis SH, Poznanski AK. Intraspinal masses: efficacy of plain spine radiography. Pediatr Neurosci. 1985;12(1):10-17.
48 Galasko CSB, Spilsbury JB. Treatment of secondary spinal tumours. In: Poitout, editor. Bone Metastases. London: Springer; 2002:115-128.
49 Jorgens J. The radiographic characteristics of carcinoma of the prostate. Surg Clin North Am. 1965;45(6):1427-1440.
50 Dorfman HD. Metastatic tumors in bone. St. Louis: Mosby, 1998.
51 Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
52 King GJ, Kostuik JP, McBroom RJ, et al. Surgical management of metastatic renal carcinoma of the spine. Spine. 1991;16(3):265-271.
53 Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
54 Sundaresan N, Scher H, DiGiacinto GV, et al. Surgical treatment of spinal cord compression in kidney cancer. J Clin Oncol. 1986;4(12):1851-1856.
55 Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29(5):645-650.
56 Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer. 1995;75(1 Suppl):154-170.
57 Brown PD, Stafford SL, Schild SE, et al. Metastatic spinal cord compression in patients with colorectal cancer. J Neurooncol. 1999;44(2):175-180.
58 Enzmann D, De La Paz RL. Tumor. In: Achenbach F, editor. Magnetic resonance of the spine. St. Louis: CV Mosby; 1990:301-422.
59 Valderrama J, Bullough PG. Solitary myeloma of the spine. J Bone Joint Surg [Br]. 1988;50B:82-90.
60 Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16(6):2038-2044.
61 Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
62 Sorensen S, Helweg-Larsen S, Mouridsen H, et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
63 Heimdal K, Hirschberg H, Slettebo H, et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
64 Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(Suppl 2):S202-S213.
65 Schiff D. Treatment and prognosis of epidural spinal cord compression, including cauda equina syndrome. UpToDate Online 2003.
66 Danzig LA, Resnick D, Akeson WH. The treatment of cervical spine metastasis from the prostate with a Halo cast. Spine. 1980;5(5):395-398.
67 Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
68 Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT and US-guided radio-frequency ablation. Radiology. 2002;224(1):87-97.
69 Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15(7):707-712.
70 Poggi G, Gatti C, Melazzini M, et al. Percutaneous ultrasound-guided radiofrequency thermal ablation of malignant osteolyses. Anticancer Res. 2003;23(6D):4977-4983.
71 Gronemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002;8(1):33-39.
72 Rosenthal DI, Hornicek FJ, Torriani M, et al. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
73 Shi H, Jin Z, Suh DC, et al. Preoperative transarterial embolization of hypervascular vertebral tumor with permanent particles. Chin Med J (Engl). 2002;115(11):1683-1686.
74 Prabhu VC, Bilsky MH, Jambhekar K, et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg Spine. 2003;98(2):156-164.
75 Treves R, Legoff JJ, Doyon D, et al. [Therapeutic or palliative embolization aimed at analgesia for bone metastases of renal origin]. Rev Rhum Mal Osteoartic. 1984;51(1):1-5.
76 O’Reilly GV, Kleefield J, Klein LA, et al. Embolization of solitary spinal metastases from renal cell carcinoma: alternative therapy for spinal cord or nerve root compression. Surg Neurol. 1989;31(4):268-271.
77 Chiras J, Adem C, Vallee JN, et al. Selective intra-arterial chemoembolization of pelvic and spine bone metastases. Eur Radiol. 2004;14(10):1774-1780.
78 Lackman R, Hosalkar HS, Khoury LD, et al. Podium presentation: Serial arterial embolization for sacral giant cell tumors. MSTS annual meeting, Long Beach, CA; 2004.
79 Lackman RD, Khoury LD, Esmail A, et al. The treatment of sacral giant-cell tumours by serial arterial embolisation. J Bone Joint Surg [Br]. 2002;84(6):873-877.
80 Barr JD, Barr MS, Lemley TJ, et al. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;25(8):923-928.
81 Deramond H, Depriester C, Toussaint P, et al. Percutaneous vertebroplasty. Sem Musculoskelet Radiol. 1997;1(2):285-296.
82 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg Spine. 2003;98(1):21-30.
83 Wenger M. Vertebroplasty for metastasis. Med Oncol. 2003;20(3):203-209.
84 Hall AJ, Mackay NN. The results of laminectomy for compression of the cord or cauda equina by extradural malignant tumour. J Bone Joint Surg [Br]. 1973;55(3):497-505.
85 Cobb CA. Indications for nonoperative treatment of spinal cord compression due to breast cancer. J Neurosurg. 1977;47(5):653-658.
86 White A, Panjabi MM. Biomechanical considerations in the surgical management of the spine. Part III. Surgical constructs employing methylmethacrylate. In: White A, Panjabi MM, editors. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1978:424-425.
87 Bilsky MH, Shannon FJ, Sheppard S, et al. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine. 2002;27(10):1062-1069.
88 Boriani S, De Lure F. Bone tumors of the spine and epidural cord compression: treatment options. Sem Spine Surg. 1995;7:317-322.
89 Vieweg U, Meyer B, Schramm J. Tumour surgery of the upper cervical spine – a retrospective study of 13 cases. Acta Neurochir (Wien). 2001;143(3):217-225.
90 Rao S, Badani K, Schildhauer T, et al. Metastatic malignancy of the cervical spine. A nonoperative history. Spine. 1992;17(10 Suppl):S407-S412.
91 Wang GJ, Reger SI, McLaughlin RE, et al. The safety of cement fixation in the cervical spine. Studies of a rabbit model. Clin Orthop. 1979;139:276-282.
92 Klimo PJr, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9(2):188-196.
93 Wai EK, Finkelstein JA, Tangente RP, et al. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28(5):508-512.
94 Wise JJ, Fischgrund JS, Herkowitz HN, et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine. 1999;24(18):1943-1951.
95 Harrington KD. Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop. 1988;233:177-197.
96 Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop. 1982;169:95-102.
97 Sundaresan N, Galicich JH, Lane JM. Harrington rod stabilization for pathological fractures of the spine. J Neurosurg. 1984;60(2):282-286.
98 Martenson JAJr, Evans RG, Lie MR, et al. Treatment outcome and complications in patients treated for malignant epidural spinal cord compression (SCC). J Neurooncol. 1985;3(1):77-84.
99 Sundaresan N, Bains M, McCormack P. Surgical treatment of spinal cord compression in patients with lung cancer. Neurosurgery. 1985;16(3):350-356.
100 McLain RF, Bell GR. Newer management options in patients with spinal metastasis. Cleve Clin J Med. 1998;65(7):356-359.
101 Ryken TC, Eichholz KM, Gerszten PC, et al. Evidence-based review of the surgical management of vertebral column metastatic disease. Neurosurg Focus. 2003;15(5):E11.
102 Ratliff JK, Cooper PR. Metastatic spine tumors. South Med J. 2004;97(3):246-253.